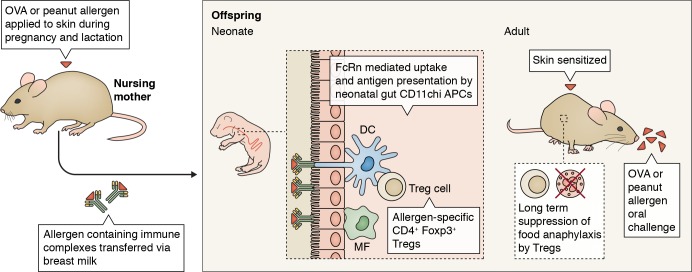
In this issue Ohsaki et al. explain how breastfeeding can prevent the onset of food allergies in offspring by instructing T reg formation via neonatal Fc receptor (FcRn)–mediated transfer and uptake of allergen-containing IgG immune complexes (Ig-ICs) by gut dendritic cells (DCs).
Abstract
In this issue Ohsaki et al. (https://doi.org/10.1084/jem.20171163) explain how breastfeeding can prevent the onset of food allergies in offspring by instructing T reg formation via neonatal Fc receptor (FcRn)–mediated transfer and uptake of allergen-containing IgG immune complexes (Ig-ICs) by gut dendritic cells (DCs).

Insight from Bart N. Lambrecht
Allergy is characterized by the presence of serum IgE antibodies to common and harmless environmental and food allergens and can lead to allergic diseases like asthma, rhinitis, food allergy, and anaphylaxis. As allergy is highly prevalent is Western societies, and there seems to be no halt to the epidemic of food allergy, there is an urgent need for preventive strategies (Lambrecht and Hammad, 2017). Breastfeeding has long been shown to have protective effects on allergy development, yet protection seems to depend on whether the nursing mother is allergic or not and by extension whether the baby has high or low risk to develop disease (Munblit and Verhasselt, 2016). Yet other studies showed that breastfeeding can also promote allergies in babies, by allowing the transfer of allergens from the mother’s diet. Maternal allergen avoidance during breastfeeding has therefore been a common recommendation to prevent the onset of allergies in high-risk babies born to allergic parents. However, recent data suggest that early life allergen avoidance during the period of breastfeeding does not pay off and that certain food allergens should be introduced early in a diet to prevent food allergies (Du Toit et al., 2015). Given these controversies, in this issue, Ohsaki et al. studied whether and how maternal allergy to ovalbumin (OVA; a major allergen in egg) or peanut affects the onset of allergy in their offspring. To induce allergy to foods, Ohsaki et al. (2018) sensitized mice via the epicutaneous route before and during pregnancy and during breastfeeding. When the offspring of these allergic mothers reached adulthood, they were also sensitized epicutaneously and given an oral challenge with allergen. Epicutaneous sensitization to food allergens is a highly relevant model, as peanut allergy in children often develops via a leaky skin barrier, and many food allergens like peanut are found in the household environment or as contaminants in baby skin lotions. The choice of OVA as a model allergen was also driven by the clinical observation that allergy to egg in early life is one of the strongest predictors of progression in the atopic march, the process by which children gradually develop severe allergic diseases like atopic dermatitis, rhinitis, and asthma. Remarkably, the offspring of allergic mothers was tolerant to food allergen challenge, whereas those born out of control nonallergic mothers developed signs of systemic anaphylaxis, a life-threatening form of food allergy. The tolerant offspring mice had developed allergen-specific Foxp3+ T reg cells that expanded in response to allergen exposure and suppressed anaphylaxis to food allergen challenge even in 3-mo-old offspring, when maternal-derived antibodies had long disappeared. Elegant setup of breeding and fostering of mice revealed that the protective T reg cells were induced mainly as a result of transfer of maternal allergen IgG immune complexes (Ig-ICs) via breastfeeding of the pups and less efficiently via direct transplacental transfer. Supplementation of lactating mice with allergen IgG1-IC was sufficient to confer protection in offspring. The neonatal Fc receptor FcRn is well known to mediate the transfer of Igs from mother to child via placenta and breastfeeding to suckling mice, implying a role of FcRn in mother and child. When only the offspring was deficient in FcRn, the induction of T reg cells and functional tolerance to food allergen offered by maternal allergen Ig-IC was abolished. Gut dendritic cells (DCs) express the FcRn receptor and were able to induce OVA-specific Foxp3+ T reg cells in response to exposure to breast milk–derived OVA Ig-ICs in vitro and ex vivo. Strikingly, mice lacking FcRn selectively in CD11chi DCs were unable to mount T reg cells and tolerance to food allergens via maternal protection. Ohsaki et al. (2018) finally made an important translational leap and fed humanized FcRn transgenic mice with breast milk of healthy nonatopic mothers. This source of breast milk was rich in OVA-specific IgG4-IC. Although this experiment was performed by oral gavage in adult mice, human breast milk suppressed the salient features of food allergy, including systemic anaphylaxis, suggesting that the mouse findings of the study likely translate to the human situation.
