Abstract
End-stage kidney disease is a strong risk factor for cardiovascular-specific mortality. Polyphenol-rich interventions may attenuate cardiovascular disease risk factors; however, this has not been systematically evaluated in the hemodialysis population. Using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, the following databases were searched: Cochrane Library (http://www.cochranelibrary.com/), MEDLINE (https://health.ebsco.com/products/medline-with-full-text), Embase (https://www.elsevier.com/solutions/embase-biomedical-research), and CINAHL (https://www.ebscohost.com/nursing/products/cinahl-databases/cinahl-complete). Meta-analyses were conducted for measures of lipid profile, inflammation, oxidative stress, and blood pressure. Risk of bias was assessed using the Cochrane Collaboration Risk of Bias tool and quality of the body of evidence was assessed by the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) methodology. Twelve studies were included for review. Polyphenol-rich interventions included soy, cocoa, pomegranate, grape, and turmeric. Polyphenol-rich interventions significantly improved diastolic blood pressure (Mean Difference (MD) −5.62 mmHg (95% Confidence Interval (CI) −8.47, −2.78); I2 = 2%; p = 0.0001), triglyceride levels (MD −26.52 mg/dL (95% CI −47.22, −5.83); I2 = 57%; p = 0.01), and myeloperoxidase (MD −90.10 (95% CI −135.84, −44.36); I2 = 0%; p = 0.0001). Included studies generally had low or unclear risks of bias. The results of this review provide preliminary support for the use of polyphenol-rich interventions for improving cardiovascular risk markers in haemodialysis patients. Due to the limited number of studies for individual polyphenol interventions, further studies are required to provide recommendations regarding individual polyphenol intervention and dose.
Keywords: polyphenol, dialysis, cardiovascular, review, meta-analysis, oxidative stress, inflammation, blood pressure, pomegranate, cocoa, soy, turmeric
1. Introduction
End-stage kidney disease (ESKD) is a major health burden worldwide, with over 2 million people estimated to be receiving renal replacement therapy [1]. Among those with ESKD, cardiovascular disease (CVD) accounts for almost 50% of all deaths, most commonly sudden cardiac death [2]. Many factors are known to influence the elevated CVD risks in ESKD, including high blood pressure, dyslipidaemia and high levels of oxidative stress [3,4]. The uraemic state, which causes increased production of pro-inflammatory cytokines and promotes oxidative stress, may trigger the onset and progression of atherosclerosis and CVD [5]. While adequate dialysis therapy ameliorates the accumulation of uremic toxin and pro-inflammatory cytokines, the dialysis process itself can induce a chronic state of inflammation [6]. This can be further compromised by the loss of key antioxidants during haemodialysis [7], which further exacerbates inflammation and therefore, increases the risk of CVD in dialysis patients.
Lifestyle modification, including adherence to a cardio-protective diet may provide potential improvements in CVD risk factors in dialysed ESKD patients [8]. However, common limitations to developing nutrition management plans in dialysis, particularly haemodialysis, arise when attempting to implement a cardio-protective diet [9]. Many nutrient restrictions placed on haemodialysis patients have the knock-on effect of limiting antioxidant vitamins (e.g., ascorbic acid, tocopherols), minerals (e.g., selenium), and various non-nutritive polyphenols, which may be attributable to the commonly higher levels of potassium in nutrient-rich fruit and vegetables [10]. Therefore, a low risk dietary intervention which may improve intake of potentially cardioprotective compounds may improve CVD outcomes in the haemodialysis patient.
A healthy dietary pattern, such as the Mediterranean and Dietary Approaches to Stop Hypertension (DASH) diet, are associated with reduced risk of death in renal disease [8,11]. One of the proposed mechanisms of mediated risk is through higher intake of fruits and vegetables, which are inherently cardio-protective due to their higher levels of dietary fibre, antioxidants, and lower renal acid load [10,11]. In addition, plant-based diets provide an abundant source for a large number of non-nutrient phytochemicals such as carotenoids and polyphenols [12,13].
Polyphenols, present only in plant-based foods, have been associated with reductions in cardiovascular disease and related chronic diseases in large observational studies [14,15,16,17]. Examples of food sources of polyphenols include various berries (hydroxybenzoic & hydroxycinnamic acids), grapes and currants (anthocyanins), onions and kale (flavonols), parsley and celery (flavones), soy products (isoflavones), and fruit juices (flavanones) [18]. The potential mechanisms of action responsible for these cardioprotective effects include their antioxidant and anti-inflammatory properties [19]. Polyphenols may also influence cholesterol levels through modulation of hepatic cholesterol metabolism [20]. Furthermore, animal studies have demonstrated reductions in blood pressure after polyphenol consumption that was associated with endothelium-dependent relaxation and induction of gene expression related to nitric oxide synthase [21].
In haemodialysis supplementation studies, key vitamins have demonstrated improvements in (non-polyphenol) antioxidant activity, such as Vitamin C [22] and Vitamin E supplementation [23,24]. While other polyphenol-rich interventions have shown promise to control oxidative stress and ameliorate inflammation in ESKD patients, for example, grape juice powder [25], pomegranate juice [26], turmeric [27], and cocoa flavanols [28].
To date, the effects of polyphenol-rich interventions on CVD risk markers is mixed and no systematic review has specifically evaluated nor pooled the effect of polyphenols on CVD outcomes in dialysis patients. Therefore, the aim of this review was to systematically evaluate the literature from existing randomised controlled trials on polyphenol-rich interventions (food and products) and how it affects CVD markers in haemodialysis populations.
2. Methods
2.1. Literature Search
This review is written in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [29]. Relevant studies were identified through a systematic search of the Cochrane Library, MEDLINE (via Scopus), Embase, and CINAHL databases for articles published since journal inception up to the 29 June 2017. Search terms (including mapping to appropriate MeSH terms where appropriate) described major polyphenol classes (‘polyphenol’; ‘phenol’; ‘flavonoid’; ‘flavone’; ‘flavonol*’; ‘isoflavon*’; ‘hydroxycinnamic’; ‘hydroxybenzoic’) and common polyphenol-rich foods (‘Juice’; ‘wine’, ‘tea’; ‘olive’; ‘cacao’; ‘berry’; ‘herb’; ‘spice’; ‘plant’; ‘soy’; ‘flax’; ‘nut’; ‘mint’); in combination with keywords relating to dialysis (‘dialysis’; ‘end-stage renal’; ‘end-stage kidney’; haemodialysis’; ‘peritoneal’; ‘renal failure’; ‘kidney failure’; ESKD’ referring to End Stage Kidney Disease; ‘ESRD’ referring to End Stage Renal Disease).
Studies were eligible for inclusion if they (1) used a double blind, randomized, placebo-controlled trial study design; (2) had no concurrent intervention; (3) examined the effect of a polyphenol-rich intervention on CVD outcomes (e.g., lipid profile, blood pressure, oxidative stress); and (4) recruited haemodialysis patients only. Other ESKD populations were excluded, in an attempt to keep the study population homogenous. We used the Phenol-Explorer 3.6 database to characterize and inform our decision on known polyphenol-rich interventions [30,31].
2.2. Data Extraction
The screening of articles was independently conducted by two review authors (J.K. and W.M.), with disagreements in judgement resolved by consensus or third reviewer (S.M.). Relevant articles titles and abstracts were initially screened. If deemed potentially eligible, studies were selected for full text review. Data was extracted from relevant studies using the following parameters: author/date, study design, sample size, total study period, population characteristics (including age, gender and co-morbidities), intervention characteristics (including type of polyphenol, dose and duration of exposure), length of follow up, and country of origin. For all included studies, mean, standard deviation (SD), standard error or 95% confident intervals (CI) for all pre-specified outcome data that were reported at baseline and follow-up were extracted for analysis if a significant difference was reported. Data was extracted by one reviewer (S.M.) and checked for accuracy by a second reviewer (S.N.).
