Abstract
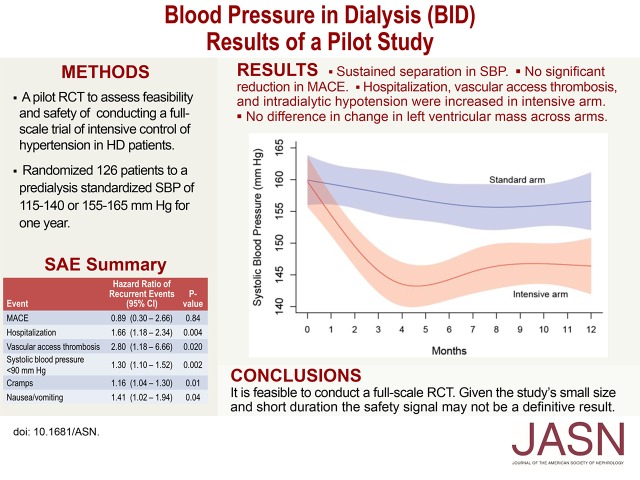
The optimal BP target for patients receiving hemodialysis is unknown. We randomized 126 hypertensive patients on hemodialysis to a standardized predialysis systolic BP of 110–140 mmHg (intensive arm) or 155–165 mmHg (standard arm). The primary objectives were to assess feasibility and safety and inform the design of a full-scale trial. A secondary objective was to assess changes in left ventricular mass. Median follow-up was 365 days. In the standard arm, the 2-week moving average systolic BP did not change significantly during the intervention period, but in the intensive arm, systolic BP decreased from 160 mmHg at baseline to 143 mmHg at 4.5 months. From months 4–12, the mean separation in systolic BP between arms was 12.9 mmHg. Four deaths occurred in the intensive arm and one death occurred in the standard arm. The incidence rate ratios for the intensive compared with the standard arm (95% confidence intervals) were 1.18 (0.40 to 3.33), 1.61 (0.87 to 2.97), and 3.09 (0.96 to 8.78) for major adverse cardiovascular events, hospitalizations, and vascular access thrombosis, respectively. The intensive and standard arms had similar median changes (95% confidence intervals) in left ventricular mass of −0.84 (−17.1 to 10.0) g and 1.4 (−11.6 to 10.4) g, respectively. Although we identified a possible safety signal, the small size and short duration of the trial prevent definitive conclusions. Considering the high risk for major adverse cardiovascular events in patients receiving hemodialysis, a full-scale trial is needed to assess potential benefits of intensive hypertension control in this population.
Keywords: hemodialysis, hypertension, randomized controlled trials, blood pressure
The Kidney Disease Outcomes Quality Initiative guideline recommending a predialysis systolic BP (SBP) <140 mmHg in patients receiving hemodialysis (HD)1 is on the basis of expert opinion.2 Although hypertension control decreases mortality in the general population,3 observational studies in patients on HD have found increased mortality among those with SBP≤140 mmHg.4–9 Foley et al.10 and the Frequent Hemodialysis Network (FHN) Study Group reported that a decrease in SBP was associated with a decrease in left ventricular mass (LVM).11 However, reducing predialysis SBP may increase the frequency of intradialytic hypotension (IDH),12,13 major adverse cardiovascular events (MACE),14,15 and vascular access thromboses (VAT).16
In a meta-analysis of randomized, controlled trials (RCT) in patients receiving dialysis, antihypertensive therapy was associated with improved survival, although the pooled decline in SBP was only 5 mmHg.17 In the Dry Weight Reduction in Hypertensive Hemodialysis Patients (DRIP) Study, a 1 kg decrease in mean estimated dry weight at 8 weeks was accompanied by a mean 6.6 mmHg decline in SBP.18 However, the short duration of the trial precluded assessing effects on clinical outcomes.
There is uncertainty regarding the optimal BP level and type of measurement. Predialysis SBP may be inferior to home BP measurements (HBPM) and ambulatory BP monitoring (ABPM) in predicting clinical outcomes.19,20 However, the long-term adherence of patients on HD with requirements for repeated HBPM and ABPM is unknown.
Recent trials in high-risk patients, without ESRD, present strong evidence of equipoise. In the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial, targeting an SBP<120 mmHg did not reduce MACE in patients with diabetes and was associated with increased hospitalization.21 In contrast, in the Systolic Blood Pressure Intervention Trial (SPRINT), conducted in high-risk nondiabetic patients, including those with an eGFR of 20–60 ml/min per 1.73 m2, intensive control of SBP reduced all-cause mortality and MACE.22 ACCORD and SPRINT relied primarily upon measurements of clinic BP.
The primary objectives of the BP in Dialysis (BID) Pilot Study were to assess the feasibility and safety of treating hypertensive patients receiving HD to a standardized predialysis SBP of 110–140 mmHg (intensive arm) versus 155–165 mmHg (standard arm) and to inform the design of a full-scale RCT. Assessing changes in LVM was a secondary objective.
Results
Participation Rates and Disposition
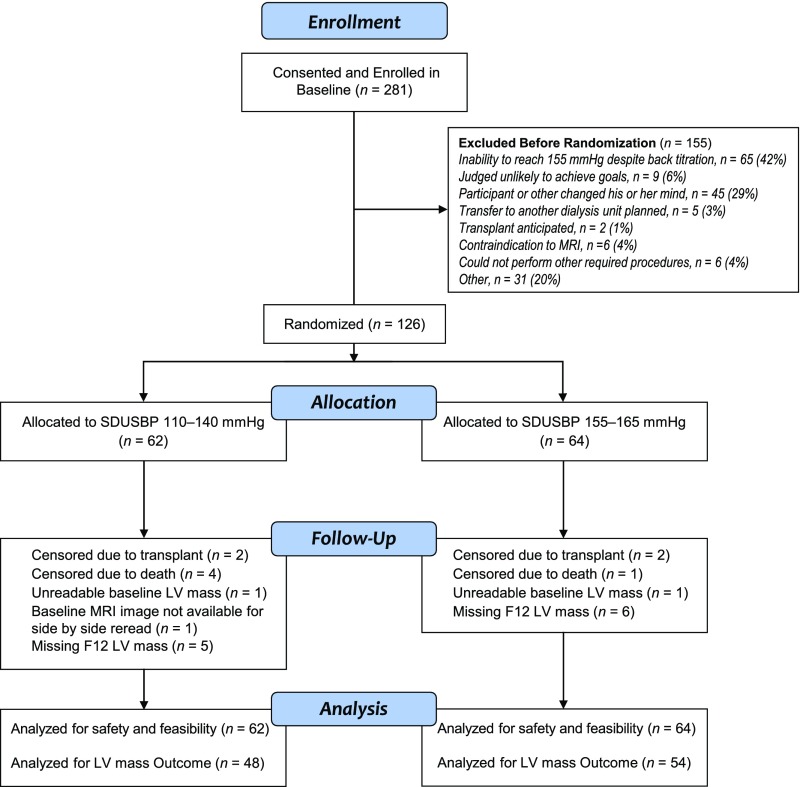
Approximately 45% of potentially eligible patients approached agreed to participate. A Consolidated Standards of Reporting Trials diagram depicts the flow of consented participants (Figure 1). We randomized 126 (45%) of the 281 participants who entered baseline, to intensive (n=62) and standard (n=64) treatment arms. The large number of dropouts during baseline reflected SBPs<155 mmHg despite back-titration of antihypertensive medications, frequent IDH, or voluntary withdrawal (Supplemental Table 2). Median follow-up was 365 days (10th and 90th percentiles, 291 and 392 days). There were four deaths in the intensive and one in the standard arm, two renal transplants in each arm, and missing cardiac magnetic resonance imaging (MRI) in six and seven participants in the standard and intensive arms, respectively. The intervention was stopped for safety concerns in one participant in the standard arm (ischemic stroke) and one in the intensive arm (repeated hospitalizations for chest pain and uncontrolled hypertension).
