Abstract
Introduction:
The incidence of type 1 diabetes (T1D) is increasing worldwide and there is a very large need for effective therapies. Essentially no therapies other than insulin are currently approved for the treatment of T1D. Drugs already in use for type 2 diabetes and many new drugs are under clinical development for T1D, including compounds with both established and new mechanisms of action.
Content of the Review:
Most of the new compounds in clinical development are currently in Phase 1 and 2. Drug classes discussed in this review include new insulins, SGLT inhibitors, GLP-1 agonists, immunomodulatory drugs including autoantigens and anti-cytokines, agents that regenerate β-cells and others.
Regulatory Considerations:
In addition, considerations are provided with regard to the regulatory environment for the clinical development of drugs for T1D, with a focus on the United States Food and Drug Administration and the European Medicines Agency. Future opportunities, such as combination treatments of immunomodulatory and beta-cell regenerating therapies, are also discussed.
Keywords: Type 1 diabetes, new drug development, regulatory environment, FDA, EMA, new mechanisms of action, insulins, anti-inflammatory drugs, immunomodulatory, islet regeneration
INTRODUCTION
The incidence and prevalence of both type 1 diabetes (T1D) and type 2 diabetes (T2D) are increasing worldwide, and in both cases there is a very large unmet medical need for pediatric and adult patients in these populations. However, over the recent past, “the most impressive changes in treatment and preventive strategies have been focused on type 2 diabetes,” leading to significantly more approved drug entities for T2D than for T1D [1]. T1D therapeutic development has attracted much less attention than T2D, not only because T2D is much more common, but also because the clinical and regulatory efficacy endpoints of T2D are better established, easier to affect, and more feasible to measure: a related 2015 review in this journal focused on development of T2D drugs [2]. However, because essentially no therapies other than insulin products have been approved for T1D, it can be argued that greater unmet therapeutic need is found among people with T1D that those with T2D. This review therefore focuses on drugs and biologics that are under development for T1D indications.
The review does not attempt to cover important progress that has been made with closed-loop insulin pumps (artificial pancreas) and encapsulated islets, either manufactured or animal-sourced. In addition advances in cell therapies are not presented. It also does not attempt to cover the wide range of approaches and large number of programs aimed at diabetic complications, e.g., nephropathy, neuropathy, retinopathy, and foot ulcers.
Pathophysiology of Type 1 Diabetes and Opportunities for Therapeutic Intervention
T1D arises from autoimmunity that has a strong but not completely defined genetic predisposition. As reflected in (Fig. 1) and in the review by Skyler and Ricordi [3], clinical onset of T1D is preceded by highly variable periods of latency and subclinical autoimmune β-cell injury. These periods also define potential points of therapeutic intervention (Fig. 2). Intervening progressively earlier than at T1D clinical onset is challenging, as exemplified by the landmark Diabetes Prevention Trial--Type 1 (DPT-1) [4]. This trial evaluated small injected doses of insulin and continues to evaluate oral doses of insulin. Randomization of approximately 800 participants required screening of about 100,000 first- and second-degree relatives of T1D patients to find about 4,000 individuals with islet cell antibodies. From that total, participants for randomization were selected to have a projected 5-year risk of clinical T1D >25% [4].
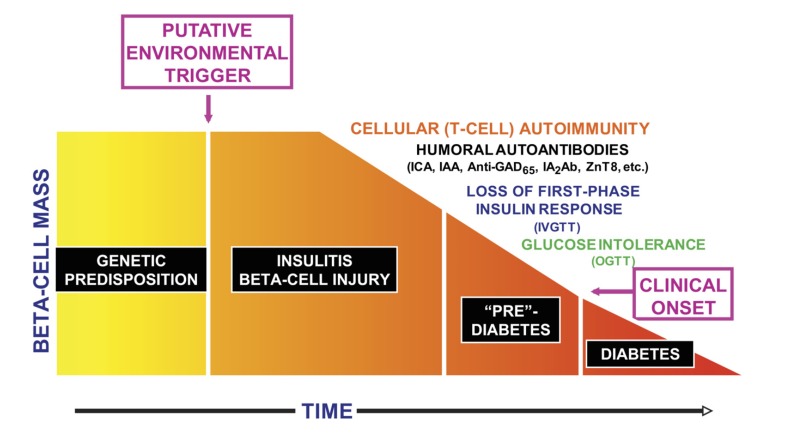
Fig. (1).
Progression of the type 1 diabetes disease process. This is a cellular autoimmune process occurring in individuals with a genetic predisposition to the disease, presumably triggered by some environmental factor. Humoral antibodies indicate that the disease process is underway, and there is then progressive impairment of -cell function manifested by progressive deterioration of glucose metabolism. The time frame is variable, so the x-axis is dimensionless. IAA, insulin autoantibody; ICA, islet cell antibody; IVGTT, intravenous glucose tolerance test; OGTT, oral glucose tolerance test (From Skyler JS and Ricordi C, 2011) [3].
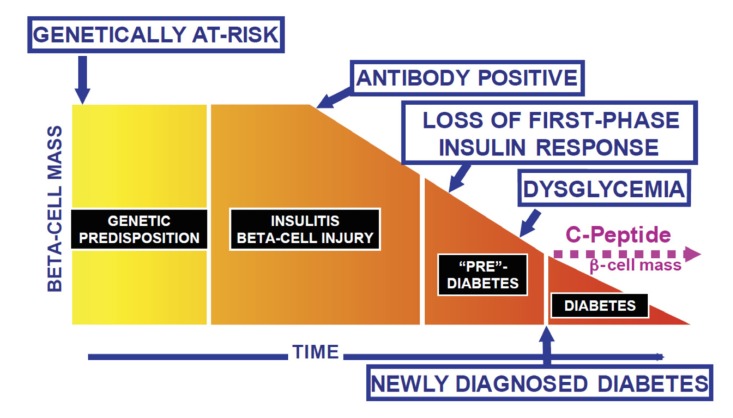
Fig. (2).
Potential time points for intervention to alter the type 1 diabetes disease process. Intervention may be attempted in the genetically at-risk to try to abrogate autoimmunity, in those with antibodies signifying that the disease process is underway, or in those with varying degrees of metabolic abnormalities, including at the time of clinical onset of type 1 diabetes (From Skyler JS and Ricordi C, 2011) [3]
People at T1D risk by familial relationship to a diagnosed patient account for only about 15% of the total population that goes on to develop T1D. HLA genotyping could be used at birth to screen the general population, but this approach has relatively low predictive value and has not been implemented [5]. For now, intervention at the root autoimmune cause of T1D is largely confined to patients who have been very recently diagnosed with T1D. Though new onset patients can feasibly be recruited into clinical trials, demonstrating efficacy in these trials is much more challenging than is the case for T2D trials. In contrast to trials that evaluate glucose-lowering therapies and use HbA1c as the primary endpoint, trials aimed at T1D autoimmunity evaluate the treatment’s effect on the decline in endogenous insulin secretion as reflected by stimulated C-peptide levels [6]. Because of the high variability in stimulated baseline C-peptide levels, these trials must be longer and larger than typical trials with HbA1c as the endpoint [7, 8].
The Unmet and Underestimated Need of T1D
In glaring contrast to the wide array of approved drugs for T2D, the only drugs approved for T1D are insulins and pramlintide: these are presented in Table 1. Miller et al. [9] discussed the limitations of available treatments for T1D, noting the need for new therapies to improve outcomes in this disease across all age groups, and observed that “only a minority of children and adults with type 1 diabetes achieve HbA1c targets,” an observation that Cefalu et al. [1] considered “a sobering reminder” of the need for better therapies to facilitate better outcomes.
Table 1.
Drugs Currently Approved for the Treatment of Type 1 Diabetes.
| Compound | Date First Approved | Comments |
|---|---|---|
| Regular human insulin | 1982 (FDA), 1984 (Europe); Bovine insulin: 1922 | Peak insulin levels 2-4 hours after injection |
| NPH insulin | Marketed 1950 | Long acting insulin |
| Insulin aspart | 1999 (EMA), 2000 (FDA) | Rapid acting insulin analogue |
| Insulin lispro | 1996 (EMA), 1996 (FDA) | Rapid acting insulin analogue |
| Insulin glulisine | 2004 (EMA), 2004 (FDA) | Rapid acting insulin analogue |
| Insulin glargine | 2000 (EMA), 2000 (FDA) | Long acting insulin analogue |
| Insulin detemir | 2004 (EMA), 2005 (FDA) | Long acting insulin analogue |
| Insulin degludec | 2013 (EMA) 2015 (FDA) |
Long acting insulin analogue |
| Insulin glargine U300 | 2015 (EMA), 2015 (FDA) | Long acting insulin analogue |
| Afrezza® inhaled insulin | 2014 (FDA) | Rapid acting insulin for inhalation |
| Pramlintide | 2005 (FDA) | Amylin analogue |
Although the 2014 United States (US) Centers for Disease Control and Prevention (CDC) report states that T1D represents about 5% of total patients with diabetes [10], this population is sizable, roughly 1.5 million people in the US alone. Even the CDC report provided very little information about T1D, instead focusing on T2D, which is undeniably a public health crisis but not a reason to neglect a large unmet clinical need in T1D. The prevalence of T1D is likely higher than suggested by reports that depend on clinical diagnoses. It was found in the United Kingdom Prospective Diabetes Trial (UKPDS) that about 10% of patients presumed to have type 2 diabetes at diagnosis had evidence of islet autoimmunity in the form of circulating ICA or GAD antibodies, and the majority progressed to insulin dependence within 6 years [11]. This syndrome was originally referred to as latent autoimmune disease in adults (LADA), but more recently has been found to occur in younger people [12]. A view is emerging that LADA is not a distinct entity, but that LADA and childhood-onset T1D are opposing ends of the same continuum of autoimmune diabetes [13]. The wider understanding of autoimmune diabetes leads to the conclusion that a substantially larger population has a form of T1D than is generally recognized. This concept also suggests a wider window of opportunity for interventions that prevent T1D or reduce the progression towards T1D.
The specific risk of greatest concern to people with T1D, which can be viewed as the basis of highest unmet need, is the risk of severe hypoglycemia that results from total dependence on injected insulin therapy [14]. This risk, typically defined as hypoglycemia requiring the assistance of another person, averages about two episodes per person per year [15]. T2D patients on insulin therapy have significant but substantially lower rates of hypoglycemia.
Despite huge unmet clinical need for therapies specifically approved for T1D and defined regulatory pathways for T1D indications [8, 16, 17], to date (and for a variety of reasons) no drug remains in Phase 3 development. Four drugs reached that stage, GAD65 (Diamyd), DiaPep277 (Andromeda), otelixizumab (GlaxoSmithKline), and teplizumab (Lilly/MacroGenics), but all have gone back to Phase 2 or have been discontinued.
Differences in Challenges and Opportunities for T1D and T2D Therapies
Development of T1D and T2D therapies differ in both their challenges and opportunities. T1D is comprised of both an established orphan indication for new-onset T1D, defined by the US Food and Drug Administration (FDA) as a disease with fewer than 200,000 individuals affected, and a general indication for T1D as a disease that affects about 1.5 million people in the US. FDA has recognized that new-onset T1D represents an orphan population and has provided orphan designations to at least 10 different drugs and biologics [18].
In part because of the smaller population involved, FDA and the European Medicines Agency (EMA) require smaller development programs for T1D therapies than are required of T2D therapies. FDA notably expects the cardiovascular safety of T2D therapies to be systematically evaluated before and after approval. This is not the case for T1D therapies. FDA has not applied the same cardiovascular outcome trial (CVOT) requirement to insulin therapies, which are indicated for both T1D and T2D, but approval of NovoNordisk’s long-acting insulin analog, insulin degludec (TresibaTM), was held up by a requirement to complete a CVOT after FDA observed an adverse signal in the original new drug application [19]. Differences in regulatory expectations and clinical trial designs for various T1D therapies are compared in Table 2.
