Abstract
The concept that individuals with the same disease and a similar clinical presentation may have very different outcomes and need very different therapies is not novel. With the development of many innovative tools derived from the omics technologies, transplant medicine is slowly entering the era of precision medicine. Biomarkers are the cornerstone of precision medicine, which aims to integrate biomarkers with traditional clinical information and tailor medical care to achieve the best outcome for an individual patient. Here, we discuss the basic concepts of precision medicine and biomarkers, with a specific focus on progress in renal transplantation. We delineate the different types of biomarkers and provide a general assessment of the current applications and shortcomings of previously proposed biomarkers. We also outline the potential of precision medicine in transplantation. Moving toward precision medicine in the field of transplantation will require transplant physicians to embrace the increased complexity and expanded decision algorithms and therapeutic options that are associated with improved disease nosology.
Keywords: kidney transplantation, transplant outcomes, acute rejection, chronic allograft failure, chronic graft deterioration
Several millennia ago, Hippocrates had the notion that individuals with a similar clinical presentation of a disease may have very different outcomes and require very different therapies: “It is far more important to know what sort of person the disease has, than what sort of disease the person has.” This makes him the father of precision medicine and illustrates that the quest for precision medicine is of all times.
Biomarkers are the cornerstone of precision medicine, which aims to integrate molecular data (biomarkers) with traditional clinical information to tailor medical care to achieve an individual patient’s best outcome. Although precision medicine is rather proactive, traditional medicine is more reactive (predict and prevent instead of diagnose and treat).
The applications of biomarkers in precision medicine range from evaluating an individual’s disease risk, detecting disease at an early stage, and diagnosing and evaluating a disease’s activity to estimating prognosis, predicting therapy responses or side effects, and guiding drug dosing.
Also, the field of transplantation is slowly entering the era of precision medicine. In this review, we discuss the basic concepts of precision medicine and biomarkers, with a specific focus on progress in renal transplantation.
Innovative Biomarkers in Kidney Transplantation
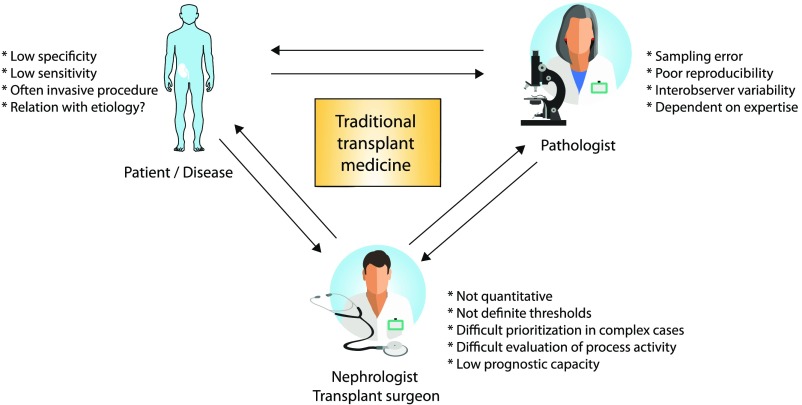
The current clinical routine in kidney transplantation uses an age-old toolbox of markers that primarily include serum creatinine/eGFR, proteinuria, and graft histology, with the more recent adjunction of donor-specific antibody (DSA) monitoring. Despite the clinical usefulness of these markers in clinical transplantation, all of these markers have inherent problems, most importantly low sensitivity and specificity, invasiveness, and clinical problems for interpretation1–4 (Figure 1).
Figure 1.
The shortcomings of currently used biomarkers in transplant medicine.
To overcome the shortcomings of traditional transplant medicine, many candidate biomarkers have been proposed and tested in recent years as summarized extensively in several review articles,5–10 most recently by ourselves (Table 1).11 Rigorous evaluation of novel biomarkers requires a multistep approach that includes a discovery phase followed by various internal and very importantly, external validation methods, taking into account the prevalence of the disease in a real life setting as we outlined in detail in our recent publication.11 In addition, multiple testing should also be taken into account to avoid type 1 error or inflated C statistics. The characteristics and performance of these individual markers are beyond the scope of this review.
Table 1.
Overview of biomarker subtypes and examples for the assessment of kidney allografts
| Biomarker Type | Biomarker Definition | Established Examples in Transplantation | Potential New Examples in Transplantation That Are Insufficiently Validated for Clinical Use |
|---|---|---|---|
| Susceptibility/risk biomarker | A biomarker that indicates the potential for developing a disease, medical condition, or sensitivity to an exposure in an individual without clinically apparent disease or medical condition | Number of HLA mismatches | Epitope mismatch load18 |
| Pretransplant PRA percentage | Urinary or serum suPAR for FSGS recurrence54 | ||
| Pretransplant DSA | FSGS recurrence panel21 | ||
| De novo DSA occurrence | Phospholipase A2 receptor and thrombospondin type 1 domain–containing 7A antibodies for recurrence of membranous glomerulopathy22,23 | ||
| Genetic assessment for atypical hemolytic uremic syndrome recurrence | Donor-reactive T cell response55 | ||
| Diagnostic biomarker | A biomarker used to identify individuals with the disease or condition of interest or define a subset of the disease | Serum creatinine/GFR | Urinary three-gene mRNA expression signature and wide range of other suggested molecules11,31 |
| Proteinuria | Wide range of urinary target proteins, like CXCL10 and CXCL911 | ||
| Hematuria | Blood 17-gene mRNA expression “kSORT”33 | ||
| DSA | Blood 200-gene mRNA expression “TruGraf”32 | ||
| Signs of hemolysis | Several blood and urine miRNAs11 | ||
| Renal ultrasound examination | Molecular microscope for allograft pathologya | ||
| Protocol or for-cause biopsy histologya | |||
| Prognostic biomarker | A biomarker used to identify likelihood of a clinical event, disease recurrence, or progression | Serum creatinine/GFR | Complement-fixing characteristics of DSA39,40 |
| Proteinuria | Edmontona classifier for graft loss42 | ||
| DSA | Edmontona “ABMR molecular score”45 | ||
| Protocol or for-cause biopsy histology (rejection subtype, chronic injury, PVAN stage, etc.)a | GOCARa 13-gene set43 | ||
| Predictive biomarker | A biomarker used to identify individuals who are more likely than similar patients without the biomarker to experience a favorable or unfavorable effect from a specific intervention or exposure | There are currently no established predictive biomarkers proposed for treatment of transplant pathologies | There are currently no new predictive biomarkers proposed in kidney transplantation |
| Suggestions made in the past are complement-fixing characteristics of DSA for use of complement inhibitors (no studies) and intrarenal C4d deposition for use of complement inhibitors (no studies) | |||
| Monitoring biomarker | A biomarker measured serially and used to detect a change in the degree or extent of disease; monitoring biomarkers may also be used to indicate toxicity, assess safety, or provide evidence of exposure, including exposures to medical products | Serum creatinine/GFR | There are currently no new monitoring biomarkers proposed in kidney transplantation |
| Proteinuria | |||
| Hematuria | |||
| Immunosuppressive drug levels | |||
| BKV PCR | |||
| Signs of hemolysis | |||
| Pharmacodynamic/response biomarker | A biomarker used to show that a biologic response has occurred in an individual who has received an intervention or exposure | CD19/CD20 count with rituximab treatment | There are currently no new pharmacodynamic/response biomarkers proposed in kidney transplantation |
| DSA mean fluorescence index after ABMR treatment | |||
| Post-treatment control biopsy histology (resolution of disease and disease activity)a | |||
| Safety biomarker | A biomarker used to indicate the presence or extent of toxicity related to an intervention or exposure | Immunosuppressive drug levels | There are currently no new safety biomarkers proposed in kidney transplantation |
| Peripheral blood cell counts | |||
| Liver tests | |||
| Diabetes occurrence | |||
| Calcineurin inhibitor nephrotoxicity (biopsy histology)a |
Definitions are derived from the Food and Drug Administration/National Institutes of Health Biomarker Working Group.56 This is not an exhaustive list.
