Whether maternal immune responses modulate tolerance in association with allergy in offspring is unknown. Ohsaki et al. demonstrate that the neonatal crystallizable fragment receptor mediates transfer and antigen presentation of maternal allergen–IgG immune complex in breast milk and drives regulatory T cell–mediated tolerance in offspring against food allergy.
Abstract
The role of maternal immune responses in tolerance induction is poorly understood. To study whether maternal allergen sensitization affects offspring susceptibility to food allergy, we epicutaneously sensitized female mice with ovalbumin (OVA) followed by epicutaneous sensitization and oral challenge of their offspring with OVA. Maternal OVA sensitization prevented food anaphylaxis, OVA-specific IgE production, and intestinal mast cell expansion in offspring. This protection was mediated by neonatal crystallizable fragment receptor (FcRn)–dependent transfer of maternal IgG and OVA immune complexes (IgG-IC) via breast milk and induction of allergen-specific regulatory T (T reg) cells in offspring. Breastfeeding by OVA-sensitized mothers or maternal supplementation with IgG-IC was sufficient to induce neonatal tolerance. FcRn-dependent antigen presentation by CD11c+ dendritic cells (DCs) in offspring was required for oral tolerance. Human breast milk containing OVA-IgG-IC induced tolerance in humanized FcRn mice. Collectively, we demonstrate that interactions of maternal IgG-IC and offspring FcRn are critical for induction of T reg cell responses and control of food-specific tolerance in neonates.
Introduction
Food allergy is a growing public health concern as it affects 5–8% of the U.S. population, has no effective cure, and can be associated with life-threatening anaphylaxis (Sicherer and Sampson, 2014). The disease is associated with CD4+ T cells that secrete Th2 cytokines, and allergen-specific IgE antibodies that activate mast cells (Metcalfe et al., 2009). Allergic reactions to foods often occur on the first known ingestion (Sicherer et al., 1998), suggesting that exposure of offspring to food allergens may occur in utero and/or through breast milk. However, how maternal factors influence food allergy in offspring remains largely unknown. For example, effects of maternal allergen exposure on development of allergies in offspring have been controversial. Past studies have identified an increased risk (Sicherer et al., 2010) or no association (Lack et al., 2003) of maternal peanut consumption with peanut sensitization in offspring. In contrast, maternal exposure and/or sensitization to food allergens could be beneficial for protection of offspring from allergic diseases in humans and in mice (Fusaro et al., 2007; López-Expósito et al., 2009; Mosconi et al., 2010; Verhasselt, 2010b; Bunyavanich et al., 2014; Frazier et al., 2014). Nevertheless, whether active tolerance is induced in offspring has not been reported in these studies.
Forkhead box protein 3 (Foxp3)+ regulatory T (T reg) cells regulate Th2 responses and food allergy in humans and in mice (Chatila, 2005; van Wijk et al., 2007; Littman and Rudensky, 2010; Ohkura et al., 2013; Noval Rivas et al., 2015). However, whether maternal factors modulate T reg cell–mediated tolerance in offspring remains elusive. Both naturally occurring thymic-derived T reg cells and inducible T reg cells derived from conventional CD4+ T cells in the presence of TGF-β and specialized dendritic cells (DCs) such as CD11c+CD103+ DCs suppress Th2 responses (Chatila, 2005; van Wijk et al., 2007; Curotto de Lafaille et al., 2008; Gri et al., 2008; Akdis and Akdis, 2011). Successful immunotherapy is associated with increased T reg cells (Karlsson et al., 2004; Shreffler et al., 2009; Akdis and Akdis, 2011; Mousallem and Burks, 2012) and allergen-specific IgG antibodies (Scadding et al., 2010; Syed et al., 2014). Although protective effects of allergen-specific IgG through competition with IgE (Schroeder and Cavacini, 2010) and binding to inhibitory Fc receptor FcγRIIB (Jarrett and Hall, 1979; Fusaro et al., 2002; Uthoff et al., 2003; Till et al., 2004; Wachholz and Durham, 2004; Mosconi et al., 2010; Verhasselt, 2010a; Burton et al., 2014a) in food allergy have been proposed, the role of IgG in protective immune regulation requires further studies.
Neonatal crystallizable fragment receptor (FcRn) is expressed in intestinal epithelial cells until weaning in mice, and throughout life in humans (Simister and Mostov, 1989; Dickinson et al., 1999). FcRn mediates the transfer of maternal IgG to rodent offspring in early life, and thus plays a key role in neonatal passive immunity (Brambell, 1969; Simister and Mostov, 1989; Leach et al., 1996; Simister et al., 1996). Recent studies identified a much broader function of FcRn beyond the neonatal period in humans and mice, including protection of IgG and albumin from catabolism (Chaudhury et al., 2003; Roopenian et al., 2003; Pyzik et al., 2015), bidirectional transport of IgG (but not IgA or IgM) between the lumen and lamina propria (LP; Antohe et al., 2001; Claypool et al., 2002; Spiekermann et al., 2002; Akilesh et al., 2008; Dickinson et al., 2008; Bai et al., 2011; Li et al., 2011), and retrieval of antigen as IgG and antigen immune complexes (IgG-IC) from lumen to APCs such as DCs and macrophages in LP (Yoshida et al., 2004, 2006). It has been proposed that after internalization of IgG-IC into APCs by Fcγ receptors (FcγRs) on the cell surface, FcRn binds to IgG-IC in acidic endosomes and controls routing of IgG-IC to late endosomes, where antigen is processed into peptide compatible with loading onto MHC molecules, facilitating antigen presentation to T cells (Yoshida et al., 2004, 2006; Qiao et al., 2008; Baker et al., 2011, 2013, 2014; Liu et al., 2011; Pyzik et al., 2015). Fc-fusion proteins that bind to FcRn induce T reg cells and have been developed as therapeutic reagents (Lei and Scott, 2005; De Groot et al., 2008; De Groot and Martin, 2009; Scott and De Groot, 2010; Rath et al., 2015). However, the role of FcRn in promoting allergen-specific T reg cell response in early life is currently underinvestigated.
Food allergy often coexists with atopic dermatitis (AD; Eigenmann et al., 1998; Sicherer and Sampson, 1999; Hill et al., 2007). AD is often the initial step in the so-called atopic march, suggesting AD as a causal risk factor for subsequent food allergy. Epidemiological data have shown that peanut oil contaminants in baby lotions predispose to peanut allergy (Lack et al., 2003) and that loss of function mutations in the Filaggrin gene that encodes the epithelial barrier protein Filaggrin associated with AD also increase risk for food allergy (Brown et al., 2011; Brough et al., 2014), implying that increased allergen penetration through the damaged skin barrier causes systemic allergen sensitization that may lead to food allergy. Given the potential role of allergen sensitization through the skin, we have previously established that epicutaneous allergen sensitization of mice through tape-stripped skin over 7 wk results in food anaphylaxis (Bartnikas et al., 2013), providing experimental support for this epidemiological hypothesis linking skin allergen sensitization and food allergy.
Little is known about the role of maternally transferred allergen and allergen-specific IgG in the development of allergic diseases in offspring. To study the effects of maternal allergen-specific immune responses on the susceptibility of offspring to food allergy, we have further refined the epicutaneous sensitization model to develop an adjuvant-free, short (9-d) protocol that retains key features of the previous model. We hypothesized that maternal allergen sensitization induces allergen-specific tolerance in offspring and protects against food allergy. Female mice were epicutaneously sensitized with allergen followed by epicutaneous sensitization and oral challenge of their offspring with the same allergen. We have demonstrated that maternal sensitization with allergen protected their offspring from the development of food-allergic responses to the same allergen. This protection was mediated by allergen-specific T reg cells in offspring. The interactions of maternal IgG-IC in breast milk and offspring FcRn are essential for the development of allergen-specific T reg cells. We demonstrate that human breast milk containing OVA-IgG-IC induced oral tolerance in humanized FcRn mice, substantiating the importance of the IgG-IC-FcRn axis in neonatal tolerance induction. Collectively, these data suggest that the IgG-IC-FcRn axis may provide a therapeutic target to induce tolerance in early life to prevent food allergy in children.
Results
Maternal allergen sensitization protects offspring against food allergy
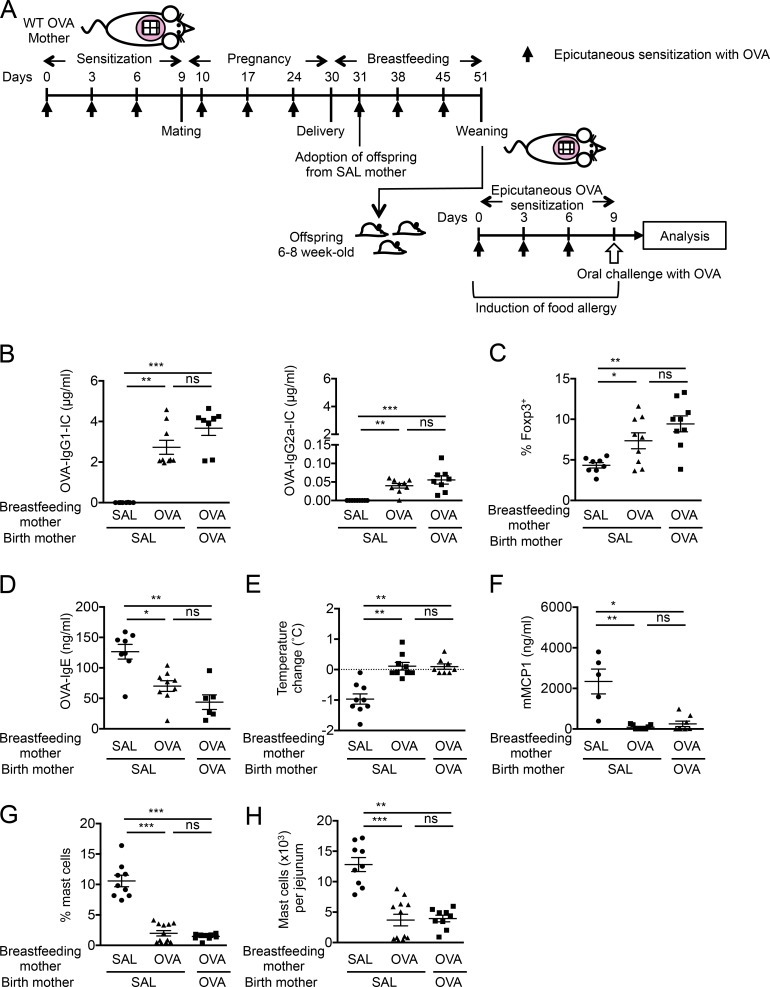
To assess whether maternal allergen-specific immune responses influence food allergic responses in offspring, we epicutaneously sensitized 6–8-wk-old BALB/c WT female mice with OVA or saline over 9 d before mating, and once weekly during pregnancy and breastfeeding. 6–8-wk-old offspring were epicutaneously sensitized with OVA or saline followed by oral OVA challenge by gavage at day 9 (Fig. 1 A). Epicutaneous sensitization with OVA of offspring from saline-exposed (unsensitized) mothers resulted in development of food-allergic reactions as indicated by an increase in serum OVA-specific IgE production. After oral OVA challenge, mice exhibited serum levels of IL-4, systemic anaphylaxis indicated by a drop in core body temperature, serum levels of mouse mast cell protease-1 (mMCP1), frequencies and numbers of jejunal mast cells, and Il13 mRNA expression in the jejunum (Fig. 1, B–I). Saline-exposed offspring from unsensitized mothers showed no detectable changes in these parameters after oral OVA challenge (Fig. 1, B–I). These results are consistent with our previous findings in mice epicutaneously sensitized over 7 wk (Bartnikas et al., 2013). In contrast to OVA-sensitized offspring of unsensitized mothers, these parameters of food-allergic responses were significantly decreased in OVA-sensitized offspring of OVA-sensitized mothers (Fig. 1, B–I). To test whether the protection of offspring from food allergy elicited by maternal allergen sensitization is applicable to a clinically relevant food allergen, mothers were sensitized with 0.45 mg commercial peanut butter (100 µg protein) followed by epicutaneous sensitization (0.45 mg) and oral challenge (225 mg) of their offspring with peanut butter. Offspring of unsensitized mothers exhibited food-allergic responses, including development of peanut-specific IgE, systemic anaphylaxis, serum mMCP1, and jejunal mast cell expansion. In contrast, these food-allergic responses were significantly impaired in offspring of peanut-sensitized mothers (Fig. S1). Collectively, these results suggest that maternal allergen sensitization results in protection of offspring from development of food-allergic response to epicutaneous sensitization and oral challenge with the same allergens.
Figure 1.
Maternal allergen sensitization protects offspring against food allergy. (A) Experimental protocol. (B) Serum OVA-IgE levels. (C) Serum IL-4 levels. (D) Core body temperature change 30 min after challenge. (E) Serum mMCP1 levels after challenge. (F) Representative flow cytometric analysis of jejunal mast cells (c-kit+IgE+lineage−CD45+) from two independent experiments. Numbers indicate percentages. (G–I) Mast cell frequencies (G), numbers (H), and Il13 mRNA (I) in the jejunum. Il13 mRNA levels are expressed as fold induction relative to jejunum of saline (SAL)-exposed offspring of saline-exposed mothers. Groups of animals were compared using nonparametric one-way ANOVA. Data are mean ± SEM of two independent experiments (B–E and G–I). *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001; ns, not significant.