Table 2.
Comparison of Clinical Development and Regulatory Expectations for Different Diabetes Therapies.
| Cell Therapy for Severe T1D | New Onset T1D Immune Therapy | New Insulin Analog | |
|---|---|---|---|
| Patient population | Late T1D with disabling hypoglycemia | All with T1D diagnosed within 3 months | Any T1D or T2D requiring insulin treatment |
| Study design | Single-arm open label | Placebo-controlled | Active-controlled (insulin comparator) |
| Total N for submission | 200 | 1000 | 4000 |
| Primary endpoint | Percent of subjects with HbA1c<6.5% and no hypoglycemia | C-Peptide | HbA1c Non-inferiority |
| Secondary endpoint | Percent off insulin treatment at one year | HbA1c, hypoglycemia, insulin dose | Hypoglycemia rates |
| Study duration to endpoint | 12 months | 24 months | 26-52 weeks |
| Long-term follow-up | Important but informal | Strongly encouraged | Only required for a subset |
| Requirement for CVOT | No | No | Only if a CV signal is detected |
| FDA Review Center | CBER | CDER | CDER |
Abbreviations: T1D, Type 1 Diabetes; T2D, Type 2 Diabetes; CVOT, Cardiovascular Outcome Trial; CV, Cardiovascular; FDA, US Food and Drug Administration; CBER, Center for Biologics Evaluation and Research; CDER, Center for Drug Evaluation and Research
Few, if any, current T2D products are more than just palliative approaches: they only reduce glucose, body weight, and/or insulin resistance. If a T2D drug regimen is removed from a patient, little, if any, benefit may remain. In contrast, many T1D therapeutic candidates, other than insulin products, have prospects to be either curative, restorative, and/or disease modifying.
Finally here, in contrast to individuals with T2D, those with T1D are generally diagnosed at a younger age, more adept with technology, more informed, and active in seeking better treatments.
NEW AND APPROVED VERSIONS OF APPROVED AGENTS/MECHANISMS OF ACTION FOR T1D DRUGS
Insulin has been, and still is, the most important treatment for patients with T1D. Currently available insulins are not entirely effective in controlling blood glucose levels, as hyper- and hypoglycemia are still common in patients with T1D. Therefore, a high medical need exists for insulins, especially with less risk for hypoglycemia. The most frequent approach in this regard is to shorten the time-action profile of rapid-acting insulin to be more similar to physiologic insulin secretory profiles, thereby ameliorating post-prandial hyperglycemia which could eventually have benefits in fully automated closed loop settings. Moreover, new methods of insulin delivery, such as oral insulin, could be attractive treatment options for patients with T1D and T2D. Oral insulin delivery could also provide more physiologic exposure of insulin to the liver, and this might lead to less hypoglycemia risk and weight gain. “Smart insulin,” insulin analogs with activity modulated by ambient blood glucose levels, is another approach for improving the therapeutic index of insulin [20].
The FDA and EMA require studies of both T1D and T2D patients for all insulin products. No indication has yet been approved for an insulin product that restricts use to one form of diabetes or the other.
Injectable Insulins
New basal insulin analogs that lead to less glucose variability and less hypoglycemia risk and/or have a longer duration of action than currently available basal analogs are in development. LY2605541 (Lilly) is a pegylated insulin Lispro (pegLispro) that is intended for once-daily injection. The pegylation of the insulin is associated with a slower absorption and reduced clearance, resulting in a longer duration of action. A Phase 2 study in participants with T1D showed that pegLispro was more effective than insulin glargine with regards to glycemic control, and reduced the insulin doses needed for meals as well as body weight. The overall incidence of hypoglycemia was increased, but measures of nocturnal hypoglycemia were reduced compared with insulin glargine. Of concern, liver enzymes (alanine aminotransferase, aspartate aminotransferase), triglycerides, and LDL-cholesterol increased while HDL-cholesterol decreased compared with insulin glargine in this study [21]. The report of increased liver fat associated with this analog has led to a delay in regulatory filings, and Eli Lilly has eventually decided to terminate its development in December 2015 [22, 23].
A once-weekly basal insulin is in development by the South Korean company Hanmi (HM12460A). A Phase 1 study in patients with T1D has already been successfully completed [24]. NovoNordisk’s once-weekly insulin LA1287 is currently in Phase 1 development for T1D (NCT01730014). Data are not yet publicly available.
No injected ultra-rapid-acting insulin product has yet been approved, but the approved pulmonary inhaled insulin product Afrezza® does have a distinctly faster time-action profile than currently available injected insulin analogs (as discussed in due course). Injected ultra-rapid acting insulins and insulin analogs are in various stages of development. BIOD-123 (Biodel) proved noninferior compared with insulin lispro with regards to HbA1c (defined as the upper bound of the 95% confidence interval around change from baseline HbA1c of <0.40%) in a Phase 2 study including 132 participants with T1D [25]. Linjeta™ (Biodel), another ultra-rapid acting insulin analog, completed Phase 3 development, but did not get approval from the FDA due to questions about efficacy, tolerability, and stability. The company, therefore, decided to go forward with a follow-on product, BIOD-123 [26]. NovoNordisk’s faster acting insulin aspart (FI-ASP), a combination of insulin aspart, nicotinamide, and arginine, showed a significantly greater glucose lowering effect within 90 minutes and an earlier onset with a similar potency compared with insulin aspart [27]. Results from a Phase 3 trial in T1D patients showed a better HbA1c reduction with FI-ASP compared with insulin aspart [28].
An ultra-fast acting insulin lispro is co-developed by Adocia and Lilly using Adocia’s Biochaperone® technology, which attaches polymers to proteins and thereby stabilizes them and protects against enzymatic degradation. A Phase 2b trial in patients with T1D was initiated in August 2015 (NCT02528396) and the companies plan to initiate a Phase 3 trial soon [29].
Another interesting approach is the development of glucose-responsive insulins (smart insulins). These insulins would automatically activate once the blood glucose is high and be inactive when the blood glucose is low. Merck’s MK-2640 is already in Phase 1 development (NCT02269735) but it will take some time until these smart insulins reach the market.
Halozyme is developing an adjuvant therapy for reducing the time-action profile of injected insulin. Halozyme’s rHuPH20 is a recombinant human hyaluronidase that can be combined with rapid acting insulin analogues. This leads to a faster and more consistent absorption [30]. Phase 2 trials showed that insulin lispro or aspart combined with rHuPH20 were noninferior to insulin lispro alone and reduced post-meal glucose excursions by 82% and hypoglycemias by 5-7% [31].
Pulmonary Insulin
The only inhaled insulin product to be approved to date is Afrezza® (Mannkind), which does have a distinctly faster time-action profile that injected insulin analogs. It is noteworthy that FDA has not permitted Afrezza® the use of the term ‘ultra-rapid’ insulin, even though its time-action profile is clearly different from fast acting insulins [32].
Another inhaled insulin product is under development by Dance Biopharm. Dance’s product consists of a pocket-sized inhaler device and insulin container. In contrast to the dry powder form of the insulin used in the Afrezza® device, the Dance insulin formulation is packaged as a liquid in a separate container. A few drops of the liquid formulation can be accurately dispensed into the reservoir on top of the device for dosing at mealtime. The Dance drug-device combination product has completed Phase 2 clinical trials and is in preparation for pivotal development [33].
Oral Insulins
Delivering insulin orally to achieve therapeutically relevant circulating insulin levels is a promising approach for improving glycemic control in patients with T1D. Oral delivery can provide a more physiologic presentation of insulin to the liver than that provided by peripherally injected insulin. Endogenous insulin is subject to a first-pass effect in the liver, which modulates the appearance of insulin in the periphery. The higher portal insulin concentration that results from pancreatic insulin secretion leads to a greater suppression of hepatic glucose production than can be achieved with peripherally injected insulin [34, 35]. If oral insulin is absorbed from the gastrointestinal tract into the portal venous system, this can lead to effects in the liver similar to those resulting from physiologic insulin secretion. This contrasts with injected insulin, which immediately exerts systemic action. Thus, enterally absorbed oral insulin treatment might be a way to smooth the glucose profile and reduce the risk of hypoglycemia compared with parenteral insulin. Although oral insulin may not replace parenteral insulin for patients with T1D, the addition of oral insulin could reduce the amount of parenteral insulin needed and improve glycemic control and risks of hypoglycemia [36]. A favorable benefit-risk profile has still to be shown in larger clinical studies, but currently available human data suggest that this might be a valuable approach for treating diabetes.
Several enterally absorbed oral insulins are in development. A short-acting oral insulin currently in Phase 2 development for T1D is ORMD0801 (Oramed). A Phase 3 study of IN-105 (Biocon) has already been conducted in India in T2D. A Phase 1 study in T1D has been initiated (NCT01035801) but there are no other public data on IN-105 available in T1D. Basal oral insulins are in earlier stages of development. As one example, OI287GT (NN1956) (NovoNordisk) is in Phase 1 development but information on studies employing participants with T1D is not yet available (NCT01809184).
Another way to administer insulin orally is buccal administration. Oral-lynTM (Generex) is a human insulin spray to deliver insulin via the buccal wall for T1D and T2D patients [37]. It is not approved by the FDA or EMA but is available in some non-US and non-EU countries. Oral-lyn was marketed in India, but its approval was rescinded in 2009 [38]. Generex announced in 2013 that its Indian licensee, Shreya Life Sciences Pvt. Ltd., had completed a local Phase III trial of the buccal insulin product (branded as Oral Recosulin® in India) and submitted the dossier to the Drugs Controller General (India) (DCGI) [39]. There is currently no information available from Generex on the status of the DCGI submission or its plans to seek FDA or EMA approval.
Hepatoselective Insulin Product
The development of oral insulin products represents one example of a parallel and more general effort to increase the insulinization of the liver, which could improve the therapeutic index of insulin treatment. Targeting of insulin to the liver or enhancing liver selectivity of insulin products has a long history [40]. A recent example of a hepatoselective insulin program is Lilly’s long-acting basal insulin, LY2605541, discussed previously. This pegylated insulin analog showed direct evidence of hepatoselectivity [41] and compared well to basal insulin glargine in clinical trials [21], but development was terminated in December 2015 related to the association of increased hepatic fat with treatment [23].
Diasome Pharmaceuticals is developing a novel means of targeting insulin delivery to the liver. Diasome’s approach consists of insulin-containing vesicles. The vesicle is coated with a ligand that is avidly taken up by the liver [42]. Preliminary clinical studies have provided evidence of increased insulin activity at the liver relative to insulin comparators [43]. The company is currently planning a Phase 2b trial in collaboration with the Joslin Clinic [44].
DRUGS APPROVED FOR T2D SEEKING AN INDICATION FOR T1D
Sodium/Glucose Cotransporter (SGLT) Protein Inhibitors
SGLT2 inhibitors reduce blood glucose levels by lowering the renal threshold of glycosuria. Given their insulin-independent mechanism of action, SGLT2 inhibitors could be helpful to the management of T1D when given as an add-on therapy to insulin. Some of these agents that are already approved for the treatment of T2D are being investigated to pursue an indication for T1D, e.g., empagliflozin, dapagliflozin and canagliflozin. Empagliflozin (Jardiance ® - Boehringer Ingelheim/Eli Lilly) was studied in a Phase 2 clinical trial (NCT01392560) in 40 participants with T1D to investigate renal hemodynamic effects and glucose efficacy as an exploratory endpoint. Treatment with empagliflozin for 8 weeks demonstrated a 0.4% decrease in average HbA1c, which was also followed by a decrease in mean fasting glucose and total daily insulin dose. Interestingly, there were also reductions in hypoglycemic events, weight (mean of 2.6 kg), and waist circumference [45].