Figure 1.
Consort diagram showing participant flow from enrollment to randomization, follow-up, and analysis. F12, month 12 post randomization; LV, left ventricular; SDUSBP, standardized dialysis unit systolic blood pressure.
Study Participants
Baseline demographic and clinical characteristics of randomized participants were similar across treatment arms (Table 1). Mean age was 56.0±12.8 years, 46.8% were black, 54.1% had diabetes as cause of ESRD, 72.2% were dialyzed with an arteriovenous fistula, and the average treatment time was 218.7±27.8 minutes. Demographic and clinical characteristics of those randomized versus those enrolled but not randomized are shown (Supplemental Table 4). Age, race, and causes of ESRD were similar among randomized versus nonrandomized participants. Hispanics were more common among randomized participants.
Table 1.
Baseline characteristics of participants
| Characteristic | Intensive Treatment Arm (n=62) | Standard Treatment Arm (n=64) | P Value |
|---|---|---|---|
| n (%) or mean±SD | n (%) or mean±SD | ||
| Age, yr | 56.7±14.5 | 55.3±10.8 | 0.55 |
| Men | 32 (51.6) | 39 (60.9) | 0.37 |
| Race | 0.85 | ||
| Native American, Aboriginal Canadian/Alaskan | 4 (6.5) | 2 (3.1) | |
| Asian | 4 (6.5) | 4 (6.3) | |
| Black, African | 30 (48.4) | 29 (45.3) | |
| White | 22 (35.5) | 28 (43.8) | |
| More than one race, part Native American | 1 (1.6) | ||
| Unknown or not reported | 1 (1.6) | 1 (1.6) | |
| Hispanic ethnicity | 21 (33.9) | 23 (35.9) | 0.85 |
| Cause of ESRD | 0.68 | ||
| Diabetic nephropathy | 30 (50.9) | 36 (57.1) | |
| Hypertensive nephrosclerosis | 22 (37.3) | 17 (27.0) | |
| GN | 4 (6.8) | 6 (9.5) | |
| Other | 3 (5.1) | 4 (6.4) | |
| Vascular access | 0.77 | ||
| Arteriovenous graft | 11 (17.7) | 11 (17.2) | |
| Arteriovenous fistula | 46 (74.2) | 45 (70.3) | |
| Central venous catheter | 5 (8.1) | 8 (12.5) | |
| Years on dialysis (from most recent dialysis start) | 3.1±2.4 | 3.5±2.8 | 0.39 |
| BMI, kg/m2 | 28.1±7.0 | 27.1±5.8 | 0.38 |
| Last baseline 2 wk running mean SBP | 161.1±9.8 | 160.5±11.9 | 0.76 |
| Last baseline 2 wk running mean DBP | 79.5±12.2 | 82.1±12.3 | 0.24 |
| Treatment time, min | 216.8±30.2 | 220.5±25.4 | 0.46 |
| FACIT score | 30.8±12.9 | 30.0±12.8 | 0.76 |
| Charlson index | 3.3±1.0 | 3.3±1.0 | 0.89 |
| Number of antihypertensive medications | 2.9±1.4 | 2.4±1.1 | 0.03 |
| History of myocardial infarction | 4 (6.5) | 5 (7.8) | 1.00 |
| History of congestive heart failure | 9 (14.5) | 9 (14.1) | 1.00 |
| History of CVA | 4 (6.5) | 3 (4.7) | 0.72 |
| History of atrial fibrillation | 2 (3.2) | 1 (1.6) | 0.62 |
| LVH (n=102) | 42 (87.5) | 43 (79.6) | 0.43 |
P values for race, cause of ESRD, and ateriovenous access types are from chi-squared test. BMI, body mass index; DBP, diastolic blood pressure; CVA, cerebrovascular accident.
Adherence with BP Measurements
Tables summarizing adherence with prescribed standardized dialysis unit BP measurements (SDUBPM) and HBPM are included in Supplemental Tables 5 and 7. We obtained ≥4 SDUBPM in 97%, 91%, 86%, and 75% of participants in months 1, 4, 8, and 12, respectively. However, in the same months, we obtained ≥1 HBPM in only 82%, 73%, 68%, and 62% and ≥4 HBPM in 36%, 33%, 25%, and 22%. We obtained ABPM in only 32%, 29%, 28%, and 58% of participants in quarters 1, 2, 3, and 4, respectively.