Maternal allergen sensitization induces allergen-specific T reg cells in offspring
We next examined whether T reg cell responses are involved in the protection of offspring against food allergy after maternal allergen sensitization. Three-week-old weaned BALB/c WT offspring of OVA-sensitized mothers exhibited an increase in the frequencies and numbers of CD4+Foxp3+ T reg cells in mesenteric lymph nodes (MLN) as compared with weaned offspring of unsensitized mothers (Fig. 2, A–C). To test whether these T reg cells are allergen-specific, offspring MLN cells were labeled with proliferation dye, stimulated in vitro with OVA or peanut extract as an irrelevant allergen, and then examined for expansion and proliferation as assessed by frequencies of CD4+Foxp3+ cells and dye dilution, respectively. In response to OVA stimulation, MLN cells from offspring of OVA-sensitized mothers exhibited higher levels of expansion (Fig. 2 D) and proliferation (Fig. 2, E and F) of CD4+Foxp3+ T reg cells as compared with MLN cells from offspring of unsensitized mothers. In contrast, MLN cells from offspring of OVA-sensitized mothers did not show expansion or proliferation of CD4+Foxp3+ T reg cells in response to stimulation with peanut extract (Fig. 2, D–F). There was no significant increase in expansion or proliferation of CD4+Foxp3+ T reg cells in MLN cells from offspring of unsensitized mothers in response to stimulation with OVA or peanut extract (Fig. 2, D–F). These results suggest that CD4+Foxp3+ T reg cells in MLN of offspring from OVA-sensitized mothers expanded and proliferated specifically to OVA. We next tested the capacity of T reg cells from offspring of OVA-sensitized versus offspring of unsensitized mothers to suppress OVA-specific T cell proliferation in vitro. CD4+CD25+ T reg cells isolated by magnetic beads from MLN of weaned BALB/c WT offspring were cocultured with CD4+CD25−DO11.10+ responder T cells labeled with proliferation dye in the presence of OVA323-339 peptide and irradiated WT splenocytes. Proliferation of DO11.10+ T cells assessed by dye dilution was significantly less in the presence of T reg cells from offspring of OVA-sensitized mothers as compared with T reg cells from offspring of unsensitized mothers (Fig. 2, G and H), indicating that OVA-specific T reg cells from offspring of OVA-sensitized mothers are capable of suppressing the OVA-specific T cell response in vitro. After epicutaneous sensitization with OVA of offspring, greater expansion of OVA-specific CD4+Foxp3+ T reg cells was observed in MLN of offspring from OVA-sensitized mothers as compared with those in similarly sensitized offspring of unsensitized mothers (Fig. 2 I). These results indicate that maternal allergen sensitization elicits T reg cell responses in offspring that are specific to the same allergen.
Figure 2.
Allergen-specific T reg cells expand in offspring of allergen-sensitized mothers. (A–C) Flow cytometric analysis (A), frequencies (B), and numbers (C) of CD4+Foxp3+ cells in offspring MLN cells. (D) Analysis of OVA-specific Foxp3+ cells expanded from offspring MLN cells. (E and F) Flow cytometric analysis (E) and frequencies (F) of proliferation among CD4+Foxp3+ MLN cells labeled with CellTrace Violet cultured in vitro for 5 d. n = 4 (D and F). (G and H) Flow cytometric analysis (G) and percent suppression (H) of CellTrace Violet proliferation in DO11.10+ T responder cells cultured with OVA323-339 peptide in the absence or presence of T reg cells isolated from offspring MLN cells. (I) Frequencies of OVA-specific Foxp3+ cells expanded from offspring MLN cells after epicutaneous sensitization and oral challenge with OVA. Representative plots from two independent experiments are shown (A, E, and G). Numbers indicate percentages (A and E). Groups of animals were compared using the Mann-Whitney U test (B, C, H, and I) or nonparametric one-way ANOVA (D and F). Data are representative of two independent experiments (B–D, F, and H–I). Data are mean ± SEM. *, P < 0.05; **, P < 0.01; ns, not significant. SAL, saline.
Allergen-specific T reg cells in offspring are required for protection against food allergy
To examine the possibility that allergen-specific T reg cells in weaned offspring reduce disease susceptibility, we tested whether inducible depletion of T reg cells in offspring of OVA-sensitized mothers abolished the protection from food allergy. We used a genetic approach in which lineage-specific expression of the diphtheria toxin (DT) receptor (DTR) gene in BALB/c Foxp3EGFP/DTR+ mice allows selective Foxp3+ T reg cell depletion by DT treatment (Haribhai et al., 2011). 4–6-wk-old Foxp3EGFP/DTR+ offspring of OVA-sensitized Foxp3EGFP/DTR+ mothers were treated with a single i.p. injection of 50 µg/kg DT to eliminate T reg cells in offspring, including OVA-specific T reg cells. Control Foxp3EGFP/DTR+ littermates were injected with PBS. After DT treatment, offspring were rested for 2 wk before OVA sensitization for the recovery of Foxp3EGFP cells (Haribhai et al., 2011) to avoid global T reg cell deficiency (Fig. 3 A). In response to epicutaneous sensitization and oral challenge with OVA, DT-treated Foxp3EGFP/DTR+ offspring of OVA-sensitized mothers exhibited an increase in disease susceptibility, as indicated by higher levels of OVA-IgE, systemic anaphylaxis, serum mMCP1, and jejunal mast cell expansion as compared with PBS-treated Foxp3EGFP/DTR+ littermates (Fig. 3, B–F). These results support the key role of allergen-specific T reg cells induced by maternal allergen sensitization in induction of tolerance in offspring.
Figure 3.
Allergen-specific T reg cells in offspring are required for protection against food allergy. (A) Experimental protocol. (B) Serum OVA-IgE levels. (C) Core body temperature change. (D) Serum mMCP1 levels. (E and F) Flow cytometric analysis of jejunal mast cell frequencies (E) and numbers (F). Groups of animals were compared using the Mann-Whitney U test (B–F). Data are representative of two independent experiments (B–F). Data are mean ± SEM. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ns, not significant. SAL, saline.
Maternal IgG–allergen immune complexes, but not free allergen, are transferred to weaned offspring via breast milk
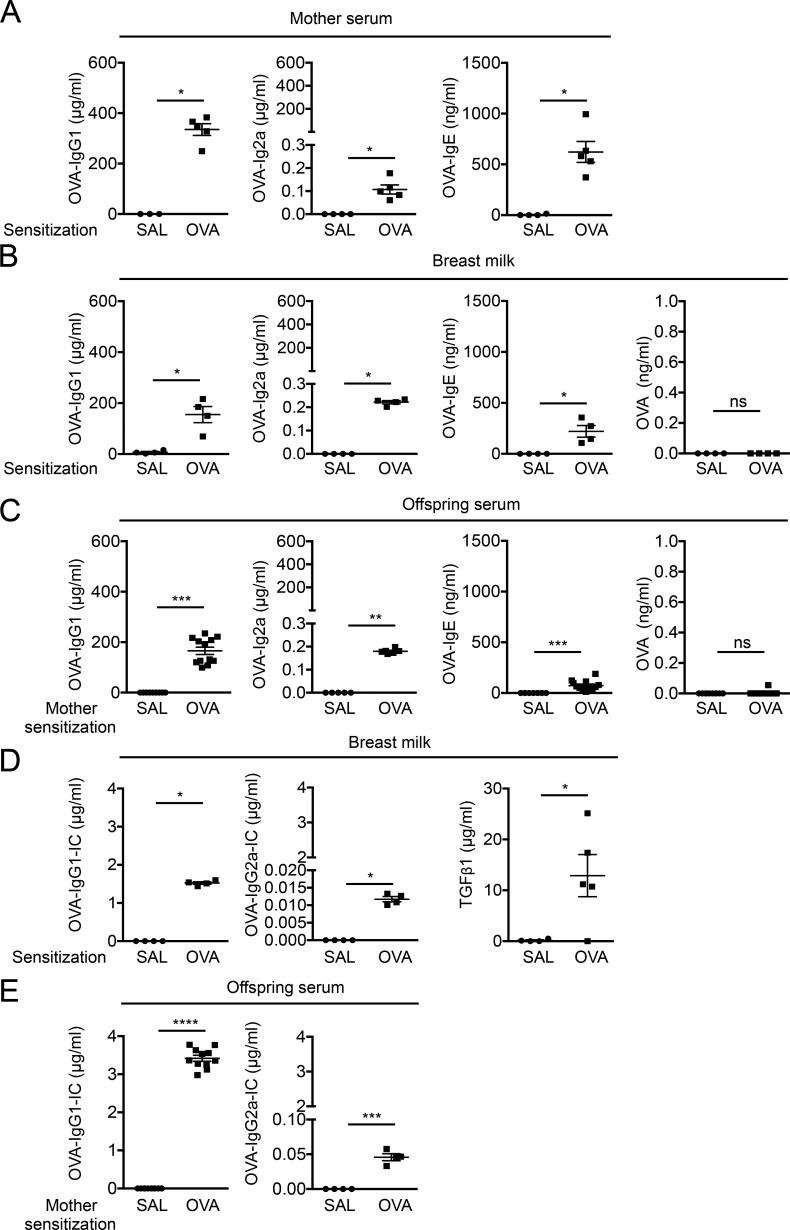
Maternal allergen and Igs are transferred to offspring and shape neonatal immune responses (Renz et al., 2011). Given the development of allergen-specific T reg cells in weaned offspring of allergen-sensitized mothers before direct exposure of offspring to allergen, we hypothesized that allergens are transferred from mothers to offspring before weaning and induce allergen-specific T reg cells. BALB/c WT female mice epicutaneously sensitized over 9 d with OVA, but not saline, showed an increase in serum OVA-specific IgG1, OVA-IgG2a, and OVA-IgE (Fig. 4 A), as previously reported (Nakajima et al., 2012; Venturelli et al., 2016). Similarly, OVA-Igs were detectable in breast milk and offspring sera of OVA-sensitized mothers, but not of unsensitized mothers (Fig. 4, B and C). Unexpectedly, levels of free OVA in breast milk or offspring sera of OVA-sensitized mothers were undetectable or close to the lower detection limit (Fig. 4, B and C). These results led us to test whether allergen is transferred from mothers to offspring as IgG-IC consisting of allergen and allergen-specific Igs. Indeed, OVA-IgG1-IC and OVA-IgG2a-IC, but not OVA-IgE-IC, were detectable in breast milk from OVA-sensitized mothers but not in breast milk from unsensitized mothers (Fig. 4 D and not depicted). Levels of TGF-β1 were also higher in breast milk from OVA-sensitized mothers than in breast milk from unsensitized mothers (Fig. 4 D). Consistently, offspring sera of OVA-sensitized mothers, but not of unsensitized mothers, exhibited OVA-IgG1-IC and OVA-IgG2a-IC (Fig. 4 E). OVA-IgA was virtually absent in sera and breast milk from OVA-sensitized mothers, and sera of offspring from OVA-sensitized mothers (not depicted). These results suggest that allergens are transferred from allergen-sensitized mothers as IgG-IC rather than as free allergen to offspring via breast milk.
Figure 4.
Maternal IgG-allergen immune complex, but not free allergen, are transferred to offspring via breast milk. (A) OVA-specific Igs in mother sera. (B and C) OVA-specific Igs and OVA in breast milk (B) and offspring sera (C). (D) OVA-IgG-ICs and TGF-β1 in breast milk. (E) OVA-IgG-ICs in offspring sera. Groups of animals were compared using the Mann-Whitney U test. Data are mean ± SEM of two independent experiments (A–E). *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001; ns, not significant. SAL, saline.
