Abstract
AKI after cardiac surgery remains strongly associated with mortality and lacks effective treatment or prevention. Preclinical studies suggest that cell-based interventions may influence functional recovery. We conducted a phase 2, randomized, double-blind, placebo-controlled trial in 27 centers across North America to determine the safety and efficacy of allogeneic human mesenchymal stem cells (MSCs) in reducing the time to recovery from AKI after cardiac surgery. We randomized 156 adult subjects undergoing cardiac surgery with evidence of early AKI to receive intra-aortic MSCs (AC607; n=67) or placebo (n=68). The primary outcome was the time to recovery of kidney function defined as return of postintervention creatinine level to baseline. The median time to recovery of kidney function was 15 days with AC607 and 12 days with placebo (25th, 75th percentile range, 10–29 versus 6–21, respectively; hazard ratio, 0.81; 95% confidence interval, 0.53 to 1.24; P=0.32). We did not detect a significant difference between groups in 30-day all-cause mortality (16.7% with AC607; 11.8% with placebo) or dialysis (10.6% with AC607; 7.4% with placebo). At follow-up, 12 patients who received AC607 and six patients who received placebo had died. Rates of other adverse events did not differ between groups. In these patients with AKI after cardiac surgery, administration of allogeneic MSCs did not decrease the time to recovery of kidney function. Our results contrast with those in preclinical studies and provide important information regarding the potential effects of MSCs in this setting.
Keywords: acute kidney injury, stem cells, cardiac surgery, cardiopulmonary bypass
AKI after cardiac surgery is strongly associated with adverse outcomes, including early mortality and late progression to CKD.1–3 In addition, rates of AKI after cardiac surgery continue to increase, and the burden on health care resources is significant.4 The cardiac surgical setting is appealing to AKI research, because the timing of insult is defined, patients are carefully followed while remaining in the acute care setting, and patients are typically seen in follow-up over the months after discharge. A substantial body of work has helped to characterize postcardiac surgery AKI, including its incidence, time trends, risk factors, predictive algorithms, and clinical consequences. However, despite the breadth of knowledge of AKI and recent consensus in terminology, successful preventive strategies and therapeutic options remain elusive. Given the poor short- and long-term outcomes in patients with postcardiac surgical AKI, there is considerable interest in identifying and evaluating interventions to not only prevent AKI but also, enhance recovery of kidney function.5,6
Several investigators have previously examined the potential value of bone marrow–derived mesenchymal stem cells (MSCs) in facilitating repair in injured kidney tissue.7–9 In the setting of acute injury, a number of organs, including the kidney, increase expression of stromal derived factor-1, which by its cognate receptor, CXCR4, on MSCs provides a homing signal, enabling MSC migration to the site of injury. It has been proposed that these stimulated MSCs secrete paracrine effector molecules, including endothelial and epithelial growth factors and anti-inflammatory cytokines that collectively encourage the injured renal tubule epithelial cells to pursue a path of cell division and recovery versus an unaltered course characterized by apoptosis and ultimate fibrosis.10 Since the early description of the putative role of bone marrow–derived stem cells in repair of ischemia and tubular toxin–mediated AKI,11 several preclinical studies have established the potential role of MSCs in AKI. Several reviews on the use of MSCs in animal models concluded that MSCs could indeed improve kidney function after AKI.12,13 The few clinical applications of MSCs in kidney disease reported to date have focused on transplant recipients, with one study reporting faster recovery of kidney function among patients treated with MSCs.14 We conducted a multicenter, randomized, controlled trial evaluating the safety and efficacy of allogeneic MSCs (AC607; AlloCure Inc., Burlington, MA) in patients with AKI after cardiac surgery to test the hypothesis that MSCs reduce the time to recovery of kidney function after postoperative AKI, defined as the return of postinterventional serum creatinine to the baseline presurgery level (trial registration: ClinicalTrials.gov no. NCT01602328).
Results
Baseline and Surgical Characteristics
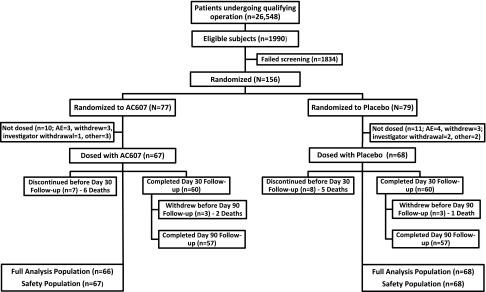
A total of 26,548 patients underwent study-qualifying cardiac operations; 1990 were eligible, and 156 patients were randomized into the study (Figure 1 and Supplemental Table 1). The protocol-defined incidence of AKI was 7.5%. Twenty-one randomized patients (ten AC607; 11 placebo) were not dosed, and 15 withdrew from the study (seven AC607; eight placebo). Sixty patients in each group completed 30 days of follow-up, and 57 in each group were followed for 90 days. Baseline characteristics of the study population are summarized in Table 1. More than one half of all patients had impaired kidney function at baseline. The median cardiopulmonary bypass (CPB) time and the time from end of surgery to study drug administration were longer in the AC607 group; otherwise, surgical data were comparable between groups.