Mice were sensitized to OVA or peanut allergen via the skin during pregnancy and lactation, which led to the formation of IgG allergen–immune complexes. These were transferred to offspring mice via breast milk and taken up by APCs like DCs and macrophages (MF) in an FcRn-dependent manner. Triggering of the FcRn on DCs led to the formation of allergen-specific CD4+ Foxp3+ T reg cells. When the offspring mice reached adulthood, they were again sensitized via the skin to the allergens and subsequently were challenged via oral gavage. In mice born to allergic mothers, the T reg cells that were generated in an FcRn-dependent manner in the neonatal period were able to suppress anaphylaxis. In mice born to nonallergic mothers, the allergen challenge led to signs of severe anaphylaxis.
Breast milk has many beneficial effects on the progeny’s developing immune system, much more than passively protecting the offspring form infectious disease by passive transfer of maternal protective antibodies and influencing the composition of the infant gut microbiome and gut barrier function. Breastfeeding can shape the neonatal repertoire of T cells and B cells by transfer of free antigen, including such potent allergens like house dust mite and peanut. The current study is not the first to report that allergen Ig-ICs from an allergic mother protect the progeny against allergic disease via breastfeeding and FcRn and induction of Foxp3+ T reg cells (Mosconi et al., 2010; Nakata et al., 2010; Bernard et al., 2014). In the past it was found that TGFβ contained in breast milk could be decisive whether or not long-lasting T reg–mediated tolerance developed, but when Ig-ICs are present in breast milk, TGFβ does not seem to be required (Verhasselt et al., 2008). So where does the current study add novelty? In previous work, the role of FcRn was merely seen as a transfer receptor mediating the transepithelial uptake of allergen Ig-ICs from the lumen to the immune system’s DCs or macrophages, a known function of this receptor (Yoshida et al., 2004). However, in mice and humans, the FcRn is also expressed by DCs and macrophages (Guilliams et al., 2014), and it turns out increasingly that FcRn is crucial in determining the immunological outcome of an Ig-IC encounter (Stapleton et al., 2015). When IgG or and Ig-IC binds to activating Fc receptors on APCs, it is internalized in endosomes that undergo a gradual drop in pH. At a lower pH 6.5, the FcRn receptor has increased affinity for the constant domains of IgG. In the case of free IgG, this leads to recycling of the IgG to the cell membrane, followed by exocytosis, thus extending the half-life of IgG considerably. Binding of Ig-IC to FcRn and interaction with the invariant chain CD74 promote the routing of the Ig-IC to the MHCII-rich processing and loading compartment for presentation of peptides on MHCII to CD4 T cells (Qiao et al., 2008). It has also been shown that FcRn in DCs promotes the cross-presentation of Ig-IC by protecting antigens in an acidic endosomal environment and allowing transfer of undigested protein epitopes to the cytoplasm for further trimming by the proteasome (Baker et al., 2011). Ohsaki et al. (2018) suggest that FcRn might also act to promote tolerance, although the mechanism of such tolerance induction requires much more detailed analysis. It will be important to study which APCs among the CD11c