Abstract
Background:
Breast cancer metastasis is a highly prevalent cause of death for European females. DNA microarray analysis has established that primary tumors, which remain localized, differ in gene expression from those that metastasize. Cross-analysis of these studies allow to revile the differences that may be used as predictive in the disease prognosis and therapy.
Objective:
The aim of the project was to validate suggested prognostic and therapeutic markers using meta-analysis of data on gene expression in metastatic and primary breast cancer tumors.
Method:
Data on relative gene expression values from 12 studies on primary breast cancer and breast cancer metastasis were retrieved from Genevestigator (Nebion) database. The results of the data meta-analysis were compared with results of literature mining for suggested metastatic breast cancer markers and vectors and consistency of their reported differential expression.
Results:
Our analysis suggested that transcriptional expression of the COX2 gene is significantly downregulated in metastatic tissue compared to normal breast tissue, but is not downregulated in primary tumors compared with normal breast tissue and may be used as a differential marker in metastatic breast cancer diagnostics. RRM2 gene expression decreases in metastases when compared to primary breast cancer and could be suggested as a marker to trace breast cancer evolution. Our study also supports MMP1, VCAM1, FZD3, VEGFC, FOXM1 and MUC1 as breast cancer onset markers, as these genes demonstrate significant differential expression in breast neoplasms compared with normal breast tissue.
Conclusion:
COX2 and RRM2 are suggested to be prominent markers for breast cancer metastasis. The crosstalk between upstream regulators of genes differentially expressed in primary breast tumors and metastasis also suggests pathways involving p53, ER1, ERB-B2, TNF and WNT, as the most promising regulators that may be considered for new complex drug therapeutic interventions in breast cancer metastatic progression.
Keywords: Breast cancer, metastasis, markers, meta-analysis, gene, differential expression
1. Introduction
The most common cause of cancer death in European females is breast cancer; the majority of breast cancer mortalities arise from metastatic spread of the cancer and not from the cancer at the primary site [1, 2]. DNA microarray studies have established that tumors which remain localized and primary tumors which metastasize differ in gene expression [3]. Hence gene-expression profiling can aid the identification of prognostic markers for patient outcomes and could potentially aid the identification of new therapeutic targets. Accurately predicting the risk of metastasis could also enrich patient quality of life, sparing those without metastatic disease from aggressive chemotherapy which invokes a range of short and long term side effects [4].
A key application of gene expression profile analysis is to identify a small number of gene signatures that classify cancer phenotypes in relation to their prognosis [5, 6]. Genes can be linked to mediating breast cancer metastasis to different areas of the body and to specific stages of metastasis. Genes such as IL3RA2, VCAM1 and MMP2 are currently associated with mediating aggressive breast cancer metastasis to the lung [7], whereas ST6GLANAC5 is a specific mediator of breast cancer metastasis to the brain [8] and cytokeratin-19 has been found as a putative marker of stem cells from the breast [9]. Tumor suppressors such as Nm23, KAI1 and BRMS1 are linked to the prevention of detachment of tumor cells from primary tumors, while KISS1 and MKK4 are linked to reduced growth at secondary sites [10]. A subgroup of metastasis-inducing proteins (MIPs), namely secreted phosphoprotein 1 (SPP1) [11], S100 calcium binding proteins A4 (S100A4) [12, 13] and S100P [14] and anterior gradient 2 (AGR2) [15] have also been shown to be overexpressed in patients exhibiting sporadic and metastatic breast cancer and are associated with reduced patient survival [14, 16-19]. Hence the up-regulation and down-regulation of identified gene signatures can be assessed in terms of prognosis.
It is a major target in breast cancer research to determine the genetic mechanisms that underpin the metastatic processes, which include: tumor cell intravasation, cell survival during circulation, extravasation into new tissues and successfully inhibited growth at a secondary site [20]. The initial step facilitating the transmission of tumors from primary to secondary sites is thought to involve epithelial-mesenchymal transmission (EMT); a process that allows epithelial cells to exhibit enhanced motility and invasion [21-24]. Many genes influence EMT, overexpression of the FOXM1 gene has been demonstrated to stimulate EMT-like changes in cells and EMT has also been shown to increase the regulation of MMP1 [25, 26]. The next step, intravasation, involves cancer cells forming circulating tumor cells (CTC’s) in the blood or lymphatics and cancer dissemination occurs either via angiogenesis or lymphangiogenesis [27-29]. The final steps involve extravasation to different parts of the body, such as the lung, bone and liver and adaptation and proliferation within new tissues [30, 31]. Cancer cells which have escaped from the primary lesion prior to its removal at surgery can remain dormant for 10 years or more before manifestation [32].
Meta-analysis of the existing gene expression data aims to facilitate the identification of a range of prognostic biomarkers for metastatic breast cancer within this project. It is proposed that a fundamental reason why genes within prognostic signatures are so unreliable is due to the difficulty in distinguishing their role as ‘passenger’ or ‘driver’ genes within the metastatic phenotype [33] and that expression of passengers may vary greatly across different cases due to many factors, such as noise in transcriptional regulation, which consequently results in differential expression [34]. Constant and reliable differential expression though may indicate a role of a gene as a driver, and the defined marker gene candidates should be experimentally validated. There is currently an abundance of online databases holding microarray and RNAseq data, which can be used in a context-query driven manner and can provide information on gene expression, giving information on up-regulation and down-regulation of genes within different tissue types and perturbations. In this study we used a number of applications for meta-analysis of gene expression, alongside interactions data to validate a general significance of already-suggested metastatic markers. We have analyzed several defined datasets representing different contrasts of gene expression in metastatic breast cancer compared to non-metastatic breast cancer and normal tissue, to define metastasis-specific cell functions distinguishing metastasis from the non-metastatic breast neoplasms.
2. Methods
Gene list compilation: 52 genes were compiled from literature, including genes up-regulated in metastasis, genes down-regulated in metastasis and genes that mediate metastasis to specific sites such as the lung and the bone. The combinations of key words ‘breast metastasis & gene’, ‘gene expression’, ‘microarray’, ‘PCR’, ‘up-regulated’ and ‘down-regulated’ were used to search for relevant publications in the NCBI database. 52 genes were selected as they had been previously reported as differentially expressed in metastasic tumors when compared to primary tumors.
Meta-analysis of Gene Expression data was performed using the Genevestigator Biomedical V4 microarray database (https://genevestigator.com/gv/biomed.jsp) [34, 35]. The software was used to identify genes that are specifically up-or down-regulated in response to a set of perturbations [34]. The conditions tool was used to identify gene sets that were differentially expressed across different breast cancer neoplasms. The Genevestigator Similarity Search tool provides co-expressed gene relationships calculated from array data [35]. More information on how the Genevestigator software can be operated is described in extensive detail in the Genevestigator user manual (https://genevestigator.com/userdocs/manual/). The top 10 significant, differentially expressed genes from the literature list associated with breast metastasis were selected for further analysis. A number of metastatic tissue datasets and corresponding independent gene expression experiments were limited to 3 compared to 9 of independent primary breast cancer gene expression analysis datasets. Moreover, according to the data source notation, lymph nodes were used as sources of the metastatic tissue, though in the case of primary breast tumors the samples were taken directly from breast. It was the only available option to compare primary and metastatic breast cancer.