Figure 1.
CONSORT (Consolidated Standards of Reporting Trials) diagram.
Table 1.
Baseline characteristics and surgical information
| Variable | AC607, n=67 | Placebo, n=68 |
|---|---|---|
| Age, yr, mean±SD | 65.6±11.9 | 67.0±9.9 |
| Sex, n | ||
| Women | 23 | 12 |
| Men | 44 | 56 |
| Weight, kg, mean±SD | 90.5±21.0 | 91.6±21.5 |
| Height, cm, mean±SD | 169.0±10.4 | 172.5±9.8 |
| Race, n | ||
| White/European | 55 | 58 |
| Black/African | 6 | 8 |
| Other | 6 | 2 |
| Diabetes, n | 27 | 33 |
| Hypertension, n | 56 | 58 |
| CHF, n | 29 | 30 |
| COPD, n | 10 | 9 |
| Preoperative albuminuria, n | 7 | 6 |
| CKD, n | ||
| Stage 1 | 12 | 8 |
| Stage 2 | 22 | 23 |
| Stage 3 | 29 | 34 |
| Stage 4 | 4 | 3 |
| Baseline eGFR, ml/min, mean±SD | 61±26 | 59±24 |
| Preoperative SCr, mg/dl, mean±SD | 1.3±0.6 | 1.4±0.6 |
| Pretreatment SCr, mg/dl, mean±SD | 2.1±0.7 | 2.2±0.6 |
| Type of surgery, n | ||
| CABG + valve | 13 | 19 |
| CABG only | 18 | 25 |
| Valve only | 31 | 22 |
| Other | 5 | 2 |
| Reoperative procedure, n | 15 | 12 |
| Elective procedure, n | 43 | 48 |
| CPB time, min, mean±SD | 168.1±84.2 | 149.0±70.2 |
| Time from end of surgery to study treatment, h, mean±SD | 36.9±10.1 | 35.5±10.5 |
CHF, congestive heart failure; COPD, chronic obstructive lung disease; SCr, serum creatinine; CABG, coronary artery bypass graft.
The Data Monitoring Committee (DMC) reviewed the safety data at prespecified intervals after 34, 50, and 119 patients were recruited. After the final meeting with 75% patient accrual, the DMC recommended the trial be halted due to futility. The results included here represent all data collected during the study, including additional follow-up information that was not available to the DMC at the time of their recommendation.
Outcomes
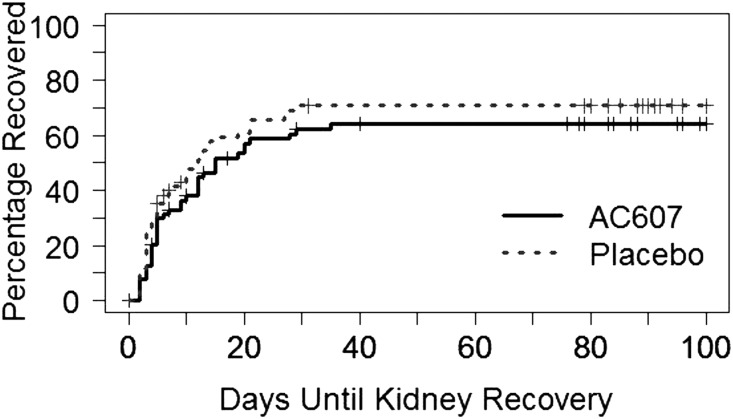
The primary analysis showed no significant difference in time to recovery of kidney function, with a median of 15 (25th, 75th percentile range, 10–29) days in the AC607 group versus 12 (interquartile range [IQR], 6–21) days in the placebo group (hazard ratio, 0.81; 95% confidence interval, 0.53 to 1.24; P=0.32) (Figure 2). Thirty-nine of 66 patients (59%) in the AC607 group and 46 of 68 (68%) in the placebo group recovered to baseline kidney function; all recovery of kidney function occurred by day 35 poststudy treatment. There were no significant differences between groups in the secondary outcomes of all-cause mortality or provision of dialysis (Table 2). Similarly, there were no differences between groups in peak rise in serum creatinine, area under the curve for serum creatinine at day 30, longer-term kidney function, in-hospital mortality, intensive care length of stay, or total hospital length of stay (Table 2).
