Abstract
The renin-angiotensin-aldosterone system has an important role in the control of fluid homeostasis and BP during volume depletion. Dietary salt restriction elevates circulating angiotensin II (AngII) and aldosterone levels, increasing levels of the Cl−/HCO3− exchanger pendrin in β-intercalated cells and the Na+-Cl− cotransporter (NCC) in distal convoluted tubules. However, the independent roles of AngII and aldosterone in regulating these levels remain unclear. In C57BL/6J mice receiving a low-salt diet or AngII infusion, we evaluated the membrane protein abundance of pendrin and NCC; assessed the phosphorylation of the mineralocorticoid receptor, which selectively inhibits aldosterone binding in intercalated cells; and measured BP by radiotelemetry in pendrin-knockout and wild-type mice. A low-salt diet or AngII infusion upregulated NCC and pendrin levels, decreased the phosphorylation of mineralocorticoid receptor in β-intercalated cells, and increased plasma aldosterone levels. Notably, a low-salt diet did not alter BP in wild-type mice, but significantly decreased BP in pendrin-knockout mice. To dissect the roles of AngII and aldosterone, we performed adrenalectomies in mice to remove aldosterone from the circulation. In adrenalectomized mice, AngII infusion again upregulated NCC expression, but did not affect pendrin expression despite the decreased phosphorylation of mineralocorticoid receptor. By contrast, AngII and aldosterone coadministration markedly elevated pendrin levels in adrenalectomized mice. Our results indicate that aldosterone is necessary for AngII-induced pendrin upregulation, and suggest that pendrin contributes to the maintenance of normal BP in cooperation with NCC during activation of the renin-angiotensin-aldosterone system by dietary salt restriction.
Keywords: aldosterone, angiotensin, Na transport
The kidney maintains systemic fluid homeostasis predominantly through the reabsorption of filtered NaCl via specialized electrolyte transport mechanisms in different nephron segments. The renin-angiotensin-aldosterone system (RAAS) plays a key role in regulating BP and fluid volume by activating renal NaCl transport mechanisms. During volume depletion, elevated angiotensin II (AngII) and aldosterone levels activate the Na+/H+ exchanger in proximal tubules,1 the Na+-K+-2Cl− cotransporter in the thick ascending limb of Henle,2 the Na+-Cl− cotransporter (NCC) in distal convoluted tubule (DCT) cells,3,4 and the epithelial Na+ channel in principal cells,5 resulting in increased NaCl reabsorption.
Pendrin, encoded by SLC26A4, is a Cl−/HCO3− exchanger expressed specifically in β-intercalated cells6 and regulates acid-base balance by excreting HCO3− in the urine. In addition to its role in HCO3− excretion, several studies have demonstrated that pendrin promotes reabsorption of Cl−,7,8 and increases Na+ reabsorption in cooperation with the Na+-dependent Cl−/HCO3− exchanger.7,9–12 Recently, we identified a phosphorylation site in the ligand-binding domain of the mineralocorticoid receptor (MR) at S843 (pMR-S843), which prevents ligand binding and MR signaling in intercalated cells.9 In addition to the ligand–receptor relationship in MR activation, there are several factors influencing pendrin expression; plasma AngII and aldosterone levels and potassium concentration ([K+]), all of which exert effects upon the other. Indeed, AngII upregulates pendrin expression,2,8,9 which is associated with dephosphorylation of pMR-S8439; however, the independent roles of AngII and aldosterone in regulating pMR-S843 levels and pendrin expression remain unclear.
With respect to the physiologic role of pendrin in BP regulation, pendrin overexpression in intercalated cells results in salt-sensitive hypertension,13 whereas acute deletion of pendrin lowers BP.14 Moreover, there has been growing interest in pendrin and NCC interactions associated with BP maintenance. However, the precise mechanisms associated with the way in which pendrin contributes to BP maintenance in cooperation with NCC remain obscure. Recent studies indicate that single deletion of pendrin or NCC does not result in a salt-losing phenotype10,15,16; however, double-knockout mice demonstrate severe salt loss and hypotension.10 Pendrin expression is elevated in the kidneys of NCC-knockout mice,6,10 raising the possibility that pendrin or NCC compensates for the loss of the other through their increased expression and activity associated with regulating NaCl reabsorption.
Given the previous finding indicating upregulation of pendrin expression during dietary salt restriction and aldosterone infusion,17,18 in this study using pendrin-knockout (PDS−/−) mice, we evaluate the role of pendrin in the regulation of BP in cooperation with NCC during RAAS activation induced by changes in salt intake from a high-salt (HS) diet to a low-salt (LS) diet. In addition, we investigate the independent roles of AngII and aldosterone on MR phosphorylation and pendrin expression in vivo compared with NCC expression. This comparison resulted in detailed insight into pendrin regulation, given that the role of the RAAS in NCC regulation has been more extensively studied.3,4,19,20
Results
Activation of RAAS by NaCl Restriction Increases Pendrin and Decreases pMR-S843 Levels
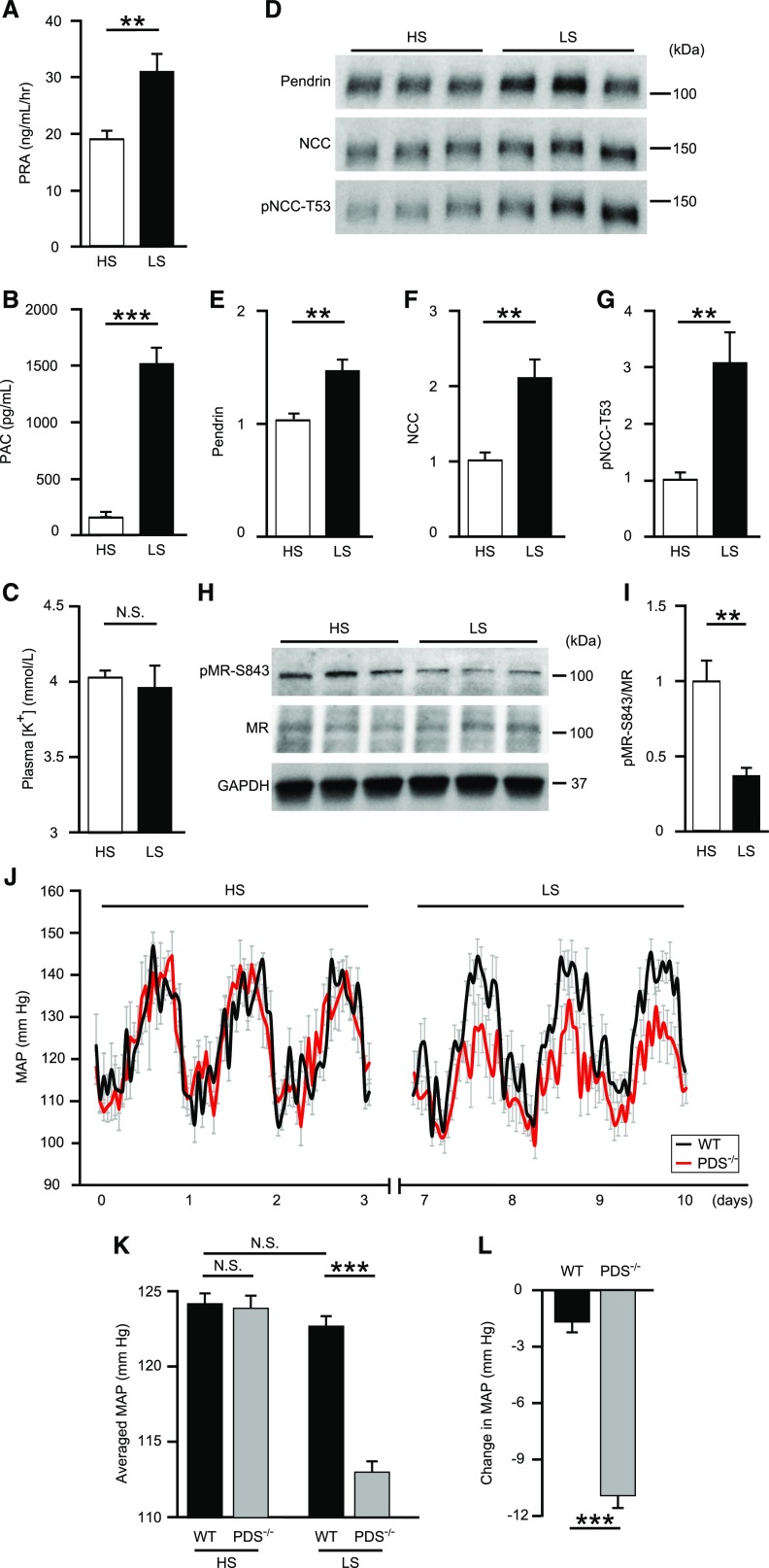
During volume depletion, the activated RAAS contributes to maintaining fluid volume through NaCl reabsorption. To modulate intravascular volume by dietary salt intake, mice were fed either an HS (8% NaCl) or LS (0.03% NaCl) diet. As expected, NaCl restriction by an LS diet increased plasma renin activity (30.8±3.2 ng/ml per hour versus 18.8±1.8 ng/ml per hour; P=0.004) (Figure 1A) and plasma aldosterone concentrations (1513±190 pg/ml versus 152±48 pg/ml; P<0.001) (Figure 1B), suggesting activation of the endogenous RAAS. Plasma [K+] was not affected by these diets (3.96±0.15 mmol/L versus 4.02±0.05 mmol/L; P=0.66) (Figure 1C). Additionally, an LS diet increased membrane-protein abundance of pendrin by 1.5-fold (P=0.004), NCC by 2.1-fold (P=0.003), and phosphorylation of NCC at threonine-53 (pNCC-T53) by 3.1-fold (P=0.01) (Figure 1, D–G). Furthermore, pMR-S843 levels, but not total MR levels (Figure 1, H and I) in whole kidney lysates were reduced by 61% (P=0.001), despite no changes in plasma [K+].