Invasive biomarkers.
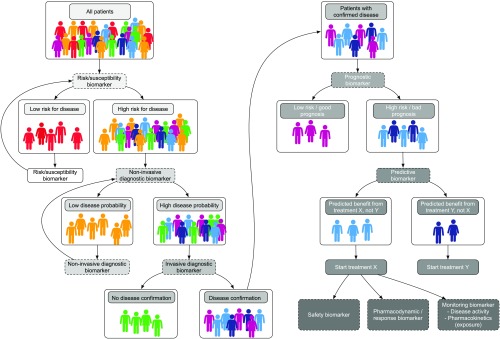
Here, we provide a general assessment of the current applications and shortcomings of previously proposed biomarkers and an outline of the potential of precision medicine in transplantation. It is very important to discriminate between the different applications of biomarkers, which can be used for risk assessment, noninvasive screening and diagnostics, invasive disease confirmation, and prognostic or predictive purposes as outlined in Figures 2 and 3.
Figure 2.
Overview of biomarker subtypes for precision transplant medicine.
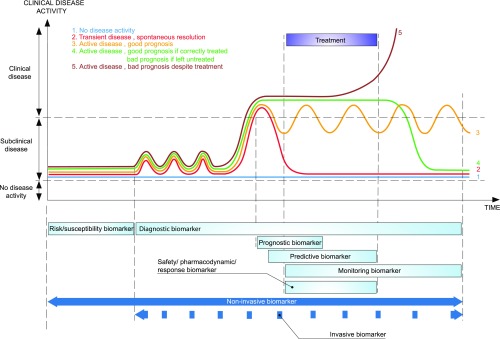
Figure 3.
Time dependency of the different types of biomarkers for precision medicine. Before any disease activity is present, risk/susceptibility biomarkers will facilitate the identification of high-risk patients who require closer follow-up examinations, which are typically performed using noninvasive diagnostic biomarkers. These biomarkers ideally also detect subclinical disease activity before clinical signs or irreversible injury become evident. After a disease process is diagnosed or its activity is assessed, a prognostic biomarker estimates the effect of the disease and the chance of spontaneous resolution versus that of irreversible injury and eventually, graft failure. A prognostic biomarker should be able to identify those patients who need treatment and those patients who will have spontaneous disease resolution or a good prognosis in the absence of therapy. If a patient with disease would have a poor outcome according to the prognostic biomarker, the search for an appropriate therapy can begin. Ideally, this therapy is not solely on the basis of the diagnosis and prognosis of the disease but also, on the basis of predictive biomarkers that predict which treatment has the highest chance of success in reversing the outcome. Disease activity should be assessed over time using monitoring biomarkers, including after therapy cessation. In addition, there are crucial differences in the nature of biomarkers. If repeated biomarker assessment is necessary (e.g., as with diagnostic biomarkers for subclinical disease, safety/pharmacodynamic/response biomarkers, and monitoring biomarkers), ideally, noninvasive tests should be developed and used. For biomarkers that are used for treatment decisions (e.g., whether to start treatment and the type of treatment), such as some diagnostic, prognostic, and predictive biomarkers, noninvasiveness is a plus but is not essential. Important treatment decisions are typically not very repetitive and can be on the basis of invasive biomarker assessments. Thus, invasive biomarkers have very different clinical applications than noninvasive biomarkers.
Risk/Susceptibility Biomarkers
In the field of transplantation, there has long been great interest in defining patients at increased risk of acute rejection or graft failure among other conditions. Typically, this risk is evaluated using not only some demographic determinants (e.g., donor/recipient age, ethnicity, socioeconomic background) but also, widely used markers, such as donor-recipient HLA mismatch and the presence or de novo occurrence of HLA DSAs.12 Despite the great importance of a correct risk assessment, the definition of the high-risk subpopulation in renal transplantation is not well standardized.
A more thorough evaluation of the donor-recipient HLA mismatch (e.g., using epitope mismatch analysis) may provide a more precise immunologic risk assessment. The tools are already available (e.g., HLAMatchmaker and EpViX),13,14 and several small studies suggest that epitope mismatch analysis is capable of identifying high-risk donor-recipient pairs.15–19 However, apart from the evaluation of acceptable mismatches in extremely sensitized patients (e.g., in the Eurotransplant region with the “Acceptable Mismatch” program), epitope mismatching has not yet been translated into a clinical strategy for the overall transplant population, and the potential benefits in risk prediction for T cell–mediated rejection (TCMR) and the occurrence of de novo DSAs remain unexplored.
Additional risk/susceptibility biomarkers that are currently used in clinical practice are derived from increasing insight into the pathophysiology of primary renal diseases. Recent advances in the understanding of the pathogenesis of atypical hemolytic uremic syndrome permit an individualized risk assessment of post-transplant disease recurrence on the basis of the mutations involved in alternative complement pathway dysregulation.20 The impressively improved risk assessment of patients with atypical hemolytic uremic syndrome is truly precision medicine; this application not only uses genetic risk/susceptibility biomarkers but also, illustrates that this strategy can be adopted rapidly in routine clinical practice if the results and outcome improvements are compelling.