SBP
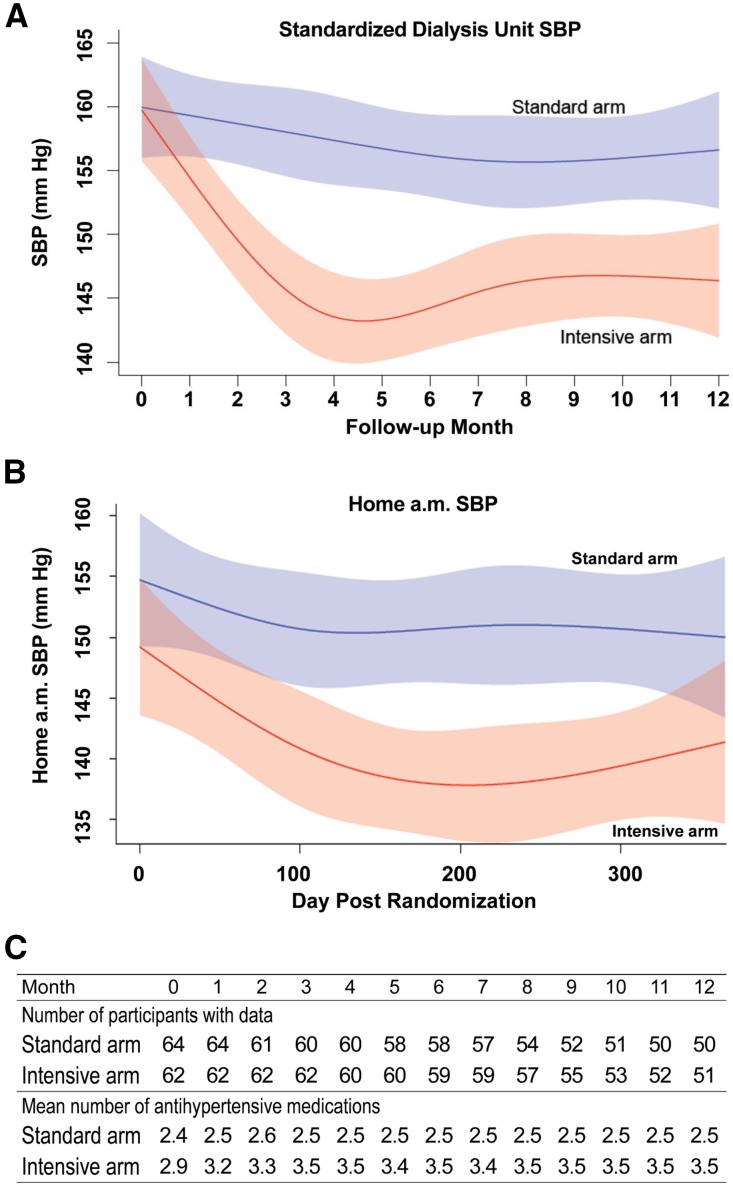
At baseline, 6, and 12 months, mean SBP in the intensive and standard arms was 159.7, 144.2, and 146.4 mmHg, and 159.9, 156.2, and 156.6 mmHg, respectively. Figure 2A shows the fitted values for 2-week moving averages of SDUBPM with 95% confidence intervals (95% CI), computed using a restricted cubic spline model. In the standard arm, the 2-week moving average SBP did not change significantly during the intervention period (horizontal line fits within the confidence band). In contrast, in the intensive arm the fitted 2-week average SBP decreased from 160 mmHg at baseline to 143 mmHg at 4.5 months. Separation in SBP between the arms was maintained from 2 months until the end of the study. Separation was greatest (14 mmHg) at 4.5 months. During months 4–12, mean separation in SBP between arms was 12.9 mmHg. The overall pattern of the fitted values for 2-week moving averages of SDUBPM and morning home SBP was similar (Figure 2B). Overall, the predialysis SBP was 6.1±0.7 mmHg higher than the home SBP. We estimated within-subject SDs for SBP obtained from SDUBPM immediately before the midweek dialysis and HBPM taken the following morning using a linear mixed model in SAS 9.4. We obtained estimates of 14.3 and 16.9 mmHg for within-subject SDs for SBP obtained in the dialysis unit and at home, respectively, using a likelihood ratio test (P<0.001) as described by Rhorscheib et al.23
Figure 2.
Standardized dialysis unit and home SBP and mean number of antihypertensive medications. (A) Fitted values for 2-week moving averages of SBP measured in the dialysis unit. (B) Fitted values for 2-week moving averages of SBP measured at home. (C) Mean number of antihypertensive medications throughout the intervention period.
The mean number of antihypertensive medications, by month, in each arm is shown in Figure 2C. The number of antihypertensive medications was greater in the intensive versus standard arm at baseline (2.9 versus 2.4), 6 months (3.5 versus 2.5), and 12 months (3.5 versus 2.5). The percentage of participants who were prescribed angiotensin converting enzyme inhibitors or angiotensin receptor blockers was higher in the intensive versus standard treatment arms (Figure 2C).
Postdialysis Weight and Interdialytic Weight Gain by Treatment Arm
During the intervention, postdialysis weight, expressed as least square means and 95% CI, decreased by 1.05 (95% CI, −1.55 to −0.55; P<0.001) kg in the standard arm but increased by 1.13 (95% CI, 0.60 to 1.66; P<0.001) kg in the intensive arm. Interdialytic weight gain after a 2-day interval decreased by 0.20 (95% CI, −0.30 to −0.10; P<0.001) kg and by 0.25 (95% CI, −0.35 to −0.15; P<0.001) kg in the intensive and standard arms, respectively. After a 3-day interval, interdialytic weight gain decreased by 0.10 (95% CI, −0.23 to 0.04; P=0.16) kg and 0.19 (95% CI, −0.32 to −0.06; P=0.003) kg in the intensive and standard arms, respectively. There were no significant changes in treatment time in either arm.
Safety Outcomes
There were four deaths in the intensive arm: one acute myocardial infarction, one cardiac arrhythmia, one vascular access hemorrhage, and one lung cancer. There was one death in the standard arm which was attributed to a combination of stroke, acute myocardial infarction, and heart failure. The incidence rate ratios (IRR) were 1.18 (95% CI, 0.40 to 3.33; P=0.78); 1.61 (95% CI, 0.87 to 2.97; P=0.13); 3.09 (95% CI, 0.96 to 8.78; P=0.06); and 0.90 (95% CI, 0.51 to 1.58; P=0.72) for MACE, all-cause hospitalizations, VATs, and emergency room visits (Table 2). The hazard ratios (HR) for time to first and to recurrent events are shown (Table 2).
Table 2.
Hospitalizations and VAT
| Event | No. Events/No. Subjects Incidence Ratea per Patient Year (95% CI) | IRR (95% CI) | P Value | HRb of Time to First Event (95% CI) | P Value | HRc of Recurrent Events (95% CI) | P Value | |
|---|---|---|---|---|---|---|---|---|
| Intensive Arm (n=62) | Standard Arm (n=64) | |||||||
| MACEd | 11/10 | 10/6 | 1.18 (0.40 to 3.33) | 0.78 | 1.76 (0.64 to 4.85) | 0.27 | 0.89 (0.30 to 2.66) | 0.84 |
| 0.20 (0.09 to 0.41) | 0.17 (0.08 to 0.37) | |||||||
| Hospitalization | 85/29 | 53/25 | 1.61 (0.87 to 2.97) | 0.13 | 1.29 (0.76 to 2.21) | 0.35 | 1.66 (1.18 to 2.34) | 0.004 |
| 1.51 (0.99 to 2.30) | 0.94 (0.60 to 1.46) | |||||||
| VAT | 19/10 | 7/7 | 3.09 (0.96 to 8.78) | 0.06 | 1.54 (0.59 to 4.04) | 0.38 | 2.80 (1.18 to 6.66) | 0.020 |
| 0.34 (0.18 to 0.66) | 0.11 (0.05 to 0.28) | |||||||
| ER visit | 50/30 | 55/27 | 0.90 (0.51 to 1.58) | 0.72 | 1.24 (0.74 to 2.09) | 0.42 | 0.94 (0.64 to 1.38) | 0.74 |
| 0.89 (0.59 to 1.34) | 0.99 (0.67 to 1.46) | |||||||
Calculated using negative binomial regression.