Figure 1.
Activation of the RAAS by NaCl restriction increases pendrin and decreases pMR-S843 levels, and pendrin contributes to maintaining normal BP in cooperation with NCC during volume depletion. (A) PRA, (B) PAC, and (C) plasma [K+] in C57BL/6J mice fed HS (n=9) or LS (n=8) diets. (D) Representative Western blots and quantification of (E) pendrin, (F) NCC, and (G) pNCC-T53 in the membrane fraction of kidneys from mice fed HS (n=6) or LS (n=7) diets. Equal loading was confirmed by parallel Coomassie-stained gels (see Supplemental Figure 3). Representative (H) Western blots and (I) quantification of pMR-S843 levels in the whole-kidney lysates from mice fed HS and LS diets. (J) Hourly averaged MAPs measured over three consecutive days in mice fed HS or LS diets, (K) averaged MAPs of mice fed HS or LS diets, and (L) changes in the MAPs of PDS−/− (n=5) and WT mice (n=5) fed HS or LS diets. MAP tracings represent averages of measurement from all animals. For (A–I), statistical comparisons were performed using an unpaired t test. For (J–L), statistical comparisons were performed using a two-way ANOVA, followed by Holm–Sidak multiple comparison tests. Data represent the mean±SEM. **P<0.01; ***P<0.001. PAC, plasma aldosterone concentration; PRA, plasma renin activity.
Dietary Salt Restriction Decreases BP in PDS−/− Mice
Activation of the RAAS by dietary salt restriction increased pendrin as well as NCC during volume depletion.15,16 To evaluate the physiologic roles of pendrin in maintaining BP during volume depletion, we investigated the effect of dietary salt restriction on BP using PDS−/− and wild-type (WT) mice. Averaged mean arterial pressures (MAPs) at baseline for an HS diet were similar in both groups (PDS−/−: 124.1±0.8 mm Hg versus WT: 124.3±0.8 mm Hg) (Figure 1, J and K). WT mice showed no significant difference between HS and LS diets (Figure 1, J and K), but PDS−/− mice exhibited significantly lower MAPs on an LS diet compared with those observed on an HS diet (Figure 1, J and K). These differences between HS and LS diets were significantly greater in PDS−/− mice relative to those observed in WT mice (PDS−/−: −11.0±0.7 mm Hg versus WT: −1.6±0.6 mm Hg; P<0.001) (Figure 1L), indicating that deletion of the pendrin gene resulted in NaCl loss and resultant BP reduction. On the basis of the previous findings of NCC upregulation in PDS−/− mice with an LS diet,18 our results suggest that activation of pendrin contributed to maintaining normal BP in cooperation with NCC during volume depletion with an LS diet, in contrast to the small role of pendrin in the regulation of BP during volume repletion with an HS diet.
AngII Infusion Increases Pendrin and Decreases pMR-S843 Levels
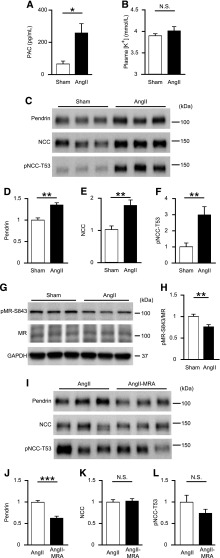
Next, we examined whether exogenous AngII also increased pendrin and decreased pMR-S843 levels. AngII infusion increased plasma aldosterone concentrations (257±60 pg/ml versus 65±19 pg/ml; P=0.01) (Figure 2A), whereas plasma [K+] was not affected by AngII infusion at day 7 (4.01±0.10 mmol/L versus 3.89±0.04 mmol/L; P=0.31) (Figure 2B). Of note, AngII infusion increased membrane-protein abundance of pendrin by 1.4-fold (P=0.001), NCC by 1.8-fold (P=0.01), and pNCC-T53 by 3.0-fold (P=0.01) (Figure 2, C–F), and reduced pMR-S843 levels by 25% (P=0.01) (Figure 2, G and H). These results suggest that both endogenously and exogenously activated RAAS increased membrane-protein abundance of pendrin, NCC, and pNCC-T53 without altering plasma [K+], which was associated with MR dephosphorylation.
Figure 2.
AngII infusion increases pendrin and decreases pMR-S843 levels. (A) PAC and (B) plasma [K+] in C57BL/6J mice undergoing either a sham operation (Sham; n=8) or AngII infusion (n=9). (C) Representative Western blots and quantifications of (D) pendrin, (E) NCC, and (F) pNCC-T53 in the membrane fraction of kidneys from Sham and AngII-infused mice (n=6 per group). Equal loading was confirmed by parallel Coomassie-stained gels (see Supplemental Figure 3). (G) Representative Western blots and (H) quantification of pMR-S843 levels in the whole-kidney lysates of Sham and AngII-infused mice. (I) Representative Western blots and quantifications of (J) pendrin, (K) NCC, and (L) pNCC-T53 in the membrane fraction of kidneys from mice treated with AngII infusion and AngII infusion with spironolactone (n=6 per group). Equal loading was confirmed by parallel Coomassie-stained gels (see Supplemental Figure 3). Statistical comparisons were performed using an unpaired t test. Data represent the mean±SEM. *P<0.05; **P<0.01; ***P<0.001. PAC, plasma aldosterone concentration.
To investigate whether AngII-induced pendrin upregulation is mediated by MR activation associated with MR dephosphorylation, we administered the MR antagonist (MRA) spironolactone to AngII-treated mice. Spironolactone administration significantly decreased membrane-protein abundance of pendrin by 35% (P<0.001) (Figure 2, I and J), suggesting that activated MR signaling contributed to increased pendrin expression in AngII-treated mice. By contrast, AngII-induced upregulation of NCC and pNCC-T53 was not reversed by spironolactone treatment (Figure 2, I, K, and L). These results suggest that AngII increased membrane-protein abundance of NCC and phosphorylated NCC in an aldosterone-independent manner, but AngII-induced pendrin expression was dependent on aldosterone-MR signaling.