Assessing the risk of glomerular disease recurrence after transplantation is less well established. Autoantibodies, including anti-CD40, have been associated with primary FSGS recurrence,21 and phospholipase A2 receptor antibodies and thrombospondin type 1 domain–containing 7A antibodies have been associated with recurrent membranous glomerulopathy.22,23 However, further work is necessary to translate these data and novel insights into clinically useful susceptibility biomarkers.22–27
Next to assessment of the immunologic risk of rejection and the risk of disease recurrence, other widely used risk/susceptibility biomarkers have been found in the field of transplant infectious diseases. Viral disease risk is classically evaluated using serology (e.g., for Epstein–Barr virus and cytomegalovirus [CMV]) and has been more recently evaluated using systematic CMV and BKV PCR assessments. Not only are these virologic tests relevant for disease risk assessments and diagnostic confirmation, viral PCR is typically also used as monitoring biomarkers of ongoing disease and response biomarkers for treatment.28,29
Noninvasive Diagnostic Biomarkers
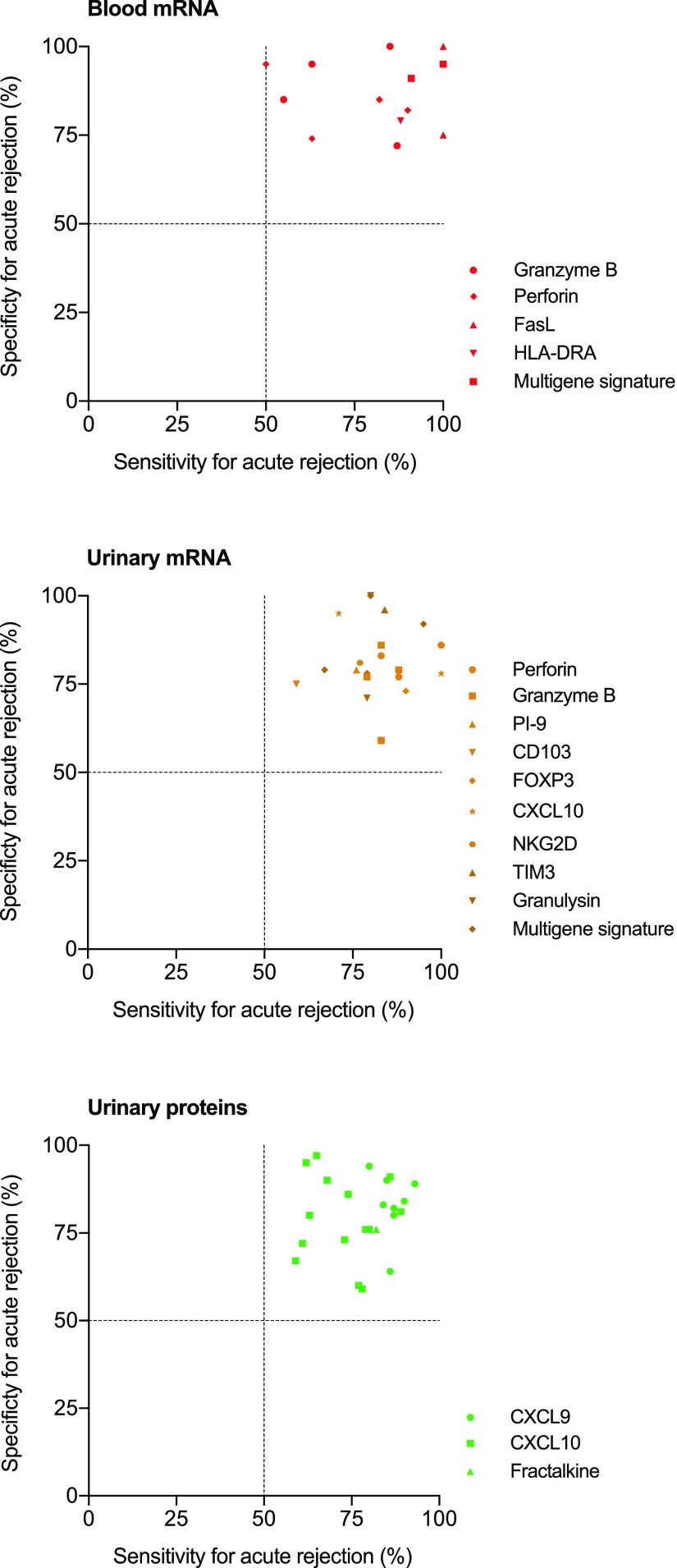
In precision medicine, which tries to be proactive and preventative, repeated assessment of the probability (screening) of ongoing subclinical injury is necessary, especially in higher-risk populations (Figures 2 and 3). The repeated character necessitates noninvasiveness to avoid complications of the screening procedure in itself. As is illustrated in Table 1, many research teams have worked on diagnostic biomarkers for kidney transplant injury, primarily acute rejection. Our recent overview11 discussed the many studies in this domain in great detail. Figure 4 provides a visual overview of the summary table that was provided in this publication.
Figure 4.
Overview of the diagnostic performance (sensitivity and specificity) of the noninvasive diagnostic biomarkers for acute rejection. Modified from ref. 11, with permission.
The vast majority of studies on diagnostic biomarkers focused on single noninvasive blood or urinary molecules for diagnosing ongoing acute rejection. Several molecules were repeatedly assessed as candidate biomarkers, such as urinary CXCL10, perforin, granzyme B and granulysin mRNA, urinary CXCL9 and CXCL10 protein, and blood granzyme B and perforin mRNA. The potential of miRNA molecules in urine and peripheral blood samples for diagnosing acute rejection or allograft dysfunction has been also evaluated.11 The summary of this research, depicted in Figure 4, illustrates that the results of these studies are not consistent. There is much heterogeneity in the diagnostic performance of these biomarkers, which can be partially explained by the low numbers included in these studies and the different phenotypes of the study populations.
Another major drawback of using urinary expression analysis is the difficulty of standardizing the samples,30 because the molecule levels depend on the overall levels of proteinuria and urinary concentration, which depend on fluid intake. Moreover, another important technical limitation is that RNA molecules are not stable in urine, which is exemplified by the fact that only 83% of urine samples passed quality control in a large cohort study.31
The intrinsic difficulties in urinary sampling are potentially less present in peripheral blood sampling. Several studies evaluated the diagnostic performance of granzyme B, perforin, and a few other molecules in single-center studies (Figure 4). Overall, the diagnostic performance remains very inconsistent across these studies, which is likely related to the small cohort sizes and perhaps, the inherent insufficient robustness of the hypothesis-driven markers.11 Overall, the clinical diagnostic value of single biomarkers remains unclear, and large multicenter studies are still needed.
