Introduction
Despite advances in the management of advanced heart failure, morbidity and mortality rates remain high.1 Because heart transplantation is available to only about 2200 patients per year,1–3 mechanical circulatory support with left ventricular assist devices (LVADs) has become an effective option not only as a bridge to transplant (BTT), but also as destination therapy (DT), prolonging life and alleviating distressing symptoms of those patients for whom transplantation is not an option.2, 4
In the US, in recent years there has been a dramatic increase in the total number of LVADs. In particular, the percentage of LVADs designated as DT at the time of implant has increased substantially. From June 2006 until December 2007, 14.7% of the 436 LVADs were designated DT.5 In contrast, from January 2011 until June 2013, 41.6% of the total 6,704 LVADs were designated DT at the time of device placement.5 Furthermore, with the increasing numbers of patients reaching late-stage HF, the continued shortage of donor hearts, and the designation of more institutions as LVAD centers, the number of LVADs for DT is projected to increase substantially.2, 5, 6
Despite improvements in survival5 and symptom management with LVADs,7–9 they are not curative; and are associated with complications such as: bleeding, infection, stroke, device malfunction, and cognitive and psychological symptoms. These complications may lead to hospital readmissions, disability, decreased quality of life, and in some cases, death.8, 10–12 It is important to note that even among patients whose LVADs were implanted as BTT between 2011 and 2013, there was only a 37% chance that they would be status post-transplant after one year. If the designation were “bridge to candidacy”, the chance was only 20%.5
A similar trend has been noted outside of the US. Although the US is currently the only country to reimburse for LVAD designated as DT at the time of implant, the worldwide donor shortage has resulted in increasing numbers of patients with LVADs who will not undergo heart transplant, ending up with LVAD-DT by default. This has resulted in other countries, such as the Netherlands13 and Australia14 reexamining their policies regarding LVADs for BTT only.
Therefore, regardless of the initial indication for LVAD, patients and family members in the US and abroad may face difficult treatment decisions at or near end-of-life. However, they typically do not engage in advance care planning (ACP) at the time of LVAD placement.15–17
Without adequate ACP, defined as discussions of both the potential impact of device-related complications on quality of life and survival, and the patient’s end-of-life treatment preferences, patients and their surrogate decision-makers are likely to be unprepared for decision-making when acute complications or worsening heart failure develops.17–19 This in turn, may lead to increased patient and family conflict and distress, 16, 20, 21 and end-of-life care inconsistent with patient preferences and values.15–17
Since October 2014, The Joint Commission has required, as a condition for disease-specific care advanced certification for VAD for DT that a palliative care clinician be part of the interdisciplinary LVAD team.22 Some VAD teams had already incorporated into their pre-VAD evaluation, a palliative care consultation consisting of discussion of a “preparedness plan”, and others will likely follow.23 However, no such requirement exists for LVAD for BTT, nor has a theory-based ACP intervention been tested in the LVAD population. Therefore, we conducted a pilot randomized trial of a theory-based ACP intervention, Sharing Patients’ Illness Representations to Increase Trust or SPIRIT(cc), in a group of LVAD patients and their surrogates. SPIRIT has been tested in cardiac surgery patients24, and in dialysis patients and their surrogate decision-makers,25–27 and has demonstrated its efficacy in improving outcomes related to preparation for end-of-life care and post-bereavement psychological distress.27
The foundational theory of SPIRIT is the Representational Approach to Patient Education.28, 29 This approach proposes that gaining an understanding of a patient’s perspectives on his/her illness and treatment, including its impact on all aspects of his/her life, and its likely course and outcomes, is a crucial first step in patient education. This understanding facilitates the delivery of targeted, highly individualized information that the recipient will likely view as pertinent and beneficial; thereby increasing the likelihood of positive behavior change.28–30
The purposes of this pilot study were to: (1) determine the feasibility and acceptability of SPIRIT-HF among patients with LVADs and their surrogate decision-makers; and (2) evaluate the preliminary, short-term effects of SPIRIT-HF on patient-surrogate congruence in goals of care, patient’s decisional conflict, and surrogate’s decision-making confidence.
Methods
Design
The study used a randomized controlled design in which patient-surrogate dyads were randomized with equal allocation (1:1) to either: SPIRIT-HF plus usual care or usual care only. Measures were assessed in person at baseline, and over the telephone at 2 weeks post-intervention.
Ethics
All research team members were in compliance with CITI training requirements. The University of North Carolina at Chapel Hill IRB approved the study.
Participants and Setting
Patients were recruited from an LVAD Program at a large academic medical center in North Carolina. The program currently serves 74 LVAD patients; with 58 (79.4%) designated as DT and 15 as BTT at the time of device implant.
Eligible patient participants were English-speaking adults, who were at least 30 days post-LVAD placement and medically stable, had access to a telephone, and had a willing surrogate to participate in the study with the patient. Patients who were hospitalized in critical condition were excluded. LVAD patients were not excluded based on DT or BTT status because both groups are at risk for life-threatening complications, neither group has likely engaged in ACP discussions, and there is insufficient evidence that they have differing perspectives regarding ACP. Eligible surrogate participants were English-speaking adults, with access to a telephone. According to guidelines for pilot trials31, our goal was to ensure at least 12 dyads per group would complete the study. We sought to recruit 15 dyads per group to ensure adequate numbers of dyads would complete the study.
