Abstract
Dendritic cells (DCs) are thought to form a dendritic network across barrier surfaces and throughout organs, including the kidney, to perform an important sentinel function. However, previous studies of DC function used markers, such as CD11c or CX3CR1, that are not unique to DCs. Here, we evaluated the role of DCs in renal inflammation using a CD11c reporter mouse line and two mouse lines with DC-specific reporters, Zbtb46-GFP and Snx22-GFP. Multiphoton microscopy of kidney sections confirmed that most of the dendritically shaped CD11c+ cells forming a network throughout the renal interstitium expressed macrophage-specific markers. In contrast, DCs marked by Zbtb46-GFP or Snx22-GFP were less abundant, concentrated around blood vessels, and round in shape. We confirmed this pattern of localization using imaging mass cytometry. Motility measurements showed that resident macrophages were sessile, whereas DCs were motile before and after inflammation. Although uninflamed glomeruli rarely contained DCs, injury with nephrotoxic antibodies resulted in accumulation of ZBTB46+ cells in the periglomerular region. ZBTB46 identifies all classic DCs, which can be categorized into two functional subsets that express either CD103 or CD11b. Depletion of ZBTB46+ cells attenuated the antibody-induced kidney injury, whereas deficiency of the CD103+ subset accelerated injury through a mechanism that involved increased neutrophil infiltration. RNA sequencing 7 days after nephrotoxic antibody injection showed that CD11b+ DCs expressed the neutrophil-attracting cytokine CXCL2, whereas CD103+ DCs expressed high levels of several anti-inflammatory genes. These results provide new insights into the distinct functions of the two major DC subsets in glomerular inflammation.
Keywords: immunology, glomerulonephritis, glomerular disease
Since their first discovery by Steinman and Cohn,1 dendritic cells (DCs) have been considered a key regulator of both innate and adaptive immune responses. This was on the basis of their ability to activate naïve T cells, their dendritic morphology, and their localization in tissue. At barrier surfaces, they are thought to form an interdigitating network of cells that allows them to function as sentinels for micro-organisms that attempt to invade tissue or breach the mucosal barrier. Recognition of pathogens by innate signaling activates DCs and promotes their migration to lymph nodes, where they activate T cells.1–4
Over the last 10 years, the distinction between monocytes, macrophages, and DCs has improved with a better understanding of the developmental origins of these cells and our ability to define subclass heterogeneity.5 Resident macrophages derive from a yolk sac or fetal liver precursors and are self-perpetuated in tissue.6 Classic dendritic cells (cDCs) and plasmacytoid DCs originate from hematopoietic stem cells starting with the macrophage and DC progenitor7 that generates the common DC progenitor.8,9 This progenitor then develops into the committed cDC progenitor, which gives rise to the CD11b+ and CD103+ cDC subsets.10,11 Monocytes also develop from the macrophage and DC progenitor and are recruited into tissues during inflammation, and they can develop into macrophages.12 All of these cell subsets can now be largely distinguished from each other by expression markers. For example, Langerhans cells in the epidermis, once considered DCs, have an embryonic and not bone marrow origin and share a transcriptional profile with microglia; thus, they are now thought to more closely resemble tissue resident macrophages than DCs.13–16
Many previous studies of the role of DCs preceded our current understanding of myeloid cell ontogeny and were often on the basis of single markers, such as CD11c and CX3CR1, both known today to lack specificity for DCs.17 Thus, in this study, we have re-evaluated the localization of DCs and their role in inflammation using the kidney as our focus. Previously, using GFP reporter mice for CD11c and CX3CR1, it was reported that DCs are highly dendritic and form a dense anatomic network extending across the renal interstitium.18–20 Ablation strategies using CD11c-specific expression of the diphtheria toxin (DTX) receptor argued that DCs are critical positive effectors of renal inflammation, because depletion of CD11c+ cells attenuated inflammation induced by antibodies to the glomerular basement membrane (nephrotoxic nephritis [NTN]).21 Other studies, however, suggest an anti-inflammatory effect of CD11c+ cells in GN.22,23
Conventional DCs or cDCs are divided into two major subsets. One subset expresses CD8, CD103, and XCR1 (cDC1), whereas the other subset expresses CD11b and SIRPα (cDC2).17 CD11c is not specific for DCs, because it also labels some macrophages, whereas DCs can be distinguished from macrophages, because they lack expression of the Fc receptor CD6424,25 and either lack or express at low levels F4/80. CD103+ cells function primarily to prime cytotoxic T cells,26 whereas CD11b+ cells prime CD4+ T cells and produce inflammatory chemokines.27 Recently, a transcription factor, ZBTB46, was identified as a specific marker of both subsets of cDCs.28,29
Here, using mice with either GFP or DTX knocked into the Zbtb46 locus, we re-evaluated the anatomic localization of all cDCs as well as their role in NTN. Furthermore, using a new GFP-reporter mouse that specifically marks the CD103+ subset on the basis of expression of Snx22 and Batf3-knockout (KO) mice, we analyzed the localization and behavior of CD103+ cDCs in vivo as well as their role in NTN. We found, surprisingly, that the dense networks of cells reported previously to be DCs on the basis of CD11c or CX3CR1-GFP reporters19,20 are actually not ZBTB46+ cDCs. Rather, these cDCs were round and localized mostly to areas around blood vessels. We confirmed this finding obtained with reporter mice with a novel imaging approach, immune mass cytometry, which allows for multiplex imaging of cells in tissue sections.30,31 Ablation of all cDCs using the Zbtb46-DTR mouse confirmed the idea that cDCs are positive effectors of kidney inflammation, but NTN induced in the absence of CD103+ cDCs showed enhanced inflammation, suggesting that CD103+ cDCs limit the inflammatory response. Gene expression analysis was consistent with a distinct role for DC subsets in regulating the recruitment of neutrophils and monocytes from the blood.
Results
CD11c+/MHC Class II+ Cells in the Kidney Represent a Mixed Population of cDCs and Other Myeloid Cells
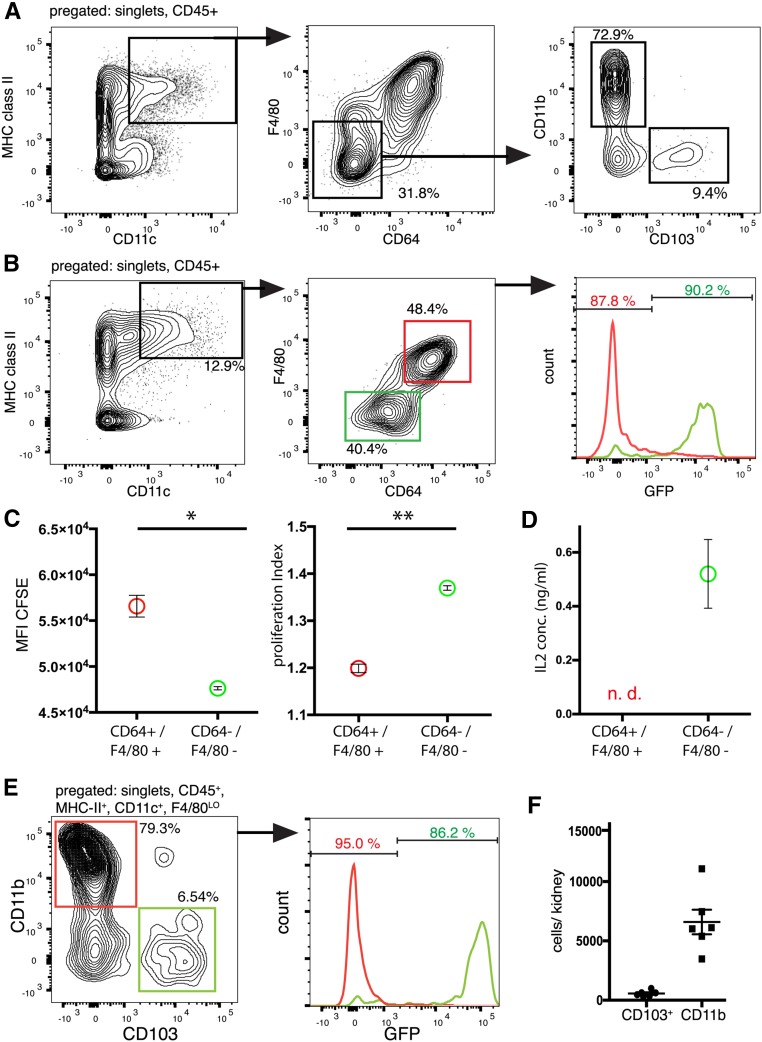
We first analyzed the CD11chigh/MHC class IIhigh myeloid population in the kidney using flow cytometry. This population was then analyzed for F4/80 and CD64, markers of macrophages and monocytes. This showed that approximately 70% of the CD11c/MHC class IIhigh cells were macrophages (Figure 1A), showing that the majority of CD11chigh cells in the kidney are macrophages as recently reported.32 Analysis of the cDC population showed that 73% were CD11b+, whereas 9% were CD103+ (Figure 1A; quantified in Figure 1F). Using the Zbtb46GFP/+ mouse that marks all cDCs with GFP, we confirmed that all of the F4/80/CD64 double-negative cells were cDCs (Figure 1B). We confirmed that these double-negative cells are DCs by comparing their ability to present antigen (ovalbumin) with the CD64/F4/80 double-positive cells. Although both populations could stimulate ovalbumin-specific T cells, the double-negative cells (cDCs) induced more proliferation and induced more IL-2 production compared with the double-positive cells (macrophages) (Figure 1, C and D). In expression studies, we found that sorting-nexin 22 (SNX22) is specifically expressed in CD103+ DCs and used homologous recombination to insert GFP into the Snx22 locus (Supplemental Figure 1A). We confirmed that Snx22GFP specifically labeled CD103+ cells and can be used as a specific reporter for kidney CD103+ DCs (cDC1) (Figure 1E, Supplemental Figure 1).
Figure 1.
Flow cytometry analysis of fluorescent reporter lines reveals distinct populations in the kidney. (A) Gating strategy for renal CD11b+ and CD103+ populations of cDCs. CD45+ singlet cells from whole kidney were analyzed as indicated. MHC class II+/CD11c+ with low expression of CD64 and F4/80 were regarded as cDCs and further separated by expression of CD103 and CD11b. (B) Kidney cells from Zbtb46GFP/+ mice were analyzed for the expression of the indicated markers. Histograms for GFP expression for the F4/80−/CD64− (green) and F4/80+/CD64+ (red) populations were overlaid. The majority of F4/80−/CD64− cells were GFP+. (C) In vitro T cell proliferation assay. CFSE-loaded OT-II cells were cocultured with sorted CD45+, MHC class II+, and CD11c+ kidney cells that were either F4/80−/CD64− or F4/80+/CD64+ and loaded with OVA. Depicted are the CFSE geometric mean fluorescence intensity (MFI) and the proliferation index of T cells after 72 hours. *P<0.05; **P<0.01. (D) The undiluted supernatant of the same cells was analyzed for IL-2 by ELISA, and the experiment was performed in duplicates. n.d., not detected. (E) Kidney cells from Snx22GFP/+ mice were pregated as indicated above the diagram, and histograms for GFP expression for the CD11b+ and CD103+ populations were overlaid (y axes for the two histograms have different scales), which show that the Snx22GFP/+ mouse is a specific reporter for CD103+ cDCs in the kidney. (F) Absolute quantification of CD11b+ and CD103+ DCs per mouse kidney under healthy conditions.
