Abstract
Bile acids are ligands for the nuclear hormone receptor farnesoid X receptor (FXR) and the G protein–coupled receptor TGR5. We have shown that FXR and TGR5 have renoprotective roles in diabetes- and obesity-related kidney disease. Here, we determined whether these effects are mediated through differential or synergistic signaling pathways. We administered the FXR/TGR5 dual agonist INT-767 to DBA/2J mice with streptozotocin-induced diabetes, db/db mice with type 2 diabetes, and C57BL/6J mice with high-fat diet-induced obesity. We also examined the individual effects of the selective FXR agonist obeticholic acid (OCA) and the TGR5 agonist INT-777 in diabetic mice. The FXR agonist OCA and the TGR5 agonist INT-777 modulated distinct renal signaling pathways involved in the pathogenesis and treatment of diabetic nephropathy. Treatment of diabetic DBA/2J and db/db mice with the dual FXR/TGR5 agonist INT-767 improved proteinuria and prevented podocyte injury, mesangial expansion, and tubulointerstitial fibrosis. INT-767 exerted coordinated effects on multiple pathways, including stimulation of a signaling cascade involving AMP-activated protein kinase, sirtuin 1, PGC-1α, sirtuin 3, estrogen-related receptor-α, and Nrf-1; inhibition of endoplasmic reticulum stress; and inhibition of enhanced renal fatty acid and cholesterol metabolism. Additionally, in mice with diet-induced obesity, INT-767 prevented mitochondrial dysfunction and oxidative stress determined by fluorescence lifetime imaging of NADH and kidney fibrosis determined by second harmonic imaging microscopy. These results identify the renal signaling pathways regulated by FXR and TGR5, which may be promising targets for the treatment of nephropathy in diabetes and obesity.
Keywords: diabetic nephropathy, metabolism, obesity
Diabetes mellitus is the leading cause of cardiovascular and renal disease in the United States.1–3 This is of increasing concern, because as many as one in four Americans are expected to become diabetic by the year 2050.4,5
The pathogenesis of diabetic complications is multifactorial and includes (1) hypertension; (2) abnormal carbohydrate metabolism; (3) increased profibrotic growth factors and vasoactive hormones, including renin, angiotensin II, aldosterone, TGF-β, and vascular endothelial growth factor; (4) upregulated inflammatory cytokines, including TNF-α, IL-1β, and IL-6; (5) increased oxidative stress and increased advanced glycation end products; and (6) abnormal lipid metabolism and accumulation of lipids, including cholesterol and triglycerides. All of these pathways contribute to the pathogenesis of diabetes-related renal and cardiovascular disease.6–13 In addition, recent studies also indicate that alterations in mitochondrial function play an essential role in diabetic kidney disease.14–17
In spite of all of the beneficial interventions implemented in patients with diabetes, including tight glucose control, stringent BP control, angiotensin-converting enzyme inhibition, angiotensin II receptor, or mineralocorticoid receptor antagonism, renal injury progresses in most of these patients.18,19 Additional treatment modalities that modulate the pathogenic pathways involved in diabetic nephropathy are, therefore, urgently needed to slow the progression of renal failure in patients with diabetes.
Bile acids activate several nuclear hormone receptors, in particular the farnesoid X receptor (FXR; also known as NR1H4) and the Takeda G protein–coupled receptor 5 (TGR5; also known as GPR131, GPBAR1, M-BAR, and BG37).20–23
Our laboratory has previously shown the renal protective role of FXR activation in (1) db/db mice, a model of type 2 diabetes mellitus24; (2) DBA/2J mice fed a Western diet, a model of diet-induced obesity and insulin resistance25; and (3) DBA/2J mice with streptozotocin (STZ)-induced diabetes.26 The renal protective effects of FXR activation are mediated through coordinated regulation of (1) lipid metabolism, (2) oxidative stress, (3) proinflammatory cytokines, and (4) profibrotic growth factors, which result in decreased albuminuria, podocyte loss, glomerular mesangial expansion, and renal lipid accumulation. Consistent with these data, FXR knockout mice with STZ-induced hyperglycemia exhibit marked renal injury, even in the nephropathy-resistant C57BL/6 genetic background.26
Our laboratory in addition has shown the renal protective role of TGR5 activation in (1) db/db mice and (2) mice with diet-induced obesity.27 The renal protective effects of TGR5 activation by treatment with the TGR5 selective agonist INT-777 were mediated through increases in renal expression of master regulators of mitochondrial biogenesis, inhibitors of oxidative stress, and inducers of fatty acid β-oxidation, including sirtuin 1 (SIRT1), SIRT3, estrogen-related receptor-α (ERR-α), and Nrf-1. Increased activity of SIRT3 was evidenced by normalization of the increased acetylation of mitochondrial SOD2 and isocitrate dehydrogenase 2 observed in untreated db/db mice. Accordingly, INT-777 decreased mitochondrial H2O2 generation and increased the activity of SOD2, which associated with decreased urinary levels of H2O2 and thiobarbituric acid reactive substances. Furthermore, INT-777 decreased renal lipid accumulation by activating mitochondrial fatty acid β-oxidation.
The molecular mechanisms underlying renal beneficial effects from FXR or TGR5 activation are not fully understood. However, using RNA-Seq to compare the samples of the treated diabetic groups with those of nontreated diabetic groups, we now find pathways that are known to be important for the pathogenesis of diabetic kidney disease but that are specifically regulated by FXR agonist or TGR5 agonist, which makes the dual FXR/TGR5 activation a potential new therapeutic target for diabetic kidney disease.
We have previously characterized the semisynthetic bile acid derivative INT-767 as the first agonist able to potently and selectively activate both FXR and TGR5.28 In this study, we have tested the efficacy of INT-767 in the treatment of kidney disease in diabetes and obesity, including in (1) DBA/2J mice with STZ-induced diabetes, (2) db/db mice with type 2 diabetes mellitus, and (3) C57BL/6J mice with diet-induced obesity. Our data indicate a protective role of INT-767 in diabetic kidney disease via regulation of multiple pathways relevant to the pathogenesis and prevention of diabetic nephropathy.
Results
FXR mRNA and Protein Expression Is Decreased in Human Nephropathy in Diabetes and Obesity
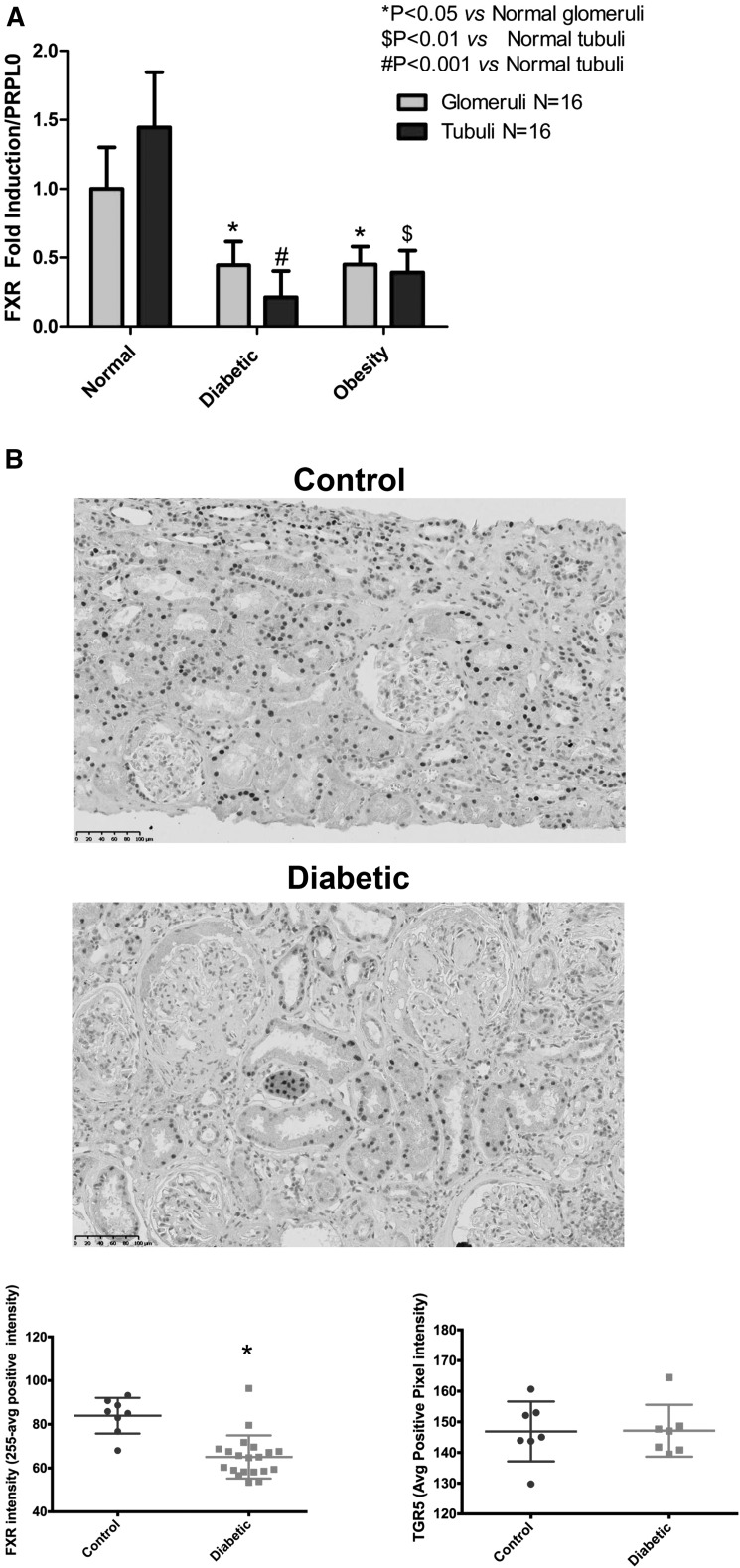
In kidney biopsies obtained from human subjects with nephropathy associated with diabetes and obesity that we have previously characterized,27 after laser capture microdissection, RNA extraction, and quantitative RT-PCR, we found that FXR mRNA is markedly reduced in both glomeruli and tubules (Figure 1A). We also performed immunohistochemistry for FXR in kidney biopsies obtained from human subjects with diabetic nephropathy. FXR staining in control subjects was concordant with the expression reported from rat tubular segments with predominant expression in the S1 segment of the proximal tubule and cortical thick ascending limb of the loop of Henle, with less expression in the S2 and S3 segment and distal convoluted tubule.29 In subjects with diabetic nephropathy, we found a significant decrease in FXR protein expression in the cortical tubular epithelium across segments, including in the proximal tubular epithelium (Figure 1B). In agreement with our earlier publication,27 we did not see changes in TGR5 protein expression (Figure 1B) in spite of a decrease in TGR5 mRNA.27
Figure 1.
FXR mRNA and protein expression are decreased in human kidney biopsies with diabetes- and obesity-related kidney disease. (A) Glomerular and tubular FXR mRNA levels are decreased in kidney biopsy samples obtained from human subjects with diabetic- and obesity-related kidney disease. Glomerular and tubular cells were obtained by laser capture microdissection. (B) FXR protein expression as determined by immunohistochemistry is decreased in kidney biopsy samples obtained from human subjects with diabetic kidney disease (n=16 subjects in each group).