Procedures
Recruitment
Participants were recruited from November 2014 through June 2015. One of the VAD coordinators approached eligible patients, and assessed their interest in learning more about the study. Interested patients were then contacted by study personnel for further screening, which included asking the patient to identify a surrogate decision-maker and provide his/her contact information. If both the patient and his/her designated surrogate were willing and able to participate, arrangements were made for a face-to-face meeting at their next VAD clinic appointment for written consent and completion of baseline measures.
Randomization
Dyads were randomized immediately after baseline measures, using a computer-generated random scheme. The interventionist was blinded to group assignment until she opened a sequentially numbered opaque envelope containing the enrolled dyad’s assignment.
Treatment Conditions
Usual care
The LVAD Program is composed of a multidisciplinary team including cardiac surgeons, cardiologists, nurse practitioners, social workers, a psychologist, a financial counselor, VAD nurse coordinators, an infectious disease specialist, pharmacists, and a nutritionist. Usual care consisted of a pre-VAD evaluation by clinicians in psychology, social work, nutrition, nursing, cardiology, and cardiac surgery. Information about advance directives is provided during the LVAD evaluation. However, palliative care consultations and ACP discussions are not part of usual care.
SPIRIT-HF
SPIRIT-HF was a structured, guided discussion, delivered by a trained interventionist in a single, approximately one-hour long session. It was composed of five steps:28–30 1) assessing representations; 2) identifying gaps and concerns; 3) creating conditions for conceptual change; 4) introducing replacement information; 5) setting goals, planning and summarizing. During SPIRIT-HF the interventionist first gained an understanding of the patient’s experiences, thoughts, attitudes, and beliefs, related to his/her HF and the LVAD, which in turn, facilitated the delivery of targeted, individualized information. The patient was given the opportunity to consider his/her values and preferences related to end-of-life care. The inclusion of the surrogate ensured that the surrogate had an opportunity to learn about the patient’s illness experiences, and end-of-life concerns, and to prepare for the role of surrogate decision-maker.28–30 Participants were provided a copy of the Goals of Care used during the discussion, a written summary of the discussion, and information on resources regarding advance directives.
Intervention delivery took place in a private room generally in the VAD clinic building. All intervention sessions were delivered by a PhD-prepared nurse with 30 years of clinical experience. She completed the training sessions used for the nurse interventionists in the previous SPIRIT studies.30, 32
Based on the experience of the first few participants, the intervention delivery process was amended, giving those patients and surrogates randomized to the intervention group, the option of receiving the intervention either immediately after completing baseline measures or at the time of their next clinic visit. All but 2 dyads elected to separate enrollment and collection of baseline measures from the intervention. In addition, due to the volume of patients seen on clinic days, it was not possible to have a room designated for enrollment or intervention delivery. Instead a room was booked on as needed basis, sometimes necessitating delivering the intervention in the patient’s exam room (3 cases), or in a large conference room in another area of the hospital (2 cases).
Measures and Data Collection
All baseline measures were collected in person. A trained research assistant (not the interventionist), assessed all outcomes by telephone.
Feasibility
Feasibility was assessed by tracking: 1) the numbers and percentages of eligible dyads versus consented dyads, and reasons for refusal to participate; 2) the number of consented dyads that completed the study; and 3) the amount of time to deliver the intervention sessions.
Acceptability
Acceptability was assessed using audiotaped, semi-structured, separate interviews with each of the dyad members. The interviews were conducted over the telephone at 2 weeks post-intervention, by one of the research assistants. Participants were asked to describe: their overall experiences with the discussion, positive/helpful and negative/less helpful aspects of the discussion, if and how this type of discussion should be incorporated into patient care, when in the heart failure trajectory these types of conversations should be introduced, and suggestions for improvement. These interviews lasted 10–45 minutes.
Short-term outcomes and measures
The tools, shown to be reliable and valid in previous studies,33, 34 including previous studies examining the effect of SPIRIT,25, 26 were used to assess the following outcomes:
Dyad congruence
Goals of Care (GOC)26 was used to assess dyad congruence about the patient’s end-of-life treatment preferences. This tool includes two end-of-life scenarios that are commonly confronted by LVAD patients. One scenario described a situation in which the patient experienced an acute event, such as sepsis, hemorrhage, or stroke, which rendered him/her gravely ill in the intensive care unit, and unable to participate in treatment decisions. The other described a scenario in which the patient was very ill, but also had advanced dementia and unable to participate in decision-making. Patients and their surrogates read the scenarios and then separately indicated the goals of care most consistent with patient preferences. The options included: 1) goals of care focused on comfort; 2) goals of care focused on extending life; 3) not sure. Patient and surrogate responses were compared to determine congruence, ranging from 0 (incongruent in both scenarios) to 2 (congruent in both scenarios). This tool has been tested with cardiac patients24 and patients on dialysis and their surrogates.25–27
Patient decisional conflict
The Decisional Conflict Scale (DCS),26, 33 was used to assess the level of difficulty experienced by patients in understanding, determining and articulating choices related to end-of-life care. It is a 13-item, 5-point Likert scale (1 = strongly agree to 5 = strongly disagree). Higher mean scores indicate greater difficulty. The internal consistency for the study sample was 0.84. Its known group validity has been previously reported.33
Surrogate decision-making confidence
The Surrogate Decision-making Confidence Scale (DMC),26, 34 was used to measure the surrogate’s level of comfort about end-of-life decision-making. The DMC is a Likert-type 5-item scale (0= not at all confident, 4= very confident). Higher mean scores indicate greater levels of confidence. The internal consistency for the study sample was 0.82. Its known group validity has been previously reported.34
Participant characteristics
Socio-demographic (e.g. age, gender, ethnicity, marital status, religious affiliation), and clinical information (e.g. presence of advance directives, number of hospital days since LVAD, co-morbidities, length of time in months on LVAD support), were also collected.