Of considerable note, the recent results of the large CVOT with empagliflozin in T2D demonstrated cardiovascular benefits, i.e., a 38% reduction in risk of cardiovascular death and a 35% relative risk reduction in hospitalization for heart failure as compared with standard-of-care. While still not completely explained, these findings indicate an advantage for the treatment of T2D with stablished cardiovascular disease, although it is too early to speculate whether empagliflozin could provide similar cardiovascular benefit in patients with T1D [46].
Improvements in measures of glucose control in T1D were also shown with dapagliflozin (Farxiga® - Astra Zeneca). In a 2-week Phase 2a trial employing 70 participants with T1D, dapagliflozin as compared with placebo showed greater numeric reductions in mean blood glucose levels over 24 hours and mean amplitude of glucose excursions measured by continuous glucose monitoring, as well as a decrease in total daily insulin dose [47]. These results from short-term treatment still need to be confirmed in longer duration studies to support a future indication of this agent for T1D.
Canagliflozin (Invokana® – Janssen) is another SGLT2 approved for T2D that is currently being tested in T1D (NCT02139943). Ipragliflozin (Suglat® - Astellas Pharma), an SGLT2 approved in Japan, was tested in animal models of T1D [48]. Among the SGLT2 inhibitors still in earlier phases of clinical development, remogliflozin (BHV Pharma) is in Phase 2 development for T2D and in Phase 1 for T1D, with initial positive safety findings from a single dose administration as an add-on to continuous insulin infusion [49].
While the anti-hyperglycemic effect of SGLT2 inhibitors is primarily due to its action at the renal tubule, SGLT1 inhibitors have been shown to target the gut absorption of glucose, which can reduce post-prandial glucose excursions. No SGLT1 inhibitor has yet been studied in T1D. Sotagliflozin (LX4211, Lexicon Pharmaceuticals) is a combined SGLT1/2 inhibitor in Phase 3 development, which has been successfully tested in T2D and T1D, and it is therefore positioned to become the first in this class to gain approval for treatment of T1D [50]. Results of the Phase 2 proof-of-concept trial of sotagliflozin in 33 participants with T1D (NCT01742208) treated for 29 days showed a superior reduction of total daily mealtime insulin dose compared with placebo (32% vs 6.4%) and improved glucose control with higher reduction of HbA1c (0.55% vs 0.06%) [51]. Continuous glucose monitoring evaluation showed reduction in time in the hyperglycemia range without increase of hypoglycemia. Continuous glucose monitoring evaluation additionally demonstrated greater percentage of time in the target glycemic range with sotagliflozin compared with placebo (68.2% vs 54.0%) and reduction in time in the hyperglycemia range (25.0% versus 40.2%), without increase of hypoglycemia [52].
Overall, SGLT inhibitors carry a promise of improving glucose control while promoting a moderate weight loss and providing some additional cardiovascular benefits, such as reduction of systolic blood pressure, renal hyperfiltration, and albuminuria [53-56]. Although less of a concern for the majority of patients with T1D than is the case for T2D patients, excess weight gain is still an important drawback of insulin therapy. Though understanding of the metabolic and hemodynamic effects of this class is incomplete, T1D patients may potentially benefit from an additional improvement in glucose control provided by an oral agent that decreases insulin requirements and thereby decreases hypoglycemia risk. Despite the optimistic efficacy results demonstrated in short-term studies, further studies are needed to assess the safety profile of these agents in people with T1D, particularly children. The clinical relevance of adverse events reported in T2D and T1D studies, such as higher rates of genitourinary infections, requires special attention in this population given the possible long term impact on clinical outcomes and quality of life. Of concern for use of SGLT2 agents in T1D are the recent reports from FDA and EMA of the association of SGLT2 agents with diabetic ketoacidosis (DKA) [57, 58]. Most of the cases were T2D patients, but a few were T1D patients. Presumably the risk of DKA in T1D patients would be at least as high as that for T2D patients, but the risks related to SGLT2 inhibitors for both T1D and T2D patients are not yet clear [59]. A recent report described 13 episodes of SGLT2 inhibitor associated diabetic ketoacidosis in 7 T1D and 2 T2D patients [60]. Studies of newer agents in this class and studies of a longer duration of marketed products will help to characterize more fully the benefits and risks of SGLT2 inhibitors in T1D.
GLP-1 Agonists
Glucagon-like peptide-1 (GLP-1) is secreted from the gut after meals and enhances glucose-induced insulin secretion, inhibits glucagon secretion, suppresses appetite, and delays the gastric-emptying rate. GLP-1 receptor agonists represent a class of drugs that has traditionally been used in the treatment of adults with T2D. GLP-1 agonists have demonstrated several advantages over previously approved therapies, including less risk of hypoglycemia compared with insulins and insulin secretegogues and promotion of weight loss.
It has been suggested that the use of GLP-1 receptor agonists in T1D may reduce excessive postprandial glucagon secretion allowing patients to reduce their total daily dose of exogenous insulin [61]. Hypoglycemia risk may also be minimized in T1D as glucagon counter-regulation can be preserved to some degree via the glucose-dependent action of the GLP-1 receptor agonists. GLP-1 agonist therapies have shown some promising effects in terms of positively affecting overall β-cell health and increasing β-cell mass, primarily in mouse models.
Achieving good glycemic control with insulin therapy in T1D patients is necessary to prevent both microvascular and macrovascular complications [62]: however, insulin therapy also promotes unwanted weight gain [63]. Given that approximately 40% of patients with T1D now also carry the diagnosis of metabolic syndrome, investigating the therapeutic employment of GLP-1 agonists as a supplemental therapy in T1D is logical: the glucose-lowering effects resulting from the inhibition of glucagon secretion and the gastric-emptying rate could be of clinical importance.
In one study, 17 participants with T1D, half of whom had residual insulin production, underwent two mixed meal tolerance tests (MMTTs) and two intravenous glucose tolerance tests (IVGTTs), with and without pre-treatment with exenatide. This study showed significant acute metabolic effects in T1D participants following an oral meal in individuals with T1D, involving reduced glucose excursions, glucagon suppression, and delayed gastric emptying, while preserving insulin secretion in participants with residual insulin production. Despite the small number of individuals studied, the antidiabetic effects observed indicate a potential value for GLP-1 analogs as adjunctive treatment in T1D, especially in the new-onset period [64].
Several small nonrandomized studies with liraglutide employing individuals with T1D have demonstrated that liraglutide, a long acting GLP-1 receptor agonist, resulted in improved glycemic control and led to weight loss [65].
A retrospective analysis of data obtained from 27 obese individuals with T1D treated with insulin and liraglutide in addition to being treated for hypertension was reported by Kuhadiya et al [66]. Results demonstrated that after 180 ± 14 days of treatment with liraglutide, mean glucose concentrations fell from 191 ± 6 to 170 ± 6 mg/dL (P = 0.002). HbA1c fell from 7.89 ± 0.13% to 7.46 ± 0.13% (P = 0.001), without an increase in frequency of hypoglycemia. Mean body weight fell from 96.20 ± 3.68 kg to 91.56 ± 3.78 kg (P<0.0001). Daily total and bolus doses of insulin fell from 73 ± 6 to 60 ± 4 units (P = 0.008) and from 40 ± 5 to 29 ± 3 units (P = 0.011), respectively. Mean systolic blood pressure fell from 130 ± 3 to 120 ± 4 mm Hg (P = 0.020).
However, NovoNordisk recently announced that it will not submit an application to expand the label of liraglutide for use in T1D due to results of two large Phase 3 trials involving 2,000 participants with T1D. Liraglutide reduced the HbA1c but showed a higher rate of symptomatic hypoglycemia events compared with placebo [67]. Currently, only exenatide is still being studied in T1D. It remains to be seen if this GLP-1 agonist will differ from liraglutide in hypoglycemia risk.
IMMUNOMODULATORY DRUGS
Interventions against the underlying autoimmunity of T1D fall into the two broad categories of auto-antigen and non-specific immunomodulatory or anti-inflammatory approaches. Auto-antigen approaches have emerged from the quest to understand the fundamental mechanisms of T1D autoimmunity. Insulin and glutamic acid decarboxylase (GAD) emerged as leading auto-antigen prospects for inducing tolerance, but trials with these auto-antigens have been largely unsuccessful.
Autoantigen Intended to Induce Tolerance
Autoantigen therapies have been suggested for induction of immune tolerance in T1D, although results so far have not been promising [68]. In the DPT-1 trial, subcutaneous low-dose insulin did not have any effect in preventing diabetes in first- and second-degree relatives of patients with T1D [69, 70], although a study of orally administered insulin continues. Similarly, intranasal insulin did not prevent or delay T1D in young children with increased genetic susceptibility to T1D [71].
Glutamic Acid Decarboxylase (GAD65) as Vaccine
Diamyd® is a vaccine-like product consisting of the 65-kD isoform of glutamic acid decarboxylase (GAD65) formulated with alum (GAD-alum). It has been studied extensively and found to have a good safety profile. The development program was discontinued in 2011 after the first Phase 3 trial failed to meet the primary endpoint of area under the curve for meal-stimulated C-peptide after 15 months of treatment [72]. Nonetheless, Diamyd Medical started further developing this vaccine in 2012. Several approaches to treat T1D are now being tested with Diamyd®, including trials started in 2015 in which this vaccine is directly injected into lymph nodes and combined with Vitamin D in newly diagnosed T1D patients (DIAGNODE-1, NCT02352974) or combined with gamma-aminobutyric acid (GABA) to preserve the residual insulin-producing capacity in 75 newly diagnosed patients aged 4-18 years (NCT02352974). In addition, a prevention trial in pediatric patients with multiple islet cell autoantibodies was initiated in 2015 (DiAPREV-IT, NCT 01122446).
TOL-3021
TOL-3021 (Tolerion Inc.) is a novel reverse vaccine that induces tolerance with the autoantigen proinsulin, thereby reducing islet autoimmunity [73]. In a Phase 2a study, 80 participants with T1D (diagnosed within 5 years) were randomized to 4 different doses of TOL-3021 or placebo (administered intramuscularly) once weekly for 12 weeks, and were followed for 6 months. At Week 15 after baseline, C-peptide levels were improved in all dose treatment arms compared with placebo, and CD8 proinsulin-reactive T cells decreased in the active treatment arms [74].
Non-specific Immunomodulatory or Anti-inflammatory Agents
The hallmark of T1D is autoimmune destruction of islet β-cells. The pathogenesis of this destruction is increasingly understood to be complex and heterogeneous. It is now known that, in addition to T-cell mediated destruction of β-cells and induction of β-cells giving rise to autoantibodies, there is neutrophil infiltration to both the endocrine and exocrine pancreas as well as involvement of dendritic cell, B1 cells, and IL17+ γδ T lymphocytes. In addition, cytokine release, such as IFNγ and inflammation, plays a key role. However, the insulitis, as well as β-cell destruction and residual function, is variable histologically, as is the rate of β-cell loss and dysfunction. Thus, the therapies discussed next target several of these pathways by which β-cell destruction may be mediated.
Alpha-1 antitrypsin (AAT)
Human AAT is a serine protease inhibitor, mainly synthesized in the liver (>80%), and in smaller quantities in the lungs, pancreas, enterocytes, and macrophages. AAT deficiency is associated with lung emphysema, intrahepatic obstruction and liver cirrhosis, panniculitis, and systemic vasculitis. An increasing amount of evidence over the last 10 years suggests that AAT has important anti-inflammatory, anti-apoptotic, and immunomodulatory properties not related to its anti-protease activity. These properties include the following: inhibition of nitric oxide (NO) production [75]; suppression of the synthesis of proinflammatory cytokines, such as IL-6, IL-8, IL-1β, and TNF-α [76]; prevention of pancreatic β-cell and bronchial epithelial cell apoptosis [77, 78]; and inhibition of caspases 3 and 6 [79]. Accordingly, administration of AAT in non-deficient individuals could potentially modify disease progression in a variety of clinical conditions, such as T1D, rheumatoid arthritis, inflammatory bowel disease, multiple sclerosis, and graft-versus-host disease [80]. Specifically for T1D, recently diagnosed individuals have decreased circulating levels of serum AAT, as well as increased enzymatic activity of neutrophil serine proteases, which could be implicated in the β-cell autoimmunity [81].