Breastfeeding by allergen-sensitized mothers protects offspring from food allergy
Our data show that the protection of offspring by maternal allergen sensitization is associated with the maternal IgG-IC transfer via breast milk and the induction of allergen-specific T reg cells in offspring. We hypothesized that maternal IgG-IC transferred through breast milk as allergen is a key factor in allergen-specific T reg cell induction in offspring. To this purpose, BALB/c WT mothers exposed to saline (unsensitized) or sensitized with OVA were coordinately mated, and a part of offspring from unsensitized mothers were fostered immediately after birth and nursed by OVA-sensitized mothers until weaning. The remaining offspring from unsensitized mothers were kept and nursed by unsensitized mothers. Offspring of OVA-sensitized mothers were kept in the same cage and nursed together with fostered offspring of unsensitized mothers (Fig. 5 A). Breastfeeding by OVA-sensitized mothers increased levels of serum OVA-IgG1-IC and OVA-IgG2a-IC as well as OVA-specific T reg cells in MLN of fostered offspring at weaning similar to offspring of OVA-sensitized mothers and higher than their littermates nursed by unsensitized mothers (Fig. 5, B and C). In response to epicutaneous sensitization and oral challenge with OVA, fostered offspring and offspring of OVA-sensitized mothers were similarly protected from food-allergic responses, as levels of serum OVA-IgE, systemic anaphylaxis, serum mMCP1, and jejunal mast cell expansion were strongly impaired as compared with OVA-sensitized offspring of unsensitized mothers (Fig. 5, D–H). These results indicate that breastfeeding by allergen-sensitized mothers promotes allergen-specific T reg cells and protection from food allergy in offspring. As maternal Igs are also transferred through the placenta (Renz et al., 2011), we have tested whether in utero–transferred IgG-IC also participates in driving tolerance in offspring. To this purpose, a part of offspring of OVA-sensitized mothers were fostered immediately after birth and nursed by unsensitized mothers (Fig. S2 A). Levels of maternal IgG-IC in fostered offspring of OVA-sensitized mothers were higher than those in offspring of unsensitized mothers and lower than in their littermates nursed by OVA-sensitized mothers (Fig. S2 B), indicating transfer of maternal IgG-IC both in utero and via breast milk. This was associated with a trend toward expansion of OVA-specific T reg cells in MLN of weaned fostered offspring that were significantly lower than their littermates nursed by OVA-sensitized mothers (Fig. S2 C). After epicutaneous sensitization and oral challenge with OVA, fostered offspring of OVA-sensitized mice showed a decrease in serum OVA-IgE levels and a trend toward lower systemic anaphylaxis, serum mMCP1, and jejunal mast cell expansion as compared with OVA-sensitized offspring of unsensitized mothers (Fig. S2, D–H), although these differences did not reach statistical significance. These results suggest that maternal IgG-IC transferred in utero to offspring may contribute to neonatal tolerance and that IgG-IC transferred during breastfeeding is necessary for optimal induction of tolerance in offspring of OVA-sensitized mothers.
Figure 5.
Breastfeeding by allergen-sensitized mothers protects offspring from food allergy. (A) Experimental protocol. (B) Serum OVA-IgG-ICs in weaned offspring. (C) Analysis of OVA-specific Foxp3+ cells expanded from offspring MLN cells. (D) Serum OVA-IgE levels. (E) Core body temperature change. (F) Serum mMCP1 levels. (G and H) Flow cytometric analysis of jejunal mast cell frequencies (G) and numbers (H). Groups of animals were compared using nonparametric one-way ANOVA. Data are mean ± SEM of two independent experiments (B–H). *, P < 0.05; **, P < 0.01; ***, P < 0.001; ns, not significant. SAL, saline.
IgG-IC supplementation to mothers protects offspring from food allergy
We next sought to directly test the role of IgG-IC in induction of neonatal tolerance by supplementing naive mothers with IgG-IC. We chose OVA-IgG1-IC based on the higher concentrations of maternal OVA-IgG1-IC relative to OVA-IgG2a-IC in breast milk and offspring sera from OVA-sensitized mothers (Fig. 4, D and E). In vitro–formed OVA-IgG1-IC consisting mouse monoclonal anti-OVA-IgG1 antibodies and OVA (Baker et al., 2013) was given i.p. to naive BALB/c WT female mice once weekly for 6 wk during pregnancy and breastfeeding (Fig. 6 A). Control BALB/c WT female mice were left untreated. Maternal IC supplementation resulted in an increase in levels of serum OVA-IgG1-IC and OVA-specific T reg cells in MLN of offspring at weaning (Fig. 6, B and C). These were associated with protection of offspring from food-allergic responses after epicutaneous sensitization and oral challenge with OVA, as indicated by a decrease in levels of serum OVA-IgE, systemic anaphylaxis, serum mMCP1, and jejunal mast cell expansion (Fig. 6, D–H). As expected, offspring of untreated mothers failed to show an increase in maternal OVA-IgG1-IC, OVA-specific T reg cells, or protection from food-allergic responses (Fig. 6, B–H). To further dissect the role of maternal IC during breastfeeding in induction of tolerance in offspring, mothers were supplemented i.p. with OVA-IgG1-IC once weekly for 3 wk only during breastfeeding (Fig. 6 I). Offspring of mothers supplemented with OVA-IgG1-IC during breastfeeding, but not offspring from untreated mothers, exhibited an increase in levels of serum OVA-IgG1-IC, OVA-specific T reg cells in MLN at weaning, and protection from food-allergic responses after epicutaneous sensitization and oral challenge with OVA (Fig. 6, J–P). To test whether IgG-IC mediates neonatal tolerance directly versus through the induction of allergen-specific T reg cells, we examined food-allergic responses in offspring after maternal IgG and IgG-IC had been cleared from circulation. Maternal OVA-Igs or OVA-IgG-ICs were undetectable in 15-wk-old BALB/c WT offspring (not depicted). 15-wk-old offspring of OVA-sensitized mothers, but not of unsensitized mothers, showed an expansion of OVA-specific T reg cells in MLN (Fig. S3 A) and decreased food-allergic responses after epicutaneous sensitization and oral challenge with OVA (Fig. S3, B–F), suggesting that maternal allergen sensitization induces long-lasting protection in offspring. Collectively, these results demonstrate that maternal allergen IgG-IC plays a critical role in establishing tolerance in offspring against food allergy through induction of allergen-specific T reg cells.
Figure 6.
IC supplementation to mothers protects offspring from food allergy. (A–H) IC supplementation during pregnancy and breastfeeding. Experimental protocol (A), serum OVA-IgG1-IC in weaned offspring (B), OVA-specific Foxp3+ cells expanded from offspring MLN cells (C), serum OVA-IgE levels (D), core body temperature change (E), serum mMCP1 levels (F), jejunal mast cell frequencies (G), and numbers (H). (I–P) IC supplementation during breastfeeding. Experimental protocol (I), serum OVA-IgG1-IC in weaned offspring (J), OVA-specific Foxp3+ cells expanded from offspring MLN cells (K), serum OVA-IgE levels (L), core body temperature change (M), serum mMCP1 levels (N), jejunal mast cell frequencies (O), and numbers (P). Groups of animals were compared using the Mann-Whitney U test. Data are mean ± SEM and representative of 2 independent experiments (B–H, J–P). *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001.
Offspring FcRn is required for protection from food allergy
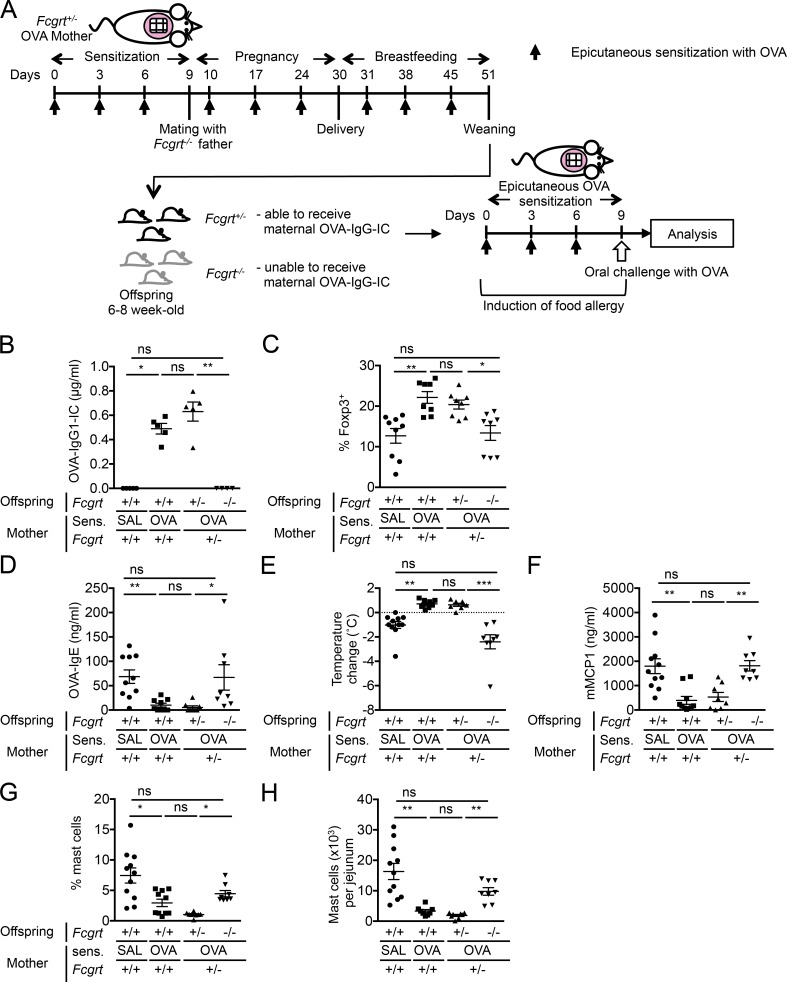
As FcRn is involved in the retrieval of IgG-IC from the lumen into LP (Yoshida et al., 2004, 2006), we examined the role of FcRn in induction of tolerance in offspring. BALB/c Fcgrt+/− females were OVA-sensitized and mated with BALB/c Fcgrt−/− males (Fig. 7 A). Fcgrt−/− females were not used as they predictably exhibit a shorter IgG half-life (Roopenian et al., 2003; Pyzik et al., 2015). Levels of serum OVA-IgG1-IC and MLN OVA-specific T reg cells in weaned Fcgrt+/− offspring were comparable to BALB/c WT offspring of OVA-sensitized WT mothers, whereas there was no increase in these parameters in Fcgrt−/− littermates (Fig. 7, B and C). After epicutaneous sensitization and oral challenge with OVA, Fcgrt+/− offspring of OVA-sensitized mothers exhibited tolerance, as indicated by a decrease in levels of OVA-IgE production, systemic anaphylaxis, and serum mMCP1, and jejunal mast cell expansion (Fig. 7, D–H), whereas OVA-sensitized Fcgrt−/− offspring of OVA-sensitized mothers failed to show tolerance against food-allergic responses (Fig. 7, D–H). Collectively, these results suggest that offspring FcRn is essential in maternal IgG-IC transfer, differentiation of allergen-specific T reg cells, and induction of tolerance in offspring, confirming the requirement of maternal IgG-IC and offspring FcRn in disease protection.
Figure 7.
Offspring FcRn is required for protection of offspring from food allergy. (A) Experimental protocol. (B) Serum OVA-IgG1-IC in weaned offspring. (C) Analysis of OVA-specific Foxp3+ cells expanded from offspring MLN cells. (D) Serum OVA-IgE levels. (E) Core body temperature change. (F) Serum mMCP1 levels. (G and H) Flow cytometric analysis of jejunal mast cell frequencies (G) and numbers (H). Groups of animals were compared using nonparametric one-way ANOVA. Data are mean ± SEM of two independent experiments (B–H). *, P < 0.05; **, P < 0.01; ***, P < 0.001; ns, not significant. SAL, saline.
Milk-borne allergen IgG-IC induces allergen-specific T reg cells via MLN CD11c+ DCs
We hypothesized that maternal IgG-IC is processed and presented by CD11c+ DCs in offspring to promote the differentiation of allergen-specific T reg cells. To test the capacity of CD11c+ DCs to present milk-borne IgG-IC and to induce allergen-specific T reg cells, CD11c+ DCs were isolated from MLN of naive BALB/c WT mice and cocultured with CD4+ T cells from DO11.10+Foxp3EGFPRag2−/− mice that lack natural T reg cells in the presence or absence of breast milk from saline- or OVA-sensitized mothers for 4 d. Breast milk of OVA-sensitized mothers, but not breast milk of saline-exposed mothers or PBS, increased the frequencies of OVA-specific Foxp3EGFP T reg cells (Fig. 8, A and B), suggesting that CD11c+ DCs process and present milk-borne allergen IgG-IC and induce allergen-specific T reg cells. We next compared the capacity of CD11c+ DCs from MLN of offspring of saline- or OVA-sensitized mothers to induce OVA-specific T reg cells in vitro without exogenous antigen. CD11c+ DCs from offspring of OVA-sensitized mothers, but not from saline-exposed mothers, induced OVA-specific Foxp3EGFP T reg cells (Fig. 8, C and D), indicating that CD11c+ DCs from offspring of OVA-sensitized mothers already acquired allergen in vivo and induced allergen-specific T reg cells. To examine the ability of CD11c+ DCs to induce tolerance in vivo, MLN CD11c+ DCs were isolated from BALB/c WT offspring of saline- or OVA-sensitized WT mothers and adoptively transferred into naive BALB/c WT recipients together with DO11.10+Foxp3EGFPRag2−/−CD4+ T cells (Fig. 8 E). After sensitization and oral challenge with OVA, recipients of CD11c+ DCs from offspring of OVA-sensitized mothers, but not of CD11c+ DCs from offspring of saline-exposed mothers, exhibited a decrease in food-allergic responses (Fig. 8, F–J). Adoptive transfer of CD11c+ DCs from offspring of OVA-sensitized mothers resulted in greater expansion of OVA-specific T reg cells in vivo, as assessed as Foxp3EGFP cells in MLN and jejunum (Fig. 8, K and L). These results indicate the critical role of CD11c+ DCs in offspring in processing maternally transferred IgG-IC and promoting T reg cell–mediated tolerance against food allergy.
Figure 8.