Multiphoton Imaging of Kidney DCs in Different Reporter Mice Reveals Differences in Cell Localization, Structure, and Motility
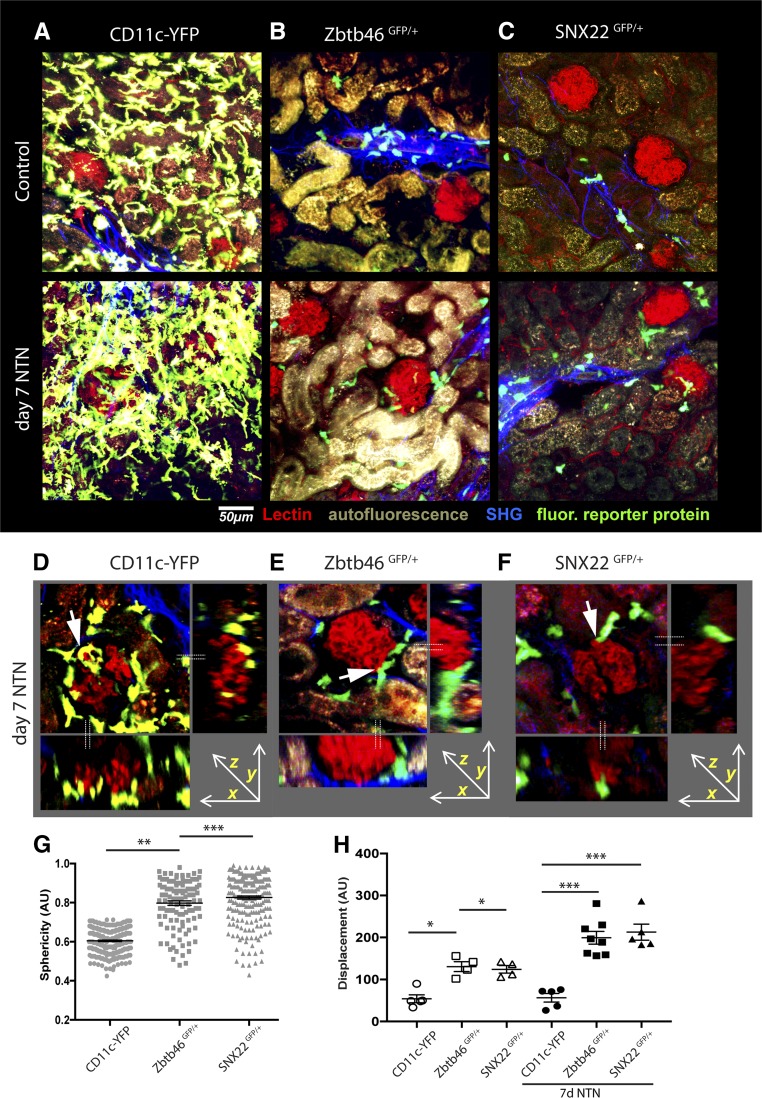
Previous studies used Cx3cr1GFP or CD11c-YFP mice19,20 to visualize DCs in the kidney. These studies suggested that DCs form a dense anatomic network across the kidney parenchyma. Because ZBTB46 and SNX22 more accurately mark cDCs, we used multiphoton microscopy to compare the distribution of GFP+ cells in the kidneys between CD11c-YFP, Zbtb46GFP, and Snx22GFP mice. Because the expression of Zbtb46 in endothelial cells potentially complicates our imaging analysis, we generated bone marrow chimeras by transferring Zbtb46GFP/+ bone marrow into irradiated wild-type (WT) mice. To ensure comparability between imaging groups, we also generated chimeras using CD11c-YFP and Snx22GFP/+ bone marrow. Flow cytometric analysis confirmed that the chimerism of the mice was >95% (Supplemental Figure 2, A–C). We then imaged GFP+/YFP+ cells in vibratome sections of the kidney by multiphoton microscopy.33
Consistent with previous reports, CD11c-YFP+ cells formed a continuous network of dendritic-shaped cells throughout the kidney, mostly within the interstitium and surrounding the tubules (Figure 2A).19,32 CD11c-YFP+ cells were very rarely detected inside glomeruli, but instead, as reported previously, they formed a dense network that surrounded Bowman’s capsule. In sharp contrast, Zbtb46-GFP+ and Snx22-GFP+ cells were mostly seen in clusters in collagen-rich areas surrounding blood vessels, such as small arteries and small arterioles, and only sparsely localized in the tubulointerstitium. The morphology of these cells was round, not dendritic. This was quantitated by a statistical analysis of cell sphericity (Figure 2G). Neither the Zbtb46-GFP+ nor the Snx22-GFP+ cells were organized into any type of continuous network of cells (Figure 2, B and C, Supplemental Figure 3, A–C). This suggests that the previously identified dendritically shaped myeloid cells in the kidney are likely macrophages.
Figure 2.
Multiphoton imaging of kidney DCs in different reporter mice reveals disparities in cell localization, structure, and motility. (A–C) Multiphoton imaging of kidney slices from bone- marrow chimeras generated by transferring bone marrow from Cd11c-YFP+, Zbtb46GFP/+, or Snx22GFP/+ donors into lethally irradiated mice was used to show localization and structure of DC populations in the steady state and on day 7 of NTN. Capillaries are depicted in red, YFP or GFP appears in green, collagen fibers are in blue (second harmonic signal), and the tubules autofluoresce. Although CD11c-YFP+ cells show a continuous network throughout the kidney, ZBTB46-GFP+ and SNX22-GFP+ cells were sparsely localized in the interstitium and in clusters in collagen-rich areas and close to larger blood vessels. ZBTB46- and SNX22-GFP cells were attracted to the periglomerular regions in the inflamed state. (D–F) Z-stack reconstructions of glomeruli and side views from CD11c-YFP, Zbtb46GFP/+, or SNX22GFP/+ bone marrow chimeras. The dotted lines represent the optical planes in the side views. Although the glomerular tuft itself was mostly free of DCs, their processes were observed to be in close proximity to the glomeruli (arrows). (G) Analysis of sphericity in Z-stack reconstructions from at least three individual mice per group reveals that the majority of ZBTB46-GFP+ and SNX22-GFP+ cells have a spherical shape in comparison with the majority of CD11c-YFP+ cells. (H) Analysis of cell motility in the uninflamed and inflamed states by analysis of fluorescence intensity changes (displacement) of Z stacks acquired every 30 seconds for 15 minutes in kidney organ slices. Zbtb46-GFP and Snx22-GFP cells showed a higher average motility at baseline and increased motility with NTN in comparison with CD11c-YFP cells, which remained stationary. Each dot represents an individual time-lapse image, and for each group, movies from at least three individual mice were analyzed. *P<0.05 as determined by one-way ANOVA with Tukey post-test; **P<0.01 as determined by one-way ANOVA with Tukey post-test; ***P<0.001 as determined by one-way ANOVA with Tukey post-test.
To assess the response of these cells to inflammation, we treated mice with a nephrotoxic serum (NTS) that induces an acute proteinuria and does not require preimmunization. We imaged after 7 days. There was a generalized increase in CD11c-YFP+ cells, with dense clusters of cells forming around glomeruli (Figure 2, A and D). At this stage of inflammation, Zbtb46- and Snx22-GFP+ cells also localized to the interstitium and generally around glomeruli (Figure 2, B, C, E, and F). Although the initial inflammatory insult was at the glomerular capillary wall, we noted that GFP+ cells were still rarely seen inside the glomerular tuft. However, long processes emanating from GFP+/YFP+ cells could be seen near the glomerulus (Figure 2, D–F, Supplemental Movies 1–6).
We analyzed the motility of cells by quantifying their fluorescent pixel intensity change over time, an unbiased method that we had developed previously.33 This revealed that the majority of the CD11c+ cells were relatively sessile, consistent with their likely identity as resident macrophages.31 In contrast, Zbtb46- and Snx22-GFP+ cells showed more active movement in the absence of inflammation. Using the same quantification approach, we found that, after induction of injury with nephrotoxic antibody, the CD11c+ population remained relatively sessile, whereas the Zbtb46-GFP+ and Snx22-GFP+ cells increased their motility but did not appear to be directionally migrating toward lymphatics (Figure 2H, Supplemental Figure 2, D–I, Supplemental Movies 1–6).
Imaging Mass Cytometry Confirms Shape and Localization of Macrophages and DCs in WT, Nonirradiated Tissue
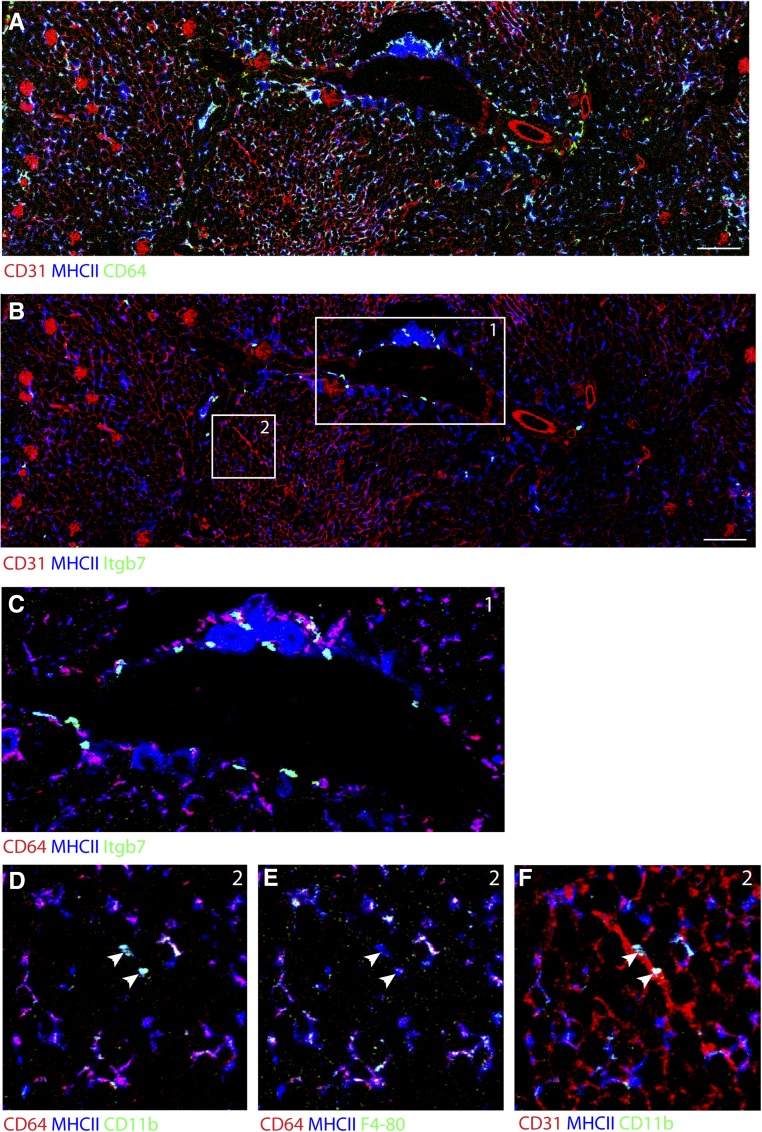
Currently, because of the requirement to use multiple cell surface markers to distinguish between DCs and macrophages, it is difficult to accurately locate and identify macrophage and DC subsets in tissue by immunostaining, which makes the use of reporter mice necessary. Conversely, modern flow cytometry can analyze large panels of markers but lacks any spatial information. We used a new imaging method that combines laser ablation with the mass cytometry technology, allowing for simultaneously tissue staining with up to 50 antibodies labeled with rare earth metals at a 1-μm resolution.30,31
Using this technique, we found that the majority of MHC class II+ cells express CD64 and F4/80, have a dendritic shape, and form an extensive network throughout the kidney, similar to the network seen with CD11c-YFP (Figure 3A) and Cx3cr1GFP mice.19 In contrast, staining for integrin-β7 and integrin-αE (not shown), the two components of CD103, identified CD103+ cDCs as round cells that were located mainly around large blood vessels (Figure 3, B and C). MHC class II+, CD11b+, F4/80- and CD64− cells were also identified to have a round shape and localized in the interstitium near smaller blood vessels (Figure 3, D–F). Thus, imaging using WT, nonirradiated kidney tissue largely confirmed our findings using reporter mice.