AngII Increases NCC, but Not Pendrin Expression in Adrenalectomized Mice Despite MR Dephosphorylation
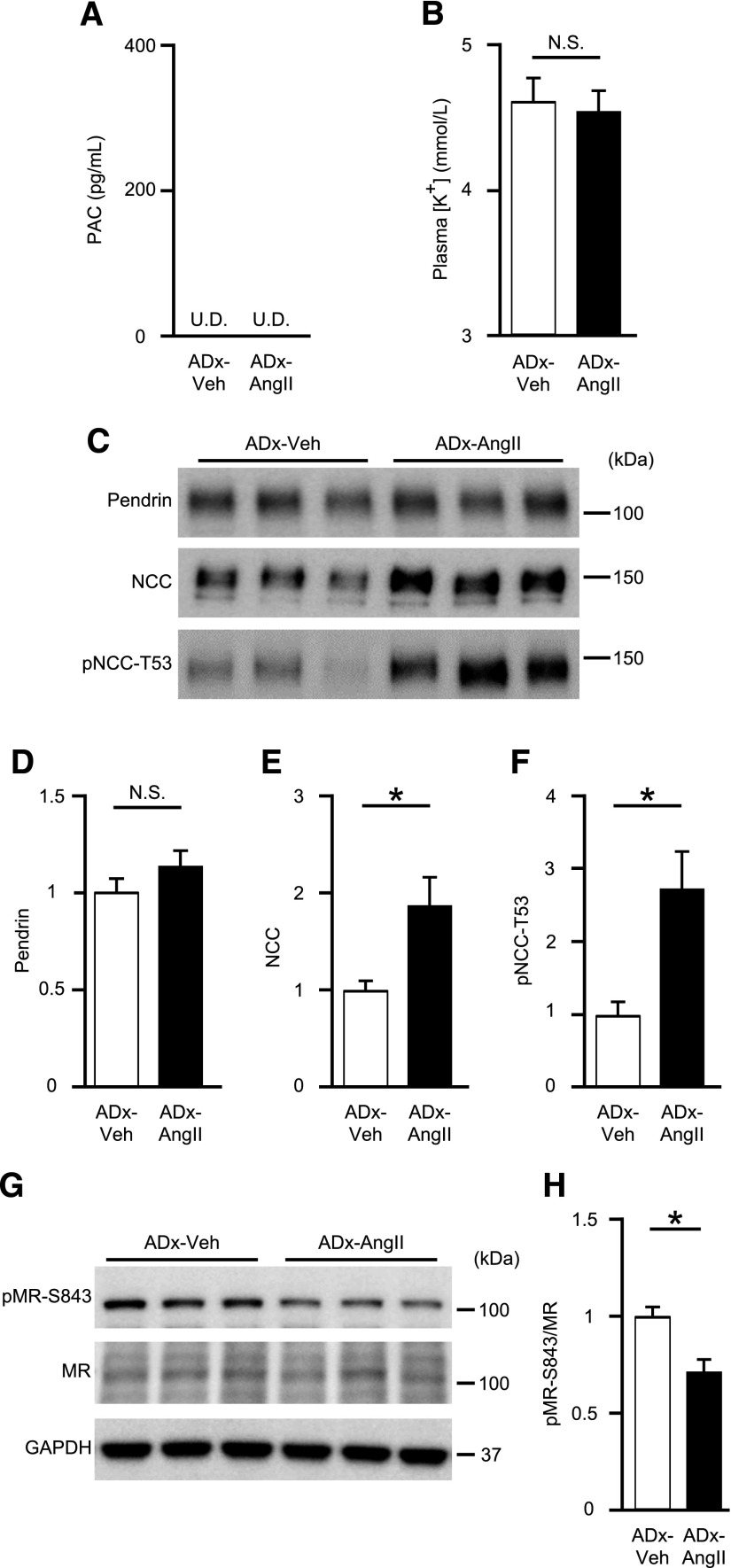
To dissect the roles of AngII and aldosterone, we performed adrenalectomies to remove aldosterone from the circulation of blood in mice, resulting in complete abolishment of aldosterone in plasma (Figure 3A). In adrenalectomized mice, plasma [K+] was unaffected by AngII infusion (4.59±0.15 mmol/L versus 4.64±0.19 mmol/L; P=0.83) (Figure 3B). AngII infusion upregulated membrane-protein abundance of NCC by 1.9-fold (P=0.04) and pNCC-T53 by 2.8-fold (P=0.03), but not that of pendrin (a 1.1-fold change; P=0.25) in adrenalectomized mice (Figure 3, C–F), although pMR-S843 levels were again reduced in the absence of aldosterone (a 30% reduction; P=0.04) (Figure 3, G and H). These results strongly supported our hypothesis that aldosterone was necessary for AngII-mediated pendrin expression, despite aldosterone-independent MR dephosphorylation induced by AngII (Figure 2, C and D).
Figure 3.
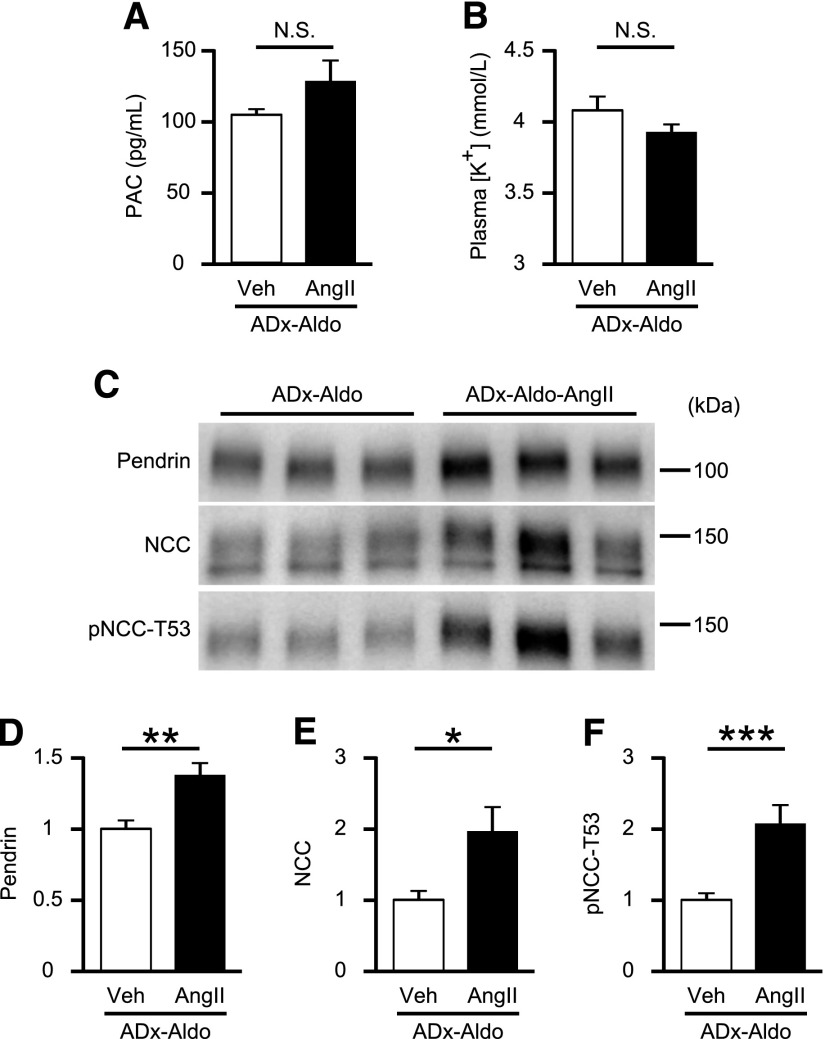
AngII increases NCC, but not pendrin levels in adrenalectomized mice. (A) PAC and (B) plasma [K+] in adrenalectomized C57BL/6J mice treated with either vehicle (ADx-Veh; n=5) or AngII infusion (ADx-AngII; n=7). (C) Representative Western blots and quantifications of (D) pendrin, (E) NCC, and (F) pNCC-T53 in the membrane fraction of kidneys in ADx-Veh (n=5) or ADx-AngII (n=7) mice. Equal loading was confirmed by parallel Coomassie-stained gels (see Supplemental Figure 3). (G) Representative Western blots and (H) quantification of pMR-S843 levels in whole-kidney lysates from ADx-Veh (n=5) or ADx-AngII (n=7) mice. Statistical comparisons were performed using an unpaired t test. Data represent the mean±SEM. *P<0.05. PAC, plasma aldosterone concentration; U.D., undetectable.