targeted cells is mediating T reg tolerance in the gut. Various APCs can mediate food tolerance in the intestine of adult mice, but T reg formation seems to be particularly induced by macrophages closely collaborating with CD103+ conventional cDC1 (Mazzini et al., 2014). FcRn often does not work in isolation, but requires collaboration with activating or inhibitory Fc receptors for signaling and internalization of the Ig-ICs. Although the inhibitory Fc receptor FcγRIIb has a role in oral tolerance induction to common foods and is expressed by conventional DCs, it was shown that it was not required for tolerance induction by breast milk Ig-ICs in suckling mice (Samsom et al., 2005; Mosconi et al., 2010). Activating Fc receptors are therefore more likely candidates, but these are poorly expressed on conventional DCs, suggesting an important contribution of monocyte-derived cells and macrophages that highly express activating Fc receptors (Guilliams et al., 2014). Finally, the translational part of the study shows that ICs containing IgG4 and allergen confer protection from food allergy in adult mice in an FcRn-dependent manner. Induction of allergen-specific IgG4 is often seen in patients undergoing successful subcutaneous or sublingual allergen immunotherapy, or those who spontaneously tolerate high loads of allergen exposure like bee keepers and cat owners, likely also leading to formation of allergen Ig-ICs. It will be very important to study whether FcRn on DCs is involved in this particular state of tolerance to allergens. Studying the molecular details of the interaction of FcRn with IgG is certainly on the radar of drug companies who use this knowledge to improve the half-life of therapeutic antibodies that recycle via FcRn. It might therefore not be too long before we see therapeutic applications of tolerance promoting immune complexes that target FcRn.
References
- Baker K., et al. Proc. Natl. Acad. Sci. USA. 2011 doi: 10.1073/pnas.1019037108. [DOI] [Google Scholar]
- Bernard H., et al. Allergy. 2014 doi: 10.1111/all.12411. [DOI] [Google Scholar]
- Du Toit G., et al. N. Engl. J. Med. 2015 doi: 10.1056/NEJMoa1414850. [DOI] [Google Scholar]
- Guilliams M., et al. Nat. Rev. Immunol. 2014 doi: 10.1038/nri3582. [DOI] [Google Scholar]
- Lambrecht B.N., and Hammad H. Nat. Immunol. 2017 doi: 10.1038/ni.3829. [DOI] [PubMed] [Google Scholar]
- Mazzini E., et al. Immunity. 2014 doi: 10.1016/j.immuni.2013.12.012. [DOI] [PubMed] [Google Scholar]
- Mosconi E., et al. Mucosal Immunol. 2010 doi: 10.1038/mi.2010.23. [DOI] [PubMed] [Google Scholar]
- Munblit D., and Verhasselt V. Curr. Opin. Allergy Clin. Immunol. 2016 doi: 10.1097/ACI.0000000000000303. [DOI] [PubMed] [Google Scholar]
- Nakata K., et al. Biochem. Biophys. Res. Commun. 2010 doi: 10.1016/j.bbrc.2010.03.170. [DOI] [Google Scholar]
- Ohsaki A., et al. J. Exp. Med. 2018 doi: 10.1084/jem.20171163. [DOI] [Google Scholar]
- Qiao S.W., et al. Proc. Natl. Acad. Sci. USA. 2008 doi: 10.1073/pnas.0801717105. [DOI] [Google Scholar]
- Samsom J.N., et al. J. Immunol. 2005 doi: 10.4049/jimmunol.174.9.5279. [DOI] [Google Scholar]
- Stapleton N.M., et al. Immunol. Rev. 2015 doi: 10.1111/imr.12331. [DOI] [PubMed] [Google Scholar]
- Verhasselt V., et al. Nat. Med. 2008 doi: 10.1038/nm1718. [DOI] [PubMed] [Google Scholar]
- Yoshida M., et al. Immunity. 2004 doi: 10.1016/j.immuni.2004.05.007. [DOI] [Google Scholar]