Distinct Pathways Linked to FXR or TGR5 Activation in Diabetic Kidneys
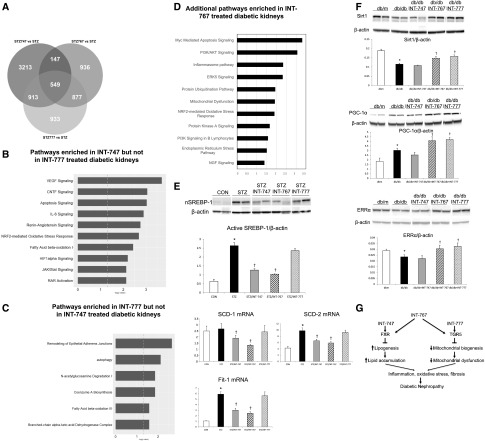
To determine if FXR versus TGR5 agonists activate differential pathways in the diabetic kidney, we treated DBA/2J mice with STZ-induced hyperglycemia with the FXR selective agonist obeticholic acid (INT-747),30 the TGR5 selective agonist INT-777,31 or the dual FXR/TGR5 agonist INT-767.28 RNA-Seq analysis indicates that INT-747 (Figure 2B) and INT-777 (Figure 2C) activate distinct pathways that are relevant for the pathogenesis and treatment of diabetic kidney disease. INT-767 can further activate an additional set of pathways (Figure 2, A and D). In the protein level, we found that both INT-747 and INT-767, but not INT-777, can regulate lipogenesis pathway mediated by SREBP-1 and targets SCD-1, SCD-2, and Fit-1 mRNA (Figure 2E). However, INT-777 and INT-767, but not INT-747, can both induce mitochondrial biogenesis pathway as shown by increases in SIRT1, PGC-1α, and ERR-α protein expression (Figure 2F). This is illustrated in a model where dual agonist INT-767 can simultaneously activate both FXR and TGR5 signaling and their nonoverlapping pathways, with potential additive effects (Figure 2G).
Figure 2.
FXR and TGR5 regulate common and distinct pathways in the kidney. Regulation of pathways by FXR and TGR5. RNA-Seq analysis (A) shows the transcript numbers regulated by INT-767 compared with INT-747 or INT-777 in the Venn diagram and indicates differential pathways that are regulated by (B) the FXR selective agonist INT-747 and (C) the TGR5-specific agonist INT-777 as well as (D) the additional pathways regulated by INT-767 that are not included in B or C. VEGF, vascular endothelial growth factor. (E and F) Western blot analysis reveals pathways specifically for FXR and not TGR5 or vice versa, but all were regulated by INT-767. (G) Proposed model showing that dual agonist INT-767 can simultaneously activate both FXR and TGR5 signaling, and their nonoverlapping pathways, with potential additive effects. CON, nondiabetic DBA/2J. *P<0.05 versus CON or db/m (n=6 mice per group); †P<0.05 versus STZ or db/db (n=6 mice per group).
INT-767 Decreases Albuminuria and Prevents Renal Histopathologic Alterations and Renal Fibrosis in Diabetic DBA/2J Mice
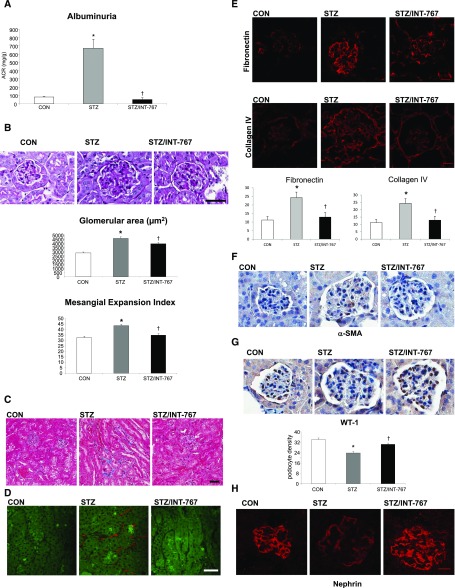
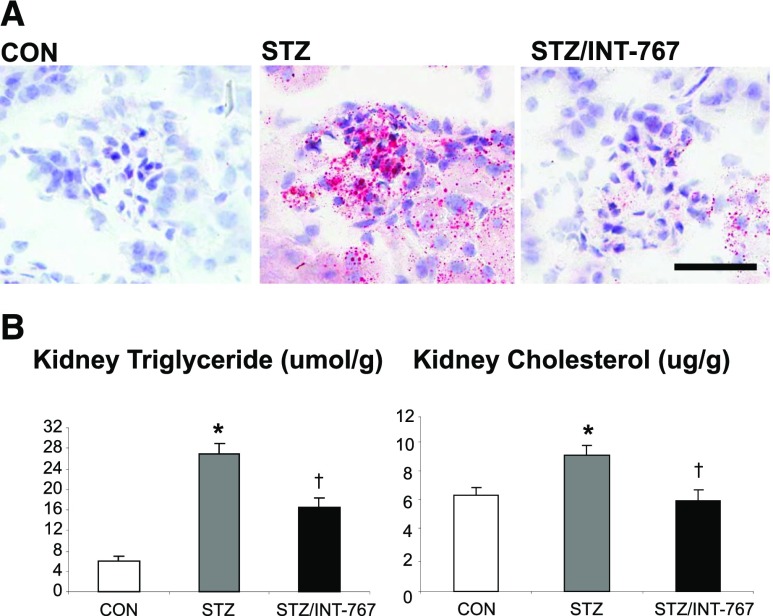
DBA/2J mice with STZ-induced hyperglycemia fed a standard chow diet (10 kcal% fat, complex carbohydrates) develop significant proteinuria, but they only develop very minimal renal histopathologic changes.32 When these mice were fed a Western diet (42 kcal% milkfat, 34% sucrose, 0.20% cholesterol; approximating the human Western diet), they developed marked albuminuria, which was normalized by INT-767 treatment (Figure 3A). Treatment with INT-767 also decreased the glomerular area and mesangial matrix expansion (Figure 3B). Masson trichrome staining shows patchy fibrosis in the tubular interstitium, which was nearly absent in the kidneys of INT-767–treated mice (Figure 3C). Label-free imaging of kidney sections with two-photon excitation and second harmonic generation (SHG) microscopy also shows accumulation of extracellular matrix proteins in the tubular interstitium, which was prevented by treatment with INT-767 (Figure 3D).
Figure 3.
INT-767 treatment prevents renal disease in diabetic DBA/2J mice. (A) Albuminuria, defined by ACR, was markedly increased in diabetic mice and normalized by INT-767 treatment. (B) Representative periodic acid–Schiff (PAS) staining of kidney sections. Mesangial expansion index was defined by the percentage of mesangial area in glomerular tuft area. The mesangial area was determined by assessment of PAS-positive and nucleus-free areas in the mesangium. Glomerular area and mesangial expansion index were increased in diabetic mice, and INT-767 treatment prevented these effects. (C) Representative Masson trichrome staining of kidney sections showing increased tubulointerstitial fibrosis (blue) in diabetic mice, which is prevented by INT-767 treatment. (D) Representative merged two-photon excitation (green)-SHG (red) images of kidney sections showing increased tubulointerstitial fibrosis (red) in diabetic mice, which is prevented by treatment with INT-767. (E) Immunofluorescence staining of kidney sections for fibronectin and collagen 4 indicating increased expression in the glomeruli of the diabetic kidney, which are prevented by INT-767 treatment. (F) α-SMA expression in kidney determined by immunohistochemical staining indicates increased expression in the diabetic kidney, which is prevented by INT-767 treatment. (G) Immunohistochemical detection of WT-1 in glomeruli. Podocyte density is presented as numbers of podocytes per glomerular area. There is decreased expression of WT-1 in the diabetic kidney, and treatment with INT-767 prevents this decrease. (H) Immunofluorescence staining of kidney sections for the podocyte marker nephrin indicates decreased expression in the diabetic kidney, which is prevented by INT-767 treatment. CON, nondiabetic DBA/2J; STZ, diabetic DBA/2J without treatment; STZ/INT-767, diabetic DBA/2J treated with INT-767. Scale bar, 50 μm in B and D; 20 μm in E and G. ACR, albumin-to-creatinine ratio. *P<0.05 versus CON (n=6 mice per group); †P<0.05 versus STZ (n=6 mice per group).
Immunofluorescence microscopy showed increased fibronectin and type 4 collagen in the glomeruli of the diabetic kidney, which were prevented by INT-767 treatment (Figure 3E). In addition, immunohistochemistry showed increased staining with α-smooth muscle actin (α-SMA), which was prevented by INT-767 as well (Figure 3F).
In diabetic DBA/2J mice, staining with WT-1, a nuclear podocyte marker, showed a significantly reduced podocyte density in diabetic mice, which was rescued by INT-767 treatment (Figure 3G). Podocyte loss was also confirmed by immunofluorescence staining of the podocyte marker nephrin in kidney sections, showing reduced expression in diabetic kidneys, which was prevented by INT-767 treatment (Figure 3H).
INT-767 Prevents Activation of Profibrotic Signaling Pathways in Diabetic DBA/2J Mice
In diabetic DBA/2J mice, INT-767 treatment blocked the increase of TGF-β expression in diabetic kidneys as well as its target gene CTGF (Table 1). Expression of two myofibroblast markers, α-SMA (Figure 3F) and FSP-1 (Table 1), was increased in diabetic DBA/2J mice and decreased after INT-767 treatment. The expression of their transcriptional regulators Snail and Zeb1 was also downregulated by INT-767 treatment (Table 1).
Table 1.
Metabolic parameters and renal gene expression in DBA/2J mice
| CON | STZ | STZ/INT-767 | |
|---|---|---|---|
| Body weight, g | 40.4±1.76 | 23.7±1.36a | 22.4±2.65 |
| Kidney weight, g | 0.54±0.03 | 0.65±0.06a | 0.61±0.05 |
| Kidney weight-to-body weight ratio, % | 1.33±0.09 | 2.73±0.28a | 2.74±0.38 |
| Plasma glucose, mg/dl | 167±9 | 823±15a | 882±29 |
| Plasma TG, mg/dl | 192±43 | 1606±242a | 744±208b |
| Plasma TC, mg/dl | 216±11 | 1337±102a | 220±24b |
| Plasma HDL-C, mg/dl | 71.4±2.7 | 91.3±17.0 | 89.2±2.4 |
| Plasma LDL-C, mg/dl | 14.0±2.1 | 654±79a | 40.5±2.5b |
| Plasma insulin, ng/ml | 10.58±2.17 | 0.22±0.02a | 0.25±0.05 |
| Kidney fibrosis marker | |||
| TGF-β | 1.19±0.05 | 3.53±0.28a | 2.34±0.25b |
| CTGF | 1.18±0.10 | 2.93±0.35a | 1.51±0.17b |
| FSP-1 | 1.50±0.20 | 2.43±0.21a | 1.48±0.15b |
| Snail | 1.26±0.09 | 2.12±0.13a | 1.66±0.13b |
| ZEB1 | 1.34±0.08 | 1.62±0.11 | 1.11±0.04b |
| Lipid metabolism | |||
| SREBP-1c | 5.74±0.60 | 3.96±0.27a | 1.61±0.14b |
| SCD-1 | 3.45±0.57 | 2.80±0.32 | 1.02±0.05b |
| SCD-2 | 0.85±0.06 | 1.99±0.23a | 1.12±0.14b |
| ChREBPα | 2.47±0.10 | 1.79±0.18a | 2.14±0.29 |
| ChREBPβ | 1.72±0.13 | 28.3±4.72a | 26.1±1.75 |
| LPK | 1.04±0.06 | 2.85±0.31a | 1.78±0.19b |
| SREBP-2 | 1.86±0.09 | 1.71±0.11 | 1.26±0.11b |
| HMGCOR | 1.63±0.10 | 1.47±0.11 | 1.52±0.04 |
| HMGCS-2 | 0.07±0.01 | 19.22±2.48a | 13.20±2.10 |
| LPL | 2.82±0.37 | 1.27±0.09a | 2.32±0.18b |
| FIT-1 | 1.19±0.08 | 7.13±0.75a | 2.34±0.24b |
| CD36 | 2.45±0.20 | 1.84±0.17 | 2.11±0.34 |
| Proinflammatory cytokines | |||
| Cox2 | 8.09±1.77 | 27.88±4.02a | 12.14±1.49b |
| ICAM-1 | 1.06±0.16 | 3.40±0.15a | 2.45±0.72b |
| CD68 | 0.91±0.03 | 3.40±0.52a | 1.46±0.18b |
| ER stress | |||
| GRP78 | 2.58±0.18 | 1.78±0.11a | 2.45±0.08b |
| XBP-1s | 4.83±0.38 | 1.56±0.12a | 2.29±0.14b |
| Chop | 2.35±0.23 | 4.07±0.27a | 3.41±0.56 |
Data are mean±SEM (n=6 mice in each group). CON, nondiabetic DBA/2J; TG, total triglycerides; TC, total cholesterol; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol; LPK, liver pyruvate kinase; ICAM-1, intercellular adhesion molecule-1; GRP78, glucose regulated/binding Ig protein-78; XBP-1s, spliced form of X-box binding protein-1.
