Abstract
Nephrin is a key structural component of the podocyte slit diaphragm, and proper expression of nephrin on the cell surface is critical to ensure integrity of the blood filtration barrier. Maintenance of nephrin within this unique cell junction has been proposed to require dynamic phosphorylation events and endocytic recycling, although the molecular mechanisms that control this interplay are poorly understood. Here, we investigated the possibility that the phosphotyrosine adaptor protein ShcA regulates nephrin turnover. Western blotting and immunostaining analysis confirmed that ShcA is expressed in podocytes. In immunoprecipitation and pulldown assays, ShcA, via its SH2 domain, was associated with several phosphorylated tyrosine residues on nephrin. Overexpression of ShcA promoted nephrin tyrosine phosphorylation and reduced nephrin signaling and cell surface expression in vitro. In a rat model of reversible podocyte injury and proteinuria, phosphorylated nephrin temporally colocalized with endocytic structures coincident with upregulation of ShcA expression. In vivo biotinylation assays confirmed that nephrin expression decreased at the cell surface and correspondingly increased in the cytosol during the injury time course. Finally, immunostaining in kidney biopsy specimens demonstrated overexpression of ShcA in several human proteinuric kidney diseases compared with normal conditions. Our results suggest that increases in ShcA perturb nephrin phosphosignaling dynamics, leading to aberrant nephrin turnover and slit diaphragm disassembly.
Keywords: nephrin, endocytosis, kidney disease, signaling, podocyte
Podocytes play a critical role in maintaining the integrity of the kidney’s blood filtration barrier. These specialized epithelial cells extend a network of interdigitating actin-based foot processes, which are bridged by a unique cell junction known as the slit diaphragm.1 A fundamental component of the slit diaphragm is the transmembrane protein nephrin. Through cis and trans interactions of its extracellular domain, nephrin contributes to the filtration pore that is largely responsible for permselectivity.2 Aberrant expression of nephrin and other slit diaphragm molecules such as podocin, leads to foot process effacement and loss of essential plasma proteins into the urine (proteinuria).1 Accumulating evidence suggests that stable expression of nephrin on the cell surface is regulated by endocytosis, and that defects in nephrin turnover might lead to proteinuria.3
Nephrin can be internalized via both clathrin-mediated endocytosis and raft-mediated endocytosis mechanisms.4 Nephrin endocytosis is reduced in podocytes lacking the endocytic effector dynamin,5 and enhanced in the presence of CIN85, which further induces its ubiquitination.6 Additionally, nephrin endocytosis appears to be triggered by its tyrosine phosphorylation,4,7 with the phosphorylation signature instructing differential binding of endocytic regulators β-arrestin and podocin.8 Nephrin contains several tyrosine residues on its intracellular tail that undergo Src family kinase (SFK)–mediated phosphorylation and serve as docking sites for Src homology 2 (SH2) domain-containing proteins.9 We have recently demonstrated that sustained phosphorylation on several of these sites is essential to stabilize foot process morphology and maintain barrier function.10 Despite these observations, the molecular mechanisms that facilitate nephrin turnover are poorly understood, and a nephrin phosphotyrosine-binding partner involved in endocytosis remains to be identified.
Here, we reveal the adaptor protein ShcA as a novel signaling molecule within the slit diaphragm. Shc family adaptors (ShcA-D) contain both a phosphotyrosine-binding (PTB) domain and a C-terminal SH2 domain, each of which can bind phosphotyrosine residues (Figure 1A).11 The central CH1 region contains three phosphorylatable tyrosine residues, in addition to a motif that binds α-adaptin, a component of the clathrin assembly complex. Splicing and alternative translation start sites produce the 46, 52, and 66 kDa isoforms of ShcA.12 The p46 and p52 isoforms are well established activators of the Ras/MAPK pathway via their recruitment of Grb2 to the central tyrosines, and they also promote clathrin-mediated endocytosis of the EGF receptor through the α-adaptin–binding motif.13 The p66 isoform uniquely controls the oxidative stress response through its extended CH2 region.14 Global knockout of all ShcA isoforms in mice causes major cardiovascular abnormalities, resulting in embryonic lethality, and ShcA-deficient fibroblasts exhibit defects in actin organization.15 In the kidney, deletion of p66 renders proximal tubule cells more resistant to oxidative stress,16 and it also suppresses hyperglycemia-induced glomerular injury17; however, the in vivo role of ShcA in podocytes has not yet been explored.