Figure 2.
Primary end point: no difference between groups in cumulative incidence curves for time to kidney recovery.
Table 2.
Secondary and exploratory outcomes
| Parameter | AC607, n=66 | Placebo, n=68 | Mean Difference (95% CI) |
|---|---|---|---|
| Secondary outcomes | |||
| All-cause mortality or dialysis, n (%) | |||
| Day 30 | 11 (16.7) | 8 (11.8) | 4.9% (−11.7 to 21.9) |
| Day 90 | 12 (18.2) | 9 (13.2) | 4.9% (−11.7 to 21.9) |
| All-cause mortality, n (%) | |||
| Day 30 | 6 (9.1) | 5 (7.4) | 1.7% (−15.1 to 19.0) |
| Day 90 | 8 (12.1) | 6 (8.8) | 3.3% (−13.4 to 20.4) |
| 3 yr | 12 (18.2) | 6 (8.8) | 9.4% (−7.3 to 26.2) |
| In-hospital mortality, n (%) | 7 (10.6) | 4 (5.9) | 4.7% (−12.1 to 21.9) |
| Dialysis at day 30, n (%) | 7 (10.6) | 5 (7.4) | 3.3% (−13.5 to 20.4) |
| Exploratory outcomes | |||
| Peak rise in SCr, mg/dl, mean±SD | 1.59±1.27 | 1.34±1.43 | 0.25 (−0.21 to 0.72) |
| AUC of SCr, mg/dl × h, mean±SD | 296.9±322.8 | 205.0±241.5 | 92.0 (−5.3 to 189.2) |
| eGFR, ml/min, mean±SD | |||
| Baseline | 61.4±26.2 | 58±24.2 | |
| 6 mo | 62.1±25.2 | 58.2±20.1 | |
| 1 yr | 52.0±26.2 | 55.7±19.7 | |
| ICU length of stay, d, mean±SD | 16.4±46.7 | 7.0±7.2 | 9.4 (−2.1 to 20.9) |
| Hospital length of stay, d, mean±SD | 19.5±15.4 | 16.3±10.6 | 3.1 (−1.5 to 7.8) |
| Hospital readmission within 30 d, n (%)a | 17/54 (31.5) | 17/61 (27.9) | 3.6% (−14.7 to 21.7) |
95% CI, 95% confidence interval; SCr, serum creatinine; AUC, area under curve; ICU, intensive care unit.
Denominator includes only patients discharged within 30 d of study treatment.
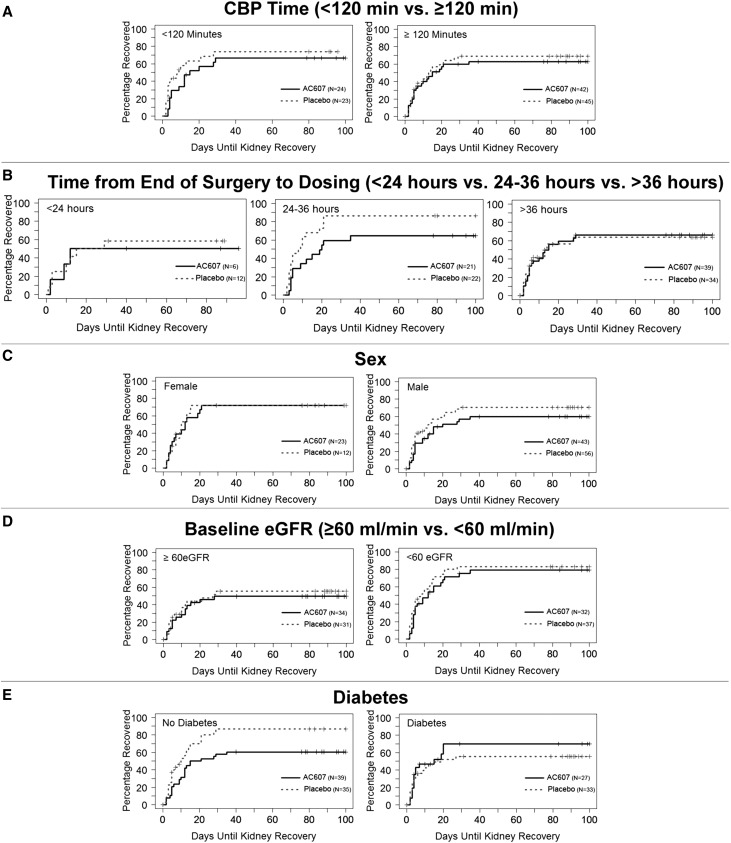
Using the alternative definition of recovery (to baseline serum creatinine +0.2 mg/dl), the median time to recovery of kidney function was 5.5 (IQR, 3.0–17.0) days in the AC607 group and 4.0 (IQR, 2.0–10.0) days in the placebo group (hazard ratio, 0.70; 95% confidence interval, 0.49 to 1.02; P=0.06), and the proportion of patients who recovered kidney function was 82% in the AC607 group and 91% in the placebo group. Exploratory prespecified subgroup analyses on the basis of duration of CPB (>120 or <120 minutes), timing of dosing (<24, 24–36, and >36 hours), baseline eGFR (≥60 versus <60 ml/min), study site, sex, or diabetes yielded results consistent with the primary efficacy analysis (Figure 3).