P<0.05 versus CON.
P<0.05 versus STZ.
INT-767 Modulates Renal Lipid Metabolism and Prevents Renal Triglyceride and Cholesterol Accumulation in Diabetic DBA/2J Mice
Oil red O staining revealed that diabetic DBA/2J mice have increased kidney neutral lipid accumulation in both glomeruli and tubulointerstitium (Figure 4A). The lipid accumulation was mediated by increases in SCD-2 as well as ChREBP-β and liver pyruvate kinase (Table 1). Biochemical analysis of kidney lipid extracts revealed increased kidney triglyceride and cholesterol accumulation, which was significantly decreased by INT-767 treatment (Figure 4B). The effects of INT-767 in decreasing renal triglyceride and cholesterol content were mediated by coordinated effects inducing (1) decreased expression of SREBP-1c and its target genes SCD-1 and SCD-2, which mediate fatty acid and triglyceride synthesis (Table 1); (2) decreased expression of liver pyruvate kinase, which also mediates fatty acid and triglyceride synthesis (Table 1); (3) decreased expression of SREBP-2, which mediates cholesterol synthesis (Table 1); (4) increased expression of lipolysis gene LPL (Table 1); and (5) decreased expression of lipid droplet formation gene FIT-1 (Table 1).
Figure 4.
INT-767 treatment prevents renal lipid accumulation in diabetic DBA/2J mice. (A) Oil red O staining of kidney sections. (B) Kidney lipid content analysis indicating increased triglyceride and cholesterol accumulation in diabetic DBA/2J kidneys, which is prevented by INT-767 treatment. CON, nondiabetic DBA/2J; STZ, diabetic DBA/2J without treatment; STZ/INT-767, diabetic DBA/2J treated with INT-767. Scale bar, 50 μm. *P<0.05 versus CON (n=6 mice per group); †P<0.05 versus STZ (n=6 mice per group).
INT-767 also decreases serum triglycerides and LDL cholesterol in diabetic DBA/2J mice. STZ treatment of DBA/2J mice fed a Western diet resulted in marked increases in serum glucose, triglyceride, and cholesterol levels, with most of the cholesterol derived from LDL (14.0±2.1 mg/dl in control versus 654±79 mg/dl in diabetic mice) (Table 1). Treatment with INT-767 did not decrease serum glucose levels in diabetic DBA/2J mice but significantly decreased plasma triglyceride, total cholesterol, and LDL cholesterol levels (654±79 mg/dl in diabetic mice versus 40.5±2.5 mg/dl in diabetic mice treated with INT-767) (Table 1).
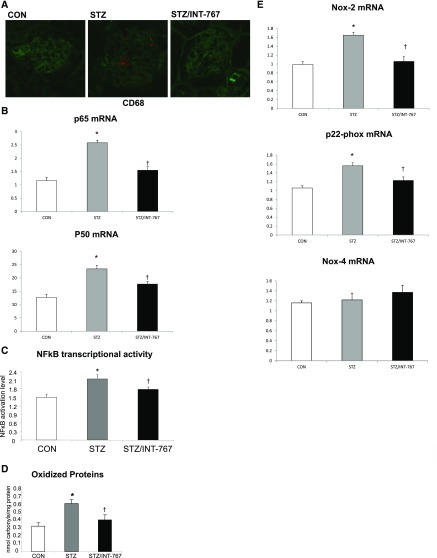
INT-767 Prevents Inflammation, Oxidative Stress, and Endoplasmic Reticulum Stress in Diabetic DBA/2J Mice
INT-767 markedly decreased the expression of macrophage marker CD68 in diabetic kidneys (Figure 5A). This was consistent with the inhibition by INT-767 treatment of NF-κB p65 and p50 heterodimeric complexes expression (Figure 5B) and NF-κB activity, the master transcription factor regulating inflammation (Figure 5C). The expression of NF-κB–dependent proinflammatory mediators, like intercellular adhesion molecule-1 and cyclooxygenase-2, was also significantly decreased by INT-767 treatment (Table 1). In addition, INT-767 modulates oxidative stress, as shown by reduced total protein carbonylation in diabetic kidneys from treated mice (Figure 5D) and decreased NADPH oxidase Nox-2 and p22-phox mRNA expression (Figure 5E). However, NADPH oxidase Nox-4 was not changed (Figure 5E). Endoplasmic reticulum (ER) stress is increased in the kidneys of diabetic mice as determined by increased expression of phospho–EIF-2α–to-total EIF-2α protein ratio (Figure 5F) and CHOP mRNA level (Table 1). Treatment with INT-767 decreased phospho–EIF-2α–to-total EIF-2α protein ratio (Figure 5F), which was also associated with increased glucose regulated/binding Ig protein-78 (BiP) and spliced form of X-box binding protein-1 mRNA levels (Table 1).
Figure 5.
INT-767 treatment prevents the increase in renal inflammation and oxidative stress in diabetic DBA/2J mice. (A) Immunofluorescence staining of kidney sections for CD68 (red) and wheat germ agglutinin (staining whole nephron; green) indicates increased CD68 staining in the diabetic kidney, which is prevented by INT-767. (B) p65 and p50 mRNA abundance is increased in the diabetic kidney, which is prevented by INT-767. (C) Renal NF-κB transcriptional activation determined by DNA binding assay is increased in the diabetic kidney, which is prevented by INT-767. (D) Oxidative carbonylation of proteins in kidney homogenate as measured by ELISA is increased in the diabetic kidney, which is prevented by INT-767. (E) Nox-2 and p22-phox mRNA are increased in the diabetic kidney, which is prevented by INT-767. Nox-4 gene is not regulated. CON, nondiabetic DBA/2J. *P<0.05 versus CON (n=6 mice per group); †P<0.05 versus STZ (n=6 mice per group).
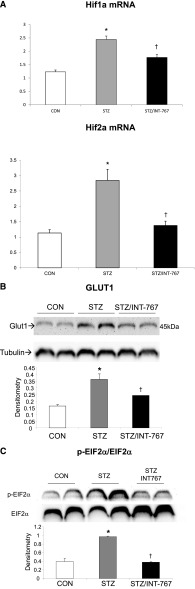
INT-767 Modulates Hypoxia Signaling in Diabetic DBA/2J Mice
The mRNA abundance of HIF-1α and HIF-2α was significantly increased in diabetic DBA/2J mice. Treatment with INT-767 prevented the increased expression of HIF-1α and HIF-2α in diabetic kidneys (Figure 6A). As a downstream target for HIF signaling, Glut1 expression was also increased in diabetic kidneys but reversed by INT-767 treatment (Figure 6B).
Figure 6.
INT-767 treatment prevents the increase in renal HIF and Glut1 expression and ER stress in diabetic DBA/2J mice. (A) HIF-1α and HIF-2α mRNA expression is increased in the diabetic kidney, which is prevented by INT-767. (B) Glut1 protein is increased in the diabetic kidney, which is prevented by INT-767. (C) INT-767 treatment prevents the increase in renal ER stress in diabetic DBA/2J mice as determined by phospho–EIF-2α versus total EIF-2α protein abundance. CON, nondiabetic DBA/2J; STZ, diabetic DBA/2J without treatment; STZ/INT-767, diabetic DBA/2J treated with INT-767. *P<0.05 versus CON (n=6 mice per group); †P<0.05 versus STZ (n=6 mice per group).
Renal Effects of INT-767 in the db/db Mouse Model of Type 2 Diabetes Mellitus and Obesity
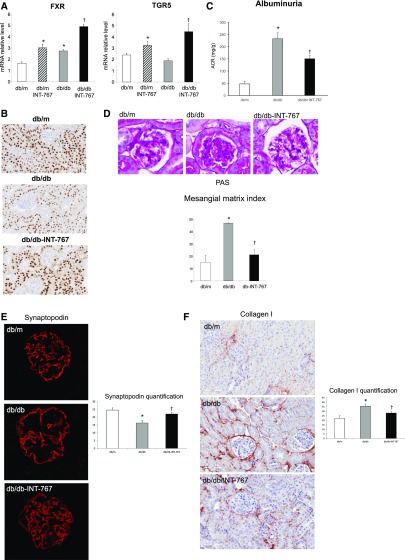
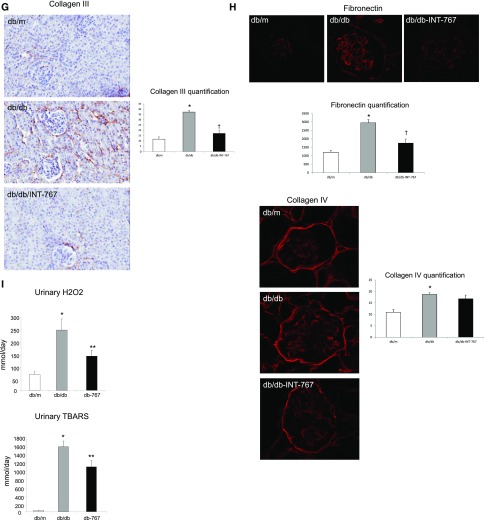
We also determined the therapeutic efficacy and renal effects of INT-767 in a well established model of type 2 diabetes mellitus associated with obesity. INT-767 stimulated FXR and TGR5 mRNA in both db/m and db/db mice (Figure 7A). Interestingly, although FXR mRNA was increased in db/db mice, FXR protein abundance as determined by FXR immunohistochemistry was decreased in db/db mice (−35.2% relative to db/m), and INT-767 treatment activated FXR protein expression to levels seen in nondiabetic db/m mice (Figure 7B). Because there are no suitable antibodies for TGR5 in mice, we could not perform TGR5 IHC. INT-767 treatment of db/db mice did not alter blood glucose levels, but as reported earlier,28 it significantly decreased plasma total cholesterol and triglyceride levels (Table 2). In addition, treatment of db/db mice with INT-767 resulted in significantly decreased albuminuria (Figure 7C). Treatment with INT-767 also decreased mesangial matrix expansion (Figure 7D), podocyte loss as shown by synaptopodin immunofluorescence microscopy (Figure 7E), renal fibrosis indicated by the decreased collagen 1 (Figure 7F), and collagen 3 (Figure 7G) protein abundance as determined by immunohistochemistry and extracellular matrix protein fibronectin, but not collagen 4, expression as determined by immunofluorescence microscopy (Figure 7H). Treatment with INT-767 decreased oxidative stress as shown by significant changes in urinary H2O2 and TBARS level (Figure 7I). We also found that treatment with INT-767 significantly increased CD163 and CD206 expression in macrophages (Table 2), suggesting that FXR/TGR5 activation enhances anti-inflammatory M2 macrophages in the db/db mice.