Calculated using Cox proportional hazards regression.
Calculated using Anderson–Gill method in Cox proportional hazards regression.
MACE defined as fatal or nonfatal myocardial infarction, stroke, or hospitalization for congestive heart failure.
Intradialytic Events and Symptoms
The frequency of IDH requiring intervention was similar in the intensive versus standard arm (IRR, 1.29; 95% CI, 0.86 to 1.94; P=0.22). The IRR and HR for other intradialytic events are summarized in Table 3. The IRRs for any intradialytic event did not differ significantly across treatment arms. However, the HR for recurring events of hypotension, cramps, and nausea/vomiting revealed significant increased risks in the intensive arm.
Table 3.
Intradialytic events
| Events | No. Events/No. Subjectsa per 100 Treatments (95% CI) | IRR (95% CI) | P Value | HRb of Time to First Event (95% CI) | P Value | HRc of Recurrent Events (95% CI) | P Value | |
|---|---|---|---|---|---|---|---|---|
| Intensive Arm (n=62) | Standard Arm (n=64) | |||||||
| SBP<90 mmHg | 332/39 | 264/45 | 1.36 (0.80 to 2.31) | 0.25 | 0.88 (0.57 to 1.35) | 0.56 | 1.30 (1.10 to 1.52) | 0.002 |
| 3.67 (2.53 to 5.31) | 2.69 (1.85 to 3.91) | |||||||
| Cramps | 641/55 | 571/51 | 1.15 (0.77 to 1.72) | 0.51 | 1.19 (0.81 to 1.75) | 0.37 | 1.16 (1.04 to 1.30) | 0.01 |
| 6.74 (5.05 to 8.96) | 5.87 (4.42 to 7.82) | |||||||
| Nausea±vomiting | 90/25 | 66/29 | 1.35 (0.70. 2.63) | 0.37 | 0.89 (0.52 to 1.53) | 0.68 | 1.41 (1.02 to 1.94) | 0.04 |
| 0.98 (0.61 to 1.57) | 0.73 (0.44 to 1.17) | |||||||
| Dizziness | 110/24 | 126/23 | 0.88 (0.40 to 1.95) | 0.76 | 1.00 (0.56 to 1.78) | >0.99 | 0.92 (0.71 to 1.19) | 0.50 |
| 1.15 (0.66 to 2.01) | 1.31 (0.75 to 2.27) | |||||||
| Dyspnea | 27/12 | 56/11 | 0.44 (0.13 to 1.45) | 0.18 | 1.13 (0.50 to 2.57) | 0.77 | 0.50 (0.31 to 0.79) | 0.003 |
| 0.28 (0.12 to 0.68) | 0.66 (0.28 to 1.50) | |||||||
| Chest pain | 11/7 | 9/7 | 1.27 (0.40 to 4.01) | 0.69 | 1.06 (0.37 to 3.03) | 0.91 | 1.26 (0.52 to 3.04) | 0.61 |
| 0.12 (0.05 to 0.26) | 0.09 (0.05 to 0.21) | |||||||
| Loss of consciousness | 4/4 | 1/1 | 4.15 (0.46 to 37.12) | 0.20 | 4.18 (0.47 to 37.43) | 0.20 | 4.10 (0.46 to 36.64) | 0.21 |
| 0.05 (0.02 to 0.12) | 0.00 (0.00 to 0.07) | |||||||
| Seizure | 2/1 | 1/1 | 2.05 (0.08 to 55.05) | 0.67 | 1.05 (0.07 to 16.84) | 0.97 | 2.08 (0.19 to 22.95) | 0.55 |
| 0.02 (0.00 to 0.19) | 0.00 (0.00 to 0.14) | |||||||
Includes all events, regardless of whether an intervention was done. Referent group: Standard treatment arm.
Calculated using negative binomial regression
Calculated using Cox proportional hazards regression
Calculated using Anderson–Gill method in Cox proportional hazards regression
LVM
At baseline, 87.5% and 79.6% of participants in the intensive and standard arms, respectively, had left ventricular hypertrophy (LVH) by LVM.24 In the intensive arm, median LVM, excluding papillary muscle, decreased from baseline 149.5 (95% CI, 115.0 to 184.3) g to 133.2 (95% CI, 110.2 to 168.7) g at month 12 (Table 4). In the standard arm, median LVM increased slightly from baseline 143.8 (95% CI, 120.4 to 176.8) g to month 12, 149.9 (95% CI, 126.8 to 176.0) g. The median differences from baseline to month 12 in the intensive arm −0.84 (95% CI, −17.1 to 10.0) g versus the standard arm 1.4 (95% CI −11.6 to 10.4) g were similar (P=0.43).
Table 4.
Effects of intensive versus standard SBP goal on LVM
| Variable | Intensive Arm (n=48) | Standard Arm (n=54) | P Value of Differences Across Arms |
|---|---|---|---|
| Baseline median (IQR) LVM (g/m) | 149.5 (115.0, 184.3) | 143.8 (120.4, 176.8) | 0.95 |
| F12 median (IQR) LVM (g/m) | 133.2 (110.2, 168.7) | 149.9 (126.8, 176.0) | 0.19 |
| Median difference (IQR) (g/m2) baseline minus month 12 | −0.84 (−17.1, 10.0) | 1.4 (−11.6, 10.4) | 0.43 |
IQR, interquartile range; F12, study visit in month 12 post randomization.
Health-Related Quality of Life
There were no differences in baseline values and changes in the Functional Assessment of Chronic Illness Therapy (FACIT) fatigue scores across treatment arms. Recovery times at baseline and follow-up were similar in each treatment arm (Supplemental Table 8). The physical and mental component scores, respectively, on the Short Form-36 (SF-36) did not differ across treatment arms at baseline or on the last administration (Supplemental Table 9).