Data Analysis
Feasibility data were summarized using descriptive statistics (frequencies, percentages, mean, etc.). Acceptability interviews were recorded and transcribed verbatim. Qualitative content analysis was used to distil the relevant aspects of the participants’ experiences of the intervention.35–37 Team members initially conducted parallel analyses. Results were compared to assess agreement, and later analyses were reviewed as a group.
Mean scores on the DCS and DMC scales were calculated accordingly for each group. To estimate the preliminary short-term effects of SPIRIT-HF on dyad congruence, patient’s decisional conflict and surrogate’s decision-making confidence, we used the generalized estimating equation method. Odds ratios (ORs) were computed for the dyad congruence and Cohen’s d were computed for the patient’s decisional conflict and surrogate’s decision-making confidence.
Results
Sample Characteristics
Of the 29 patients in the final sample, the largest percentage were married or partnered (n = 26, 89.6%), white (n = 19, 65.5%), and male (n = 20, 67.0%), with multiple co-morbidities, including dyslipidemia, coronary artery disease, diabetes, kidney disease, depression, and chronic obstructive pulmonary disease. Eight had advance directives in the health record. The majority of patients were designated as LVAD for DT at the time of device placement. However, 4 patients were re-classified: 1 from BTT to DT, and 3 from DT to bridge to candidacy; and 2 of the patient participants died, both LVAD for DT patients. Compared to the patients in the control group, those in the intervention group had a significantly shorter average time of LVAD support (11 ± 5.4 months versus 23.2 ± 13.6, p-value = 0.005). The designated surrogate decision-makers were generally female (n = 25, 86.2%), spouses/partners (n = 22, 75.9%) of the patients. (Table 1)
Table 1.
Patient and Surrogate Sample Characteristics
| Patients Control (n = 15) | Intervention (n = 14) | Surrogates Control (n = 15) | Intervention (n = 14) | |
|---|---|---|---|---|
|
|
||||
| Characteristic/Variable | n (%) M ± SD (range) |
n (%) M ± SD (range) |
n (%) M ± SD (range) |
n (%) M ± SD (range) |
| Gender | ||||
| Male | 9 (60) | 11 (78.6) | 2 (13.3) | 2 (14.3) |
| Female | 6 (40) | 3 (21.4) | 13 (86.7) | 12 (85.7) |
| Age | 62.3 ± 12.3 (43–85) | 62.6 ± 7.6 (44–74) | 56.5 ± 17.6 (28–85) | 56.2 ± 12.4 |
| Race | ||||
| African-American | 6 (40) | 5 (35.7) | 6 (40) | 5 (35.7) |
| Caucasian | 9 (60) | 9 (64.3) | 9 (60) | 9 (64.3) |
| Marital Status | ||||
| Never married | – | – | 3 (20) | 2 (14.3) |
| Currently married/partnered | 12 (80) | 14 (100) | 11 (73.4) | 11 (78.6) |
| Widowed | 2 (13.3) | – | – | 1 (7.1) |
| Separated or divorced | 1 (6.7) | – | 1 (6.7) | – |
| Patient/surrogate relationship Surrogate is … | ||||
| Spouse or partner | – | – | 10 (66.7) | 12 (85.7) |