Figure 1.
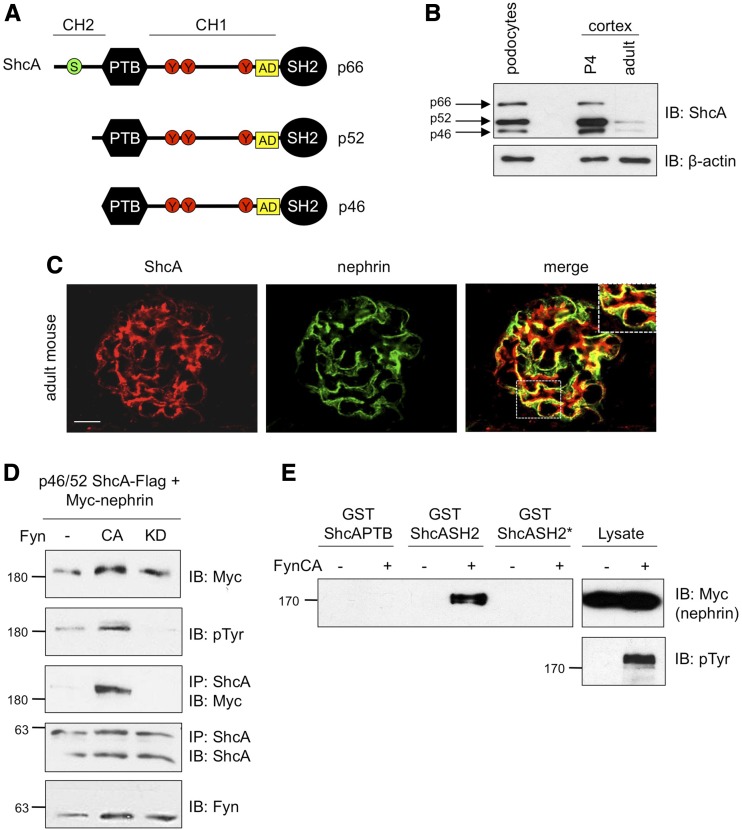
ShcA is expressed in podocytes and binds tyrosine phosphorylated nephrin. (A) Schematic of ShcA isoforms and signaling domains including an N-terminal PTB domain, a C-terminal SH2 domain, three central tyrosine residues (red circles), an α-adaptin (AD)–binding motif (yellow square), and a serine residue in p66 key for oxidative stress signaling (green circle). (B) Confirmation of ShcA protein expression in lysates prepared from cultured mouse podocyte cells and kidney cortex of postnatal day 4 (P4) and adult mice by immunoblotting (IB). (C) Dual immunofluorescence staining showing overlapping expression between ShcA (red) and nephrin (green) on kidney sections from adult mice. Scale bar, 10 μm. (D) Lysates from HEK293T cells transiently coexpressing p46/52 ShcA-Flag, Myc-nephrin, and constitutively active (CA) or kinase dead (KD) Fyn were immunoprecipitated (IP) with ShcA and immunoblotted (IB) as indicated. ShcA coimmunoprecipitated with phosphorylated nephrin. (E) Lysates from HEK293T cells overexpressing Myc-nephrin with or without CA-Fyn were incubated with immobilized GST fusion proteins corresponding to the PTB, SH2, or mutant SH2 (R397K, SH2*) domains of ShcA. Pulldown complexes immunoblotted for Myc indicate binding between the ShcA SH2 domain but not ShcA SH2* with phosphorylated nephrin. pTyr, phosphotyrosine.