Figure 7.
INT-767 treatment regulates nephropathy in db/db mice. INT-767 (A) increases FXR and TGR5 mRNA in db/m and db/db mice, (B) prevents the decrease in FXR protein expression in db/db mice as determined by immunohistochemistry, (C) prevents the increase in urinary albumin excretion in db/db mice, (D) prevents the increase in mesangial matrix as determined by periodic acid–Schiff (PAS) staining, (E) prevents the decrease in the podocyte marker synaptopodin expression as determined by immunofluorescence microscopy, (F and G) prevents increases in periglomerular and tubulointerstitial accumulation of (F) type 1 collagen and (G) type 3 collagen as determined by immunohistochemistry, (H) prevents the increase in glomerular accumulation of fibronectin as determined by immunofluorescence microscopy, and (I) prevents the increases in urinary H2O2 and urinary TBARS. ACR, albumin-to-creatinine ratio. *P<0.05 db/db versus db/m (n=6 mice per group); †P<0.05 db/db + INT-767 versus db/db (n=6 mice per group).
Table 2.
Metabolic parameters and renal gene expression in db/db mice
| db/m | db/m/INT-767 | db/db | db/db/INT-767 | |
|---|---|---|---|---|
| Plasma glucose, mg/dl | 198±7 | 227±14 | 892±63 | 807±52 |
| Plasma TG, mg/dl | 63.4±7.31 | 39.4±3.18a | 141.4±19.0a | 56.01±11.9b |
| Plasma TC, mg/dl | 84.6±4.61 | 42.0±2.73a | 102.3±4.12a | 39.2±2.88b |
| Mitochondrial biogenesis | ||||
| Nampt | 1.71±0.07 | 1.82±0.10 | 1.43±0.10a | 2.04±0.19b |
| Sirt1 | 2.66±0.16 | 2.64±0.11 | 1.44±0.10a | 2.48±0.24b |
| Sirt3 | 1.88±0.10 | 2.12±0.10 | 1.44±0.11a | 2.44±0.41b |
| PGC-1α | 2.06±0.10 | 2.36±0.10 | 1.73±0.15a | 3.26±0.55b |
| ERR-α | 1.92±0.13 | 2.00±0.11 | 1.38±0.06a | 2.04±0.19b |
| Nrf1 | 5.24±0.19 | 4.31±0.70 | 3.65±0.32a | 5.80±1.20b |
| PDK4 | 1.45±0.09 | 1.33±0.12 | 2.55±0.37a | 5.76±1.05b |
| CPT-1a | 1.41±0.08 | 1.68±0.12 | 1.96±0.18a | 2.78±0.26b |
| AceS2 | 1.79±0.08 | 2.31±0.15a | 2.03±0.21 | 2.51±0.37b |
| LCAD | 2.10±0.09 | 1.96±0.11 | 1.20±0.08a | 1.63±0.23b |
| M2 macrophage markers | ||||
| CD163 | 2.41±0.28 | 2.23±0.61 | 1.41±0.40 | 3.35±0.86b |
| CD206 | 4.42±0.40 | 4.15±0.33 | 2.19±0.17a | 3.27±0.44b |
Data are mean±SEM (n=6 mice in each group). TG, total triglycerides; TC, total cholesterol; Nampt, nicotinamide phosphoribosyl transferase; PDK4, pyruvate dehydrogenase kinase 4; CPT-1a, carnitine palmitoyltransferase-1a; AceS2, acetyl CoA synthetase 2; LCAD, long-chain acyl CoA dehydrogenase.
P<0.05 versus db/m.
P<0.05 versus db/db.
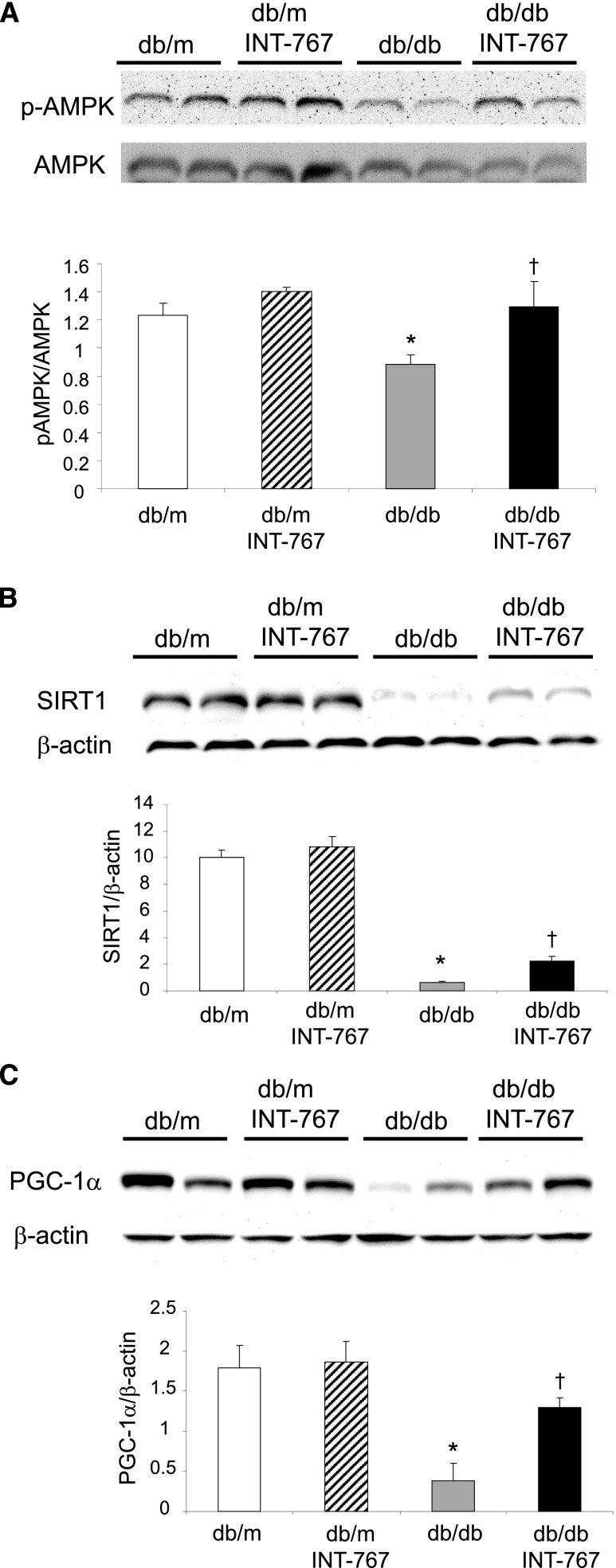
INT-767 Induces Mitochondrial Biogenesis and Metabolism Pathways in db/db Mouse Model of Type 2 Diabetes Mellitus and Obesity
Treatment of db/db mice with INT-767 increased phospho-AMPK–to-AMPK protein ratio (Figure 8A), nicotinamide phosphoribosyl transferase, the rate-limiting enzyme in NAD+ biosynthesis (Table 2), SIRT1 mRNA (Table 2) and protein (Figure 8B), and SIRT3 mRNA (Table 2). INT-767 treatment also increased PGC-1α mRNA (Table 2) and protein (Figure 8C), ERR-α, and Nrf1 mRNA (Table 2), the transcriptional regulators of mitochondrial biogenesis and activity, as well as several enzymes that mediate fatty acid and glucose oxidation, including carnitine palmitoyltransferase-1A, pyruvate dehydrogenase kinase 4, long-chain acyl CoA dehydrogenase, and acetyl CoA synthetase 2 (Table 2).
Figure 8.
INT-767 treatment regulates renal mitochondrial biogenesis and metabolism pathways in db/db mice. INT-767 prevents (A) the decreased expression of phospho-AMPK versus total AMPK, (B) SIRT1, and (C) PGC-1α as determined by Western blotting. *P<0.05 db/db versus db/m (n=6 mice per group); †P<0.05 db/db + INT-767 versus db/db (n=6 mice per group).
INT-767 Prevents Mitochondrial Dysfunction, Oxidative Stress, Inflammation, and Fibrosis in Mice with Diet-Induced Obesity
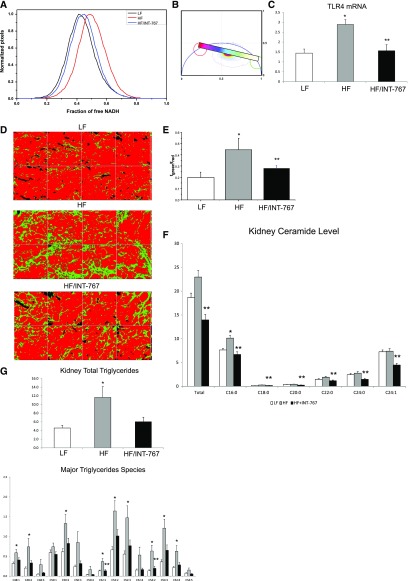
In mice fed a high-fat diet, there was an increase in free, nonmitochondria-bound NADH as determined by label-free imaging with fluorescence lifetime imaging microscopy (FLIM) (Figure 9, A and B). The increase in free NADH is indicative of mitochondrial dysfunction and oxidative stress.33 There was also increased expression of the proinflammatory TLR4 (Figure 9C). These alterations were associated with increased renal fibrosis as determined by label-free imaging with SHG-FLIM (Figure 9, D and E). Treatment with INT-767 prevented the increase in NADH free fraction (Figure 9A), TLR4 expression (Figure 9C), and fibrosis (Figure 9, D and E).
Figure 9.
INT-767 treatment regulates mitochondrial function, inflammation, fibrosis, and lipid metabolism in high-fat diet–induced obesity mice. INT-767 (A and B) prevents the increase in free NADH in HF diet–fed mice as determined FLIM and the phasor plot, (C) prevents the increase in the proinflammatory TLR4 mRNA, (D and E) prevents the increase in fibrosis in HF diet–fed mice as determined by SHG and FLIM, (F) total and major ceramide species accumulation as determined by liquid chromatography-tandem mass spectrometry (LC-MS/MS), and (G) total triglycerides and major triglycerides species accumulation as determined by LC-MS/MS. LF, low fat; HF, high fat. *P<0.05 HF versus LF (n=6 mice per group); **P<0.05 HF + INT-767 compared to HF; †P<0.05 HF + INT-767 versus LF (n=6 mice per group).
INT-767 Prevents Ceramide and Triglyceride Accumulation and Alters Ceramide and Triglyceride Composition in Mice with Diet-Induced Obesity
In mice fed a high-fat diet, there was a significant increase in C16:0 ceramide level (Figure 9F). Treatment with INT-767 prevented the increases in total and individual ceramide species levels (Figure 9F). In mice fed a high-fat diet, there were also significant increases in total and individual triglyceride species levels (Figure 9G). Treatment with INT-767 prevented the increases of most triglyceride species but most significantly, the C52:1 and C54:2 triglyceride species levels (Figure 9G).