| Adult child | – | – | 2 (13.3) | 2 (14.3) |
| Sibling/other family member | – | – | 3 (20) | – |
| Educational background | ||||
| Grade school | 1 (6.7) | – | – | – |
| High School | 7 (46.7) | 6 (42.9) | 8 (53.3) | 5 (35.7) |
| Associates level college/trade school | 3 (20) | 4 (28.6) | 2 (13.3) | 6 (42.9) |
| Bachelors level college | 2 (13.3) | 1 (7.1) | 4 (26.7) | 3 (21.4) |
| Graduate school | 2 (13.3) | 3 (21.4) | 1 (6.7) | – |
| Employment status | ||||
| Full time | 1 (6.7) | 1 (7.1) | 4 (26.7) | 6 (42.9) |
| Part time | 1 (6.7) | – | 2 (13.3) | – |
| Unemployed | – | – | 4 (26.7) | 1 (7.1) |
| Retired | 5 (33.3) | 6 (42.9) | 5 (33.3) | 5 (35.7) |
| Disabled/unable to work | 8 (53.3) | 7 (50) | – | 2 (14.3) |
| Religious Affiliation | ||||
| None | 1 (6.7) | 1 (7.1) | 2 (13.3) | 1 (7.1) |
| Catholic | 1 (6.7) | – | 1 (6.7) | – |
| Protestant | 6 (40) | 10 (71.4) | 8 (53.3) | 10 (71.4) |
| Other (Buddhist, Muslim, Nondenominational, Christian etc.) | 7 (46.7) | 3 (21.4) | 4 (26.7) | 3 (21.4) |
| Total gross annual household income | ||||
| < $10,000 | 2 (13.3) | 1 (7.1) | 1 (6.7) | – |
| $10,000 – $19,999 | 4 (26.7) | 1 (7.1) | 6 (40) | – |
| $20,000 – $29,999 | 4 (26.7) | 3 (21.4) | 3 (20) | 4 (28.6) |
| $30,000 – $49,999 | 1 (6.7) | – | 1 (6.7) | 1 (7.1) |
| ≥ $50,000 | 4 (26.7) | 9 (64.3) | 4 (26.7) | 9 (64.3) |
| Months on VAD support* | 23.2 ± 13.6 (10–55) | 11 ± 5.4 (2–20) | – | – |
| LVAD purpose at time of device placement | ||||
| Bridge to transplant | 5 (33.3) | 3 (21.4) | – | – |
| Destination therapy | 10 (66.7) | 11 (78.6) | – | – |
| Advance Directives in Health Record | – | – | ||
| Yes | 2 (13.3) | 6 (42.8) | – | – |
| No | 13 (86.7) | 8 (57.2) | – | – |
Statistically significant difference between intervention and control groups, p = .005
Feasibility
Forty-six patients were assessed for eligibility, of those, 38 (83%) were deemed eligible for the study. Of those, 8 (21%) declined. The 4 patients who actively refused were asked, via an open-ended question, to provide a reason for refusal. Reasons included, “being too stressed or tired”, “living too far from clinic”, and having “too much else going on”. The other 4 patients were passive refusals, meaning they did not return phone calls or answer emails. One surrogate did not meet as arranged to complete enrollment. The patient therefore, was deemed ineligible. Twenty-nine dyads (76%) provided consent and were randomized; 15 to the control group, 14 to the intervention group. All 29 dyads completed the study. (Figure 1)
Figure 1.
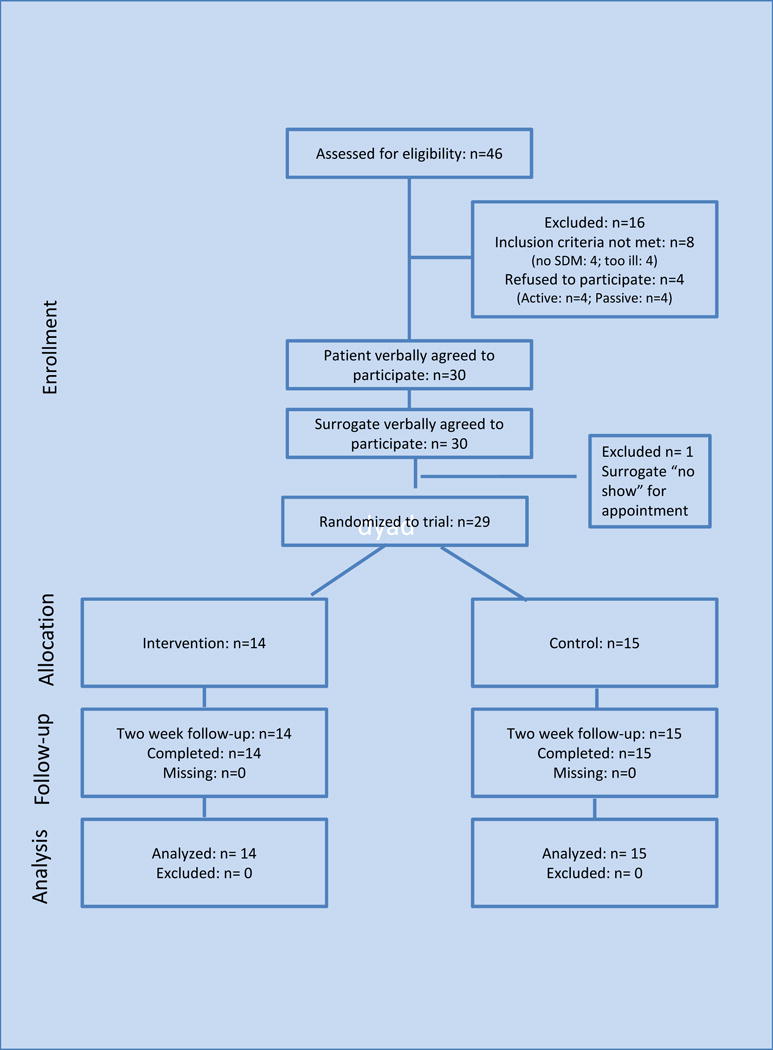
Participants Progress in the Study
All dyads randomized to the intervention group received the SPIRIT-HF session. Intervention sessions lasted 68 (SD = 17) minutes on average, with a median of 66 minutes (range 44–96 minutes). No sessions were stopped because of participants’ emotional distress.