Discussion
The BID Pilot demonstrated the feasibility of recruiting and retaining hypertensive patients receiving HD in an RCT of intensive versus standard control of SBP. Similar to ACCORD21 and SPRINT,22 the achieved average SBP in the intensive arm was slightly above the target. However, we achieved a sustained ≥10 mmHg separation in both predialysis and morning home SBP, the magnitude required to detect a 20% relative reduction in MACE.25 The magnitude of the separation in SBP across treatment arms was similar to that in previous trials. The African American Study of Kidney Disease achieved an average (SD) BP 128/78 (12/8) mmHg in the intensive versus 141/85 (12/7) mmHg in the standard arm.26,27 DRIP, an RCT of aggressive ultrafiltration versus usual care, achieved a 6.6 (95% CI, 12.2 to 1.0 mmHg; P=0.02) mmHg reduction in SBP at 8 weeks among patients randomized to aggressive ultrafiltration.18 In the FHN Trial, the decrease in mean SBP from baseline to 12 months was 10.1 (95% CI, 6.0 to 14.3) mmHg greater in the six versus three times weekly HD arm.10
BID encouraged site investigators to challenge estimated dry weight as the initial step in reducing SBP. Because many participants were intolerant to or refused these challenges, changes in antihypertensive medications were mainly responsible for achieving separation in SBP across treatment arms. This is similar to the experience in an RCT of atenolol versus lisinopril in which mean postdialysis weight increased by 0.9 kg and decreased by 1.5 kg in the atenolol and lisinopril arms, respectively.28 However, in DRIP there was a −1 (95% CI, −1.6 to −0.5) kg change in postdialysis weight at 8 weeks in the aggressive ultrafiltration arm, which was the sole intervention.18 Because DRIP lasted only 8 weeks it did not test long-term feasibility of aggressive ultrafiltration.
Although volume is undoubtedly important, the pathogenesis of hypertension in patients on HD is multifactorial. A German study of 500 hypertensive patients receiving HD used bioimpedance to estimate extracellular volume. Less than 50% of hypertensive participants had evidence of volume expansion.29 In a recent observational study of patients receiving HD treated in facilities operated by Fresenius Medical Care, sustained fluid overload was strongly associated with increased mortality across BP categories.30 However, because the study was observational it did not establish causation. Several studies have shown that aggressive ultrafiltration is associated with increased frequency of IDH,31 VAT,16 loss of residual renal function,32 and increased mortality.33–35
As in ACCORD21 and SPRINT,22 the rate of serious adverse events (SAEs) was higher in the intensive versus the standard treatment arm. BID identified a possible safety signal because deaths, all-cause hospitalizations, and VATs were more frequent in the intensive arm. However, there were no significant differences in the number of patients hospitalized or experiencing VATs. The frequency of MACE or emergency room (ER) visits, respectively, was similar across treatment arms. Overall, hospitalizations in BID were not more frequent than reported by the US Renal Data Systems (USRDS).36 The frequency of IDH was higher in the intensive versus standard treatment arm. Nevertheless, the frequency of IDH in the intensive arm (3.7%) was lower than in the baseline period of the Hemodialysis (HEMO) study (11.3%),13 a large United States dialysis provider organization (9.7%),13 and the conventional (10.9%) and frequent (13.6%) arms of FHN.10 VATs were more frequent in the intensive versus the standard arm. These results are consistent with data from a subset of HEMO participants, in which low predialysis SBP and frequent IDH were associated with an increase in VAT.16 In BID, VATs were less common than in the control arm of contemporary prospective trials for arteriovenous grafts37 and fistulas.38 However, the increase in VATs in the intensive arm of BID and the daily arm of FHN speaks to the need to ascertain the risk-benefit ratios of these interventions in RCTs powered for all-cause mortality and MACE.
In BID, LVM tended to decrease in the intensive arm and increase in the standard arm but these differences did not attain statistical significance. Although we were underpowered for this outcome, it is possible that targeting a lower SBP may not reduce LVM in hypertensive patients receiving HD dialyzed thrice weekly. Nevertheless, multiple studies have shown that decreases in dialysis unit BP are associated with decreases in LVM.10,11,39
We chose predialysis SDUBPM rather than HBPM to guide therapy, in anticipation that adherence with HBPM would be low. Some investigators have postulated that HBPM and ABPM are superior to predialysis BP in predicting LVH.19,20 However, the majority of these reports compared only 2 weeks of predialysis BP, 1 week of HBPM, and a single 44-hour ABPM. Moreover, there was considerable overlap in the 95% CI of the area under the receiver operator characteristic curves for predialysis BP, HBPM, and ABPM.19 Conion et al.40 and Zoccali et al.39 reported that multiple BP readings averaged over a month are as good as ABPM in predicting an increase in LVM.
Strengths and Limitations
BID has several important strengths. It was the first RCT to randomize patients receiving HD to different predialysis SBP targets. Participants were demographically diverse and treated in for-profit and not-for-profit facilities. BP measurements were made in accord with American Heart Association (AHA) guidelines. A National Institutes Health (NIH)–appointed Review Panel and a Data Safety Monitoring Board reviewed the study before its start. An experienced Outcomes Committee (A.S.L. and A.K.S.) adjudicated outcomes. The main limitations relate to the small size and short duration, which constrained statistical power and generalizability, and the lack of an objective tool for assessing volume. Although some may argue that the use of SDUBPM instead of HBPM or ABPM to drive therapy is a limitation,19,20 the enhanced adherence with SDUBPM likely overrides this potential limitation. The mean age of participants (56.0 years) was slightly lower than that of prevalent patients in the USRDS 2016 Annual Data Report (59.4 years);41–43 25% were ≥65 years of age. We did not perform pill counts or HPLC of antihypertensive medication metabolites.44,45
Informing the Design of a Full-Scale Trial
In a full-scale trial, it may be necessary to protocolize challenges to postdialysis weights, including use of frequent small decrements; repeated measurements of volume via a practical, accurate, and reliable method;30 and participant agreement to adhere to dialysis prescriptions. Despite their putative advantages, use of HBPM or ABPM to drive therapy would require innovative solutions to ensure adequate adherence. Although SDUBPM added approximately 8 minutes to the treatment time, which some staff and participants found burdensome, adherence was excellent and indicates it could be used in a full-scale trial. Vascular access monitoring should be incorporated into a full-scale trial to minimize risk for VAT. The use of cooled dialysate may be considered to decrease the risk for IDH and VAT.
BID demonstrated the feasibility and safety of conducting a full-scale RCT to test the hypothesis that intensive SBP lowering in patients receiving HD reduces MACEs and all-cause mortality. However, the pilot study did identify a potential safety signal. Although the deaths in the intensive arm did not appear to be protocol related, all-cause hospitalizations, VAT, and IDH, analyzed as recurrent events, were increased in the intensive arm. Given the small size and short duration of BID, these findings represent a safety signal, not a definitive result. Given the high risk for adverse cardiovascular events in patients receiving HD and the potential benefits of intensive SBP lowering observed in SPRINT, conducting a large-scale trial is warranted.
Concise Methods
The study protocol has been previously described in Gul et al.46
The Study Protocol is currently available at: http://qhsapps.ccf.org/bid/protocol/Protocol.pdf and will become available on the National Institute of Diabetes and Digestive and Kidney Diseases repository website.