The Ingenuity Pathway Analysis (IPA) (Ingenuity Systems, Inc., Redwood City, CA, USA) (www.qiagen.com/ingenuity) [36] tool was used to identify the regulators upstream of the gene set and to reconstruct the relational functional networks. A set of gene IDs were entered into the software for a Core Analysis. The tool produces graphical networks from the uploaded gene dataset and the molecules associated with this dataset in IPA Knowledge base, the Upstream Regulator Analysis can be used to suggest transcriptional factors ranked by the z-scores reflecting the number of associated genes in the dataset.
3. Results
3.1. Functional Analysis of Literature-Derived List of Genes Associated with Breast Cancer Metastasis
Genes were identified as relevant to metastasis via literature mining and analyzed using the Genevestigator software. The perturbations tool was used to visualize the expression of genes over a variety of conditions including the expression of genes across different breast cancer types (Genevestigator database). 10 top differentially expressed genes which were consistently reported in the literature as up or down regulated in metastatic cancer progression (Table 1) were selected for further analysis. Although all of these genes showed slight differential expression across different breast cancer samples, p-values for each gene within a set of different perturbations were not significant. Insignificance could occur due to the way samples were collected; metastasis samples were extracted from the lymph nodes of patients whilst non-metastatic samples were taken from primary tumors of breast ductal carcinomas with no sign of distant metastases, inconsistencies could also arise due to a limited number of metastatic samples [37]. An extensive literature search was performed to validate a potential functional input of selected genes with detected insignificant p-values (Table 2).
Table 1.
Genes Identified by literature mining and their corresponding mRNA expression.
| Study | MMP1 | VCAM1 | FZD3 | VEGFC | COX2 | DEPDC1 | NUSAP1 | RRM2 | FOXM1 | MUC1 |
|---|---|---|---|---|---|---|---|---|---|---|
| Metastatic vs. normal breast tissue | + log2=0.15 fold=1.09 pval=0.620 |
+ log2=0.51 fold=1.59 pval=0.348 |
+ log2=2.71 fold=5.9 pval=0.006 |
- log2=-2.33 fold=-0.09 pval=0.012 |
- log2=-1.06 fold=-2.09 pval=<0.001 |
+ log2=0.24 fold=1.18 pval=0.146 |
+ log2=0.43 fold=1.42 pval=0.237 |
+ log2=1.32 fold=1.84 pval=0.398 |
+ log2=0.13 fold=1.00 pval=0.806 |
+ log2=2.50 fold=5.78 pval=0.059 |
| Metastatic vs. primary breast cancer | - log2=-2.73 fold=-14.61 pval=0.098 |
+ log2=1.72 fold=2.93 pval=0.048 |
+ log2=0.41 fold=1.11 pval=0.562 |
- log2=-0.09 fold=-1.13 pval=0.901 |
- log2=-1.04 fold=-2.08 pval=<0.001 |
- log2=-0.53 fold=-1.66 pval=0.332 |
- log2=-1.02 fold=-3.22 pval=0.368 |
- log2=-1.84 fold=-3.36 pval=0.038 |
- log2=-2.35 fold=-9.59 pval=0.076 |
- log2=-0.68 fold=-2.47 pval=0.617 |
| Primary breast cancer vs. normal breast tissue | + log2=2.87 fold=15.91 pval=0.017 |
- log2=-1.21 fold=-1.84 pval=0.039 |
+ log2=2.30 fold=5.36 pval=<0.001 |
- log2=-2.24 fold=-4.04 pval=<0.001 |
- log2=-0.02 fold=-1.00 pval=0.892 |
+ log2=0.77 fold=1.96 pval=0.056 |
+ log2=1.45 fold=4.56 pval=0.076 |
+ log2=3.16 fold=6.18 pval=<0.001 |
+ log2=2.48 fold=9.63 pval=0.012 |
+ log2=3.18 fold=14.25 pval=0.004 |
Log(2)- ratios, fold change values and p values. Log(2)-ratios (log2), fold change values (fold) and p-values (pval) from Genevestigator analysis are shown. Up-regulation of genes is indicated by (+), down-regulation of genes is indicated by (-).
Table 2.
Validated Genevestigator results.
| MMP1 | VCAM1 | FZD3 | VEGFC | COX2 | DEPDC1 | NUSAP1 | RRM2 | FOXM1 | MUC1 | |
|---|---|---|---|---|---|---|---|---|---|---|
|
Metastatic vs. normal breast tissue |
✓ + [38] |
✓ + [39] |
SIGNIFICANT ✓+ |
SIGNIFICANT ✓- |
SIGNIFICANT ✓- |
✓ + [40] |
✓ + [40] |
✓ + [41] [42] |
✓ + [43] |
✓ + [40] |
| Metastatic vs. primary breast cancer |
✗ + [44 [7] [45 [46] |
SIGNIFICANT ✓+ |
✓ + [47] |
✗ + [48] [49] [50] [51] [52] |
SIGNIFICANT ✓- |
✗ + [53] [40] |
✗ + [54] [40] [55] |
SIGNIFICANT ✓- |
✗ + [43] [56] |
✗ + [57] [58 |
| Primary breast vs. normal breast tissue |
SIGNIFICANT ✓+ |
SIGNIFICANT ✓- |
SIGNIFICANT ✓+ |
SIGNIFICANT ✓- |
✗ + [59] [60] [61] [62] |
✓ + [63] [40] |
✓ + [40] [64] [65 |
SIGNIFICANT ✓+ |
SIGNIFICANT ✓+ |
SIGNIFICANT ✓+ |
Instances where Genevestigator results were significant (p<0.05) were automatically indicated by (✓). Instances where p >0.05 were consistently confirmed by literature (✓), or have consistently contradicted to published data (X). Consistency here, by a rule for an initial selection of these genes from a literature gene set, means that an opposite vector of differential expression for a gene hasn’t been reported.
Due to the fact that metastatic samples used for the original microarray experiments were derived from lymph nodes and that contamination by immune cells can occur, we could not rely on the promising marker contrasts where increment in gene differential expression being characteristic only for metastatic neoplasms (i.e. in the case of VCAM1). From our analysis, VCAM1 appears to be the best potential marker for metastatic breast cancer and hence should be validated via analysis of the gene expression in metastatic tissue samples, where contamination with immune cells has been avoided. We can also dismiss gene expression changes that occurred only in primary neoplasms when contrasted against normal tissues, as these represent markers for primary breast cancer. The FZD3 gene however, may be an exception, its expression is thought to be insignificant but is still high in metastatic tumors when compared to primary tumors and this tendency is supported by literature (Table 2). Gene expression contrasts observed between metastatic tissues and primary breast tumors (DEPDC1, NUSAP1, FOXM1, MUC1) demonstrate good prognostic markers for metastatic cancer development.
Our analysis suggested that the expression of the COX2 gene is significantly downregulated in metastatic tissue compared to both primary tumors and normal tissue, but not in primary tumors compared with normal breast tissue and may be used as a differential marker in metastatic cancer diagnostics.
RRM2 expression decreases in metastatic breast cancer progression and can be suggested as a marker to monitor breast cancer signatures. Our study also supports MMP1, VCAM1, FZD3, VEGFC, FOXM1 and MUC1 as breast cancer onset markers as these genes demonstrate significant differential expression in breast neoplasms when compared with normal breast tissue (Table 1).