Acceptability
Nearly all (25 of 28) participants in the intervention group reported that their experience of SPIRIT-HF was a positive one. However, 2 patients and 1 surrogate, characterized their experience as “mixed” as the conversation “stirred up some emotions” that were “tough to deal with”. Despite this, they too felt the conversation was beneficial.
The most positive or helpful aspects of SPIRIT-HF included: being able to express or clarify their, or their loved ones’, preferences for end-of-life care; learning about common scenarios requiring end-of-life decision-making in LVAD patents; and having the opportunity to make sense of their experiences and thoughts about heart failure in general, and LVADs in particular. For example, one patient said, “it was a really good time to clarify what it is you mean when you say something like, ‘no heroic measures’…cause although we like to think it’s black and white; so often it’s gray.” According to another patient’s surrogate, it was very helpful to “inform my mom of the LVAD situations that might come…to get the information that we needed to be prepared for decisions…to talk about what she wants or not, so we can know ahead of time.” The wife of another patient reported, “it was so good being able to voice what we’ve been through, not just [patient]’s side, but the caregiver side too.”
The most commonly cited barriers to the discussion were time and scheduling constraints. As one surrogate responded, “It was worth it, but it made a long day…I have a long drive, and work…That’s a lot of wear and tear on my body.” Another patient said, “The day ran very long. We didn’t even get to see [interventionist] until after 5 pm.”
All of the participants reported that conversations like SPIRIT-HF are very important and nearly all said these types of discussions should be part of patient care. Many spoke of the importance of being prepared for the “what ifs”, as illustrated by the following excerpt from a surrogate’s interview: “I feel like I could make those right decisions for him if he were unable….It’s something everybody needs to think about and talk about. It will open your eyes and make you think. Everybody needs it. Two big words: ‘what if’. Be prepared”.
When asked about where in the heart failure or LVAD trajectory ACP conversations would be most helpful, answers among the patients varied. Several suggested, as demonstrated by the following quotes, that before LVAD placement would be best: “We should have delved into it prior to the operation, because that’s important stuff.” “People are not prepared…might be better to have this (conversation) before you actually have the VAD.” Other patient participants disagreed, saying, “Doing it closer to when the LVAD is placed would add to the confusion. You’re overwhelmed at that point…best to let it simmer for a while…”
Surrogates tended to be more consistent in their suggestions of having detailed ACP discussions before LVAD placement. As one daughter said, “before his surgery…when he was still a candidate…We weren’t prepared…and you definitely need to be prepared.” Regardless of the timing participants nearly all agreed that it was important to assess the patient’s and family’s “readiness” for the conversation and as one wife said, “make allowances for how they want to do it.”
Participants offered few suggestions for improvement. One surrogate suggested, “Having the choice of somebody coming out to the home…and if a family wants to continue the conversations, they could have that too.” Another surrogate suggested, “Having them in pieces…as you go along; at different stages…”
Preliminary Effects
Both groups demonstrated improvement in dyad congruence on GOC. However, the SPIRIT-HF group demonstrated greater improvement, with 5 dyads congruent at baseline in each group, and 13 out of 14 congruent at time 2 in the SPIRIT-HF group; and 9 out of 15 congruent in the control group. The difference between the groups was not statistically significant (p = 0.064). There were no significant differences between the groups on patient decisional conflict or surrogate decision-making confidence.
Intervention effect sizes for all 3 outcomes were calculated and are presented in Table 2. The effect size (odds ratio or OR) of SPIRIT-HF on dyad congruence was large (OR = 8.7), indicating that intervention dyads were nearly 9 times as likely as controls to achieve congruence in patient goal of care. The effect size (Cohen’s d) for patient decisional conflict, as measured by Patient DCS, was moderate (0.53). SPIRIT-HF showed no effect on surrogate decision-making confidence (Cohen’s d = 0.02).
Table 2.
Preliminary Effects of SPIRIT-HF
| Outcomes | SPIRIT (n = 14) | Control (n = 15) | Effect size (Odds ratio or Cohen’s d) |
|---|---|---|---|
| Dyad congruent, No. (%) | |||
| Baseline | 5 (35.7) | 5 (33.3) | |
| 2-week follow-up | 13 (92.9) | 9 (60.0) | 8.7 |
| Patient DCS*, mean (SD) | |||
| Baseline | 1.9 (0.4) | 2.2 (0.6) | |
| 2-week follow-up | 1.6 (0.5) | 1.9 (0.5) | 0.53 |
| Surrogate DMC**, mean (SD) | |||
| Baseline | 3.4 (0.7) | 3.4 (0.8) | |
| 2-week follow-up | 3.7 (0.4) | 3.7 (0.5) | 0.02 |
Decisional Conflict Scale (higher scores indicate greater distress)
Decision-Making Confidence (higher scores greater confidence)
Discussion
The findings from this pilot indicate that recruiting LVAD patients and their surrogates for a study of an ACP intervention and conducting SPIRIT-HF in an outpatient clinic was feasible and that participants perceive SPIRIT-HF positively. In fact, all of the participants who engaged in the SPIRIT-HF discussion, completed all components, and were unanimous in their contention that it is beneficial to patients and families, and is an important, yet currently overlooked, part of care. Both of these findings, the lack of detailed advance care planning discussions,15–17, 38 and the willingness of patients and surrogates to engage in them,19, 39, 40 are consistent with previously reported findings. However, the interview data provide additional information about the participants’ perspectives on the most positive aspects, and the immediate outcomes of the conversation. Inviting them to share their thoughts and beliefs, and including situation-specific scenarios and outcomes of treatment, were greatly appreciated. This underscores the importance of listening and understanding “where a patient is coming from” and of offering information, specific to his/her situation, which are main components of the Representational Approach.28–30 Similarly, centering the goals of care part of the discussion on scenarios that the participants felt are relevant to LVAD patients, and encouraging the patients to explain “why” they selected the goals of care they did, increased feelings of preparedness especially in the surrogates.
