Abstract
The comparative effectiveness of partial nephrectomy versus radical nephrectomy to preserve kidney function has not been well established. We determined the risk of clinically significant (stage 4 and higher) CKD after radical or partial nephrectomy among veterans treated for kidney cancer in the Veterans Health Administration (2001–2013). Among patients with preoperative eGFR≥30 ml/min per 1.73 m2, the incidence of CKD stage 4 or higher after radical (n=9759) or partial nephrectomy (n=4370) was 7.9% overall. The median time to stage 4 or higher CKD after surgery was 5 months, after which few patients progressed. In propensity score–matched cohorts, partial nephrectomy associated with a significantly lower relative risk of incident CKD stage 4 or higher (hazard ratio, 0.34; 95% confidence interval [95% CI], 0.26 to 0.43, versus radical nephrectomy). In a parallel analysis of patients with normal or near-normal preoperative kidney function (eGFR≥60 ml/min per 1.73 m2), partial nephrectomy was also associated with a significantly lower relative risk of incident CKD stage 3b or higher (hazard ratio, 0.15; 95% CI, 0.11 to 0.19, versus radical nephrectomy) in propensity score–matched cohorts. Competing risk regression models produced consistent results. Finally, patients treated with a partial nephrectomy had reduced risk of mortality (hazard ratio, 0.55; 95% CI, 0.49 to 0.62). In conclusion, compared with radical nephrectomy, partial nephrectomy was associated with a marked reduction in the incidence of clinically significant CKD and with enhanced survival. Postoperative decline in kidney function occurred mainly in the first year after surgery and appeared stable over time.
Keywords: kidney cancer, Nephrectomy, chronic kidney disease
Radical nephrectomy was historically the standard approach to treat localized kidney cancer (renal cell carcinoma).1 Elective partial nephrectomy has become more common with increased detection of small renal masses (<4 cm).2,3 In these patients, partial nephrectomy offers equivalent oncologic outcomes while preserving nephron mass.4–6 There is increasing concern that surgically induced CKD will be associated with an increased risk of hospitalization, cardiovascular events, and death, as has been shown in CKD from all causes.7–10 Furthermore, CKD is independently associated with postoperative death and cardiovascular events in patients undergoing elective noncardiac surgery.11 Consequently, partial nephrectomy has been increasingly adopted in order to minimize surgically induced CKD and is now considered a standard surgical approach for early-stage kidney cancer.12,13
The Veterans Health Administration (VHA) is the largest integrated national health care system in the United States. Veterans treated in the VHA tend to be older and sicker than patients treated in the community and, as a result, may be more sensitive to changes in kidney function after kidney surgery.14,15 We have previously shown that the use of partial nephrectomy in the VHA has been increasing in parallel with the rest of the country.16
We sought to determine the postoperative change in kidney function among patients undergoing radical or partial nephrectomy in the VHA. We hypothesized that partial nephrectomy would better preserve kidney function when compared with radical nephrectomy after accounting for preoperative kidney function and relevant patient, clinical, and tumor factors.
Results
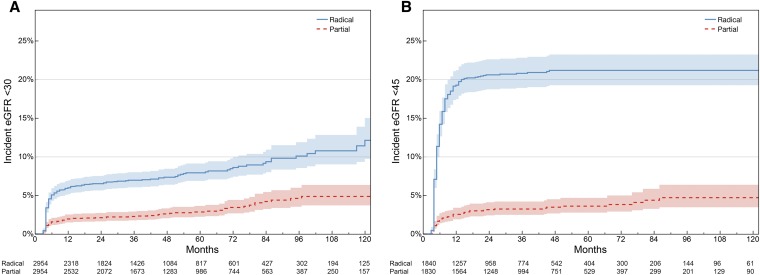
In the full cohort (n=14,129), a total of 1121 (7.9%) patients experienced incident stage 4 or higher CKD, including 183 patients after partial nephrectomy and 938 patients after radical nephrectomy. Kaplan–Meier plots demonstrate the freedom from stage 4 or higher CKD for all patients and from stage 3b or higher CKD for patients with normal or near-normal preoperative kidney function (Figure 1). For patients experiencing a kidney function outcome, most (75%) occurred within the first 9 months after surgery, with a median time from surgery of 5 months (25th, 75th percentile, 4–8 months). In the full cohort, the average postoperative eGFR measurements declined slowly after partial nephrectomy (−0.41 ml/min per 1.73 m2 per year), and increased slightly after radical nephrectomy (0.25 ml/min per 1.73 m2 per year) (Figure 2, Supplemental Figure 1). The unadjusted patient demographics of the full cohort are included in Supplemental Table 1.
Figure 1.
The incidence of kidney function outcomes was greater among patients treated with radical nephrectomy when compared with patients receiving partial nephrectomy in the full analytic cohort (n=14,129). (A) Over a median follow-up of 40 months (25th, 75th percentile, 15–74 months), 183 of 4370 (4.2%) patients experienced stage 4 or higher CKD after partial nephrectomy compared with 938 of 9759 (9.6%) after radical nephrectomy. (B) In 8089 patients with normal or near-normal kidney function, 96 of 2849 (3.7%) experienced stage 3b CKD or higher after partial nephrectomy compared with 1053 of 5240 (20.1%) after radical nephrectomy. (C) The median time to kidney function event was 5 months (25th, 75th percentile, 4–9 months) in the full cohort. (D) In patients with normal or near-normal preoperative kidney function, the median time to stage 3b CKD was 5 months (25th, 75th percentile, 4–8 months).
Figure 2.