2.3. Assessment of Study and Evidence Quality
Bias assessment was preformed based on the Cochrane Risk of Bias tool [32]. This tool provides criteria for assessing the quality of the included studies. All studies were included in the review regardless of bias rating. A score of ‘high’ indicated a high risk of all bias categories. A score of ‘unclear’ was given when information available was inadequate to correctly comment. A score of ‘low’ indicated low risk of all forms of bias and was the most desirable outcome.
The certainty in the body of evidence for each CVD outcome category was assessed using the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) assessment tool [33] Certainty in the body of evidence was informed by considering risk of bias, inconsistency, indirectness, imprecision, publication bias, effect size, dose-response and plausible confounding. Based on the pooled or combined data across studies informing these considerations, the certainty in the body of evidence was conserved as very low, low, moderate or high [34]. Determination of the GRADE level of evidence was determined independently by two reviewers (S.M. and J.K.), with disagreements managed by consensus and discussion with a third reviewer (W.M.).
2.4. Data Analysis
The overall treatment effect on primary and secondary outcomes was calculated as the difference between the intervention and comparison groups’ from change scores from baseline to the end of follow-up, or end of intervention values, permitting no significant differences observed at baseline between groups.
Quantitative analysis was conducted for sufficiently homogeneous and adequately reported outcome measures by pooling data into Review Manager (Version 5.3, The Cochrane Collaboration 2014) for meta-analysis using raw data. The appropriate variance from each individual study was used, either as the SD or calculated from the standard error of the mean (SEM) or 95% CI. Studies that reported on Median and Inter-Quartile-Ranges were assumed to not be normally distributed data and therefore not included in the meta-analysis. Meta-analysis was performed using the DerSimonian and Laird random-effects model [35]
The I2 statistic was used to assess the inconsistencies between studies and describe the percentage of variability in effect and data was checked using the fixed-effect model to ensure robustness and susceptibility to potential outliers. Heterogeneity was considered substantial if the I2 statistic was ≥50%. A statistically significant (p < 0.05) result was considered evidence of an effect.
3. Results
3.1. Study Selection
As shown in Figure 1, the literature search identified 3521 citations after the removal of duplicates. Initial screening identified 50 papers as potentially relevant for full text review. From this, 39 studies were excluded as they did not meet the inclusion criteria. Hand searching identified 1 additional study for inclusion, leaving 12 total studies included in the review.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) study flow diagram describing process of study selection.
3.2. Study Characteristics
Table 1 provides a summary of the study designs of included studies. The total sample size of the included studies was 520 participants, with individual study sample sizes ranging from 27 to 101 participants. All studies used a double-blind, randomized placebo-controlled parallel study design.
Table 1.
Summary of included studies.
| Study | Setting | Study Design | Population |
|---|---|---|---|
| Soy protein (contains isoflavones) | |||
| Fanti et al. 2006 [40] |
|
|
|
| Chen et al. 2005 [42] |
|
|
|
| Chen et al. 2006 [41] |
|
|
|
| Siefker & DiSilvestro 2006 [43] |
|
|
|
| Grape (contains various polyphenols) | |||
| Janiques et al. 2014 [25] |
|
|
|
| Turmeric (contains curcuminoids) | |||
| Pakfetrat et al. 2014 [38] |
|
|
|
| Pakfetrat et al. 2015 a [27] |
|
|
|
| Cocoa (contains flavanols) | |||
| Rassaf et al. 2016 [28] |
|
|
|
| Pomegranate (Phenolic acids & Flavanoids) | |||
| Shema-Didi et al. 2012 [37] |
|
|
|
| Shema-Didi et al. 2014 [36] | As per Shema-Didi et al. 2012 [37] | Shema-Didi et al. 2012 [37] | Shema-Didi et al. 2012 [37] |
| Shema-Didi et al. 2013 [26] |
|
|
|
| Wu et al. 2015 [44] |
|
|
|
CVD, Cardiovascular Disease; CG, control group; DM, Diabetes Mellitus; HTN, hyptertension; IG, intervention group; N/A, Not Applicable; a It is unclear if the sample and study is the same as that described in Pakfetrat et al. 2014 [38]. The samples appear to be the same; where Pakfetrat et al. [27] excluded some participants after recruitment, which could explain slight differences in sample characteristics. However, Pakfetrat et al. 2015 [27] does not refer to the earlier study at all, and therefore it is not clear.
3.3. Interventions
A variety of interventions were investigated including pomegranate juice (standardized to 0.7 mmol/L polyphenols) [26,36,37], pomegranate extract (standardized to 600–755 mg gallic acid equivalents), cacao (900 mg cocoa-flavanols) [28], turmeric (22.1 mg curcumin) [27,38], grape (500 mg total polyphenols) [25], green tea (455 mg total catechins) [39], and soy (26–54 mg isoflavones) (Table 2) [40,41,42,43]. The duration of the study interventions varied from an acute, one day study [26] to 12 months [36,37]. Interventions were delivered in a juice [26,36,37]; a fortified jelly [25]; drink, cereal or protein bar [40]; powder [28,41,42,43]; or capsules [27,38,44]. Most studies required participants to consume the intervention each day of the study duration with the exception of one acute study where participants consumed pomegranate juice once during the first hour of a haemodialysis session and two long term studies where the intervention was given three or four times per week [26,37,43]. One study reported results as Median and Inter-Quartile-Ranges and were assumed to not be normally distributed data and not included in the meta-analysis [40].
Table 2.
Included Study Intervention Details and Results.
| Study | Intervention | Results |
|---|---|---|
| Soy protein (contains isoflavones) | ||
| Fanti et al. 2006 [40] | Intervention: Isoflavone-containing soy-based a protein powder mixed into a drink (54 mg isoflavones), a protein bar (26 mg isoflavones) or cereal product (26 mg isoflavones). Comparator: Isoflavone-free milk-based protein powder mixed into a drink, a protein bar or a cereal product. Energy, protein, CHO, fat, Na and K intake equal to intervention product. Dose: one supplement item daily (powder 3 times per week; bar or cereal 4 times per week). Duration: 8-weeks. |