It was interesting to note that both groups demonstrated improvement in congruence. A possible explanation for this is what Song and Ward41 describe as “assessment effects in educational and psychosocial intervention trials”. In this particular case, all participants completed baseline measures which included the GOC assessment. It is quite possible that completing the survey motivated the participants in both groups to discuss the patients’ goals of care; thereby increasing the congruence between them.
The findings have important research implications. It will be important to consider assessment effects in any future intervention, whether the findings support the research hypothesis or not.38 Employing a variety of outcome measures, both quantitative and qualitative would likely be helpful in understanding and explaining intervention effects.
In addition, it is likely that patients who have engaged in detailed, specific ACP discussions with their loved ones and health care providers, and have a shared understanding of the patient’s preferences for end-of-life care would derive less benefit from the intervention than those who have not. However, at present we do not have a consistent, reliable method of determining who fits this criterion. Similarly, we do not yet know if and how LVAD patients’ perspectives on ACP and related issues differ based on the indication for device implant. Research in these areas would be helpful.
Study findings also have implications for clinical practice. Both patient and surrogate participants appreciated the inclusion of both in the discussion, suggesting that clinicians should check to ensure that the appropriate people are present for ACP discussions. Participants also stressed the importance of assessing patient and family “readiness” to engage in ACP, and of providing information according to their needs and desires. Finally, because these conversations can be lengthy and tiring, consideration should be given to beginning them during times of relative medical stability and continuing them over time.
Our study has several limitations. As this was a pilot study only a small number of patients from one outpatient VAD service in an academic medical center in the US was included. They may not be representative of patients in other parts of the US or in other countries. The study was not powered to test the intervention effects. We evaluated only short-term effects and thus the impact of SPIRIT-HF on longer term outcomes, including actual end-of-life decision-making experiences was not examined. Also, our control condition was usual care, not an active control. In addition, although the perspectives of the SPIRIT-HF group did not differ based on LVAD designation as DT or BTT, there were only three patients with BTT. We cannot assume that in a larger sample that this would hold true.
Conclusion
In summary, both patients and surrogates felt they benefitted from the SPIRIT-HF discussion. This study, and others like it reinforce the importance of improving communication among patients and their loved ones about patient preferences for end- of-life care. The findings from this study will inform a larger scale trial to evaluate the effects of SPIRIT-HF in this population.
Acknowledgments
Research Supported by: Sigma Theta Tau International/Hospice and Palliative Nurses Foundation End of Life Nursing Care Research Grant; National Center for Advancing Translational Sciences (NCATS), National Institutes of Health, through Grant Award Number UL1TR001111; Sigma Theta Tau International, Alpha Alpha Chapter Postdoc Award. During the conduct of this research project, Dr. Metzger was a postdoctoral trainee on Interventions to Prevent or Manage Chronic Illness (5T32NR007091), Dr. Sheila Judge Santacroce, Director.
The authors would like to thank Research Assistants, Stephanie Devane-Johnson and Jill Polk, the VAD nurse coordinators, Mandy Bowen, Danielle Miller, and Sharon Wesner, and the rest of the VAD team at UNC Hospitals; and the study particpants.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Identifier removed:
The University of North Carolina at Chapel Hill IRB approved the study.
Setting and Sample
Patients were recruited from an LVAD Program at a large academic medical center in North Carolina.