AngII and Aldosterone Coadministration in Adrenalectomized Mice Increases Pendrin Expression along with MR Dephosphorylation
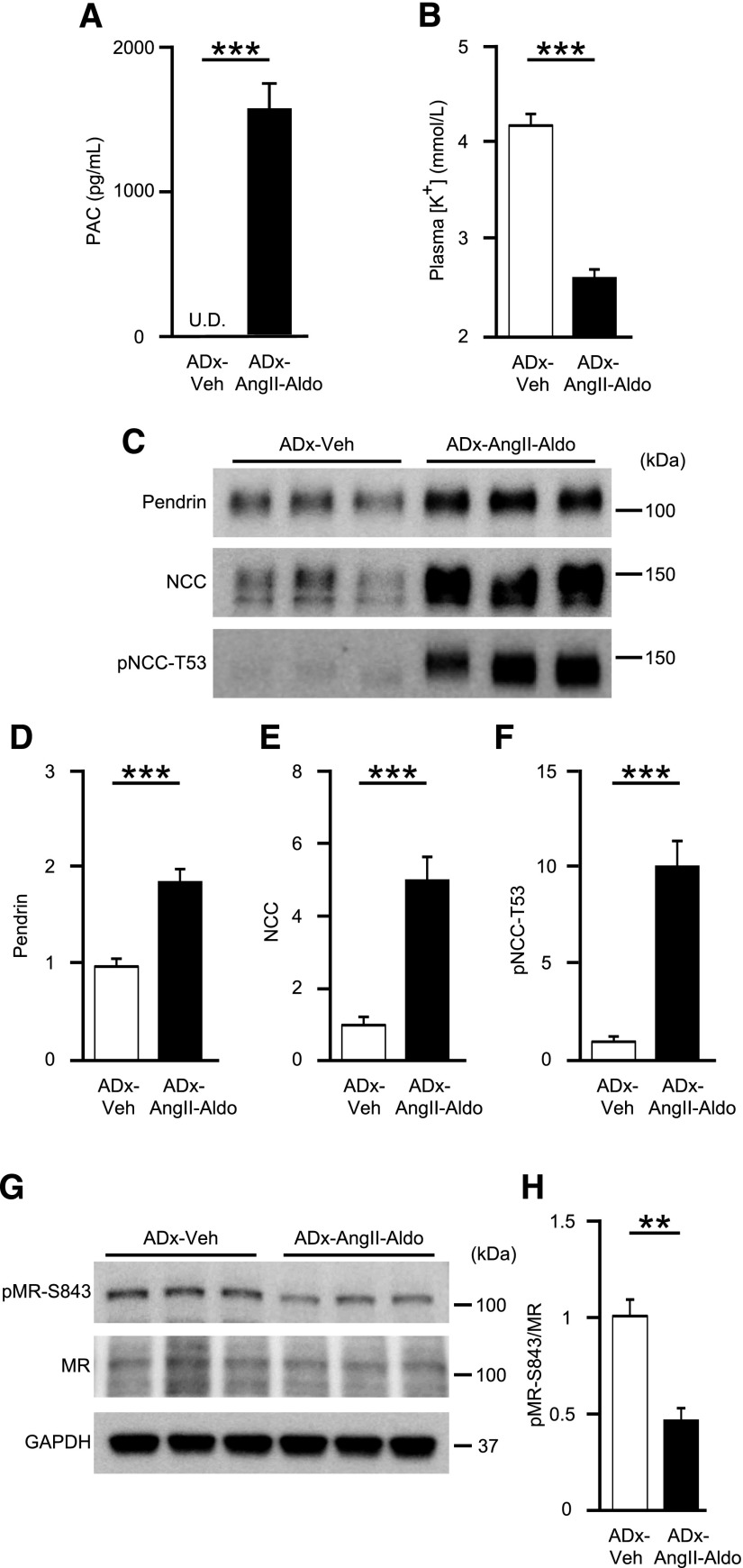
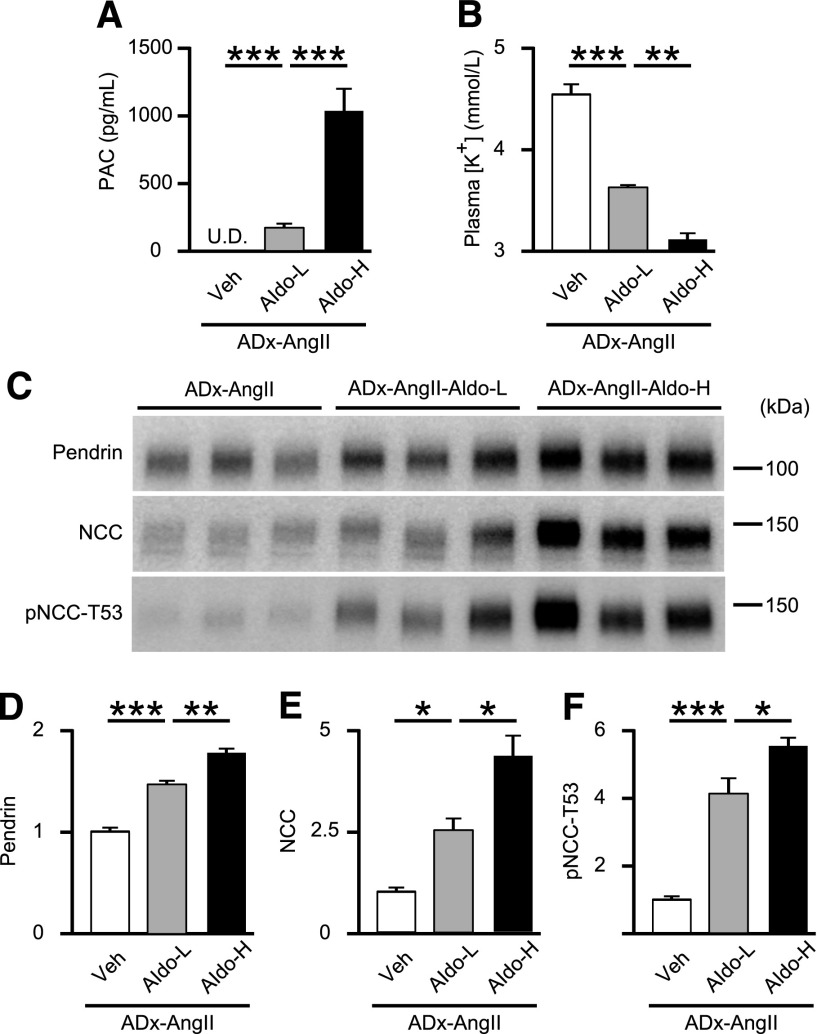
To confirm that aldosterone is essential for AngII-induced pendrin upregulation, we supplemented adrenalectomized mice with either vehicle (ADx-Veh) or AngII with aldosterone (ADx-AngII-Aldo). Aldosterone levels during exogenous supplementation were similar to those in mice on an LS diet (Figures 1B and 4A). Additionally, plasma [K+] was significantly lowered (2.64±0.88 mmol/L versus 4.25±0.13 mmol/L; P<0.001) (Figure 4B). Of note, AngII and aldosterone coadministration to adrenalectomized mice markedly upregulated levels of pendrin by 1.9-fold (P<0.001), NCC by 5.0-fold (P<0.001), and pNCC-T53 by 10.1-fold (P<0.001) (Figure 4, C–F), along with a 53% reduction in pMR-S843 levels (P=0.001) (Figure 4, G and H). To further evaluate whether AngII and aldosterone act synergistically to stimulate pendrin or NCC, we tried to compare membrane-protein abundance of pendrin and NCC in the kidney of aldosterone-treated adrenalectomized mice with or without AngII infusion; expressions of both pendrin and NCC were significantly higher in aldosterone-treated adrenalectomized mice with AngII infusion (Figure 5, C–F), associated with comparable plasma [K+] (Figure 5B). Aldosterone levels during exogenous supplementation were almost two times higher than those in mice on an HS diet (Figures 5A and 2A). When high-dose and low-dose aldosterone were administered to AngII-treated adrenalectomized mice, aldosterone-induced increases in pendrin expression occurred in a dose-dependent fashion (Figure 6, C–F), in accompaniment with increased plasma aldosterone and decreased plasma [K+] (Figure 6, A and B), suggesting that there was a synergy between AngII and aldosterone to promote expressions of pendrin as well as NCC.
Figure 4.
AngII and aldosterone coadministration to adrenalectomized mice increases pendrin levels along with MR dephosphorylation. (A) PAC and (B) plasma [K+] in adrenalectomized C57BL/6J mice treated with either vehicle (ADx-Veh; n=6) or AngII and aldosterone (1.0 μg/d) coadministration (ADx-AngII-Aldo; n=8). (C) Representative Western blots and quantifications of (D) pendrin, (E) NCC, and (F) pNCC-T53 in the membrane fraction of kidneys from ADx-Veh (n=6) and ADx-AngII-Aldo (n=8) mice. Equal loading was confirmed by parallel Coomassie-stained gels (see Supplemental Figure 3). (G) Representative Western blots and (H) quantification of pMR-S843 levels in whole-kidney lysates from ADx-Veh (n=6) and ADx-AngII-Aldo (n=8) mice. Statistical comparisons were performed using an unpaired t test. Data represent the mean±SEM. **P<0.01; ***P<0.001. PAC, plasma aldosterone concentration; U.D., undetectable.
Figure 5.
AngII infusion increases pendrin levels in aldosterone-treated adrenalectomized mice. (A) PAC and (B) plasma [K+] in aldosterone (0.1 μg/d)-treated adrenalectomized C57BL/6J mice received either vehicle (ADx-Aldo) or AngII infusion (ADx-Aldo-AngII) (n=5 per group). (C) Representative Western blots and quantifications of (D) pendrin, (E) NCC, and (F) pNCC-T53 in the membrane fraction of kidneys from ADx-Aldo and ADx-Aldo-AngII mice (n=5 per group). Equal loading was confirmed by parallel Coomassie-stained gels (see Supplemental Figure 3). Statistical comparisons were performed using an unpaired t test. Data represent the mean±SEM. *P<0.05; **P<0.01; ***P<0.001. PAC, plasma aldosterone concentration.
Figure 6.