A contour plot of the density of 440,582 postoperative eGFR measurements after either radical or partial nephrectomy illustrates the stability of kidney function measurements over time in the full cohort. The density of eGFR measurements is illustrated by each contour. The linear trend for kidney function over time is reflected by the regression lines.
Preoperative kidney function was inversely associated with the development of clinically significant CKD, whereas older age at the time of surgery was directly associated with the development of clinically significant CKD. Similarly, preoperative kidney function and patient age were associated with the development of stage 3b or greater CKD for patients with normal or near-normal preoperative kidney function (Supplemental Figures 2 and 3). Patients with larger tumors (T2) treated with partial nephrectomy did show increased incidence of clinically significant postoperative CKD when compared with patients with T1 tumors (Supplemental Figure 4).
Propensity Score–Matched Cohorts
Propensity score matching balanced important patient characteristics (Supplemental Table 2, Table 1). Similar to the full cohort, among patients in the propensity score–matched cohorts, 83 patients (2.8%) experienced incident stage 4 or higher CKD after partial nephrectomy compared with 212 patients (7.2%) after radical nephrectomy. In the propensity score–matched cohort with normal or near-normal kidney function, 62 patients (3.4%) developed stage 3b or higher CKD compared with 354 (19.2%) patients after radical nephrectomy (Figure 3).
Table 1.
Patient demographics of the propensity score–matched cohorts
| Characteristic | Preoperative Kidney Function eGFR>30 ml/min per 1.73 m2 | Preoperative Kidney Function eGFR>60 ml/min per 1.73 m2 | ||
|---|---|---|---|---|
| Radical Nephrectomy n=2954 | Partial Nephrectomy n=2954 | Radical Nephrectomy n=1840 | Partial Nephrectomy n=1840 | |
| Age, mean (25th–75th %) | 64.0 (59–69) | 63.9 (59–69) | 62.2 (58–67) | 62.2 (58–66) |
| Age group, n (%) | ||||
| <60 | 898 (30.4) | 912 (30.9) | 687 (37.3) | 699 (38.0) |
| 60–64 | 713 (24.1) | 711 (24.1) | 481 (26.1) | 461 (25.1) |
| 65–69 | 707 (23.9) | 695 (23.5) | 426 (23.2) | 434 (23.6) |
| 70–74 | 329 (11.1) | 323 (10.9) | 155 (8.4) | 160 (8.7) |
| 75–79 | 207 (7.0) | 215 (7.3) | 71 (3.9) | 64 (3.5) |
| ≥80 | 100 (3.4) | 98 (3.3) | 20 (1.1) | 22 (1.2) |
| Sex, n (%) | ||||
| Men | 2938 (99.5) | 2938 (99.5) | 1832 (99.6) | 1832 (99.6) |
| Women | 16 (0.5) | 16 (0.5) | 8 (0.4) | 8 (0.4) |
| Race/ethnicity, n (%) | ||||
| White | 2494 (84.4) | 2494 (84.4) | 1542 (83.8) | 1542 (83.8) |
| Black | 334 (11.3) | 223 (11.3) | 228 (12.4) | 228 (12.4) |
| Other or unknown | 126 (4.3) | 126 (4.3) | 70 (3.8) | 70 (3.8) |
| Preoperative serum creatinine, mean (25th–75th %) | 1.11 (0.95–1.24) | 1.11 (0.94–1.24) | 0.98 (0.90–1.07) | 0.98 (0.89–1.07) |
| Preoperative eGFR, mean (25th–75th %) | 73.4 (61.6–84.5) | 73.3 (61.2–84.7) | 82.4 (73.2–90.0) | 82.5 (73.2–89.8) |
| Preoperative eGFR, n (%) | ||||
| 30–45 | 110 (3.7) | 100 (3.4) | 0 (0) | 0 (0) |
| 46–60 | 558 (18.9) | 548 (18.6) | 12 (0.7) | 6 (0.3) |
| 61–90 | 1814 (61.4) | 1830 (61.9) | 1367 (74.3) | 1383 (75.2) |
| >90 | 472 (16.0) | 476 (16.1) | 461 (25.1) | 451 (24.5) |
| Charlson score, mean | 2.02 | 1.98 | 1.84 | 1.87 |
| Charlson score, n (%) | ||||
| 0 | 675 (22.9) | 675 (22.9) | 477 (25.9) | 477 (25.9) |
| 1 | 384 (13.0) | 384 (13.0) | 230 (12.5) | 230 (12.5) |
| 2 | 888 (30.1) | 888 (30.1) | 591 (32.1) | 591 (32.1) |
| ≥3 | 1007 (34.1) | 1007 (34.1) | 542 (29.5) | 542 (29.5) |
| Year of surgery, n (%) | ||||
| 2001 | 73 (2.5) | 73 (2.5) | 32 (1.7) | 32 (1.7) |
| 2002 | 90 (3.0) | 90 (3.0) | 50 (2.7) | 50 (2.7) |
| 2003 | 130 (4.4) | 130 (4.4) | 65 (3.5) | 65 (3.5) |
| 2004 | 163 (5.5) | 163 (5.5) | 73 (4.0) | 73 (4.0) |
| 2005 | 196 (6.6) | 196 (6.6) | 99 (5.4) | 99 (5.4) |
| 2006 | 231 (7.8) | 231 (7.8) | 113 (6.1) | 113 (6.1) |
| 2007 | 226 (7.7) | 226 (7.7) | 151 (8.2) | 151 (8.2) |
| 2008 | 288 (9.7) | 288 (9.7) | 168 (9.1) | 168 (9.1) |
| 2009 | 286 (9.7) | 286 (9.7) | 200 (10.9) | 200 (10.9) |
| 2010 | 367 (12.4) | 367 (12.4) | 256 (13.9) | 256 (13.9) |
| 2011 | 340 (11.5) | 340 (11.5) | 234 (12.7) | 234 (12.7) |
| 2012 | 379 (12.8) | 379 (12.8) | 258 (14.0) | 258 (14.0) |
| 2013 | 203 (6.9) | 203 (6.9) | 141 (7.7) | 141 (7.7) |
Figure 3.