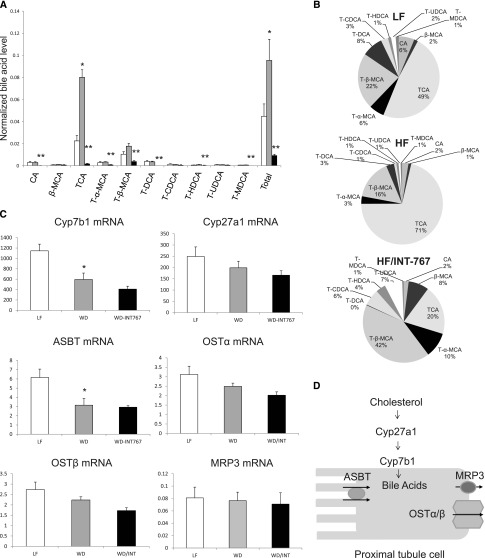
INT-767 Prevents Bile Acid Accumulation and Alters Bile Acid Composition in Mice with Diet-Induced Obesity
In mice fed a high-fat diet, there was a significant increase in total bile acid levels (Figure 10A). The increase in absolute and relative trichloroacetic acid levels was most marked and significant (Figure 10, A and B). Treatment with INT-767 induced significant decreases in total bile acid levels and absolute and relative trichloroacetic acid, T-α-MCA, T-β-MCA, T-DCA, and T-HDCA levels (Figure 10, A and B). To explore for potential mechanisms of these changes in total and individual bile acid composition, we measured the expression of the renal bile acid synthesis and bile acid transporter genes. Interestingly, high-fat diet induced decreases in Cyp7B1 mRNA, which mediates bile acid synthesis, and ASBT mRNA, which mediates bile acid transport from the urine (Figure 10, C and D). Treatment with INT-767 did not cause any significant changes in Cyp7B1, ASBT mRNA, or other bile acid synthesis and bile acid transporter genes (Figure 10, C and D).
Figure 10.
INT-767 treatment regulates kidney bile acid composition, bile acid synthesis, and bile acid transporters in high-fat diet–induced obesity mice. (A and B) In mice fed a high-fat diet, there was a significant increase in total bile acid levels. The increases in absolute and relative trichloroacetic acid (TCA) levels were most marked and significant. Treatment with INT-767 induced significant decreases in total bile acid levels and absolute and relative TCA, T-α-MCA, T-β-MCA, T-DCA, and T-HDCA levels. (C and D) High-fat diet induced decreases in Cyp7B1 mRNA, which mediates bile acid synthesis, and ASBT mRNA, which mediates bile acid transport from the urine. Treatment with INT-767 did not cause any significant changes in Cyp7B1 or ASBT mRNA or other bile acid synthesis and bile acid transporter genes. LF, low fat; HF= high fat. WD, Western diet. *P< 0.05 WD compared to LF, **P<0.05 WD/INT compared to WD.
Discussion
We have previously shown25,27 that FXR and TGR5 agonists modulate differential signaling pathways in the kidney. The purpose of this study was to define more precisely the pathways involved and determine the effects of the dual FXR/TGR5 bile acid receptor agonist INT-767 in kidney disease in diabetes and obesity. These data show significant decrease in renal injury by INT-767 treatment in diabetic DBA/2J mice, diabetic db/db mice, and C57BL/6J mice with diet-induced obesity.
Using RNA-Seq analysis, we found that the selective FXR agonist obeticholic acid modulates signaling pathways related to vascular endothelial growth factor, CNTF, apoptosis, IL-6, renin-angiotensin, NRF2-mediated oxidative stress response, fatty acid β-oxidation I, HIF-1α, JAK/STAT, and RAR. Conversely, the specific TGR5 agonist INT-777 modulates signaling pathways related to remodeling of epithelial adherens junction, autophagy, N-acetylglucosamine degradation, CoA biosynthesis, fatty acid oxidation III, and branched chain α-keto acid dehydrogenase complex. We also identified additional pathways regulated by INT-767 but not INT-747 or INT-777 from RNA-Seq. Furthermore, we checked protein level expression and found that both INT-747 and INT-767 but not INT-777 regulated renal lipogenesis pathway and that both INT-777 and INT-767 but not INT-747 regulated renal mitochondrial biogenesis pathway. These findings suggest that FXR activation and TGR5 activation lead to differential pathway regulation and that dual FXR/TGR5 activation combines effects of both singular activation of FXR and TGR5, with additional effects not seen in either activation. Therefore, these data provided the rationale for determining the effects of the dual FXR/TGR5 agonist in kidney disease.
In a further attempt to determine the mechanisms by which INT-767 exerts its nephroprotective effects, we found that multiple pathways are involved in the beneficial actions of INT-767. INT-767 effectively reduced renal expression of profibrotic factors, proinflammatory mediators, and oxidative stress as well as accumulation of lipids. In addition, our data show several novel findings, showing that INT-767 (1) stimulates AMPK-SIRT1-PGC-1α-SIRT3-ERR-α signaling, (2) inhibits ER stress, and (3) inhibits HIF signaling and Glut1 transporter. These findings were associated with INT-767 treatment in diabetic kidneys but not in control, nondiabetic kidneys (Figure 8, Supplemental Figure 1).
Glucose transport in the diabetic kidney is upregulated, and this has been implicated in the pathogenesis of progressive diabetic nephropathy.34,35 In particular, overexpression of Glut1 has shown pathogenic effects in mesangial cells, although not in podocytes.34,35 The regulation of renal glucose transporter Glut1 by INT-767 suggests a direct renal mechanism, whereby bile acid receptor signaling targets intracellular glucose uptake to reduce the adverse effect of hyperglycemia. Glut1 is also a bona fide transcriptional target of HIF-1α, mediating high glucose–induced matrix protein expression.36 The inhibition of Glut1 in INT-767–treated kidney is, therefore, indicative of a potential mechanism, whereby HIF-1 signaling acts through activation of bile acid receptors. These effects are independent of alterations in serum glucose and insulin; in this study, we did not observe a systemic effect of INT-767 on blood glucose level or the low insulin level in DBA/2J mice with STZ-induced diabetes.
INT-767 inhibits in the kidney the expression of NF-κB and proinflammatory cytokines and enhances expression of M2 macrophages, which have been proposed to reduce renal injury.37,38 We have seen similar actions of INT-767 in the livers of db/db mice that develop nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. In that study, treatment with INT-767 significantly improved the histologic features of nonalcoholic steatohepatitis. Furthermore, treatment increased the proportion of intrahepatic monocytes with the anti-inflammatory Ly6C(low) phenotype and increased intrahepatic expression of genes expressed by alternatively activated macrophages, including CD206, Retnla, and Clec7a.39
ER stress has been shown to be induced in diabetes and associated with renal injury.40 Furthermore, ER stress is associated with mitochondrial dysfunction and may result in increased rate of apoptosis.41–44 We found that INT-767 decreases ER stress in the kidney. Previous studies have shown that the bile acid metabolites and derivatives ursodeoxycholic acid and tauroursodeoxycholic acid decrease ER stress by acting as chemical chaperones.45 It is not clear if the mechanism of action of INT-767 is similar to ursodeoxycholic acid and tauroursodeoxycholic acid, but treatment with INT-767 stimulates the ER chaperone protein glucose regulated/binding Ig protein-78, which play a major role in the defense against progression of ER stress to renal injury.46–48 The effects of INT-767 to prevent ER stress may be related to its effects on ceramide levels, because ceramides have been shown to modulate ER stress.49,50 The effects of INT-767 to prevent ER stress may also be related to the effects of INT-767 to prevent the increases in SREBP-1 and SREBP-2, because both SREBP-1 and SREBP-2 can be activated by ER stress.51
In addition, we have found that INT-767 induces increased activity of AMPK, SIRT1, PGC-1α, SIRT3, and ERR-α, which are major regulators of metabolism and mitochondrial biogenesis.52,53 Indeed, we have also found increased expression of the mitochondrial transcription factor Nrf1.54,55 The activation of the AMPK-SIRT1-PGC-1α-SIRT3-ERR-α axis is associated with increased expression of the mitochondrial fatty acid oxidation genes carnitine palmitoyltransferase-1A, pyruvate dehydrogenase kinase 4, long-chain acyl CoA dehydrogenase, and acetyl CoA synthetase 2 as recently shown in other tissues and cells.56 These results are of particular significance, because alterations in mitochondrial function have been implicated as a major mediator of diabetic nephropathy.14–17 Our results indicate that INT-767 induced increased activity of AMPK, SIRT1, PGC-1α, SIRT3, and ERR-α, which are major regulators of metabolism and mitochondrial biogenesis and play a major role in the prevention of diabetic nephropathy. Indeed, in high-fat diet–induced obesity mice, treatment of INT-767 prevented the increase in free NADH as determined by FLIM, which is a further indication that INT-767 prevents mitochondrial dysfunction.33
Because INT-767 is a dual FXR and TGR5 agonist, we compared it with the two single agonists in the treatment of diabetic kidney disease. All three agonists showed the similar level of renoprotection in our study. This is best illustrated by the observation that treatment of diabetic db/db mice with the FXR agonist INT-747, the TGR5 agonist INT-777, or the dual FXR/TGR5 agonist INT-767 results in a significant reduction of urinary albumin excretion (Supplemental Figure 2). We did not find the dual agonism by INT-767 with further protection in the structural level. This is partly because of the limitations with the diabetic mouse models used in these studies, which compared with human diabetic nephropathy, represent early diabetic changes in the kidney. However, dual agonism by INT-767 does target different metabolic pathways that are important for diabetic kidney disease. Our results clearly indicate that, although some signaling pathways are specifically regulated by TGR5, others are specifically activated or inhibited by FXR activation (Figure 2). Our results suggest that FXR and TGR5 have nonredundant effects in the kidney. This is consistent with our study in FXR knockout mice, indicating that in the absence of FXR, an intact TGR5 is not able to compensate for the absence of FXR.26
In summary, both TGR5 and FXR regulate multiple complementary as well as nonoverlapping signaling and metabolic pathways that are important for the pathogenesis and prevention of diabetic nephropathy. The dual agonist INT-767 not only regulates pathways from both single agonisms but may also provide additional mechanisms. This could add extra renoprotection for INT-767 in more robust animal models with more features observed in human diabetic nephropathy.
Concise Methods
Animal Models
DBA/2J Mice
Eight-week-old male DBA2/J mice were obtained from the Jackson Laboratories (Bar Harbor, ME). They were maintained on a 12-hour light/12-hour dark cycle. Mice were injected with STZ (Sigma-Aldrich, St. Louis, MO) intraperitoneally (40 mg/kg in 50 mM sodium citrate buffer, pH 4.5) for 5 consecutive days or 50 mM sodium citrate solution only. Tail vein blood glucose levels were measured 1 week after the last STZ injection, and mice with glucose levels >250 mg/dl were considered diabetic. DBA/2J mice were fed with a Western diet (21% milkfat, 0.15% cholesterol; TD88137; Harlan-Teklad, Madison, WI). Mice were treated for 8 weeks with (1) Western diet only; (2) the semisynthetic dual FXR/TGR5 agonist INT-767 (Intercept Pharmaceuticals, New York, NY)28: 30 mg/kg body wt/d admixed with Western diet; (3) INT-747: 20 mg/kg body wt/d admixed with Western diet; or (4) INT-777: 30 mg/kg body wt/d admixed with Western diet.
db/db Mice
Six-week-old male db/m and db/db mice (BLKS/J) were obtained from the Jackson Laboratories. They were maintained on a 12-hour light/12-hour dark cycle. They were on (1) a regular chow diet, (2) INT-767 (30 mg/kg body wt/d), (3) INT-747 (20 mg/kg body wt/d), or (4) INT-777 (30 mg/kg body wt/d) admixed with chow for 2 weeks.