Study Population
Each site’s institutional review board approved the study. Our goal was to recruit 120 participants from five geographic hubs, which included dialysis units operated by Dialysis Clinic, Inc. (DCI), Centers for Dialysis Care, and DaVita. Eligibility criteria included ≥18 years of age, treatment with HD for ≥3 months, upper arm suitable for measuring BP, and a 2-week average predialysis SBP≥155 mmHg. Exclusion criteria included unscheduled dialysis treatments for congestive heart failure and IDH requiring hospitalization in the 3 months before enrollment.46
Baseline Period
Antihypertensive medications were sometimes reduced (back-titrated) to achieve a 2-week average predialysis SBP≥155 mmHg. Comorbidity was classified using the Charlson Index.47 The FACIT48 and the recovery question were administered.49 LVM was measured using MRI. We used web-based randomization with random-sized blocks and stratification by geographic site to ensure unpredictable treatment allocation and balance within sites.
Study Visits
At each study visit, participants were asked if they had a recent hospitalization, ER visit, VAT, or IDH. VAT was defined by the inability to use the access for dialysis due to thrombosis with an urgent need for intervention to restore flow. IDH was defined as an intradialytic SBP<90 mmHg.
BP Control
In planning the study, we were aware of the putative superiority of HBPM and ABPM compared with predialysis BP for predicting outcomes including LVH.19,20 However, the primary objectives of the BID pilot were to assess the safety, feasibility, and inform the design of a full-scale trial to determine optimal dialysis unit SBP. Also, there is considerable evidence that dialysis unit SBP can predict changes in LVM. Because we had significant concerns about adherence with HBPM and ABPM during a 1-year intervention, the BID investigators and DSMB decided that the possible slightly stronger predictive value of HBPM and ABPM versus SDUBPM would be offset by poorer adherence.
Standardized predialysis BP was measured in accord with AHA guidelines.50 Although we used SDUBPM to drive therapy, the study protocol included morning and afternoon HBPM the day after each midweek dialysis46 and (in four geographic hubs) quarterly ABPM.46 We encouraged site investigators to challenge postdialysis weights as the initial step in attaining the assigned target SBP. This was followed by addition of antihypertensive agents. Blockade of the renin-angiotensin system was the preferred first-line antihypertensive drug therapy, unless there was an indication for a β-adrenergic blocker. The DCI pharmacy provided all study medications.
Assessing Outcomes
We reviewed discharge summaries and coded primary diagnoses using a study-specific checklist. The Outcomes Committee adjudicated all deaths, MACE, VATs, and a random sample of noncardiovascular SAEs. The NIH-appointed Data and Safety Monitoring Board reviewed all SAEs annually. Baseline and 12-month cardiac MRIs were read side-by-side, in blinded fashion, by a cardiologist using a standardized protocol, at Brigham and Women’s Hospital (Supplemental Appendix 5). We assessed health-related quality of life by administering the FACIT, recovery question, and SF-36 at baseline and at the end of the intervention.
Statistical Analyses
Feasibility was assessed by number of participants randomized, separation in 2-week running average of predialysis SBP between arms, and participant retention. Prespecified safety outcomes included deaths, all-cause hospitalizations, MACE, VAT, and IDH. We calculated incidence rates with corresponding 95% CIs for all-cause hospitalizations, MACE, and ER visits. Incidence rates were calculated by negative binomial regression from the MASS51 package in R52 after using Akaike information criterion and Vuong’s test from the PSCI53 package to eliminate Poisson regression and zero-inflated versions of Poisson and negative binomial regression. We tested for differences across treatment arms by computing HR and corresponding 95% CI with proportional hazards regression for time to first event and for recurring events (Anderson–Gill model).
To assess the longitudinal trends of standardized SBP in each treatment arm we examined 2-week moving averages for each participant. We used a linear mixed model with autoregressive error structure to model trends in SBP moving averages throughout the study. Time was fit using restricted cubic splines to allow for nonlinear trends in the data.54 To assess changes in LVM by arm, we specified a Wilcoxon–Mann–Whitney rank sum test before testing because the data were skewed. We recognized that the small size of the trial severely constrained our power to detect a difference between arms for change in LVM. We had only 80% power to detect a ≥21.5 g difference, which was almost twice the difference observed in the Daily Trial in FHN.10 Analyses were performed using SAS 9.4 (SAS Institute, Cary, NC) and R Core Team 2016.52.
For the SF-36 analysis, we compared between-group mean changes in scores from baseline to month 12 for the physical component summary scores and mental component summary scores using linear mixed effects models with unstructured covariance matrix incorporating baseline and 12-month scores for each metric. We adjusted for baseline score, clinical center, and the interactions of these factors with treatment time. ClinicalTrials.gov Identifier: NCT01421771.
Disclosures
None.
Supplementary Material
Acknowledgments
Supported by National Institute of Diabetes and Digestive and Kidney Diseases (R01DK083424) and Dialysis Clinic, Inc.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2017020135/-/DCSupplemental.