AAT is commercially available as an intravenous infusion in various countries, prepared from pooled plasma of blood donors: Omnio Bio Pharmaceuticals has announced the first-in-class recombinant version of human AAT.
A Phase 1/2 study of Glassia® (a plasma-derived AAT produced by Kamada Pharma) in 24 pediatric participants with recently diagnosed T1D showed that the treatment was safe and decreased HbA1c from a mean of 8.43% to 7.09% [82]. A pivotal placebo-controlled Phase 2/3 trial in 192 participants with new-onset T1D is currently ongoing (NCT02005848). A Phase 2 study with another AAT preparation (Prolastin-C®, Grifols Therapeutics, NCT02093221) is currently ongoing, but data are not yet available.
Anti-CD3 Antibodies
CD3 is an essential component of the T-cell receptor complex and transduces signals to activate mature T-cells. Therefore, inhibiting CD3 is a plausible way to reduce T1D autoimmunity. Several anti-CD3 antibodies have been approved for acute and/or chronic rejection of solid organ transplants, including the first ever approved monoclonal antibody for human use, muromonab-CD3. Newer anti-CD3 antibodies, teplizumab, visilizumab, and otelixizumab have been developed for T1D. Visilizumab was in Phase 2/3 development for T1D but was terminated due to less favorable safety and efficacy profiles compared with other members of its drug class.
Teplizumab (MacroGenics) is an anti-CD3 epsilon chain monoclonal antibody in development for prevention of T1D. Studies have shown teplizumab increases CD8, CD25+ Tregs, thus inhibiting the CD4+ effector cells [83]. In the last decade, there were multiple trials demonstrating the efficacy of teplizumab in preserving post-MMTT C-peptide levels. These included smaller trials employing participants diagnosed with T1D within 6 weeks of enrolment and followed for 2-5 years (NCT00378508) [84, 85]. Also, the Phase 2 open-label AbATE trial, which enrolled 81 participants with T1D diagnosed within 6 weeks prior to enrolment, showed preservation of post-MMTT C-peptide by 75% in the treatment arm compared with placebo one year later (NCT00129259) [86]. A follow-up observational trial of AbATE is ongoing (NCT02067923).
However, the development of teplizumab for the treatment of T1D was discontinued after the Phase 3 554-participant PROTÉGÉ trial did not meet the composite endpoints of HbA1c (<6.5%) and insulin dose reduction (<0.5 U/kg/day) after one year (NCT00385697) [87]. Despite this, teplizumab was effective in maintaining post-MMTT C-peptide levels in subpopulations with higher C-peptide level and lower insulin dose at the start of the trial, a more recent diagnosis (6 weeks rather than 12 weeks), and younger age. Teplizumab was well tolerated, with the main adverse reactions being rash and transient decreased lymphocytes. Similar results were reported after two years [88].
In conjunction with nonclinical data, these results led to a now-ongoing trial examining teplizumab therapy for T1D prevention, targeting pre-diabetic individuals who have two or more auto-antibodies and are relatives of T1D patients (NCT01030861). There is also a Phase 3, 250-participant PROTÉGÉ ENCORE trial studying the efficacy of teplizumab in treating T1D with two 14-day courses (NCT00920582). The trial is completed but results are not yet released.
Otelixizumab (GlaxoSmithKline) is another anti-CD3 monoclonal antibody that is back in development after its Phase 3 program was discontinued. Earlier trials had shown efficacy using 48 mg cumulative dose and follow-up as long as 48 months (NCT00627146) [89, 90]. However, the pivotal Phase 3 trial (DEFEND-1) enrolled 281 participants with T1D diagnosed less than 90 days prior to enrolment and administered 3.1 mg total dose of otelixizumab to avoid the higher-dose-induced EBV mononucleosis and cytokine release syndrome (NCT00678886) [91]. While there were no EBV reactivations, the efficacy endpoint of post-MMTT C-peptide was not achieved. Following this, the second Phase 3 trial DEFEND-2 was terminated early after 12 months and the results were similar (NCT011230830) [92].
Currently, the ongoing Phase 2 trial is aimed to examine the safety and tolerability of otelixizumab between 9 mg and 36 mg with post-MMTT C-peptide as a secondary endpoint. This trial will enroll 40 participants in Belgium, and last 5 years.
Abatacept
Abatacept modulates T-cell co-stimulation and prevents full T-cell activation. In this manner it could have an influence on the progression of T1D. It was shown that Abatacept can delay the C-peptide decline in patients with recent-onset T1D during 2 years of treatment [93]. This effect is sustained for at least 1 year after cessation of treatment [94]. Currently, abatacept is evaluated for prevention of abnormal glucose tolerance in patients at risk for T1D (NCT01773707).
Rituximab
Treatment with the selective β-lymphocyte depletion agent rituximab, an anti-CD20 monoclonal antibody, for 1 year delayed the C-peptide decline in patients with recent-onset T1D [95]. However, this approach for T1DM treatment is not pursued anymore as it could not significantly influence its pathophysiology.
DiaPep277
DiaPep277 (Hyperion Therapeutics) is a 24 amino acid peptide corresponding to positions 437-460 in heat shock protein 60 (HSP60). The peptide has been show to modulate immunological attack on β-cells in the NOD mouse model of type 1 diabetes [96]. Phase 2 studies showed that this peptide can inhibit the decline in stimulated C-peptide secretion in adult T1D patients [97]. The first of two Phase 3 studies was published in 2014. However, the publication was retracted and the second Phase 3 trial results have not been released. The development of DiaPep was discontinued after Hyperion Therapeutics, Inc., which acquired Andromeda Biotech in June 2014, uncovered evidence of study misconduct during this Phase 3 study [98].
Dendritic Cells
Dendritic cells (DC) are antigen-presenting cells that can initiate the immune response, while also participating in peripheral T-cell tolerance [99]. Tolerogenic DCs could potentially be useful in the management of autoimmune conditions, such as rheumatoid arthritis, solid organ transplantation, and T1D. Creusot et al. [100] provided a review of the use of DCs in T1D.
DiaVacs Inc. has developed vaccine DV0100, which aims to reverse recent-onset T1D. DV0100 obtained orphan-drug status from FDA in 2014. The vaccine is based in leukapheresis-harvested autologous DCs, engineered via incubation with antisense DNA oligonucleotides that target CD40, CD80, and CD86, followed by their administration in the peri-umbilical area of the individual. These cells are called “immunoregulatory” DCs (iDCs).
A Phase 1 study in 10 participants with T1D duration greater than 5 years reported no safety issues for this vaccine [101]. Phase 2 studies targeting patients earlier after their diagnosis (<6 months) are currently planned: no data are yet available (NCT00445913, NCT01947569).
Anti-Cytokines
Anti-IL-17
PF-06342674 (Pfizer) is a monoclonal antibody antagonizing both IL-7 and IL-17 receptors. IL-7 stimulates the proliferation of lymphoid cells, whereas IL-17 stimulates production of many chemokines important for chemotaxis of myeloid cells. A Phase 1 trial (NCT01740609) employing 80 healthy participants and assessing the compound’s safety and tolerability has been completed but results are not publicly available. In 2014, a Phase 2 trial (NCT02038764) in T1D adults diagnosed within two years of randomization was initiated to assess safety, tolerability, pharmacokinetics, and immunogenicity by subcutaneous administration.
IL-1 Modulators
The IL-1 pathway is involved in autoimmune and auto-inflammatory diseases [102]. IL1-α activation leads to synthesis of the more potent IL-1β in many cell types. Both IL-1α and IL-1β bind to IL-1 receptor 1 (IL-1R1) and activate multiple intracellular signalling cascades leading to activation of NFκB and AP-1. Furthermore, hyperglycemia and high circulating free fatty acids activate β-cells to increase IL-1β by more than 100-fold. This leads to β-cell dysfunction and death.
Canakinumab (Novartis) is approved for cryopyrin-associated periodic syndromes, gouty arthritis, and juvenile rheumatoid arthritis. Canakinumab binds selectively to IL-1β and competitively prevent its binding to the IL-1 receptors. The drug has been shown to be well tolerated in multiple clinical trials for inflammatory diseases. However, a Phase 2 trial designed to assess the change in C-peptide levels after MMTT in participants with T1D diagnosed within the previous 3 months was terminated in 2011. In 2013, Moran and co-workers reported on behalf of the Type 1 Diabetes TrialNet Study Group that monthly injection of canakinumab for 12 months in individuals with newly diagnosed T1D did not lead to statistically significant improvement in post-MMTT C-peptide level compared with placebo [103]. Currently, the compound’s development for T1D has been terminated. However, although monotherapy may not be effective, this drug may be useful in combination therapy with other immune-modulators.
Gevokizumab (Xoma), a monthly injectable drug, is being developed in Phase 2 in Switzerland for T1D. It binds selectively to IL-1β and allosterically reduces its binding affinity to the IL-1 receptors, but not its decoy receptor that leads to its degradation [104]. It is being developed in a Phase 3 program for non-infectious uveitis and has been found to be safe and well tolerated. Two Phase 2 trials (NCT01788033, NCT00998699) have been completed for T1D. Both are randomized double-blind, placebo-controlled trials evaluating the effect of gevokizumab after 4 months of monthly subcutaneous injection on post-MMTT C-peptide levels in adults with T1D on stable dose of insulin and diagnosed more than two years prior. The results are not yet published.
Anti IL-12 and IL-23
Ustekinumab is a human monoclonal antibody directed against IL-12 and IL-23 and is indicated for the treatment of psoriasis. It is currently being investigated in a Phase 1/2 study for the treatment of recently onset T1D [105] and in combination with the islet regeneration agent, INGAP Peptide [106].
Non-specific Immunosuppressant
Antithymocyte globulin (ATG) (Genzyme) has been approved for transplant rejection and aplastic anemia. It leads to lysis of T-cells as well as altered T-cell function, thus reducing immune competence. It has been in Phase 2 development for T1D. The Phase 2 trial, Study of Antithymocyte Globulin for Treatment of New-onset T1DM (START) (NCT00515099), was terminated due to slow recruitment [107]. This was a 2-year study in newly diagnosed diabetic patients comparing ATG with placebo. ATG was given by infusion and required hospitalization for 5-8 days. Results showed no difference between the ATG and placebo groups for 2- or 4-hour AUC C-peptide production after a MMTT both 1 and 2 years after infusion. While there was no difference in insulin usage, there was a trend toward better HbA1c at both one and two years in favor of ATG. Additionally, the ATG group had increased adverse reactions, including serum sickness and cytokine release syndrome.
Another Phase 2a trial (NCT01106157) focused on T1D diagnosed between 4 months and 2 years before enrolment to assess the preservation of β-cell function comparing combination treatment of ATG plus GCSF with placebo [108]. Results showed stable post-MMTT C-peptide in the treatment group comparing with declining C-peptide in the placebo group after 6 and 12 months, with no statistical significant difference in daily insulin requirement or HbA1c. Currently, a Phase II trial (NCT02215200) is recruiting participants with T1D diagnosed within 100 days of enrolment with residual β-cell function to assess the effect of antithmocyte globulin alone and in combination with granulocyte colony stimulating factor (GCSF) on C-peptide production.
Miscellaneous Approaches
Imatinib (Tyrosine Kinase Inhibitor)
Imatinib is marketed by Novartis as Gleevec® and is a tyrosine-kinase inhibitor used in the treatment of multiple cancers, most notably Philadelphia chromosome-positive (Ph+) chronic myelogenous leukemia (CML). It has been shown that imatinib can reverse T1D in prediabetic and new onset diabetic mice [109]. This is further investigated in a currently ongoing Phase 2 study in patients with a recent onset of T1D (NCT01781975).