We now report that ShcA interacts via its SH2 domain with phosphorylated nephrin to regulate nephrin tyrosine phosphorylation and surface expression. We further demonstrate that ShcA is upregulated in a reversible podocyte injury model coincident with relocalization of phosphorylated nephrin into endocytic structures, and that nephrin is removed from the cell surface in this model. Finally, we show increased expression of ShcA in humans with proteinuric kidney disease. We conclude that ShcA is a central regulator of nephrin phosphosignaling dynamics, which in turn, modulates filtration barrier function via direct control of nephrin turnover at the plasma membrane.
Results
ShcA Is Expressed in Podocytes and Interacts with Nephrin via its SH2 Domain
ShcA is among a select few SH2 domain-containing proteins predicted by DomPep modeling18 to interact with phosphorylated nephrin (Supplemental Table 1). As ShcA is widely expressed, we first established the presence of this adaptor protein in podocytes via Western immunoblot analysis of protein lysates obtained from cultured podocytes and neonatal and adult mouse kidney cortex. All ShcA isoforms are found within podocytes, and their expression declines once glomerulogenesis is complete, with the p46/52 isoforms predominating over the p66 isoform in both the immature and mature kidney (Figure 1B). We also performed immunostaining of mouse kidney cryosections, where ShcA was distributed throughout the glomerulus and robustly localized with nephrin in podocytes (Figure 1C). To next investigate whether ShcA interacts with nephrin, Flag-tagged p46/52 ShcA was coexpressed with Myc-tagged, full-length nephrin in HEK293T cells. Nephrin coprecipitated with ShcA, and this effect was enhanced upon coexpression of constitutively active Fyn, but not a kinase dead variant of this SFK (Figure 1D), indicating a requirement for nephrin tyrosine phosphorylation in the nephrin-ShcA interaction. We confirmed that p66 ShcA could similarly associate with phosphorylated nephrin (Supplemental Figure 1A). ShcA contains both PTB and SH2 domains, thus we next wanted to determine which phosphotyrosine-binding module might mediate interaction with nephrin. Pulldown assays of nephrin in the presence or absence of constitutively active Fyn, using glutathione S-transferase (GST) fused to each domain, revealed that the SH2 domain but not the PTB domain is required for nephrin binding, and mutation of the phosphotyrosine-binding pocket of the SH2 domain (R397K, SH2*) disrupted this interaction (Figure 1E).
Nephrin Phosphotyrosine Residues 1176, 1193, and 1217 Mediate Binding to ShcA
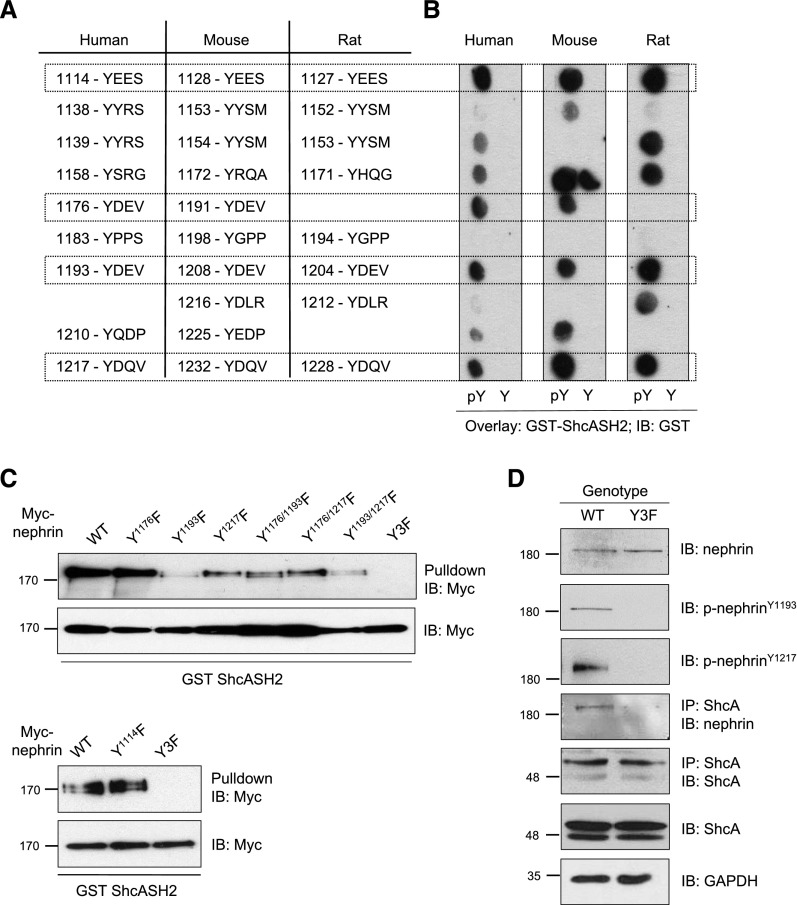