References
- 1.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, et al. Heart disease and stroke Statistics—2013 update: A report from the American Heart Association. Circulation. 2013;127(1):e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Byram EK. Upstream palliative care for the patient with a left ventricular assist device as destination therapy. Dimensions of Crit Care Nurs: DCCN. 2012;31(1):18–24. doi: 10.1097/DCC.0b013e31823a537c. [DOI] [PubMed] [Google Scholar]
- 3.Lietz K. Destination therapy: Patient selection and current outcomes. J Card Surg. 2010;25(4):462–471. doi: 10.1111/j.1540-8191.2010.01050.x. [DOI] [PubMed] [Google Scholar]
- 4.Slaughter MS, Meyer AL, Birks EJ. Destination therapy with left ventricular assist devices: Patient selection and outcomes. Current Opinion in Cardiology. 2011;26(3):232–236. doi: 10.1097/HCO.0b013e328345aff4. [DOI] [PubMed] [Google Scholar]
- 5.Kirklin JK, Naftel DC, Pagani FD, Kormos RL, Stevenson LW, Blume ED, et al. Sixth INTERMACS annual report: A 10,000-patient database. J Heart Lung Transplant. 2014;33(6):555–564. doi: 10.1016/j.healun.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 6.Vitale CA, Chandekar R, Rodgers PE, Pagani FD, Malani PN. A call for guidance in the use of left ventricular assist devices in older adults. J Am Geriatr Soc. 2012;60(1):145–150. doi: 10.1111/j.1532-5415.2011.03740.x. [DOI] [PubMed] [Google Scholar]
- 7.Rose EA, Gelijns AC, Moskowitz AJ, Heitjan DF, Stevenson LW, Dembitsky W, et al. Long-term mechanical left ventricular assistance for end-stage heart failure. NEJM. 2001;345(20):1435–1443. doi: 10.1056/NEJMoa012175. [DOI] [PubMed] [Google Scholar]
- 8.Slaughter MS, Rogers JG, Milano CA, Russell SD, Conte JV, Feldman D, et al. Advanced heart failure treated with continuous-flow left ventricular assist device. NEJM. 2009;361(23):2241–2251. doi: 10.1056/NEJMoa0909938. [DOI] [PubMed] [Google Scholar]
- 9.Rogers JG, Aaronson KD, Boyle AJ, Russell SD, Milano CA, Pagani FD, et al. Continuous flow left ventricular assist device improves functional capacity and quality of life of advanced heart failure patients. J Am Coll Cardiol. 2010;55(17):1826–1834. doi: 10.1016/j.jacc.2009.12.052. [DOI] [PubMed] [Google Scholar]
- 10.Stern DR, Kazam J, Edwards P, Maybaum S, Bello RA, D’Alessandro DA, et al. Increased incidence of gastrointestinal bleeding following implantation of the HeartMate II LVAD. J Cardiac Surg. 2010;25(3):352–356. doi: 10.1111/j.1540-8191.2010.01025.x. [DOI] [PubMed] [Google Scholar]
- 11.Park SJ, Milano CA, Tatooles AJ, Rogers JG, Adamson RM, Steidley DE, et al. Outcomes in advanced heart failure patients with left ventricular assist devices for destination therapy. Circulation. 2012;5(2):241–248. doi: 10.1161/CIRCHEARTFAILURE.111.963991. [DOI] [PubMed] [Google Scholar]
- 12.Yuan N, Arnaoutakis GJ, George TJ, Allen JG, Ju DG, Schaffer JM, et al. The spectrum of complications following left ventricular assist device placement. J Cardiac Surg. 2012;27(5):630–638. doi: 10.1111/j.1540-8191.2012.01504.x. [DOI] [PubMed] [Google Scholar]
- 13.Manintveld OC. Left ventricular assist device for end-stage heart failure: results of the first LVAD destination therapy program in the Netherlands. Towards LVAD destination therapy in the Netherlands. Neth Heart J. 2015;23:100–101. doi: 10.1007/s12471-014-0609-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hayward C, Jansz P. Mechanical circulatory support for the failing heart: Progress, pitfalls, and promises. Heart, Lung and Circulation. 2015;24:527–531. doi: 10.1016/j.hlc.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 15.Brush S, Budge D, Alharethi R, McCormick AJ, MacPherson JE, Reid BB, et al. End-of-life decision making and implementation in recipients of a destination left ventricular assist device. J Heart Lung Transplant. 2010;29(12):1337–1341. doi: 10.1016/j.healun.2010.07.001. 10.1016/j.healun.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 16.Petrucci RJ, Benish LA, Carrow BL, Prato L, Hankins SR, Eisen HJ, et al. Ethical considerations for ventricular assist device support: A 10-point model. ASAIO Journal. 2011;57(4):268–273. doi: 10.1097/MAT.0b013e3182223269. [DOI] [PubMed] [Google Scholar]
- 17.Swetz KM, Mueller PS, Ottenberg AL, Dib C, Freeman MR, Sulmasy DP. The use of advance directives among patients with left ventricular assist devices. Hospital Practice. 2011;39(1):78–84. doi: 10.3810/hp.2011.02.377. [DOI] [PubMed] [Google Scholar]
- 18.Feldman D, Pamboukian SV, Teuteberg JJ, Birks E, Lietz K, Moore SA, et al. The 2013 international society for heart and lung transplantation guidelines for mechanical circulatory support: Executive summary. J Heart Lung Transplant. 2013;32(2):157–187. doi: 10.1016/j.healun.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 19.Metzger M, Norton SA, Quinn JR, Gramling R. Patient and family members’ perceptions of palliative care in heart failure. Heart Lung. 2013;42(2):112–119. doi: 10.1016/j.hrtlng.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.MacIver J, Ross HJ. Withdrawal of ventricular assist device support. J Palliat Care. 2005;21(3):151–156. [PubMed] [Google Scholar]