It is important to note that differential gene expression vectors across breast cancer neoplasms were sometimes contradictory when comparing the results of the Genevestigator analysis and sources of literature. A factor which could largely contribute to discrepancies in the results are the sample types used within different studies. For metastatic samples, the Genevestigator analysis used samples solely from the lymph nodes, whereas in other instances, samples were taken from surgically resected breast tumors [36] while some studies used a combination of freshly frozen human breast tumor samples and transgenic mice models [40]. Differences in cell lines used for each study may contribute to inconsistencies in results.
3.2. Extending the Gene List by Co-Expressed Genes
The list of genes strongly and differentially expressed in metastasis (Table 1) was used as a seed to identify other relevant genes potentially associated with metastatic breast cancer.
Thus, a co-expression analysis was performed for each of the top 10 differentially expressed genes listed in Table 1. A co-expression value was calculated as a Pearson’s correlation coefficient and co-expressed genes which possessed a Pearson’s correlation coefficient of >0.8 were included in further analysis (Table 3). The aim of identifying other genes, from microarray data, that exhibit strong co-differential expression with members of our initial gene list (Table 1) was to facilitate prediction of the upstream regulators associated with metastatic process.
Table 3.
Genes co-expressed with the genes in the literature-derived dataset (Table 2) obtained by the Genevestigator co-expression analysis.
| Gene Co-Expressed with a Gene in the Dataset | Gene (Literature Analysis Dataset) | Pearson’s Correlation Coefficient |
|---|---|---|
| GPX8 | VEGFC | 0.85 |
| FST | VEGFC | 0.84 |
| LOX | VEGFC | 0.84 |
| PXDN | VEGFC | 0.84 |
| EHD2 | VEGFC | 0.82 |
| HNRNPM | COX2 | 0.93 |
| ATP6 | COX2 | 0.93 |
| DCAF6 | COX2 | 0.91 |
| ND2 | COX2 | 0.87 |
| ND3 | COX2 | 0.86 |
| GTSE1 | DEPDC1 | 0.82 |
| HJURP | DEPDC1 & FOXM1 | 0.8 & 0.92 |
| KIF2C | DEPDC1 | 0.8 |
| MKI67 | NUSAP1 | 0.77 |
| TPX2 | FOXM1 | 0.9 |
| DLGAP5 | FOXM1 | 0.9 |
| AURKB | NUSAP1 | 0.82 |
| CCNA2 | NUSAP1 | 0.81 |
| UBE2C | NUSAP1 | 0.79 |
| KIF4A | FOXM1 | 0.9 |
| ELF3 | MUC1 | 0.86 |
| AGR2 | MUC1 | 0.83 |
| PIGR | MUC1 | 0.83 & 0.84 |
| TMC4 | MUC1 | 0.82 & 0.88 |
| RASEF | MUC1 | 0.8 & 0.84 |
| TMC5 | AGR2 | 0.89 |
| SLC44A4 | AGR2 | 0.88 |
| KRT19 | MUC1 | 0.79 |
| TSPAN1 | AGR2 | 0.87 |
| C9orf152 | AGR2 | 0.89 |
| ST6GALNAC1 | AGR2 | 0.88 |
| LOC100505989 | AGR2 | 0.86 |
| KIAA0101 | RRM2 | 0.93 |
| TOP2A | RRM2 & NUSAP1 | 0.93 & 0.82 |
| ZWINT | RRM2 | 0.93 |
| DTL | RRM2 | 0.93 |
| CCNB2 | RRM2 & FOXM1 | 0.92 & 0.88 |
| DLGAP5 | FOXM1 | 0.9 |
| Gene Co-Expressed with a Gene in the Dataset | Gene (Literature Analysis Dataset) | Pearson’s Correlation Coefficient |
| HMMR | DEPDC1 | 0.84 |
| MELK | RRM2 | 0.91 |
| BIRC5 | DEDPC1 & FOXM1 & NUSAP1 | 0.79 & 0.91 & 0.78 |
| ASPM | FOXM1 | 0.87 |
| NUF2 | RRM2 | 0.89 |
Pearson’s Correlation Coefficient is shown for each pair of gene profiles.
3.3. Functional Analysis of the Metastasis-Specific Gene Set Extended by their Co-Expression Partners
The merged list of genes retrieved from the literature and co-expression analysis (Table 1 & Table 3) was uploaded in IPA for functional interrogation. Table 4 presents the top-ranked results of analysis of upstream regulators based on their activation z scores calculated by IPA predictive algorithm. The list of potential regulators may be biased by our initial selection of genes. We however discuss a relevance, based on literature analysis, of predicted factors to metastatic breast cancer in our Discussion section.
Table 4.
IPA Upstream regulators associated with the genes selected from literature analysis and co-expressed genes.
| Upstream Regulator | Molecule Type | P-Value | Target Molecules in Integrated Dataset |
|---|---|---|---|
| CDKN1A | Kinase | 6.31E-09 | BIRC5,CCNA2,FOXM1,KIAA0101,KRT18, MMP1,TOP2A (7) |
| AR | ligand-dependent nuclear receptor | 7.80E-09 | BIRC5,CCNA2,KIF2C,MUC1,NUSAP1, PGR,PIGR,TPX2,UBE2C,ZWINT (10) |
| ERBB2 | Kinase | 1.38E-08 | ASPM,BIRC5,CCNA2,CCNB2,MKI67, MMP1,MUC1,TOP2A,VEGFC,ZWINT (10) |
| FOXO1 | transcription regulator | 3.86E-08 | ASPM,BIRC5,CCNB2,DEPDC1, DLGAP5,MMP1,NUSAP1 (7) |
| TNF | Cytokine | 2.30E-07 | ADIPOQ,BIRC5,ELF3,FST,HLA-DRB4, MMP1,MT-CO2,MUC1,PIGR,PPP2R1B,RRM2, VCAM1,VEGFC (13) |
| FOXM1 | transcription regulator | 1.70E-06 | AURKB,BIRC5,CCNA2,FOXM1, GTSE1 (5) |
| estrogen receptor | Group | 5.25E-06 | KRT18,KRT19,MMP1,MT-CO2, MUC1,PGR,PRLR,VEGFC (8) |
| ESR1 | ligand-dependent nuclear receptor | 7.09E-07 | ASPM,BIRC5,CCNA2,FST,KRT19, MKI67,MMP1,PDZK1,PGR,PRLR,RAMP3 (11) |
| ESR2 | ligand-dependent nuclear receptor | 5.67E-04 | BIRC5,MMP1,PGR (3) |
| LGALS3 | Other | 1.70E-06 | CCNB2,KRT18,KRT19,MUC1,VCAM1 (5) |
| TP53 | transcription regulator | 3.06E-04 | AURKB,BIRC5,CCNA2,HMMR, KIAA0101,RRM2,TOP2A,TPX2,UBE2C (9) |
P-value measures whether there is a statistically significant overlap between dataset genes and the downstream targets of a regulator. The number of different proteins that each upstream regulator has been reported to be associated with is indicated in brackets. The top genes differentially expressed in metastasis (Table 2) are highlighted in red. In bold are TFs that may be responsible for regulation of suggested prognostic marker, RRM2.