Figure 3.
Exploratory subgroup analyses were consistent with the primary efficacy analysis. (A) Duration of CPB, (B) timing of dosing, (C) sex, (D) baseline eGFR, and (E) diabetes.
A total of 12 patients received dialysis (seven AC607; five placebo), and 11 died (six AC607; five placebo) within 30 days of treatment (Table 2). By the end of study follow-up, 12 patients on AC607 and six patients on placebo had died; the range of time to death after study treatment was 0–219 days in the AC607 group and 9–85 days in the placebo group (Supplemental Material and Supplemental Table 2). There were no adverse events (AEs) of special interest or AEs that led to discontinuation of study treatment (Table 3). Similarly, there were no reports of development of cancer or manifestations of ectopic MSC differentiation. Data pertaining to treatment emergent serious AEs up to 90 days post-treatment are provided in Supplemental Table 3. The groups were comparable in terms of vital signs and clinical laboratory results (Supplemental Tables 4 and 5).
Table 3.
Treatment emergent AEs up to 30 d post-treatment
| Parameter | AC607, n=67 | Placebo, n=68 |
|---|---|---|
| Patients with at least one TEAE (%) | ||
| At any time after study drug | 63 (94.0) | 65 (95.6) |
| During hospitalization | 61 (91.0) | 59 (86.8) |
| From discharge to day 90 | 25/55 (45.5) | 29/61 (47.5) |
| Patients with at least one study drug–related TEAE (%) | 7 (10.4) | 6 (8.8) |
| Patient with at least one serious TEAE (%) | 31 (46.3) | 30 (44.1) |
| Patients with at least one TEAE leading to death (%) | 7 (10.4) | 5 (7.4) |
| Patients with at least one TEAE leading to treatment discontinuation (%) | 0 | 0 |
| Patients with at least one AE of special interest (%) | 0 | 0 |
TEAE, treatment emergent adverse event.
Discussion
In this phase 2, multicenter, randomized, controlled trial, we found that treatment of early postcardiac surgery AKI with MSCs did not reduce the time to recovery of kidney function, provision of dialysis, or mortality compared with placebo. Although not statistically significant, there were numerically more patients treated with AC607 who received dialysis or died, and there was a longer time to recovery of kidney function in either the primary analysis or a sensitivity analysis with a modified definition of the primary outcome.
AKI is a common complication of cardiac surgery, has been shown consistently to be associated with morbidity and mortality, consumes precious health care resources, and has been increasing in incidence over the last several decades.4 Cardiac surgery–associated AKI is an appropriate model to test interventions aimed at either mitigating injury or accelerating recovery, because the timing of the insult is predictable and because patients can be readily followed during their inpatient recovery from surgery. Most interventional studies of prevention strategies have failed to show any significant reduction in incidence or severity of AKI, most likely due to the many potential mechanisms of cardiac surgery–related injury and the low number of events compromising statistical power. The advantage of studying the recovery of kidney function after proven AKI is in the targeting of patients who are more likely to have an adverse clinical outcome. Strategies that promote endogenous repair or that limit further injury may be as or more effective than those that focus purely on prevention.6
The ability to mobilize endogenous progenitor cells that can mitigate injury and participate in repair is well described.15,16 However, clinically relevant injury could overwhelm endogenous repair mechanisms. Studies have, therefore, focused on how injured organs may be supplied with progenitor cells by either stimulating mobilization of endogenous stores or administration of exogenous cells. Bone marrow–derived progenitor cells have been known to play an important role in repair and recovery from injury in several organs, including the kidney.12 MSCs are multipotent, nonhematopoietic stromal cells that reside in the perivascular niches of various organs; can form bone, cartilage, and fat; and can participate in repair of injured tissue principally through paracrine mechanisms.9 Bone marrow–derived MSCs have been shown to reduce the severity of experimental kidney injury and accelerate recovery at variable rates depending on the delivery route and timing of administration relative to the injury.12