More recent attempts to establish blood-based multigene diagnostic biomarkers for acute rejection could perhaps become more successful. Two groups32,33 recently developed interesting but insufficiently validated peripheral blood gene expression tests (the TruGraf and kSORT tests, respectively; details are in our recent overview11). These tests are now being developed commercially, although further validation in prospectively collected and unselected samples is warranted for the further development of these diagnostic biomarkers.
A major issue regarding the recently proposed noninvasive markers for acute rejection is the fact that these markers are essentially developed for the kidney transplant population as a whole. The low risk of acute rejection automatically leads to low PPVs and high NPVs.5,11 A better development strategy could be to restrict the use and validation of these noninvasive diagnostic biomarkers to the subgroup of patients at a higher immunologic risk (e.g., patients with DSAs). This is the patient population with the highest medical need and most likely to benefit from improved monitoring and early diagnosis, primarily of antibody-mediated rejection. Studying candidate biomarkers in higher-risk individuals will automatically increase the PPVs to desirably higher levels. A high NPV is useful in clinical practice to rule out disease activity but not if the chance of the disease is intrinsically very low. The development of noninvasive diagnostics should, therefore, go along with the establishment of advanced risk assessment as outlined above.
This brings us to another drawback of the currently used noninvasive diagnostic biomarkers for rejection. None of these biomarkers were developed specifically for ABMR, and most studies did not even make a distinction between TCMR and ABMR. The number of patients with ABMR is likely very low in these studies, which calls into question the validity of the markers for this highly relevant injury process. The development of biomarkers for patients at high risk of ABMR using molecules able to diagnose subclinical and active ABMR is urgently needed to improve patient outcomes.
Invasive Diagnostic Biomarkers for Disease Reclassification
In the precision medicine scheme (Figure 2), invasive diagnostics are often necessary to confirm the diagnosis in patients with cases in which noninvasive diagnostics show a high probability of active disease. Also, when there are no sensitive noninvasive markers (e.g., in kidney transplantation), invasive biomarkers are necessary to detect subclinical disease (e.g., protocol biopsies). In addition, invasive diagnostics help in refining the exact diagnosis (e.g., discriminating TCMR from ABMR), which is often not possible with the noninvasive markers.
The Banff consensus on renal transplant pathology typically discusses the invasive diagnostic criteria of transplant pathology and includes sufficiently validated markers in updates of the Banff classification, including molecular diagnostics.34,35 Until today, the evolving Banff consensus was mainly reached through clinicopathologic studies describing the association between histologic features and clinical outcomes. Since the conception of the Banff system >20 years ago in 1993, very few histologic markers have been added to the Banff scheme. Clearly, this strategy has been very successful in describing the phenotype of antibody-mediated rejection in the past decade, but other more common inflammatory processes (tubulitis, interstitial inflammation, inflammation in areas of atrophy, etc.) remain ill defined. The complexity of the scheme for antibody-mediated rejection illustrates that the Banff classification is reaching its limits, and important further progress cannot be expected from this very descriptive approach of linking histologic changes to clinical end points.
The associations of histologic and intrarenal mRNA expression changes with transplant outcomes have been extensively studied to improve the classification of transplant injuries.36,37 This research led to the suggestion that intrarenal mRNA markers of endothelial activation could also be used as diagnostic criteria in the Banff classification for ABMR,38 although it is acknowledged that these diagnostic biomarkers require additional validation before being clinically used.34,35 The potential application of intrarenal mRNA expression analysis for the diagnosis of endothelial activation or other markers of ongoing disease activity is currently limited to a small number of centers, but it is likely to become more important as the molecular analysis of biopsy tissue becomes more technically and economically feasible.
The approach to enrich histologic diagnoses with molecular data (“molecular microscope”) has several benefits, including increased reproducibility, probabilistic data, and fewer problems with sampling error and tissue heterogeneity.36,37 However, extensive validation studies are necessary to show that the molecular biomarker approach is better for diagnostics than the current gold standard histologic assessments performed by expert pathologists. The clinical usefulness of this approach will be shown when molecular diagnosis improves the diagnostic accuracy, with expert histologic classification as the gold standard (in comparison with local, nonexpert pathology), or when molecular diagnosis improves the (prediction of) patient outcomes.
This topic relates to a crucial characteristic of disease reclassification: reclassification should translate into either a more accurate assessment of outcomes (better prognostic capacity) or a better assessment of treatment opportunities (better predictive capacity). The introduction of new disease classifiers in the Banff classification (C4d deposition, DSA presence, and peritubular capillaritis score) was preceded by studies illustrating that these parameters were independently associated with outcome. The association of a marker with outcome could make it a useful diagnostic addition; however, the significant association of a marker with outcome does not automatically make it a useful prognostic biomarker, which we outline below.
Prognostic Biomarkers: Treat or Do Not Treat?
After having established a diagnosis using noninvasive and invasive disease classification tools, it is necessary to elucidate the prognosis of the disease (Figure 2). A good prognostic biomarker requires more than just an association with outcome. A prognostic biomarker should provide an improved assessment of which patients with the disease will have a poor outcome without treatment and thus, need specific treatment. Vice versa, a prognostic biomarker should provide information regarding which patients and disease subtypes have a good prognosis, even without treatment, to avoid treatment (and related side effects). Therefore, a prognostic biomarker helps answer the following question: “treat or do not treat?”
Currently used prognostic biomarkers in kidney transplantation are serum creatinine/eGFR, proteinuria, DSA, and of course, graft histology with the updated Banff classification. Each of these markers is associated with graft outcome and currently used in routine clinical practice for not only diagnosis but also, decisions on which patients need treatment for, for example, rejection or recurrent glomerular disease. As discussed above, the current Banff classification integrates prognostic aspects in the diagnostic framework and is, therefore, partly prognostic. This is clearly the case for ABMR, but other diagnoses (like TCMR) lack this prognostic capacity and need attention in future updates of the Banff consensus. Multidimensional clinicopathologic prognostic algorithms (integrating histologic lesions with graft functional parameters and antibody status) could be constructed and become useful for clinical decision making, but they are currently not available. Previous efforts at developing prognostic systems on the basis of combinations of functional and histologic parameters have been hampered by small sample sizes, absence of proper validation, and limited phenotypic detail.
The complement binding capacity of DSA, determined using C1q or C3d flow bead assays, has recently been suggested as a prognostic biomarker. In patients with DSA, a positive test identified those patients at an increased risk of graft failure with moderate accuracy, whereas patients with a negative test did not have this increased risk.39–41 The discussion of whether the complement binding characteristics are relevant independent of the mean fluorescence index is not yet closed, and it remains unclear whether the test improves prognostic accuracy in patients who are actually diagnosed with ABMR. Further evaluation of this potential is necessary before the complement binding capacity of DSA could become a clinically accessible prognostic biomarker.