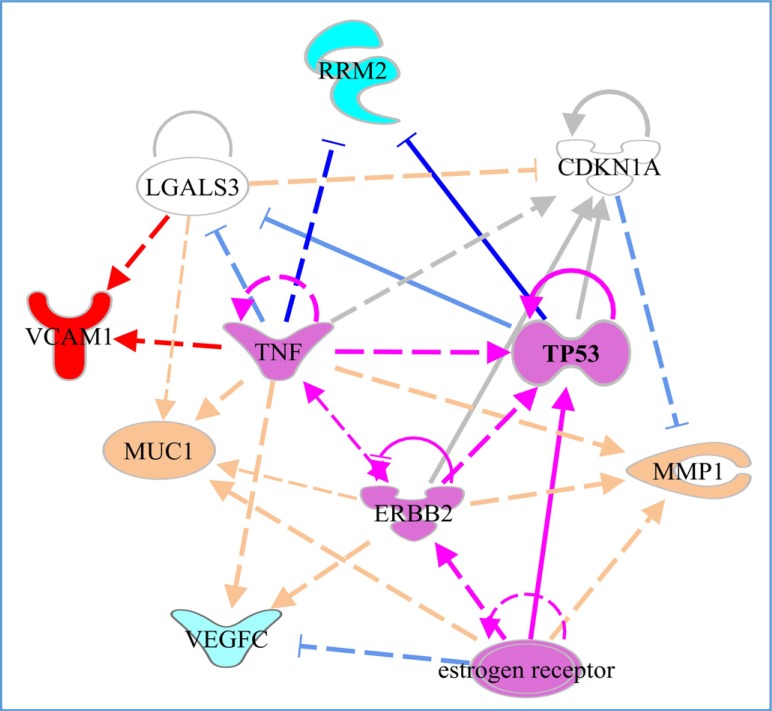
Fig. (1) reconstructs its predicted regulatory interactions and shows regulatory interactions potentially leading to activation of a positive metastatic breast cancer prognostic marker, VCAM1, and two positive prognostic markers for breast cancer, which do not show specific links to metastatic transition in our analysis, MMP1 and MUC1. Interestingly, only RRM2, which shows decreased expression in metastasis in comparison to primary breast cancer, is downregulated by TNF and p53, according to results based on IPA knowledge-base data. However, TNF up-regulates VCAM1. ERB2 up-regulates primary breast cancer markers and rather pro-metastatic TNF and p53 genes. The oestrogen receptor may also modulate expression of p53. The core predicted regulators form an interacting circuit that may regulate primary breast cancer markers and lead to differential expression of genes involved in the metastatic transition.
Fig. (1).
Functional interactions between top breast cancer metastasis markers (bright blue, red) and primary breast cancer prognostic markers (pastel blue red) with the IPA-predicted upstream regulators. Highlighted in purple-regulatory circuit that may lead to development of metastatic transition. Arrows indicate activation, blocked lines-inhibition. Bright line colours correspond to regulatory interactions that potentially lead to differential expression of the metastatic markers. Dashed lines indicate predicted direct interactions between proteins, depicting protein-protein binding and regulatory actions. Validated transcriptional regulation is shown by solid lines.
4. Discussion
Gene expression profiling is widely used to define potential disease biomarkers, however individual experiments and small cohorts may lead to predictions that are not sustained on a larger scale. An integration of available data in meta-analysis gives an opportunity to validate the consistency of relationships between gene expression and physiological conditions, and our analysis aimed for the validation of such relationships between predicted gene markers and metastatic breast cancer.
The availability of proper datasets for metastatic cancer tissue limits our understanding of the specificity of the detected associations. We assume, for instance, that there is a high degree of probability that immune cells could contaminate the metastatic tissue derived from lymph nodes. This restricts our ability to suggest genes, which are highly prevalent in metastasis when compared to primary breast cancer. Thus, we would rather focus on genes which are strongly downregulated in metastatic tissue. However, even the sustainable predictions supported by the literature, need to be validated in a clearly designed experimental setting. Our analysis suggests that expression of COX2 is significantly downregulated in metastatic tissues, but not in primary tumors when compared with normal breast tissue and expression of RRM2 also decreases during the evolution of primary breast cancer into the metastatic one. Therefore, these genes may be suggested for disease monitoring and diagnostics. Our study supports MMP1, VCAM1, FZD3, VEGFC, FOXM1 and MUC1 as breast cancer onset markers, as these genes have a significant differential expression in breast cancer neoplasms, compared with normal breast tissue (based on data from 9 microarray experiments/Genevestigator), however it does not show confident evidence of their differential expression in metastatic tissue compared to primary tumors (based on data from 3 microarray experiments/ Genevestigator).
Several potential regulators and functional pathways of the differential gene expression in breast metastasis were suggested by our analysis. CDKN1A [66-68] is identified as the top-ranked regulator of MMP1 and FOXM1 (Table 4), two genes where up-regulation is associated with breast cancer metastasis and primary tumors. CDKN1A may act downstream of c-ERBB-2 [68], and c-ERBB-2 also increases the expression of MMP1 [69, 70]. In agreement with the results of our upstream analysis performed via IPA, c-ERBB-2 was shown to regulate expression of VEGFC and MUC1 [71, 72] and associated with poor prognosis in breast cancer [73].
TNF alpha is predicted to up-regulate the expression of several top breast cancer and metastasis markers (Table 4). TNF has indeed been shown to increase the expression of MMP1 via up-regulation of IL4 [74] and to induce the upregulation of MUC1 [75] and VEGFC [76]. Interestingly, RRM2 is also on this list of TNF-regulated genes, as well as on the list of genes regulated by p53 function (Table 4). The protein product of the RRM2 gene is known to inhibit WNT signaling [77] and may have a direct role in WNT signaling regulation during the metastatic process. One of the components of WNT signaling, The Frizzled protein, FZD3, is up-regulated in breast cancer metastasis [78] via the WNT pathway and was also defined in our analysis as a potential prognostic marker. Crosstalk between TNF and WNT signaling can be suggested for future experimental analysis as one of the promising interactions driving or strongly reflecting the metastatic process. The multi-step process of tumor development and expansion driven by accumulation of new mutations may explain a transition from TNF-dependent primary tumor to less proliferating metastatic cells due to altered p53 function and dysregulated LGALS3 and CDKN1A downstream pathways (Fig. 1).
The product of the Lectin Galactoside-Binding soluble 3 (LGALS3) gene is involved in the regulation of MUC1 and in up-regulation of VCAM1 [79, 80]; it also activates VEGFC expression, which is linked to tumor angiogenesis [81]. Expression of VEGF factors and VCAM1 is also controlled by NFKB [81, 82] stimulating leukocyte recruitment and leukocyte infiltration of a tumor [83-85].
The Forkhead box M1 (FOXM1) gene was linked to the up-regulation of several genes within the gene set (Table 2). It was also shown to increase the expression of estrogen receptor alpha (ER1) [86] predicted to be upstream of a number of genes within the gene set. Estrogen has been shown to increase the expression of MMP1 [87], but also to suppress the expression of VEGFC [88, 89], that correlates with the vector of differential expression of these genes in breast cancer contrasted to normal breast tissue and metastatic tissue contrasted to primary breast tumor (Table 2).