- 21.Retrum JH, Nowels CT, Bekelman DB. Patient and caregiver congruence: The importance of dyads in heart failure care. The Journal of Cardiovascular Nursing. 2013;8(2):129–136. doi: 10.1097/JCN.0b013e3182435f27. [DOI] [PubMed] [Google Scholar]
- 22.Joint Commission. Ventricular assist device for destination therapy requirements. Joint Commission Perspectives. 2014;34(2) Accessed October 5,2015 http://www.jointcommission.org/assets/1/18/Ventricular_Assist_Devices_Destination_Therapy.pdf. [PubMed] [Google Scholar]
- 23.Swetz KM, Freeman MR, Abouezzeddine OF, Carter KA, Boilson BA, Ottenberg AL, et al. Palliative medicine consultation for preparedness planning in patients receiving left ventricular assist devices as destination therapy. Mayo Clinic Proceedings. 2011;86(6):493–500. doi: 10.4065/mcp.2010.0747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Song M, Kirchhoff KT, Douglas J, Ward S, Hammes B. A randomized, controlled trial to improve advance care planning among patients undergoing cardiac surgery. Med Care. 2005;43(10):1049–1053. doi: 10.1097/01.mlr.0000178192.10283.b4. [DOI] [PubMed] [Google Scholar]
- 25.Song MK, Ward SE, Happ MB, Piraino B, Donovan HS, Shields AM, et al. Randomized controlled trial of SPIRIT: An effective approach to preparing African-American dialysis patients and families for end of life. Res Nurs Health. 2009;32(3):260–273. doi: 10.1002/nur.20320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Song MK, Donovan HS, Piraino BM, Choi J, Bernardini J, Verosky D, et al. Effects of an intervention to improve communication about end-of-life care among African Americans with chronic kidney disease. Applied Nurs Res. 2010;23:65–72. doi: 10.1016/j.apnr.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 27.Song MK, Ward SE, Fine JP, Hanson LC, Lin FC, Hladik GA, et al. Advance care planning and end-of-life decision-making in dialysis: A randomized controlled trial targeting patients and their surrogates. American Journal of Kidney Diseases. doi: 10.1053/j.ajkd.2015.05.018. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Donovan HS, Ward S. Clinical scholarship: A representational approach to patient education. J Nurs Scholarship. 2001;33(3):211–216. doi: 10.1111/j.1547-5069.2001.00211.x. [DOI] [PubMed] [Google Scholar]
- 29.Donovan HS, Ward SE, Song MK, Heidrich SM, Gunnarsdottir S, Phillips CM. An update on the representational approach to patient education. J Nurs Scholarship. 2007;39(3):259–265. doi: 10.1111/j.1547-5069.2007.00178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Song M, Ward S. Making visible a theory-guided advance care planning intervention. Journal of Nursing Scholarship. 2015;47(5):389–396. doi: 10.1111/jnu.12156. [DOI] [PubMed] [Google Scholar]
- 31.Julious SA. Sample size of 12 per group rule of thumb for a pilot study. Pharm Stats. 2005;4:287–291. [Google Scholar]
- 32.Song M, Happ MB, Sadelowski M. Development of a tool to assess fidelity to a psycho-educational intervention. Journal of Advanced Nursing. 2010;66(3):673–682. doi: 10.1111/j.1365-2648.2009.05216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O’Connor AM. Validation of a decisional conflict scale. Medical Decision-Making. 1995;15:25–30. doi: 10.1177/0272989X9501500105. [DOI] [PubMed] [Google Scholar]
- 34.Song M, Sereika SM. An evaluation of the decisional conflict scale for measuring the quality of end-of-life decision-making. Patient Education and Counseling. 2006;61(3):397–404. doi: 10.1016/j.pec.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 35.Kearney MH. Levels and applications of qualitative research evidence. Research in Nursing and Health. 2001;24:145–153. doi: 10.1002/nur.1017. [DOI] [PubMed] [Google Scholar]
- 36.Miles MB, Huberman AM. Qualitative data analysis: An expanded sourcebook. 2nd. Thousand Oaks, CA: Sage; 1994. [Google Scholar]
- 37.Averill JB. Matrix analysis as a complementary analytic strategy in qualitative inquiry. Qualitative Health Research. 2002;12:855–866. doi: 10.1177/104973230201200611. [DOI] [PubMed] [Google Scholar]
- 38.Habal MV, Micevski V, Greenwood S, Delgado DH, Ross HJ. How aware of advanced care directives are heart failure patients, and are they using them? Canadian Journal of Cardiology. 2011;27:376–381. doi: 10.1016/j.cjca.2010.12.067. [DOI] [PubMed] [Google Scholar]
- 39.Selman L, Harding R, Beynon T, Hodson F, Coady E, Hazeldine C, et al. Improving end-of-life care for patients with chronic heart failure: “Let’s hope it’ll get better, when I know in my heart of hearts it won’t”. Heart. 2007;93:963–967. doi: 10.1136/hrt.2006.106518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Horne G, Payne S. Removing the boundaries: Palliative care for patients with heart failure. Palliative Medicine. 2004;18:291–296. doi: 10.1191/0269216304pm893oa. [DOI] [PubMed] [Google Scholar]
- 41.Song MK, Ward SE. Assessment effects in educational and psychosocial intervention trials: An important but often-overlooked problem. Research in Nursing and Health. 2015;38:241–247. doi: 10.1002/nur.21651. [DOI] [PMC free article] [PubMed] [Google Scholar]