References
- 1.K/DOQI Workgroup : K/DOQI clinical practice guidelines for cardiovascular disease in dialysis patients. Am J Kidney Dis 45[Suppl 3]: S1–S153, 2005 [PubMed] [Google Scholar]
- 2.Kjeldsen SE, Hedner T, Jamerson K, Julius S, Haley WE, Zabalgoitia M, Butt AR, Rahman SN, Hansson L: Hypertension optimal treatment (HOT) study: Home blood pressure in treated hypertensive subjects. Hypertension 31: 1014–1020, 1998 [DOI] [PubMed] [Google Scholar]
- 3.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R; Prospective Studies Collaboration : Age-specific relevance of usual blood pressure to vascular mortality: A meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 360: 1903–1913, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Hannedouche T, Roth H, Krummel T, London GM, Jean G, Bouchet JL, Drüeke TB, Fouque D; French Observatory : Multiphasic effects of blood pressure on survival in hemodialysis patients. Kidney Int 90: 674–684, 2016 [DOI] [PubMed] [Google Scholar]
- 5.Kalantar-Zadeh K, Kilpatrick RD, McAllister CJ, Greenland S, Kopple JD: Reverse epidemiology of hypertension and cardiovascular death in the hemodialysis population: The 58th annual fall conference and scientific sessions. Hypertension 45: 811–817, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Port FK, Hulbert-Shearon TE, Wolfe RA, Bloembergen WE, Golper TA, Agodoa LY, Young EW: Predialysis blood pressure and mortality risk in a national sample of maintenance hemodialysis patients. Am J Kidney Dis 33: 507–517, 1999 [DOI] [PubMed] [Google Scholar]
- 7.Robinson BM, Tong L, Zhang J, Wolfe RA, Goodkin DA, Greenwood RN, Kerr PG, Morgenstern H, Li Y, Pisoni RL, Saran R, Tentori F, Akizawa T, Fukuhara S, Port FK: Blood pressure levels and mortality risk among hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study. Kidney Int 82: 570–580, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tentori F, Hunt WC, Rohrscheib M, Zhu M, Stidley CA, Servilla K, Miskulin D, Meyer KB, Bedrick EJ, Johnson HK, Zager PG: Which targets in clinical practice guidelines are associated with improved survival in a large dialysis organization? J Am Soc Nephrol 18: 2377–2384, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Zager PG, Nikolic J, Brown RH, Campbell MA, Hunt WC, Peterson D, Van Stone J, Levey A, Meyer KB, Klag MJ, Johnson HK, Clark E, Sadler JH, Teredesai P: “U” curve association of blood pressure and mortality in hemodialysis patients. Medical Directors of Dialysis Clinic, Inc. Kidney Int 54: 561–569, 1998 [DOI] [PubMed] [Google Scholar]
- 10.Chertow GM, Levin NW, Beck GJ, Depner TA, Eggers PW, Gassman JJ, Gorodetskaya I, Greene T, James S, Larive B, Lindsay RM, Mehta RL, Miller B, Ornt DB, Rajagopalan S, Rastogi A, Rocco MV, Schiller B, Sergeyeva O, Schulman G, Ting GO, Unruh ML, Star RA, Kliger AS; FHN Trial Group : In-center hemodialysis six times per week versus three times per week. N Engl J Med 363: 2287–2300, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foley RN, Parfrey PS, Harnett JD, Kent GM, Murray DC, Barre PE: Impact of hypertension on cardiomyopathy, morbidity and mortality in end-stage renal disease. Kidney Int 49: 1379–1385, 1996 [DOI] [PubMed] [Google Scholar]
- 12.Daugirdas JT: Measuring intradialytic hypotension to improve quality of care. J Am Soc Nephrol 26: 512–514, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flythe JE, Xue H, Lynch KE, Curhan GC, Brunelli SM: Association of mortality risk with various definitions of intradialytic hypotension. J Am Soc Nephrol 26: 724–734, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sands JJ, Usvyat LA, Sullivan T, Segal JH, Zabetakis P, Kotanko P, Maddux FW, Diaz-Buxo JA: Intradialytic hypotension: Frequency, sources of variation and correlation with clinical outcome. Hemodial Int 18: 415–422, 2014 [DOI] [PubMed] [Google Scholar]
- 15.Stefánsson BV, Brunelli SM, Cabrera C, Rosenbaum D, Anum E, Ramakrishnan K, Jensen DE, Stålhammar NO: Intradialytic hypotension and risk of cardiovascular disease. Clin J Am Soc Nephrol 9: 2124–2132, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang TI, Paik J, Greene T, Desai M, Bech F, Cheung AK, Chertow GM: Intradialytic hypotension and vascular access thrombosis. J Am Soc Nephrol 22: 1526–1533, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heerspink HJ, Ninomiya T, Zoungas S, de Zeeuw D, Grobbee DE, Jardine MJ, Gallagher M, Roberts MA, Cass A, Neal B, Perkovic V: Effect of lowering blood pressure on cardiovascular events and mortality in patients on dialysis: A systematic review and meta-analysis of randomised controlled trials. Lancet 373: 1009–1015, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Agarwal R, Alborzi P, Satyan S, Light RP: Dry-weight reduction in hypertensive hemodialysis patients (DRIP): A randomized, controlled trial. Hypertension 53: 500–507, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agarwal R, Andersen MJ, Light RP: Location not quantity of blood pressure measurements predicts mortality in hemodialysis patients. Am J Nephrol 28: 210–217, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alborzi P, Patel N, Agarwal R: Home blood pressures are of greater prognostic value than hemodialysis unit recordings. Clin J Am Soc Nephrol 2: 1228–1234, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Cushman WC, Evans GW, Byington RP, Goff DC Jr, Grimm RH Jr, Cutler JA, Simons-Morton DG, Basile JN, Corson MA, Probstfield JL, Katz L, Peterson KA, Friedewald WT, Buse JB, Bigger JT, Gerstein HC, Ismail-Beigi F; ACCORD Study Group : Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 362: 1575–1585, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wright JT Jr, Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, Reboussin DM, Rahman M, Oparil S, Lewis CE, Kimmel PL, Johnson KC, Goff DC Jr, Fine LJ, Cutler JA, Cushman WC, Cheung AK, Ambrosius WT; SPRINT Research Group : A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 373: 2103–2116, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rohrscheib MR, Myers OB, Servilla KS, Adams CD, Miskulin D, Bedrick EJ, Hunt WC, Lindsey DE, Gabaldon D, Zager PG; DCI Medical Directors : Age-related blood pressure patterns and blood pressure variability among hemodialysis patients. Clin J Am Soc Nephrol 3: 1407–1414, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kawel-Boehm N, Maceira A, Valsangiacomo-Buechel ER, Vogel-Claussen J, Turkbey EB, Williams R, Plein S, Tee M, Eng J, Bluemke DA: Normal values for cardiovascular magnetic resonance in adults and children. J Cardiovasc Magn Reson 17: 29, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cushman WC, Grimm RH Jr, Cutler JA, Evans GW, Capes S, Corson MA, Sadler LS, Alderman MH, Peterson K, Bertoni A, Basile JN; ACCORD Study Group : Rationale and design for the blood pressure intervention of the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Am J Cardiol 99[12A]: 44i–55i, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Appel LJ, Wright JT Jr, Greene T, Agodoa LY, Astor BC, Bakris GL, Cleveland WH, Charleston J, Contreras G, Faulkner ML, Gabbai FB, Gassman JJ, Hebert LA, Jamerson KA, Kopple JD, Kusek JW, Lash JP, Lea JP, Lewis JB, Lipkowitz MS, Massry SG, Miller ER, Norris K, Phillips RA, Pogue VA, Randall OS, Rostand SG, Smogorzewski