A gene expression profiling study similar to ours analyzed microarray data from 189 invasive breast carcinomas combined with data from three published gene expression datasets. The study identified genes in parallel with this study such as FOXM1 and BIRC5 as genes that are overexpressed in grade 3 tumors providing strength for our findings [90].
Conclusion
The number of genes that were suggested in publications as markers for breast cancer metastasis (Table 1) did not show consistent differential expression in association with metastatic conditions, their differential expression vectors mentioned in the literature were also contradictory. Without dismissing the potential biomarker role of the genes that did not show confident differential signal in our meta-analysis, we advocate a re-evaluation of their differential association with metastatic breast cancer and the usefulness of their expression level measurement in diagnostics. On the other hand, further analysis and validation is required for the gene-markers and interactions suggested in this study. Crosstalk between the regulatory pathways and transcription factors (Fig. 1) is likely to define a complex landscape for realization of the metastatic cell program in different gene and environmental contexts and should be taken into account for complex therapies and a personalized medicine approach.
ACKNOWLEDGEMENTS
Declared none.
Abbreviations
- IPA
Ingenuity Pathway Analysis
- ILK
Integrin-Linked Kinase
- NF-KB
Nuclear Factor Kappa Beta
- WNT
Wingless-Int
- CXCL17
Chemokine (CXC motif) ligand 17
- CCL19
Chemokine (C-C motif) ligand 19
- CCL21
Chemokine (C-C motif) ligand 21
- MIP
Metastasis-Inducing Proteins
- IL3RA2
Interlukin 13 Receptor Alpha 2
- VCAM1
Vascular Cell Adhesion protein 1
- MMP2
Matrix Metalloproteinase-2
- ST6GALNAC5
ST6 (Alpha-N-Acetyl-Neuraminyl-2,3-Beta-Galactosyl-1,3)-N-Acetylgalactosaminide Alpha -2, 6-Sialytransferase 5
- Nm23
Nonmetastatic gene 23
- BRMS1
Breast Cancer Metastasis Supressor 1
- KISS1
Kisspeptin
- MKK4
Mitogen-activated protein Kinase Kinase 4
- EMT
Epithelial-Mesenchymal Transition
- IL-1RA
Interlukin Receptor Antagonist 1
- IL12
Interlukin 12
- IL-23
Interlukin 23
- Th17
T helper type-17 cell
- IL-17
Interlukin 17
- CD8
Cluster of Differentiation 8
- CD4
Cluster of Differentiation 4
- S100A4
S100 calcium binding protein A4
- AGR2
Anterior Gradient 2
- SPP1
Secreted Phosphoprotein 1
- MAP
Molecular Activity Predictor
- MMP1
Matrix Metalloproteinase-1
- FZD3
Frizzled Class Receptor 3
- VEGFC
Vascular Endothelial Growth Factor C
- COX2
Cyclooxygenase-2
- DEPDC1
DEP Domaining Containing 1
- NUSAP1
Nuclear and Spindle Associated Protein 1
- FOXM1
Forkhead Box M1
- MUC1
Mucin 1
- CDKN1A
Cyclin-Dependent Kinase Inhibitor 1A
- AR
Androgen Receptor
- ERBB2
Erb-B2 Receptor Tyrosine Kinase 2
- FOXO1
Forkhead Box O1
- TNF
Tumor Necrosis Factor
- ESR1
Estrogen Receptor 1
- ESR2
Estrogen Receptor 2
- LGALS3
Lectin, Galactoside-Binding, Soluble 3
- TP53
Tumor Protein P53
- KRT18
Keratin 18
- PGR
Progesterone Receptor
- HLA-DRB4
Major Histocompatability Complex, Class II, DR Beta 4
- IL-4
Interlukin 4
- PRLR
Prolactin-Receptor
- RRM2
Ribonucleotide Reductase M2
- DVL1
Dishevelled Segment Polarity Protein 1
- CTNN
Catenin
- APC
Adenomatous Polyposis Coli
- GSK-3
Glycogen Synthase Kinase 3
- PTGER
Prostaglandin E Receptor
- PP1R1B
Protein Phosphatase 1, Regulatory (inhibitor) Subunit 1B
- SNAI2
Snail Family Zinc Finger 2
- AKT
Protein Kinase B
- mTOR
Mechanistic Target Of Rapamycin
- VEGFA
Vascular Endothelial Growth Factor A
- VEGFD
Vascular Endothelial Growth Factor D
- DKK1
Dickkopf WNT Signaling Pathway Inhibitor 1
- PPP2R1B
Protein Phosphatase 2, Regulatory Subunit A, Beta
- STC1
Stanniocalcin 1
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
No Animals/Humans were used for studies that are base of this research.
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Ferlay J., Soejomataram I., Dikshit R., et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A., Bray F., Center M.M., Ferlay M.E., Ward E., Forman D. Global Cancer Statistics. CA Cancer J. Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 3.Montel V., Huang T., Mose E., Pestonjamasp K., Tarin D. Expression Profiling of Primary Tumors and Matched Lymphatic and Lung Metastases in a Xenogeneic Breast Cancer Model. Am. J. Pathol. 2005;166:1565–1579. doi: 10.1016/S0002-9440(10)62372-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Selwood K., Gibson R., Soanes L. Cancer in Children and Young People: Acute Nursing Care. John Wiley & Sons, Ltd.; 2008. Side effects of chemotherapy. [Google Scholar]
- 5.Alizadeh A.A., Eisen M.B., Davis R.E., et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000;403:503–511. doi: 10.1038/35000501. [DOI] [PubMed] [Google Scholar]
- 6.Golub T.R., Slonim D.K., Tamayo P., et al. Molecular classification of cancer: class discovery and class prediction by gene expression monitoring. Science. 1999;286:531–537. doi: 10.1126/science.286.5439.531. [DOI] [PubMed] [Google Scholar]
- 7.Minn A.J., Gupta G.P., Siegel P.M., et al. Genes that mediate breast cancer metastasis to lung. Nature. 2005;436:518–524. doi: 10.1038/nature03799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bos P.D., Zhang X.H., Nadal C., et al. Genes that mediate breast cancer metastasis to the brain. Nature. 2009;459:1005–1009. doi: 10.1038/nature08021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gudjonsson T., Villadsen R., Nielsen H.L., Rønnov-Jessen L., Bissell M.J., Petersen O.W. Isolation, immortalization, and characterization of a human breast epithelial cell line with stem cell properties. Genes Dev. 2002;16:693–706. doi: 10.1101/gad.952602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stark A.M., Tongers K., Maass N., Mehdorn H.M., Held-Feindt J. Reduced metastasis-suppressor gene mRNA-expression in breast cancer brain metastases. J. Cancer Res. Clin. Oncol. 2005;131:191–198. doi: 10.1007/s00432-004-0629-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oates A.J., Barraclough R., Rudland P.S. The identification of osteopontin as a metastasis-related gene product in a rodent mammary tumor model. Oncogene. 1996;13:97–104. [PubMed] [Google Scholar]
- 12.Davies B.R., Davies M.P., Gibbs F.E., Barraclough R., Rudland P.S. Induction of the metastatic phenotype by transfection of a benign rat mammary epithelial cell line with the gene for p9Ka, a rat calcium-binding protein, but not with the oncogene EJ-ras-1. Oncogene. 1993;8:999–1008. [PubMed] [Google Scholar]