The incidence of kidney function outcomes following either partial or radical nephrectomy for the propensity score–matched cohort (n=5908). (A) Over a median follow-up of 38 months (25th, 75th percentile, 16–68 months), 83 patients experienced stage 4 or higher CKD after partial nephrectomy compared with 212 after radical nephrectomy (log-rank P<0.001). (B) In the propensity score–matched cohort of 3680 patients with normal or near-normal kidney function, 62 experienced stage 3b or higher CKD after partial nephrectomy compared with 354 after radical nephrectomy (log-rank P<0.001).
In propensity score–matched cohorts, the relative hazard (partial versus radical nephrectomy) of incident CKD stage 4 or higher was significantly reduced (0.34; 95% confidence interval [95% CI], 0.26 to 0.43) (Table 2). The association remained after controlling for tumor stage (0.28; 95% CI, 0.18 to 0.43) (Supplemental Table 3). Among patients with normal or near-normal kidney function, the relative hazard of incident CKD stage 3b or higher was also significantly reduced (0.15; 95% CI, 0.11 to 0.19); this association was unchanged after further adjustment for tumor stage (0.13; 95% CI, 0.08 to 0.20).
Table 2.
Proportional hazards models estimating the risk of renal function events in the propensity score–matched cohorts after kidney cancer surgery
| Characteristic | Preoperative Kidney Function eGFR>30 ml/min per 1.73 m2 | Preoperative Kidney Function eGFR>60 ml/min per 1.73 m2 | ||
|---|---|---|---|---|
| Unadjusted HR (95% CI) | Adjusted HR (95% CI) | Unadjusted HR (95% CI) | Adjusted HR (95% CI) | |
| Age (per 10 yr increase) | 1.69 (1.47 to 1.94)a | 1.18 (1.02 to 1.38)a | 1.60 (1.40 to 1.82)a | 1.64 (1.43 to 1.89)a |
| Sex (female versus male) | 0.56 (0.08 to 4.0) | 2.13 (0.29 to 15.5) | 0.59 (0.08 to 4.23) | 6.62 (0.9 to 48.6) |
| Race/ethnicity | ||||
| Black versus white | 1.07 (0.76 to 1.52) | 0.71 (0.5 to 1.02) | 0.85 (0.63 to 1.16) | 0.36 (0.26 to 0.51)a |
| Other/unknown versus white | 1.44 (0.88 to 2.35) | 1.41 (0.86 to 2.32) | 0.67 (0.37 to 1.23) | 0.75 (0.41 to 1.37) |
| Serum creatinine (per 0.1 mg/dl increase) | 1.40 (1.35 to 1.45)a | 1.40 (1.35 to 1.45)a | 1.55 (1.44 to 1.66)a | 1.90 (1.74 to 2.08)a |
| Partial versus radical nephrectomy | 0.37 (0.28 to 0.47)a | 0.34 (0.26 to 0.43)a | 0.16 (0.12 to 0.21)a | 0.15 (0.11 to 0.19)a |
| Charlson score | ||||
| 1 versus 0 | 1.42 (0.87 to 2.31) | 1.24 (0.76 to 2.03) | 1.05 (0.74 to 1.50) | 0.99 (0.69 to 1.41) |
| 2 versus 0 | 1.91 (1.30 to 2.79)a | 1.56 (1.06 to 2.30)a | 1.10 (0.84 to 1.43) | 0.97 (0.74 to 1.27) |
| ≥3 versus 0 | 2.87 (2.0 to 4.12)a | 2.07 (1.43 to 3.01)a | 1.47 (1.14 to 1.90)a | 1.45 (1.11 to 1.90)a |
| Surgery year (versus 2001) | ||||
| 2002 | 1.49 (0.69 to 3.22) | 1.70 (0.78 to 3.68) | 1.08 (0.47 to 2.46) | 1.36 (0.59 to 3.12) |
| 2003 | 1.29 (0.61 to 2.72) | 1.26 (0.60 to 2.67) | 0.67 (0.28 to 1.59) | 0.62 (0.26 to 1.48) |
| 2004 | 1.21 (0.58 to 2.52) | 1.23 (0.59 to 2.57) | 0.45 (0.18 to 1.12) | 0.44 (0.17 to 1.12) |
| 2005 | 0.92 (0.44 to 1.93) | 1.05 (0.50 to 2.21) | 0.75 (0.34 to 1.64) | 0.76 (0.35 to 1.67) |
| 2006 | 1.28 (0.63 to 2.62) | 1.22 (0.59 to 2.49) | 1.07 (0.51 to 2.23) | 1.06 (0.50 to 2.22) |
| 2007 | 1.02 (0.49 to 2.13) | 1.24 (0.59 to 2.60) | 0.85 (0.41 to 1.77) | 0.87 (0.42 to 1.82) |
| 2008 | 0.94 (0.45 to 1.95) | 1.08 (0.52 to 2.26) | 0.99 (0.48 to 2.02) | 0.98 (0.48 to 2.02) |
| 2009 | 1.05 (0.51 to 2.17) | 1.70 (0.82 to 3.52) | 0.93 (0.46 to 1.91) | 0.92 (0.45 to 1.89) |
| 2010 | 0.69 (0.33 to 1.46) | 1.18 (0.55 to 2.51) | 0.82 (0.40 to 1.66) | 0.83 (0.41 to 1.69) |
| 2011 | 0.68 (0.32 to 1.46) | 0.97 (0.45 to 2.09) | 0.98 (0.49 to 1.99) | 1.06 (0.52 to 2.14) |
| 2012 | 0.97 (0.47 to 2.01) | 1.61 (0.77 to 3.35) | 0.85 (0.42 to 1.74) | 0.96 (0.47 to 1.95) |
| 2013 | 1.30 (0.56 to 2.99) | 1.97 (0.85 to 4.55) | 1.45 (0.68 to 3.09) | 1.50 (0.70 to 3.20) |
HR, Hazard Ratio.