Milk-borne IgG-IC induces allergen-specific T reg cells via MLN CD11c+ DCs. (A and B) Flow cytometric analysis (A) and frequencies (B) of CD4+DO11.10+Foxp3EGFP+ T reg cells. MLN CD11c+ cells were isolated from naive WT mice and cultured with CD4+DO11.10+Foxp3EGFP- cells in the presence or absence of breast milk. (C and D) Flow cytometric analysis (C) and frequencies (D) of CD4+DO11.10+Foxp3EGFP+ T reg cells. MLN CD11c+ cells were isolated from offspring of SAL or OVA mothers and cultured with CD4+DO11.10+Foxp3EGFP- cells without exogenous allergens. (E–L) Adoptive transfer of MLN CD11c+ cells from offspring of SAL or OVA mothers. Experimental protocol (E), OVA-IgE (F), core body temperature change (G), serum mMCP1 levels (H), jejunal mast cell frequencies (I) and numbers (J), and flow cytometric analysis of CD4+DO11.10+Foxp3EGFP+ T reg cells in MLN (K) and jejunum (L) of recipients. Representative plots from two independent experiments shown (A and C). Numbers indicate percentages (A and C). Groups of animals were compared using nonparametric one-way ANOVA (B) and the Mann-Whitney U test (D and F–L). Data are mean ± SEM of two independent experiments (B and D) or representative of two independent experiments (F–L). *, P < 0.05; **, P < 0.01; ns, not significant. SAL, saline.
FcRn in CD11c+ DCs is critical for induction of tolerance in offspring
FcRn is expressed in DCs and macrophages throughout life in humans and mice (Zhu et al., 2001). FcRn in APCs mediates antigen presentation of IgG-IC more efficiently than soluble antigen alone (Qiao et al., 2008; Baker et al., 2011). Our results suggest that maternally transferred OVA-IgG-IC, but not free OVA, processed and presented by CD11c+ DCs likely provides the basis for the development of allergen-specific T reg cell responses in offspring. We hypothesized that FcRn in offspring DCs contributes to tolerance induction in offspring by facilitating IgG-IC presentation to promote the induction of allergen-specific T reg cells during this period of life. To this purpose, we examined the capacity of CD11c+ DCs from MLN of naive BALB/c Fcgrt−/− mice to induce OVA-specific T reg cells in vitro in the presence of breast milk from OVA-sensitized BALB/c WT mothers. Unlike WT CD11c+ DCs, Fcgrt−/− CD11c+ DCs failed to induce OVA-specific T reg cells in response to breast milk from OVA-sensitized mothers (Fig. 9 A). WT and Fcgrt−/− CD11c+ DCs were comparable in their capacity of inducing OVA-specific T reg cells after in vitro stimulation with exogenous OVA323-339 peptide and TGF-β1 (Fig. 9 A), indicating that the impaired capacity of Fcgrt−/− CD11c+ DCs in inducing OVA-specific T reg cells was not a result of their general failure to present allergens to CD4+ T cells. These results suggest that FcRn in CD11c+ DCs is required for maternal allergen presentation and induction of antigen-specific T reg cells. To further examine the role of FcRn in CD11c+ DCs in induction of tolerance in vivo, we used mice bearing a floxed Fcgrt gene (Fcgrtfl/fl) crossed with CD11c-cre mice on C57BL/6 background to specifically delete FcRn in CD11c+ DCs (C57BL/6 ItgaxcreFcgrtfl/fl). Saline- or OVA-sensitized Fcgrtfl/fl females were mated with ItgaxcreFcgrtfl/fl males (Fig. 9 B). Weaned ItgaxcreFcgrtfl/fl offspring of OVA-sensitized mothers exhibited a decrease in induction of OVA-specific T reg cells as compared with Fcgrtfl/fl littermates (Fig. 9 C). Consistently, ItgaxcreFcgrtfl/fl offspring of OVA-sensitized mothers failed to exhibit tolerance to food allergy, as ItgaxcreFcgrtfl/fl mice developed greater levels of OVA-IgE, systemic anaphylaxis, and jejunal mast cell expansion than their Fcgrtfl/fl littermates in response to epicutaneous sensitization and oral challenge with OVA (Fig. 9, D–G).
Figure 9.
FcRn in CD11c+ DCs is critical for induction of tolerance in offspring. (A) Flow cytometric analysis of CD4+DO11.10+Foxp3EGFP+ T reg cells. MLN CD11c+ DCs were isolated from WT or Fcgrt−/− mice and cultured with CD4+DO11.10+Foxp3EGFP- cells with breast milk from OVA-sensitized WT mothers or with exogenous TGF-β1 and OVA323-339 peptide. (B) Experimental protocol. (C) Analysis of OVA-specific Foxp3+ cells expanded from weaned offspring MLN cells. (D) Serum OVA-IgE levels. (E) Core body temperature change. (F and G) Flow cytometric analysis of jejunal mast cell frequencies (F) and numbers (G). Groups of animals were compared using the Mann-Whitney U test (A, C–G). Data are mean ± SEM of two independent experiments (A) or 3 independent experiments (C–G). *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001; ns, not significant. SAL, saline.
IgG-IC in human breast milk protects humanized FcRn mice from food allergy
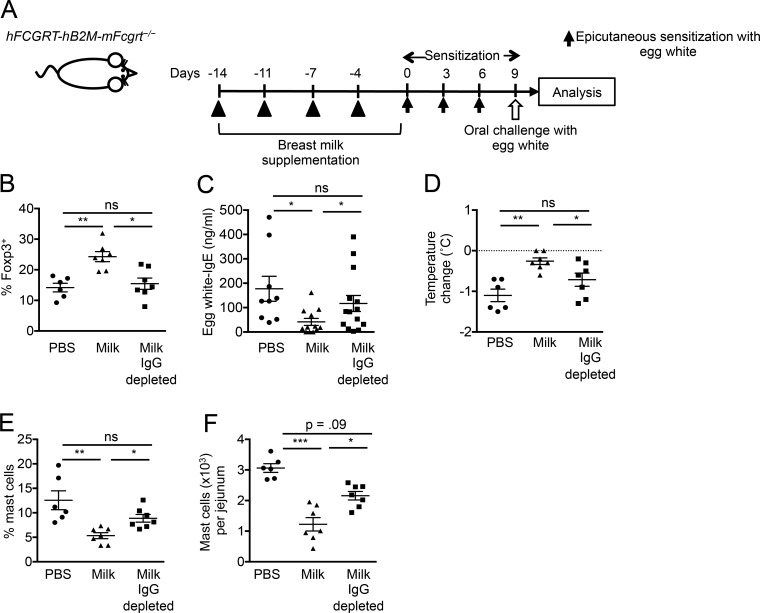
Food-specific IgG and food allergen–immune complexes are present in sera and breast milk of healthy subjects (Husby et al., 1985; Rumbo et al., 1998; Hirose et al., 2001; Bernard et al., 2014; Hochwallner et al., 2014; Schwarz et al., 2016). Elevated levels of allergen-specific IgG, especially IgG4, are associated with successful allergen-specific immunotherapy (Skripak et al., 2008; Caubet et al., 2012; James et al., 2012). The presence of OVA-specific IgG4 and OVA-IgG4-IC was assessed in breast milk samples from 16 nonatopic mothers by ELISA. OVA-IgG4 was detectable in 10 (62.5%) milk samples with a median value among positive samples of 95.8 ng/ml (Table S1). OVA-IgG4-IC was present in 8 subjects among 10 subjects that were positive for OVA-IgG4 (Table S1). OVA-IgE was undetectable in all samples. These results together with the findings in our mouse models suggest that maternal food-specific IgG in breast milk may promote the development of neonatal tolerance if transferred to neonates together with food allergen. To examine the capacity of IgG-IC from human breast milk to promote induction of neonatal tolerance and to demonstrate a direct link between human FcRn and neonatal tolerance induction, we used BALB/c mice that constitutively express human FcRn and β2-microglobulin (β2M) and are deficient in mouse FcRn (hFCGRT-hB2M-mFcgrt−/−) and thus have only the human form of the receptor (Roopenian et al., 2003; Yoshida et al., 2004). BALB/c hFCGRT-hB2M-mFcgrt−/− mice were supplemented by gavage 2 times a week for 2 wk with pooled human breast milk samples positive for OVA-IgG4-IC followed by epicutaneous sensitization and oral challenge with egg white protein (Fig. 10 A). Treatment of humanized FcRn mice with OVA-IgG4-IC–containing breast milk resulted in oral tolerance, as indicated by an increase in allergen-specific T reg cells and a decrease in allergen-specific IgE, oral anaphylaxis, and jejunal mast cells (Fig. 10, B–F). The hypothesis that OVA-IgG4-IC mediated the observed protection in humanized FcRn mice from food allergy was tested by treatment of humanized FcRn mice with the same milk after IgG depletion. Removal of IgG from OVA-IgG4-IC–containing milk abrogated the differentiation of allergen-specific T reg cells as well as suppressive effects of milk on food-allergic responses (Fig. 10, B–F). Collectively, these results indicate that allergen-specific IgG-IC in breast milk drive allergen-specific T reg cells and that food-specific IgG antibodies exert an induction of tolerance against food allergy. These data further support the concept that the IgG-IC-FcRn axis contributes to neonatal tolerance induction and may extend to humans.
Figure 10.
IgG-IC in human breast milk protects humanized FcRn mice from food allergy. (A) Experimental protocol. (B) Analysis of allergen-specific Foxp3+ cells expanded from MLN cells. (C) Serum egg white-IgE levels. (D) Core body temperature change. (E and F) Flow cytometric analysis of jejunal mast cell frequencies (E) and numbers (F). Groups of animals were compared using a nonparametric one-way ANOVA. Data are mean ± SEM and representative of two independent experiments (B–F). *, P < 0.05; **, P < 0.01; ***, P < 0.001; ns, not significant.
Discussion
In this study, we demonstrate that the interactions of maternal food allergen–specific IgG-IC and offspring FcRn in induction of allergen-specific T reg cell responses are fundamental to establishment of an effective, long-lasting neonatal tolerance to foods. Food allergen–specific IgG-IC is also observed in human breast milk from nonatopic mothers and sufficient to reduce disease susceptibility in humanized FcRn mice.
By using a physiological mouse model that shares features of human food allergy, including a portal of exposure (the skin) that is important for disease development, and response to oral challenge with IgE- and mast cell–mediated anaphylaxis, we demonstrated that maternal sensitization with food allergen induces T reg cells in offspring that are specific to the same allergen, which mediate the protection from food allergy. Allergen-specific T reg cells were induced by maternal IgG-IC in breast milk, which was transferred to offspring in an FcRn-dependent manner, followed by FcRn-mediated antigen presentation by CD11c+ DCs in offspring. These findings outline a cascade of cellular and molecular events that underlie the induction of neonatal tolerance toward food allergens elicited by maternal immune responses.
BALB/c WT offspring of unsensitized mothers developed food-allergic responses after epicutaneous sensitization over 9 d and oral challenge with OVA, with increased levels of serum OVA-IgE, systemic anaphylaxis, serum mMCP1, and jejunal mast cell expansion. Our adjuvant-free model eliminates in mothers and offspring potentially confounding effects of adjuvants such as alum and cholera toxin (Oyoshi et al., 2014) that were used in previous studies examining maternal effects on offspring allergies (Leme et al., 2006; Matson et al., 2009; Mosconi et al., 2010). It is also highly versatile as it is operative in different backgrounds including BALB/c and C57BL/6. We demonstrate that maternal allergen sensitization results in protection of offspring from development of food allergy, as the disease features were strongly attenuated in offspring of OVA-sensitized mothers. This protection was long-lasting, as food-allergic responses were suppressed in 15-wk-old offspring from OVA-sensitized mothers.
This protection of offspring against food allergy was associated with an increase in allergen-specific Foxp3+ T reg cells in offspring at weaning, as indicated by the capacities of these T reg cells to expand, proliferate, and suppress T cell proliferation in an allergen-specific manner. Although natural or type 1 T reg cells could also suppress food allergy, the critical importance of allergen-specific T reg cells in offspring in mediating neonatal tolerance was evident in in vivo models, as depletion of allergen-specific T reg cells in Foxp3EGFP/DTR+ offspring of allergen-sensitized mothers abolished tolerance against food allergy.
The differentiation of allergen-specific T reg cells in offspring at weaning reflected the role of maternally transferred IgG-IC via breast milk. Maternal OVA-Igs were increased in sera and breast milk from OVA-sensitized mothers, which were also observed in offspring sera of OVA-sensitized mothers. Although maternal OVA-IgE was detectable in offspring of OVA-sensitized mothers, oral OVA challenge of saline-exposed offspring of OVA-sensitized mothers did not result in systemic anaphylaxis, consistent with the observations in humans and in mice that the presence of allergen-specific IgE is not always associated with food allergy symptoms (Bartnikas et al., 2013; Valenta et al., 2015). Although mothers were constantly exposed to OVA from preconception through weaning, levels of free OVA in breast milk or sera of offspring from OVA-sensitized mothers were negligible, whereas similar ELISA protocols readily detected IgG-IC and OVA-Igs not bound to OVA in the same samples. Although it is possible that undetectable levels of OVA in breast milk could also be transferred to offspring, these results suggest that transfer of free antigen to the pups during breastfeeding period is likely minimal. Our results suggest that in utero–transferred IgG-IC may also participate in driving tolerance in offspring and that IgG-IC transferred during breastfeeding is necessary for optimal induction of tolerance. This was further supported by the findings that breastfeeding by OVA-sensitized mothers was sufficient to increase IgG-IC levels and allergen-specific T reg cells, as well as protection against food allergy in fostered offspring of unsensitized mothers, similar to offspring of OVA-sensitized mothers.