Several other prognostic markers have been proposed. A few years ago, Einecke et al.42 described a biopsy-based “molecular classifier” for graft failure at the time of graft dysfunction (i.e., at the time of a for-cause biopsy). The test was not further validated or developed as a biomarker, and it remains unclear how the test relates to the histologic diagnosis of a biopsy, complicating the assessment of its clinical utility as a prognostic marker in conjunction with standard routine markers and histologic data.
More recently, the GoCAR Consortium proposed a 13-gene expression panel measured in mRNA extracted from post-transplant biopsies at 3 months as a prognostic biomarker with a good ROC AUC of 0.81–0.87.43 Apart from the necessity for further and independent validation, problems for this prognostic test are that it remains unclear how the 13-gene set relates to diagnostic categories and whether this biomarker is also of value at the time of, for example, TCMR or ABMR diagnosis. In other words, will this biomarker be helpful in deciding which patients need treatment for the diagnosed disease process? Further research is necessary to answer this pertinent question.44
Few studies have evaluated prognostic tools for patients who have already received a specific diagnosis, such as ABMR. The team in Edmonton built a biopsy mRNA-based “ABMR molecular score” that improved the stratification of patients at high risk for graft loss both in addition to and compared with conventional (histologic) assessments in patients with kidney transplants and ABMR.45,46 Although the significant improvement in stratification that seems to be offered by this test is promising, the actual prognostic performance of this test (PPV and NPV for graft failure in patients diagnosed with ABMR) has not yet been evaluated. Extensive further validation and assessment of the potential clinical usefulness of the ABMR molecular score are necessary.
Finally, it is remarkable that, for most other potentially reversible transplant pathologies, such as TCMR, polyomavirus nephropathy, and de novo or recurrent glomerulopathy, no prognostic tools have been proposed to decide which patients could benefit from specific treatment.
In clinical trials, prognostic biomarkers can be used to assess the effect of an intervention on clinical outcome. The use of these so-called surrogate markers as replacement end points for clinically meaningful end points may be of interest to reduce the size and duration of clinical trials.47 In transplantation, if many clinical parameters (e.g., delayed graft function and acute TCMR) serve as qualitative parameters in evaluating patient or graft outcome, their performance as surrogate markers for outcome is either poorly evaluated or critically depends on the context in which they appear.48,49 A good prognostic marker does not necessarily translate into a valid surrogate end point accepted for drug approval by health authorities, which was detailed in the viewpoint of Schold and Kaplan.50
Predictive Biomarkers = Which Treatment?
The difference between a prognostic biomarker and a predictive biomarker could, at first, seem to be a semantic difference, but it is not (Figures 2 and 3). A prognostic biomarker identifies the likelihood of a clinical event, such as disease recurrence or progression (e.g., the likelihood of graft failure). A predictive biomarker is used to identify individuals who are more likely than similar patients without the biomarker to experience a favorable or unfavorable effect from a specific intervention or exposure. A predictive biomarker facilitates decisions regarding which therapeutic intervention would help improve the outcome of a disease.
An example of a predictive marker in transplant infectious diseases is CMV genotyping to determine viral mutations that cause resistance to antiviral agents, which guides the choice of second-line treatments for ganciclovir-resistant CMV infections.51
However, for renal allograft pathologies, no predictive biomarkers are available to facilitate treatment decisions. After the diagnosis of, for example, ABMR, TCMR, or PVAN is made, treatment decisions are on the basis of the diagnosis and prognosis but not on the basis of predictive tests. A predictive marker that could guide therapeutic decisions would be very useful in diseases where there is substantial heterogeneity in the responses to treatment, such as is the case in allograft rejection.
Predictive markers that have recently been suggested in kidney transplantation but that lack supportive data are the complement binding capacity of DSA or C4d deposition in peritubular capillaries as a marker of complement activation for facilitating decisions regarding the use of complement inhibitors to treat ABMR.
Monitoring Biomarkers: Treatment Success or Failure?
Monitoring biomarkers are serially measured and used to detect a change in the degree or extent of disease after a diagnosis is made. Monitoring markers can provide information on when to stop treatment, whether to continue treatment, and when treatment is no longer efficacious. The frequent serial measurement of monitoring markers is only feasible if these markers are noninvasive. Monitoring biomarkers that are currently used in clinical kidney transplantation are serum creatinine/eGFR, proteinuria, hematuria, signs of intravascular hemolysis, and BKV viral load.
The problem with using these markers for monitoring is their nonspecificity for renal pathology and their poor sensitivity for subclinical ongoing disease; thus, they are intrinsically insufficient for assessing disease activity.
To date, no specific and sensitive disease activity monitoring markers have been developed for use in kidney transplantation. Potential markers, such as the blood-based diagnostic biomarkers kSORT and TruGraf or urinary profiling (see above),31–33 require much more work in terms of studying their kinetics over time, including during and after treatment, before they can be proposed as monitoring markers.
Pharmacodynamic/Response Biomarkers and Safety Biomarkers: What Dose?
Monitoring biomarkers may also be used to indicate toxicity, assess safety, or provide evidence of exposure, including exposure to medical products. These markers are often more specifically called “safety biomarkers” or “pharmacokinetics/pharmacodynamics biomarkers.”
In transplantation, because of the narrow therapeutic index of most immunosuppressive agents and because of the nephrotoxicity of, for example, calcineurin inhibitors, these markers are widely used and comprise peripheral blood drug-level monitoring, peripheral blood cell counts, liver function tests, and follow-up tests for glycemia, BP, cholesterol level, and kidney function.
Clearly, monitoring these biomarkers is essential after transplantation. However, apart from renal function assessments, there are no biomarkers for calcineurin inhibitor nephrotoxicity, and even the histologic diagnosis of this entity remains cumbersome given the lack of lesion specificity.52 A comprehensive review of the side effects and monitoring of immunosuppressive agents is beyond the scope of this review but can be found elsewhere.53
Conclusion
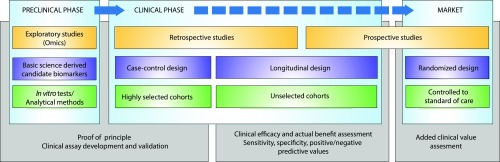
The development of biomarkers and precision medicine remains a vast task, and transplant physicians are now facing an increasing number of new, innovative tools entering the market. Surprisingly, very few of them have been or will be truly implemented in the clinic. Our view is that the lack of validation (the discovery step often inflates predictive values) and the lack of demonstrations of improved clinical outcomes for individual patients are the main driving forces for the lack of adoption by physicians and/or funders. Figure 5 illustrates the complexity of and the ideal pipeline for developing clinically usable and useful biomarkers in renal transplantation.