We conducted this multicenter, phase 2 trial with the objective of assessing the effect of allogeneic MSCs on functional recovery of the kidneys after clinically relevant postoperative AKI. The absence of a significant recovery signal after AKI in patients treated with MSCs compared with placebo may be attributable to several factors. First, MSCs could be less effective in treating than in preventing AKI, which was also suggested by the difference between this study and a small phase 1 study in which 16 high-risk patients given MSCs before cardiac surgery had a lower incidence of postoperative AKI than a historical control.13,17 Second, mechanisms of cardiac surgery–related injury and recovery may be distinct from rodent models of ischemia reperfusion, and the complexity of the postcardiac surgery clinical setting may hinder our ability to observe a modest clinical effect with MSCs or other agents.18,19 Third, longer durations of CPB in the MSC group might have masked a beneficial effect, and some patients in this trial may have had continuing renal insults (e.g., infection, low cardiac output, or inflammation) that counterbalanced any beneficial effect of MSCs administered at a single time point along the injury continuum. In addition, any significant recovery signal may have been insufficient to be clinically evident, limited to a small subset of patients with AKI and a suitable biochemical environment compatible with MSC-mediated repair, or obscured by the complexities of their postoperative course. Another potential reason that the therapy failed is because renal blood flow may be decreased during this phase of postcardiac AKI, thus limiting the delivery of the MSCs to the kidney. Furthermore, many of the patients in this study had impaired kidney function before surgery, and in the setting of already compromised kidney function, any beneficial effect of mesenchymal stromal cells could be attenuated. Finally, the sample size may have been insufficient to detect a small but meaningful acceleration of kidney recovery. However, the observed data and findings of our sensitivity analysis do not suggest a strong likelihood of benefit. Finally, we should consider the possibility that bone marrow–derived MSCs could induce harm, thereby delaying recovery—precisely the opposite of what we had hypothesized. The lack of a beneficial effect of MSCs has been shown in some clinical settings, especially those where chronic inflammation was a significant underlying modifier, such as rheumatoid arthritis or inflammatory bowel disease.20,21 Although considered immunoprivileged, human MSCs can have significant in vivo immunomodulatory effects or may even become immunogenic on differentiation. The biologic activity of MSCs seems to be dependent on the underlying inflammatory state, such that, in the presence of proinflammatory conditions, MSCs can differentiate into an anti-inflammatory phenotype and vice versa. It is possible that the proinflammatory phenotype may contribute to AKI as suggested by preclinical and clinical studies of MSCs in kidney transplantation.22–24 Although we do not have data on either biomarkers of chronic inflammation or differentiation of administered MSCs, it could be speculated that these factors may have played a role in mitigating a beneficial effect of MSCs in our patient population or even exerted a negative influence.
Among the strengths of this study was its rigorous design of a treatment strategy for AKI in a relatively homogenous population of patients undergoing cardiac surgery. However, there are some important limitations associated with the use of human allogeneic MSCs and their administration. One assumption in this trial was that the delivered MSCs were sufficient and viable at the time of administration. The dose used in this trial (2×106 MSCs per 1 kg) is consistent with what has been used in other clinical settings and supported by both preclinical and phase 1 data. Cell viability was tested during the manufacturing procedure, before storage in inventory, after post-thaw reconstitution in an experimental setting, and after the shipping of AC607 to validate the shipping containers. During the study, quality control testing was performed at each enrolling site to ensure that reconstitution protocols were strictly followed and yielded a final product that met the release specifications. After the termination of the study, viability and cell count studies were performed on returned site inventory before destruction. In all cases, cell viability ranged between 93% and 96%. As a result, we presume that the majority of cells were viable at the time of administration. The intra-arterial route was selected to maximize the likelihood of exposure of injured kidneys to the delivered MSC population in the first pass and minimize filtration in the lungs, although we were unable to determine amount and distribution of MSCs to the kidneys. Preclinical studies indicate that MSCs exert a beneficial effect on the kidneys in ischemia-reperfusion models via paracrine effects, even when administered MSCs are not seen to persist in host kidney tissue.9 We are, therefore, confident that the intra-arterial route was the optimal method for ensuring maximal delivery of viable MSCs in sufficient numbers to injured kidneys. It is possible that intra-arterial administration might have resulted in vascular injury or cholesterol embolization in some patients, although one would expect similar effects in patients randomized to both groups.