Pro-inflammatory markers: 8-weeks post-baseline: Serum CRP
|
| Chen et al. 2005 [42] | Intervention: 30 g isolated soy protein (36.3 mg isoflavone content; as reported in Chen et al. 2006 [41]) fortified with calcium, mixed with 200 mL fluid. Comparator: 30 g milk protein, with equivalent amounts of calcium, energy, carbohydrate and fat; mixed with 200 mL fluid. Dose: one supplement daily Duration: 12-weeks. |
Normolipidemic Subjects—Lipid profile: 12-weeks post-baseline: Fasting serum TG:
Fasting serum TG:
|
| Chen et al. 2006 [41] | Supplement: 30 g isolated soy protein (36.3 mg isoflavone content) fortified with calcium, mixed with 200 mL fluid. Comparator: 30 g milk protein, with equivalent amounts of protein, calcium, energy, carbohydrate and fat; mixed with 200 mL fluid. Dose: one supplement daily Duration: 12-weeks. |
Lipid profile: 12-weeks post-baseline: Fasting serum TG:
|
| Siefker & DiSilvestro 2006 [43] | Intervention: soy protein powder (52 mg isoflavone content) mixed with fluid, artificial chocolate flavoured. Comparator: whey protein powder mixed with fluid, artificial chocolate flavoured. Dose: one supplement 4 times per week Duration: 4-weeks. |
Oxidative stress markers: 4-weeks post-baseline: Oxidized LDL-C:
TNF-α:
|
| Grape (contains various polyphenols) | ||
| Janiques et al. 2014 [25] | Intervention: 12 g (500 mg total polyphenols) grape powder mixed into grape jelly. Comparator: grape jelly/placebo. Dose: 1 tablespoon of jelly daily consumed in the afternoon. Duration: 5-weeks. |
Anti-inflammatory markers: Not clear when measured; assumed to be measured at end of treatment (5-weeks post-baseline): Plasma GSH-Px:
Plasma CRP:
|
| Turmeric (contains curcuminoids) | ||
| Pakfetrat et al. 2014 [38] | Intervention: capsule of 500 mg (22.1 mg active ingredient curcumin, a polyphenol) turmeric powder Comparator: capsule of starch/placebo. Dose: 3 capsules per day, one with each main meal. Duration: 8-weeks |
Pro-inflammatory markers: Not clear when measured; assumed to be measured at end of treatment (8-weeks post-baseline): High-sensitivity CRP:
|
| Pakfetrat et al. 2015 [27] | Intervention: capsule of 500 mg (22.1 mg active ingredient curcumin, a polyphenol) turmeric powder Comparator: capsule of starch/placebo. Dose: 3 capsules per day, one with each main meal. Duration: 8-weeks |
Oxidative stress: 8-weeks post-baseline: Red blood cell catalase (CAT):
|
| Cocoa (contains flavanols) | ||
| Rassaf et al. 2016 [28] | Intervention: 450 mg cocoa-flavanol containing low-energy fruit-flavoured powder to be mixed as a drink. Comparator: low-energy fruit-flavoured powder to be mixed as a drink, matched for energy, protein, CHO, micronutrients, theobromine and caffeine. Dose: Two sachets (900 mg cocoa-flavanols) daily Duration: 30 days |
Haemodynamics markers: 30-days post-baseline: Flow-mediated vasodilation of the brachial artery:
Systolic blood pressure: Acute
Diastolic blood pressure:
Systolic blood pressure:
IL-6:
Oxidized LDL-C:
|
| Pomegranate (Phenolic acids & Flavonoids) | ||
| Shema-Didi et al. 2012 [37] | Intervention: Commercial pomegranate juice (0.7 mmol polyphenols/100 mL juice). Comparator: Placebo juice containing artificial pomegranate extract, confirmed no polyphenol content. Dose: 100 mL, three times per week. Duration: 12-months. |
Oxidative stress: 12-months post-baseline: CD11b:
Serum IL-6:
Common carotid intima-media thickness:
|
| Shema-Didi et al. 2014 [36] | Shema-Didi et al. 2012 [37] |
Haemodynamics markers: 12-months post-baseline: Systolic blood pressure:
LDL-C:
|
| Shema-Didi et al. 2013 [26] | Intervention: Commercial pomegranate juice (0.7 mmol polyphenols/100 mL juice). Comparator: Placebo juice containing artificial pomegranate extract, confirmed no polyphenol content. Dose: 100 mL Duration: Once during the first hour of a haemodialysis session. |
Oxidative stress markers: At the end of the dialysis session: Serum myeloperoxidase (MPO):
Serum polymorphonuclear leukocytes (PMNLs):
|
| Wu et al. 2015 [44] | Intervention: 1000 mg capsule of purified pomegranate polyphenol extract with 600–755 mg gallic acid equivalents. Comparator: Noncaloric placebo capsule not further described. Dose: 1 capsule daily Duration:6-months |
Haemodynamics markers: 6-months post-baseline: Systolic blood pressure:
Oxygen radical absorbance capacity (ORAC)
CRP:
Total cholesterol:
|
AGE CML, Advanced Glycation End product carboxymethyl lysine; CG, Control Group; CRP, C-Reactive Protein; GSH-Px, Glutathione peroxidase; Hb, haemoglobin; IL, Interleukin; IG, Intervention Group; HDL-C, High Density Lipoprotein Cholesterol; LDL-C, Low Density Lipoprotein Cholesterol; TNF-α, Tumour Necrosis Factor Alpha; Total-C, Total Cholesterol; TG, Triglycerides.
3.4. Study Results
3.4.1. Oxidative Stress
Seven studies reported measures of oxidative stress [26,27,28,37,43,44]. A variety of measures were used to assess oxidative stress including advanced oxidation protein products [26,37,44], polymorphonuclear leukocyte priming [26,37], oxidized fibrinogen [37], oxidized LDL-C (Low Density Lipoprotein Cholesterol) [28,43,44] , malondialdehyde (MDA) [27,37], oxygen radical absorbance capacity (ORAC) assay [44], 8-hydroxy-20-deoxyguanosine (8-OHdG) [44], red blood cell catalase [27], glutathione reductase [27], glutathione peroxidase [25,27], and myeloperoxidase [26,37].
Pomegranate juice and extract improved markers of oxidative stress in three studies [26,37,44]. Two studies reported significant reductions in advanced oxidation protein products, polymorphonuclear leukocyte priming, myeloperoxidase [26,37]. One study reported significant reductions in oxidized fibrinogen (p = 0.03) and MDA (p < 0.001) [37], and another reported a significant interaction effect (group × time) for a measure of HDL-C (High Density Lipoprotein Cholesterol; p value not reported) associated paranoxose-1 activity [44].
Soy supplementation reduced oxidized LDL-C in one study (p < 0.05) [43], and one study reported turmeric supplementation to improve measures of catalase (p = 0.039) and MDA (p = 0.040) [27]. No other significant between-group differences in measures of oxidative stress were reported.
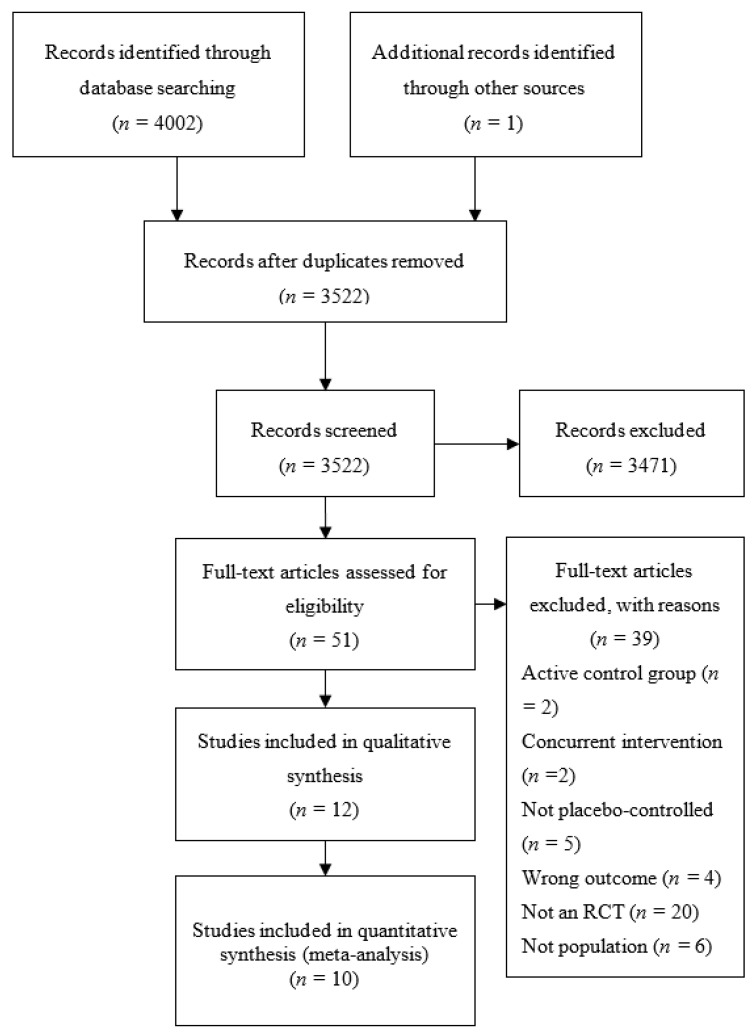
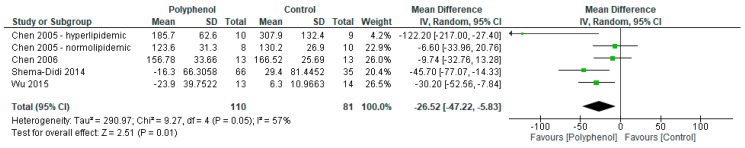
Meta-analyses reported significant improvements in myeloperoxidase (MD −90.10 (95% CI −135.84, −44.36); I2 = 0%; p = 0.0001; n = 2 studies; 1 polyphenol-rich intervention; n = 126 participants; Figure 2). There was insufficient data to conduct a meta-analysis on any other measure of oxidative stress due to insufficient numbers of common outcomes reported in the included studies.