Hazard ratios met a statistical significance threshold of P<0.05.
Competing Risks Analysis
The adjusted competing risks regression model is shown in Supplemental Table 4. More patients in the propensity score–matched cohort died (n=1107, 18.7%) than experienced clinically significant CKD (n=295, 5.0%). Partial nephrectomy was independently associated with a lower relative hazard of CKD in the propensity score–matched cohort (subdistribution relative hazard, 0.36; 95% CI, 0.28 to 0.47). This association was preserved when controlling for tumor T stage (0.28; 95% CI, 0.18 to 0.44). Among propensity score–matched patients with normal or near-normal preoperative kidney function, 502 patients (13.6%) died whereas 416 (11.3%) experienced decline in kidney function to eGFR<45 ml/min per 1.73 m2. Partial nephrectomy was associated with a lower relative hazard of kidney function decline (0.15; 95% CI, 0.12 to 0.20); an effect that persisted after adjusting for tumor T stage (0.13; 95% CI, 0.0 to 0.21).
Overall Survival Analysis
The unadjusted differences in overall survival in each propensity score–matched cohort are shown in Figure 4. Over a median follow-up of 47 months (25th, 75th percentile, 24–77 months), more patients died after radical nephrectomy (760, 25.7%) than partial nephrectomy (472, 16.0%) in the primary propensity score–matched cohort. Similarly, more patients died after radical nephrectomy (212, 11.5%) than partial nephrectomy (83, 4.5%) among patients with normal or near-normal preoperative kidney function. These differences were consistent in propensity score–matched cohorts that included clinical tumor T stage as matching criteria. The hazard of death was significantly reduced in fully adjusted proportional hazards models performed in the primary propensity score–matched cohort (Hazard Ratio, 0.55; 95% CI, 0.49 to 0.62) and in the cohort of patients with normal or near-normal preoperative kidney function (Hazard Ratio, 0.52; 95% CI, 0.44 to 0.61) (Table 3).
Figure 4.
Patients treated with partial nephrectomy demonstrated improved overall survival in each of the propensity score–matched cohorts. (A) The primary propensity score–matched cohort (preoperative kidney function eGFR>30 ml/min per 1.73 m2); (B) additionally matched by clinical T stage; (C) patients with normal or near-normal kidney function (eGFR>60 ml/min per 1.73 m2); and (D) additionally matched by tumor T stage. PSM, Propensity Score-Matched.
Table 3.
Multivariable proportional hazards models estimating the risk of death in the propensity score-matched cohorts after kidney cancer surgery
| Characteristic | Preoperative Kidney Function eGFR>30 ml/min per 1.73 m2 | Preoperative Kidney Function eGFR>60 ml/min per 1.73 m2 | ||
|---|---|---|---|---|
| Adjusted HR (95% CI) | Adjusted with T Stage HR (95% CI) | Adjusted HR (95% CI) | Adjusted with T Stage HR (95% CI) | |
| Age (per 10 yr increase) | 1.44 (1.34 to 1.55)a | 1.39 (1.22 to 1.58)a | 1.34 (1.21 to 1.48)a | 1.36 (1.11 to 1.65)a |
| Sex (female versus male) | 0.33 (0.08 to 1.40) | |||
| Race/ethnicity | ||||
| Black versus white | 0.83 (0.68 to 1.01) | 0.75 (0.53 to 1.07) | 0.99 (0.75 to 1.31) | 1.05 (0.66 to 1.69) |
| Other/unknown versus white | 1.12 (0.87 to 1.45) | 0.94 (0.55 to 1.61) | 0.88 (0.57 to 1.36) | 0.39 (0.11 to 1.31) |
| Serum creatinine (per 0.1 mg/dl increase) | 1.01 (0.98 to 1.03) | 1.02 (0.98 to 1.07) | 0.88 (0.82 to 0.95)a | 0.84 (0.74 to 0.95)a |
| Partial versus radical nephrectomy | 0.55 (0.49 to 0.62)a | 0.68 (0.57 to 0.82)a | 0.52 (0.44 to 0.61)a | 0.69 (0.52 to 0.90)a |
| Charlson score | ||||
| 1 versus 0 | 1.25 (1.01 to 1.56)a | 1.69 (1.12 to 2.55)a | 1.25 (0.93 to 1.69) | 1.45 (0.82 to 2.57) |
| 2 versus 0 | 1.25 (1.05 to 1.49)a | 1.71 (1.25 to 2.34)a | 1.15 (0.91 to 1.46) | 1.34 0.86 to 2.08) |
| ≥3 versus 0 | 2.04 (1.73 to 2.41)a | 3.03 (2.25 to 4.09)a | 2.07 (1.64 to 2.60)a | 2.74 (1.82 to 4.13)a |
| Surgery year (versus 2001) | ||||
| 2002 | 0.97 (0.71 to 1.31) | 0.68 (0.42 to 1.11) | 1.88 (1.11 to 3.17) | 0.92 (0.37 to 2.32) |
| 2003 | 0.97 (0.74 to 1.29) | 0.71 (0.45 to 1.13) | 1.87 (1.12 to 3.12) | 1.41 (0.64 to 3.08) |
| 2004 | 0.98 (0.74 to 1.29) | 0.62 (0.39 to 0.98)a | 2.13 (1.29 to 3.52) | 0.85 (0.37 to 1.96) |
| 2005 | 0.83 (0.62 to 1.10) | 0.72 (0.48 to 1.09) | 1.37 (0.83 to 2.28) | 1.09 (0.53 to 2.25) |
| 2006 | 1.01 (0.77 to 1.33) | 0.78 (0.50 to 1.20) | 1.95 (1.19 to 3.20) | 1.41 (0.69 to 2.88) |
| 2007 | 0.86 (0.65 to 1.15) | 0.63 (0.40 to 0.99)a | 1.45 (0.87 to 2.40) | 1.11 (0.53 to 2.32) |
| 2008 | 0.82 (0.62 to 1.09) | 0.86 (0.57 to 1.31) | 1.58 (0.96 to 2.59) | 1.51 (0.76 to 3.00) |