In summary, in a phase 2, multicenter, randomized, controlled trial, we found no significant difference in time to recovery of kidney function after treatment of early postoperative AKI with allogeneic MSCs compared with placebo. There were also no significant differences noted in any of the secondary outcomes, although numerical imbalances favored placebo. These results contrast with the favorable effects of MSCs in preclinical studies and could be attributed to overwhelming or ongoing injury, an insufficient or undetectable beneficial effect, or possible harm. Despite the potential for benefit suggested by preclinical studies and relative safety of early-phase human trials, several unanswered questions, such as optimal timing, preparation, and method of delivery and whether any subsets of patients may benefit, would need to be addressed before continued development of MSCs for the treatment of established AKI in patients undergoing cardiac surgery.
Concise Methods
In accordance with the International Conference on Harmonization Good Clinical Practice guidelines, we conducted this phase 2, randomized, double-blind, placebo-controlled trial in patients undergoing cardiac surgery with laboratory evidence of postoperative AKI after site-specific regulatory approval in 27 tertiary care institutions in the United States and Canada from January 10, 2013 to March 30, 2014.
We included adults (>21 years of age) undergoing cardiac surgery using CPB who had a baseline serum creatinine measured within 30 days before surgery and who developed postoperative AKI (defined as a postoperative rise in serum creatinine >0.5 mg/dl from baseline within 48 hours of removal from CPB). We excluded patients with stage 5 CKD, active cancer or active treatment for cancer, surgery for thoracoabdominal aortic aneurysm, presence of a medical condition or device that would preclude or compromise femoral artery catheter placement, or current or expected receipt of dialysis within 24 hours of enrollment or dosing. All inclusion and exclusion criteria are provided in Supplemental Table 1.
We randomized enrolled subjects to receive AC607 (2×106 cells per 1 kg body weight) or placebo in a 1:1 ratio. We performed laboratory assessments at screening, daily during the postoperative hospital stay from the day of randomization to discharge, and at 30 and 90 days after study drug administration.
We administered AC607 or placebo as a single dose within 48 hours of removal from CPB via an intra-aortic catheter placed in the femoral artery with the tip located proximal to the origin of the renal arteries. AC607 was manufactured, processed, packaged, and labeled by Lonza Walkersville, Inc. (Walkersville, MD). Clinical trials teams at each institution were blinded to the preparation of AC607 or placebo.
The primary end point was the time to recovery of kidney function after postoperative AKI defined by the time in days from dosing to the first of two postdosing serum creatinine samples that returned to the patient’s baseline level. Secondary efficacy end points included all-cause mortality or provision of dialysis at 30 and 90 days poststudy drug administration, all-cause mortality at 30 and 90 days postadministration, and proportion of patients who required dialysis during the 30-day evaluation period.
We performed safety and efficacy analyses on all patients who underwent cardiac surgery, provided informed consent, and were treated with AC607 or placebo. We constructed Kaplan–Meier product limit plots and determined relative hazard (AC607 versus placebo) using proportional hazards (Cox) regression with treatment group as a dependent variable. We calculated the difference between treatment groups in proportions using the exact Clopper–Pearson methodology. We conducted all statistical analyses using SAS software, version 9.2 or higher (SAS Institute, Cary, NC).
Disclosures
V.P. and R.M.B. were employed by AlloCure, Inc. (Burlington, MA). No other authors have financial disclosures relevant to this work.
Supplementary Material
Acknowledgments
This study was funded by AlloCure Inc. (Burlington, MA).
The Data Monitoring Committee included Adeera Levin (chair), James E. Udelson, and Thomas R. Fleming. AC607 Trial in Acute Kidney Injury (ACT-AKI) Investigators who recruited at least one patient are M.S., M.S.-S., F. Willem Lombard, Jacob Schroder, Joanne Kurtzberg, and Tiffany Bisnar (Duke University); M.G.A., John Conte, Jeffrey Dodd-o, Hamid Rabb, Nevin Katz, Ashish Shah, and Elizabeth Huyette-Arrizza (Johns Hopkins University); S.M. and Chris Bellot (University of Alabama at Birmingham); Robert Kramer and Betsey Tolson (Maine Medical Center); Richard Solomon (Fletcher Allen Health Care); Charles Brooks (University of Virginia); Christina Mora-Mangano and Jimmy Wong (Stanford University); Kianoush Kashani (Mayo Clinic); Yoshifumi Naka (Columbia University); Kausik Umanath and Jerry Yee (Henry Ford Hospital); Ahmet Kilic (The Ohio State University); Stewart Lecker (Beth Israel Deaconess Medical Center); Gyorgy Frendl (Brigham & Women's Hospital); Burkhard Mackensen (University of Washington); F.L., Mathieu Simon, Francois Dagenais, Marie-Claude Ferland, and Pierre-Alexandre Bouchard (Institut Universitaire de Cardiologie et de Pneumologie de Québec); A.F.-R., Richard Whitlock, Craig Ainsworth, and Ellen McDonald (McMaster-Hamilton General Hospital/Thrombosis and Atherosclerosis Research Institute); R.W., S.V., C.D.M., Gerard Curley, Sanjay Yagnik, and Charmagne Crescini (St. Michael's Hospital); André Ferland and Karen Maier (Libin Cardiovascular Institute of Alberta Foothills Hospital); André Denault and Hung Ly (Montréal Heart Institute); Daniel Bainbridge and Tracey Bentall (London Health Sciences Centre, University Hospital); Jean-François Légaré (Capital District Health Authority Queen Elizabeth II Health Sciences Centre); and Hilary Grocott (St. Boniface Hospital, University of Manitoba).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Mesenchymal Stromal Cells for AKI after Cardiac Surgery,” on pages 7–9.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2016101150/-/DCSupplemental.