- 13.Davies M.P., Rudland P.S., Robertson L., Parry E.W., Jolicoeur P., Barraclough R. Expression of the calcium-binding protein S100A4 (p9Ka) in MMTV-neu transgenic mice induces metastasis of mammary tumors. Oncogene. 1996;13:1631–1637. [PubMed] [Google Scholar]
- 14.Wang G., Platt-Higgins A., Carroll J., et al. Induction of metastasis by S100P in a rat mammary model and its association with poor survival of breast cancer patients. Cancer Res. 2006;66:1199–1207. doi: 10.1158/0008-5472.CAN-05-2605. [DOI] [PubMed] [Google Scholar]
- 15.Liu D., Rudland P.S., Sibson D.R., Platt-Higgins A., Barraclough R. Human homologue of cement gland protein, a novel metastasis inducer associated with breast carcinomas. Cancer Res. 2005;65:3796–3805. doi: 10.1158/0008-5472.CAN-04-3823. [DOI] [PubMed] [Google Scholar]
- 16.Rudland P.S., Platt-Higgins A., El-Tanani M., et al. Prognostic significance of the metastasis-associated protein osteopontin in human breast cancer. Cancer Res. 2002;62:3417–3427. [PubMed] [Google Scholar]
- 17.Rudland P.S., Platt-Higgins A., Renshaw C., et al. Prognostic significance of the metastasis-inducing protein S100A4 (p9Ka) in human breast cancer. Cancer Res. 2000;60:1595–1603. [PubMed] [Google Scholar]
- 18.Innes H.E., Liu D., Barraclough R., et al. Significance of the metastasis-inducing protein AGR2 for outcome in hormonally treated breast cancer patients. Br. J. Cancer. 2006;94:1057–1065. doi: 10.1038/sj.bjc.6603065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barraclough D.L., Platt-Higgins A., de Silva Rudland S., et al. The metastasis-associated anterior gradient 2 protein is correlated with poor survival of breast cancer patients. Am. J. Pathol. 2009;175:1848–1857. doi: 10.2353/ajpath.2009.090246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chambers A.F., Groom A.C., MacDonald I.C. Metastasis: dissemination and growth of cancer cells in metastatic sites. Nat. Rev. Cancer. 2002;2:563–572. doi: 10.1038/nrc865. [DOI] [PubMed] [Google Scholar]
- 21.Acloque H., Adams M.S., Fishwick K., Bronner-Fraser M., Nieto M.A. Epithelial-mesenchymal transitions: the importance of changing cell state in development and disease. J. Clin. Invest. 2009;119:1438–1449. doi: 10.1172/JCI38019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peinado H., Olmeda D., Cano A. Snail, Zeb and bHLH factors in tumor progression: an alliance against the epithelial phenotype? Nat. Rev. Cancer. 2007;7:415–428. doi: 10.1038/nrc2131. [DOI] [PubMed] [Google Scholar]
- 23.Thiery J.P., Sleeman J.P. Complex networks orchestrate epithelial–mesenchymal transitions. Nat. Rev. Mol. Cell Biol. 2006;7:131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 24.Bailey J.M., Singh P.K., Hollingsworth M.A. Cancer metastasis facilitated by developmental pathways: Sonic hedgehog, Notch, and bone morphogenic proteins. J. Cell. Biochem. 2007;102:829–839. doi: 10.1002/jcb.21509. [DOI] [PubMed] [Google Scholar]
- 25.Park H.J., Gusarova G., Wang Z., et al. Deregulation of FoxM1b leads to tumor metastasis. EMBO Mol. Med. 2011;3:21–34. doi: 10.1002/emmm.201000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Uttamsingh S., Bao X., Nguyen K.T., et al. Synergistic effect between EGF and TGF-β1 in inducing oncogenic properties of intestinal epithelial cells. Oncogene. 2008;27:2626–2634. doi: 10.1038/sj.onc.1210915. [DOI] [PubMed] [Google Scholar]
- 27.Folkman J. Tumor angiogenesis: therapeutic implications. N. Engl. J. Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 28.Donegan W.L. Tumor‐related prognostic factors for breast cancer. CA Cancer J. Clin. 1997;47:28–51. doi: 10.3322/canjclin.47.1.28. [DOI] [PubMed] [Google Scholar]
- 29.Fisher B., Bauer M., Wickerham D.L., et al. Relation of number of positive axillary nodes to the prognosis of patients with primary breast cancer. An NSABP update. Cancer. 1983;52:1551–1557. doi: 10.1002/1097-0142(19831101)52:9<1551::aid-cncr2820520902>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 30.Gupta G.P., Nguyen D.X., Chiang A.C., et al. Mediators of vascular remodelling co-opted for sequential steps in lung metastasis. Nature. 2007;446:765–770. doi: 10.1038/nature05760. [DOI] [PubMed] [Google Scholar]
- 31.Lee Y.T. Breast carcinoma: pattern of metastasis at autopsy. J. Surg. Oncol. 1983;23:175–180. doi: 10.1002/jso.2930230311. [DOI] [PubMed] [Google Scholar]
- 32.Marsden C.G., Wright M.J., Carrier L., Moroz K., Rowan B.G. Disseminated breast cancer cells acquire a highly malignant and aggressive metastatic phenotype during metastatic latency in the bone. PLoS One. 2012;7:e47587. doi: 10.1371/journal.pone.0047587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lim W.K., Lyashenko E., Califano A. Master regulators used as breast cancer metastasis classifier. Pac. Symp. Biocomput. 2009;•••:504–515. [PMC free article] [PubMed] [Google Scholar]
- 34.Hruz T, Laule O, Szabo G, et al. Genevestigator v3: a reference expression database for the meta-analysis of transcriptomes. 2008. [DOI] [PMC free article] [PubMed]
- 35.Zimmermann P., Hennig L., Gruissem W. Gene-expression analysis and network discovery using Genevestigator. Trends Plant Sci. 2005;10:407–409. doi: 10.1016/j.tplants.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 36.Krämer A., Green J., Pollard J., Tugendreich S. Causal analysis approaches in ingenuity pathway analysis (ipa). Bioinformatics. 2013;30:523–530. doi: 10.1093/bioinformatics/btt703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hawthorn L., Luce J., Stein L., Rothschild J. Integration of transcript expression, copy number and LOH analysis of infiltrating ductal carcinoma of the breast. BMC Cancer. 2010;10:460. doi: 10.1186/1471-2407-10-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Merdad A., Karim S., Schulten H.J., et al. Expression of matrix metalloproteinases (MMPs) in primary human breast cancer: MMP-9 as a potential biomarker for cancer invasion and metastasis. Anticancer Res. 2014;34:1355–1366. [PubMed] [Google Scholar]
- 39.O’Hanlon D.M., Fitzsimons H., Lynch J., Tormey S., Malone C., Given H.F. Soluble adhesion molecules (E-selectin, ICAM-1 and VCAM-1) in breast carcinoma. Eur. J. Cancer. 2002;38:2252–2257. doi: 10.1016/s0959-8049(02)00218-6. [DOI] [PubMed] [Google Scholar]