| 2009 | 0.78 (0.57 to 1.05) | 0.69 (0.44 to 1.09) | 1.15 (0.68 to 1.93) | 1.00 (0.47 to 2.13) |
| 2010 | 0.76 (0.56 to 1.02) | 0.81 (0.52 to 1.28) | 1.40 (0.84 to 2.32) | 1.40 (0.68 to 2.88) |
| 2011 | 0.74 (0.53 to 1.04) | 0.85 (0.50 to 1.47) | 1.30 (0.75 to 2.26) | 1.51 (0.68 to 3.35) |
| 2012 | 0.60 (0.40 to 0.89)a | 0.54 (0.26 to 1.11) | 0.79 (0.41 to 1.53) | 0.29 (0.06 to 1.36) |
| 2013 | 0.45 (0.20 to 0.97)a | 0.75 (0.22 to 2.51) | 0.41 (0.10 to 1.80) | 0.73 (0.09 to 5.84) |
| Clinical T stage | ||||
| T2 versus T1 | 0.94 (0.58 to 1.52) | 1.09 (0.45 to 2.67) | ||
| T3 versus T1 | 2.08 (1.56 to 2.76)a | 3.06 (1.96 to 4.79)a | ||
HR, Hazard Ratio.
Hazard ratios met a statistical significance threshold of P<0.05.
Discussion
The likelihood of decline in kidney function after kidney cancer surgery is an important consideration when treating patients with a renal mass. Previous reports have explored the decline in kidney function after kidney cancer surgery using administrative claims for ESRD or receipt of dialysis,17 using laboratory results in clinical trials18 or data from single institutions. In this study, we measured changes in kidney function after kidney cancer surgery using electronic health record data from the largest nation-wide integrated health care system in the United States. These data show that a decline in kidney function after kidney cancer surgery is relatively common. Nearly 8% of patients experienced incident stage 4 or higher CKD, and 14.2% of patients with normal or near-normal kidney function experienced incident stage 3b or higher CKD.
These data help establish and quantify the comparative effectiveness of partial nephrectomy versus radical nephrectomy in preserving kidney function in an era of increasing utilization of elective partial nephrectomy. To account for selection bias on the basis of observed characteristics,16 we used propensity score matching and fitted multivariate proportional hazards and competing risk regression models. Our results suggest that receipt of partial nephrectomy was associated with a >60% reduction of the hazard of developing advanced (stage 4 or higher) CKD. For patients with normal or near-normal preoperative kidney function, partial nephrectomy offered an even greater 80% reduction in the hazard of eGFR decline to stage 3b or higher CKD.
These data build on previous single institution reports documenting the role of partial nephrectomy in preserving kidney function.19,20 Although administrative data does not include direct measurements of kidney function, Miller et al.17 and Sun et al.21 both reported that partial nephrectomy was protective against incident claims for CKD or RRT in the Surveillance, Epidemiology, and End Results–Medicare data. Kidney function outcomes have also been measured as a secondary outcome in a prospective randomized, controlled trial.18 In this trial, patients with normal preoperative creatinine levels treated with partial nephrectomy were less likely to reach a postoperative eGFR<60 ml/min per 1.73 m2 (38.4% versus 58.7%) or a postoperative eGFR<30 ml/min per 1.73 m2 (3.5% versus 6.6%) than those receiving radical nephrectomy. A growing number of reports have documented the safety of partial nephrectomy in older patients,22,23 and provide evidence for a survival benefit in older patients undergoing partial nephrectomy.24 Our analysis of propensity score–matched cohorts also suggests a survival benefit for patients who receive a partial nephrectomy. Together, these studies support the contemporary use of partial nephrectomy in older patients and those with impaired preoperative kidney function when feasible.
This report also illustrates how patient age and preoperative kidney function can be used for preoperative counseling. Each 0.1 mg/dl increase in the preoperative serum creatinine concentration was associated with a 40% increase in the hazard of incident stage 4 or higher CKD, and a 90% increase in the hazard of stage 3b or higher CKD for patients with normal or near-normal preoperative kidney function. For every 10-year increase in age, the hazard of development of stage 4 or greater CKD increased 18%, and among patients with normal or near-normal kidney function, the hazard of developing stage 3b or greater CKD increased 64%. The association of age with decline in kidney function was particularly pronounced after radical nephrectomy, where 1.4%, 6.2%, 8.9%, and 15.1% of patients aged <50, 50–60, 60–70, and older than 70 years, respectively, experienced stage 4 or greater CKD, suggesting a loss of renal reserve.