Figure 3.
Imaging mass cytometry confirms shape and localization of macrophages and DCs in WT, nonirradiated tissue. (A) A kidney section was stained with rare earth metal–labeled antibodies and laser ablated with a resolution of 1×1 μm. The image shows expression of MHC class II, CD64, and CD31 (as markers for blood vessels in a cortex and medulla). (B) The same section was analyzed for integrin-β7 (Itgβ7), which reveals the localization of CD103+ DCs around larger blood vessels predominantly at the cortex-medulla border. Scale bar, 200 μm in A and B. (C) Magnification of area 1 shows the perivascular localization of CD103+ DCs in detail. (D–F) Magnification of area 2 shows macrophages and CD11b+ DCs. Coexpression of CD64 and F4/80 in dendritic-shaped MHC class II+ cells in the tissue represents macrophages. A smaller population of MHC class II+, CD11b+, CD64−, and F4/80− cells that are round in shape and most likely represent CD11b+ DCs in the tissue (arrowheads) was found (E) in the interstitium and (F) in close proximity to smaller blood vessels.
Depletion of ZBTB46+ DCs Ameliorates the Course of GN in Mice
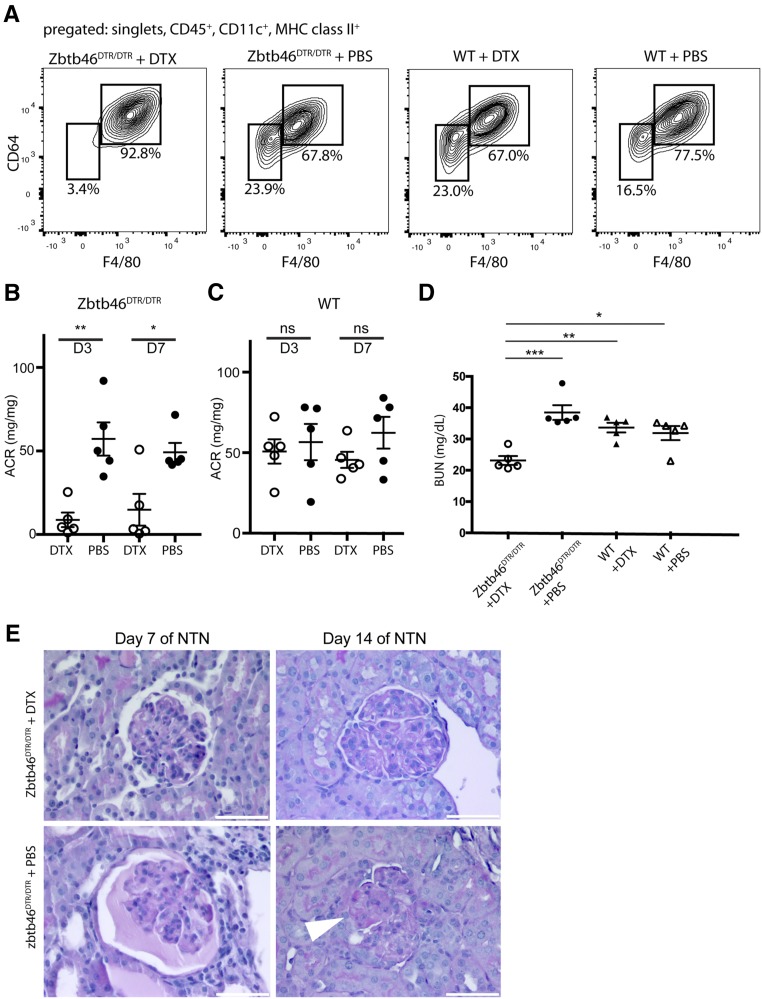
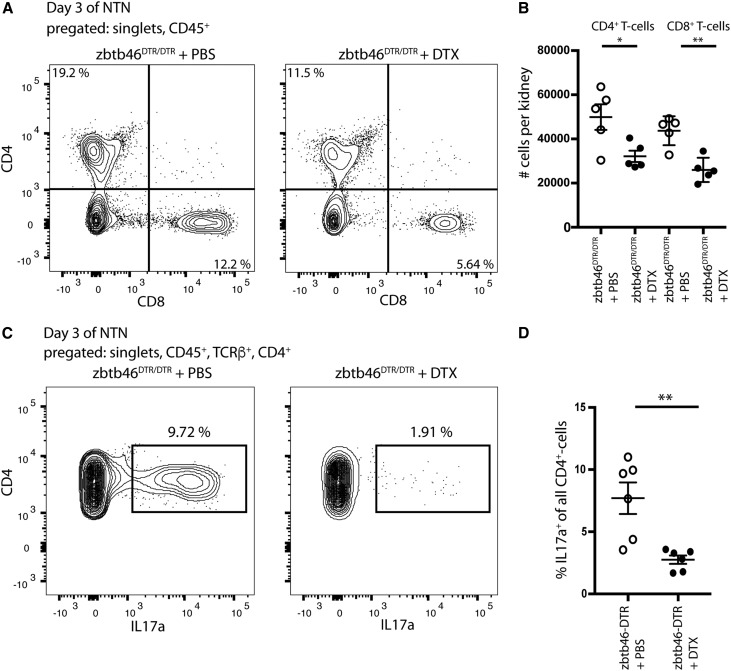
Because we have established that CD11c is not an accurate marker of kidney DCs and because previous studies inferring the role of DCs in kidney inflammation used CD11c-DTR mice,21–23,34 we repeated these studies using the Zbtb46DTR/DTR mouse to specifically deplete cDCs.29 We again used bone marrow chimeras for our experiments to mitigate potential issues with the expression of Zbtb46 in endothelial cells (Supplemental Figure 4A). Mice were treated with DTX or PBS 4 days before injection of NTS.35 Flow cytometry confirmed that DTX treatment depleted predominantly cDCs. (Figure 4A, statistics in Supplemental Figure 4B). The decrease seen in the macrophage population (F4/80HI/CD64HI) was not statistically significant (Supplemental Figure 4C).
Figure 4.
Depletion of ZBTB46+ DCs ameliorates the course of GN in mice. (A) FACS analysis of kidney cells from Zbtb46DTR/DTR and WT bone marrow chimeras treated with DTX or PBS. Loss of cells expressing Zbtb46 selectively depletes the F4/80LO/CD64LO cDC population. Mice were euthanized on day 14 of NTN. (B and C) Urinary albumin-to-creatinine ratios (ACRs) of (B) NTS-injected Zbtb46DTR/DTR or (C) WT bone marrow chimeras treated with DTX or PBS. (D) BUN levels in cDC-depleted and control mice on day 14 of NTN. (E) Periodic acid–Schiff staining shows a reduction of glomerular damage in cDC-depleted mice versus controls on days 7 and 14 of NTN (arrowhead points at adhesion). *P<0.05 determined by t test in B and C and one-way ANOVA with Tukey post-test in D; **P<0.01 determined by t test in B and C and one-way ANOVA with Tukey post-test in D; ***P<0.01 determined by one-way ANOVA with Tukey post-test in D.
On day 3, during the heterologous phase, cDC-depleted mice (Zbtb46DTR/DTR mice + DTX) showed a significant reduction of albuminuria in comparison with bone marrow chimeras treated only with PBS (Figure 4B). On day 14, during the autologous phase, cDC-depleted mice had significantly lower BUN levels compared with the three control groups (Figure 4D), consistent with better renal function in the cDC-depleted animals. Histologic examination showed that cDC-depleted bone marrow chimeras had fewer protein casts and fewer sclerotic glomerular lesions in comparison with the control groups (Figure 4E). DTX treatment of WT bone marrow chimeras had no effect on the number of cDCs, and it did not have a significant effect on proteinuria in comparison with untreated animals (Figure 4C), showing that the results were not due to nonspecific effects of DTX.
Analysis of the inflammatory infiltrate on days 3 and 7 after administration of NTS showed similar numbers of macrophages and neutrophils. The number of T cells was, however, reduced in cDC-depleted mice (Figure 5, A and B). Although both CD4+ and CD8+ T cells were reduced on day 3, by day 7, only CD4+ T cells were reduced (Supplemental Figure 5). Additional analysis showed also a reduction in TH17 memory cells (Figure 5, C and D). This suggests that cDCs can function to either recruit central memory TH17 cells or expand resident memory TH17 cells.
Figure 5.
Depletion of ZBTB46+ DCs leads to a reduction of CD4+ and CD8+ T cells and percentage of IL-17–producing T-helper cells. (A) FACS analysis of kidney cells from Zbtb46DTR/DTR bone marrow chimeras on day 3 of NTN reveals reduction in CD4+ and CD8+ T lymphocytes when treated with DTX. (B) Quantification of absolute CD4+ and CD8+ T cell numbers per kidney on day 3 of NTN (n=5 per group). (C) Kidney cells from Zbtb46DTR/DTR bone marrow chimeras (day 3 of NTN) treated with DTX or PBS were isolated and cultured with PMA/Ionomycin + Brefeldin for 4 hours, and then, they were stained for intracellular IL-17. (D) Quantification of the percentage of IL-17–producing CD4+ T cells on day 3 of NTN shows a reduction in cDC-depleted mice. *P<0.05 determined by t test; **P<0.01 determined by t test.