- 40.Kretschmer C., Sterner-Kock A., Siedentopf F., Schoenegg W., Schlag P.M., Kemmner W. Identification of early molecular markers for breast cancer. Mol. Cancer. 2011;10:15. doi: 10.1186/1476-4598-10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ma X.J., Salunga R., Tuggle J.T., et al. Gene expression profiles of human breast cancer progression. Proc. Natl. Acad. Sci. USA. 2003;100:5974–5979. doi: 10.1073/pnas.0931261100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ma X.J., Dahiya S., Richardson E., Erlander M., Sgroi D.C. Gene expression profiling of the tumor microenvironment during breast cancer progression. Breast Cancer Res. 2009;11:R7. doi: 10.1186/bcr2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bektas N., Haaf A.T., Veeck J., et al. Tight correlation between expression of the Forkhead transcription factor FOXM1 and HER2 in human breast cancer. BMC Cancer. 2008;8:42. doi: 10.1186/1471-2407-8-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Przybylowska K., Kluczna A., Zadrozny M., et al. Polymor-phisms of the promoter regions of matrix metalloproteinases genes MMP-1 and MMP-9 in breast cancer. Breast Cancer Res. Treat. 2006;95:65–72. doi: 10.1007/s10549-005-9042-6. [DOI] [PubMed] [Google Scholar]
- 45.Boström P., Söderström M., Vahlberg T., et al. MMP-1 expression has an independent prognostic value in breast cancer. BMC Cancer. 2011;11:348. doi: 10.1186/1471-2407-11-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mannello F. What does matrix metalloproteinase-1 expression in patients with breast cancer really tell us? BMC Med. 2011;9:95. doi: 10.1186/1741-7015-9-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Franci C., Zhou J., Jiang Z., et al. Biomarkers of residual disease, disseminated tumor cells, and metastases in the MMTV-PyMT breast cancer model. PLoS One. 2013;8:e58183. doi: 10.1371/journal.pone.0058183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kinoshita J., Kitamura K., Kabashima A., Saeki H., Tanaka S., Sugimachi K. Clinical significance of vascular endothelial growth factor‐C (VEGF‐C) in breast cancer. Breast Cancer Res. Treat. 2001;66:159–164. doi: 10.1023/a:1010692132669. [DOI] [PubMed] [Google Scholar]
- 49.Zhang X.H., Huang D.P., Guo G.L., et al. Coexpression of VEGF-C and COX-2 and its association with lymphangio-genesis in human breast cancer. BMC Cancer. 2008;8:4. doi: 10.1186/1471-2407-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Skobe M., Hawighorst T., Jackson D.G., et al. Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nat. Med. 2001;7:192–198. doi: 10.1038/84643. [DOI] [PubMed] [Google Scholar]
- 51.Timoshenko A.V., Rastogi S., Lala P.K. Migration-promoting role of VEGF-C and VEGF-C binding receptors in human breast cancer cells. Br. J. Cancer. 2007;97:1090–1098. doi: 10.1038/sj.bjc.6603993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang C.A., Harrell J.C., Iwanaga R., Jedlicka P., Ford H.L. Vascular endothelial growth factor C promotes breast cancer progression via a novel antioxidant mechanism that involves regulation of superoxide dismutase 3. Breast Cancer Res. 2014;16:462. doi: 10.1186/s13058-014-0462-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Girardini J.E., Napoli M., Piazza S., et al. A Pin1/mutant p53 axis promotes aggressiveness in breast cancer. Cancer Cell. 2011;20:79–91. doi: 10.1016/j.ccr.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 54.Ryu B., Kim D.S., DeLuca A.M., Alani R.M. Comprehensive expression profiling of tumor cell lines identifies molecular signatures of melanoma progression. PLoS One. 2007;2:e594. doi: 10.1371/journal.pone.0000594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen X., Wan J., Liu J., et al. Increased IL-17-producing cells correlate with poor survival and lymphangiogenesis in NSCLC patients. Lung Cancer. 2010;69:348–354. doi: 10.1016/j.lungcan.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 56.Ahmad A., Wang Z., Kong D., et al. FoxM1 down-regulation leads to inhibition of proliferation, migration and invasion of breast cancer cells through the modulation of extra-cellular matrix degrading factors. Breast Cancer Res. Treat. 2010;122:337–346. doi: 10.1007/s10549-009-0572-1. [DOI] [PubMed] [Google Scholar]
- 57.Lillehoj E.P., Han F., Kim K.C. Mutagenesis of a Gly-Ser cleavage site in MUC1 inhibits ectodomain shedding. Biochem. Biophys. Res. Commun. 2003;307:743–749. doi: 10.1016/s0006-291x(03)01260-9. [DOI] [PubMed] [Google Scholar]
- 58.Sachdeva M., Mo Y.Y. MicroRNA-145 suppresses cell invasion and metastasis by directly targeting mucin 1. Cancer Res. 2010;70:378–387. doi: 10.1158/0008-5472.CAN-09-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ristimäki A., Sivula A., Lundin J., et al. Prognostic significance of elevated cyclooxygenase-2 expression in breast cancer. Cancer Res. 2002;62:632–635. [PubMed] [Google Scholar]
- 60.Soslow R.A., Dannenberg A.J., Rush D., et al. COX‐2 is expressed in human pulmonary, colonic, and mammary tumors. Cancer. 2000;89:2637–2645. doi: 10.1002/1097-0142(20001215)89:12<2637::aid-cncr17>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 61.Half E., Tang X.M., Gwyn K., Sahin A., Wathen K., Sinicrope F.A. Cyclooxygenase-2 expression in human breast cancers and adjacent ductal carcinoma in situ. Cancer Res. 2002;62:1676–1681. [PubMed] [Google Scholar]
- 62.Costa C., Soares R., Reis-Filho J.S., Leitao D., Amendoeira I., Schmitt F.C. Cyclo-oxygenase 2 expression is associated with angiogenesis and lymph node metastasis in human breast cancer. J. Clin. Pathol. 2002;55:429–434. doi: 10.1136/jcp.55.6.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sparano J.A., Goldestin L.J., Childs B.H., et al. Genotypic characterization of phenotypically defined triple-negative breast cancer. J. Clin. Oncol. 2009;27:500. [Google Scholar]
- 64.Bosch A.V., Raemaekers T., Denayer S., et al. NuSAP is essential for chromatin-induced spindle formation during early embryogenesis. J. Cell Sci. 2010;123:3244–3255. doi: 10.1242/jcs.063875. [DOI] [PubMed] [Google Scholar]
- 65.Lauss M., Kriegner A., Vierlinger K., et al. Consensus genes of the literature to predict breast cancer recurrence. Breast Cancer Res. Treat. 2008;110:235–244. doi: 10.1007/s10549-007-9716-3. [DOI] [PubMed] [Google Scholar]