The direct contribution of maternal IgG-IC to tolerance induction was evident as maternal IC supplementation during pregnancy and breastfeeding promoted neonatal tolerance against food allergy. Maternal IgG-IC supplementation only during breastfeeding resulted in lower levels of IgG-IC in offspring as compared with maternal IgG-IC supplementation during pregnancy and breastfeeding, likely reflecting the total IgG-IC dose given to mothers and the contribution of in utero IgG-IC transfer. Although physiological dose requirements for IgG-IC to induce neonatal tolerance are unknown, maternal IgG-IC supplementation during breastfeeding alone still provided resistance toward food allergy in offspring. This further supports the critical role of IgG-IC in induction of neonatal tolerance. The essential role of offspring FcRn in tolerance induction was shown in an in vivo model. BALB/c Fcgrt+/− offspring of OVA-sensitized Fcgrt+/− mothers showed comparable levels of maternal IgG-IC transfer, allergen-specific T reg cell induction, and protection against food allergy as in WT controls. Impaired formation of allergen-specific T reg cells and failure of tolerance induction in BALB/c Fcgrt−/− littermates of OVA-sensitized Fcgrt+/− mothers were associated with their inability to receive and respond to maternal IgG-IC, consistent with a previous study that offspring FcRn is required for maternal IgG-IC transfer and protection against experimental asthma (Mosconi et al., 2010). A previous study evaluated mothers and offspring immunized through a nonphysiological route (i.p.) with an artificial adjuvant, alum, which induces allergic airway inflammation that is independent of IgE or mast cells (Williams and Galli, 2000). In this study, mothers were exposed to OVA aerosols after adoption of offspring, raising a possibility that mothers and offspring ingested OVA by licking their fur. Thus, their results of neonatal tolerance induction may have reflected exposure of mothers and offspring to OVA by both airway and oral routes. The capacity of IgG-IC to induce T reg cell–mediated tolerance and the role of FcRn in DCs were not directly addressed in the previous study. Furthermore, the mechanisms suggested using a mouse model of allergic airway inflammation do not necessarily extend to the mechanisms applicable to a model of food allergy. Our model is likely to be more relevant to naturally occurring sensitization to food allergens in that it is adjuvant-free, uses skin as a route of exposure that is important for development of clinical food allergy, is applicable to a clinically relevant peanut allergen, and assesses responses to oral challenge together with IgE- and mast cell–mediated anaphylaxis (Bartnikas et al., 2013; Galand et al., 2016).
The mechanism driving the differentiation of allergen-specific T reg cells involved maternal antigen presentation by CD11c+ DCs in offspring. CD11c+ DCs from MLN of naive BALB/c WT mice supported induction of allergen-specific T reg cells in the presence of breast milk from OVA-sensitized mothers in vitro. In addition, CD11c+ DCs from offspring of OVA-sensitized mothers induced allergen-specific T reg cells in vitro without addition of exogenous antigen, indicating that these cells acquired maternally transferred antigens in vivo, likely as IgG-IC, given the absence of free OVA in breast milk from OVA-sensitized mothers. We further demonstrated that CD11c+ DCs from MLN in offspring promote differentiation of allergen-specific T reg cells that mediate tolerance in vivo. Adoptive transfer of CD11c+ DCs from offspring of OVA-sensitized mothers, but not CD11c+ DCs from offspring of unsensitized mothers, transferred tolerance in recipients.
In addition to mediating maternal IgG-IC transfer to offspring through intestinal epithelial cells as supported by our previous studies (Yoshida et al., 2004), FcRn in CD11c+ DCs in offspring was also critical for maternal IgG-IC processing and antigen presentation to induce allergen-specific T reg cells. Unlike WT CD11c+ DCs, Fcgrt−/− CD11c+ DCs were incapable of presenting OVA-IgG-IC in breast milk from OVA-sensitized mothers to induce allergen-specific T reg cells in vitro. These results are consistent with the distinctive role of FcRn in CD11c+ DCs in processing IgG-IC, rather than soluble antigen, to prime T cell responses (Qiao et al., 2008; Baker et al., 2011) and further support our concept that IgG-IC in breast milk provides the main basis of allergen-specific T reg cell differentiation. The requisite role of FcRn in CD11c+ DCs in determining the tolerogenic environment was further demonstrated by us in vivo in mice with CD11c+ cell–specific FcRn deficiency, which failed to increase allergen-specific T reg cells and tolerance induction by maternal allergen sensitization. Previous studies have linked FcRn-dependent antigen processing to the induction of Th1 responses via induction of IL-12 production by CD11c+ cells (Baker et al., 2013). Our studies here thus extend these observations by showing that FcRn in CD11c+ cells in response to IgG-IC also regulates the induction of FoxP3+ T reg cells. Whether this is a result of the activities of FcRn in specific subsets of APCs and in a tissue- and/or age-dependent manner remains to be addressed. Our study delineates for the first time the critical role of FcRn in DCs in promoting allergen-specific T reg cell responses, which together provides a greater understanding of the role of FcRn in modulating protective immune regulation in food allergy. Our results thus provide a novel role of FcRn in mediating allergen-specific T reg cell responses in mediating neonatal tolerance, on the top of its known role in the maternal IgG transfer.
FcRn may thus control tolerance induction at many points of life. These include the role played by FcRn in controlling the transport of IgG-IC across the placenta antenatally into the developing fetus (Leach et al., 1996). FcRn function in intestinal epithelial cells of the neonatal animal may be particularly important in the rodent during neonatal life when FcRn expression and function are robust (Gill et al., 1999). Although never directly addressed, FcRn in intestinal epithelial cells may play a role in the antigen presentation functions of this cell type in the induction of tolerance (Kaiserlian et al., 1989). These possibilities are of potential future interest through conditional deletion of Fcgrt in the intestinal epithelium, for example.
As IgG-IC processing also requires FcγR (Baker et al., 2011), the contribution of each FcγR to IgG-IC uptake by CD11c+ DCs leading to the T reg cell induction is not clear and an important future area of study. One recent study demonstrated a critical role for FcγRIIb in mediating a protective effect of allergen-specific IgG against food allergy (Burton et al., 2017). Nevertheless, it may be hypothesized that after the internalization of IgG-IC by FcγRs, FcRn is the only intracellular receptor that is known to control the routing of IgG-IC to an antigen-processing pathway to prime T cells (Qiao et al., 2008; Baker et al., 2011, 2013, 2014; Liu et al., 2011; Guilliams et al., 2014).
We demonstrate in the current study that food-specific IgG4 is detectable in breast milk from nonatopic mothers, consistent with the previous studies (Bernard et al., 2014; Rekima et al., 2017). Furthermore, OVA-IgG4-IC was commonly present in breast milk samples that were positive for OVA-IgG4. The importance of the milk-borne IgG-IC-FcRn axis in tolerance induction was strongly corroborated by the impaired food-allergic responses in humanized FcRn mice supplemented with breast milk from nonatopic mothers containing OVA-IgG-IC, but not with IgG-depleted milk. These results further validate that the concept of inducing oral tolerance by the IgG-IC pathway is also effective when human breast milk IgG-IC is used, providing a particularly important piece of evidence for the potential clinical relevance of our observations in mice for humans.
Our study is in line with recent findings that maternal exposure to food allergens decreases allergy in offspring in humans and in mice (Fusaro et al., 2007; López-Expósito et al., 2009; Mosconi et al., 2010; Verhasselt, 2010b; Bunyavanich et al., 2014; Frazier et al., 2014). Prior human studies examining the effect of maternal diets during pregnancy on peanut allergy have shown inconsistent results. A prospective US study showed no benefit of maternal and early childhood avoidance of milk, egg, or peanut in preventing food allergies (Zeiger et al., 1989). Our results provide experimental support for recent decisions to withdraw recommendations of allergen avoidance during pregnancy and breastfeeding, and support potential beneficial effects of maternal allergen exposure to protect offspring from food allergy. A recent study suggesting that early food introduction might decrease the risk of food allergy development (Perkin et al., 2016) underscored the potential benefit of food allergen transfer through breast milk as this may be the first food exposure for the infant. Our data indicate that both offspring of sensitized mothers and offspring of unsensitized mothers supplemented with IgG-IC exhibited tolerance against food allergy, highlighting the critical role of maternal IgG-IC in tolerance induction in offspring regardless of the sensitization status of mothers in our mouse model. Our findings that food allergen–specific IgG-IC is present in human breast milk from nonatopic mothers and sufficient to reduce disease susceptibility in humanized FcRn mice support that nonatopic mothers may induce oral tolerance through the IgG-IC pathway. Analysis of how the atopic status of mothers may influence tolerance induction in offspring in humans will be an important future question to be addressed.
In summary, this study has provided novel experimental evidence supporting a critical role of maternal allergen-specific immune responses in establishing effective tolerance that prevents food allergy in offspring, extending well beyond the previously known roles of maternal antibodies and FcRn in providing passive immunity. Our experimental approaches that combine cellular and in vivo animal investigations as well as human ex vivo samples demonstrate that the interactions of IgG-IC and FcRn are critical in the development of neonatal tolerance, namely FcRn-dependent transfer of maternal IgG-IC and FcRn-dependent IgG-IC processing and antigen presentation by CD11c+ DCs to induce allergen-specific T reg cells in offspring (Fig. S4), thus identifying multiple roles for FcRn in neonatal tolerance. Our results also provide a rationale for measures that improve the development of allergen-specific T reg cells through maternal allergen exposure. This previously unrecognized tolerance pathway could suggest a potential for IgG-IC as an immunotherapy to improve oral tolerance and may lead to improved therapeutic strategies to induce tolerance in early life to prevent food allergy in children.
Materials and methods
Study participants
Nonatopic breastfeeding mothers were recruited as defined by no personal or family history of atopic diseases (food allergy, environmental allergy, eczema, asthma) in first-degree relatives. These subjects had no other chronic diseases or mastitis during the preceding 4 wk. Informed consent was obtained from the subjects and the study was approved by the Boston Children’s Hospital Institutional Review Board. Milk samples were centrifuged (400 g, 15 min), fat was removed, and supernatant was collected, frozen, and stored at −20°C until use.
Mice
BALB/c WT mice were purchased from Taconic. BALB/c Foxp3EGFP/DTR+ and BALB/c DO11.10+Rag2−/−Foxp3EGFP mice bred onto a BALB/c background for >10 generations were a gift from T. Chatila (Boston Children’s Hospital, Boston, MA). Fcgrt−/− (Roopenian et al., 2003) and hFCGRT-hB2M-mFcgrt−/− (Roopenian et al., 2003; Yoshida et al., 2004) mice were bred onto a BALB/c background for more than 10 generations. C57BL/6 Fcgrtfl/fl mice were kindly provided by E.S. Ward (University of Texas Southwestern Medical Center, Dallas, TX; Yoshida et al., 2004; Montoyo et al., 2009). All mice were bred in the animal facility of Boston Children’s Hospital, kept in a specific pathogen-free environment, and fed an OVA-free diet. All procedures were performed in accordance with the Animal Care and Use Committee of Boston Children’s Hospital.
Allergen sensitization and anaphylaxis
Epicutaneous sensitization of mice was performed as previously described (Venturelli et al., 2016). In brief, the dorsal skin of anesthetized 6–8-wk-old female mice was shaved and tape-stripped six times with Tegaderm (Westnet Inc.). Then 100 µg OVA (Grade V; Sigma) in 100 µl of normal saline, or placebo (100 µl of normal saline), was placed on a patch of sterile gauze (1 × 1 cm), which was secured to the dorsal skin with Tegaderm. Each mouse had a total of three 1-d exposures to the patch separated by 2-d intervals over 9 d before mating. Female mice received epicutaneous sensitization with OVA once weekly during pregnancy and breastfeeding. Mothers did not receive oral OVA challenge. 6–8-wk-old offspring were epicutaneously sensitized with OVA or saline over 9 d followed by a bolus oral challenge with 100 mg OVA at day 9 (Bartnikas et al., 2013). Temperature changes were measured by using the DAS-6006 Smart Probe and transponders (Biomedic Data Systems) injected subcutaneously. Mice were euthanized 24 h after challenge to harvest tissues. For T reg cell depletion by DT, Foxp3EGFPDTR+ mice were treated i.p. with 50 µg/kg DT (List Biological Laboratory).
Fostering of offspring
Mothers sensitized with saline or OVA were coordinately mated, and offspring of saline- or OVA-exposed mothers were fostered and nursed by OVA- or saline-sensitized mothers. Offspring of OVA- or saline-sensitized mothers were kept in the original cage and nursed by OVA- or saline-sensitized mothers together with fostered offspring of saline- or OVA-exposed mothers.
Breast milk collection
Mouse breast milk was collected after i.p. injection of 4 IU oxytocin (Sigma) 24 h after OVA sensitization of breastfeeding mothers.