Aldosterone infusion increases pendrin levels in AngII-treated adrenalectomized mice in a dose-dependent fashion. (A) PAC and (B) plasma [K+] in AngII-treated adrenalectomized C57BL/6J mice with the treatment of vehicle (ADx-AngII; n=5), low-dose (0.3 μg/d) aldosterone (ADx-AngII-Aldo-L; n=4), and high-dose (1.0 μg/d) aldosterone (ADx-AngII-Aldo-H; n=5). (C) Representative Western blots and quantifications of (D) pendrin, (E) NCC, and (F) pNCC-T53 in the membrane fraction of kidneys from ADx-AngII (n=5), ADx-AngII-Aldo-L (n=4), and ADx-AngII-Aldo-H (n=5) mice. Equal loading was confirmed by parallel Coomassie-stained gels (see Supplemental Figure 3). Statistical comparisons were performed using a one-way ANOVA, followed by Tukey–Kramer multiple comparison tests. Data represent the mean±SEM. *P<0.05; **P<0.01; ***P<0.001. PAC, plasma aldosterone concentration; U.D., undetectable.
AngII and Aldosterone Coadministration in Adrenalectomized Mice Increases Immunostaining of Pendrin in β-Intercalated Cells
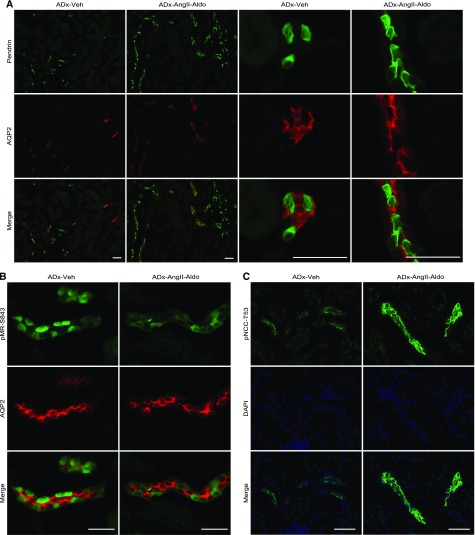
We then performed immunofluorescence analysis by costaining mouse kidneys with pendrin or pMR-S843 and aquaporin 2 (AQP2), a marker of principal cells in the connecting tubule and collecting duct. Immunofluorescence results showed increased pendrin labeling in β-intercalated cells from ADx-AngII-Aldo mice compared with that observed from ADx-Veh mice (Figure 7A). Importantly, in contrast to the diffuse cytoplasmic and weak apical-membrane pendrin-labeling signals observed in β-intercalated cells from ADx-Veh mice, intense pendrin labeling was observed in the apical regions of β-intercalated cells from ADx-AngII-Aldo mice (Figure 7A). These results suggested that AngII and aldosterone coadministration promoted membrane-trafficking processes and increased membrane-protein abundance of pendrin. No immunostains of pMR-S843 in principal cells stained with AQP2 confirmed that pMR-S843 existed exclusively in intercalated cells,9 and that pMR-S843 labeling was markedly reduced in ADx-AngII-Aldo mice (Figure 7B), consistent with the results of Western blots. Along with pendrin staining, pNCC-T53 labeling was significantly increased in ADx-AngII-Aldo mice (Figure 7C). These data indicated that AngII was capable of upregulating NCC independent of aldosterone, whereas aldosterone was essential for AngII-induced pendrin upregulation.
Figure 7.
AngII and aldosterone coadministration in adrenalectomized mice increases and decreases immunostaining of pendrin and pMR, respecrtively, in β-intercalated cells and increases immunostaining of pNCC in DCT cells. (A) Immunofluorescence analyses for detection of pendrin (green), AQP2 (red), and a merged image in kidneys from adrenalectomized C57BL/6J mice treated with either vehicle (ADx-Veh) or AngII and aldosterone (1.0 μg/d) coadministration (ADx-AngII-Aldo). (B) Immunofluorescence analyses for detection of pMR-S843 (green), AQP2 (red), and a merged image in kidneys from ADx-Veh or ADx-AngII-Aldo mice. (C) Immunofluorescence analyses for detection of pNCC-T53 (green), nuclei (DAPI; blue), and a merged image in kidneys from ADx-Veh or ADx-AngII-Aldo mice. Scale bars represent 50 μm. DAPI, 4′,6-diamidino-2-phenylindole.
Given that the well known genomic action of aldosterone is mediated by nuclear translocation of the aldosterone-MR complex in principal cells, we have evaluated whether cytoplasmic MR in β-intercalated cells is also translocated into the nucleus when AngII and aldosterone are coadministered to adrenalectomized mice. We found the nuclear translocation of MR in β-intercalated cells (Figure 8) as well as in principal cells (Supplemental Figure 1) with AngII and aldosterone coadministration, whereas it was not affected by AngII alone.
Figure 8.
AngII and aldosterone coadministration in adrenalectomized mice induces nuclear translocation of MR in β-intercalated cells. Immunofluorescence analyses of MR (green), pendrin (red), nuclei (DAPI; blue), and a merged image in kidneys from adrenalectomized C57BL/6J mice treated with either vehicle (ADx-Veh), AngII (ADx-AngII), or AngII and aldosterone (1.0 μg/d) coadministration (ADx-AngII-Aldo). Arrowheads indicate β-intercalated cells. Scale bars represent 25 μm. DAPI, 4′,6-diamidino-2-phenylindole.
Discussion
In this study, we demonstrated that pendrin expression in β-intercalated cells was regulated by the combination of AngII and aldosterone. In DCT cells, AngII increased NCC expression independent of aldosterone; however, in β-intercalated cells, AngII alone was insufficient to increase pendrin levels, although it promoted MR dephosphorylation. In adrenalectomized mice, AngII alone did not increase pendrin levels despite dephosphorylation of pMR-S843, until the addition of aldosterone. These results clearly show that aldosterone is essential to increase pendrin expression. As a consequence, during volume depletion, pendrin contributed to maintaining normal BP in cooperation with NCC through NaCl reabsorption (Figure 9).
Figure 9.
Schematic illustrating the hypothetical mechanism associated with maintaining normal BP via pendrin and NCC cooperation during volume depletion. Volume depletion elevates circulating AngII and aldosterone levels. In DCT cells, AngII directly increases NCC expression independent of aldosterone; however, in β-intercalated cells, unlike its regulation of NCC, AngII promotes dephosphorylation of pMR-S843, thereby increasing the ability of MR binding to aldosterone, results in the potentiation of pendrin expression. As a consequence, during volume depletion, pendrin contributes to maintaining normal BP in cooperation with NCC through NaCl reabsorption. β-IC, β-intercalated cell.
Mice with genetic deletion of pendrin and humans carrying inactivating mutations in the pendrin gene do not demonstrate salt wasting under basal conditions.10,15,21 Similarly, NCC-knockout mice also do not demonstrate salt wasting under basal conditions10,16; however, NCC-knockout mice,16 as well as PDS−/− mice,15 demonstrate salt wasting and resultant volume depletion during NaCl restriction. Furthermore, a recently identified pendrin inhibitor has no effect on the basal condition when administered alone, although this compound increases the natriuretic effects of furosemide.22 These findings indicate that pendrin and NCC are predominantly active during volume depletion associated with RAAS activation.
Volume depletion increases AngII levels, which stimulate the adrenal gland to produce aldosterone in vivo.23 Under these conditions, kidneys maximize NaCl reabsorption in response to AngII and aldosterone. Recently, in DCT cells, the independent roles of AngII and aldosterone on NCC expression and activity were reported; in contrast to the direct effect of AngII on NCC,4,19 the effect of aldosterone is indirect. Aldosterone-induced activation of NCC is mediated by hypokalemia, on the basis of the inverse correlation between plasma [K+] and p-NCC expression.20 In both mice receiving an LS diet and AngII infusion, with no changes in plasma [K+], pendrin as well as NCC were upregulated (Figures 1 and 2). In adrenalectomized mice, however, AngII-induced upregulation of pendrin was not observed (Figure 3), despite the definite increase of NCC expression, suggesting that aldosterone was indispensable for AngII-induced upregulation of pendrin expression, in contrast to aldosterone-independent activation of NCC induced by AngII. Thus, on an LS diet, increased AngII decreases phosphorylation of pMR-S843, and in turn, increased the ability of MR binding to aldosterone, resulted in the potentiation of pendrin expression. Moreover, our results suggest that AngII and aldosterone act synergistically to stimulate pendrin or NCC (Figures 5 and 6). However, further studies are needed to clarify how MR phosphorylation is involved in aldosterone-induced upregulation of pendrin.