- 66.Nonomura Y., Kohsaka H., Nagasaka K., Miyasaka N. Gene transfer of a cell cycle modulator exerts anti-inflammatory effects in the treatment of arthritis. J. Immunol. 2003;171:4913–4919. doi: 10.4049/jimmunol.171.9.4913. [DOI] [PubMed] [Google Scholar]
- 67.Chang B.D., Swift M.E., Shen M., Fang J., Broude E.V., Roninson I.B. Molecular determinants of terminal growth arrest induced in tumor cells by a chemotherapeutic agent. Proc. Natl. Acad. Sci. USA. 2002;99:389–394. doi: 10.1073/pnas.012602599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang Z., Banerjee S., Kong D., Li Y., Sarkar F.H. Down-regulation of Forkhead Box M1 transcription factor leads to the inhibition of invasion and angiogenesis of pancreatic cancer cells. Cancer Res. 2007;67:8293–8300. doi: 10.1158/0008-5472.CAN-07-1265. [DOI] [PubMed] [Google Scholar]
- 69.García-Castillo J., Pedersen K., Angelini P.D., et al. HER2 carboxyl-terminal fragments regulate cell migration and cortactin phosphorylation. J. Biol. Chem. 2009;284:25302–25313. doi: 10.1074/jbc.M109.001982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hartman Z.C., Yang X.Y., Glass O., et al. HER2 overexpression elicits a proinflammatory IL-6 autocrine signaling loop that is critical for tumorigenesis. Cancer Res. 2011;71:4380–4391. doi: 10.1158/0008-5472.CAN-11-0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Su J.L., Shih J.Y., Yen M.L., et al. Cyclooxygenase-2 induces EP1- and HER-2/Neu-dependent vascular endothelial growth factor-C up-regulation: a novel mechanism of lymphan-giogenesis in lung adenocarcinoma. Cancer Res. 2004;64:554–564. doi: 10.1158/0008-5472.can-03-1301. [DOI] [PubMed] [Google Scholar]
- 72.Gullick W.J., Love S.B., Wright C., et al. c-erbB-2 protein overexpression in breast cancer is a risk factor in patients with involved and uninvolved lymph nodes. Br. J. Cancer. 1991;63:434–438. doi: 10.1038/bjc.1991.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Adriance M.C., Gendler S.J. Downregulation of Muc1 in MMTV-c-Neu tumors. Oncogene. 2004;23:697–705. doi: 10.1038/sj.onc.1207165. [DOI] [PubMed] [Google Scholar]
- 74.Chizzolini C., Rezzonico R., De Luca C., Burger D., Dayer J.M. Th2 cell membrane factors in association with IL-4 enhance matrix metalloproteinase-1 (MMP-1) while decreasing MMP-9 production by granulocyte-macrophage colony-stimulating factor-differentiated human monocytes. J. Immunol. 2000;164:5952–5960. doi: 10.4049/jimmunol.164.11.5952. [DOI] [PubMed] [Google Scholar]
- 75.Lagow E.L., Carson D.D. Synergistic stimulation of MUC1 expression in normal breast epithelia and breast cancer cells by interferon‐γ and tumor necrosis factor‐α. J. Cell. Biochem. 2002;86:759–772. doi: 10.1002/jcb.10261. [DOI] [PubMed] [Google Scholar]
- 76.Banno T., Gazel A., Blumenberg M. Effects of tumor necrosis factor-α (TNFα) in epidermal keratinocytes revealed using global transcriptional profiling. J. Biol. Chem. 2004;279:32633–32642. doi: 10.1074/jbc.M400642200. [DOI] [PubMed] [Google Scholar]
- 77.Tang L.Y., Deng N., Wang L.S., et al. Quantitative phosphoproteome profiling of Wnt3a-mediated signaling network indicating the involvement of ribonucleoside-diphosphate reductase M2 subunit phosphorylation at residue serine 20 in canonical Wnt signal transduction. Mol. Cell. Proteomics. 2007;6:1952–1967. doi: 10.1074/mcp.M700120-MCP200. [DOI] [PubMed] [Google Scholar]
- 78.Fodde R., Smits R., Clevers H. APC, signal transduction and genetic instability in colorectal cancer. Nat. Rev. Cancer. 2001;1:55–67. doi: 10.1038/35094067. [DOI] [PubMed] [Google Scholar]
- 79.Mazurek N., Sun Y.J., Price J.E., et al. Phosphorylation of galectin-3 contributes to malignant transformation of human epithelial cells via modulation of unique sets of genes. Cancer Res. 2005;65:10767–10775. doi: 10.1158/0008-5472.CAN-04-3333. [DOI] [PubMed] [Google Scholar]
- 80.Chen P., Shen W.Z., Karnik P. Suppression of malignant growth of human breast cancer cells by ectopic expression of integrin‐linked kinase. Int. J. Cancer. 2004;111:881–891. doi: 10.1002/ijc.20340. [DOI] [PubMed] [Google Scholar]
- 81.Okuno Y., Nakamura-Ishizu A., Otsu K., Suda T., Kubota Y. Pathological neoangiogenesis depends on oxidative stress regulation by ATM. Nat. Med. 2012;18:1208–1216. doi: 10.1038/nm.2846. [DOI] [PubMed] [Google Scholar]
- 82.Hinata K., Gervin A.M., Zhang Y.J., Khavari P.A. Divergent gene regulation and growth effects by NF-κB in epithelial and mesenchymal cells of human skin. Oncogene. 2003;22:1955–1964. doi: 10.1038/sj.onc.1206198. [DOI] [PubMed] [Google Scholar]
- 83.Wang H., Lafdil F., Kong X., Gao B. Signal transducer and activator of transcription 3 in liver diseases: a novel therapeutic target. Int. J. Biol. Sci. 2011;7:536–550. doi: 10.7150/ijbs.7.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hanada T., Yoshimura A. Regulation of cytokine signaling and inflammation. Cytokine Growth Factor Rev. 2002;13:413–421. doi: 10.1016/s1359-6101(02)00026-6. [DOI] [PubMed] [Google Scholar]
- 85.Cavender D., Haskard D., Yu C.L., et al. Pathways to chronic inflammation in rheumatoid synovitis. Fed. Proc. 1987;46:113–117. [PubMed] [Google Scholar]
- 86.Madureira P.A., Varshochi R., Constantinidou D., et al. The Forkhead box M1 protein regulates the transcription of the estrogen receptor α in breast cancer cells. J. Biol. Chem. 2006;281:25167–25176. doi: 10.1074/jbc.M603906200. [DOI] [PubMed] [Google Scholar]
- 87.Zhao C., Matthews J., Tujague M., et al. Estrogen receptor β2 negatively regulates the transactivation of estrogen receptor α in human breast cancer cells. Cancer Res. 2007;15(67):3955–3962. doi: 10.1158/0008-5472.CAN-06-3505. [DOI] [PubMed] [Google Scholar]
- 88.Webb P., Lopez G.N., Uht R.M., Kushner P.J. Tamoxifen activation of the estrogen receptor/AP-1 pathway: potential origin for the cell-specific estrogen-like effects of antiestrogens. Mol. Endocrinol. 1995;9:443–456. doi: 10.1210/mend.9.4.7659088. [DOI] [PubMed] [Google Scholar]
- 89.Al Saleh S., Al Mulla F., Luqmani Y.A. Estrogen receptor silencing induces epithelial to mesenchymal transition in human breast cancer cells. PLoS One. 2011;6:e20610. doi: 10.1371/journal.pone.0020610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sotiriou C., Wirapati P., Loi S., et al. Gene expression profiling in breast cancer: understanding the molecular basis of histologic grade to improve prognosis. J. Natl. Cancer Inst. 2006;98:262–272. doi: 10.1093/jnci/djj052. [DOI] [PubMed] [Google Scholar]