Diet-Induced Obesity Mice
Six-week-old male C57BL/6J mice were obtained from the Jackson Laboratories. They were maintained on a 12-hour light/12-hour dark cycle. They were fed a matched (1) control diet (10 kcal% fat), (2) a high-fat diet (60 kcal% fat; Research Diets), or (3) a high-fat diet containing the dual FXR/TGR5 agonist INT-767 (Intercept Pharmaceuticals)28: 30 mg/kg body wt/d admixed in the diet.
Animal studies and relative protocols were approved by the Animal Care and Use Committee at the University of Colorado Denver.
Blood and Urine Chemistry
Blood glucose levels were measured with a Glucometer (Elite XL; Bayer, Tarrytown, NY). Plasma lipid levels were measured with kits (Wako Chemical, Richmond, VA). Urine albumin and creatinine concentrations were determined with kits (Exocell, Philadelphia, PA).
RNA Extraction and RNA-Seq
Approximately 300–500 ng RNA was used to generate barcoded RNA libraries using Lexogen’s Quant-sEquation 3′ mRNA seq kit modified per the manufacturer’s recommendations for low input/partial degradation (Lexogen, Vienna, Austria). ERCC controls were spiked in at the manufacturer’s recommended concentrations (Life Technologies, Carlsbad, CA). Library quantification and quality control were performed using the High Sensitivity DNA Kit and the Agilent 2100 Bioanalyzer (Agilent, Santa Clara, CA). Precise library quantification was performed using the Ion Library Quantitation Kit (Life Technologies), which uses Taqman qPCR chemistry to quantify adapter ligated library fragments; these results were used to determine library input into the template reaction, and templates for sequencing were prepared using the 200-bp v3 OT2 kit and the Ion One Touch 2 platform (Life Technologies) with sequencing performed on an Ion Proton with signal processing and base calling using Ion Torrent Suite, version 5.0.4 (Life Technologies). Raw sequence was mapped to Ampliseq-supported mm10 transcriptome. Quality control metrics and normalized read counts per million were generated using the RNA-Seq Analysis plugin (v. 5.0.3.0; Ion Torrent community; Life Technologies).
Quantitative Real-Time PCR
Quantitative real-time PCRs were performed as previously described.57–60 Primer sequences are available in our previous publications57–60 and Supplemental Table 1.
Western Blotting
Cortical homogenate protein content was measured by BCA assay (Thermo Fisher Scientific, Waltham, MA). Equal amounts of total protein were separated by SDS-PAGE gels and transferred onto PVDF membranes. The antibodies against SREBP-1 (catalog no. H-160; Santa Cruz Biotechnology, Dallas, TX), Glut1 (catalog no. 07–1401; Cell Signaling, Danvers, MA), p-AMPK/AMPK (catalog nos. 4184 and 2795; Cell Signaling), SIRT1 (catalog no. 07–131; Millipore, Billerica, MA), PGC-1α (catalog no. AB3242; Millipore), and pEIF-2α/EIF-2α (catalog nos. 9721 and 9722; Millipore) were used for Western blotting. After HRP-conjugated secondary antibodies, the immune complexes were detected by chemiluminescence captured on the UVP Biospectrum 500 Imaging System (Upland, CA), and the densitometry was performed with ImageJ software.
Lipid Extraction and Measurement of Lipid Composition
Lipids from the kidneys were extracted by the method of Bligh and Dyer, which we have previously described.57–60 Triglyceride and cholesterol composition were measured by gas chromatography (Agilent Technologies, Wilmington, DE).
Liquid Chromatography-Tandem Mass Spectrometry Techniques for Ceramide Species, Triglyceride Species, and Bile Acid Composition
For Kidney Ceramide and Triglyceride Composition
Kidney tissue was homogenized with 500 μl methanol:H2O (4:3, vol/vol) solution and then extracted using 700 μl chloroform containing SM (17:0), PC (17:0), and CER (17:0) at 1 μM as internal standards. The homogenate was shaken and incubated at 37°C for 20 minutes followed by centrifugation at 15,000×g for another 15 minutes. The lower organic phase was collected and evaporated to dryness under vacuum. The residue was then suspended with 100 μl chloroform:methanol (1:1, vol/vol) solution and then diluted with isopropanol:acetonitrile:H2O (2:1:1, vol/vol/vol) solution before injection. Lipidomics analysis was performed on an Acquity UPLC/Synapt G2 Si HDMS QTOFMS system (Waters Corp., Milford, MA) equipped with electrospray ionization (ESI) source. Separation was achieved on an Acquity UPLC CSH C18 column (100×2.1-mm internal diameter, 1.7 mm; Waters Corp.). The mobile phase was a mixture of acetonitrile/water (60/40, vol/vol; A) and isopropanol/acetonitrile (90/10, vol/vol; B), and both A and B contained 10 mM ammonium acetate and 0.1% formic acid. The gradient elution program consisted of a 2-minute linear gradient of 60% A to 57% A to 50% A at 2.1 minutes, a linear decrease to 46% A at 12 minutes to 30% A at 12.1 minutes, and a linear decrease to 1% A at 18 minutes before returning to initial conditions at 18.5 minutes to equilibrate the column. The column temperature was maintained at 55°C, and the flow rate was 0.4 ml/min. Mass spectrometry data were acquired in the both positive and negative ESI modes at a range of m/z 100–1200.
For Kidney Bile Acid Composition
Kidney tissue was homogenized with 200 μl acetonitrile containing 1 μM trichloroacetic acid-d5. The samples were centrifuged at 15,000×g for 15 minutes at 4°C; 40 μl supernatant was collected and diluted ten times with 0.1% formic acid before analysis. Concentrations of bile acids were determined by an Acquity UPLC/Xevo G2 QTOFMS system (Waters Corp.) with an ESI source. An Acquity BEH C18 column (100×2.1-mm internal diameter, 1.7 mm; Waters Corp.) was applied for chromatographic separation. A mixture of 0.1% formic acid in water (A) and 0.1% formic acid in acetonitrile (B) was used as the mobile phase. The gradient elution was started from 80% A for 4 minutes, decreased linearly to 60% A over 11 minutes, to 40% A over the next 5 minutes, and to 10% A for the succeeding 1 minute, and finally, increased to 80% A for 4 minutes to re-equilibrate the column. Column temperature was maintained at 45°C, and the flow rate was 0.4 ml/min. Mass spectrometry detection was operated in negative mode. A mass range of m/z 50–1000 was acquired.61–66
NF-κB Transcriptional Activity Assay
Nuclear protein extracts were prepared from kidney tissue as we have previously described.24,25,57–60 The nuclear extracts were used for the measurement of NF-κB transcriptional activity with a kit from Marligen Biosciences (Rockville, MD) according to the manufacturer’s instructions.
Oxidized Protein Analyses
The amount of oxidized proteins in kidney homogenates was determined by using an OxyElisa Oxidized Protein Quantitation Kit (Millipore) according to the manufacturer’s instructions.
Histology Staining and Immunofluorescence Microscopy
Sections (4-μm-thick) cut from 10% formalin-fixed, paraffin-embedded kidney samples were used for periodic acid–Schiff staining and Masson trichrome staining. Frozen sections were used for oil red O staining of neutral lipid deposits or immunostaining for nephrin (a gift from Larry Holzman, University of Pennsylvania, Philadelphia, PA), synaptopodin (Sigma-Aldrich), fibronectin (Sigma-Aldrich), CD68 (AbD Serotec, Raleigh, NC), and α-SMA (Sigma-Aldrich) and imaged with a laser-scanning confocal microscope (LSM 510; Zeiss, Jena, Germany). The expression level was quantified as sum of pixel values per glomerular area using ImageJ (version 1.44) image analysis software.
Quantification of Morphology
All quantifications were performed in a masked manner. Using coronal sections of the kidney, 30 consecutive glomeruli per mouse with six mice per group were examined for evaluation of glomerular mesangial expansion. The index of the mesangial expansion was defined as the ratio of mesangial area to glomerular tuft area. The mesangial area was determined by assessment of the periodic acid–Schiff-positive and nucleus-free area in the mesangium using ScanScope image analyzer (Aperio Technologies, Vista, CA).
Autofluorescence FLIM, SHG, and Third Harmonic Generation Measurements Using the Deep Imaging via Enhanced-Photon Recovery Microscope
Autofluorescence FLIM, SHG, and third harmonic generation (THG) signals were acquired using the Deep Imaging via Enhanced-Photon Recovery microscope developed at the Laboratory of Fluorescence Dynamics, University of California, Irvine. This microscope is especially useful for harmonic imaging, both second and third harmonic, because of its transmission geometry. The details of this microscope have been described elsewhere67–71. Briefly, the system is on the basis of a custom-made upright laser-scanning microscope equipped with the special Deep Imaging via Enhanced-Photon Recovery detector. A short pulsed two-photon laser (Insight Deep See; Spectra-Physics) is used as the excitation source, and an Acousto-Optic Modulator (AA Opto-Electronic) is used to modulate excitation power. The samples were excited with a 40×, 0.8 NA water objective (Olympus) for harmonic and FLIM measurements. The sample is placed directly on top of the detector assembly input window below the objective, and two photon–induced fluorescence, SHG, and THG are detected by a large area photomultiplier (PMT; R7600P-300; Hamamatsu). The detector assembly consists of a sealed chamber with the filter wheel/shutter inside and the housing with PMT. The refractive index matching liquid filled the inside of the housing, and it removes loss of photons due to internal reflections and thus, achieves efficient collection of photons. Two BG39 filters serve as input and output windows of the chamber and block NIR excitation light from entering PMT and transmitting UV and visible fluorescence as well as harmonic signals. The only optical elements in the detector assembly are BG39 filters and the glass filter of the filter wheel, allowing detection of emitted photons from 320- to 650-nm-wavelength range. The transmission geometry of detection system allows more efficient detection of SHG and THG signals due to forward propagating nature of the harmonic signals.
The signal from the PMT is collected using an FLIMBox and directly transferred to the phasor plot. Details of the phasor approach toward FLIM analysis have been explained elsewhere.72–74 Briefly, this method of lifetime analysis involves transferring the fluorescence intensity decays in the Fourier space and plotting the Fourier components against each other. This results in a phasor plot shown in Figure 9B, where each point results from a pixel of the image. The SHG and THG signals are coherent, and the signal resembles that of the laser pulse, which is measured as zero lifetime; hence, the phasor points originating from SHG appear at s=0, g=1 in the phasor plot. However, the autofluorescence from the tissue has a nonzero lifetime and appears inside the semicircle. A distribution in the phasor plot can then be selected using either a cursor or a continuous distribution cursor, and the images can be colored accordingly. Phasor approach toward FLIM is a fitfree approach, and it increases the speed of the analysis and decreases the computational difficulty associated with FLIM technique. Free NADH and bound NADH have lifetimes of 0.4 and approximately 3.4 ns, respectively, and their individual fluorescence intensity decay seems close to the universal circle.75 In Figure 9B, the phasor positions of free and bound NADH are represented by the blue and the red cursors, respectively and the line joining the two cursors is called the metabolic trajectory. The distribution of the points along the metabolic trajectory can be then transformed to a distribution depicting the number of pixels belonging to a certain fraction of free NADH using the law of linear addition in phasor space.76 The distribution of free NADH can then be compared with the difference in metabolism of different samples. The methodology of this type of phasor analysis has been shown elsewhere.77
The state of fibrosis was analyzed by a ratiometric method.71 In this new method, a ratio of the area covered by SHG to the area covered by FLIM is calculated. This analysis takes into account of both collagen accumulation due to fibrosis and the changes in tissue architecture. The higher the value of this ratio, the higher the fibrosis. The green and red colors in Figure 9B were used to show the SHG and FLIM signals, respectively, and thus, the ratio has been defined as fgreen/fred. The data collection and analysis were carried out by using SimFCS developed by Enrico Gratton at the Laboratory for Fluorescence Dynamics, University of California, Irvine.