Figure 2.
Forest plot of polyphenol-rich interventions on myeloperoxidase.
3.4.2. Inflammation
Eight studies reported measures of inflammation [25,26,28,37,38,40,43,44]. Analyzed markers include C-reactive protein [25,28,38,40,43,44], interleukin-6 [28,37,40,44], tumor necrosis factor alpha (TNF-α) [37,40,43], 8-iso-prostaglandin F2α [43]; and advanced glycation end products [28].
One study reported turmeric to reduce CRP (p = 0.012) [38] and one study reported pomegranate juice to reduce IL-6 (p < 0.001) and TNF-α (p = 0.03) [37] No other significant between-group differences in inflammatory measures were reported.
Meta-analyses reported no significant difference in CRP (MD 1.31 mg/dL (95% CI −1.11, 3.74); I2 = 85%; p = 0.29; n = 5 studies; 5 polyphenol-rich interventions; n = 195 participants), IL-6 (MD −0.94 mg/dL (95% CI −2.73, 0.85); I2 = 88%; p = 0.30; n = 3 studies; 2 polyphenol-rich interventions; n = 128 participants), and AOPP (MD −17.70 mg/dL (95% CI −46.46, 11.06); I2 = 81%; p = 0.23; n = 3 studies; 1 polyphenol-rich intervention; n = 153 participants). See Supplemental material for forest plots of each non-significant analysis.
3.4.3. Hemodynamic Measures
Four studies measured at least one of the following haemodynamic measures: flow mediated dilatation [28], augmentation index [44], pulse wave velocity [28,44], pulse pressure [36], intima-media thickness [28,37,44], and measures of blood pressure (i.e., systolic, diastolic, aortic blood pressure) [28,36,44].
Pomegranate extract significantly improved systolic and diastolic blood pressure, and mean arterial pressure (p < 0.05 reported for all measures) [44]. Cocoa flavanols significantly improved flow-mediated dilatation (p < 0.001) [28]. No other significant between-group differences were reported.
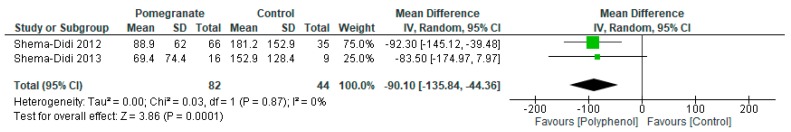
Meta-analyses reported significant improvements in diastolic blood pressure (MD −5.62 mmHg (95% CI −8.47, −2.78); I2 = 2%; p = 0.0001; n = 4 studies; 2 polyphenol-rich interventions; n = 245 participants; Figure 3) but not systolic blood pressure (MD mmHg −10.02 (95% CI −21.39, 1.35); I2 = 66%; p = 0.08; n = 4 studies; 2 polyphenol-rich interventions; n = 193 participants)
Figure 3.
Forest plot of polyphenol-rich interventions on diastolic blood pressure.
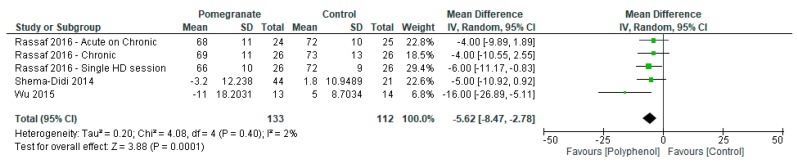
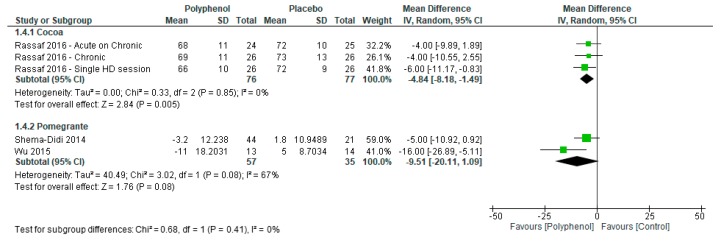
Sensitivity analyses were conducted to determine the effect of individual polyphenol-rich interventions on systolic and diastolic blood pressure. Pomegranate (−19.22 (−30.94, −7.49)) had a greater effect on systolic blood pressure compared to cacao (−0.99 (−9.65, 7.67); p = 0.01; Figure 4) whereas there was no subgroup difference between pomegranate (−9.51 (−20.11, 1.09)) and soy (−4.84 (−8.18, −1.49)) for diastolic blood pressure (p = 0.41; Figure 5).
Figure 4.
Sensitivity analysis of polyphenol-rich interventions on systolic blood pressure.
Figure 5.
Forest plot of polyphenol-rich interventions on diastolic blood pressure.
3.4.4. Lipid Profiles
Four studies reported on changes to cholesterol profile (i.e., total-C, HDL-C, LDL-C and triglycerides) following pomegranate [36,44], and soy supplementation [41,42]. Pomegranate had no significant between-group differences on participant lipid profiles except for one study that reported significant improvements in HDL-C (p = 0.03) and triglycerides (p = 0.008) for a subset of participants with low HDL-C or high triglycerides, respectively [36]. Soy supplementation improved fasting total cholesterol (p < 0.05) in one study and another study reported significant improvements on fasting triglycerides and total cholesterol in a subset of hyperlipidemic participants only (p < 0.05) [41,42].
Meta-analyses reported significant improvements in triglycerides (MD −26.52 mg/dL (95% CI −47.22, −5.83); I2 = 57%; p = 0.01; n = 4 studies; 2 polyphenol-rich interventions; n = 191 participants; Figure 6) but no significant difference in total (MD −11.24 mg/dL (95% CI −24.81, 2.34); I2 = 76%; p = 0.10; n = 4 studies; 2 polyphenol-rich interventions; n = 191 participants), HDL-C (MD 2.38 mg/dL (95% CI −0.05, 4.82); I2 = 23%; p = 0.06; n = 4 studies; 2 polyphenol-rich interventions; n = 191 participants), and LDL-C (MD mg/dL −3.31 (95% CI −14.45, 7.84); I2 = 43%; p = 0.13; n = 4 studies; 2 polyphenol-rich interventions; n = 191 participants).
Figure 6.
Forest plot of polyphenol-rich interventions on triglyceride levels.
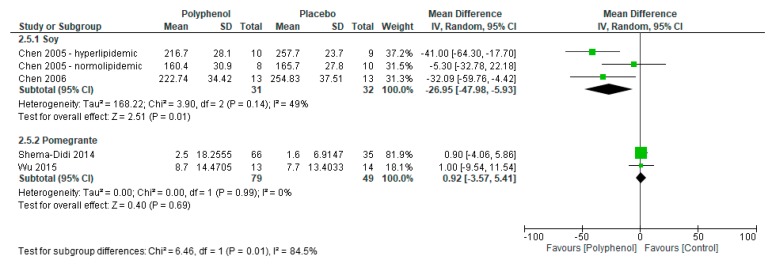
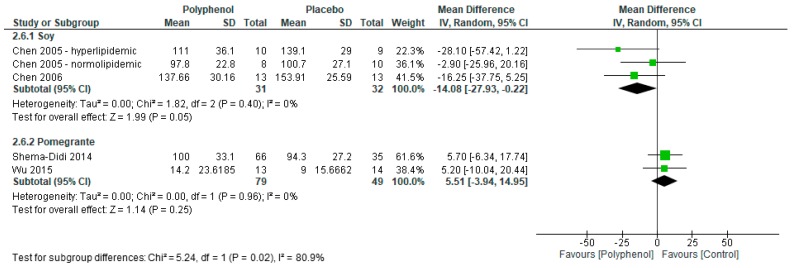
Sensitivity analyses were conducted to determine the effect of individual polyphenol-rich interventions on lipid markers. Soy had a greater effect on total cholesterol (−26.95 (−47.98, −5.93) vs. 0.92 (−3.57, 5.41); p = 0.01; Figure 7) and LDL-C (−14.08 (−27.93, −0.22) vs. 5.51 (−3.94, 14.95); p = 0.02; Figure 8) compared to pomegranate. There were no significant subgroup differences between soy and pomegranate for HDL-C (1.56 (−1.91, 5.03) vs. 3.16 (−2.23, 8.54), respectively; p = 0.63) and triglycerides (−20.29 (−54.32, 13.73) vs. −35.42 (−53.63, −17.21), respectively; p = 0.44).