IC supplementation
OVA-IgG1-IC was formed as previously described (Baker et al., 2011). In brief, mouse monoclonal anti-OVA-IgG1 antibodies purified using Affi-Gel Protein A MAPS II kit (Biorad) from hybridoma (clone 01–4), a kind gift of H. Karasuyama (Tokyo Medical and Dental University, Tokyo, Japan), was incubated at 1:2 molar ratio of anti-OVA-IgG1 to OVA at 37°C for 30 min. OVA-IgG1-IC (100 µg) was given i.p. to BALB/c WT female mice once weekly for 6 wk during pregnancy and breastfeeding, or for 3 wk during breastfeeding.
Human breast milk supplementation
3–5-wk-old hFCGRT-hB2M-mFcgrt−/− mice were treated by gavage twice a week for 2 wk with 300 µl pooled human breast milk samples containing OVA-IgG4-IC, the same breast milk samples depleted of IgG, or PBS. IgG depletion was performed with the Nab Protein G Spin kit (Thermo Scientific) according to the manufacturer’s instructions. Mice were epicutaneously sensitized with 200 µg egg white extract (Greer) and orally challenged with 200 mg egg white protein (Nutriom LLC) as described above (Allergen sensitization and anaphylaxis).
ELISA
Analysis of mMCP1, mouse IL-4, OVA-specific IgE, IgG1, and IgG2a were performed as described previously (Oyoshi et al., 2011; Bartnikas et al., 2013). Biotin-conjugated peanut extract or egg white extract (Greer) was used to detect peanut- or egg white–specific IgE. TGF-β1 was measured per the manufacturer’s instructions (Affymetrix). For analysis of OVA-IgG1 and OVA-IgG2a immune complexes or free OVA, ELISA plates were coated with goat anti-OVA polyclonal antibody (MP Biomedicals), saturated nonspecific binding, and incubated with serum or breast milk, followed by incubation with biotin-conjugated anti–mouse IgG1 or IgG2a antibodies (BD) or rabbit anti-OVA polyclonal antibody (Abcam). HRP-conjugated avidin (Affymetrix) was used for detection. As a reference standard, adjacent wells on the same plates were coated with OVA (grade V, Sigma), and anti-OVA mouse IgG1 (Sigma) or anti-OVA mouse IgG2a (Biolegend) were added. For analysis of human OVA-IgG4 or OVA-IgG4 immune complexes, ELISA plates were coated with OVA or goat anti-OVA antibody and incubated with breast milk followed by biotin-conjugated anti–human IgG4 (BD Biosciences) with total human IgG4 as a reference standard.
Cell cultures
Allergen-specific T cells were identified by allergen-induced ex vivo proliferation as previously described (Burton et al., 2014b). In brief, MLN cells were labeled with CellTrace Violet (Life Technologies) and cultured in complete RPMI 1640 (Invitrogen) supplemented by 10% FCS, 0.05 mM 2-mercaptoethanol, and penicillin/streptomycin with or without allergen (200 µg/ml OVA or peanut extract) for 5 d. Cells undergoing proliferation (dye dilution) were considered allergen-specific, a conclusion supported by a lack of proliferation in the absence of allergen or in allergen-stimulated cells from unsensitized mice. For OVA-specific T reg cell differentiation, CD11c+ DCs were enriched from MLN of 3–5-wk-old WT or Fcgrt−/− mice by CD11c+ Microbeads (Miltenyi). Naive WT mice or offspring of epicutaneously sensitized mothers with saline or OVA 1 day after last maternal exposure (day 46; Fig. 1 A) were used. Naive CD4+ T cells were enriched from spleen of DO11.10+Rag2−/−Foxp3EGFP mice by a naive CD4+ T cell kit (Miltenyi). Naive CD4+ T cells (2.5 × 105) were cultured with CD11c+ DCs (0.5 × 105) in the presence or absence of 40× diluted breast milk, or 1 µM OVA323-339 peptide and 5 ng/ml recombinant human TGF-β1 (R&D Systems). After 3 d, allergen-specific induced T reg cells were analyzed by flow cytometry on the basis of EGFP fluorescence.
Adoptive transfer
CD11c+ DCs were enriched as above (Cell cultures) from MLN of offspring. CD11c+ DCs (2 × 105) and naive CD4+DO11.10+Foxp3EGFP- T cells (2 × 106) prepared as above (Cell cultures) were injected intravenously into naive recipients. After 5 d, recipients were epicutaneously sensitized over 9 d, then orally challenged with OVA as above (Allergen sensitization and anaphylaxis).
Suppression assay
CD4+CD25+ T reg cells isolated by magnetic beads (Miltenyi) from MLN of BALB/c WT offspring were cocultured with naive CD4+ T cells isolated from DO11.10+Rag2−/−Foxp3EGFP mice (2.5 × 104 cells/well) labeled with CellTrace Violet at 1:1 T reg/responder ratio in the presence of 1 µM OVA323-339 peptide and irradiated splenocytes (5 × 104 cells/well). After 4 d, cell proliferation was evaluated by dye dilution. The percent suppression was calculated using the following formula:
Flow cytometry
T reg cells were stained with fluorochrome-conjugated antibodies for Foxp3, CD3, CD4, and CD25 using the Foxp3 staining kit (eBioscience). For lamina propria lymphocyte isolation, a jejunum section of the small intestine was harvested, and the tissue was prepared as previously described (Galand et al., 2016). Single-cell suspensions were stained with fluorochrome-conjugated mAbs for IgE, c-kit, CD3, CD11c, CD19, CD45, and NKp46 (purchased from BioLegend, BD Biosciences, or eBioscience). Dead cells were routinely excluded from analysis by Fixable Viability Dye staining (eBioscience). Mouse mast cells were identified as live, CD45+lin−c-kit+IgE+ cells. Cells were analyzed on LSRFortessa (BD), and the data were analyzed using FlowJo software (Tree Star Inc.).
Quantitative PCR
Quantitative real-time PCR was performed as described previously (Oyoshi et al., 2011).
Statistical analysis
A Mann-Whitney U test (between two groups) or nonparametric one-way ANOVA (between multiple groups) was used to compare the distribution of each outcome. All analyses were performed with Prism software, version 6.0 (GraphPad Software). A p-value of <0.05 was considered to indicate statistical significance.
Online supplemental material
Fig. S1 shows that maternal sensitization protects offspring from peanut allergy. Fig. S2 shows the contribution of in utero transferred maternal IgG-IC to induction of neonatal tolerance. Fig. S3 shows maternal sensitization induces long-lasting protection in offspring. Fig. S4 summarizes the mechanisms of neonatal tolerance via a maternal IgG-IC-FcRn axis. Table S1 summarizes the characteristics of human breast milk.
Supplementary Material
Acknowledgments
We thank Dr. Talal A. Chatila for his kind gifts of Foxp3EGFP/DTR+ and DO11.10+Rag2−/−Foxp3EGFP mice and scientific discussion. We thank Dr. Hans C. Oettgen for scientific advice and reading the manuscript, Ms. Kimberly H. Barbas for facilitating clinical sample collection, and Ms. Shelly Zing Chin Lum and Ms. Jacqueline Beaupre for technical assistance.
This work was supported by the Food Allergy Research and Education (FARE), the HOPE APFED/ARTrust Pilot Grant, the William F. Milton Fund, the Harvard Catalyst Clinical and Translational Research Center (NCATS grant no. 8UL 1TR000170), and the Boston Children’s Hospital Pediatric Associates Award to M.K. Oyoshi and a National Institutes of Health grant (no. DK053056) and a Harvard Digestive Diseases Center grant (no. P30DK034854) to R.S. Blumberg.
R.S. Blumberg consults for and has received equity in Syntimmune Pharmaceuticals, which is developing therapies that target FcRn. The rest of the authors declare no competing financial interests.
Author contributions: A. Ohsaki, N. Venturelli, and M.K. Oyoshi designed and performed the experiments; T.M. Buccigrosso, S.K. Osganian, J. Lee, and M.K. Oyoshi collected clinical samples; R.S. Blumberg provided critical mice; and A. Ohsaki, R.S. Blumberg, and M.K. Oyoshi wrote the manuscript.
References
- Akdis C.A., and Akdis M.. 2011. Mechanisms of allergen-specific immunotherapy. J. Allergy Clin. Immunol. 127:18–27. 10.1016/j.jaci.2010.11.030 [DOI] [PubMed] [Google Scholar]
- Akilesh S., Huber T.B., Wu H., Wang G., Hartleben B., Kopp J.B., Miner J.H., Roopenian D.C., Unanue E.R., and Shaw A.S.. 2008. Podocytes use FcRn to clear IgG from the glomerular basement membrane. Proc. Natl. Acad. Sci. USA. 105:967–972. 10.1073/pnas.0711515105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antohe F., Rădulescu L., Gafencu A., Gheţie V., and Simionescu M.. 2001. Expression of functionally active FcRn and the differentiated bidirectional transport of IgG in human placental endothelial cells. Hum. Immunol. 62:93–105. 10.1016/S0198-8859(00)00244-5 [DOI] [PubMed] [Google Scholar]
- Bai Y., Ye L., Tesar D.B., Song H., Zhao D., Björkman P.J., Roopenian D.C., and Zhu X.. 2011. Intracellular neutralization of viral infection in polarized epithelial cells by neonatal Fc receptor (FcRn)-mediated IgG transport. Proc. Natl. Acad. Sci. USA. 108:18406–18411. 10.1073/pnas.1115348108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker K., Qiao S.W., Kuo T.T., Aveson V.G., Platzer B., Andersen J.T., Sandlie I., Chen Z., de Haar C., Lencer W.I., et al. . 2011. Neonatal Fc receptor for IgG (FcRn) regulates cross-presentation of IgG immune complexes by CD8-CD11b+ dendritic cells. Proc. Natl. Acad. Sci. USA. 108:9927–9932. 10.1073/pnas.1019037108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker K., Rath T., Flak M.B., Arthur J.C., Chen Z., Glickman J.N., Zlobec I., Karamitopoulou E., Stachler M.D., Odze R.D., et al. . 2013. Neonatal Fc receptor expression in dendritic cells mediates protective immunity against colorectal cancer. Immunity. 39:1095–1107. 10.1016/j.immuni.2013.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker K., Rath T., Pyzik M., and Blumberg R.S.. 2014. The Role of FcRn in Antigen Presentation. Front. Immunol. 5:408 10.3389/fimmu.2014.00408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartnikas L.M., Gurish M.F., Burton O.T., Leisten S., Janssen E., Oettgen H.C., Beaupre J., Lewis C.N., Austen K.F., Schulte S., et al. . 2013. Epicutaneous sensitization results in IgE-dependent intestinal mast cell expansion and food-induced anaphylaxis. J. Allergy Clin. Immunol. 131:451–460. 10.1016/j.jaci.2012.11.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard H., Ah-Leung S., Drumare M.F., Feraudet-Tarisse C., Verhasselt V., Wal J.M., Créminon C., and Adel-Patient K.. 2014. Peanut allergens are rapidly transferred in human breast milk and can prevent sensitization in mice. Allergy. 69:888–897. 10.1111/all.12411 [DOI] [PubMed] [Google Scholar]
- Brambell F.W. 1969. The transmission of immune globulins from the mother to the foetal and newborn young. Proc. Nutr. Soc. 28:35–41. 10.1079/PNS19690007 [DOI] [PubMed] [Google Scholar]
- Brough H.A., Simpson A., Makinson K., Hankinson J., Brown S., Douiri A., Belgrave D.C., Penagos M., Stephens A.C., McLean W.H., et al. . 2014. Peanut allergy: effect of environmental peanut exposure in children with filaggrin loss-of-function mutations. J. Allergy Clin. Immunol. 134:867–875. 10.1016/j.jaci.2014.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S.J., Asai Y., Cordell H.J., Campbell L.E., Zhao Y., Liao H., Northstone K., Henderson J., Alizadehfar R., Ben-Shoshan M., et al. . 2011. Loss-of-function variants in the filaggrin gene are a significant risk factor for peanut allergy. J. Allergy Clin. Immunol. 127:661–667. 10.1016/j.jaci.2011.01.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunyavanich S., Rifas-Shiman S.L., Platts-Mills T.A., Workman L., Sordillo J.E., Camargo C.A. Jr., Gillman M.W., Gold D.R., and Litonjua A.A.. 2014. Peanut, milk, and wheat intake during pregnancy is associated with reduced allergy and asthma in children. J. Allergy Clin. Immunol. 133:1373–1382. 10.1016/j.jaci.2013.11.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton O.T., Logsdon S.L., Zhou J.S., Medina-Tamayo J., Abdel-Gadir A., Noval Rivas M., Koleoglou K.J., Chatila T.A., Schneider L.C., Rachid R., et al. . 2014a Oral immunotherapy induces IgG antibodies that act through FcgammaRIIb to suppress IgE-mediated hypersensitivity. J. Allergy Clin. Immunol. 134:1310–1317. 10.1016/j.jaci.2014.05.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton O.T., Noval Rivas M., Zhou J.S., Logsdon S.L., Darling A.R., Koleoglou K.J., Roers A., Houshyar H., Crackower M.A., Chatila T.A., and Oettgen H.C.. 2014b Immunoglobulin E signal inhibition during allergen ingestion leads to reversal of established food allergy and induction of regulatory T cells. Immunity. 41:141–151. 10.1016/j.immuni.2014.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton O.T., Tamayo J.M., Stranks A.J., Koleoglou K.J., and Oettgen H.C.. 2017. Allergen-specific IgG antibodies signaling via FcgammaRIIb promote food tolerance. J. Allergy Clin. Immunol. 10.1016/j.jaci.2017.03.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caubet J.C., Bencharitiwong R., Moshier E., Godbold J.H., Sampson H.A., and Nowak-Węgrzyn A.. 2012. Significance of ovomucoid- and ovalbumin-specific IgE/IgG(4) ratios in egg allergy. J. Allergy Clin. Immunol. 129:739–747. 10.1016/j.jaci.2011.11.053 [DOI] [PubMed] [Google Scholar]