The differences in spatial accumulation of all of the lipids and the long lifetime species, which are representative of ROS and lipid oxidation, are imaged through the THG and FLIM, respectively.33,78
SHG imaging was carried out with a 710-nm pulsed laser for excitation and a combination of UG-11 and BG39 filters for the efficient detection of 355-nm SHG photons. The combination of BG39 and UG-11 creates a spectral window of observation with maxima around 355 nm. Thus, the SHG signal (green) when excited at 710 nm can be very efficiently collected using these two filters. THG was achieved by exciting the samples at 1050 nm and detecting the THG signal at 1050/3=350 nm using the same filter settings as those of SHG. Two-photon autofluorescence FLIM was achieved by using the same 710-nm excitation as that of the SHG and the BG39 filter of the detection assembly (λEM=300–650 nm). Each of the images was taken with a 360-μm field of view, 32-μs pixel dwell time, and 20 repeat scans.
Statistical Analyses
Results are presented as the mean±SEM for at least three independent experiments. Data were analyzed by ANOVA and Student–Newman–Keuls tests for multiple comparisons or t test for unpaired data between two groups. Statistical significance was accepted at the P<0.05 level.
Disclosures
L.A. and M.P. are with Intercept Pharmaceuticals (New York, NY). S.R. and M.L. have received an Intercept Medical School grant. Other authors declared no competing interests.
Supplementary Material
Acknowledgments
This work was supported by an Intercept Medical School grant (to S.R. and M.L.); National Institutes of Health grants P41GM103540 (to E.G.), P50GM076516 (to E.G.), 1R01DK098336 (to M.L.), and R01AG026529 (to M.L.); Intramural Research Program, National Institute of Diabetes, Digestive and Kidney Disease, the National Institutes of Health (NIH) (to J.B.K.), National Cancer Institute, NIH (to F.J.G.); and VA Merit Review grant 1I01BX001954 (to M.L.).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2017020222/-/DCSupplemental.
References
- 1.Thomas MC, Brownlee M, Susztak K, Sharma K, Jandeleit-Dahm KA, Zoungas S, Rossing P, Groop PH, Cooper ME: Diabetic kidney disease. Nat Rev Dis Primers 1: 15018, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reidy K, Kang HM, Hostetter T, Susztak K: Molecular mechanisms of diabetic kidney disease. J Clin Invest 124: 2333–2340, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Breyer MD, Coffman TM, Flessner MF, Fried LF, Harris RC, Ketchum CJ, Kretzler M, Nelson RG, Sedor JR, Susztak K; Kidney Research National Dialogue (KRND) : Diabetic nephropathy: A national dialogue. Clin J Am Soc Nephrol 8: 1603–1605, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boyle JP, Thompson TJ, Gregg EW, Barker LE, Williamson DF: Projection of the year 2050 burden of diabetes in the US adult population: Dynamic modeling of incidence, mortality, and prediabetes prevalence. Popul Health Metr 8: 29, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gregg EW, Li Y, Wang J, Burrows NR, Ali MK, Rolka D, Williams DE, Geiss L: Changes in diabetes-related complications in the United States, 1990-2010. N Engl J Med 370: 1514–1523, 2014 [DOI] [PubMed] [Google Scholar]
- 6.Forbes JM, Coughlan MT, Cooper ME: Oxidative stress as a major culprit in kidney disease in diabetes. Diabetes 57: 1446–1454, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Qian Y, Feldman E, Pennathur S, Kretzler M, Brosius FC 3rd: From fibrosis to sclerosis: Mechanisms of glomerulosclerosis in diabetic nephropathy. Diabetes 57: 1439–1445, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu Y, Usui HK, Sharma K: Regulation of transforming growth factor beta in diabetic nephropathy: Implications for treatment. Semin Nephrol 27: 153–160, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bobulescu IA: Renal lipid metabolism and lipotoxicity. Curr Opin Nephrol Hypertens 19: 393–402, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gurley SB, Coffman TM: The renin-angiotensin system and diabetic nephropathy. Semin Nephrol 27: 144–152, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Hunley TE, Ma LJ, Kon V: Scope and mechanisms of obesity-related renal disease. Curr Opin Nephrol Hypertens 19: 227–234, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weinberg JM: Lipotoxicity. Kidney Int 70: 1560–1566, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Stadler K, Goldberg IJ, Susztak K: The evolving understanding of the contribution of lipid metabolism to diabetic kidney disease. Curr Diab Rep 15: 40, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qi H, Casalena G, Shi S, Yu L, Ebefors K, Sun Y, Zhang W, D’Agati V, Schlondorff D, Haraldsson B, Böttinger E, Daehn I: Glomerular endothelial mitochondrial dysfunction is essential and characteristic of diabetic kidney disease susceptibility. Diabetes 66: 763–778, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharma K, Karl B, Mathew AV, Gangoiti JA, Wassel CL, Saito R, Pu M, Sharma S, You YH, Wang L, Diamond-Stanic M, Lindenmeyer MT, Forsblom C, Wu W, Ix JH, Ideker T, Kopp JB, Nigam SK, Cohen CD, Groop PH, Barshop BA, Natarajan L, Nyhan WL, Naviaux RK: Metabolomics reveals signature of mitochondrial dysfunction in diabetic kidney disease. J Am Soc Nephrol 24: 1901–1912, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharma K: Mitochondrial hormesis and diabetic complications. Diabetes 64: 663–672, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sas KM, Kayampilly P, Byun J, Nair V, Hinder LM, Hur J, Zhang H, Lin C, Qi NR, Michailidis G, Groop PH, Nelson RG, Darshi M, Sharma K, Schelling JR, Sedor JR, Pop-Busui R, Weinberg JM, Soleimanpour SA, Abcouwer SF, Gardner TW, Burant CF, Feldman EL, Kretzler M, Brosius FC 3rd, Pennathur S: Tissue-specific metabolic reprogramming drives nutrient flux in diabetic complications. JCI Insight 1: e86976, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Boer IH, Rue TC, Hall YN, Heagerty PJ, Weiss NS, Himmelfarb J: Temporal trends in the prevalence of diabetic kidney disease in the United States. JAMA 305: 2532–2539, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosolowsky ET, Skupien J, Smiles AM, Niewczas M, Roshan B, Stanton R, Eckfeldt JH, Warram JH, Krolewski AS: Risk for ESRD in type 1 diabetes remains high despite renoprotection. J Am Soc Nephrol 22: 545–553, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kawamata Y, Fujii R, Hosoya M, Harada M, Yoshida H, Miwa M, Fukusumi S, Habata Y, Itoh T, Shintani Y, Hinuma S, Fujisawa Y, Fujino M: A G protein-coupled receptor responsive to bile acids. J Biol Chem 278: 9435–9440, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Maruyama T, Miyamoto Y, Nakamura T, Tamai Y, Okada H, Sugiyama E, Nakamura T, Itadani H, Tanaka K: Identification of membrane-type receptor for bile acids (M-BAR). Biochem Biophys Res Commun 298: 714–719, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Thomas C, Pellicciari R, Pruzanski M, Auwerx J, Schoonjans K: Targeting bile-acid signalling for metabolic diseases. Nat Rev Drug Discov 7: 678–693, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Tiwari A, Maiti P: TGR5: An emerging bile acid G-protein-coupled receptor target for the potential treatment of metabolic disorders. Drug Discov Today 14: 523–530, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Jiang T, Wang XX, Scherzer P, Wilson P, Tallman J, Takahashi H, Li J, Iwahashi M, Sutherland E, Arend L, Levi M: Farnesoid X receptor modulates renal lipid metabolism, fibrosis, and diabetic nephropathy. Diabetes 56: 2485–2493, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Wang XX, Jiang T, Shen Y, Adorini L, Pruzanski M, Gonzalez FJ, Scherzer P, Lewis L, Miyazaki-Anzai S, Levi M: The farnesoid X receptor modulates renal lipid metabolism and diet-induced renal inflammation, fibrosis, and proteinuria. Am J Physiol Renal Physiol 297: F1587–F1596, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang XX, Jiang T, Shen Y, Caldas Y, Miyazaki-Anzai S, Santamaria H, Urbanek C, Solis N, Scherzer P, Lewis L, Gonzalez FJ, Adorini L, Pruzanski M, Kopp JB, Verlander JW, Levi M: Diabetic nephropathy is accelerated by farnesoid X receptor deficiency and inhibited by farnesoid X receptor activation in a type 1 diabetes model. Diabetes 59: 2916–2927, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang XX, Edelstein MH, Gafter U, Qiu L, Luo Y, Dobrinskikh E, Lucia S, Adorini L, D’Agati VD, Levi J, Rosenberg A, Kopp JB, Gius DR, Saleem MA, Levi M: G protein-coupled bile acid receptor TGR5 activation inhibits kidney disease in obesity and diabetes. J Am Soc Nephrol 27: 1362–1378, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rizzo G, Passeri D, De Franco F, Ciaccioli G, Donadio L, Rizzo G, Orlandi S, Sadeghpour B, Wang XX, Jiang T, Levi M, Pruzanski M, Adorini L: Functional characterization of the semisynthetic bile acid derivative INT-767, a dual farnesoid X receptor and TGR5 agonist. Mol Pharmacol 78: 617–630, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee JW, Chou CL, Knepper MA: Deep sequencing in microdissected renal tubules identifies nephron segment-specific transcriptomes. J Am Soc Nephrol 26: 2669–2677, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pellicciari R, Fiorucci S, Camaioni E, Clerici C, Costantino G, Maloney PR, Morelli A, Parks DJ, Willson TM: 6Alpha-ethyl-chenodeoxycholic acid (6-ECDCA), a potent and selective FXR agonist endowed with anticholestatic activity. J Med Chem 45: 3569–3572, 2002 [DOI] [PubMed] [Google Scholar]
- 31.Thomas C, Gioiello A, Noriega L, Strehle A, Oury J, Rizzo G, Macchiarulo A, Yamamoto H, Mataki C, Pruzanski M, Pellicciari R, Auwerx J, Schoonjans K: TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab 10: 167–177, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brosius FC 3rd, Alpers CE, Bottinger EP, Breyer MD, Coffman TM, Gurley SB, Harris RC, Kakoki M, Kretzler M, Leiter EH, Levi M, McIndoe RA, Sharma K, Smithies O, Susztak K, Takahashi N, Takahashi T; Animal Models of Diabetic Complications Consortium : Mouse models of diabetic nephropathy. J Am Soc Nephrol 20: 2503–2512, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Datta R, Heylman C, George SC, Gratton E: Label-free imaging of metabolism and oxidative stress in human induced pluripotent stem cell-derived cardiomyocytes. Biomed Opt Express 7: 1690–1701, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Y, Heilig K, Saunders T, Minto A, Deb DK, Chang A, Brosius F, Monteiro C, Heilig CW: Transgenic overexpression of GLUT1 in mouse glomeruli produces renal disease resembling diabetic glomerulosclerosis. Am J Physiol Renal Physiol 299: F99–F111, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang H, Schin M, Saha J, Burke K, Holzman LB, Filipiak W, Saunders T, Xiang M, Heilig CW, Brosius FC 3rd: Podocyte-specific overexpression of GLUT1 surprisingly reduces mesangial matrix expansion in diabetic nephropathy in mice. Am J Physiol Renal Physiol 299: F91–F98, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nayak BK, Shanmugasundaram K, Friedrichs WE, Cavaglierii RC, Patel M, Barnes J, Block K: HIF-1 mediates renal fibrosis in OVE26 type 1 diabetic mice. Diabetes 65: 1387–1397, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ricardo SD, van Goor H, Eddy AA: Macrophage diversity in renal injury and repair. J Clin Invest 118: 3522–3530, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vernon MA, Mylonas KJ, Hughes J: Macrophages and renal fibrosis. Semin Nephrol 30: 302–317, 2010 [DOI] [PubMed] [Google Scholar]