VGX-1027
VGX-1027 (Inovio Pharmaceuticals) is an orally active small molecule that inhibits the NF-kB and p38 MAPK pathways while not affecting JNK and Ap-1 pathways. It also suppresses toll-like-receptor-mediated production of TNF-α, IL-1β, and IL-10. Nonclinical research showed that VGX-1027 reduced the onset of autoimmune diabetes in non-obese diabetes mice and inhibited diabetes induced by streptozotocin [110]. Two Phase 1 trials have been completed (NCT00627120, NCT00760396), showing a safe and tolerable profile [111].
Ladarixin
Ladarixin (Dompé) is an orally active sulfonamide that allosterically inhibits IL8a/CXCR1 and IL-8b/CXCR2 receptors [112]. IL8/CXCL8 is a potent chemo-attractant of neutrophils and other cells contributing to inflammation. In mice T1D models, blocking the CXCL8 pathway with ladarixin decreases infiltration of immune cells, improves islet cell survival, and delays onset of diabetes [113].
Therapies to Reduce β-cell Stress
Some evidence suggest that stress to the endoplasmic reticulum (ER) may have a role in mediating autoimmune β-cell destruction. Tauroursodeoxycholic acid (TUDCA) significantly reduced the T1D incidence in mice at risk for T1D, and this effect was attributed to reduction of ER stress [114]. The effect of TUDCA on the progression of new-onset T1D is now being tested in a clinical study (NCT02218619).
Polyamines appear to play a role in the progression of cellular inflammation. Depletion of intracellular polyamines with diflouoromethylornithine (DFMO) reduced the incidence of diabetes in mouse models of autoimmune diabetes [115]. DFMO is now being tested in a clinical trial in children with recent-onset T1DM (NCT02384889).
AGENTS THAT REGENERATE Β-CELLS
Pathogenesis of T1D clearly involves the autoimmune destruction of pancreatic β-cells with consequent insulin deficiency. Most T1D disease-modifying therapies under development target the autoimmune process, but relatively little attention has been given to research of agents inducing β-cell regeneration or islet neogenesis to restore pancreatic mass and insulin secretion. The islet neogenesis associated protein (INGAP) is a protein extracted from obstructed pancreatic ducts of hamsters that has been found to induce in vitro pancreatic cell neogenesis [116]. INGAP belongs to the REG family of cell surface binding proteins encoded by REG genes expressed in gastrointestinal tissues of several species (mouse, hamster, rat, and humans). REG gene expression is mainly linked to islet formation during fetal development, but these genes can also be expressed later in life in response to pancreatic cell injury and are associated with islet regeneration [117]. INGAP peptide (INGAPP) corresponds to the 15 amino-acid sequence that retains the islet regeneration activity of INGAP [118].
In nonclinical studies, INGAPP (Exsulin Corporation) has been shown to result in a dose-dependent stimulation of proliferation of β-cell mass in different species (rodents, dogs, and cynomolgus monkeys) [119] and increased insulin secretion in response to glucose in pancreatic rat islets, as well as induced transcription of several islet genes involved in the β-cell metabolism [120]. In Phase 2 studies, once-daily subcutaneous infusions of INGAPP for 90 days led to an increase in C-peptide secretion in T1D (NCT00071409) and modest decreases in HbA1c and mean glucose in T2D individuals (NCT00071422) at the highest dose level, although adverse events were a main reason for discontinuation (12% in T1D and 9% in T2D) [121].
Exsulin conducted trial E-201 in T1D patients (NCT00995540) to evaluate tolerability and efficacy of tid administration of 300 mg and 600 mg for 12 weeks. The study was terminated due to lack of observed efficacy with the tid regimen. Trial E-203 to test safety and efficacy of a combination of INGAP with ustekinumab, an IL-12 antagonist approved for the treatment of plaque psoriasis (NCT02204397) has just started [122]. Despite the promising effects in T1D and T2D, issues with short half-life and low bioefficacy may necessitate large doses, leading to injection site reactions, low tolerability, and elevated costs.
Another REG-encoded peptide, the human proislet peptide (HIP), has also been identified and associated with enhanced in vitro insulin production in human pancreatic ductal tissue and increased islet cell mass with improved glucose control in diabetic mice [123]. HIP2B (PancreateTM, CureDM/Sanofi) is a stabilized form of HIP that demonstrated positive nonclinical results and is currently in Phase 1b in T2D individuals (NCT01933256). The results of this study may indicate whether this can also become a potential therapeutic target for T1D.
Another peptide with similar properties, PRL-002 (Perle Bioscence), is reported to be in nonclinical development, and an IND application is expected in 2016. The encouraging initial results from the few compounds in development in this group, as well as an acceptable safety profile, still need to be confirmed in later-phase clinical trials before one can be confident that the concept of islet regeneration is a promising approach for T1D. Even if not completely curative alone, pharmacologic induction of islet regeneration may restore some level of insulin secretion sufficient to improve treatment outcomes.
CONCLUDING COMMENTS
Patients with T1D need additional treatment options, and ultimately a cure. A recent prospective cohort study from 2008 through 2010 of all individuals alive in Scotland with T1D who were aged 20 years or older and were in a nationwide registry found that the average man with type T1D subsequently had an estimated life expectancy loss of approximately 11 years; the respective figure for women was approximately 13 years [124]. Regulatory agencies recognize the need for effective and timely advances within this particular therapeutic area. In July 2015, FDA issued a report entitled “Targeted Drug Development: Why Are Many Diseases Lagging Behind?” [125]. In this report, diabetes in general, and T1D specifically, is identified as an area of great importance. Regarding diabetes, the reports reads as follows:
More basic research is needed to increase scientists’ understanding of the interaction between genetic, immunologic, metabolic, and environmental factors that cause specific subsets of patients to develop the disease and why the progress, signs, and symptoms of the disease are variable from patient to patient. Scientists still need to understand much more about why and how the immune system attacks the pancreas, to allow development of treatments that target the specific auto-immune process rather than suppressing the entire immune system, which carries serious risks. Further research is also needed to find biomarkers for susceptibility to specific complications of diabetes (as opposed to the disease itself).
FDA and EMA stand ready to approve disease modifying therapies for T1D. and have expressed reasonable expectations for demonstrating efficacy of therapies aimed at preserving insulin secretion in new onset patients. It is unclear what minimum treatment effect on preservation of C-peptide secretion, the regulatory primary efficacy endpoint, would be considered clinically meaningful for a new onset intervention. A small effect size (10-20% at two years) might be enough if the safety profile is very benign. Therapies that result in significant immune deficits or other toxicities would require much higher treatment effects. To power a two-year trial adequately to detect a 15% treatment effect would require several hundred participants in each treatment group. Larger effect sizes enable much smaller trials, but the statistical power of the typical Phase 3 trial to date [126] potentially leaves clinically meaningful effects on the table. FDA and EMA will also require some evidence for positive effects among important secondary outcomes such as HbA1c, rates of hypoglycemia, and total daily injected insulin dose. However, there are substantial challenges in new onset T1D trials to demonstrating robust effects on these secondary endpoints [127].
Going Forward
A single silver bullet for safely eliminating T1D autoimmunity is not on the horizon and perhaps will never be found. For the foreseeable future, a combination of therapies will be needed to control β-cell destruction, analogous to the paradigm of T2D treatment. T1D combination therapy is not a novel concept [128]. Combination clinical trials have been attempted and continue to be initiated [106, 129, 130]. Combinations of two or more agents have been shown to induce remission in the standard NOD mouse model of established T1D [131, 132]. However, mice and humans differ substantially in both T1D autoimmunity and islet biology. The lack of a dependable animal model is a major impediment to identifying combinations of agents that could feasibly tested in humans [133]. Given the minimum necessary trial size and duration to achieve an efficacy readout for new onset T1D trials, the evaluation of more than two agents in a factorial design becomes impractical. More reliable nonclinical models are needed to winnow the number of multiple agents to what can feasibly be evaluated in patients. While FDA has provided a guidance for the co-development of experimental therapies [134], this does not make the task of developing combination therapies any easier. Regulatory authorities will continue to ask that the contributions of individual components be demonstrated [135]. The empiric, sequential paradigm utilized to evolve combinations of cancer chemotherapy agents over the past five decades could be employed but this would require a larger timeframe and use of resources than is available.
If effective control of T1D autoimmunity is achieved without a means of restoring endogenous insulin secretion, only people with new onset T1D or with an identifiably high-risk status for developing T1D will benefit. Residual insulin secretion is still present in many T1D patients diagnosed more than 3 years in the past [136]. Therefore, the identification of safe and effective islet regeneration agents holds great promise and will extend the benefits of autoimmunity control to the general 1.5 million people in the US alone.
From a clinical evaluation standpoint, the development of islet regeneration agents is much easier than the case for evaluating immunomodulatory agents in new onset patients. People with established T1D have little or no endogenous insulin secretion. Restoration of some insulin secretion can therefore be convincing even in a single person with established T1D. Presumably, regenerated islets quickly come under autoimmune attack. Evidence of this was seen in the Phase 2 study of INGAP Peptide, in which some of the patient showed evidence of increased anti-islet activity [121]. These considerations suggest that even a minimally active islet regeneration agent can be used to facilitate the development of autoimmunity agents. A small factorial clinical study design incorporating as few as 24 established T1D patients treated with a regeneration agent and an autoimmunity agent could demonstrate the activity of the autoimmunity agent perhaps within 2 months, as opposed to the 2 years required to detect a modest effect in new onset patients.
In this sense, the co-development of immune and regeneration agents can be synergistic on multiple levels, starting with the pharmacological and extending to the commercial.
Conclusion
People with T1D are not satisfied with the current technologies for managing their condition. They will welcome the advances in mechanical and biologic products that provide glucose-sensitive, autoregulated insulin delivery. However, these approaches will be expensive and demanding of their users. People with T1D will also welcome treatments that can be used to prevent or reduce the loss of insulin secretion in their family members, before or at onset of T1D. But, people living everyday with T1D will not be satisfied until they have a “practical cure of T1D” [137]. The quest for such a treatment has been among the most elusive in the annals of medicine. Substantial progress has been made and more progress is foreseeable. Accelerated progress will require sustained, concerted, and systematic efforts from academia, industry, government, and patients with T1D as well as their caregivers.
ACKNOWLEDGEMENTS
Declared none.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
DISCLOSURES
All authors had a significant role in the conceptualization, writing, and review of this manuscript. There are no funding sources to declare for support of this work, and no editorial support was used. All authors except Dr Fleming have declared that they are employees of Quintiles: Dr Fleming is an employee of Kinexum. Dr Turner has disclosed that he is a shareholder of Quintiles.
Dr Fleming has declared that he is a Panel Member/Consultant for Arisaph, BCT, Becton Dickinson, BioCon, Diasome, Dompe, Exsulin, Fisher Scientific, Gilead, Islet Sciences, Lexicon, Johnson and Johnson, MannKind Corporation, Mediwound, Mbiome, N-Gene, NuSirt, Pfizer, Retrophin, Rhythm, Sanofi, SkyePharma, SynAgile, Takeda Pharmaceuticals, Teva, Thermalin, Thetis, ThromboGenics, VeroScience, He is a Stock/Shareholder of Ammonett Pharma, Exsulin Corporation, Locemia, Synagile, and Thetis.