To evaluate the physiologic role of pendrin in BP regulation during volume depletion with an LS diet, we evaluated BP by radiotelemetry. Despite no difference in MAPs between PDS−/− and WT mice on an HS diet, an LS diet significantly decreased MAPs in PDS−/− mice but not in WT mice (Figure 1), as firstly shown by Wall and colleagues.17,18 Notably, under basal conditions BP is similar or slightly reduced in PDS−/− relative to WT mice,17,18 which is consistent with the clinical study showing that basal levels of BP in people with pendrin syndrome are lower than in controls.24 Thus, NaCl intake must be high to eliminate the fall in BP by pendrin gene ablation observed in our study, and this depressor effect of pendrin gene ablation is greater when dietary NaCl is restricted. Taken together, activation of RAAS by volume depletion with an LS diet increased pendrin expression, and in turn, activated pendrin contributed to maintaining normal BP in cooperation with NCC through NaCl reabsorption.
Plasma [K+] affects aldosterone release independent of AngII.23 Previous studies have suggested roles for [K+] in pendrin regulation,9,25 and we recently found that pendrin expression was regulated by the interaction of plasma aldosterone and [K+], with hypokalemia allowing aldosterone-induced pendrin expression associated with MR dephosphorylation.26 In our study, neither an LS diet (Figure 1) nor AngII infusion (Figures 2 and 3) altered plasma [K+], despite increased plasma aldosterone, as previously reported.3,27 Thus, dephosphorylation of pMR-S843 induced by an LS diet and AngII infusion might be attributable solely to elevated AngII. As a result, activated MR binding to aldosterone, its agonist, resulted in increased pendrin expression. Moreover, this finding of nuclear MR translocation by AngII and aldosterone coadministration in β-intercalated cells as well as in principal cells leads to the assumption that upregulation of pendrin expression might be attributable to MR-dependent transcription of pendrin gene, although the precise mechanism of nuclear MR translocation in β-intercalated cells is still unclear.
Both AngII and aldosterone enhance NCC expression and activity.3,4,28 Recently, Terker et al.20,29 reported that hypokalemia increased NCC independent of aldosterone-MR signaling. In our study, endogenously elevated AngII and aldosterone (by LS diet) and exogenously elevated AngII and aldosterone (by AngII infusion) increased NCC levels and NCC phosphorylation, despite unchanged plasma [K+]. Additionally, AngII-induced upregulation of NCC and NCC phosphorylation was not reversed by blocking aldosterone-MR signaling after spironolactone treatment. Furthermore, AngII in the absence of aldosterone increased NCC and NCC phosphorylation. These results suggest that elevated AngII by an LS diet increases NCC levels and NCC phosphorylation in an aldosterone- and potassium-independent manner.
In both LS diet-fed mice and AngII-administered mice, we observed increases in both pendrin and NCC expression accompanied by no changes in plasma [K+]; however, adrenalectomized mice coadministered AngII and aldosterone exhibited phenotypes associated with hypokalemia. According to hypokalemia-induced NCC expressions, both hypokalemia and AngII contributed to marked increases in NCC (5.0-fold) and p-NCC (10.1-fold) levels in adrenalectomized mice coadministered AngII and aldosterone (Figure 4). Given levels of pMR-S843 and pendrin expressions affected by plasma [K+],26 the greater decrease in pMR-S843 levels in the adrenalectomized mice coadministered AngII and aldosterone as compared with those administered AngII alone (53% [Figure 4] versus 30% [Figure 3]) might be a consequence of hypokalemia, whereas we observed no difference in pMR-S843 levels between mice administered the same dosages of AngII with and without adrenalectomy (30% [Figure 3] versus 25% [Figure 2]). Moreover, despite similar plasma aldosterone levels, we observed a larger increase in pendrin expression in the adrenalectomized mice coadministered AngII and aldosterone compared with that observed in mice fed an LS diet (1.9 fold [Figure 4] versus 1.5 fold [Figure 1]) as a result of hypokalemia (plasma [K+]; 2.64 mmol/L versus 3.96 mmol/L). These observations indicate that several factors influence pendrin; plasma AngII, aldosterone, and [K+], all of which exert effects on the other. Further studies are needed to clarify how these factors are involved in the abnormal functions of pendrin and NCC related to disturbances in fluid homeostasis and hypertension.
As a clinical implication, pseudohypoaldosteronism type II model mice showed volume-dependent hypertension associated with NCC activation, but no changes in pendrin expression despite dephosphorylation of pMR-S843,9 because of intrarenal AngII signal activation but decreased plasma aldosterone levels. According to the dissociation of pendrin expression from AngII signaling by suppression of plasma aldosterone during volume expansion, our results involving adrenalectomized mice clearly revealed that aldosterone was essential for AngII-induced upregulation of pendrin expression. Furthermore, our findings associated with PDS−/− mice provide evidence supporting the critical role played by pendrin in maintaining normal BP during volume depletion, but not during volume expansion. Taken together, these findings offer respective therapeutic targets of NCC and pendrin for treating volume-dependent hypertension with intrarenal activation of AngII signaling, and disturbances in fluid balance with increased activity of circulating RAAS.
Concise Methods
Animals and Experimental Design
Animal care and treatment complied with the standards described in the Guidelines for the Care and Use of Laboratory Animals of the University of Tokyo (Tokyo, Japan). All studies were approved by the Institutional Animal Care and Use Committee of the University of Tokyo (RAC 150402). Male C57BL/6J mice were obtained from Tokyo Laboratory Animals Science (Tokyo, Japan). PDS−/− mice with a 129/Sv background were developed by Everett and colleagues17,30 and were obtained from The Jackson Laboratory (Bar Harbor, ME). WT mice with a 129/Sv background were also obtained from The Jackson Laboratory. All mice had free access to drinking water and diet under temperature-controlled conditions and a 12-hour light/dark cycle. All mice used for experiments were from 8- to 14-weeks-old.
In protocol 1, C57BL/6J mice were fed either an HS (8% NaCl) diet (n=9) or an LS (0.03% NaCl) diet (n=8) for 13 days.
In protocol 2, BP was measured by radiotelemetry in PDS−/− mice (n=5) and WT mice (n=5). Mice were fed an HS diet for 10 days (days −7 to 3). At day 3, mice began eating an LS diet, which continued for 7 days.
In protocol 3, C57BL/6J mice received either a sham operation (n=8) or AngII infusion (n=9) for 7 days.
In protocol 4, C57BL/6J mice received either AngII infusion (n=7) or AngII infusion with the MRA spironolactone (0.2 g/kg chow9; n=6) for 7 days.
In protocol 5, adrenalectomized C57BL/6J mice received either vehicle (ADx-Veh; n=5) or AngII infusion (ADx-AngII; n=7) for 7 days.
In protocol 6, adrenalectomized C57BL/6J mice received either vehicle (ADx-Veh; n=6) or AngII and aldosterone (1.0 μg/d) coadministration (ADx-AngII-Aldo; n=8) for 7 days.
In protocol 7, aldosterone-treated (0.1 μg/d) adrenalectomized C57BL/6J mice received either vehicle (ADx-Aldo; n=5) or AngII infusion (ADx-Aldo-AngII; n=5) for 7 days.
In protocol 8, AngII-treated adrenalectomized C57BL/6J mice received either vehicle (ADx-AngII; n=5), low-dose (0.3 μg/d) aldosterone (ADx-AngII-Aldo-L; n=4) or high-dose (1.0 μg/d) aldosterone (ADx-AngII-Aldo-H; n=5).
Animal Diets
In protocols 1 and 2, mice were fed either an HS or LS diet, prepared by modifying the AIN-76A semipurified diet (Oriental Yeast, Tokyo, Japan). In protocols 3–8, mice were fed an HS diet (Oriental Yeast).
AngII Infusion
In protocols 3–8, osmotic minipumps (ALZET model 2002; DURECT Corporation, Cupertino, CA) were implanted subcutaneously to infuse AngII at a dose of 400 μg/kg per day.4
Adrenalectomy
In protocols 5–8, bilateral adrenalectomy was performed through a dorsal incision under isoflurane anesthesia as previously described.31 Mice were fed an HS diet until the end of the studies to compensate for natriuresis after adrenalectomy. After a 7-day recovery from surgery, all animals were implanted with osmotic minipumps (ALZET) to administer either vehicle, AngII, aldosterone, or AngII and aldosterone coadministration. All minipumps also contained dexamethasone (12 μg/kg per day) for glucocorticoid replacement. The doses of aldosterone and dexamethasone were chosen to provide near-physiologic replacement, on the basis of published studies.31,32
BP Measurements in Conscious Mice
Radiotelemetry devices were inserted into PDS−/− and WT mice by arterial catheterization of the left carotid artery under isoflurane anesthesia, with the telemetry body positioned in a subcutaneous pocket on the right flank. Throughout the measurements, arterial BP in a conscious mouse was directly monitored by radiotelemetry using a PA-C10 transmitter, RPC-1 receiver, APR-1 ambient-pressure monitor, and a Data-Quest-ART-Silver 4.2 acquisition system (Data Sciences International, New Brighton, MN). Continuous measurement of MAPs in 10-second intervals was recorded every 15 minutes, and hourly MAPs were calculated by averaging four sequential MAP records. Baseline measurements were recorded for three consecutive days (days 0–3) in mice fed an HS diet. At day 7, post-treatment measurements were recorded for the final three consecutive days of the LS diet (days 7–10).