Figure 7.
Sensitivity analysis of polyphenol-rich interventions on total cholesterol.
Figure 8.
Sensitivity analysis of polyphenol-rich interventions on LDL-C.
3.5. Adverse Events
Eight studies provided data on measured adverse events or irregular biochemistry [26,28,37,38,39,40,45,46]. Most studies did not report any adverse events during the intervention period. Minor gastrointestinal symptoms (e.g., constipation or diarrhea) were reported in one study [25] Severity of adverse events was only reported in one study, which reported one serious adverse event (bleeding) within the intervention group [28]. No study reported a statistical analysis to determine significant differences in adverse event rates between control and interventions arms. Three studies reported changes in potassium levels with all three studies reporting no change after grape powder [25], soy [43], and cacao [28].
3.6. Risk of Bias
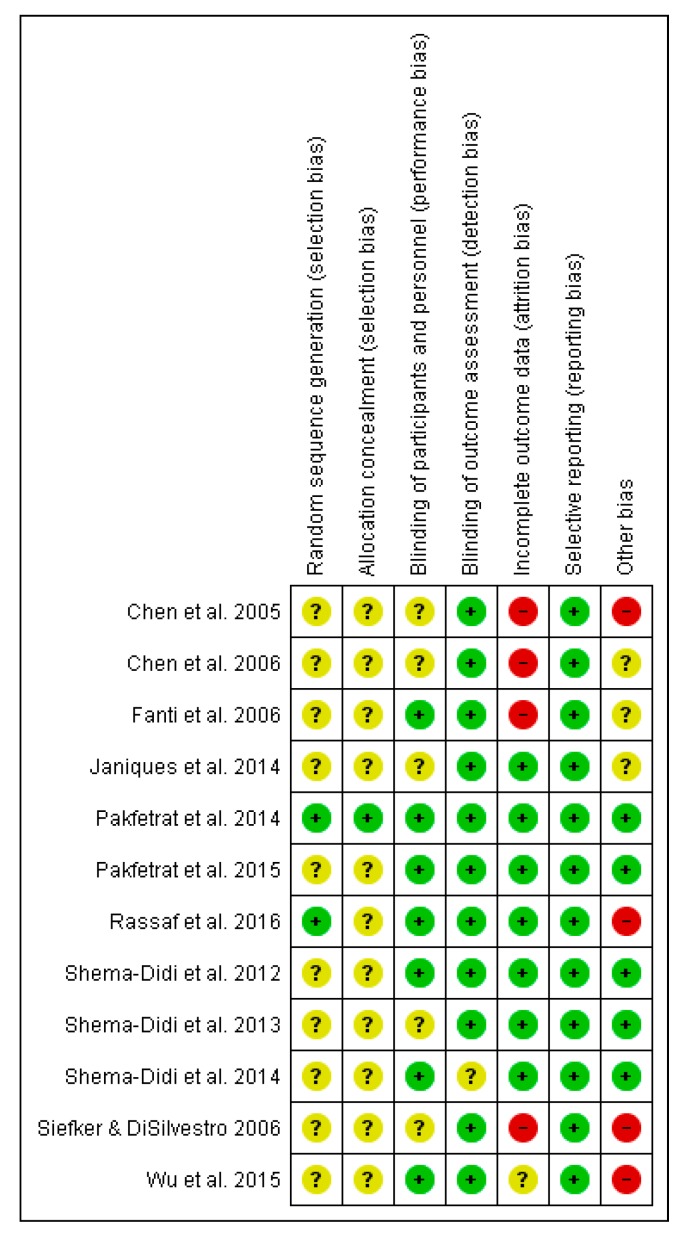
Risk of bias was low or unclear for most studies in the following domains: detection (11/12 rated as low) and reporting bias (12/12 rated as low), selection bias (10/13 and 11/12 rated as unclear). Three studies reported high risks of attrition bias and three studies were determined to have other risks of bias such as possible or undeclared conflicts of interest (Figure 9).
Figure 9.
Risk of bias summary across the included studies. Unclear risk of bias: “?”, Low of risk of bias: “+”, High risk of bias: “-“.
3.7. Quality of Evidence
Using the GRADE tool, most outcomes were rated at moderate quality (4/12) or very low (5/12) quality with inconsistency and imprecision being the most common reasons for downgrading (Table 3). Of the pooled data with significant findings, there was moderate quality of evidence for the effect on myeloperoxidase (oxidative stress marker); high quality for the effect on diastolic blood pressure, and very low quality for the effect on triglycerides.
Table 3.
Grading of Recommendations, Assessment, Development and Evaluation (GRADE) assessment of polyphenol supplementation compared to placebo for improving cardiovascular risk factors in haemodialysis patients.
| Quality Assessment | Number of Patients | Effect | Quality | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Number of Studies | Study Design | Risk of Bias | Inconsistency | Indirectness | Imprecision | Other Considerations | Polyphenol | Placebo | Absolute (95% CI) | |
| Oxidative stress (myeloperoxidase) | ||||||||||
| 2 | Randomised trials | Not serious | Not serious | Not serious | Serious a | Strong effect | 82 | 44 | MD 90.1 SD lower (135.8 lower to 44.4 lower) | MODERATE |
| Oxidative stress (other markers—not pooled) | ||||||||||
| 5 | Randomised trials | Not serious | serious b | Not serious | Serious c | None | - | - | See comment | MODERATE |
| Inflammation (CRP) | ||||||||||
| 5 | Randomised trials | Not serious | very serious d | Not serious | Serious e | None | 96 | 99 | MD 1.9 mg/dL higher (2.2 lower to 6.1 higher) | VERY LOW |
| Inflammation (IL-6) | ||||||||||
| 2 | Randomised trials | Not serious | very serious d | Not serious | Serious e | Both pomegranate studies | 79 | 49 | MD 1.6 mg/dL lower (5.1 lower to 1.96 higher) | VERY LOW |
| Inflammation (AOPP) | ||||||||||
| 3 | Randomised trials | Not serious | very serious d | Not serious | Serious e | All pomegranate studies | 96 | 57 | MD 17.7 mg/dL lower (46.5 lower to 11.1 higher) | VERY LOW |
| Diastolic blood pressure | ||||||||||
| 4 | Randomised trials | Not serious | Not serious | Not serious | Not serious | None | 133 | 112 | MD 5.6 mmHg lower (8.47 lower to 2.78 lower) | HIGH |
| Systolic blood pressure | ||||||||||
| 4 | Randomised trials | Not serious | Serious f | Not serious | Serious e | None | 107 | 86 | MD 10 mmHg lower (21.4 lower to 1.4 higher) | LOW |
| Hemodynamic measures (other markers—not pooled) | ||||||||||
| 4 | Randomised trials | Not serious | Serious g | Not serious | Serious h | None | - | - | Not pooled. See Table 2 | LOW |
| Lipid profile (TG) | ||||||||||
| 4 | Randomised trials | Not serious | Serious f | Not serious | Very serious e | None | 110 | 81 | MD 26.5 mg/dL lower (47.2 lower to 5.8 lower) | VERY LOW |
| Lipid profile (Total-C) | ||||||||||
| 4 | Randomised trials | Not serious | Very serious d | Not serious | Very serious e | None | 110 | 81 | MD 11.2 mg/dL lower (24.8 lower to 2.3 higher) | VERY LOW |
| Lipid profile (HDL-C) | ||||||||||
| 4 | Randomised trials | Not serious | Not serious | Not serious | Serious e | None | 110 | 81 | MD 2.4 mg/dL higher (0.1 lower to 4.8 higher) | MODERATE |
| Lipid profile (LDL-C) | ||||||||||
| 4 | Randomised trials | Not serious | Not serious | Not serious | Serious e | None | 110 | 81 | MD 3.3 mg/dL lower (14.5 lower to 7.8 higher) | MODERATE |
CI: Confidence interval; dL: decilitre; HDL-C, High Density Lipoprotein Cholesterol; LDL-C, Low Density Lipoprotein Cholesterol; MD: Mean difference; Total-C, Total Cholesterol; TG, Triglycerides. a Two studies were pooled in a meta-analysis for the outcome of myeloperoxidase. The 95%CI for the outcome was substantial (−135 to −44). b Although most measures of oxidative stress showed improvement from baseline in the intervention group; a number of control groups also showed improvement in this outcome. Therefore, decreasing confidence in the consistency of results for this outcome. c Although some had narrow measures of variance, other did show substantial imprecision in their measures of variance. d Heterogeneity was high (>70%). e The 95%CI was substantial. f Heterogeneity was substantial (50–70%). g Studies showed some improvement in mean arterial pressure and flow-mediated dilation; but other studies found no improvement. No improvement was seen in other measures. h Although individual studies and outcomes did not show significant variance; there is overall a small number of participants for each outcome as well as combined. This decreases confidence in the precision of the effects.