Absence of CD103+ DCs Aggravates NTN
We next tested the role of the CD103+ subset of cDCs using BATF3-deficient mice.26
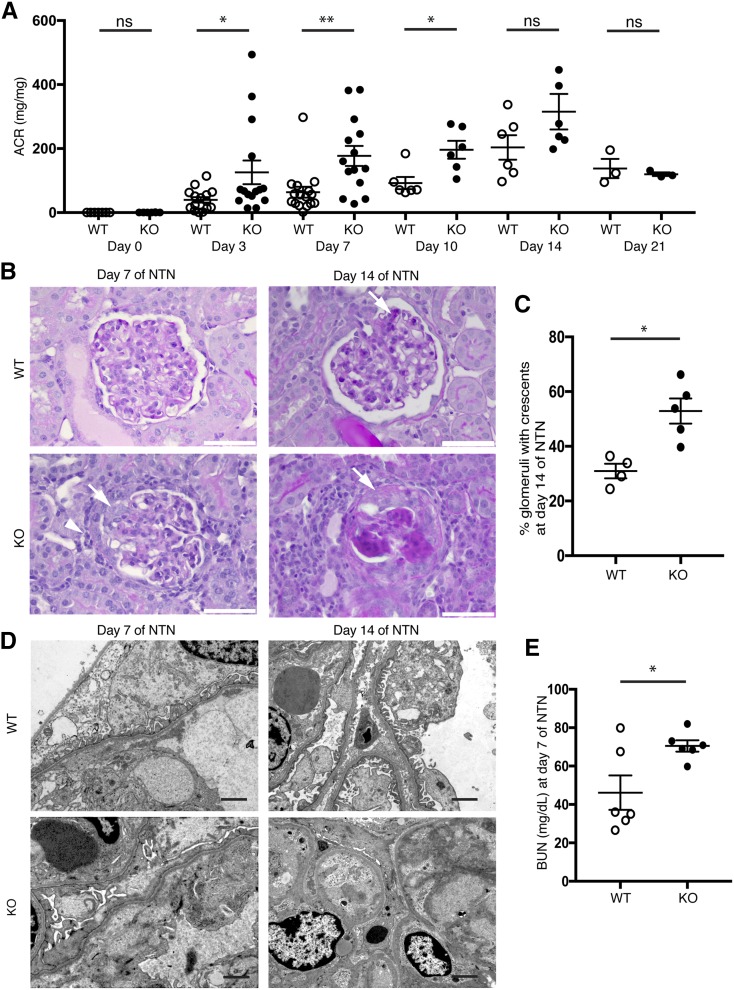
Consistent with a previous report,36 Batf3-KO mice had normal renal function but were more sensitive to NTS-induced injury and developed levels of albuminuria on days 3, 7, and 10 that were much higher than NTS-treated WT animals (Figure 6A). Histologic analysis showed increased inflammation in Batf3-KO mice with increased glomerular cellularity and crescent formation on days 7 and 14. On day 14, KO mice showed severe global glomerulosclerosis, with crescents in 53% of glomeruli, whereas WT mice showed only focal glomerulosclerosis, with crescents visible in 31% of glomeruli (Figure 6, B and C). Electron micrographs revealed that foot process effacement developed in both genotypes but was much more severe in the KO mice on day 7. On day 14, individual foot processes were visible again in the WT mouse, whereas capillary loops were frequently obliterated in the Batf3-KO mice (Figure 6D). BUN levels were significantly elevated in KO mice (Figure 6E).
Figure 6.
The absence of CD103+ DCs in Batf3-KO mice aggravates proteinuria and promotes crescent formation in NTN. (A) Urinary albumin-to-creatinine ratios (ACRs) in Batf3-KO and WT mice at baseline and after injection of NTS. (B) As shown by Periodic acid–Schiff staining on day 7 of NTN, KO mice presented with more periglomerular infiltrating cells (arrowhead) and adhesions (arrow) of the glomerular tuft. On day 14, WT mice showed minor scarring (arrow), whereas KO mice showed prominent glomerulosclerosis with crescents (arrow). (C) Quantification of crescentic glomeruli in WT and Batf3-KO mice on day 14 of NTN. (D) Batf3-KO mice showed more severe foot process effacement on day 7 of NTN. By day 14, WT mice had largely regained their normal foot processes, whereas glomeruli from KO mice showed severe scarring with obliterated capillary lumens. (E) BUN levels (milligrams per deciliter) in WT and KO mice on day 7 of NTN. *P<0.05 determined by t test; **P<0.01 determined by t test.
Neutrophil Infiltration of the Kidney Is Pathogenic in Batf3-KO Mice
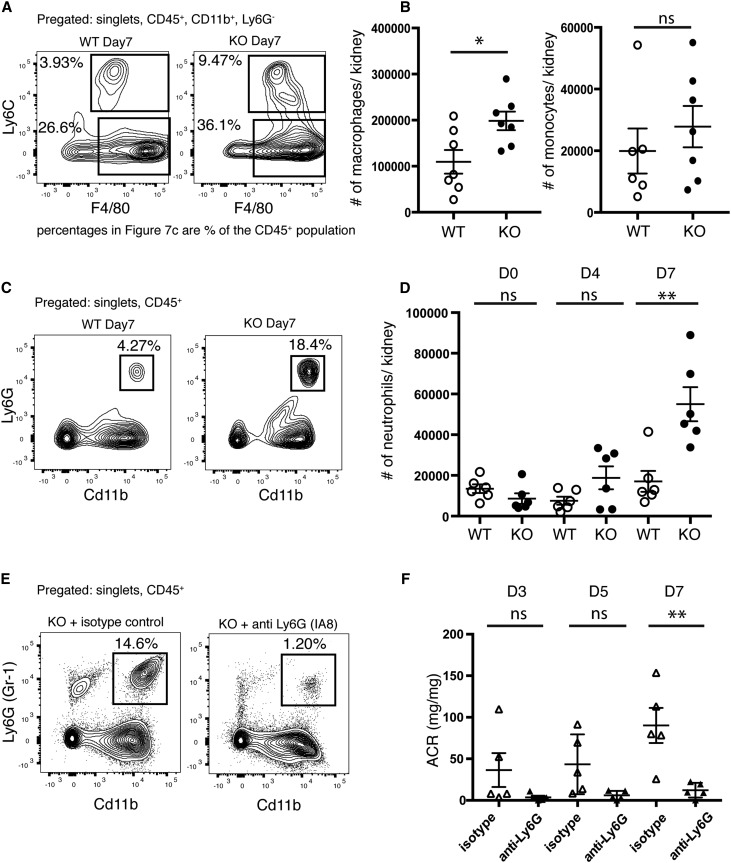
Analysis of the inflammatory infiltrate in WT and Batf3-KO mice showed that, on day 7, there was a fourfold increase of neutrophils in KO mice in comparison with WT controls (Figure 7, C and D) as well as a twofold increase of macrophages (Figure 7, A and B). Of note, CD4+ and CD8+ T cell numbers were unchanged in BATF3-KOs, with only a slight increase in Tregs in BATF3-KOs on day 7 (Supplemental Figure 6, A–D). To test the hypothesis that the increase in neutrophils contributed to increased kidney injury in Batf3-KO mice, we depleted neutrophils using an antibody against Ly6G (Figure 7E). Depletion of neutrophils significantly attenuated proteinuria in comparison with isotype antibody controls (Figure 7F). No significant differences in other inflammatory cells were noted after treatment with neutrophil-depleting antibodies (data not shown). Depletion of CD4+ T cells had no significant effect on the course of the disease in WT or KO mice (Supplemental Figure 6, E–G). In conclusion, the presence of CD103+ cDCs attenuated the severity of GN by limiting recruitment of neutrophils.
Figure 7.
Neutrophil infiltration into the kidney is pathogenic in Batf3-KO mice. (A) FACS plots for macrophages (CD45+, CD11b+, F4/80HI, and Ly6CLO) and monocytes (CD45+, CD11b+, F4/80INT, and Ly6CHI) in WT and Batf3-KO kidneys on day 7 of NTN. Shown are the percentages of the CD45+ population. (B) Quantification of absolute macrophage and monocyte numbers per kidney on the basis of the gating strategy shown in A on day 7. (C) FACS analysis for infiltrating neutrophils (CD45+, CD11b+, and Ly6G+) in WT and Batf3-KO kidneys on day 7 of NTN shows the percentages of the CD45+ parental population. (D) Quantification of total neutrophil numbers in kidneys of WT and KO mice through day 7 of NTN on the basis of the gating strategy shown in C. (E) Representative FACS plots for neutrophils (CD45+, CD11b+, and Gr-1+) from a Batf3-KO mouse and a WT mouse on day 7 of NTN treated with a neutrophil-depleting antibody (anti-Ly6G, clone 1A8) or isotype control. (F) Urinary albumin-to-creatinine ratios (ACRs) from Batf3-KO mice treated with the neutrophil-depleting antibody or isotype control on days 3, 5, and 7 of NTN. *P<0.05 determined by t test; **P<0.01 determined by t test.
CD11b+ and CD103+ DCs Each Show a Unique Transcriptional Signature
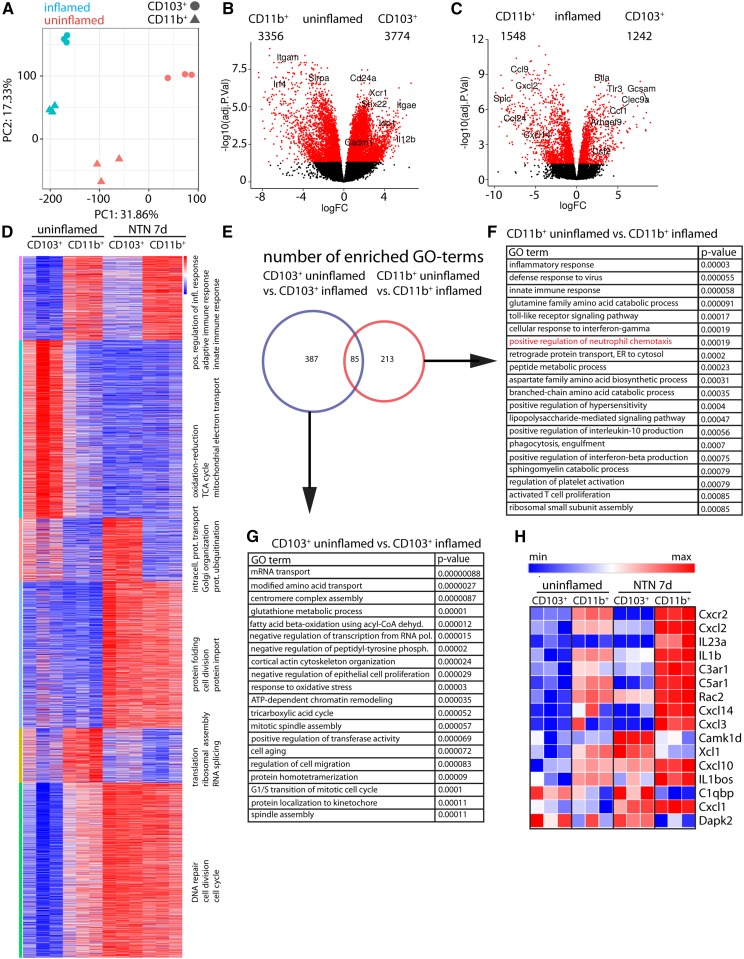
The anti-inflammatory function of CD103+ cDCs suggested that the proinflammatory effect of the ZBTB46+ population was driven by the CD11b+ population. To investigate differences between these two cell populations that could explain their contrasting roles in GN, we performed gene expression analysis by RNA sequencing. CD103+ and CD11b+ cDCs were FACS sorted under healthy conditions or 7 days after injection of NTS (Supplemental Figure 7 A-C). Unbiased principal component analysis clustered the replicates into their DC subsets as well as into the uninflamed or inflamed condition (Figure 8A). Differential expression analysis revealed that, at baseline, CD103+ and CD11b+ cDCs had distinct transcriptional profiles, with 3774 genes specific to CD103+ and 3356 genes specific to CD11b+ (FDR-adjusted P value <0.05) (Figure 8B). As expected, Itgae (CD103), Snx22, and Xcr1 were among the most differentially expressed genes in CD103+ DCs, and Itgam (CD11b), Sirpa, and Irf4 were among the most differentially expressed genes in CD11b+ cells. After inflammation, the profiles became more similar, because the number of significantly regulated genes dropped to 1242 genes in the CD103+ cells and 1548 genes in the CD11b+ cells (Figure 8C). k-Means clustering of the 8000 highest expressed genes using one minus the Pearson correlation coefficient as the dissimilarity measure revealed six differentially expressed gene clusters between the four experimental groups (Figure 8D).