Our results suggest that surgically induced reductions in nephron mass may manifest differently than loss of kidney function associated with medical causes. One of the more striking findings of this study was that the majority (nearly 80%) of eGFR decline occurred within the first year of surgery. After reaching a new (postoperative) level of baseline function, few patients progressed to develop advanced CKD. In the full cohort, patients had a mean decline in postoperative eGFR of 0.41 ml/min per 1.73 m2 per year (0.64% decline per year) after partial nephrectomy and an increase of 0.25 ml/min per 1.73 m2 per year after radical nephrectomy (0.53% increase per year). In contrast, medical conditions have been associated with steeper downward trajectories in kidney function.25 This is notable given the cohort’s relatively low baseline preoperative kidney function, and the presence of comorbidities commonly associated with impaired kidney function. Our observation of stable kidney function is also similar to the long-term kidney function outcomes after partial nephrectomy from a large, single-institution series.26 More generally, these findings raise the question of whether the decline in kidney function associated with a surgical reduction in nephron mass should be considered CKD—a controversy similar to that raised in the setting of living kidney donors.27
In this study, patients that received a partial nephrectomy had a significantly reduced risk of mortality (30%–45% reduction) when compared with patients who received a radical nephrectomy. Partial nephrectomy has been associated with improved survival outcomes in many single institution series. In 2012, Tan et al.24 also identified improved survival after partial nephrectomy among Medicare patients using an instrumental variable analysis. However, the only available randomized clinical trial comparing radical and partial nephrectomy did not demonstrate any difference in survival at a median follow-up of 9 years.18 Most of the patients in this trial were healthy, with no chronic medical conditions and normal or near-normal preoperative kidney function. We have shown previously that patients who undergo partial nephrectomy in the VHA are generally younger, with better kidney function, compared with those who undergo radical nephrectomy.16 Future research is required to determine if the survival difference seen among veterans receiving care in the VHA reflects residual selection bias, and if surgically induced kidney function loss is associated with other morbidities, such as cardiovascular disease.Our study has some notable strengths. Most importantly, we were able to determine the presence or absence of CKD at baseline and during follow-up using laboratory data rather than diagnosis codes, which may under-ascertain and/or misclassify CKD or CKD stage. Our study also has some limitations. Although we were able to adjust for numerous clinical characteristics, accounted for differential utilization of partial versus radical nephrectomy using propensity score matching, and considered the role of competing risks, some residual confounding is likely. Nevertheless, the point estimates and upper ends of the 95% CIs were well below unity. It is unlikely that residual confounding could change the qualitative results. These data were derived from the VHA population, which is older and almost entirely male. Veterans with cancer receiving care in the VHA are known to have lower performance status and higher rates of severe comorbidity.28 Consequently, our results may not be fully generalizable to women, or younger, healthier patients. Despite these concerns, veterans treated in the VHA are an ideal population to evaluate kidney function outcomes after kidney surgery. Kidney cancer is twice as likely to occur in men, and more than half of patients diagnosed with kidney cancer are between 55 and 75 years of age.29 Not surprisingly, kidney cancer is one of the most common cancers diagnosed among veterans enrolled in the VHA.30 Outcomes for patients receiving cancer care in the VHA have been shown to be comparable or better than in the community.28 Because the VHA maintains a national electronic health record and research data, the VHA is also uniquely positioned to assess kidney function over time in a nationally distributed “real world” setting. Partial nephrectomy was associated with a lower risk of developing clinically significant CKD than radical nephrectomy. Postoperative decline in kidney function occurs most commonly in the first 12 months after surgery and appears stable over time. Tumor stage, patient age, and preoperative kidney function are predictors of incident CKD after kidney cancer surgery.
Concise Methods
The study was approved by the Veterans Affairs Palo Alto Health Care System Research and Development Committee and the Stanford University Institutional Review Board.
Analytic Cohort and Patient Characteristics
The VHA national utilization files include information for all veterans receiving VHA care. We identified 19,006 patients who had a radical or partial nephrectomy in the VHA from 2001 to 2013 (Supplemental Table 5). We excluded patients with more than one kidney cancer surgical code during the study cohort (n=957). Comorbidity was ascertained using the Romano adaptation of the Charlson–Deyo comorbidity score.31
Cancer-Specific Information
We extracted tumor-specific data from the Corporate Data Warehouse Oncology data when available using the 2010 TNM staging system. Patients with metastatic kidney cancer (clinical T stage=4, N stage >0, or M stage >0) were excluded from the analytic cohort. More than two-thirds of all patients (9422 patients, 67% of the total cohort; 5663 patients, 70% of patients with normal or near-normal kidney function) had available tumor information.
Pre- and Postoperative Measures of Kidney Function
We identified serum creatinine measurements using the Managerial Cost Accounting laboratory data file, including 117,763 preoperative serum creatinine measurements during the 12-month period before the surgery date. We excluded patients with no available preoperative serum creatinine values (n=1200) and those patients with two or more preoperative eGFR values <30 ml/min per 1.73 m2 or dialysis procedure codes (n=1453). We calculated the eGFR using the four-variable Modification of Diet in Renal Disease study equation.32
We identified 440,582 postoperative serum creatinine measurements. We excluded serum creatinine measurements in the first 3 months after surgery in order to avoid detection of transient postoperative AKI. We excluded patients with no postoperative serum creatinine measurements (n=696), resulting in a total analytic cohort of 14,129 patients. To determine the risk of clinically significant CKD among patients with normal or near-normal kidney function preoperatively, we also performed a parallel analysis of 8089 patients with preoperative eGFR values ≥60 ml/min per 1.73 m2.