- Chatila T.A. 2005. Role of regulatory T cells in human diseases. J. Allergy Clin. Immunol. 116:949–959. 10.1016/j.jaci.2005.08.047 [DOI] [PubMed] [Google Scholar]
- Chaudhury C., Mehnaz S., Robinson J.M., Hayton W.L., Pearl D.K., Roopenian D.C., and Anderson C.L.. 2003. The major histocompatibility complex-related Fc receptor for IgG (FcRn) binds albumin and prolongs its lifespan. J. Exp. Med. 197:315–322. 10.1084/jem.20021829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claypool S.M., Dickinson B.L., Yoshida M., Lencer W.I., and Blumberg R.S.. 2002. Functional reconstitution of human FcRn in Madin-Darby canine kidney cells requires co-expressed human beta 2-microglobulin. J. Biol. Chem. 277:28038–28050. 10.1074/jbc.M202367200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curotto de Lafaille M.A., Kutchukhidze N., Shen S., Ding Y., Yee H., and Lafaille J.J.. 2008. Adaptive Foxp3+ regulatory T cell-dependent and -independent control of allergic inflammation. Immunity. 29:114–126. 10.1016/j.immuni.2008.05.010 [DOI] [PubMed] [Google Scholar]
- De Groot A.S., and Martin W.. 2009. Reducing risk, improving outcomes: bioengineering less immunogenic protein therapeutics. Clin. Immunol. 131:189–201. 10.1016/j.clim.2009.01.009 [DOI] [PubMed] [Google Scholar]
- De Groot A.S., Moise L., McMurry J.A., Wambre E., Van Overtvelt L., Moingeon P., Scott D.W., and Martin W.. 2008. Activation of natural regulatory T cells by IgG Fc-derived peptide “Tregitopes”. Blood. 112:3303–3311. 10.1182/blood-2008-02-138073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson B.L., Badizadegan K., Wu Z., Ahouse J.C., Zhu X., Simister N.E., Blumberg R.S., and Lencer W.I.. 1999. Bidirectional FcRn-dependent IgG transport in a polarized human intestinal epithelial cell line. J. Clin. Invest. 104:903–911. 10.1172/JCI6968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson B.L., Claypool S.M., D’Angelo J.A., Aiken M.L., Venu N., Yen E.H., Wagner J.S., Borawski J.A., Pierce A.T., Hershberg R., et al. . 2008. Ca2+-dependent calmodulin binding to FcRn affects immunoglobulin G transport in the transcytotic pathway. Mol. Biol. Cell. 19:414–423. 10.1091/mbc.E07-07-0658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eigenmann P.A., Sicherer S.H., Borkowski T.A., Cohen B.A., and Sampson H.A.. 1998. Prevalence of IgE-mediated food allergy among children with atopic dermatitis. Pediatrics. 101:E8 10.1542/peds.101.3.e8 [DOI] [PubMed] [Google Scholar]
- Frazier A.L., Camargo C.A. Jr., Malspeis S., Willett W.C., and Young M.C.. 2014. Prospective study of peripregnancy consumption of peanuts or tree nuts by mothers and the risk of peanut or tree nut allergy in their offspring. JAMA Pediatr. 168:156–162. 10.1001/jamapediatrics.2013.4139 [DOI] [PubMed] [Google Scholar]
- Fusaro A.E., Maciel M., Victor J.R., Oliveira C.R., Duarte A.J., and Sato M.N.. 2002. Influence of maternal murine immunization with Dermatophagoides pteronyssinus extract on the type I hypersensitivity response in offspring. Int. Arch. Allergy Immunol. 127:208–216. 10.1159/000053865 [DOI] [PubMed] [Google Scholar]
- Fusaro A.E., Brito C.A., Victor J.R., Rigato P.O., Goldoni A.L., Duarte A.J., and Sato M.N.. 2007. Maternal-fetal interaction: preconception immunization in mice prevents neonatal sensitization induced by allergen exposure during pregnancy and breastfeeding. Immunology. 122:107–115. 10.1111/j.1365-2567.2007.02618.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galand C., Leyva-Castillo J.M., Yoon J., Han A., Lee M.S., McKenzie A.N., Stassen M., Oyoshi M.K., Finkelman F.D., and Geha R.S.. 2016. IL-33 promotes food anaphylaxis in epicutaneously sensitized mice by targeting mast cells. J. Allergy Clin. Immunol. 138:1356–1366. 10.1016/j.jaci.2016.03.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill R.K., Mahmood S., Sodhi C.P., Nagpaul J.P., and Mahmood A.. 1999. IgG binding and expression of its receptor in rat intestine during postnatal development. Indian J. Biochem. Biophys. 36:252–257. [PubMed] [Google Scholar]
- Gri G., Piconese S., Frossi B., Manfroi V., Merluzzi S., Tripodo C., Viola A., Odom S., Rivera J., Colombo M.P., and Pucillo C.E.. 2008. CD4+CD25+ regulatory T cells suppress mast cell degranulation and allergic responses through OX40-OX40L interaction. Immunity. 29:771–781. 10.1016/j.immuni.2008.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilliams M., Bruhns P., Saeys Y., Hammad H., and Lambrecht B.N.. 2014. The function of Fcγ receptors in dendritic cells and macrophages. Nat. Rev. Immunol. 14:94–108. 10.1038/nri3582 [DOI] [PubMed] [Google Scholar]
- Haribhai D., Williams J.B., Jia S., Nickerson D., Schmitt E.G., Edwards B., Ziegelbauer J., Yassai M., Li S.H., Relland L.M., et al. . 2011. A requisite role for induced regulatory T cells in tolerance based on expanding antigen receptor diversity. Immunity. 35:109–122. 10.1016/j.immuni.2011.03.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill D.J., Heine R.G., Hosking C.S., Brown J., Thiele L., Allen K.J., Su J., Varigos G., and Carlin J.B.. 2007. IgE food sensitization in infants with eczema attending a dermatology department. J. Pediatr. 151:359–363. 10.1016/j.jpeds.2007.04.070 [DOI] [PubMed] [Google Scholar]
- Hirose J., Ito S., Hirata N., Kido S., Kitabatake N., and Narita H.. 2001. Occurrence of the major food allergen, ovomucoid, in human breast milk as an immune complex. Biosci. Biotechnol. Biochem. 65:1438–1440. 10.1271/bbb.65.1438 [DOI] [PubMed] [Google Scholar]
- Hochwallner H., Alm J., Lupinek C., Johansson C., Mie A., Scheynius A., and Valenta R.. 2014. Transmission of allergen-specific IgG and IgE from maternal blood into breast milk visualized with microarray technology. J. Allergy Clin. Immunol. 134:1213–1215. 10.1016/j.jaci.2014.08.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husby S., Oxelius V.A., Teisner B., Jensenius J.C., and Svehag S.E.. 1985. Humoral immunity to dietary antigens in healthy adults. Occurrence, isotype and IgG subclass distribution of serum antibodies to protein antigens. Int. Arch. Allergy Appl. Immunol. 77:416–422. 10.1159/000233819 [DOI] [PubMed] [Google Scholar]
- James L.K., Bowen H., Calvert R.A., Dodev T.S., Shamji M.H., Beavil A.J., McDonnell J.M., Durham S.R., and Gould H.J.. 2012. Allergen specificity of IgG(4)-expressing B cells in patients with grass pollen allergy undergoing immunotherapy. J. Allergy Clin. Immunol. 130:663–670. 10.1016/j.jaci.2012.04.006 [DOI] [PubMed] [Google Scholar]
- Jarrett E., and Hall E.. 1979. Selective suppression of IgE antibody responsiveness by maternal influence. Nature. 280:145–147. 10.1038/280145a0 [DOI] [PubMed] [Google Scholar]
- Kaiserlian D., Vidal K., and Revillard J.P.. 1989. Murine enterocytes can present soluble antigen to specific class II-restricted CD4+ T cells. Eur. J. Immunol. 19:1513–1516. 10.1002/eji.1830190827 [DOI] [PubMed] [Google Scholar]
- Karlsson M.R., Rugtveit J., and Brandtzaeg P.. 2004. Allergen-responsive CD4+CD25+ regulatory T cells in children who have outgrown cow’s milk allergy. J. Exp. Med. 199:1679–1688. 10.1084/jem.20032121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lack G., Fox D., Northstone K., and Golding J.. Avon Longitudinal Study of Parents and Children Study Team . 2003. Factors associated with the development of peanut allergy in childhood. N. Engl. J. Med. 348:977–985. 10.1056/NEJMoa013536 [DOI] [PubMed] [Google Scholar]
- Leach J.L., Sedmak D.D., Osborne J.M., Rahill B., Lairmore M.D., and Anderson C.L.. 1996. Isolation from human placenta of the IgG transporter, FcRn, and localization to the syncytiotrophoblast: implications for maternal-fetal antibody transport. J. Immunol. 157:3317–3322. [PubMed] [Google Scholar]
- Lei T.C., and Scott D.W.. 2005. Induction of tolerance to factor VIII inhibitors by gene therapy with immunodominant A2 and C2 domains presented by B cells as Ig fusion proteins. Blood. 105:4865–4870. 10.1182/blood-2004-11-4274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leme A.S., Hubeau C., Xiang Y., Goldman A., Hamada K., Suzaki Y., and Kobzik L.. 2006. Role of breast milk in a mouse model of maternal transmission of asthma susceptibility. J. Immunol. 176:762–769. 10.4049/jimmunol.176.2.762 [DOI] [PubMed] [Google Scholar]
- Li Z., Palaniyandi S., Zeng R., Tuo W., Roopenian D.C., and Zhu X.. 2011. Transfer of IgG in the female genital tract by MHC class I-related neonatal Fc receptor (FcRn) confers protective immunity to vaginal infection. Proc. Natl. Acad. Sci. USA. 108:4388–4393. 10.1073/pnas.1012861108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littman D.R., and Rudensky A.Y.. 2010. Th17 and regulatory T cells in mediating and restraining inflammation. Cell. 140:845–858. 10.1016/j.cell.2010.02.021 [DOI] [PubMed] [Google Scholar]
- Liu X., Lu L., Yang Z., Palaniyandi S., Zeng R., Gao L.Y., Mosser D.M., Roopenian D.C., and Zhu X.. 2011. The neonatal FcR-mediated presentation of immune-complexed antigen is associated with endosomal and phagosomal pH and antigen stability in macrophages and dendritic cells. J. Immunol. 186:4674–4686. 10.4049/jimmunol.1003584 [DOI] [PubMed] [Google Scholar]
- López-Expósito I., Song Y., Järvinen K.M., Srivastava K., and Li X.M.. 2009. Maternal peanut exposure during pregnancy and lactation reduces peanut allergy risk in offspring. J. Allergy Clin. Immunol. 124:1039–1046. 10.1016/j.jaci.2009.08.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matson A.P., Thrall R.S., Rafti E., and Puddington L.. 2009. Breastmilk from allergic mothers can protect offspring from allergic airway inflammation. Breastfeed. Med. 4:167–174. 10.1089/bfm.2008.0130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalfe D.D., Peavy R.D., and Gilfillan A.M.. 2009. Mechanisms of mast cell signaling in anaphylaxis. J. Allergy Clin. Immunol. 124:639–646, quiz: 647–648. 10.1016/j.jaci.2009.08.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montoyo H.P., Vaccaro C., Hafner M., Ober R.J., Mueller W., and Ward E.S.. 2009. Conditional deletion of the MHC class I-related receptor FcRn reveals the sites of IgG homeostasis in mice. Proc. Natl. Acad. Sci. USA. 106:2788–2793. 10.1073/pnas.0810796106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosconi E., Rekima A., Seitz-Polski B., Kanda A., Fleury S., Tissandie E., Monteiro R., Dombrowicz D.D., Julia V., Glaichenhaus N., and Verhasselt V.. 2010. Breast milk immune complexes are potent inducers of oral tolerance in neonates and prevent asthma development. Mucosal Immunol. 3:461–474. 10.1038/mi.2010.23 [DOI] [PubMed] [Google Scholar]