4. Discussion
The aim of this systematic literature review was to synthesise results from existing randomized controlled trials to evaluate the effect of polyphenol-rich interventions on cardiovascular markers in haemodialysis patients. The results of individual studies included in this review indicate that polyphenol-rich interventions may improve cardiovascular risk in patients on haemodialysis by improving various markers of inflammation (i.e., CRP, IL-6, TNF-α), lipid profile (i.e., HDL-C and triglycerides), blood pressure, and oxidative stress (i.e., advanced oxidation protein products, polymorphonuclear leukocyte priming, myeloperoxidase, oxidized fibrinogen, catalase, glutathione peroxidase, and MDA); with varying effect sizes and precision across studies.
Despite individual studies reporting significant improvements, pooled results report no effect for most outcomes excepting myeloperoxidase, diastolic blood pressure and triglycerides. Only myeloperoxidase, a measure of oxidative stress, had a large pooled effect size. In addition, using the GRADE assessment, most outcomes were rated as moderate or very low quality which provides limited confidence that the effect sizes reported in the existing evidence is representative of the true effect. The exception is for diastolic blood pressure, which was rated as high quality.
Individual studies that investigated cacao [28], pomegranate [26,36,37,44], turmeric [27,38], and soy [41,43], reported significant improvements in cardiovascular measures. Sensitivity analyses indicate that some polyphenol-rich interventions may provide greater improvements in cardiovascular markers. However, due to the small number of available studies investigating individual interventions in the haemodialysis population, it is premature to conclude superiority of one polyphenol-rich intervention over another at this time. In addition, while polyphenol-rich interventions reported significant improvements in numerous cardiovascular markers, there was little consistency in reported outcomes between studies that measured the same outcome and/or used the same intervention (e.g., blood pressure in [36,37]). Hence, future studies are required to expand the currently limited evidence base and to address such limitations.
The low baseline levels of some cardiovascular markers may be a possible explanation for the null findings and/or small effect sizes reported in some included studies and pooled data as it may be unlikely that further reductions are possible. For example, Janiques et al. [25] reported no significant difference in CRP; however, reported baseline levels (range: 2.6–2.6 mg/dL) were in the normal range (<3 mg/dL). In contrast, Paketrat et al. [38] reported significant reductions in CRP in participants that had CRP levels above the normal range (range: 7.0–10.8 mg/dL). This is also supported by the results of Wu et al. [44], Shema-did et al. [36], and Chen et al. [42] that reported greater decreases in blood pressure or cholesterol measures in hypertensive or hyperlipidemic participants, respectively.
Due to the large number of foods that contain appreciable levels of polyphenols [30], the habitual diet of participants may be a significant influence on study results, if not appropriately controlled for. Few studies included in this review implemented measures to control for this; however, future studies may benefit from implementing methods such as recording habitual diet throughout the study through the use of food diaries and research dietitians as well as educating participants on high polyphenol foods to avoid during the trial duration.
Few adverse events (predominantly gastrointestinal complains, one significant bleeding event reported [28]) were reported during the included trials which provide preliminary evidence for polyphenol-rich interventions being relatively safe within the haemodialysis population. However, due to the additional dietary restrictions present in this population, close monitoring for adverse events are required with clinical use and future trials are required to further evaluate their safety. In particular, although not reported to significantly affect patients in the included studies, consumption of certain polyphenol-rich food items, such as pomegranate juice, can significantly increase potassium intake beyond what would be typically advised for dialysis patients and therefore, care should be taken with people with history of or at higher risks of hyperkalaemia.
A further consideration for future research is to address the poor bioavailability of specific polyphenols. Resveratrol and curcumin (found in turmeric) [45,46], for example, have been demonstrated in pharmacokinetic studies to have poor bioavailability and a short half-life which has been addressed in several studies by using various methods such as nanoencapsulation, lipid emulsions, and co-administering active compounds that interact with liver enzymes involved in drug metabolism [46,47]. Addressing limitations with bioavailability may provide greater treatment efficacy.
A related research area is to elucidate potential inter-individual differences in polyphenol metabolism as this will inform which patients are likely to benefit from polyphenol-rich interventions. Individual differences in gastrointestinal microbiota appear to significantly influence the metabolism of certain polyphenols [48]. For example, the soy isoflavone, daidzein, is metabolised to (S)-equol in only 25–60% of the population [49]. Metabolism of ellagic acid, found in foods such as pomegranate and berries, can also be affected by microbiota composition, affecting timing, quantity, and types of metabolites excreted [50]. The role of microbiota on polyphenol metabolism in patients with kidney disease may be further complicated due to the possible influence of chronic kidney disease on intestinal microbiota [51,52].
This review includes studies that have used polyphenol-rich interventions. However, food interventions are comprised of several bioactive nutritive (e.g., vitamins and mineral) and non-nutritive compounds (e.g., polyphenols) and therefore, the results of the included studies may have been influenced by these additional compounds. Future trials that use standardized polyphenol extracts are recommended to control for the influence of non-polyphenol compounds.
The findings of this study provide preliminary evidence regarding polyphenol-rich interventions; however, results and conclusions are limited by the heterogeneity of interventions, dosages, and durations as well as variability in the cardiovascular risk of included participants. Although polyphenol-rich interventions have reported benefits in non-ESKD patients, considering the inclusion criteria of this review, generalising results to patients with ESKD or chronic kidney disease who are not receiving dialysis should be avoided until further studies are conducted. Furthermore, studies with large sample sizes are required to sufficiently evaluate the adverse events of polyphenol-rich interventions in this population group.
5. Conclusions
This review evaluated the clinical evidence of various polyphenol-rich interventions for patients with ESKD receiving haemodialysis from double-blind placebo-controlled randomized trials. The results of this review provide preliminary support for the use of polyphenol-rich interventions as part of cardiovascular disease prevention and/or management in haemodialysis patients. At this stage, no specific polyphenol-rich intervention appears superior, which is likely due to the small number of available studies, small sample sizes, and lack of control of habitual diet. With this in mind, clinical recommendations are premature until further evidence addresses these limitations.
Author Contributions
W.M. was involved in all processes of the review, J.K. was provided the meta-analyses and GRADE assessment, S.M. contributed to the GRADE assessment and data extraction, S.N. contributed to the initial literature search, K.C. provided content support into the manuscript, C.I. was involved in the design of the study. All authors contributed to the development of the study manuscript.
Conflicts of Interest
The authors have no conflicts of interest to declare.