Figure 5.
Ideal pipeline for developing clinically usable and useful biomarkers in renal transplantation. The clinical assessment of medical devices is on the basis of completely different methods from those applied to medicinal products; consequently, new tools might be marketed after a very limited validation process, which may alter the capacity of the medical device to be reimbursed by National Health Insurance, with reimbursement being guided on the basis of assessments of the actual benefit and the added clinical value. Therefore, a strong multistep validation is required to avoid concerns that new diagnostic tests may increase health care expenditures and skepticism about their benefits in terms of improving clinical management.
Increasing the complexity of transplant medicine may also limit the enthusiasm of transplant physicians for using innovative biomarkers. Nevertheless, because we need to move toward precision medicine in the field of kidney transplantation, we are to embrace the increased complexity and the expanded decision algorithms and therapeutic options that are associated with improved disease nosology.
Disclosures
None.
Acknowledgments
M.N. is supported by grants TBM-150199 and 130758 from The Research Foundation, Flanders and grant IWT-130758 from Flanders Innovation and Entrepreneurship (Agentschap Innoveren en Ondernemen) of the Flemish Government. D.A. is supported by the Emmanuel Boussard Foundation and the Day Solvay Foundation. This work is part of the BIOMARGIN (BIOMArkers of Renal Graft INjuries) European Research Network (Collaborative Project) supported by European Commission grant 305499 under the Health Cooperation Work Programme of the Seventh Framework Programme.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Hariharan S, McBride MA, Cherikh WS, Tolleris CB, Bresnahan BA, Johnson CP: Post-transplant renal function in the first year predicts long-term kidney transplant survival. Kidney Int 62: 311–318, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Naesens M, Lerut E, Emonds MP, Herelixka A, Evenepoel P, Claes K, Bammens B, Sprangers B, Meijers B, Jochmans I, Monbaliu D, Pirenne J, Kuypers DR: Proteinuria as a noninvasive marker for renal allograft histology and failure: An Observational Cohort study. J Am Soc Nephrol 27: 281–292, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaplan B, Schold J, Meier-Kriesche HU: Poor predictive value of serum creatinine for renal allograft loss. Am J Transplant 3: 1560–1565, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Lefaucheur C, Loupy A, Hill GS, Andrade J, Nochy D, Antoine C, Gautreau C, Charron D, Glotz D, Suberbielle-Boissel C: Preexisting donor-specific HLA antibodies predict outcome in kidney transplantation. J Am Soc Nephrol 21: 1398–1406, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lo DJ, Kaplan B, Kirk AD: Biomarkers for kidney transplant rejection. Nat Rev Nephrol 10: 215–225, 2014 [DOI] [PubMed] [Google Scholar]
- 6.Amrouche L, Rabant M, Anglicheau D: MicroRNAs as biomarkers of graft outcome. Transplant Rev (Orlando) 28: 111–118, 2014 [DOI] [PubMed] [Google Scholar]
- 7.Naesens M, Sarwal MM: Molecular diagnostics in transplantation. Nat Rev Nephrol 6: 614–628, 2010 [DOI] [PubMed] [Google Scholar]
- 8.van de Vrie M, Deegens JK, Eikmans M, van der Vlag J, Hilbrands LB: Urinary microRNA as biomarker in renal transplantation. Am J Transplant 17: 1160–1166, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heidt S, San Segundo D, Shankar S, Mittal S, Muthusamy AS, Friend PJ, Fuggle SV, Wood KJ: Peripheral blood sampling for the detection of allograft rejection: Biomarker identification and validation. Transplantation 92: 1–9, 2011 [DOI] [PubMed] [Google Scholar]
- 10.Ho J, Rush DN, Nickerson PW: Urinary biomarkers of renal transplant outcome. Curr Opin Organ Transplant 20: 476–481, 2015 [DOI] [PubMed] [Google Scholar]
- 11.Anglicheau D, Naesens M, Essig M, Gwinner W, Marquet P: Establishing biomarkers in transplant medicine: A critical review of current approaches. Transplantation 100: 2024–2038, 2016 [DOI] [PubMed] [Google Scholar]
- 12.Lebranchu Y, Baan C, Biancone L, Legendre C, Morales JM, Naesens M, Thomusch O, Friend P: Pretransplant identification of acute rejection risk following kidney transplantation. Transpl Int 27: 129–138, 2014 [DOI] [PubMed] [Google Scholar]
- 13.Anunciação FA, Sousa LC, da Silva AS, Marroquim MS, Coelho AG, Willcox GH, de Andrade JM, Corrêa BM, Guimarães EL, do Monte SJ: EpViX: A cloud-based tool for epitope reactivity analysis and epitope virtual crossmatching to identify low immunologic risk donors for sensitized recipients. Transpl Immunol 33: 153–158, 2015 [DOI] [PubMed] [Google Scholar]
- 14.Duquesnoy RJ: Should epitope-based HLA compatibility be used in the kidney allocation system? Hum Immunol 78: 24–29, 2017 [DOI] [PubMed] [Google Scholar]
- 15.Dankers MK, Witvliet MD, Roelen DL, de Lange P, Korfage N, Persijn GG, Duquesnoy R, Doxiadis II, Claas FH: The number of amino acid triplet differences between patient and donor is predictive for the antibody reactivity against mismatched human leukocyte antigens. Transplantation 77: 1236–1239, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Kosmoliaptsis V, Mallon DH, Chen Y, Bolton EM, Bradley JA, Taylor CJ: Alloantibody responses after renal transplant failure can be better predicted by donor-recipient HLA amino acid sequence and physicochemical disparities than conventional HLA matching. Am J Transplant 16: 2139–2147, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sapir-Pichhadze R, Tinckam K, Quach K, Logan AG, Laupacis A, John R, Beyene J, Kim SJ: HLA-DR and -DQ eplet mismatches and transplant glomerulopathy: A nested case-control study. Am J Transplant 15: 137–148, 2015 [DOI] [PubMed] [Google Scholar]