Contributor Information
Collaborators: Madhav Swaminathan, Mark Stafford-Smith, F. Willem Lombard, Jacob Schroder, Joanne Kurtzberg, Tiffany Bisnar, Mohamed Atta, John Conte, Jeffrey Dodd-o, Hamid Rabb, Nevin Katz, Ashish Shah, Elizabeth Huyette-Arrizza, Spencer Melby, Chris Bellot, Robert Kramer, Betsey Tolson, Richard Solomon, Charles Brooks, Christina Mora-Mangano, Jimmy Wong, Kianoush Kashani, Yoshifumi Naka, Kausik Umanath, Jerry Yee, Ahmet Kilic, Stewart Lecker, Gyorgy Frendl, Burkhard Mackensen, Glenn M. Chertow, David G. Warnock, Viken Paragamian, Robert M. Brenner, Ravindra L. Mehta, Francois Lellouche, Mathieu Simon, Francois Dagenais, Marie-Claude Ferland, Pierre-Alexandre Bouchard, Alison Fox-Robichaud, Richard Whitlock, Craig Ainsworth, Ellen McDonald, C. David Mazer, Ron Wald, Subodh Verma, Gerard Curley, Sanjay Yagnik, Charmagne Crescini, Andre Ferland, Karen Maier, Andre Denault, Hung Ly, Daniel Bainbridge, Tracey Bentall, Jean-Francois Legare, Hilary Grocott, Adeera Levin, James E. Udelson, and Thomas R. Fleming
References
- 1.Parikh CR, Coca SG, Thiessen-Philbrook H, Shlipak MG, Koyner JL, Wang Z, Edelstein CL, Devarajan P, Patel UD, Zappitelli M, Krawczeski CD, Passik CS, Swaminathan M, Garg AX; TRIBE-AKI Consortium : Postoperative biomarkers predict acute kidney injury and poor outcomes after adult cardiac surgery. J Am Soc Nephrol 22: 1748–1757, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hobson CE, Yavas S, Segal MS, Schold JD, Tribble CG, Layon AJ, Bihorac A: Acute kidney injury is associated with increased long-term mortality after cardiothoracic surgery. Circulation 119: 2444–2453, 2009 [DOI] [PubMed] [Google Scholar]
- 3.Bihorac A, Yavas S, Subbiah S, Hobson CE, Schold JD, Gabrielli A, Layon AJ, Segal MS: Long-term risk of mortality and acute kidney injury during hospitalization after major surgery. Ann Surg 249: 851–858, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Swaminathan M, Shaw AD, Phillips-Bute BG, McGugan-Clark PL, Archer LE, Talbert S, Milano CA, Patel UD, Stafford-Smith M: Trends in acute renal failure associated with coronary artery bypass graft surgery in the United States. Crit Care Med 35: 2286–2291, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Schmitt R, Coca S, Kanbay M, Tinetti ME, Cantley LG, Parikh CR: Recovery of kidney function after acute kidney injury in the elderly: A systematic review and meta-analysis. Am J Kidney Dis 52: 262–271, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Swaminathan M, Hudson CC, Phillips-Bute BG, Patel UD, Mathew JP, Newman MF, Milano CA, Shaw AD, Stafford-Smith M: Impact of early renal recovery on survival after cardiac surgery-associated acute kidney injury. Ann Thorac Surg 89: 1098–1104, 2010 [DOI] [PubMed] [Google Scholar]
- 7.Herrera MB, Bussolati B, Bruno S, Fonsato V, Romanazzi GM, Camussi G: Mesenchymal stem cells contribute to the renal repair of acute tubular epithelial injury. Int J Mol Med 14: 1035–1041, 2004 [PubMed] [Google Scholar]
- 8.Lange C, Tögel F, Ittrich H, Clayton F, Nolte-Ernsting C, Zander AR, Westenfelder C: Administered mesenchymal stem cells enhance recovery from ischemia/reperfusion-induced acute renal failure in rats. Kidney Int 68: 1613–1617, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Tögel F, Hu Z, Weiss K, Isaac J, Lange C, Westenfelder C: Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am J Physiol Renal Physiol 289: F31–F42, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Tögel F, Weiss K, Yang Y, Hu Z, Zhang P, Westenfelder C: Vasculotropic, paracrine actions of infused mesenchymal stem cells are important to the recovery from acute kidney injury. Am J Physiol Renal Physiol 292: F1626–F1635, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Kale S, Karihaloo A, Clark PR, Kashgarian M, Krause DS, Cantley LG: Bone marrow stem cells contribute to repair of the ischemically injured renal tubule. J Clin Invest 112: 42–49, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barnes CJ, Distaso CT, Spitz KM, Verdun VA, Haramati A: Comparison of stem cell therapies for acute kidney injury. Am J Stem Cells 5: 1–10, 2016 [PMC free article] [PubMed] [Google Scholar]
- 13.Tögel FE, Westenfelder C: Mesenchymal stem cells: A new therapeutic tool for AKI. Nat Rev Nephrol 6: 179–183, 2010 [DOI] [PubMed] [Google Scholar]
- 14.Monsel A, Zhu YG, Gennai S, Hao Q, Liu J, Lee JW: Cell-based therapy for acute organ injury: Preclinical evidence and ongoing clinical trials using mesenchymal stem cells. Anesthesiology 121: 1099–1121, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shi Q, Rafii S, Wu MH, Wijelath ES, Yu C, Ishida A, Fujita Y, Kothari S, Mohle R, Sauvage LR, Moore MA, Storb RF, Hammond WP: Evidence for circulating bone marrow-derived endothelial cells. Blood 92: 362–367, 1998 [PubMed] [Google Scholar]
- 16.Orlic D, Kajstura J, Chimenti S, Limana F, Jakoniuk I, Quaini F, Nadal-Ginard B, Bodine DM, Leri A, Anversa P: Mobilized bone marrow cells repair the infarcted heart, improving function and survival. Proc Natl Acad Sci U S A 98: 10344–10349, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gooch A, Doty J, Flores J, Swenson L, Toegel FE, Reiss GR, Lange C, Zander AR, Hu Z, Poole S, Zhang P, Westenfelder C: Initial report on a phase I clinical trial: Prevention and treatment of post-operative acute kidney injury with allogeneic mesenchymal stem cells in patients who require on-pump cardiac surgery. Cell Ther Transplant 1: 31–35, 2008 [Google Scholar]
- 18.Shabbir A, Cox A, Rodriguez-Menocal L, Salgado M, Van Badiavas E: Mesenchymal stem cell exosomes induce proliferation and migration of normal and chronic wound fibroblasts, and enhance angiogenesis in vitro. Stem Cells Dev 24: 1635–1647, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pal R, Gopinath C, Rao NM, Banerjee P, Krishnamoorthy V, Venkataramana NK, Totey S: Functional recovery after transplantation of bone marrow-derived human mesenchymal stromal cells in a rat model of spinal cord injury. Cytotherapy 12: 792–806, 2010 [DOI] [PubMed] [Google Scholar]
- 20.Chen B, Hu J, Liao L, Sun Z, Han Q, Song Z, Zhao RC: Flk-1+ mesenchymal stem cells aggravate collagen-induced arthritis by up-regulating interleukin-6. Clin Exp Immunol 159: 292–302, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nam YS, Kim N, Im KI, Lim JY, Lee ES, Cho SG: Negative impact of bone-marrow-derived mesenchymal stem cells on dextran sulfate sodium-induced colitis. World J Gastroenterol 21: 2030–2039, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nasef A, Ashammakhi N, Fouillard L: Immunomodulatory effect of mesenchymal stromal cells: Possible mechanisms. Regen Med 3: 531–546, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Casiraghi F, Perico N, Cortinovis M, Remuzzi G: Mesenchymal stromal cells in renal transplantation: Opportunities and challenges. Nat Rev Nephrol 12: 241–253, 2016 [DOI] [PubMed] [Google Scholar]
- 24.Chen C, Hou J: Mesenchymal stem cell-based therapy in kidney transplantation. Stem Cell Res Ther 7: 16, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.