References
- 1.Couser W.G., Remuzzi G., Mendis S., Tonelli M. The contribution of chronic kidney disease to the global burden of major noncommunicable diseases. Kidney Int. 2011;80:1258–1270. doi: 10.1038/ki.2011.368. [DOI] [PubMed] [Google Scholar]
- 2.De Jager D.J., Grootendorst D.C., Jager K.J., van Dijk P.C., Tomas L.M., Ansell D., Collart F., Finne P., Heaf J.G., De Meester J., et al. Cardiovascular and noncardiovascular mortality among patients starting dialysis. JAMA. 2009;302:1782–1789. doi: 10.1001/jama.2009.1488. [DOI] [PubMed] [Google Scholar]
- 3.Stenvinkel P., Carrero J.J., Axelsson J., Lindholm B., Heimbürger O., Massy Z. Emerging biomarkers for evaluating cardiovascular risk in the chronic kidney disease patient: How do new pieces fit into the uremic puzzle? Clin. J. Am. Soc. Nephrol. 2008;3:505–521. doi: 10.2215/CJN.03670807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stenvinkel P., Larsson T.E. Chronic kidney disease: A clinical model of premature aging. Am. J. Kidney Dis. 2013;62:339–351. doi: 10.1053/j.ajkd.2012.11.051. [DOI] [PubMed] [Google Scholar]
- 5.Santoro A., Mancini E. Cardiac effects of chronic inflammation in dialysis patients. Nephrol. Dial. Transplant. 2002;17:10–15. doi: 10.1093/ndt/17.suppl_8.10. [DOI] [PubMed] [Google Scholar]
- 6.Carrero J.J., Stenvinkel P. Inflammation in End-Stage Renal Disease—What Have We Learned in 10 Years? Semin. Dial. 2010;23:498–509. doi: 10.1111/j.1525-139X.2010.00784.x. [DOI] [PubMed] [Google Scholar]
- 7.Morena M., Delbosc S., Dupuy A.M., Canaud B., Cristol J.P. Overproduction of reactive oxygen species in end-stage renal disease patients: A potential component of hemodialysis-associated inflammation. Hemodial. Int. 2005;9:37–46. doi: 10.1111/j.1492-7535.2005.01116.x. [DOI] [PubMed] [Google Scholar]
- 8.Kelly J.T., Palmer S.C., Wai S.N., Ruospo M., Carrero J.J., Campbell K.L., Strippoli G.F. Healthy Dietary Patterns and Risk of Mortality and ESRD in CKD: A Meta-Analysis of Cohort Studies. Clin. J. Am. Soc. Nephrol. 2017;12:272–279. doi: 10.2215/CJN.06190616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luis D., Zlatkis K., Comenge B., Garcia Z., Navarro J.F., Lorenzo V., Carrero J.J. Dietary Quality and Adherence to Dietary Recommendations in Patients Undergoing Hemodialysis. J. Ren. Nutr. 2016;26:190–195. doi: 10.1053/j.jrn.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 10.Kelly J.T., Rossi M., Johnson D.W., Campbell K.L. Beyond Sodium, Phosphate and Potassium: Potential Dietary Interventions in Kidney Disease. Semin. Dial. 2017;30:197–202. doi: 10.1111/sdi.12580. [DOI] [PubMed] [Google Scholar]
- 11.Chan M., Kelly J., Tapsell L. Dietary Modeling of Foods for Advanced CKD Based on General Healthy Eating Guidelines: What Should Be on the Plate? Am. J. Kidney Dis. 2017;69:436–450. doi: 10.1053/j.ajkd.2016.09.025. [DOI] [PubMed] [Google Scholar]
- 12.Grassi D., Desideri G., Ferri C. Flavonoids: Antioxidants Against Atherosclerosis. Nutrients. 2010;2:889. doi: 10.3390/nu2080889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang X., Ouyang Y., Liu J., Zhu M., Zhao G., Bao W., Hu F.B. Fruit and vegetable consumption and mortality from all causes, cardiovascular disease, and cancer: Systematic review and dose-response meta-analysis of prospective cohort studies. BMJ. 2014;349 doi: 10.1136/bmj.g4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peterson J.J., Dwyer J.T., Jacques P.F., McCullough M.L. Associations between flavonoids and cardiovascular disease incidence or mortality in European and US populations. Nutr. Rev. 2012;70:491–508. doi: 10.1111/j.1753-4887.2012.00508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tresserra-Rimbau A., Rimm E.B., Medina-Remón A., Martínez-González M.A., López-Sabater M.C., Covas M.I., Corella D., Salas-Salvadó J., Gómez-Gracia E., Lapetra J., et al. Polyphenol intake and mortality risk: A re-analysis of the PREDIMED trial. BMC Med. 2014;12:77. doi: 10.1186/1741-7015-12-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tresserra-Rimbau A., Rimm E.B., Medina-Remón A., Martínez-González M.A., de la Torre R., Corella D., Salas-Salvadó J., Gómez-Gracia E., Lapetra J., Arós F., et al. Inverse association between habitual polyphenol intake and incidence of cardiovascular events in the PREDIMED study. Nutr. Metab. Cardiovasc. Dis. 2014;24:639–647. doi: 10.1016/j.numecd.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 17.Rienks J., Barbaresko J., Nöthlings U. Association of Polyphenol Biomarkers with Cardiovascular Disease and Mortality Risk: A Systematic Review and Meta-Analysis of Observational Studies. Nutrients. 2017;9:415. doi: 10.3390/nu9040415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manach C., Scalbert A., Morand C., Rémésy C., Jiménez L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004;79:727–747. doi: 10.1093/ajcn/79.5.727. [DOI] [PubMed] [Google Scholar]
- 19.Joseph S.V., Edirisinghe I., Burton-Freeman B.M. Fruit polyphenols: A review of anti-inflammatory effects in humans. Crit. Rev. Food Sci. Nutr. 2016;56:419–444. doi: 10.1080/10408398.2013.767221. [DOI] [PubMed] [Google Scholar]
- 20.Zern T.L., West K.L., Fernandez M.L. Grape polyphenols decrease plasma triglycerides and cholesterol accumulation in the aorta of ovariectomized guinea pigs. J. Nutr. 2003;133:2268–2272. doi: 10.1093/jn/133.7.2268. [DOI] [PubMed] [Google Scholar]
- 21.Diebolt M., Bucher B., Andriantsitohaina R. Wine polyphenols decrease blood pressure, improve NO vasodilatation, and induce gene expression. Hypertension. 2001;38:159–165. doi: 10.1161/01.HYP.38.2.159. [DOI] [PubMed] [Google Scholar]
- 22.Abdollahzad H., Eghtesadi S., Nourmohammadi I., Khadem-Ansari M., Nejad-Gashti H., Esmaillzadeh A. Effect of vitamin C supplementation on oxidative stress and lipid profiles in hemodialysis patients. Int. J. Vitam. Nutr. Res. 2009;79:281–287. doi: 10.1024/0300-9831.79.56.281. [DOI] [PubMed] [Google Scholar]
- 23.Bhogade R., Suryakar A., Joshi N., Patil R. Effect of vitamin E supplementation on oxidative stress in hemodialysis patients. Indian J. Clin. Biochem. 2008;23:233–237. doi: 10.1007/s12291-008-0052-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baldi S., Innocenti M., Frascerra S., Nannipieri M., Lippi A., Rindi P., Ferrannini E. Effects of hemodialysis and vitamin E supplementation on low-density lipoprotein oxidizability in end-stage renal failure. J. Nephrol. 2013;26:549–555. doi: 10.5301/jn.5000190. [DOI] [PubMed] [Google Scholar]
- 25.Janiques A.G., Leal Vde O., Stockler-Pinto M.B., Moreira N.X., Mafra D. Effects of grape powder supplementation on inflammatory and antioxidant markers in hemodialysis patients: A randomized double-blind study. J. Bras. Nefrol. 2014;36:496–501. doi: 10.5935/0101-2800.20140071. [DOI] [PubMed] [Google Scholar]
