In this issue of JEM, Sulciner et al. (https://doi.org/10.1084/jem.20170681) provide evidence that therapy-induced cancer cell death can, paradoxically, stimulate and accelerate the growth of surviving malignant cells by fueling tumor-promoting inflammation. Resolvins, a class of lipid mediators, counteract this effect, representing an attractive target for therapeutic intervention.
Abstract
In this issue of JEM, Sulciner et al. (https://doi.org/10.1084/jem.20170681) provide evidence that therapy-induced cancer cell death can, paradoxically, stimulate and accelerate the growth of surviving malignant cells by fueling tumor-promoting inflammation. Resolvins, a class of lipid mediators, counteract this effect, representing an attractive target for therapeutic intervention.

Insight from Eduardo Bonavita, Victoria S. Pelly, and Santiago Zelenay
Cytotoxic therapies such as chemotherapy or radiotherapy constitute the standard of care for most advanced and/or unresectable malignancies. Tumor shrinkage after cytotoxic therapy occurs through both direct killing of malignant cells as well as through changes in the tumor immune microenvironment triggered by therapy-induced cell death. Whether dead cell–driven inflammatory responses promote or inhibit cancer progression remains a matter of debate.
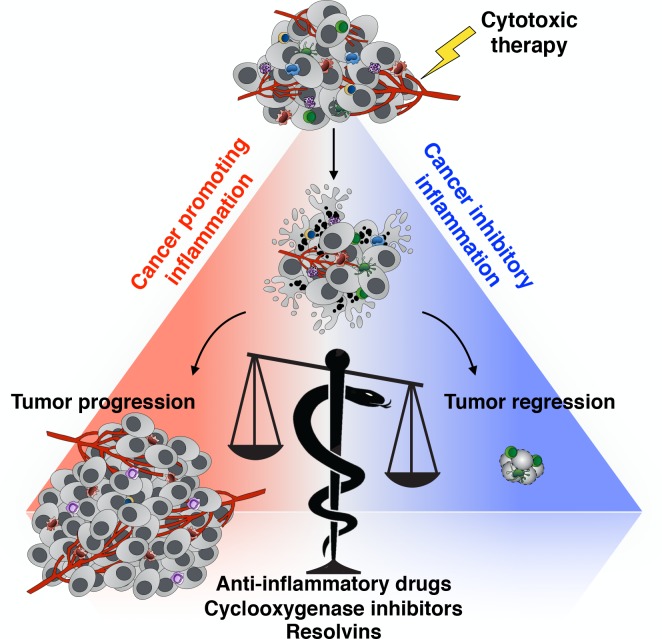
Extensive evidence has demonstrated a beneficial and often essential role for the inflammatory response triggered by cancer cell death. Numerous preclinical and clinical studies indicate that classic molecular and cellular mediators of innate and adaptive immunity underlie the mid- and long-term efficacy of mainstream anticancer clinical practices. This phenomenon is ascribed to the ability of these treatments to trigger so-called immunogenic cell death and to prime and enhance T cell responses against cancer dead cell–containing tumor-associated antigens (see figure; Kroemer et al., 2013; Galluzzi et al., 2017). The study of the features of immunogenic cell death and its consequences has underscored a major role for the immune system in determining the outcome of conventional anticancer therapies.
Responses elicited by therapy-driven cancer cell death have been shown to either promote or restrain cancer growth, mirroring the antagonistic role of inflammation in cancer. Recent studies, including the study by Sulciner et al. (2018), postulate that one way to tip the balance toward cancer control is to target tumor-promoting inflammation. The snake of the Greek god of medicine Asclepius, winding around and inclining the balance, represents a hypothetical way to achieve this therapeutically, by either blocking inflammation or actively promoting its resolution.
In contrast to the view that dead cell–mediated inflammatory responses have profound cancer-inhibitory effects, several studies indicate that dying or dead cancer cells generated in response to anticancer therapies can, conversely, promote tumor growth (see figure). These protumorigenic effects of dead cells are pleiotropic and frequently linked with the induction of immunological tolerance and/or the stimulation of wound healing–like inflammatory responses that incite many features of malignant cancer growth (Barker et al., 2015; Ichim and Tait, 2016). Indeed, tumor relapse and repopulation during or after cytotoxic and targeted therapy remains a major cause of treatment failure in cancer patients, implying therapy-induced immune (re)activation is often neither potent nor durable.
Several cellular and molecular inflammatory mediators typically found in clinically apparent tumors are well known for having protumorigenic effects, whereas others, and occasionally the very same factors, can restrain cancer growth and correlate with a favorable prognosis. Thus, although the underlying bases for the opposing effects of therapy-driven cancer cell death are still not clear, they are reminiscent of, and likely coupled to, the dual antagonistic role of inflammation in cancer (Mantovani et al., 2008).
In this issue of JEM, Sulciner et al. present an extraordinary amount of data supporting the notion that cancer cell death after cytotoxic therapy can have undesirable tumorigenic properties. Consistent with a phenomenon first described in the 1950s known as the Révész effect (Révész, 1956), the authors show that dead cancer cells generated in response to chemotherapy or targeted therapy could stimulate the growth of a subthreshold inoculum of viable cancer cells that alone failed, or took much longer, to form a progressive tumor. This cancer-promoting effect of tumor cell debris was found to be macrophage dependent but remained intact in immunocompromised hosts lacking an adaptive immune system. Furthermore, cancer cell lines and chemotherapeutic drugs previously shown to give rise to immunogenic cell death also supported the growth of viable cancer cells. Finally, cell debris of any given cancer type could also stimulate the growth of unrelated cancer cells, in accordance with a mechanism independent of adaptive immunity.