- 18.Wiebe C, Nickerson P: Strategic use of epitope matching to improve outcomes. Transplantation 100: 2048–2052, 2016 [DOI] [PubMed] [Google Scholar]
- 19.Wiebe C, Pochinco D, Blydt-Hansen TD, Ho J, Birk PE, Karpinski M, Goldberg A, Storsley LJ, Gibson IW, Rush DN, Nickerson PW: Class II HLA epitope matching-A strategy to minimize de novo donor-specific antibody development and improve outcomes. Am J Transplant 13: 3114–3122, 2013 [DOI] [PubMed] [Google Scholar]
- 20.Zuber J, Le Quintrec M, Morris H, Frémeaux-Bacchi V, Loirat C, Legendre C: Targeted strategies in the prevention and management of atypical HUS recurrence after kidney transplantation. Transplant Rev (Orlando) 27: 117–125, 2013 [DOI] [PubMed] [Google Scholar]
- 21.Delville M, Sigdel TK, Wei C, Li J, Hsieh SC, Fornoni A, Burke GW, Bruneval P, Naesens M, Jackson A, Alachkar N, Canaud G, Legendre C, Anglicheau D, Reiser J, Sarwal MM: A circulating antibody panel for pretransplant prediction of FSGS recurrence after kidney transplantation. Sci Transl Med 6: 256ra136, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sprangers B, Kuypers DR: Recurrence of glomerulonephritis after renal transplantation. Transplant Rev (Orlando) 27: 126–134, 2013 [DOI] [PubMed] [Google Scholar]
- 23.Tomas NM, Hoxha E, Reinicke AT, Fester L, Helmchen U, Gerth J, Bachmann F, Budde K, Koch-Nolte F, Zahner G, Rune G, Lambeau G, Meyer-Schwesinger C, Stahl RA: Autoantibodies against thrombospondin type 1 domain-containing 7A induce membranous nephropathy. J Clin Invest 126: 2519–2532, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barbour S, Gill JS: Advances in the understanding of complement mediated glomerular disease: Implications for recurrence in the transplant setting. Am J Transplant 15: 312–319, 2015 [DOI] [PubMed] [Google Scholar]
- 25.Cook HT, Pickering MC: Histopathology of MPGN and C3 glomerulopathies. Nat Rev Nephrol 11: 14–22, 2015 [DOI] [PubMed] [Google Scholar]
- 26.Floege J, Amann K: Primary glomerulonephritides. Lancet 387: 2036–2048, 2016 [DOI] [PubMed] [Google Scholar]
- 27.Wyld ML, Chadban SJ: Recurrent IgA nephropathy after kidney transplantation. Transplantation 100: 1827–1832, 2016 [DOI] [PubMed] [Google Scholar]
- 28.Kotton CN: Management of cytomegalovirus infection in solid organ transplantation. Nat Rev Nephrol 6: 711–721, 2010 [DOI] [PubMed] [Google Scholar]
- 29.Kuypers DR: Management of polyomavirus-associated nephropathy in renal transplant recipients. Nat Rev Nephrol 8: 390–402, 2012 [DOI] [PubMed] [Google Scholar]
- 30.Galichon P, Amrouche L, Hertig A, Brocheriou I, Rabant M, Xu-Dubois YC, Ouali N, Dahan K, Morin L, Terzi F, Rondeau E, Anglicheau D: Urinary mRNA for the diagnosis of renal allograft rejection: The issue of normalization. Am J Transplant 16: 3033–3040, 2016 [DOI] [PubMed] [Google Scholar]
- 31.Suthanthiran M, Schwartz JE, Ding R, Abecassis M, Dadhania D, Samstein B, Knechtle SJ, Friedewald J, Becker YT, Sharma VK, Williams NM, Chang CS, Hoang C, Muthukumar T, August P, Keslar KS, Fairchild RL, Hricik DE, Heeger PS, Han L, Liu J, Riggs M, Ikle DN, Bridges ND, Shaked A; Clinical Trials in Organ Transplantation 04 (CTOT-04) Study Investigators : Urinary-cell mRNA profile and acute cellular rejection in kidney allografts. N Engl J Med 369: 20–31, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kurian SM, Williams AN, Gelbart T, Campbell D, Mondala TS, Head SR, Horvath S, Gaber L, Thompson R, Whisenant T, Lin W, Langfelder P, Robison EH, Schaffer RL, Fisher JS, Friedewald J, Flechner SM, Chan LK, Wiseman AC, Shidban H, Mendez R, Heilman R, Abecassis MM, Marsh CL, Salomon DR: Molecular classifiers for acute kidney transplant rejection in peripheral blood by whole genome gene expression profiling. Am J Transplant 14: 1164–1172, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roedder S, Sigdel T, Salomonis N, Hsieh S, Dai H, Bestard O, Metes D, Zeevi A, Gritsch A, Cheeseman J, Macedo C, Peddy R, Medeiros M, Vincenti F, Asher N, Salvatierra O, Shapiro R, Kirk A, Reed EF, Sarwal MM: The kSORT assay to detect renal transplant patients at high risk for acute rejection: Results of the multicenter AART study. PLoS Med 11: e1001759, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haas M, Sis B, Racusen LC, Solez K, Glotz D, Colvin RB, Castro MC, David DS, David-Neto E, Bagnasco SM, Cendales LC, Cornell LD, Demetris AJ, Drachenberg CB, Farver CF, Farris AB 3rd, Gibson IW, Kraus E, Liapis H, Loupy A, Nickeleit V, Randhawa P, Rodriguez ER, Rush D, Smith RN, Tan CD, Wallace WD, Mengel M; Banff meeting report writing committee : Banff 2013 meeting report: Inclusion of c4d-negative antibody-mediated rejection and antibody-associated arterial lesions. Am J Transplant 14: 272–283, 2014 [DOI] [PubMed] [Google Scholar]
- 35.Loupy A, Haas M, Solez K, Racusen L, Glotz D, Seron D, Nankivell BJ, Colvin RB, Afrouzian M, Akalin E, Alachkar N, Bagnasco S, Becker JU, Cornell L, Drachenberg C, Dragun D, De Kort H, Gibson IW, Kraus ES, Lefaucheur C, Legendre C, Liapis H, Muthukumar T, Nickeleit V, Orandi B, Park W, Rabant M, Randhawa P, Reed EF, Roufosse C, Seshan SV, Sis B, Singh HK, Schinstock C, Tambur A, Zeevi A, Mengel M: The Banff 2015 kidney meeting report: Current challenges in rejection classification and prospects for adopting molecular pathology. Am J Transplant 17: 28–41, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Halloran PF, Famulski KS, Reeve J: Molecular assessment of disease states in kidney transplant biopsy samples. Nat Rev Nephrol 12: 534–548, 2016 [DOI] [PubMed] [Google Scholar]