Figure 8.
CD11b+ and CD103+ DCs show a unique transcriptional signature. (A) Principal component analysis of the 12 datasets shows clustering into the experimental groups. (B) Volcano plot comparison of differential gene expression in CD103+ versus CD11b+ DCs in the steady state. Red dots highlight differentially expressed genes with an FDR-adjusted P value of >0.05. Numbers of significantly enriched genes per subset are depicted. (C) Volcano plot comparison of differential gene expression in CD103+ versus CD11b+ DCs on day 7 of NTN. Red dots highlight differentially expressed genes with an FDR-adjusted P value of <0.05. (D) Six differentially expressed clusters were identified by k-means clustering of the 8000 highest expressed genes using one minus Pearson correlation coefficient as the dissimilarity measure. The top three GO terms (biologic function) for each cluster are depicted on the right of each cluster. (E) Venn diagram of the significantly enriched GO terms for biologic processes in uninflamed versus inflamed CD103+ cDCs (blue) and uninflamed versus inflamed CD11b+ cDCs (red). (F) List of the top 20 significantly enriched GO terms in the comparison of CD11b+ DCs in the uninflamed state with those in the inflamed state. (G) List of the top 20 significantly enriched GO terms in the comparison of CD103+ DCs in the uninflamed state with those in the inflamed state. (H) Heat map of genes contained in GO term 0090023 (positive regulation of neutrophil chemotaxis) in all datasets.
We identified the most significantly enriched GO terms (biologic processes) in each set and then compared the GO terms in a Venn diagram (Figure 8E). There were 387 terms uniquely enriched in CD103+ DCs and 213 terms uniquely enriched in CD11b+ DCs. The top 20 most significantly enriched GO terms for each group are shown in Figure 8, F and G.
In the CD11b+ subset, GO terms associated with the activation of the immune response were significantly enriched, supporting the proinflammatory role of these cells. Consistent with this, the GO term “positive regulation of neutrophil chemotaxis” (GO:0090023) was among the highest enriched GO terms in this analysis, suggesting that CD11b+ cDCs might be important in recruitment of neutrophils in GN. We next analyzed individual genes from this GO term and compared them with the CD103+ cells in both the uninflamed and the inflamed conditions (Figure 8H). The neutrophil-attracting chemokine CXCL237 was among the most differentially expressed genes in the inflamed CD11b+ cells compared with all of the other subsets.
The most significantly enriched GO terms in CD103+ DCs were associated with cell proliferation and cell metabolism as well as “negative regulation of epithelial proliferation” (GO:2001110). Although several anti-inflammatory genes were more highly expressed in inflamed CD103+ versus inflamed CD11b+ cDCs, such as Btla (14-fold upregulated; adjusted P<0.001), Ido1 (20-fold; adjusted P<0.001), and Serpinf1 (ninefold; P<0.001), there were no GO terms identified that could globally explain the suppressive phenotype of the CD103+ cells (Supplemental Figure 7E). Thus, the suppressive function of CD103+ cDCs is likely to be context dependent.
Cxcl2 Is Upregulated in CD11b+ cDCs, and Neutralization of CXCL2 Ameliorates the Course of NTN in Batf3-KO Mice
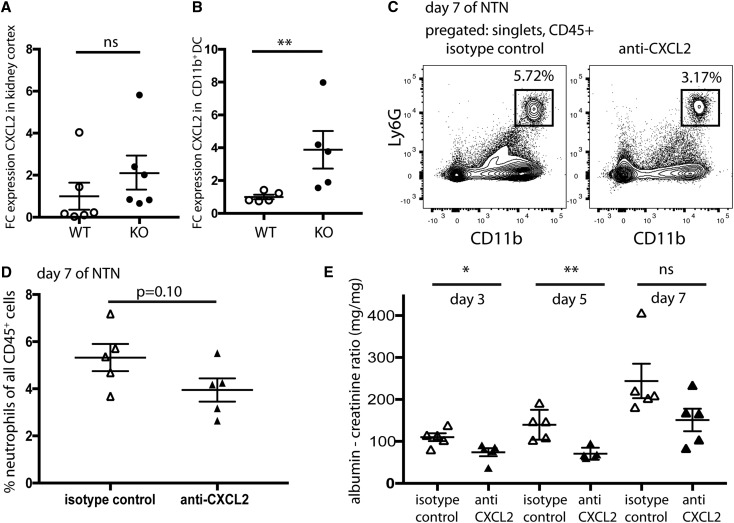
Because Cxcl2 showed one of the highest fold changes of all differentially expressed genes (115-fold change; adjusted P value <0.001) (Figure 8H), we investigated whether the absence of CD103+ DCs affected Cxcl2 expression. CD11b+ DCs were isolated from Batf3-KO and WT kidneys, and Cxcl2 expression was measured by quantitative PCR (qPCR). On day 4, a fourfold increase in Cxcl2 expression was measured in CD11b+ cells from Batf3-KO mice compared with the WT controls (Figure 9B). This shows that CD103+ DCs function to attenuate Cxcl2 expression levels in CD11b+ DCs during NTN. Cxcl2 expression in whole-kidney cortex RNA of WT and KO mice showed no significant differences, suggesting that location and cell type expressing Cxcl2 could be important (Figure 9A). Administration of a neutralizing antibody to CXCL2 into Batf3-KO mice ameliorated proteinuria, confirming the role of neutrophils. This suggests that CD103+ cDCs help to regulate the expression of Cxcl2 in CD11b+ cDCs (Figure 9, C–E).
Figure 9.
CXCL2 is upregulated in CD11b+ cDCs, and neutralization of CXCL2 ameliorates the course of NTN in Batf3-KO mice. (A) qPCR on cDNA from whole-kidney cortex of Batf3-KO and WT mice. Shown is the fold change difference in comparison with the mean expression in the WT control; each dot represents an individual animal. (B) qPCR on cDNA from FACS-sorted CD11b+ cDCs from Batf3-KO and WT mice. Shown is the fold change difference in comparison with the mean expression in the WT control; each dot represents an individual animal. *P<0.01 determined by a Mann–Whitney U test. (C) Representative FACS analyses of neutrophils in Batf3-KO mice treated with either anti-CXCL2 antibody or isotype control; cells were isolated on day 7 of NTN. (D) Percentage of neutrophils (gated as CD11b+ and Ly6G+) of all CD45+ immune cells on day 7 of NTN in the kidneys of anti-CXCL2 or isotype control–treated Batf3-KO mice. (E) Urinary albumin-to-creatinine ratios in anti-CXCL2 or isotype-matched IgG-treated Batf3-KO mice. Depicted are days 3, 5, and 7 after injection of the NTS. *P<0.05 determined by t test; **P<0.01 determined by t test.
Discussion
DCs play important roles in coordinating both innate and adaptive immune responses. They function as sentinels for the innate immune system, interrogating the environment for the presence of pathogens by macropinocytosis and phagocytosis and alerting the adaptive immune system by migrating to secondary lymphoid organs for antigen presentation. Although DCs have been studied for over 30 years, only in the last 10 years have we begun to appreciate the relationship between subset heterogeneity and specialized functions as well as their distinct ontology.6 cDCs derive from the common DC precursor in the bone marrow and differentiate in tissue into two different subsets of cDCs as well as into the plasmacytoid DC.17
DCs, originally described by Steinman and Cohn,1 were defined by their unique morphology, their strong ability to activate T cells, and their lack of expression of Fc receptors for IgG. Largely on the basis of studies of Langerhans cells that were considered a model DC, DCs were thought to have a dendritic shape in vivo and generate an anatomic network that spread across barrier surfaces as well as within some organs.38 This idea is largely supported by imaging studies using CD11c-YFP39 and Cx3cr1GFP/+40 mice that show a network of fluorescent cells in diverse tissues and at barrier surfaces.4,19,20 It is clear today, however, that CD11c and CX3CR1 are expressed on a wide variety of myeloid cells and that they are imperfect markers of DCs.17
Using Zbtb46GFP and Snx22GFP knock-in mice for multiphoton imaging, we found that GFP+ cells were only sparsely localized in the kidney tubulointerstitium; instead, they were mainly localized in clusters in the collagen-rich areas around blood vessels. They also were not dendritic but mostly round in shape. Our data are largely consistent with a recent study showing that approximately 50% of CD45+ cells in the kidney are resident macrophages on the basis of CD11b, F4/80, and CX3CR1 expression.31 These macrophages are highly dendritic and localized in the interstitium adjacent to tubulointerstitial capillaries.32 SNX22+ and ZBTB46+ DCs were motile cells at baseline and increased their motility on inflammation, whereas CD11c-YFP+ cells (mostly macrophages) were stationary before and after initiation of inflammation. This is consistent with long-standing data that macrophages are largely sessile, whereas the role of DCs is to transfer antigen from tissue to lymph node.5 Although DC motility was increased after inflammation, we did not observe directional movement of these cells toward or into lymphatics.
We validated our data by performing imaging mass cytometry (IMC), a new imaging technique that allows for the staining of multiple biomarkers at a single-cell level in tissue sections.31 Traditional immunohistochemistry methods are often limited to two or three antibodies, precluding sophisticated cell identification that often requires the concurrent analysis of multiple markers. The IMC method allowed us to stain with 20 antibodies simultaneously and distinguish specific expression of CD11b, CD11c, CD64, F4/80, ITGAE, and ITGB7 markers, thus validating the shape and localization of cDCs seen with the Zbtb46GFP mouse.
A previous study used a CD11c-DTR mouse to show that DCs are important effector cells in an autologous model of NTN.21 Because this treatment is likely to target macrophages in addition to DCs, we repeated these experiments using Zbtb46DTR/DTR bone marrow chimeras. Our results largely confirmed a proinflammatory role for cDCs. We should note that the NTS used in this study does not require immunization against sheep antibodies, lymphocytes, or complement,34 and we have only focused on the early heterologous phase of NTN, which is likely an innate immune-mediated process for this antiserum.