REFERENCES
- 1.Cefalu W.T., Tamborlane W.V., Skyler J.S. Type 1 diabetes at a crossroads! Diabetes Care. 2015;38:968–970. doi: 10.2337/dc15-0615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mittermayer F., Caveney E., De Oliveira C., et al. Addressing unmet medical needs in type 2 diabetes mellitus: a review of new drugs under development. Curr. Diabetes Rev. 2015;11:17–31. doi: 10.2174/1573399810666141224121927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Skyler J.S., Ricordi C. Stopping type 1 diabetes: attempts to prevent or cure type 1 diabetes in man. Diabetes. 2011;60:1–8. doi: 10.2337/db10-1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Skyler J.S., Krischer J.P., Wolfsdorf J., et al. Effects of oral insulin in relatives of patients with type 1 diabetes: The Diabetes Prevention Trial--Type 1. Diabetes Care. 2005;28:1068–1076. doi: 10.2337/diacare.28.5.1068. [DOI] [PubMed] [Google Scholar]
- 5.Insel R.A., Dunne J.L., Atkinson M.A., et al. Staging Presymptomatic Type 1 Diabetes: A Scientific Statement of JDRF, the Endocrine Society, and the American Diabetes Association. Diabetes Care. 2015;38:1964–1974. doi: 10.2337/dc15-1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Palmer J.P., Fleming G.A., Greenbaum C.J., et al. C-peptide is the appropriate outcome measure for type 1 diabetes clinical trials to preserve beta-cell function: report of an ADA workshop. Diabetes. 2004;53:250–264. doi: 10.2337/diabetes.53.1.250. [DOI] [PubMed] [Google Scholar]
- 7.Fleming G.A., Klonoff D.C. Glutamic Acid Decarboxylase Therapy for Recent-Onset Type 1 Diabetes: Are We at the End or the Beginning of Finding a Cure? J. Diabetes Sci. Technol. 2009;3:215–218. doi: 10.1177/193229680900300201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fleming G.A. Regulatory and policy issues for T1DM immunotherapy. Hum. Vaccin. 2011;7:1–6. doi: 10.4161/hv.7.1.14527. [DOI] [PubMed] [Google Scholar]
- 9.Miller K.M., Foster N.C., Beck R.W., et al. Current state of Type 1 diabetes treatment in the U.S.: updated data from the T1D Exchange Clinic Registry. Diabetes Care. 2015;38:971–978. doi: 10.2337/dc15-0078. [DOI] [PubMed] [Google Scholar]
- 10.US Center for Disease Control and Prevention http://www.cdc.gov/diabetes/pubs/statsreport14/diabetes-infographic.pdf
- 11.Turner R., Stratton I., Horton V., et al. UKPDS 25: autoantibodies to islet-cell cytoplasm and glutamic acid decarboxylase for prediction of insulin requirement in type 2 diabetes. UK Prospective Diabetes Study Group. Lancet. 1997;350:1288–1293. doi: 10.1016/s0140-6736(97)03062-6. [DOI] [PubMed] [Google Scholar]
- 12.Fourlanos S., Dotta F., Greenbaum C.J., et al. Latent autoimmune diabetes in adults (LADA) should be less latent. Diabetologia. 2005;48:2206–2212. doi: 10.1007/s00125-005-1960-7. [DOI] [PubMed] [Google Scholar]
- 13.Redondo M.J. LADA: Time for a New Definition. Diabetes. 2013;62:339–340. doi: 10.2337/db12-1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wild D., von Maltzahn R., Brohan E., Christensen T., Clauson P., Gonder-Frederick L. A critical review of the literature on fear of hypoglycemia in diabetes: Implications for diabetes management and patient education. Patient Educ. Couns. 2007;68:10–15. doi: 10.1016/j.pec.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 15.Seaquist E.R., Anderson J., Childs B., et al. Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and The Endocrine Society. Diabetes Care. 2013;36:1384–1395. doi: 10.2337/dc12-2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.US FDA Guidance for Industry Diabetes Mellitus: Developing Drugs and Therapeutic Biologics for Treatment and Prevention . 2015 http://www.fda.gov/downloads/Drugs/Guidances/ucm071624.pdf
- 17.EMA Guideline on clinical investigation of medicinal products in the treatment or prevention of diabetes mellitus. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2012/06/WC500129256.pdf
- 18.US FDA Developing Products for Rare Diseases & Conditions. http://www.accessdata.fda.gov/scripts/opdlisting/oopd/OOPD_Results_2.cfm
- 19.FDA Rejects Novo Nordisk’s Insulin Degludec 2015 http://www.medscape.com/viewarticle/779077
- 20.More Brains Exploring the Possibility of 2015 http://www.healthline.com/diabetesmine/more-brains-smart-insulin
- 21.Rosenstock J., Bergenstal R.M., Blevins T.C., et al. Better glycemic control and weight loss with the novel long-acting basal insulin LY2605541 compared with insulin glargine in type 1 diabetes: a randomized, crossover study. Diabetes Care. 2013;36:522–528. doi: 10.2337/dc12-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lilly delays filing of Lantus rival on safety concerns. 2015 http://www.pmlive.com/pharma_news/lilly_delays_filing_of_lantus_rival_on_safety_concerns_661029
- 23.Lilly Ends Basal Insulin Peglispro Development Program 2016 https://investor.lilly.com/releasedetail.cfm?ReleaseID=945541
- 24.Hompesch M. The Ultra-Long-Acting Insulin HM12460A Demonstrates Safety and Efficacy in Patients with Type 1 Diabetes: A Phase 1 Single Dose Explorative Glucose Clamp Study. Abstract 894-P, 74th Scientific Sessions of the American Diabetes Association, San Francisco; 2014. [Google Scholar]
- 25.Krasner A., Brazg R.L., Blevins T.C., et al. et al. Safety and efficacy of ultra-rapid-acting human insulin formulation BIOD-123 in patients with type 1 diabetes. Abstract 130-OR, 74th Scientific Sessions of the American Diabetes Association, San Francisco; 2014. [Google Scholar]
- 26.2015 http://www.biodel.com/content/ pipeline/rhi-based-ultra-rapid-acting-insulin.htm
- 27.Heise T., Hövelmann U., Brøndsted L., Adrian C.L., Nosek L., Haahr H. Faster-acting insulin aspart: earlier onset of appearance and greater early pharmacokinetic and pharmacodynamic effects than insulin aspart. Diabetes Obes. Metab. 2015;17:682–688. doi: 10.1111/dom.12468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.NovoNordisk Company Annoucement https://www.novonordisk.com/bin/getPDF.1906174.pdf Accessed on: 24 November 2015. 2015
- 29.2015 http://www.adocia.fr/WP/news-pressreleases/
- 30.Vaughn D.R., Morrow L., Hompesch M., Muchmore D.B. Human Hyaluronidase (rHuPH20) Provides Consistent Ultrafast Insulin Absorption and Action Over 3 Days of Continuous Subcutaneous Infusion. Abstract 905-P 72nd Scientific Sessions of the American Diabetes Association, Philadelphia; 2012. [Google Scholar]
- 31.Hirsch I.B., Skyler J., Garg S., Blevins T., Vaughn D.E., Muchmore D.B. Human Hyaluronidase + Rapid Analog Insulin (RAI) Improves Postprandial Glycemic Control in Type 1 Diabetes (T1DM) Compared to Insulin Lispro Alone. Abstract 353-OR, 72nd Scientific Sessions of the American Diabetes Association, Philadelphia; 2012. [Google Scholar]
- 32.Nuffer W., Trujillo J.M., Ellis S.L. Technosphere insulin (Afrezza): a new, inhaled prandial insulin. Ann. Pharmacother. 2015;49:99–106. doi: 10.1177/1060028014554648. [DOI] [PubMed] [Google Scholar]
- 33.Dance Biopharma website. 2015 http://dancebiopharm.com/
- 34.Taylor R., Magnusson I., Rothman D.L., et al. Direct assessment of liver glycogen storage by 13C nuclear magnetic resonance spectroscopy and regulation of glucose homeostasis after a mixed meal in normal subjects. J. Clin. Invest. 1996;97:126–132. doi: 10.1172/JCI118379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Singhal P., Caumo A., Carey P.E., Cobelli C., Taylor R. Regulation of endogenous glucose production after a mixed meal in type 2 diabetes. Am. J. Physiol. Endocrinol. Metab. 2002;283:E275–E283. doi: 10.1152/ajpendo.00424.2001. [DOI] [PubMed] [Google Scholar]
- 36.Eldor R., Arbit E., Corcos A., Kidron M. Glucose-reducing effect of the ORMD-0801 oral insulin preparation in patients with uncontrolled type 1 diabetes: a pilot study. PLoS One. 2013;8:e59524. doi: 10.1371/journal.pone.0059524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oral-lyn fact sheet: http://www.generex.com/UserFiles/File/Oral-LynFactSheet.pdf
- 38.Shreya Life pulls its new diabetes drug from market. 2016 http://www.livemint.com/Home-Page/152R6RvV4QHwdyxby NOfZM/Shreya-Life-pulls-its-new-diabetes-drug-from-market.html
- 39.Generex Provides Update on Generex Oral-lyn™ Conference Call. 2016 http://investor.generex.com/releasedetail.cfm?releaseid=793636
- 40.Herring R., Jones R.H., Russell-Jones L. Hepatoselectivity and the evolution of insulin. Diabetes Obes. Metab. 2014;16:1–8. doi: 10.1111/dom.12117. [DOI] [PubMed] [Google Scholar]
- 41.Moore M.C., Smith M., Sinha V.P., et al. Novel PEGylated basal insulin LY2605541 has a preferential hepatic effect on glucose metabolism. Diabetes. 2014;63:494–504. doi: 10.2337/db13-0826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Geho W.B., Geho H.C., Lau J.R., Gana T.J. Hepatic-directed vesicle insulin: a review of formulation development and preclinical evaluation. J. Diabetes Sci. Technol. 2009;3:1451–1459. doi: 10.1177/193229680900300627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Davis S.N., Geho B., Tate D., Galassetti P., Lau J., Granner D., Mann S. The effects of HDV-insulin on carbohydrate metabolism in Type 1 diabetic patients. J. Diabetes Complications. 2001;15:227–233. doi: 10.1016/s1056-8727(01)00154-4. [DOI] [PubMed] [Google Scholar]
- 44.Diasome Pharmaceuticals Announces Clinical Trial Design Collaboration Accessed on: 2015 http://diasomepharmaceuticals.com/wp-content/uploads/ 2015/07/Joslin-Diasome-Press-Release-Final.pdf
- 45.Perkins B.A., Cherney D.Z., Partridge H., et al. Sodium-glucose cotransporter 2 inhibition and glycemic control in type 1 diabetes: results of an 8-week open-label proof-of-concept trial. Diabetes Care. 2014;37:1480–1483. doi: 10.2337/dc13-2338. [DOI] [PubMed] [Google Scholar]
- 46.Zinman B., Wanner C., Lachin J.M., et al. EMPA-REG OUTCOME Investigators Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N. Engl. J. Med. 2016;374(11):1094. doi: 10.1056/NEJMc1600827. [DOI] [PubMed] [Google Scholar]
- 47.Henry R.R., Rosenstock J., Edelman S., et al. Exploring the potential of the SGLT2 inhibitor dapagliflozin in type 1 diabetes: a randomized, double-blind, placebo-controlled pilot study. Diabetes Care. 2015;38:412–419. doi: 10.2337/dc13-2955. [DOI] [PubMed] [Google Scholar]
- 48.Tahara A., Kurosaki D., Yokono M., et al. Effects of sodium-glucose cotransporter 2 selective inhibitor ipragliflozin on hyperglycemia, oxidative Stress, inflammation and liver injury in streptozotocin-induced type 1 diabetic rats. J. Pharm. Pharmacol. 2014;66:975–987. doi: 10.1111/jphp.12223. [DOI] [PubMed] [Google Scholar]