Propensity Score Matching
We constructed propensity score–matched cohorts to adjust for the previously described differences in characteristics between patients treated with partial and radical nephrectomy.16 We included patient characteristics (age, sex, race/ethnicity, Charlson score, mean preoperative serum creatinine, and year of surgery) as matching variables. We then used a two-digit greedy matching algorithm resulting in a cohort of 5908 patients with a preoperative eGFR≥30 ml/min per 1.73 m2 and a parallel cohort of 3680 patients with a preoperative eGFR≥60 ml/min per 1.73 m2. Separate propensity score–matched cohorts were also created for patients with available tumor information.
Kidney Function Outcomes
Our primary outcome was the time to incident clinically significant CKD, which we defined as the first eGFR<30 ml/min per 1.73 m2 or any claim for initiation of dialysis. Among patients with normal or near-normal preoperative kidney function, we evaluated the time to the first eGFR<45 ml/min per 1.73 m2 or the receipt of dialysis. For time-to–kidney function outcomes, patient follow-up was censored at the time of the last available serum creatinine measurement.
Mortality and Competing Risk Regression
Patient survival was determined using the Veterans Affairs (VA) Vital Status File using deaths recorded before January 1, 2014. We defined overall survival as the time from the date of surgery to the date of death. We censored patient follow-up on January 1, 2014 for patients who were alive on or after the study end date. We fitted multivariable competing risk regression models using the Fine and Gray modification of proportional hazards regression models within the propensity score–matched cohorts.33
Statistical Analyses
Preoperative characteristics were compared between radical and partial nephrectomy groups using the Mann–Whitney test for continuous variables and the chi-squared test for categoric variables. We generated Kaplan–Meier product limit estimates to compare the unadjusted association of surgery type, preoperative kidney function, and tumor characteristics on kidney function outcomes. We fitted adjusted proportional hazards regression models to test patient and clinical factors associated with decline in kidney function outcomes in propensity score–matched cohorts. We included baseline serum creatinine concentrations in the proportional hazards models, instead of eGFR, to avoid the inclusion of age and race in multiple model parameters. All statistical analyses were conducted within the VA Informatics and Computing Infrastructure (VINCI) platform using SAS v9.4 (Cary, NC) and figures were generated using JMP Pro v13 (Cary, NC).
Disclosures
None.
Supplementary Material
Acknowledgments
The authors were supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (DK089086 to J.T.L. and DK085446 to G.M.C.) at the National Institutes of Health.
R.W.L. received second place in the 2015 Miley B. Wesson Resident Essay Contest at the Western Section of the American Urological Association for an abridged version of this manuscript.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2017020136/-/DCSupplemental.
References
- 1.Robson CJ, Churchill BM, Anderson W: The results of radical nephrectomy for renal cell carcinoma. J Urol 101: 297–301, 1969 [DOI] [PubMed] [Google Scholar]
- 2.Patel SG, Penson DF, Pabla B, Clark PE, Cookson MS, Chang SS, Herrell SD, Smith JA Jr, Barocas DA: National trends in the use of partial nephrectomy: A rising tide that has not lifted all boats. J Urol 187: 816–821, 2012 [DOI] [PubMed] [Google Scholar]
- 3.Bjurlin MA, Walter D, Taksler GB, Huang WC, Wysock JS, Sivarajan G, Loeb S, Taneja SS, Makarov DV: National trends in the utilization of partial nephrectomy before and after the establishment of AUA guidelines for the management of renal masses. Urology 82: 1283–1289, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Butler BP, Novick AC, Miller DP, Campbell SA, Licht MR: Management of small unilateral renal cell carcinomas: Radical versus nephron-sparing surgery. Urology 45: 34–40; discussion 40–31, 1995 [DOI] [PubMed]
- 5.Fergany AF, Hafez KS, Novick AC: Long-term results of nephron sparing surgery for localized renal cell carcinoma: 10-year followup. J Urol 163: 442–445, 2000 [PubMed] [Google Scholar]
- 6.Belldegrun A, Tsui KH, deKernion JB, Smith RB: Efficacy of nephron-sparing surgery for renal cell carcinoma: Analysis based on the new 1997 tumor-node-metastasis staging system. J Clin Oncol 17: 2868–2875, 1999 [DOI] [PubMed] [Google Scholar]
- 7.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Drey N, Roderick P, Mullee M, Rogerson M: A population-based study of the incidence and outcomes of diagnosed chronic kidney disease. Am J Kidney Dis 42: 677–684, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Culleton BF, Larson MG, Wilson PW, Evans JC, Parfrey PS, Levy D: Cardiovascular disease and mortality in a community-based cohort with mild renal insufficiency. Kidney Int 56: 2214–2219, 1999 [DOI] [PubMed] [Google Scholar]
- 10.Vanholder R, Massy Z, Argiles A, Spasovski G, Verbeke F, Lameire N; European Uremic Toxin Work Group : Chronic kidney disease as cause of cardiovascular morbidity and mortality. Nephrol Dial Transplant 20: 1048–1056, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Mathew A, Devereaux PJ, O’Hare A, Tonelli M, Thiessen-Philbrook H, Nevis IF, Iansavichus AV, Garg AX: Chronic kidney disease and postoperative mortality: A systematic review and meta-analysis. Kidney Int 73: 1069–1081, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Campbell SC, Novick AC, Belldegrun A, Blute ML, Chow GK, Derweesh IH, Faraday MM, Kaouk JH, Leveillee RJ, Matin SF, Russo P, Uzzo RG; Practice Guidelines Committee of the American Urological Association : Guideline for management of the clinical T1 renal mass. J Urol 182: 1271–1279, 2009 [DOI] [PubMed] [Google Scholar]