Consistent with a recent report,36 we found that NTN in the Batf3-KO resulted in a significant acceleration of renal injury with increased inflammation. The course of the disease was characterized by high proteinuria, rapid crescent formation, and increased neutrophils. Neutrophils are known to be recruited acutely in WT mice, but they are largely absent by day 7, replaced by monocyte-derived cells.41,42 In contrast, neutrophils were still numerous on day 7 in the Batf3-KO. Although we did not detect a decrease in Tregs in Batf3-KO mice as reported,36 an important difference could be that we focus only on heterologous NTN, whereas the previous study used an autologous model of NTN.
A role for CD103+ cells in the negative regulation of neutrophilia is supported by a recent studying showing neutrophilia in livers of Batf3-KO mice in the steady state.17 No differences, however, were detected in neutrophils in Batf3-KO kidneys in the steady state. Our data further suggest that CD11b+ DCs are important for neutrophil recruitment and that CD103+ DCs serve to regulate this function. Specifically, we found that the neutrophil chemokine, Cxcl2, was one of the highest expressed genes in the CD11b+ subset and that the presence of CD103+ DCs resulted in a significant reduction of Cxcl2 expression in CD11b+ cDCs. We confirmed the critical role of CXCL2 by rescuing the Batf3-KO phenotype with a CXCL2 neutralizing antibody. This is consistent with a recent report that showed a critical role of CXCL2 for neutrophil migration to the site of inflammation in urogenital tract infection.37
Our imaging studies confirm that the periglomerular region and the tubulointerstitium are the main sites of inflammation, because the density of interstitial myeloid cells increases acutely, likely due to the recruitment of monocytes from the blood. We also detected the recruitment of cDCs to the abluminal surface of Bowman’s capsule with dendritic extensions in close proximity to the glomerular tuft, possibly sensing cellular injury. This could potentially explain how inflammatory signals are conferred to the interstitium when the glomerulus is the primary site of injury. We suspect that the corecruitment of both subsets of cDCs may allow these cells to communicate with each other. Although we did not detect cells in the glomerulus using CD11c, ZBTB46, or SNX22 reporter mice, MHC class II+, CD64+, and F4/80+ cells were detected in glomeruli using IMC (Supplemental Figure 3D) consistent with previous studies showing macrophages in mouse and rat glomeruli.19,43
Although a large body of work has studied the role of DCs in kidney inflammation, our study is the first to address this question using new tools that can distinguish between DCs and macrophages. Our results are surprising, because they contradict long-standing ideas about DCs in tissue. We found that DCs do not form a dendritic network across the kidney and that they are not dendritic in shape. The shape of DCs was originally on the basis of the morphology of activated DCs plated on glass or plastic and the morphology of Langerhans cells in the skin.1 Furthermore, the inactive, immature DC is not adherent, and Langerhans cells are resident macrophages on the basis of their embryonic origin.38 We also found that the role of DCs in kidney inflammation is complex, with CD11b+ cDCs playing a proinflammatory role and CD103+ cDCs playing a regulatory role. Importantly, our studies suggest crosstalk between the two cDC subsets. We suspect that their ability to migrate toward sites of inflammation and the sequential timing of their recruitment could be important in shaping the acute immune response. In future studies, it will be important to define the role of the resident as well as the monocyte-derived macrophages in both the inflammatory and repair phases after kidney injury. A better understanding of distinct myeloid subsets and their function in kidney inflammation and repair is likely to help generate new strategies that will improve understanding and treatment of kidney disease.
Concise Methods
Mice, NTN, and DTX-Mediated Depletion
Batf3−/−, Zbtb46GFP/+, Zbtb46DTR/DTR, and CD11c-YFP mice have been described previously.26,28,29,39 CD45.1-expressing mice served as recipients for bone marrow transfer and were purchased from Charles River Labs (strain B6.Ly5.1/Cr; code 564). Snx22GFP/+ knock-in mice were produced by standard homologous recombination in ES cells (N.M. Kretzer, C.G. Briseno, D.J. Theisen and K.M. Murphy unpublished data).
All animal studies were approved by the Washington University Animal Studies Committee. All mice were housed under specific pathogen–free conditions. Male mice between 8 and 12 weeks of age were used for most NTN studies, but experiments in bone marrow chimeras (all male) were performed 8–10 weeks after γ-irradiation and transplantation.
NTN was induced by a single body weight–adapted intravenous injection of NTS (9 μl/g body wt) produced by J.B.K. as described previously.35 For bone marrow chimeras, a higher dose of NTS had to be used to induce GN (16 μl/g body wt), but it was administered in two injections (50% of the total dose each) performed 8 hours apart. This was necessary, because bone marrow chimeras were prone to acute anaphylactic reactions after a single injection of the full dose.
For imaging studies and in vivo depletion of DCs by DTX, bone marrow chimeras were created. Zbtb46DTR/DTR or WT donor mice were euthanized, and a single-cell suspension of marrow from the femur and tibia was obtained using 70-μm cell strainers. Recipient mice were ordered at 6 weeks of age and lethally γ irradiated with 1100 rad. Eight hours after irradiation, 5×106 bone marrow cells were injected intravenously, and mice were analyzed 8–10 weeks later. Chimerism was verified by staining for CD45.1- and CD45.2-expressing immune cells in the blood before and in kidney single-cell suspensions after the experiment. For depletion of cDCs during NTN, mice were injected intraperitoneally with 20 ng/g DTX 3 days before injection of the NTS, and doses of 5 ng/g were given every 2 days thereafter to maintain depletion.
Antibody-Mediated Depletion
For depletion of CD4+ T cells, antibody clone GK1.5 or the equivalent dose of isotype-matched IgG (both from Leinco, St. Louis, MO) was injected intraperitoneally (250 μg per mouse) 2 days before injection of NTS. Injections were repeated every 7 days until the end of the experiment. For depletion of neutrophils, antibody clone 1A8 or the equivalent dose of isotype-matched IgG was injected intraperitoneally (500 μg per mouse) 1 day before injection of NTS. For neutralization of CXCL2, 100 μg clone 40605 (R&D Systems, Minneapolis, MN) or isotype-matched IgG (Leinco) was injected intravenously 2 days after injection of NTS.
Histology
Periodic acid–Schiff staining was performed using standard protocols by the histology core of the Department of Developmental Biology on 5-μm-thick paraffin sections.
Flow Cytometry of Murine Kidney Single-Cell Suspensions
For the preparation of kidney single-cell suspensions, mice were anesthetized and perfused intracardially with cold PBS. Kidneys were cut into small pieces and then stirred for 30 minutes at 37°C in 5 ml complete RPMI (Thermo Fisher Scientific) with 30 U/ml DNase I and 250 μg/ml collagenase B (Sigma-Aldrich). For removal of debris, cells were passed through a 100-μm strainer before red blood cells were lysed with ammonium chloride-potassium bicarbonate lysis buffer (150 mM NH4Cl, 10 mM KHCO3, and 0.1 mM EDTA) for 3 minutes at room temperature and then passed through a 70-μm cell strainer. After treatment with Fc block (clone 2.4G2; BD Biosciences, San Jose, CA) diluted in FACS buffer (0.5% BSA and 2 mM EDTA in PBS), cells were stained for 30–60 minutes at 4°C with fluorochrome-labeled antibodies in FACS buffer.
For intranuclear staining of transcription factors, the FOXP3 staining kit (eBioscience, San Diego, CA) was used. For intracellular staining of cytokines, cells were first treated with BD Pharmingen Leukocyte Activation Cocktail for 4 hours in complete RPMI at 37°C. After Fc block and staining of surface markers as described above, cells were fixed with 4% PFA and then stained in in the presence of 0.3% saponin (Sigma-Aldrich) diluted in FACS buffer for intracellular cytokines.
Cells were analyzed on an LSR Fortessa flow cytometer (BD Biosciences). Count bright absolute counting beads (Thermo Fisher Scientific) were used for absolute quantification of cell numbers. For DC sorting experiments, immune cells were first enriched with anti-mouse CD45 beads and LS columns (Miltenyi Biotec, San Diego, CA) according to the manufacturer’s instructions before proceeding to surface staining as described above. Cell sorting was performed on an FACS Aria II flow cytometer (BD Biosciences).
The following antibodies were used for flow cytometry: Biolegend: CD45 (30-F11), CD45.1 (A20), CD45.2 (104), CD3 (145–2C11), CD4 (GK1.5), CD4 (RM4–5), CD8 (53–6.7), TCRβ (H57–597), TCRγ/δ (GL3), CD44 (IM70), CD62L (MEL-14), I-A/I-E (M5/114.15.2), CD11c (N418), F4/80 (BM8), CD64 (X54–5/71), CD103 (2E7), CD11b (M1/70), Gr1 (RB8–8C5), Ly6C (HK1.4), Ly6G (1A8), IL-17a (TC11–18H10.1), and CD19 (6D5); eBiosciences: Foxp3 (FJK-16s).
IMC
Kidneys were fixed by cardiac perfusion of C57 BL/6 mice with 1% PFA/PBS. Kidneys were embedded in OCT after 30% sucrose replacement overnight; 10-μm cryosections were blocked with 3% BSA in PBS for 45 minutes. Samples were incubated overnight at 4°C with a final concentration of 5–10 μg/ml metal-conjugated antibody cocktails diluted in PBS/0.5% BSA (174Yb-IA-IE (M5/114.12.2), 165Ho-CD31 (390), 159Tb-CD103 (2E7), 151Eu-CD64 (X54–5/7.1), 148Nd-CD11b (M1/70), 146Nd-F4–80 (BM8), 142Nd-CD11c (N418), and 162Dy-integrin-β7 (FIB504; all Fluidigm Inc.). Samples were then washed with 2× PBS/0.1% Triton X-100 and 2× PBS and exposed to 25 μM Ir-Intercalator (Fluidigm) for 30 minutes at room temperature for nuclei staining. The samples were rinsed twice in distilled water and air dried before IMC analysis. The slides were inserted into the Hyperion Imaging System (Fluidigm). Data processing was performed using MCD Viewer (Fluidigm) and ImageJ (Fiji, version 1.0).
In Vitro T Cell Proliferation Assay and IL-2 ELISA
Kidneys from four animals (C57BL/6JRj; Janvier) were isolated, decapsulated, and dissociated in RPMI with DNAse and Collagenase for 40 minutes. Single-cell suspensions were filtered with a 100-μm cell strainer. CD11c+ cells were enriched by magnetic beads separation (CD11c microbeads; Miltenyi Biotec) according to the manufacturer’s protocol and then stained for CD45, MHC class II, CD11b, CD11c, F4/80, CD64, and Hoechst. Cells were then sorted on a BD FACS AriaIII cell sorter. Equal cell numbers of CD45+, MHC class II+, CD11c+, F4/80+, and CD64+ or CD45+, MHC class II+, CD11c+, F4/80−, and CD64− cells were incubated with 500 μg/ml OVA (323–339) for 2 hours at 37°C (with 5% CO2). OT-II T cells were isolated from spleen and lymph nodes of a B6.Cg-Tg(TcraTcrb)425Cbn/J mouse with a mouse CD4+ T cell isolation kit (Miltenyi Biotec) and stained with CFSE (1 μM) for 11 minutes at 37°C (with 5% CO2). OT-II cells were washed and counted, and equal numbers were added to wells containing the sorted APCs. After 72 hours, cells were stained for CD4, CD45, and Hoechst and analyzed for CFSE fluorescence.