Blood Collection and Electrolyte Measurements
At the end of the experiments, we extracted blood from the inferior vena cava under anesthesia. Blood was immediately transferred into heparinized tubes, and 80 μl was loaded into an EC8+ cartridge for electrolyte measurement using an i-STATR-1 analyzer (Abbott, Abbott Park, IL). The remaining blood volume was centrifuged at 5000 rpm for 15 minutes at 4°C, and plasma was removed and stored at −20°C. Plasma aldosterone concentrations and plasma renin activities were measured by radioimmunoassay (SRL, Tokyo, Japan).
Western Blot Analysis
Kidneys were removed, snap frozen in liquid nitrogen, and stored at −80°C until homogenization. Total membrane fraction was isolated using a Minute plasma membrane-protein isolation kit (Invent Biotechnologies, Eden Prairie, MN) according to the manufacturer’s instructions. Enrichment of membrane proteins, but not cytoplasmic proteins, was validated in the laboratory (see Supplemental Figure 2). Briefly, kidney tissues were lysed in buffer A containing protease inhibitors and phosphatase inhibitors (Roche Diagnostics, Basel, Switzerland) and placed in a filter cartridge. After centrifugation at 14,000 rpm for 30 seconds, pellets were resuspended and centrifuged at 3000 rpm for 1 minute. The supernatant was collected and centrifuged again at 14,000 rpm for 30 minutes, and the resulting pellet was resuspended in buffer containing 40 mM Tris (pH 7.9), 260 mM sucrose, and 1% Triton X-100 and used as the total membrane-protein sample. Total cell lysate was isolated with extraction buffer containing 10 mM Tris-HCl (pH 7.8), 150 mM NaCl, 1% IGEPAL CA630, 1 mM EDTA, and protease inhibitors (Complete; Roche Diagnostics) and phosphatase inhibitors (PhosSTOP; Roche Diagnostics) and centrifuged at 14,000 rpm for 30 minutes to obtain the cellular proteins in the supernatant. Protein concentrations were determined using a BCA protein assay (Pierce, Rockford, IL) in duplicate.
Western blotting was performed as previously described,33 with some modifications. Briefly, equal amounts of protein were mixed with 2× Laemmli sample buffer, boiled for 10 minutes (or incubated for 30 minutes at room temperature for membrane proteins), separated on polyacrylamide gels, and transferred to polyvinylidene fluoride membranes. The membranes were blocked with polyvinylidene fluoride–blocking reagent (Toyobo, Osaka, Japan) for 30 minutes at room temperature and incubated with primary and peroxidase-conjugated secondary antibodies, followed by imaging using enhance chemiluminescence reagents (GE Healthcare, Waukesha, WI). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH; for total cell lysates) and Coomassie brilliant blue (Bio-Rad, Hercules, CA) staining of polyacrylamide gels (for membrane proteins, because of the lack of appropriate endogenous controls for membrane proteins; see Supplemental Figure 3) were used to confirm equal protein loading for different samples.34 Signal intensity was quantitated using ImageJ 1.46r software (National Institutes of Health, Bethesda, MD).
Immunofluorescence Staining
Immunofluorescence staining was performed as previously described,9 with some modifications. Briefly, tissues were harvested and fixed in 4% paraformaldehyde in PBS (pH 7.4) overnight at 4°C. Tissues were then incubated in 20% sucrose in PBS overnight at 4°C and mounted in Tissue-Tek OTC compound (Sakura Finetek, Torrance, CA) for sectioning. Subsequently, 5-μm-thick tissue sections were used for immunofluorescence staining. The sections were blocked with 5% donkey serum in PBS and stained with the indicated primary antibodies overnight at 4°C. The following day, sections were incubated for 1 hour with affinity-purified secondary antibodies conjugated to Alexa 488 (green) and Alexa 555 (red) fluorophores (Jackson ImmunoResearch Laboratories, West Grove, PA) and 4′,6-diamidino-2-phenylindole (1:5000) for 5 minutes at room temperature. MR immunostaining was performed using a mouse-on-mouse immunodetection kit (M.O.M. Kit; Vector Laboratories, Burlingame, CA). Images were acquired by microscopy (DM14000B; Leica, Deerfield, IL) using the software LAS AF (Leica).
Antibodies
Primary antibodies included those for pendrin (1:20,000 for Western blotting and 1:2000 for immunofluorescence staining; a gift from Peter Aronson, Yale University, New Haven, CT), NCC (1:3000 for Western blotting; Millipore, Bedford, MA), pNCC-T53 (1:5000 for Western blotting and 1:500 for immunofluorescence staining; a gift from Joyannes Loffing, University of Zurich, Zurich, Switzerland),35 MR (1:500 for Western blotting; Perseus Proteomics, Tokyo, Japan), MR (clone 12A7; 1:50 for immunofluorescence staining; a gift from Celso Gomez-Sanchez, University of Mississippi Medical Center, Jackson, MS),36 AQP2 (1:200 for immunofluorescence staining; Santa Cruz Biotechnology, Dallas, TX), and GAPDH (1:5000 for Western blotting; Abcam, Cambridge, UK). The antibody for pMR-S843 (1:1000 for Western blotting and 1:100 for immunofluorescence staining) was created and characterized as described previously.9
Statistical Analyses
Data are presented as the mean±SEM. Statistical comparisons were performed using the unpaired t test or ANOVA as indicated in the figure legends. P<0.05 was considered statistically significant.
Disclosures
None.
Supplementary Material
Acknowledgments
The authors thank Peter Aronson for the pendrin antibody, Johannes Loffing for the phosphorylated NCC antibody, and Celso Gomez-Sanchez for the MR antibody.
This work was supported by JSPS KAKENHI (grants 15H05788 and 17K16074), the AMED-CREST from Japan Agency for Medical Research and development, AMED, and the Charitable Trust Araki Medical and Biochemical Research Memorial Fund.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2017030243/-/DCSupplemental.