- 49.Mudaliar S., Armstrong D.A., Mavian A.A., et al. Remogliflozin etabonate, a selective inhibitor of the sodium-glucose transporter 2, improves serum glucose profiles in type 1 diabetes. Diabetes Care. 2012;35:2198–2200. doi: 10.2337/dc12-0508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zambrowicz B., Freiman J., Brown P.M., et al. LX4211, a dual SGLT1/SGLT2 inhibitor, improved glycemic control in patients with type 2 diabetes in a randomized, placebo-controlled trial. Clin. Pharmacol. Ther. 2012;92:158–169. doi: 10.1038/clpt.2012.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lexicon Pharmaceuticals 14 April 2014. LX4211 achieves positive results in type 1 diabetes trial. 2015 http://www.lexgen.com/news/press-releases/2321-lx4211-achieves-positive-results-in-type-1-diabetes-clinical-trial.html
- 52.Sands A.T., Zambrowicz B.P., Rosenstock J., et al. Sotagliflozin, a dual SGLT1 and SGLT2 inhibitor, as adjunct to insulin in type 1 diabetes. Diabetes Care. 2015;38:1181–1188. doi: 10.2337/dc14-2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cherney D.Z., Perkins B.A., Soleymanlou N., et al. renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation. 2014;129:587–597. doi: 10.1161/CIRCULATIONAHA.113.005081. [DOI] [PubMed] [Google Scholar]
- 54.Oliva R.V., Bakris G.L. Blood pressure effects of sodium-glucose co-transport 2 (SGLT2) inhibitors. J. Am. Soc. Hypertens. 2014;8:330–339. doi: 10.1016/j.jash.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 55.Tikkanen I., Narko K., Zeller C., et al. EMPA-REG BP Investigators. Empagliflozin reduces blood pressure in patients with type 2 diabetes and hypertension. Diabetes Care. 2015;38:420–428. doi: 10.2337/dc14-1096. [DOI] [PubMed] [Google Scholar]
- 56.Yale J.F., Bakris G., Cariou B., et al. Efficacy and safety of canagliflozin in subjects with type 2 diabetes and chronic kidney disease. Diabetes Obes. Metab. 2013;15:463–473. doi: 10.1111/dom.12090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.U.S. Food and Drug Administration Drug Safety Communication: FDA warns that SGLT2 inhibitors for diabetes may result in a serious condition of too much acid in the blood [Internet], 2015 http://www.fda.gov/downloads/Drugs/DrugSafety/UCM446954.pdf
- 58.European Medicines Agency Review of diabetes medicines called SGLT2 inhibitors started: risk of diabetic ketoacidosis to be examined [Internet], 2015 http://www.ema.europa.eu/docs/ en_GB/document_library/Referrals_document/SGLT2_inhibitors__20/Procedure_started/WC500187926.pdf
- 59.Rosenstock J., Ferrannini E. Euglycemic Diabetic Ketoacidosis: A Predictable, Detectable, and Preventable Safety Concern With SGLT2 Inhibitors. Diabetes Care. 2015;38:1638–1642. doi: 10.2337/dc15-1380. [DOI] [PubMed] [Google Scholar]
- 60.Peters A.L., Buschur E.O., Buse J.B., Cohan P., Diner J.C., Hirsch I.B. Euglycemic diabetic ketoacidosis: a potential complication of treatment with sodium-glucose cotransporter 2 inhibition. Diabetes Care. 2015;38:1687–1693. doi: 10.2337/dc15-0843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kielgast U., Holst J.J., Madsbad S. Antidiabetic actions of endogenous and exogenous GLP-1 in type 1 diabetic patients with and without residual β-cell function. Diabetes. 2011;60:1599–1607. doi: 10.2337/db10-1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N. Engl. J. Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. No authors listed. [DOI] [PubMed] [Google Scholar]
- 63.Russell-Jones D., Khan R. Insulin-associated weight gain in diabetes--causes, effects and coping strategies. Diabetes Obes. Metab. 2007;9:799–812. doi: 10.1111/j.1463-1326.2006.00686.x. [DOI] [PubMed] [Google Scholar]
- 64.Ghazi T., Rink L., Sherr J., Herold K. Acute metabolic effects of exenatide in patients with type 1 diabetes with and without residual insulin to oral and IV glucose challenges. Diabetes Care. 2014;37:210–216. doi: 10.2337/dc13-1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Varanasi A., Bellini N., Rawal D., et al. Liraglutide as additional treatment for type 1 diabetes. Eur. J. Endocrinol. 2011;165:77–84. doi: 10.1530/EJE-11-0330. [DOI] [PubMed] [Google Scholar]
- 66.Kuhadiya N.D., Malik R., Bellini N.J., et al. Liraglutide as additional treatment to insulin in obese patients with type 1 diabetes mellitus. Endocr. Pract. 2013;19:963–967. doi: 10.4158/EP13065.OR. [DOI] [PubMed] [Google Scholar]
- 67.NovoNordisk Company Announcement. 2015 https://www.novonordisk.com/bin/getPDF.1947182.pdf
- 68.Ludvigsson J., Linköping Diabetes Immune Intervention Study Group The role of immunomodulation therapy in autoimmune diabetes. J. Diabetes Sci. Technol. 2009;3:320–330. doi: 10.1177/193229680900300213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Diabetes Prevention Trial--Type 1 Diabetes Study Group. Effects of insulin in relatives of patients with type 1 diabetes mellitus. N. Engl. J. Med. 2002;346:1685–1691. doi: 10.1056/NEJMoa012350. [DOI] [PubMed] [Google Scholar]
- 70.Skyler J.S., Krischer J.P., Wolfsdorf J., et al. Effects of oral insulin in relatives of patients with type 1 diabetes: The Diabetes Prevention Trial--Type 1. Diabetes Care. 2005;28:1068–1076. doi: 10.2337/diacare.28.5.1068. [DOI] [PubMed] [Google Scholar]
- 71.Näntö-Salonen K., Kupila A., Simell S., et al. Nasal insulin to prevent type 1 diabetes in children with HLA genotypes and autoantibodies conferring increased risk of disease: a double-blind, randomised controlled trial. Lancet. 2008;372:1746–1755. doi: 10.1016/S0140-6736(08)61309-4. [DOI] [PubMed] [Google Scholar]
- 72.Ludvigsson J., Krisky D., Casas R., et al. GAD65 antigen therapy in recently diagnosed type 1 diabetes mellitus. N. Engl. J. Med. 2012;366:433–442. doi: 10.1056/NEJMoa1107096. [DOI] [PubMed] [Google Scholar]
- 73. http://www.tolerioninc.com
- 74.Roep B.O., Solvason N., Gottlieb P.A., et al. Plasmid-encoded proinsulin preserves C-peptide while specifically reducing proinsulin-specific CD8+ T cells in type 1 diabetes. Sci. Transl. Med. 2013;5:191ra82. doi: 10.1126/scitranslmed.3006103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chan E.D., Pott G.B., Silkoff P.E., et al. Alpha-1-antitrypsin inhibits nitric oxide production. J. Leukoc. Biol. 2012;92:1251–1260. doi: 10.1189/jlb.0212071. [DOI] [PubMed] [Google Scholar]
- 76.Pott G.B., Chan E.D., Dinarello C.A., Shapiro L. Alpha-1-antitrypsin is an endogenous inhibitor of proinflammatory cytokine production in whole blood. J. Leukoc. Biol. 2009;85:886–895. doi: 10.1189/jlb.0208145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang B., Lu Y., Campbell-Thompson M., et al. Alpha1-antitrypsin protects beta-cells from apoptosis. Diabetes. 2007;56:1316–1323. doi: 10.2337/db06-1273. [DOI] [PubMed] [Google Scholar]
- 78.Greene C.M., Miller S.D., Carroll T.P., et al. Anti-apoptotic effects of Z alpha1-antitrypsin in human bronchial epithelial cells. Eur. Respir. J. 2010;35:1155–1163. doi: 10.1183/09031936.00191908. [DOI] [PubMed] [Google Scholar]
- 79.Lockett A.D., Van Demark M., Gu Y., et al. Effect of cigarette smoke exposure and structural modifications on the α-1 Antitrypsin interaction with caspases. Mol. Med. 2012;18:445–454. doi: 10.2119/molmed.2011.00207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lewis E.C. Expanding the clinical indications for α(1)-antitrypsin therapy. Mol. Med. 2012;18:957–970. doi: 10.2119/molmed.2011.00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang Y., Xiao Y., Zhong L., et al. Increased neutrophil elastase and proteinase 3 and augmented NETosis are closely associated with β-cell autoimmunity in patients with type 1 diabetes. Diabetes. 2014;63:4239–4248. doi: 10.2337/db14-0480. [DOI] [PubMed] [Google Scholar]
- 82.Rachmiel M., Strauss P., Dror N., et al. Alpha-1 antitrypsin therapy is safe and well tolerated in children and adolescents with recent onset type 1 diabetes mellitus. Pediatr. Diabetes. 2016;17(5):351–359. doi: 10.1111/pedi.12283. [DOI] [PubMed] [Google Scholar]
- 83.Ablamunits V1 Bisikirska B, Herold KC. Acquisition of regulatory function by human CD8(+) T cells treated with anti-CD3 antibody requires TNF. Eur. J. Immunol. 2010;40:2891–2901. doi: 10.1002/eji.201040485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Herold K.C., Gitelman S.E., Masharani U., et al. A single course of anti-CD3 monoclonal antibody hOKT3gamma1(Ala-Ala) results in improvement in C-peptide responses and clinical parameters for at least 2 years after onset of type 1 diabetes. Diabetes. 2005;54:1763–1769. doi: 10.2337/diabetes.54.6.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Herold K.C., Gitelman S., Greenbaum C., et al. Treatment of patients with new onset Type 1 diabetes with a single course of anti-CD3 mAb Teplizumab preserves insulin production for up to 5 years. Clin. Immunol. 2009;132:166–173. doi: 10.1016/j.clim.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Herold K.C., Gitelman S.E., Ehlers M.R., et al. Teplizumab (anti-CD3 mAb) treatment preserves C-peptide responses in patients with new-onset type 1 diabetes in a randomized controlled trial: metabolic and immunologic features at baseline identify a subgroup of responders. Diabetes. 2013;62:3766–3774. doi: 10.2337/db13-0345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sherry N., Hagopian W., Ludvigsson J., et al. Teplizumab for treatment of type 1 diabetes (Protégé study): 1-year results from a randomised, placebo-controlled trial. Lancet. 2011;378:487–497. doi: 10.1016/S0140-6736(11)60931-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hagopian W., Ferry R.J., Jr, Sherry N., et al. Teplizumab preserves C-peptide in recent-onset type 1 diabetes: two-year results from the randomized, placebo-controlled Protégé trial. Diabetes. 2013;62:3901–3908. doi: 10.2337/db13-0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Keymeulen B., Vandemeulebroucke E., Ziegler A.G., et al. Insulin needs after CD3-antibody therapy in new-onset type 1 diabetes. N. Engl. J. Med. 2005;352:2598–2608. doi: 10.1056/NEJMoa043980. [DOI] [PubMed] [Google Scholar]
- 90.Keymeulen B., Walter M., Mathieu C., et al. Four-year metabolic outcome of a randomised controlled CD3-antibody trial in recent-onset type 1 diabetic patients depends on their age and baseline residual beta cell mass. Diabetologia. 2010;53:614–623. doi: 10.1007/s00125-009-1644-9. [DOI] [PubMed] [Google Scholar]
- 91.Aronson R., Gottlieb P.A., Christiansen J.S., et al. Low-dose otelixizumab anti-CD3 monoclonal antibody DEFEND-1 study: results of the randomized phase III study in recent-onset human type 1 diabetes. Diabetes Care. 2014;37:2746–2754. doi: 10.2337/dc13-0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ambery P., Donner T.W., Biswas N., et al. Efficacy and safety of low-dose otelixizumab anti-CD3 monoclonal antibody in preserving C-peptide secretion in adolescent type 1 diabetes: DEFEND-2, a randomized, placebo-controlled, double-blind, multi-centre study. Diabet. Med. 2014;31:399–402. doi: 10.1111/dme.12361. [DOI] [PubMed] [Google Scholar]