Undiluted cell culture supernatants were analyzed for IL-2 72 hours after the start of the APC and OT-II coculture; 96-well plates were coated with anti-mouse IL-2 antibody (clones JES6–1A12 from eBioscience). Standards and samples were incubated in duplicates at 4°C overnight. After washing, biotinylated anti-mouse IL-2 antibody (clone JES6–5H4; eBiosciences) was added for 2 hours at 37°C. After washing, HRP-coupled streptavidin (S2438–25046; Sigma-Aldrich) was added for 1 hour at room temperature. After development with ODP substrate, the ELISA was analyzed on a plate reader at 490 nm.
RNA Preparation, qPCR, and RNA Sequencing
For qPCR using total kidney cortex RNA, 1×2-mm-sized pieces of kidney cortex were immersed in 1 ml Trizol lysis reagent (Thermo Fisher Scientific), frozen in liquid nitrogen, and stored at −80°C. RNA was prepared by mixing the kidney homogenate with 200 μl chloroform. RNA in the aqueous phase was precipitated with isopropanol, washed in 75% ethanol, and suspended in water. cDNA was synthesized using the High Capacity cDNA Reverse Transcription Kit, and a Taqman Assay (probes Mm03024075_m1 and Mm00436450_m1) was performed on a StepOne plus qPCR machine (all Thermo Fisher Scientific).
For RNA sequencing and qPCR of sorted DCs, kidney single-cell suspensions were prepared and stained as described above, and they were sorted directly into the lysis buffer of the RNAqueous micro isolation kit (Thermo Fisher Scientific). Lysates were immediately frozen on dry ice and stored at −80°C until RNA preparation and DNase treatment according to the manufacturer’s standard protocol.
Library preparation was performed with total RNA after integrity was determined using an Agilent bioanalyzer. Double-stranded cDNA was prepared using the SMARTer Ultra Low RNA kit for Illumina Sequencing (Takara Bio USA, Mountain View, CA) per the manufacturer’s protocol. cDNA was fragmented using a Covaris E210 sonicator using duty cycle 10, intensity 5, cycles per burst 200, and time 180 seconds. cDNA was blunt ended, had an adenine added to the 3′ ends, and then, had Illumina sequencing adapters ligated to the ends. Ligated fragments were then amplified for 12 cycles using primers incorporating unique index tags. Fragments were sequenced on an Illumina HiSeq-2500 (Illumina, San Diego, CA) using single reads extending 50 bases.
Changes in expression for selected genes were validated with Taqman probes Mm00492590_m1, Mm00441270_m1, and Mm00616981_m1.
RNA-Seq Data Acquisition, Quality Control, and Processing
RNA-Seq reads were aligned to the Ensembl release 76 assembly with STAR, version 2.0.4b. Gene counts were derived from the number of uniquely aligned unambiguous reads by Subread:featureCount, version 1.4.5. Transcript counts were produced by Sailfish, version 0.6.3. Sequencing performance was assessed for total number of aligned reads, total number of uniquely aligned reads, genes and transcripts detected, ribosomal fraction known junction saturation, and read distribution over known gene models with RSeQC, version 2.3.
All gene-level and transcript counts were then imported into the R/Bioconductor package EdgeR, and TMM normalization size factors were calculated to adjust for samples for differences in library size. Genes or transcripts not expressed in any sample were excluded from further analysis. The TMM size factors and the matrix of counts were then imported into R/Bioconductor package Limma, and weighted likelihoods on the basis of the observed mean-variance relationship of every gene/transcript were then calculated for all samples with the Voom function. Performance of the samples was assessed with a Spearman correlation matrix and multidimensional scaling plots. Gene/transcript performance was assessed with plots of residual SD of every gene to their average log count with a robustly fitted trend line of the residuals. Generalized linear models with robust dispersion estimates were then created to test for gene/transcript-level differential expression. Differentially expressed genes and transcripts were then filtered for FDR-adjusted P values ≤0.05.
To assess potential batch effects in the dataset, a principal component analysis was used. k-Means clustering with k=6 was performed using the 8000 most expressed genes and one minus the Pearson correlation coefficient as the dissimilarity measure, and number of clusters was determined by the elbow method on explained variance. Gene set enrichment methods were used to enhance the biologic interpretation of differentially expressed genes. The TopGO package for R was used for GO term enrichment analysis (Adrian Alexa and Jorg Rahnenfuhrer, 2016, topGO: Enrichment Analysis for Gene Ontology; R package, version 2.26.0.). For differentially expressed genes, we used the elimKS method to perform improved scoring of functional groups,44 and for cluster annotation, the elimFisher method was used.
Two-Photon Imaging of Kidney Slices
Images were collected using a customized Leica SP8 Two-Photon Microscope (Leica, Wetzlar, Germany) equipped with a 25× and 0.95 numerical aperture water-immersion objective and a Mai Tai HP DeepSee Laser (Spectra-Physics, Mountain View, CA) tuned to 895 nm. Fluorescence emission was guided directly to supersensitive external hybrid photodetectors. For signal separation, dichroic beam splitters by Semrock were used. To mark capillaries, animals were injected with 60 μl DyLight594-labeled Tomato Lectin (Vector Laboratories, Burlingame, CA).
For kidney slice imaging, freshly isolated kidneys were cut into 1-mm-thick sections using a vibratome (Leica) in CO2-independent medium and then immediately imaged in an incubation chamber (37°C RPMI medium; Thermo Fisher Scientific; 95% O2 and 5% CO2). Only glomeruli with an intact Bowman’s capsule that were surrounded by at least one layer of tubular epithelium were imaged to minimize the possibility of cutting artifacts.
Quantification of DC Shape and Movement
For analysis of movement, Z stacks were imaged with a time interval of 30 seconds between a Z stack and for at least 15 minutes. Using Imaris software (Bitplane, Zurich, Switzerland), the image data were then three-dimensionally corrected for tissue drift to reduce any artificial movement. When Imaris was unable to correct for drift, the images were removed from analysis. After correcting for drift, the images were projected to two dimensions using Fiji and then loaded into Matlab. The images were then segmented using Otsu’s method available in Matlab to separate the cells from the background noise. Objects that had an area in pixels <0.01% of the total image area were removed from the analysis. The fluorescence displacement between images was calculated by taking the absolute value of the differences in images and then summing the differences for each image stack. The sum of the absolute differences is a final image of the total displacement of the cells over time. This final image was then compared with the starting image to determine the ratio of displacement in comparison, which better allows for comparing displacements. The total pixel area of all cells or objects in the image of the sum of the final image and first image is then divided by the cells or objects in the first image. This is the displacement ratio, which is the increased movement from the first image to the last image in the stack. Using Imaris software, we analyzed cells for their sphericity as a measure of their three-dimensional cell shape.
Statistical Analyses
For comparison of two groups, the t test was used to analyze statistical significance. For comparison of qPCR data, the Mann–Whitney U test was used to analyze statistical significance. When three or more groups were compared, we used a one-way ANOVA with a Tukey post-test. Statistical tests were performed using Prism (Graphpad, La Jolla, CA). RNAseq data were analyzed as stated above.
Disclosures
M.N. and A.S.S are employees of Genentech (South San Francisco, CA). O.O. and Q.C. are employees of Fluidigm Inc. (Markham, ON, Canada). J.H.M. has received grants from Hoffmann-La Roche (Basel, Switzerland) and RGDI3, Inc. (Boston, MA); has provided consultation to Third Rock Ventures (Boston, MA); and has received licensing fees from Eli Lilly (Indianapolis, IN) and Genentech (South San Francisco, CA).
Supplementary Material
Acknowledgments
We thank C. Stander, L. LaFata, and J. Richardson for excellent technical support. We also thank the Genome Technology Access Center in the Department of Genetics at Washington University School of Medicine for help with genomic analysis.
This study was funded by scholarship DFG BR4917/1-1 from the Deutsche Forschungsgemeinschaft and the Köln Fortune Program of the University of Cologne (to S.B.) and National Institutes of Health (NIH) grant R01DK058366 (to J.H.M. and A.S.S.). H.S. was supported by a Career Development Fellowship from the Nephrotic Syndrome Study Network Consortium (funded by NIH grant U54DK083912) and Scientist Development grant 17SDG33420069 from the American Heart Association. K.Z. was supported by Government of Russian Federation grant 074-U01. J.B.K. was supported by the Intramural Research Program, the National Institute of Diabetes and Digestive and Kidney Diseases, the NIH. The Genome Technology Access Center is partially supported by National Cancer Center Support grant P30CA91842 (to the Siteman Cancer Center); Institute for Clinical and Translational Sciences/Clinical and Translational Science Award grant UL1TR000448 from the National Center for Research Resources (NCRR), a component of the NIH; and the NIH Roadmap for Medical Research.
This publication is solely the responsibility of the authors and does not necessarily represent the official view of the NCRR or the NIH.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Renal Dendritic Cells: The Long and Winding Road,” on pages 4–7.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2017030270/-/DCSupplemental.