- 13.Ljungberg B, Bensalah K, Canfield S, Dabestani S, Hofmann F, Hora M, Kuczyk MA, Lam T, Marconi L, Merseburger AS, Mulders P, Powles T, Staehler M, Volpe A, Bex A: EAU guidelines on renal cell carcinoma: 2014 update. Eur Urol 67: 913–924, 2015 [DOI] [PubMed] [Google Scholar]
- 14.Kazis LE, Miller DR, Skinner KM, Lee A, Ren XS, Clark JA, Rogers WH, Spiro A 3rd, Selim A, Linzer M, Payne SM, Mansell D, Fincke RG: Patient-reported measures of health: The Veterans Health study. J Ambul Care Manage 27: 70–83, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Rogers WH, Kazis LE, Miller DR, Skinner KM, Clark JA, Spiro A 3rd, Fincke RG: Comparing the health status of VA and non-VA ambulatory patients: The veterans’ health and medical outcomes studies. J Ambul Care Manage 27: 249–262, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Leppert JT, Mittakanti HR, Thomas IC, Lamberts RW, Sonn GA, Chung BI, Skinner EC, Wagner TH, Chertow GM, Brooks JD: Contemporary use of partial nephrectomy: Are older patients with impaired kidney function being left behind? Urology 100: 65–71, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller DC, Schonlau M, Litwin MS, Lai J, Saigal CS; Urologic Diseases in America Project : Renal and cardiovascular morbidity after partial or radical nephrectomy. Cancer 112: 511–520, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Scosyrev E, Messing EM, Sylvester R, Campbell S, Van Poppel H: Renal function after nephron-sparing surgery versus radical nephrectomy: Results from EORTC randomized trial 30904. Eur Urol 65: 372–377, 2014 [DOI] [PubMed] [Google Scholar]
- 19.Huang WC, Levey AS, Serio AM, Snyder M, Vickers AJ, Raj GV, Scardino PT, Russo P: Chronic kidney disease after nephrectomy in patients with renal cortical tumours: A retrospective cohort study. Lancet Oncol 7: 735–740, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mashni JW, Assel M, Maschino A, Russo M, Masi B, Bernstein M, Huang WC, Russo P: New chronic kidney disease and overall survival after nephrectomy for small renal cortical tumors. Urology 86: 1137–1143, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun M, Bianchi M, Hansen J, Trinh QD, Abdollah F, Tian Z, Sammon J, Shariat SF, Graefen M, Montorsi F, Perrotte P, Karakiewicz PI: Chronic kidney disease after nephrectomy in patients with small renal masses: A retrospective observational analysis. Eur Urol 62: 696–703, 2012 [DOI] [PubMed] [Google Scholar]
- 22.An JY, Ball MW, Gorin MA, Hong JJ, Johnson MH, Pavlovich CP, Allaf ME, Pierorazio PM: Partial vs radical nephrectomy for T1-T2 renal masses in the elderly: Comparison of complications, renal function, and oncologic outcomes. Urology 100: 151–157, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Corman JM, Penson DF, Hur K, Khuri SF, Daley J, Henderson W, Krieger JN: Comparison of complications after radical and partial nephrectomy: Results from the National Veterans Administration Surgical Quality Improvement Program. BJU Int 86: 782–789, 2000 [DOI] [PubMed] [Google Scholar]
- 24.Tan HJ, Norton EC, Ye Z, Hafez KS, Gore JL, Miller DC: Long-term survival following partial vs radical nephrectomy among older patients with early-stage kidney cancer. JAMA 307: 1629–1635, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klahr S, Levey AS, Beck GJ, Caggiula AW, Hunsicker L, Kusek JW, Striker G; Modification of Diet in Renal Disease Study Group : The effects of dietary protein restriction and blood-pressure control on the progression of chronic renal disease. N Engl J Med 330: 877–884, 1994 [DOI] [PubMed] [Google Scholar]
- 26.Lane BR, Campbell SC, Demirjian S, Fergany AF: Surgically induced chronic kidney disease may be associated with a lower risk of progression and mortality than medical chronic kidney disease. J Urol 189: 1649–1655, 2013 [DOI] [PubMed] [Google Scholar]
- 27.Tan JC, Ho B, Busque S, Blouch K, Derby G, Efron B, Myers BD: Imprecision of creatinine-based GFR estimates in uninephric kidney donors. Clin J Am Soc Nephrol 5: 497–502, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Landrum MB, Keating NL, Lamont EB, Bozeman SR, Krasnow SH, Shulman L, Brown JR, Earle CC, Rabin M, McNeil BJ: Survival of older patients with cancer in the Veterans Health Administration versus fee-for-service Medicare. J Clin Oncol 30: 1072–1079, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Howlader N, Noone AM, Krapcho M, Miller D, Bishop K, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA: SEER cancer statistics review, 1975-2014, Bethesda, MD, National Cancer Institute, 2017. Available at: https://seer.cancer.gov/csr/1975_2014/ [Google Scholar]
- 30.Zullig LL, Jackson GL, Dorn RA, Provenzale DT, McNeil R, Thomas CM, Kelley MJ: Cancer incidence among patients of the U.S. Veterans Affairs Health Care System. Mil Med 177: 693–701, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Romano PS, Roos LL, Jollis JG: Adapting a clinical comorbidity index for use with ICD-9-CM administrative data: Differing perspectives. J Clin Epidemiol 46: 1075–1079, discussion 1081–1090, 1993 [DOI] [PubMed] [Google Scholar]
- 32.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D; Modification of Diet in Renal Disease Study Group : A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Ann Intern Med 130: 461–470, 1999 [DOI] [PubMed] [Google Scholar]
- 33.Fine JP, Gray RJ: A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 94: 496–509, 1999 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.