MJ, Toto RD, Wang X; AASK Collaborative Research Group : Intensive blood-pressure control in hypertensive chronic kidney disease. N Engl J Med 363: 918–929, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wright JT Jr, Bakris G, Greene T, Agodoa LY, Appel LJ, Charleston J, Cheek D, Douglas-Baltimore JG, Gassman J, Glassock R, Hebert L, Jamerson K, Lewis J, Phillips RA, Toto RD, Middleton JP, Rostand SG; African American Study of Kidney Disease and Hypertension Study Group : Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: Results from the AASK trial. JAMA 288: 2421–2431, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Agarwal R, Sinha AD, Pappas MK, Abraham TN, Tegegne GG: Hypertension in hemodialysis patients treated with atenolol or lisinopril: A randomized controlled trial. Nephrol Dial Transplant 29: 672–681, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wabel P, Moissl U, Chamney P, Jirka T, Machek P, Ponce P, Taborsky P, Tetta C, Velasco N, Vlasak J, Zaluska W, Wizemann V: Towards improved cardiovascular management: The necessity of combining blood pressure and fluid overload. Nephrol Dial Transplant 23: 2965–2971, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Zoccali C, Moissl U, Chazot C, Mallamaci F, Tripepi G, Arkossy O, Wabel P, Stuard S: Chronic fluid overload and mortality in ESRD. J Am Soc Nephrol 28: 2491–2497, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garg N, Fissell WH: Intradialytic hypotension: A case for going slow and looking carefully. Nephrol Dial Transplant 28: 247–249, 2013 [DOI] [PubMed] [Google Scholar]
- 32.Mathew AT, Fishbane S, Obi Y, Kalantar-Zadeh K: Preservation of residual kidney function in hemodialysis patients: Reviving an old concept. Kidney Int 90: 262–271, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Assimon MM, Flythe JE: Variability may be the “Law of Life,” but blood pressure variability may forebode a shorter life. Am J Kidney Dis 67: 830–833, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Flythe JE, Kimmel SE, Brunelli SM: Rapid fluid removal during dialysis is associated with cardiovascular morbidity and mortality. Kidney Int 79: 250–257, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Flythe JE, Brookhart MA: Fluid management: The challenge of defining standards of care. Clin J Am Soc Nephrol 9: 2033–2035, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.United States Renal Data System : 2015 USRDS annual data report: Epidemiology of kidney disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2015 [Google Scholar]
- 37.Lok CE, Moist L, Hemmelgarn BR, Tonelli M, Vazquez MA, Dorval M, Oliver M, Donnelly S, Allon M, Stanley K; Fish Oil Inhibition of Stenosis in Hemodialysis Grafts (FISH) Study Group : Effect of fish oil supplementation on graft patency and cardiovascular events among patients with new synthetic arteriovenous hemodialysis grafts: A randomized controlled trial. JAMA 307: 1809–1816, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Polkinghorne KR, Lau KK, Saunder A, Atkins RC, Kerr PG: Does monthly native arteriovenous fistula blood-flow surveillance detect significant stenosis--a randomized controlled trial. Nephrol Dial Transplant 21: 2498–2506, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Zoccali C, Mallamaci F, Tripepi G, Benedetto FA, Cottini E, Giacone G, Malatino L: Prediction of left ventricular geometry by clinic, pre-dialysis and 24-h ambulatory BP monitoring in hemodialysis patients: CREED investigators. J Hypertens 17: 1751–1758, 1999 [DOI] [PubMed] [Google Scholar]
- 40.Conion PJ, Walshe JJ, Heinle SK, Minda S, Krucoff M, Schwab SJ: Predialysis systolic blood pressure correlates strongly with mean 24-hour systolic blood pressure and left ventricular mass in stable hemodialysis patients. J Am Soc Nephrol 7: 2658–2663, 1996 [DOI] [PubMed] [Google Scholar]
- 41.Chan CT, Greene T, Chertow GM, Kliger AS, Stokes JB, Beck GJ, Daugirdas JT, Kotanko P, Larive B, Levin NW, Mehta RL, Rocco M, Sanz J, Schiller BM, Yang PC, Rajagopalan S; Frequent Hemodialysis Network (FHN) Trial Group : Determinants of left ventricular mass in patients on hemodialysis: Frequent Hemodialysis Network (FHN) trials. Circ Cardiovasc Imaging 5: 251–261, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kotanko P, Garg AX, Depner T, Pierratos A, Chan CT, Levin NW, Greene T, Larive B, Beck GJ, Gassman J, Kliger AS, Stokes JB; FHN Trial Group : Effects of frequent hemodialysis on blood pressure: Results from the randomized frequent hemodialysis network trials. Hemodial Int 19: 386–401, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.United States Renal Data System : 2016 USRDS annual data report: Epidemiology of kidney disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2016 [Google Scholar]
- 44.Sternlicht H, Bakris GL: Resistant hypertension: A refractory disease or refractory patient. Hypertension 69: 582–583, 2017 [DOI] [PubMed] [Google Scholar]
- 45.Tomaszewski M, White C, Patel P, Masca N, Damani R, Hepworth J, Samani NJ, Gupta P, Madira W, Stanley A, Williams B: High rates of non-adherence to antihypertensive treatment revealed by high-performance liquid chromatography-tandem mass spectrometry (HP LC-MS/MS) urine analysis. Heart 100: 855–861, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gul A, Miskulin D, Gassman J, Harford A, Horowitz B, Chen J, Paine S, Bedrick E, Kusek JW, Unruh M, Zager P; BID Pilot Study Investigators : Design of the blood pressure goals in dialysis pilot study. Am J Med Sci 347: 125–130, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Charlson ME, Pompei P, Ales KL, MacKenzie CR: A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis 40: 373–383, 1987 [DOI] [PubMed] [Google Scholar]
- 48.Cella DF, Tulsky DS, Gray G, Sarafian B, Linn E, Bonomi A, Silberman M, Yellen SB, Winicour P, Brannon J: The functional assessment of cancer therapy scale: Development and validation of the general measure. J Clin Oncol 11: 570–579, 1993 [DOI] [PubMed] [Google Scholar]
- 49.Lindsay RM, Heidenheim PA, Nesrallah G, Garg AX, Suri R; Daily Hemodialysis Study Group London Health Sciences Centre : Minutes to recovery after a hemodialysis session: A simple health-related quality of life question that is reliable, valid, and sensitive to change. Clin J Am Soc Nephrol 1: 952–959, 2006 [DOI] [PubMed] [Google Scholar]
- 50.Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves J, Hill MN, Jones DW, Kurtz T, Sheps SG, Roccella EJ: Recommendations for blood pressure measurement in humans and experimental animals: Part 1: Blood pressure measurement in humans: A statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation 111: 697–716, 2005 [DOI] [PubMed] [Google Scholar]
- 51.Venables WN, Ripley BD: Modern Applied Statistics with S, 4th Ed., New York, Springer, 2002 [Google Scholar]
- 52.R Core Team: R: A language and environment for statistical computing, Vienna, Austria, R Foundation for Statistical Computing, 2016. Available at: http://www.R-project.org/. Accessed April 14, 2016
- 53.Zeileis A, Kleiber C, Jackman S: Regression models for count data in R. J Stat Softw 27: 1–25, 2008 [Google Scholar]
- 54.Harrell FE, Jr: Regression Modeling Strategies, Berlin, Springer, 2016 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.