- 37.Halloran PF, Reeve J, Akalin E, Aubert O, Bohmig GA, Brennan D, Bromberg J, Einecke G, Eskandary F, Gosset C, Duong Van Huyen JP, Gupta G, Lefaucheur C, Malone A, Mannon RB, Seron D, Sellares J, Weir M, Loupy A: Real time central assessment of kidney transplant indication biopsies by microarrays: The INTERCOMEX study [published online ahead of print April 27, 2017]. Am J Transplant 10.1111/ajt.14329 [DOI] [PubMed] [Google Scholar]
- 38.Sis B, Mengel M, Haas M, Colvin RB, Halloran PF, Racusen LC, Solez K, Baldwin WM 3rd, Bracamonte ER, Broecker V, Cosio F, Demetris AJ, Drachenberg C, Einecke G, Gloor J, Glotz D, Kraus E, Legendre C, Liapis H, Mannon RB, Nankivell BJ, Nickeleit V, Papadimitriou JC, Randhawa P, Regele H, Renaudin K, Rodriguez ER, Seron D, Seshan S, Suthanthiran M, Wasowska BA, Zachary A, Zeevi A: Banff ’09 meeting report: Antibody mediated graft deterioration and implementation of Banff working groups. Am J Transplant 10: 464–471, 2010 [DOI] [PubMed] [Google Scholar]
- 39.Loupy A, Lefaucheur C, Vernerey D, Prugger C, Duong van Huyen JP, Mooney N, Suberbielle C, Frémeaux-Bacchi V, Méjean A, Desgrandchamps F, Anglicheau D, Nochy D, Charron D, Empana JP, Delahousse M, Legendre C, Glotz D, Hill GS, Zeevi A, Jouven X: Complement-binding anti-HLA antibodies and kidney-allograft survival. N Engl J Med 369: 1215–1226, 2013 [DOI] [PubMed] [Google Scholar]
- 40.Sicard A, Ducreux S, Rabeyrin M, Couzi L, McGregor B, Badet L, Scoazec JY, Bachelet T, Lepreux S, Visentin J, Merville P, Fremeaux-Bacchi V, Morelon E, Taupin JL, Dubois V, Thaunat O: Detection of C3d-binding donor-specific anti-HLA antibodies at diagnosis of humoral rejection predicts renal graft loss. J Am Soc Nephrol 26: 457–467, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Viglietti D, Lefaucheur C, Glotz D: Evidence for an important role of both complement-binding and noncomplement-binding donor-specific antibodies in renal transplantation. Curr Opin Organ Transplant 21: 433–440, 2016 [DOI] [PubMed] [Google Scholar]
- 42.Einecke G, Reeve J, Sis B, Mengel M, Hidalgo L, Famulski KS, Matas A, Kasiske B, Kaplan B, Halloran PF: A molecular classifier for predicting future graft loss in late kidney transplant biopsies. J Clin Invest 120: 1862–1872, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O’Connell PJ, Zhang W, Menon MC, Yi Z, Schröppel B, Gallon L, Luan Y, Rosales IA, Ge Y, Losic B, Xi C, Woytovich C, Keung KL, Wei C, Greene I, Overbey J, Bagiella E, Najafian N, Samaniego M, Djamali A, Alexander SI, Nankivell BJ, Chapman JR, Smith RN, Colvin R, Murphy B: Biopsy transcriptome expression profiling to identify kidney transplants at risk of chronic injury: A multicentre, prospective study. Lancet 388: 983–993, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yazdani S, Naesens M: Foretelling graft outcome by molecular evaluation of renal allograft biopsies: The GoCAR study. Transplantation 101: 5–7, 2017 [DOI] [PubMed] [Google Scholar]
- 45.Loupy A, Lefaucheur C, Vernerey D, Chang J, Hidalgo LG, Beuscart T, Verine J, Aubert O, Dubleumortier S, Duong van Huyen JP, Jouven X, Glotz D, Legendre C, Halloran PF: Molecular microscope strategy to improve risk stratification in early antibody-mediated kidney allograft rejection. J Am Soc Nephrol 25: 2267–2277, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sellarés J, Reeve J, Loupy A, Mengel M, Sis B, Skene A, de Freitas DG, Kreepala C, Hidalgo LG, Famulski KS, Halloran PF: Molecular diagnosis of antibody-mediated rejection in human kidney transplants. Am J Transplant 13: 971–983, 2013 [DOI] [PubMed] [Google Scholar]
- 47.Fleming TR, Powers JH: Biomarkers and surrogate endpoints in clinical trials. Stat Med 31: 2973–2984, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Naesens M, Lerut E: Belatacept and long-term outcomes in kidney transplantation. N Engl J Med 374: 2598–2599, 2016 [DOI] [PubMed] [Google Scholar]
- 49.Vincenti F, Rostaing L, Grinyo J, Rice K, Steinberg S, Gaite L, Moal MC, Mondragon-Ramirez GA, Kothari J, Polinsky MS, Meier-Kriesche HU, Munier S, Larsen CP: Belatacept and long-term outcomes in kidney transplantation. N Engl J Med 374: 333–343, 2016 [DOI] [PubMed] [Google Scholar]
- 50.Schold JD, Kaplan B: The elephant in the room: Failings of current clinical endpoints in kidney transplantation. Am J Transplant 10: 1163–1166, 2010 [DOI] [PubMed] [Google Scholar]
- 51.Kotton CN, Kumar D, Caliendo AM, Asberg A, Chou S, Danziger-Isakov L, Humar A; Transplantation Society International CMV Consensus Group : Updated international consensus guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation 96: 333–360, 2013 [DOI] [PubMed] [Google Scholar]
- 52.Naesens M, Kuypers DR, Sarwal M: Calcineurin inhibitor nephrotoxicity. Clin J Am Soc Nephrol 4: 481–508, 2009 [DOI] [PubMed] [Google Scholar]
- 53.Wiseman AC: Immunosuppressive medications. Clin J Am Soc Nephrol 11: 332–343, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Franco Palacios CR, Lieske JC, Wadei HM, Rule AD, Fervenza FC, Voskoboev N, Garovic VD, Zand L, Stegall MD, Cosio FG, Amer H: Urine but not serum soluble urokinase receptor (suPAR) may identify cases of recurrent FSGS in kidney transplant candidates. Transplantation 96: 394–399, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Crespo E, Bestard O: Biomarkers to assess donor-reactive T-cell responses in kidney transplant patients. Clin Biochem 49: 329–337, 2016 [DOI] [PubMed] [Google Scholar]
- 56.FDA-NIH Biomarker Working Group: BEST (Biomarkers, EndpointS, and Other Tools) Resource edited by the Food and Drug Administration (Silver Spring, MD) and the National Institutes of Health (Bethesda, MD), 2016. Available at: https://www.ncbi.nlm.nih.gov/books/NBK326791/. Accessed April 28, 2016 [PubMed] [Google Scholar]