Further experiments in the study support a model in which exposure of phosphatidylserine by dead cells leads to macrophage-dependent inflammatory cytokine production that fuels tumor growth. Accordingly, the tumor-promoting role of cancer cell debris was impaired in the absence of macrophages or after the blockade of phosphatidylserine. Remarkably, antibody-mediated blockade of the individual inflammatory cytokines and chemokines TNF, IL-6, CCL4, and CCL5 each diminished the dead cell–driven tumor growth enhancement. Moreover, combined blockade of all these four factors fully abrogated the cancer-enhancing features of dead cells. These findings put forward the hypothesis that neutralization of many inflammatory mediators and/or the use of broad-spectrum anti-inflammatory drugs might limit the detrimental effects of therapy-induced cell death and tip the balance toward suppression of tumor growth (see figure).
Applying their expertise in the field of inflammation resolution and resolvins (Serhan, 2014), the investigators went on to further uncover a nonredundant function for this class of pro-resolution inflammatory lipids in dampening tumor-promoting inflammation. Using both gain- and loss-of-function experiments, Sulciner et al. (2018) demonstrate that resolvins, through encouraging the uptake of cancer cell debris by macrophages and limiting cancer-promoting inflammation, inhibit therapy-mediated accelerated tumor growth in their various experimental systems.
These observations have evident and direct therapeutical implications because targeting resolvins and/or their receptors could prove beneficial in increasing the efficacy of anticancer therapies. In light of the remarkable results obtained over the recent years in cancer treatment with therapies aimed at harnessing the anticancer properties of the immune system (Sharma and Allison, 2015; Topalian et al., 2015), assessing the role of resolvins in enhancing antitumor immunity would be of great interest. In this regard, it remains to be established how resolvins influence the natural and therapy-induced immunogenic properties of dying or dead cancer cells. It would be especially relevant to assess whether targeting tumor-promoting inflammation through modulation of the resolvin pathway could increase the efficacy of immunotherapies such as those based on adoptive T cell therapy or immune checkpoint blockade.
Previous studies have implicated another inflammatory lipid, prostaglandin E2 (PGE2), as a key mediator of chemoresistance and tumor repopulation after cytotoxic therapy (Huang et al., 2011; Kurtova et al., 2015). PGE2 production and release has been linked with the process of cell death (Huang et al., 2011; Kurtova et al., 2015; Hangai et al., 2016) and with diverse aspects of malignant tumor growth (Wang and Dubois, 2010). Moreover, reduction in PGE2 levels at the tumor site was shown to enhance natural or therapy-induced antitumor immunity (Zelenay et al., 2015; Hou et al., 2016). Notably, combination of PD-1 blockade and nonsteroidal antiinflammatory drugs synergizes to promote immune-dependent tumor eradication in preclinical models. Because arguably all cancer therapies rely on immunity, targeting pivotal elements that shift the balance from cancer-promoting to -inhibitory inflammation could represent the key to increasing the number of complete and long-term responders. The hypothesis that modulating tumor-promoting inflammation with antiinflammatory drugs, blocking specific mediators or boosting resolution of inflammation, can enhance the efficacy of anticancer therapies, particularly in the context of immunotherapy, awaits to be tested in a clinical trial.
References
- Barker H.E., et al. Nat. Rev. Cancer. 2015 doi: 10.1038/nrc3958. [DOI] [Google Scholar]
- Galluzzi L., et al. Nat. Rev. Immunol. 2017 doi: 10.1038/nri.2016.107. [DOI] [Google Scholar]
- Hangai S., et al. Proc. Natl. Acad. Sci. USA. 2016 doi: 10.1073/pnas.1602023113. [DOI] [Google Scholar]
- Hou W., et al. Cancer Cell. 2016 doi: 10.1016/j.ccell.2016.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Q., et al. Nat. Med. 2011 doi: 10.1038/nm.2385. [DOI] [Google Scholar]
- Ichim G., and Tait S.W.G. Nat. Rev. Cancer. 2016 doi: 10.1038/nrc.2016.58. [DOI] [PubMed] [Google Scholar]
- Kroemer G., et al. Annu. Rev. Immunol. 2013 doi: 10.1146/annurev-immunol-032712-100008. [DOI] [PubMed] [Google Scholar]
- Kurtova A.V., et al. Nature. 2015 doi: 10.1038/nature14034. [DOI] [Google Scholar]
- Mantovani A., et al. Nature. 2008 doi: 10.1038/nature07205. [DOI] [Google Scholar]
- Révész L. Nature. 1956 doi: 10.1038/1781391a0. [DOI] [Google Scholar]
- Serhan C.N. Nature. 2014 doi: 10.1038/nature13479. [DOI] [Google Scholar]
- Sharma P., and Allison J.P. Cell. 2015 doi: 10.1016/j.cell.2015.03.030. [DOI] [Google Scholar]
- Sulciner M.L., et al. J. Exp. Med. 2018 doi: 10.1084/jem.20170681. [DOI] [Google Scholar]
- Topalian S.L., et al. Cancer Cell. 2015 doi: 10.1016/j.ccell.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., and Dubois R.N. Nat. Rev. Cancer. 2010 doi: 10.1038/nrc2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelenay S., et al. Cell. 2015 doi: 10.1016/j.cell.2015.08.015. [DOI] [Google Scholar]