- 39.McMahan RH, Wang XX, Cheng LL, Krisko T, Smith M, El Kasmi K, Pruzanski M, Adorini L, Golden-Mason L, Levi M, Rosen HR: Bile acid receptor activation modulates hepatic monocyte activity and improves nonalcoholic fatty liver disease. J Biol Chem 288: 11761–11770, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cunard R, Sharma K: The endoplasmic reticulum stress response and diabetic kidney disease. Am J Physiol Renal Physiol 300: F1054–F1061, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vannuvel K, Renard P, Raes M, Arnould T: Functional and morphological impact of ER stress on mitochondria. J Cell Physiol 228: 1802–1818, 2013 [DOI] [PubMed] [Google Scholar]
- 42.Grimm S: The ER-mitochondria interface: The social network of cell death. Biochim Biophys Acta 1823: 327–334, 2012 [DOI] [PubMed] [Google Scholar]
- 43.Marchi S, Patergnani S, Pinton P: The endoplasmic reticulum-mitochondria connection: One touch, multiple functions. Biochim Biophys Acta 1837: 461–469, 2014 [DOI] [PubMed] [Google Scholar]
- 44.Malhotra JD, Kaufman RJ: ER stress and its functional link to mitochondria: Role in cell survival and death. Cold Spring Harb Perspect Biol 3: a004424, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, Görgün CZ, Hotamisligil GS: Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science 313: 1137–1140, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ji C, Kaplowitz N, Lau MY, Kao E, Petrovic LM, Lee AS: Liver-specific loss of glucose-regulated protein 78 perturbs the unfolded protein response and exacerbates a spectrum of liver diseases in mice. Hepatology 54: 229–239, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kammoun HL, Chabanon H, Hainault I, Luquet S, Magnan C, Koike T, Ferré P, Foufelle F: GRP78 expression inhibits insulin and ER stress-induced SREBP-1c activation and reduces hepatic steatosis in mice. J Clin Invest 119: 1201–1215, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Park SW, Zhou Y, Lee J, Lu A, Sun C, Chung J, Ueki K, Ozcan U: The regulatory subunits of PI3K, p85alpha and p85beta, interact with XBP-1 and increase its nuclear translocation. Nat Med 16: 429–437, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Senkal CE, Ponnusamy S, Bielawski J, Hannun YA, Ogretmen B: Antiapoptotic roles of ceramide-synthase-6-generated C16-ceramide via selective regulation of the ATF6/CHOP arm of ER-stress-response pathways. FASEB J 24: 296–308, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Senkal CE, Ponnusamy S, Manevich Y, Meyers-Needham M, Saddoughi SA, Mukhopadyay A, Dent P, Bielawski J, Ogretmen B: Alteration of ceramide synthase 6/C16-ceramide induces activating transcription factor 6-mediated endoplasmic reticulum (ER) stress and apoptosis via perturbation of cellular Ca2+ and ER/Golgi membrane network. J Biol Chem 286: 42446–42458, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ye J, Rawson RB, Komuro R, Chen X, Davé UP, Prywes R, Brown MS, Goldstein JL: ER stress induces cleavage of membrane-bound ATF6 by the same proteases that process SREBPs. Mol Cell 6: 1355–1364, 2000 [DOI] [PubMed] [Google Scholar]
- 52.Cantó C, Auwerx J: AMP-activated protein kinase and its downstream transcriptional pathways. Cell Mol Life Sci 67: 3407–3423, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jornayvaz FR, Shulman GI: Regulation of mitochondrial biogenesis. Essays Biochem 47: 69–84, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Giguère V: Transcriptional control of energy homeostasis by the estrogen-related receptors. Endocr Rev 29: 677–696, 2008 [DOI] [PubMed] [Google Scholar]
- 55.Villena JA, Kralli A: ERRalpha: A metabolic function for the oldest orphan. Trends Endocrinol Metab 19: 269–276, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hirschey MD, Shimazu T, Goetzman E, Jing E, Schwer B, Lombard DB, Grueter CA, Harris C, Biddinger S, Ilkayeva OR, Stevens RD, Li Y, Saha AK, Ruderman NB, Bain JR, Newgard CB, Farese RV Jr., Alt FW, Kahn CR, Verdin E: SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature 464: 121–125, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jiang T, Wang Z, Proctor G, Moskowitz S, Liebman SE, Rogers T, Lucia MS, Li J, Levi M: Diet-induced obesity in C57BL/6J mice causes increased renal lipid accumulation and glomerulosclerosis via a sterol regulatory element-binding protein-1c-dependent pathway. J Biol Chem 280: 32317–32325, 2005 [DOI] [PubMed] [Google Scholar]
- 58.Proctor G, Jiang T, Iwahashi M, Wang Z, Li J, Levi M: Regulation of renal fatty acid and cholesterol metabolism, inflammation, and fibrosis in Akita and OVE26 mice with type 1 diabetes. Diabetes 55: 2502–2509, 2006 [DOI] [PubMed] [Google Scholar]
- 59.Sun L, Halaihel N, Zhang W, Rogers T, Levi M: Role of sterol regulatory element-binding protein 1 in regulation of renal lipid metabolism and glomerulosclerosis in diabetes mellitus. J Biol Chem 277: 18919–18927, 2002 [DOI] [PubMed] [Google Scholar]
- 60.Wang Z, Jiang T, Li J, Proctor G, McManaman JL, Lucia S, Chua S, Levi M: Regulation of renal lipid metabolism, lipid accumulation, and glomerulosclerosis in FVBdb/db mice with type 2 diabetes. Diabetes 54: 2328–2335, 2005 [DOI] [PubMed] [Google Scholar]
- 61.Xie C, Jiang C, Shi J, Gao X, Sun D, Sun L, Wang T, Takahashi S, Anitha M, Krausz KW, Patterson AD, Gonzalez FJ: An intestinal farnesoid X receptor-ceramide signaling axis modulates hepatic gluconeogenesis in mice. Diabetes 66: 613–626, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gonzalez FJ, Jiang C, Patterson AD: An intestinal microbiota-farnesoid X receptor axis modulates metabolic disease. Gastroenterology 151: 845–859, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jiang C, Xie C, Lv Y, Li J, Krausz KW, Shi J, Brocker CN, Desai D, Amin SG, Bisson WH, Liu Y, Gavrilova O, Patterson AD, Gonzalez FJ: Intestine-selective farnesoid X receptor inhibition improves obesity-related metabolic dysfunction. Nat Commun 6: 10166, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jiang C, Xie C, Li F, Zhang L, Nichols RG, Krausz KW, Cai J, Qi Y, Fang ZZ, Takahashi S, Tanaka N, Desai D, Amin SG, Albert I, Patterson AD, Gonzalez FJ: Intestinal farnesoid X receptor signaling promotes nonalcoholic fatty liver disease. J Clin Invest 125: 386–402, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Qi Y, Jiang C, Cheng J, Krausz KW, Li T, Ferrell JM, Gonzalez FJ, Chiang JY: Bile acid signaling in lipid metabolism: Metabolomic and lipidomic analysis of lipid and bile acid markers linked to anti-obesity and anti-diabetes in mice. Biochim Biophys Acta 1851: 19–29, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li F, Jiang C, Krausz KW, Li Y, Albert I, Hao H, Fabre KM, Mitchell JB, Patterson AD, Gonzalez FJ: Microbiome remodelling leads to inhibition of intestinal farnesoid X receptor signalling and decreased obesity. Nat Commun 4: 2384, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Crosignani V, Dvornikov A, Aguilar JS, Stringari C, Edwards R, Mantulin WW, Gratton E: Deep tissue fluorescence imaging and in vivo biological applications. J Biomed Opt 17: 116023, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Crosignani V, Jahid S, Dvornikov AS, Gratton E: A deep tissue fluorescence imaging system with enhanced SHG detection capabilities. Microsc Res Tech 77: 368–373, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ranjit S, Dvornikov A, Stakic M, Hong SH, Levi M, Evans RM, Gratton E: Imaging fibrosis and separating collagens using second harmonic generation and phasor approach to fluorescence lifetime imaging. Sci Rep 5: 13378, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ranjit S, Dvornikov A, Levi M, Furgeson S, Gratton E: Characterizing fibrosis in UUO mice model using multiparametric analysis of phasor distribution from FLIM images. Biomed Opt Express 7: 3519–3530, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ranjit S, Dobrinskikh E, Montford J, Dvornikov A, Lehman A, Orlicky DJ, Nemenoff R, Gratton E, Levi M, Furgeson S: Label-free fluorescence lifetime and second harmonic generation imaging microscopy improves quantification of experimental renal fibrosis. Kidney Int 90: 1123–1128, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stringari C, Nourse JL, Flanagan LA, Gratton E: Phasor fluorescence lifetime microscopy of free and protein-bound NADH reveals neural stem cell differentiation potential. PLoS One 7: e48014, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stringari C, Cinquin A, Cinquin O, Digman MA, Donovan PJ, Gratton E: Phasor approach to fluorescence lifetime microscopy distinguishes different metabolic states of germ cells in a live tissue. Proc Natl Acad Sci U S A 108: 13582–13587, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Digman MA, Caiolfa VR, Zamai M, Gratton E: The phasor approach to fluorescence lifetime imaging analysis. Biophys J 94: L14–L16, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jameson DM: Introduction to Fluorescence, Boca Raton, FL, CRC Press, Taylor & Francis Group, 2014 [Google Scholar]
- 76.Marcu L, French PMW, Elson DS: Fluorescence Lifetime Spectroscopy and Imaging: Principles and Applications in Biomedical Diagnostics, Boca Raton, FL, CRC Press/Taylor & Francis Group, 2014 [Google Scholar]
- 77.Aguilar-Arnal L, Ranjit S, Stringari C, Orozco-Solis R, Gratton E, Sassone-Corsi P: Spatial dynamics of SIRT1 and the subnuclear distribution of NADH species [published online ahead of print October 24, 2016]. Proc Natl Acad Sci USA [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Datta R, Alfonso-García A, Cinco R, Gratton E: Fluorescence lifetime imaging of endogenous biomarker of oxidative stress. Sci Rep 5: 9848, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.