- 26.Shema-Didi L., Kristal B., Ore L., Shapiro G., Geron R., Sela S. Pomegranate juice intake attenuates the increase in oxidative stress induced by intravenous iron during hemodialysis. Nutr. Res. 2013;33:442–446. doi: 10.1016/j.nutres.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 27.Pakfetrat M., Akmali M., Malekmakan L., Dabaghimanesh M., Khorsand M. Role of turmeric in oxidative modulation in end-stage renal disease patients. Hemodial. Int. 2015;19:124–131. doi: 10.1111/hdi.12204. [DOI] [PubMed] [Google Scholar]
- 28.Rassaf T., Rammos C., Hendgen-Cotta U.B., Heiss C., Kleophas W., Dellanna F., Floege J., Hetzel G.R., Kelm M. Vasculoprotective Effects of Dietary Cocoa Flavanols in Patients on Hemodialysis: A Double–Blind, Randomized, Placebo–Controlled Trial. Clin. J. Am. Soc. Nephrol. 2016;11:108–118. doi: 10.2215/CJN.05560515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gøtzsche P.C., Ioannidis J.P.A., Clarke M., Devereaux P.J., Kleijnen J., Moher D. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration. PLOS Med. 2009;6:e1000100. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perez-Jimenez J., Neveu V., Vos F., Scalbert A. Identification of the 100 richest dietary sources of polyphenols: An application of the Phenol-Explorer database. Eur. J. Clin. Nutr. 2010;64:S112–S120. doi: 10.1038/ejcn.2010.221. [DOI] [PubMed] [Google Scholar]
- 31.Rothwell J.A., Perez-Jimenez J., Neveu V., Medina-Remón A., M’Hiri N., García-Lobato P., Manach C., Knox C., Eisner R., Wishart D.S., et al. Phenol-Explorer 3.0: A major update of the Phenol-Explorer database to incorporate data on the effects of food processing on polyphenol content. Database. 2013;2013 doi: 10.1093/database/bat070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Higgins J.P.T., Green S. Cochrane Handbook for Systematic Reviews of Interventions. Volume 5 Wiley Online Library; Hoboken, NJ, USA: 2008. [Google Scholar]
- 33.Guyatt G.H., Oxman A.D., Schünemann H.J., Tugwell P., Knottnerus A. GRADE guidelines: A new series of articles in the Journal of Clinical Epidemiology. J. Clin. Epidemiol. 2011;64:380–382. doi: 10.1016/j.jclinepi.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 34.Handbook for Grading the Quality of Evidence and the Strength of Recommendations Using the GRADE Approach. [(accessed on 12 April 2017)]; Updated October 2013. Available online: http://gdt.guidelinedevelopment.org.
- 35.DerSimonian R., Laird N. Meta-analysis in clinical trials. Controll. Clin. Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 36.Shema-Didi L., Kristal B., Sela S., Geron R., Ore L. Does Pomegranate intake attenuate cardiovascular risk factors in hemodialysis patients? Nutr. J. 2014;13:18. doi: 10.1186/1475-2891-13-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shema-Didi L., Sela S., Ore L., Shapiro G., Geron R., Moshe G., Kristal B. One year of pomegranate juice intake decreases oxidative stress, inflammation, and incidence of infections in hemodialysis patients: A randomized placebo-controlled trial. Free Radic. Biol. Med. 2012;53:297–304. doi: 10.1016/j.freeradbiomed.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 38.Pakfetrat M., Basiri F., Malekmakan L., Roozbeh J. Effects of turmeric on uremic pruritus in end stage renal disease patients: A double-blind randomized clinical trial. J. Nephrol. 2014;27:203–207. doi: 10.1007/s40620-014-0039-2. [DOI] [PubMed] [Google Scholar]
- 39.Hsu S.P., Wu M.S., Yang C.C., Huang K.C., Liou S.Y., Hsu S.M., Chien C.T. Chronic green tea extract supplementation reduces hemodialysis-enhanced production of hydrogen peroxide and hypochlorous acid, atherosclerotic factors, and proinflammatory cytokines. Am. J. Clin. Nutr. 2007;86:1539–1547. doi: 10.1093/ajcn/86.5.1539. [DOI] [PubMed] [Google Scholar]
- 40.Fanti P., Asmis R., Stephenson T.J., Sawaya B.P., Franke A.A. Positive effect of dietary soy in ESRD patients with systemic inflammation—Correlation between blood levels of the soy isoflavones and the acute-phase reactants. Nephrol. Dial. Transplant. 2006;21:2239–2246. doi: 10.1093/ndt/gfl169. [DOI] [PubMed] [Google Scholar]
- 41.Chen S., Chen J., Yang C., Peng S., Ferng S. Effect of soya protein on serum lipid profile and lipoprotein concentrations in patients undergoing hypercholesterolaemic haemodialysis. Br. J. Nutr. 2006;95:366–371. doi: 10.1079/BJN20051646. [DOI] [PubMed] [Google Scholar]
- 42.Chen S., Ferng S., Yang C., Peng S., Lee H., Chen J. Variable effects of soy protein on plasma lipids in hyperlipidemic and normolipidemic hemodialysis patients. Am. J. Kidney Dis. 2005;46:1099–1106. doi: 10.1053/j.ajkd.2005.08.031. [DOI] [PubMed] [Google Scholar]
- 43.Siefker K., DiSilvestro R.A. Safety and antioxidant effects of a modest soy protein intervention in hemodialysis patients. J. Med. Food. 2006;9:368–372. doi: 10.1089/jmf.2006.9.368. [DOI] [PubMed] [Google Scholar]
- 44.Wu P.T., Fitschen P.J., Kistler B.M., Jeong J.H., Chung H.R., Aviram M., Phillips S.A., Fernhall B., Wilund K.R. Effects of Pomegranate Extract Supplementation on Cardiovascular Risk Factors and Physical Function in Hemodialysis Patients. J. Med. Food. 2015;18:941–949. doi: 10.1089/jmf.2014.0103. [DOI] [PubMed] [Google Scholar]
- 45.Walle T., Hsieh F., DeLegge M.H., Oatis J.E., Jr., Walle U.K. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab. Dispos. 2004;32:1377–1382. doi: 10.1124/dmd.104.000885. [DOI] [PubMed] [Google Scholar]
- 46.Anand P., Kunnumakkara A.B., Newman R.A., Aggarwal B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007;4:807–818. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- 47.Johnson J.J., Nihal M., Siddiqui I.A., Scarlett C.O., Bailey H.H., Mukhtar H., Ahmad N. Enhancing the bioavailability of resveratrol by combining it with piperine. Mol. Nutr. Food Res. 2011;55:1169–1176. doi: 10.1002/mnfr.201100117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Manach C., Milenkovic D., Van de Wiele T., Rodriguez-Mateos A., de Roos B., Garcia-Conesa M.T., Landberg R., Gibney E.R., Heinonen M., Tomás-Barberán F., et al. Addressing the inter-individual variation in response to consumption of plant food bioactives: Towards a better understanding of their role in healthy aging and cardiometabolic risk reduction. Mol. Nutr. Res. 2017;61 doi: 10.1002/mnfr.201600557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Setchell K.D.R., Clerici C. Equol: History, Chemistry, and Formation. J. Nutr. 2010;140:1355S–1362S. doi: 10.3945/jn.109.119776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Marín L., Miguélez E.M., Villar C.J., Lombó F. Bioavailability of Dietary Polyphenols and Gut Microbiota Metabolism: Antimicrobial Properties. BioMed Res. Int. 2015;2015:18. doi: 10.1155/2015/905215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vaziri N.D., Wong J., Pahl M., Piceno Y.M., Yuan J., DeSantis T.Z., Ni Z., Nguyen T.H., Andersen G.L. Chronic kidney disease alters intestinal microbial flora. Kidney Int. 2013;83:308–315. doi: 10.1038/ki.2012.345. [DOI] [PubMed] [Google Scholar]
- 52.Ramezani A., Massy Z.A., Meijers B., Evenepoel P., Vanholder R., Raj D.S. Role of the Gut Microbiome in Uremia: A Potential Therapeutic Target. Am. J. Kidney Dis. 2016;67:483–498. doi: 10.1053/j.ajkd.2015.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]