References
- 1.Saccomani G, Mitchell KD, Navar LG: Angiotensin II stimulation of Na(+)-H+ exchange in proximal tubule cells. Am J Physiol 258: F1188–F1195, 1990 [DOI] [PubMed] [Google Scholar]
- 2.Gonzalez-Villalobos RA, Janjoulia T, Fletcher NK, Giani JF, Nguyen MT, Riquier-Brison AD, Seth DM, Fuchs S, Eladari D, Picard N, Bachmann S, Delpire E, Peti-Peterdi J, Navar LG, Bernstein KE, McDonough AA: The absence of intrarenal ACE protects against hypertension. J Clin Invest 123: 2011–2023, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chiga M, Rai T, Yang SS, Ohta A, Takizawa T, Sasaki S, Uchida S: Dietary salt regulates the phosphorylation of OSR1/SPAK kinases and the sodium chloride cotransporter through aldosterone. Kidney Int 74: 1403–1409, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Castañeda-Bueno M, Cervantes-Pérez LG, Vázquez N, Uribe N, Kantesaria S, Morla L, Bobadilla NA, Doucet A, Alessi DR, Gamba G: Activation of the renal Na+:Cl- cotransporter by angiotensin II is a WNK4-dependent process. Proc Natl Acad Sci U S A 109: 7929–7934, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Masilamani S, Kim GH, Mitchell C, Wade JB, Knepper MA: Aldosterone-mediated regulation of ENaC alpha, beta, and gamma subunit proteins in rat kidney. J Clin Invest 104: R19–R23, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wall SM, Lazo-Fernandez Y: The role of pendrin in renal physiology. Annu Rev Physiol 77: 363–378, 2015 [DOI] [PubMed] [Google Scholar]
- 7.Pech V, Kim YH, Weinstein AM, Everett LA, Pham TD, Wall SM: Angiotensin II increases chloride absorption in the cortical collecting duct in mice through a pendrin-dependent mechanism. Am J Physiol Renal Physiol 292: F914–F920, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Verlander JW, Hong S, Pech V, Bailey JL, Agazatian D, Matthews SW, Coffman TM, Le T, Inagami T, Whitehill FM, Weiner ID, Farley DB, Kim YH, Wall SM: Angiotensin II acts through the angiotensin 1a receptor to upregulate pendrin. Am J Physiol Renal Physiol 301: F1314–F1325, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shibata S, Rinehart J, Zhang J, Moeckel G, Castañeda-Bueno M, Stiegler AL, Boggon TJ, Gamba G, Lifton RP: Mineralocorticoid receptor phosphorylation regulates ligand binding and renal response to volume depletion and hyperkalemia. Cell Metab 18: 660–671, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Soleimani M, Barone S, Xu J, Shull GE, Siddiqui F, Zahedi K, Amlal H: Double knockout of pendrin and Na-Cl cotransporter (NCC) causes severe salt wasting, volume depletion, and renal failure. Proc Natl Acad Sci U S A 109: 13368–13373, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leviel F, Hübner CA, Houillier P, Morla L, El Moghrabi S, Brideau G, Hassan H, Parker MD, Kurth I, Kougioumtzes A, Sinning A, Pech V, Riemondy KA, Miller RL, Hummler E, Shull GE, Aronson PS, Doucet A, Wall SM, Chambrey R, Eladari D: The Na+-dependent chloride-bicarbonate exchanger SLC4A8 mediates an electroneutral Na+ reabsorption process in the renal cortical collecting ducts of mice. J Clin Invest 120: 1627–1635, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sinning A, Radionov N, Trepiccione F, López-Cayuqueo KI, Jayat M, Baron S, Cornière N, Alexander RT, Hadchouel J, Eladari D, Hübner CA, Chambrey R: Double knockout of the Na+-driven Cl-/HCO3- exchanger and Na+/Cl- cotransporter induces hypokalemia and volume depletion. J Am Soc Nephrol 28: 130–139, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacques T, Picard N, Miller RL, Riemondy KA, Houillier P, Sohet F, Ramakrishnan SK, Büsst CJ, Jayat M, Cornière N, Hassan H, Aronson PS, Hennings JC, Hübner CA, Nelson RD, Chambrey R, Eladari D: Overexpression of pendrin in intercalated cells produces chloride-sensitive hypertension. J Am Soc Nephrol 24: 1104–1113, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trepiccione F, Soukaseum C, Baudrie V, Kumai Y, Teulon J, Villoutreix B, Cornière N, Wangemann P, Griffith AJ, Byung Choi Y, Hadchouel J, Chambrey R, Eladari D: Acute genetic ablation of pendrin lowers blood pressure in mice. Nephrol Dial Transplant 32: 1137–1145, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wall SM, Kim YH, Stanley L, Glapion DM, Everett LA, Green ED, Verlander JW: NaCl restriction upregulates renal Slc26a4 through subcellular redistribution: Role in Cl- conservation. Hypertension 44: 982–987, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Schultheis PJ, Lorenz JN, Meneton P, Nieman ML, Riddle TM, Flagella M, Duffy JJ, Doetschman T, Miller ML, Shull GE: Phenotype resembling Gitelman’s syndrome in mice lacking the apical Na+-Cl- cotransporter of the distal convoluted tubule. J Biol Chem 273: 29150–29155, 1998 [DOI] [PubMed] [Google Scholar]
- 17.Verlander JW, Hassell KA, Royaux IE, Glapion DM, Wang ME, Everett LA, Green ED, Wall SM: Deoxycorticosterone upregulates PDS (Slc26a4) in mouse kidney: Role of pendrin in mineralocorticoid-induced hypertension. Hypertension 42: 356–362, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Kim YH, Pech V, Spencer KB, Beierwaltes WH, Everett LA, Green ED, Shin W, Verlander JW, Sutliff RL, Wall SM: Reduced ENaC protein abundance contributes to the lower blood pressure observed in pendrin-null mice. Am J Physiol Renal Physiol 293: F1314–F1324, 2007 [DOI] [PubMed] [Google Scholar]
- 19.van der Lubbe N, Lim CH, Fenton RA, Meima ME, Jan Danser AH, Zietse R, Hoorn EJ: Angiotensin II induces phosphorylation of the thiazide-sensitive sodium chloride cotransporter independent of aldosterone. Kidney Int 79: 66–76, 2011 [DOI] [PubMed] [Google Scholar]
- 20.Terker AS, Yarbrough B, Ferdaus MZ, Lazelle RA, Erspamer KJ, Meermeier NP, Park HJ, McCormick JA, Yang CL, Ellison DH: Direct and indirect mineralocorticoid effects determine distal salt transport. J Am Soc Nephrol 27: 2436–2445, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Everett LA, Glaser B, Beck JC, Idol JR, Buchs A, Heyman M, Adawi F, Hazani E, Nassir E, Baxevanis AD, Sheffield VC, Green ED: Pendred syndrome is caused by mutations in a putative sulphate transporter gene (PDS). Nat Genet 17: 411–422, 1997 [DOI] [PubMed] [Google Scholar]
- 22.Cil O, Haggie PM, Phuan PW, Tan JA, Verkman AS: Small-molecule inhibitors of pendrin potentiate the diuretic action of furosemide. J Am Soc Nephrol 27: 3706–3714, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spät A, Hunyady L: Control of aldosterone secretion: A model for convergence in cellular signaling pathways. Physiol Rev 84: 489–539, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Kim BG, Yoo TH, Yoo JE, Seo YJ, Jung J, Choi JY: Resistance to hypertension and high Cl(-) excretion in humans with SLC26A4 mutations. Clin Genet 91: 448–452, 2017 [DOI] [PubMed] [Google Scholar]
- 25.Wagner CA, Finberg KE, Stehberger PA, Lifton RP, Giebisch GH, Aronson PS, Geibel JP: Regulation of the expression of the Cl-/anion exchanger pendrin in mouse kidney by acid-base status. Kidney Int 62: 2109–2117, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Xu N, Hirohama D, Ishizawa K, Chang WX, Shimosawa T, Fujita T, Uchida S, Shibata S: Hypokalemia and pendrin induction by aldosterone. Hypertension 69: 855–862, 2017 [DOI] [PubMed] [Google Scholar]
- 27.Veiras LC, Han J, Ralph DL, McDonough AA: Potassium supplementation prevents sodium chloride cotransporter stimulation during angiotensin II hypertension. Hypertension 68: 904–912, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sandberg MB, Riquier AD, Pihakaski-Maunsbach K, McDonough AA, Maunsbach AB: ANG II provokes acute trafficking of distal tubule Na+-Cl(-) cotransporter to apical membrane. Am J Physiol Renal Physiol 293: F662–F669, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Terker AS, Zhang C, McCormick JA, Lazelle RA, Zhang C, Meermeier NP, Siler DA, Park HJ, Fu Y, Cohen DM, Weinstein AM, Wang WH, Yang CL, Ellison DH: Potassium modulates electrolyte balance and blood pressure through effects on distal cell voltage and chloride. Cell Metab 21: 39–50, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Royaux IE, Wall SM, Karniski LP, Everett LA, Suzuki K, Knepper MA, Green ED: Pendrin, encoded by the Pendred syndrome gene, resides in the apical region of renal intercalated cells and mediates bicarbonate secretion. Proc Natl Acad Sci U S A 98: 4221–4226, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kawarazaki W, Nagase M, Yoshida S, Takeuchi M, Ishizawa K, Ayuzawa N, Ueda K, Fujita T: Angiotensin II- and salt-induced kidney injury through Rac1-mediated mineralocorticoid receptor activation. J Am Soc Nephrol 23: 997–1007, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Crowley SD, Gurley SB, Oliverio MI, Pazmino AK, Griffiths R, Flannery PJ, Spurney RF, Kim HS, Smithies O, Le TH, Coffman TM: Distinct roles for the kidney and systemic tissues in blood pressure regulation by the renin-angiotensin system. J Clin Invest 115: 1092–1099, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mu S, Shimosawa T, Ogura S, Wang H, Uetake Y, Kawakami-Mori F, Marumo T, Yatomi Y, Geller DS, Tanaka H, Fujita T: Epigenetic modulation of the renal β-adrenergic-WNK4 pathway in salt-sensitive hypertension. Nat Med 17: 573–580, 2011 [DOI] [PubMed] [Google Scholar]
- 34.Gueutin V, Vallet M, Jayat M, Peti-Peterdi J, Cornière N, Leviel F, Sohet F, Wagner CA, Eladari D, Chambrey R: Renal β-intercalated cells maintain body fluid and electrolyte balance. J Clin Invest 123: 4219–4231, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sorensen MV, Grossmann S, Roesinger M, Gresko N, Todkar AP, Barmettler G, Ziegler U, Odermatt A, Loffing-Cueni D, Loffing J: Rapid dephosphorylation of the renal sodium chloride cotransporter in response to oral potassium intake in mice. Kidney Int 83: 811–824, 2013 [DOI] [PubMed] [Google Scholar]
- 36.Gomez-Sanchez CE, Warden M, Gomez-Sanchez MT, Hou X, Gomez-Sanchez EP: Diverse immunostaining patterns of mineralocorticoid receptor monoclonal antibodies. Steroids 76: 1541–1545, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.