- Mousallem T., and Burks A.W.. 2012. Immunology in the Clinic Review Series; focus on allergies: immunotherapy for food allergy. Clin. Exp. Immunol. 167:26–31. 10.1111/j.1365-2249.2011.04499.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima S., Igyarto B.Z., Honda T., Egawa G., Otsuka A., Hara-Chikuma M., Watanabe N., Ziegler S.F., Tomura M., Inaba K., et al. . 2012. Langerhans cells are critical in epicutaneous sensitization with protein antigen via thymic stromal lymphopoietin receptor signaling. J. Allergy Clin. Immunol. 129:1048–1055. 10.1016/j.jaci.2012.01.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noval Rivas M., Burton O.T., Wise P., Charbonnier L.M., Georgiev P., Oettgen H.C., Rachid R., and Chatila T.A.. 2015. Regulatory T cell reprogramming toward a Th2-cell-like lineage impairs oral tolerance and promotes food allergy. Immunity. 42:512–523. 10.1016/j.immuni.2015.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkura N., Kitagawa Y., and Sakaguchi S.. 2013. Development and maintenance of regulatory T cells. Immunity. 38:414–423. 10.1016/j.immuni.2013.03.002 [DOI] [PubMed] [Google Scholar]
- Oyoshi M.K., Elkhal A., Scott J.E., Wurbel M.A., Hornick J.L., Campbell J.J., and Geha R.S.. 2011. Epicutaneous challenge of orally immunized mice redirects antigen-specific gut-homing T cells to the skin. J. Clin. Invest. 121:2210–2220. 10.1172/JCI43586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyoshi M.K., Oettgen H.C., Chatila T.A., Geha R.S., and Bryce P.J.. 2014. Food allergy: Insights into etiology, prevention, and treatment provided by murine models. J. Allergy Clin. Immunol. 133:309–317. 10.1016/j.jaci.2013.12.1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkin M.R., Logan K., Tseng A., Raji B., Ayis S., Peacock J., Brough H., Marrs T., Radulovic S., Craven J., et al. EAT Study Team . 2016. Randomized Trial of Introduction of Allergenic Foods in Breast-Fed Infants. N. Engl. J. Med. 374:1733–1743. 10.1056/NEJMoa1514210 [DOI] [PubMed] [Google Scholar]
- Pyzik M., Rath T., Lencer W.I., Baker K., and Blumberg R.S.. 2015. FcRn: The Architect Behind the Immune and Nonimmune Functions of IgG and Albumin. J. Immunol. 194:4595–4603. 10.4049/jimmunol.1403014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao S.W., Kobayashi K., Johansen F.E., Sollid L.M., Andersen J.T., Milford E., Roopenian D.C., Lencer W.I., and Blumberg R.S.. 2008. Dependence of antibody-mediated presentation of antigen on FcRn. Proc. Natl. Acad. Sci. USA. 105:9337–9342. 10.1073/pnas.0801717105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rath T., Baker K., Dumont J.A., Peters R.T., Jiang H., Qiao S.W., Lencer W.I., Pierce G.F., and Blumberg R.S.. 2015. Fc-fusion proteins and FcRn: structural insights for longer-lasting and more effective therapeutics. Crit. Rev. Biotechnol. 35:235–254. 10.3109/07388551.2013.834293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rekima A., Macchiaverni P., Turfkruyer M., Holvoet S., Dupuis L., Baiz N., Annesi-Maesano I., Mercenier A., Nutten S., and Verhasselt V.. 2017. Long-term reduction in food allergy susceptibility in mice by combining breastfeeding-induced tolerance and TGF-β-enriched formula after weaning. Clin. Exp. Allergy. 47:565–576. 10.1111/cea.12864 [DOI] [PubMed] [Google Scholar]
- Renz H., Brandtzaeg P., and Hornef M.. 2011. The impact of perinatal immune development on mucosal homeostasis and chronic inflammation. Nat. Rev. Immunol. 12:9–23. 10.1038/nri3112 [DOI] [PubMed] [Google Scholar]
- Roopenian D.C., Christianson G.J., Sproule T.J., Brown A.C., Akilesh S., Jung N., Petkova S., Avanessian L., Choi E.Y., Shaffer D.J., et al. . 2003. The MHC class I-like IgG receptor controls perinatal IgG transport, IgG homeostasis, and fate of IgG-Fc-coupled drugs. J. Immunol. 170:3528–3533. 10.4049/jimmunol.170.7.3528 [DOI] [PubMed] [Google Scholar]
- Rumbo M., Chirdo F.G., Añón M.C., and Fossati C.A.. 1998. Detection and characterization of antibodies specific to food antigens (gliadin, ovalbumin and beta-lactoglobulin) in human serum, saliva, colostrum and milk. Clin. Exp. Immunol. 112:453–458. 10.1046/j.1365-2249.1998.00587.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scadding G.W., Shamji M.H., Jacobson M.R., Lee D.I., Wilson D., Lima M.T., Pitkin L., Pilette C., Nouri-Aria K., and Durham S.R.. 2010. Sublingual grass pollen immunotherapy is associated with increases in sublingual Foxp3-expressing cells and elevated allergen-specific immunoglobulin G4, immunoglobulin A and serum inhibitory activity for immunoglobulin E-facilitated allergen binding to B cells. Clin. Exp. Allergy. 40:598–606. 10.1111/j.1365-2222.2010.03462.x [DOI] [PubMed] [Google Scholar]
- Schroeder H.W. Jr., and Cavacini L.. 2010. Structure and function of immunoglobulins. J. Allergy Clin. Immunol. 125(2, Suppl 2):S41–S52. 10.1016/j.jaci.2009.09.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz A., Panetta V., Cappella A., Hofmaier S., Hatzler L., Rohrbach A., Tsilochristou O., Bauer C.P., Hoffmann U., Forster J., et al. . 2016. IgG and IgG4 to 91 allergenic molecules in early childhood by route of exposure and current and future IgE sensitization: Results from the Multicentre Allergy Study birth cohort. J. Allergy Clin. Immunol. 138:1426–1433. 10.1016/j.jaci.2016.01.057 [DOI] [PubMed] [Google Scholar]
- Scott D.W., and De Groot A.S.. 2010. Can we prevent immunogenicity of human protein drugs? Ann. Rheum. Dis. 69(Suppl 1):i72–i76. 10.1136/ard.2009.117564 [DOI] [PubMed] [Google Scholar]
- Shreffler W.G., Wanich N., Moloney M., Nowak-Wegrzyn A., and Sampson H.A.. 2009. Association of allergen-specific regulatory T cells with the onset of clinical tolerance to milk protein. J. Allergy Clin. Immunol. 123:43–52. 10.1016/j.jaci.2008.09.051 [DOI] [PubMed] [Google Scholar]
- Sicherer S.H., and Sampson H.A.. 1999. Food hypersensitivity and atopic dermatitis: pathophysiology, epidemiology, diagnosis, and management. J. Allergy Clin. Immunol. 104:S114–S122. 10.1016/S0091-6749(99)70053-9 [DOI] [PubMed] [Google Scholar]
- Sicherer S.H., and Sampson H.A.. 2014. Food allergy: Epidemiology, pathogenesis, diagnosis, and treatment. J. Allergy Clin. Immunol. 133:291–307. 10.1016/j.jaci.2013.11.020 [DOI] [PubMed] [Google Scholar]
- Sicherer S.H., Burks A.W., and Sampson H.A.. 1998. Clinical features of acute allergic reactions to peanut and tree nuts in children. Pediatrics. 102:e6 10.1542/peds.102.1.e6 [DOI] [PubMed] [Google Scholar]
- Sicherer S.H., Wood R.A., Stablein D., Lindblad R., Burks A.W., Liu A.H., Jones S.M., Fleischer D.M., Leung D.Y., and Sampson H.A.. 2010. Maternal consumption of peanut during pregnancy is associated with peanut sensitization in atopic infants. J. Allergy Clin. Immunol. 126:1191–1197. 10.1016/j.jaci.2010.08.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simister N.E., and Mostov K.E.. 1989. An Fc receptor structurally related to MHC class I antigens. Nature. 337:184–187. 10.1038/337184a0 [DOI] [PubMed] [Google Scholar]
- Simister N.E., Story C.M., Chen H.L., and Hunt J.S.. 1996. An IgG-transporting Fc receptor expressed in the syncytiotrophoblast of human placenta. Eur. J. Immunol. 26:1527–1531. 10.1002/eji.1830260718 [DOI] [PubMed] [Google Scholar]
- Skripak J.M., Nash S.D., Rowley H., Brereton N.H., Oh S., Hamilton R.G., Matsui E.C., Burks A.W., and Wood R.A.. 2008. A randomized, double-blind, placebo-controlled study of milk oral immunotherapy for cow’s milk allergy. J. Allergy Clin. Immunol. 122:1154–1160. 10.1016/j.jaci.2008.09.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiekermann G.M., Finn P.W., Ward E.S., Dumont J., Dickinson B.L., Blumberg R.S., and Lencer W.I.. 2002. Receptor-mediated immunoglobulin G transport across mucosal barriers in adult life: functional expression of FcRn in the mammalian lung. J. Exp. Med. 196:303–310. 10.1084/jem.20020400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed A., Garcia M.A., Lyu S.C., Bucayu R., Kohli A., Ishida S., Berglund J.P., Tsai M., Maecker H., O’Riordan G., et al. . 2014. Peanut oral immunotherapy results in increased antigen-induced regulatory T-cell function and hypomethylation of forkhead box protein 3 (FOXP3). J. Allergy Clin. Immunol. 133:500–510. 10.1016/j.jaci.2013.12.1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Till S.J., Francis J.N., Nouri-Aria K., and Durham S.R.. 2004. Mechanisms of immunotherapy. J. Allergy Clin. Immunol. 113:1025–1034. 10.1016/j.jaci.2004.03.024 [DOI] [PubMed] [Google Scholar]
- Uthoff H., Spenner A., Reckelkamm W., Ahrens B., Wölk G., Hackler R., Hardung F., Schaefer J., Scheffold A., Renz H., and Herz U.. 2003. Critical role of preconceptional immunization for protective and nonpathological specific immunity in murine neonates. J. Immunol. 171:3485–3492. 10.4049/jimmunol.171.7.3485 [DOI] [PubMed] [Google Scholar]
- Valenta R., Hochwallner H., Linhart B., and Pahr S.. 2015. Food allergies: the basics. Gastroenterology. 148:1120–1131. 10.1053/j.gastro.2015.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wijk F., Wehrens E.J., Nierkens S., Boon L., Kasran A., Pieters R., and Knippels L.M.. 2007. CD4+CD25+ T cells regulate the intensity of hypersensitivity responses to peanut, but are not decisive in the induction of oral sensitization. Clin. Exp. Allergy. 37:572–581. 10.1111/j.1365-2222.2007.02681.x [DOI] [PubMed] [Google Scholar]
- Venturelli N., Lexmond W.S., Ohsaki A., Nurko S., Karasuyama H., Fiebiger E., and Oyoshi M.K.. 2016. Allergic skin sensitization promotes eosinophilic esophagitis via the IL-33-basophil axis in mice. J. Allergy Clin. Immunol. 138:1367–1380.e5. 10.1016/j.jaci.2016.02.034 [DOI] [PubMed] [Google Scholar]
- Verhasselt V. 2010a Neonatal tolerance under breastfeeding influence. Curr. Opin. Immunol. 22:623–630. 10.1016/j.coi.2010.08.008 [DOI] [PubMed] [Google Scholar]
- Verhasselt V. 2010b Oral tolerance in neonates: from basics to potential prevention of allergic disease. Mucosal Immunol. 3:326–333. 10.1038/mi.2010.25 [DOI] [PubMed] [Google Scholar]
- Wachholz P.A., and Durham S.R.. 2004. Mechanisms of immunotherapy: IgG revisited. Curr. Opin. Allergy Clin. Immunol. 4:313–318. 10.1097/01.all.0000136753.35948.c0 [DOI] [PubMed] [Google Scholar]
- Williams C.M., and Galli S.J.. 2000. Mast cells can amplify airway reactivity and features of chronic inflammation in an asthma model in mice. J. Exp. Med. 192:455–462. 10.1084/jem.192.3.455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M., Claypool S.M., Wagner J.S., Mizoguchi E., Mizoguchi A., Roopenian D.C., Lencer W.I., and Blumberg R.S.. 2004. Human neonatal Fc receptor mediates transport of IgG into luminal secretions for delivery of antigens to mucosal dendritic cells. Immunity. 20:769–783. 10.1016/j.immuni.2004.05.007 [DOI] [PubMed] [Google Scholar]
- Yoshida M., Kobayashi K., Kuo T.T., Bry L., Glickman J.N., Claypool S.M., Kaser A., Nagaishi T., Higgins D.E., Mizoguchi E., et al. . 2006. Neonatal Fc receptor for IgG regulates mucosal immune responses to luminal bacteria. J. Clin. Invest. 116:2142–2151. 10.1172/JCI27821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeiger R.S., Heller S., Mellon M.H., Forsythe A.B., O’Connor R.D., Hamburger R.N., and Schatz M.. 1989. Effect of combined maternal and infant food-allergen avoidance on development of atopy in early infancy: a randomized study. J. Allergy Clin. Immunol. 84:72–89. 10.1016/0091-6749(89)90181-4 [DOI] [PubMed] [Google Scholar]
- Zhu X., Meng G., Dickinson B.L., Li X., Mizoguchi E., Miao L., Wang Y., Robert C., Wu B., Smith P.D., et al. . 2001. MHC class I-related neonatal Fc receptor for IgG is functionally expressed in monocytes, intestinal macrophages, and dendritic cells. J. Immunol. 166:3266–3276. 10.4049/jimmunol.166.5.3266 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.