- 93.Orban T., Bundy B., Becker D.J., et al. Co-stimulation modulation with abatacept in patients with recent-onset type 1 diabetes: a randomised, double-blind, placebo-controlled trial. Lancet. 2011;378:412–419. doi: 10.1016/S0140-6736(11)60886-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Orban T., Bundy B., Becker D.J., et al. Costimulation modulation with abatacept in patients with recent-onset type 1 diabetes: follow-up 1 year after cessation of treatment. Diabetes Care. 2014;37:1069–1075. doi: 10.2337/dc13-0604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pescovitz M.D., Greenbaum C.J., Krause-Steinrauf H., et al. Rituximab, B-lymphocyte depletion, and preservation of beta-cell function. N. Engl. J. Med. 2009;361:2143–2152. doi: 10.1056/NEJMoa0904452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Elias D., Meilin A., Blamunits V., et al. Hsp60 peptide therapy of NOD mouse diabetes induces a Th2 cytokine burst and downregulates autoimmunity to various beta-cell antigens. Diabetes. 1997;46:758–764. doi: 10.2337/diab.46.5.758. [DOI] [PubMed] [Google Scholar]
- 97.Eldor R., Kassem S., Raz I. Immune modulation in type 1 diabetes mellitus using DiaPep277: a short review and update of recent clinical trial results. Diabetes Metab. Res. Rev. 2009;25:316–320. doi: 10.1002/dmrr.942. [DOI] [PubMed] [Google Scholar]
- 98.[No Authors Listed] Treatment of recent-onset type 1 diabetic patients with DiaPep277: results of a double-blind, placebocontrolled, randomized phase 3 trial. Diabetes Care 2014; 37: 1392-400. DOI: 10.2337/dc13-1391. Diabetes Care 2015; 38(1): 178. 2014. [No Authors Listed]
- 99.Ohnmacht C., Pullner A., King S.B., et al. Constitutive ablation of dendritic cells breaks self-tolerance of CD4 T cells and results in spontaneous fatal autoimmunity. J. Exp. Med. 2009;206:549–559. doi: 10.1084/jem.20082394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Creusot R.J., Giannoukakis N., Trucco M., Clare-Salzler M.J., Fathman C.G. It’s time to bring dendritic cell therapy to type 1 diabetes. Diabetes. 2014;63:20–30. doi: 10.2337/db13-0886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Giannoukakis N., Phillips B., Finegold D., Harnaha J., Trucco M. Phase I (safety) study of autologous tolerogenic dendritic cells in type 1 diabetic patients. Diabetes Care. 2011;34:2026–2032. doi: 10.2337/dc11-0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dinarello C.A. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood. 2011;117:3720–3732. doi: 10.1182/blood-2010-07-273417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Moran A., Bundy B., Becker D.J., et al. Interleukin-1 antagonism in type 1 diabetes of recent onset: two multicentre, randomised, double-blind, placebo-controlled trials. Lancet. 2013;381:1905–1915. doi: 10.1016/S0140-6736(13)60023-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pafili K., Papanas N., Maltezos E. Gevokizumab in type 1 diabetes mellitus: extreme remedies for extreme diseases? Expert Opin. Investig. Drugs. 2014;23:1277–1284. doi: 10.1517/13543784.2014.947026. [DOI] [PubMed] [Google Scholar]
- 105.Pilot Clinical Trial of Ustekinumab in Patients With New-onset T1D (UST1D) https://clinicaltrials.gov/ct2/show/NCT02117765
- 106.Exploratory Study on Tolerability and Safety of Adding Ustekinumab to INGAP Peptide for 12 Weeks in Adult Patients With Type 1 Diabetes Mellitus . https://clinicaltrials.gov/ct2/show/
- 107.Gitelman S.E., Gottlieb P.A., Rigby M.R., et al. Antithymocyte globulin treatment for patients with recent-onset type 1 diabetes: 12-month results of a randomised, placebo-controlled, phase 2 trial. Lancet Diabetes Endocrinol. 2013;1:306–316. doi: 10.1016/S2213-8587(13)70065-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Haller M.J., Gitelman S.E., Gottlieb P.A., et al. Anti-thymocyte globulin/G-CSF treatment preserves β-cell function in patients with established type 1 diabetes. J. Clin. Invest. 2015;125:448–455. doi: 10.1172/JCI78492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Louvet C., Szot G.L., Lang J., et al. Proc. Natl. Acad. Sci. USA. 2008;105:18895–18900. doi: 10.1073/pnas.0810246105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Stosic-Grujicic S., Cvetkovic I., Mangano K., et al. A potent immunomodulatory compound, (S,R)-3-Phenyl-4,5-dihydro-5-isoxazole acetic acid, prevents spontaneous and accelerated forms of autoimmune diabetes in NOD mice and inhibits the immunoinflammatory. diabetes induced by multiple low doses of streptozotocin in CBA/H mice. J. Pharmacol. Exp. Ther. 2007;320:1038–1049. doi: 10.1124/jpet.106.109272. [DOI] [PubMed] [Google Scholar]
- 111.Lee J.C., Menacherry S., Diehl M.C., et al. Safety, bioavailability and pharmacokinetics of VGX-1027 - a novel oral anti-inflammatory drug in healthy human subjects. Clin. Pharmacol. Drug Dev. 2016;5(2):91–101. doi: 10.1002/cpdd.193. [DOI] [PubMed] [Google Scholar]
- 112.Bertini R., Barcelos L.S., Beccari A.R., et al. Receptor binding mode and pharmacological characterization of a potent and selective dual CXCR1/CXCR2 non-competitive allosteric inhibitor. Br. J. Pharmacol. 2012;165:436–454. doi: 10.1111/j.1476-5381.2011.01566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Citro A., Valle A., Cantarelli E., et al. CXCR1/2 inhibition blocks and reverses type 1 diabetes in mice. Diabetes. 2015;64:1329–1340. doi: 10.2337/db14-0443. [DOI] [PubMed] [Google Scholar]
- 114.Engin F., Yermalovich A., Nguyen T., et al. Restoration of the unfolded protein response in pancreatic β cells protects mice against type 1 diabetes. Sci. Transl. Med. 2013;5:211ra156. doi: 10.1126/scitranslmed.3006534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tersey S.A., Colvin S.C., Maier B., Mirmira R.G. Protective effects of polyamine depletion in mouse models of type 1 diabetes: implications for therapy. Amino Acids. 2014;46:633–642. doi: 10.1007/s00726-013-1560-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Fleming A., Rosenberg L. Prospects and challenges for islet regeneration as a treatment for diabetes: a review of islet neogenesis associated protein. J. Diabetes Sci. Technol. 2007;1:231–244. doi: 10.1177/193229680700100214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Parikh A., Stephan A-F., Tzanakis E.S. Regenerating proteins and their expression, regulation and signaling. Biomol. Concepts. 2012;3:57–70. doi: 10.1515/bmc.2011.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rafaeloff R., Pittenger G.L., Barlow S.W., et al. Cloning and sequencing of the pancreatic islet neogenesis sssociated protein (INGAP) gene and its expression in islet neogenesis in hamsters. J. Clin. Invest. 1997;9:2100–2109. doi: 10.1172/JCI119383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lipsett M., Hanley S., Castellarin M., et al. The role of islet neogenesis-associated protein (INGAP) in islet neogenesis. Cell Biochem. Biophys. 2007;48:127–137. doi: 10.1007/s12013-007-0028-3. [DOI] [PubMed] [Google Scholar]
- 120.Barbosa H., Bordin S., Stoppiglia L., et al. Islet neogenesis associated protein (INGAP) modulates gene expression in cultured neonatal rat islets. Regul. Pept. 2006;136:78–84. doi: 10.1016/j.regpep.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 121.Dungan K.M., Buse J.B., Ratner R.E. Effects of therapy in type 1 and type 2 diabetes mellitus with a peptide derived from islet neogenesis associated protein (INGAP). Diabetes Metab. Res. Rev. 2009;25:558–565. doi: 10.1002/dmrr.999. [DOI] [PubMed] [Google Scholar]
- 122. Landmark clinical trial of a novel combination treatment for type 1 diabetes. 2015 http://www.eurekalert.org/pub_releases/2015-11/mu-lct112515.php
- 123.Levetan C.S., Upham L.V., Deng S., et al. Discovery of a human peptide sequence signaling islet neogenesis. Endocr. Pract. 2008;14:1075–1083. doi: 10.4158/EP.14.9.1075. [DOI] [PubMed] [Google Scholar]
- 124.Livingstone S.J., Levin D., Looker H.C., et al. Scottish Diabetes Research Network epidemiology group; Scottish Renal Registry. Estimated life expectancy in a Scottish cohort with type 1 diabetes, 2008-2010. JAMA. 2015;313:37–44. doi: 10.1001/jama.2014.16425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.US FDA Targeted Drug Development: Why Are Many Diseases Lagging Behind? 2015 http://www.fda.gov/AboutFDA/ ReportsManualsForms/Reports/ucm454955.htm
- 126.Ludvigsson J., Krisky D., Casas R., et al. GAD65 antigen therapy in recently diagnosed type 1 diabetes mellitus. N. Engl. J. Med. 2012;366:433–442. doi: 10.1056/NEJMoa1107096. [DOI] [PubMed] [Google Scholar]
- 127.Palmer J.P., Fleming G.A., Greenbaum C.J., et al. C-peptide is the appropriate outcome measure for type 1 diabetes clinical trials to preserve beta-cell function: report of an ADA workshop, 21-22 October 2001. Diabetes. 2004;53:250–264. doi: 10.2337/diabetes.53.1.250. [DOI] [PubMed] [Google Scholar]
- 128.Matthews J.B., Staeva T.P., Bernstein P.L., et al. Developing combination immunotherapies for type 1 diabetes: recommendations from the ITN-JDRF Type 1 Diabetes Combination Therapy Assessment Group. Clin. Exp. Immunol. 2010;160:176–184. doi: 10.1111/j.1365-2249.2010.04153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Rother K.I., Spain L.M., Wesley R.A., et al. Effects of exenatide alone and in combination with daclizumab on beta-cell function in long-standing type 1 diabetes. Diabetes Care. 2009;32:2251–2257. doi: 10.2337/dc09-0773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Diamyd press release. 2015 http://www.diamyd.com/ docs/pressClip.aspx?section=investor&ClipID=738266
- 131.Xue S., Posgai A., Wasserfall C., et al. Combination Therapy Reverses Hyperglycemia in NOD Mice With Established Type 1 Diabetes. Diabetes. 2015;64:3873–3884. doi: 10.2337/db15-0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Tersey S.A., Carter J.D., Rosenberg L., Taylor-Fishwick D.A., Mirmira R.G., Nadler J.L. Amelioration of type 1 diabetes following treatment of non-obese diabetic mice with INGAP and lisofylline. J. Diabetes Mellitus. 2012;2:251–257. doi: 10.4236/jdm.2012.22040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Atkinson M.A. Evaluating preclinical efficacy. Sci. Transl. Med. 2011;3:96cm22. doi: 10.1126/scitranslmed.3002757. [DOI] [PubMed] [Google Scholar]
- 134.US FDA Guidance for Industry, Codevelopment of Two or More New Investigational Drugs for Use in Combination; 2015 http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm236669.pdf
- 135.US FDA Code of Federal Regulations Title 21; 2015 http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRsearch.cfm?fr=300.50
- 136.Davis A.K., DuBose S.N., Haller M.J., et al. Prevalence of detectable C-Peptide according to age at diagnosis and duration of type 1 diabetes. Diabetes Care. 2015;38:476–481. doi: 10.2337/dc14-1952. [DOI] [PubMed] [Google Scholar]
- 137.Website diabetic connect; 2015 http://www.diabeticconnect.com/ diabetes-information-articles/general/707-finding-a-practical-cure-for-diabetes