References
- 1.Steinman RM, Cohn ZA: Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J Exp Med 137: 1142–1162, 1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nussenzweig MC, Steinman RM, Gutchinov B, Cohn ZA: Dendritic cells are accessory cells for the development of anti-trinitrophenyl cytotoxic T lymphocytes. J Exp Med 152: 1070–1084, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Durai V, Murphy KM: Functions of murine dendritic cells. Immunity 45: 719–736, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Niess JH, Brand S, Gu X, Landsman L, Jung S, McCormick BA, Vyas JM, Boes M, Ploegh HL, Fox JG, Littman DR, Reinecker H-C: CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science 307: 254–258, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Guilliams M, Ginhoux F, Jakubzick C, Naik SH, Onai N, Schraml BU, Segura E, Tussiwand R, Yona S: Dendritic cells, monocytes and macrophages: A unified nomenclature based on ontogeny. Nat Rev Immunol 14: 571–578, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ginhoux F, Guilliams M: Tissue-resident macrophage ontogeny and homeostasis. Immunity 44: 439–449, 2016 [DOI] [PubMed] [Google Scholar]
- 7.Fogg DK, Sibon C, Miled C, Jung S, Aucouturier P, Littman DR, Cumano A, Geissmann F: A clonogenic bone marrow progenitor specific for macrophages and dendritic cells. Science 311: 83–87, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Onai N, Obata-Onai A, Schmid MA, Ohteki T, Jarrossay D, Manz MG: Identification of clonogenic common Flt3+M-CSFR+ plasmacytoid and conventional dendritic cell progenitors in mouse bone marrow. Nat Immunol 8: 1207–1216, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Naik SH, Sathe P, Park H-Y, Metcalf D, Proietto AI, Dakic A, Carotta S, O’Keeffe M, Bahlo M, Papenfuss A, Kwak J-Y, Wu L, Shortman K: Development of plasmacytoid and conventional dendritic cell subtypes from single precursor cells derived in vitro and in vivo. Nat Immunol 8: 1217–1226, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Liu K, Victora GD, Schwickert TA, Guermonprez P, Meredith MM, Yao K, Chu F-F, Randolph GJ, Rudensky AY, Nussenzweig M: In vivo analysis of dendritic cell development and homeostasis. Science 324: 392–397, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ginhoux F, Liu K, Helft J, Bogunovic M, Greter M, Hashimoto D, Price J, Yin N, Bromberg J, Lira SA, Stanley ER, Nussenzweig M, Merad M: The origin and development of nonlymphoid tissue CD103+ DCs. J Exp Med 206: 3115–3130, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K: Development of monocytes, macrophages, and dendritic cells. Science 327: 656–661, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Y, Szretter KJ, Vermi W, Gilfillan S, Rossini C, Cella M, Barrow AD, Diamond MS, Colonna M: IL-34 is a tissue-restricted ligand of CSF1R required for the development of Langerhans cells and microglia. Nat Immunol 13: 753–760, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greter M, Lelios I, Pelczar P, Hoeffel G, Price J, Leboeuf M, Kündig TM, Frei K, Ginhoux F, Merad M, Becher B: Stroma-derived interleukin-34 controls the development and maintenance of langerhans cells and the maintenance of microglia. Immunity 37: 1050–1060, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schulz C, Gomez Perdiguero E, Chorro L, Szabo-Rogers H, Cagnard N, Kierdorf K, Prinz M, Wu B, Jacobsen SEW, Pollard JW, Frampton J, Liu KJ, Geissmann F: A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science 336: 86–90, 2012 [DOI] [PubMed] [Google Scholar]
- 16.Hoeffel G, Wang Y, Greter M, See P, Teo P, Malleret B, Leboeuf M, Low D, Oller G, Almeida F, Choy SHY, Grisotto M, Renia L, Conway SJ, Stanley ER, Chan JKY, Ng LG, Samokhvalov IM, Merad M, Ginhoux F: Adult Langerhans cells derive predominantly from embryonic fetal liver monocytes with a minor contribution of yolk sac-derived macrophages. J Exp Med 209: 1167–1181, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiao J, Dragomir AC, Kocabayoglu P, Rahman AH, Chow A, Hashimoto D, Leboeuf M, Kraus T, Moran T, Carrasco-Avino G, Friedman SL, Merad M, Aloman C: Central Role of Conventional Dendritic Cells in Regulation of Bone Marrow Release and Survival of Neutrophils. J Immunol 192(7): 3374–3382, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krüger T, Benke D, Eitner F, Lang A, Wirtz M, Hamilton-Williams EE, Engel D, Giese B, Müller-Newen G, Floege J, Kurts C: Identification and functional characterization of dendritic cells in the healthy murine kidney and in experimental glomerulonephritis. J Am Soc Nephrol 15: 613–621, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Soos TJ, Sims TN, Barisoni L, Lin K, Littman DR, Dustin ML, Nelson PJ: CX3CR1+ interstitial dendritic cells form a contiguous network throughout the entire kidney. Kidney Int 70: 591–596, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Yatim KM, Gosto M, Humar R, Williams AL, Oberbarnscheidt MH: Renal dendritic cells sample blood-borne antigen and guide T-cell migration to the kidney by means of intravascular processes. Kidney Int 90: 818–827, 2016 [DOI] [PubMed] [Google Scholar]
- 21.Hochheiser K, Engel DR, Hammerich L, Heymann F, Knolle PA, Panzer U, Kurts C: Kidney dendritic cells become pathogenic during crescentic glomerulonephritis with proteinuria. J Am Soc Nephrol 22: 306–316, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scholz J, Lukacs-Kornek V, Engel DR, Specht S, Kiss E, Eitner F, Floege J, Groene H-J, Kurts C: Renal dendritic cells stimulate IL-10 production and attenuate nephrotoxic nephritis. J Am Soc Nephrol 19: 527–537, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Riedel J-H, Paust H-J, Turner J-E, Tittel AP, Krebs C, Disteldorf E, Wegscheid C, Tiegs G, Velden J, Mittrücker H-W, Garbi N, Stahl RAK, Steinmetz OM, Kurts C, Panzer U: Immature renal dendritic cells recruit regulatory CXCR6(+) invariant natural killer T cells to attenuate crescentic GN. J Am Soc Nephrol 23: 1987–2000, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Langlet C, Tamoutounour S, Henri S, Luche H, Ardouin L, Grégoire C, Malissen B, Guilliams M: CD64 expression distinguishes monocyte-derived and conventional dendritic cells and reveals their distinct role during intramuscular immunization. J Immunol 188: 1751–1760, 2012 [DOI] [PubMed] [Google Scholar]
- 25.Guilliams M, Dutertre C-A, Scott CL, McGovern N, Sichien D, Chakarov S, Van Gassen S, Chen J, Poidinger M, De Prijck S, Tavernier SJ, Low I, Irac SE, Mattar CN, Sumatoh HR, Low GHL, Chung TJK, Chan DKH, Tan KK, Hon TLK, Fossum E, Bogen B, Choolani M, Chan JKY, Larbi A, Luche H, Henri S, Saeys Y, Newell EW, Lambrecht BN, Malissen B, Ginhoux F: Unsupervised high-dimensional analysis aligns dendritic cells across tissues and species. Immunity 45: 669–684, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hildner K, Edelson BT, Purtha WE, Diamond M, Matsushita H, Kohyama M, Calderon B, Schraml BU, Unanue ER, Diamond MS, Schreiber RD, Murphy TL, Murphy KM: Batf3 deficiency reveals a critical role for CD8alpha+ dendritic cells in cytotoxic T cell immunity. Science 322: 1097–1100, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dudziak D, Kamphorst AO, Heidkamp GF, Buchholz VR, Trumpfheller C, Yamazaki S, Cheong C, Liu K, Lee H-W, Park CG, Steinman RM, Nussenzweig MC: Differential antigen processing by dendritic cell subsets in vivo. Science 315: 107–111, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Satpathy AT, Kc W, Albring JC, Edelson BT, Kretzer NM, Bhattacharya D, Murphy TL, Murphy KM: Zbtb46 expression distinguishes classical dendritic cells and their committed progenitors from other immune lineages. J Exp Med 209: 1135–1152, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meredith MM, Liu K, Darrasse-Jeze G, Kamphorst AO, Schreiber HA, Guermonprez P, Idoyaga J, Cheong C, Yao K-H, Niec RE, Nussenzweig MC: Expression of the zinc finger transcription factor zDC (Zbtb46, Btbd4) defines the classical dendritic cell lineage. J Exp Med 209: 1153–1165, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Giesen C, Wang HAO, Schapiro D, Zivanovic N, Jacobs A, Hattendorf B, Schüffler PJ, Grolimund D, Buhmann JM, Brandt S, Varga Z, Wild PJ, Günther D, Bodenmiller B: Highly multiplexed imaging of tumor tissues with subcellular resolution by mass cytometry. Nat Methods 11: 417–422, 2014 [DOI] [PubMed] [Google Scholar]
- 31.Chang Q, Ornatsky OI, Siddiqui I, Straus R, Baranov VI, Hedley DW: Biodistribution of cisplatin revealed by imaging mass cytometry identifies extensive collagen binding in tumor and normal tissues. Sci Rep 6: 36641, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stamatiades EG, Tremblay M-E, Bohm M, Crozet L, Bisht K, Kao D, Coelho C, Fan X, Yewdell WT, Davidson A, Heeger PS, Diebold S, Nimmerjahn F, Geissmann F: Immune monitoring of trans-endothelial transport by kidney-resident macrophages. Cell 166: 991–1003, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brähler S, Yu H, Suleiman H, Krishnan GM, Saunders BT, Kopp JB, Miner JH, Zinselmeyer BH, Shaw AS: Intravital and kidney slice imaging of podocyte membrane dynamics. J Am Soc Nephrol 27: 3285–3290, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heymann F, Meyer-Schwesinger C, Hamilton-Williams EE, Hammerich L, Panzer U, Kaden S, Quaggin SE, Floege J, Gröne H-J, Kurts C: Kidney dendritic cell activation is required for progression of renal disease in a mouse model of glomerular injury. J Clin Invest 119: 1286–1297, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yo Y, Braun MC, Barisoni L, Mobaraki H, Lu H, Shrivastav S, Owens J, Kopp JB: Anti-mouse mesangial cell serum induces acute glomerulonephropathy in mice. Nephron, Exp Nephrol 93: e92–e106, 2003 [DOI] [PubMed] [Google Scholar]
- 36.Evers BDG, Engel DR, Böhner AMC, Tittel AP, Krause TA, Heuser C, Garbi N, Kastenmüller W, Mack M, Tiegs G, Panzer U, Boor P, Ludwig-Portugall I, Kurts C: CD103+ kidney dendritic cells protect against crescentic GN by maintaining IL-10-producing regulatory T cells. J Am Soc Nephrol 27: 3368–3382, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schiwon M, Weisheit C, Franken L, Gutweiler S, Dixit A, Meyer-Schwesinger C, Pohl J-M, Maurice NJ, Thiebes S, Lorenz K, Quast T, Fuhrmann M, Baumgarten G, Lohse MJ, Opdenakker G, Bernhagen J, Bucala R, Panzer U, Kolanus W, Gröne H-J, Garbi N, Kastenmüller W, Knolle PA, Kurts C, Engel DR: Crosstalk between sentinel and helper macrophages permits neutrophil migration into infected uroepithelium. Cell 156: 456–468, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Merad M, Ginhoux F, Collin M: Origin, homeostasis and function of Langerhans cells and other langerin-expressing dendritic cells. Nat Rev Immunol 8: 935–947, 2008 [DOI] [PubMed] [Google Scholar]
- 39.Lindquist RL, Shakhar G, Dudziak D, Wardemann H, Eisenreich T, Dustin ML, Nussenzweig MC: Visualizing dendritic cell networks in vivo. Nat Immunol 5: 1243–1250, 2004 [DOI] [PubMed] [Google Scholar]
- 40.Jung S, Aliberti J, Graemmel P, Sunshine MJ, Kreutzberg GW, Sher A, Littman DR: Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol Cell Biol 20: 4106–4114, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mayadas TN, Rosetti F, Ernandez T, Sethi S: Neutrophils: Game changers in glomerulonephritis? Trends Mol Med 16: 368–378, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cochrane CG, Unanue ER, Dixon FJ: A role of polymorphonuclear leukocytes and complement in nephrotoxic nephritis. J Exp Med 122: 99–116, 1965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schreiner GF, Kiely JM, Cotran RS, Unanue ER: Characterization of resident glomerular cells in the rat expressing Ia determinants and manifesting genetically restricted interactions with lymphocytes. J Clin Invest 68: 920–931, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alexa A, Rahnenführer J, Lengauer T: Improved scoring of functional groups from gene expression data by decorrelating GO graph structure. Bioinformatics 22: 1600–1607, 2006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.