Nephrin possesses a series of intracellular tyrosine residues that are highly conserved between the human, mouse, and rat protein sequences (Figure 2A). To identify which residues might mediate the interaction with ShcA, a spot peptide array harboring phosphorylated (pY) and nonphosphorylated (Y) tyrosine-based sequences from each species was incubated with purified GST-SH2 domain of ShcA. The SH2 domain bound consistently to phosphorylated Y1114, Y1176, Y1193, and Y1217 (human numbering system), and not the nonphosphorylated peptide counterparts (Figure 2B). To validate this binding in the context of full-length nephrin, we performed pulldown assays of the ShcA GST-SH2 domain with nephrin variants bearing single or multiple tyrosine (Y) to phenylalanine (F) mutations at these sites. The Y1193F and Y1217F mutations had the greatest reduction on ShcA binding compared with wild type (WT), whereas Y1114F surprisingly had no effect (Figure 2C). Compound mutation of Y1176F, Y1193F, and Y1217F (referred to as Y3F mutation) completely abolished interaction with ShcA (Figure 2C). Lastly, we confirmed the specificity of ShcA binding to nephrin in vivo in mouse glomerular lysates, and determined that this interaction was disrupted in mice bearing the nephrin-Y3F mutation (Figure 2D). Altogether, these findings demonstrate that the ShcA adaptor interacts via its SH2 domain with multiple tyrosine phosphorylated sites on nephrin.
Figure 2.
ShcA binds multiple phosphotyrosine residues on nephrin in vitro and in vivo. (A) Conserved tyrosine residues in human, mouse, and rat nephrin. Boxes indicate residues demonstrated to be phosphorylated in vitro and/or in vivo. (B) Spot peptide arrays centered around tyrosine (Y) or phosphotyrosine (pY) of all phosphosites in human, mouse, and rat nephrin were incubated with purified GST-ShcA SH2 domain and immunoblotted with GST. Boxes indicate sites with positive anti-GST signal across all species. (C) Lysates from HEK293T cells overexpressing CA-Fyn with WT Myc-nephrin or variants with single, double, or triple mutations at tyrosine sites identified in (B) were incubated with GST-ShcA SH2 domain. Pulldown complexes immunoblotted for Myc indicate reduced binding of the ShcA SH2 domain to nephrin variants lacking Y1193, and a complete loss of binding with the Y3F variant. (D) Immunoblot (IB) for nephrin and ShcA in anti-ShcA immunoprecipitants (IP) from nephrinWT and nephrinY3F mouse glomerular lysates, confirming binding of ShcA and nephrin in vivo, and disruption of this interaction in mice lacking phosphorylated nephrin.
ShcA Enhances Src-Dependent Nephrin Phosphorylation and Promotes Nephrin Endocytosis
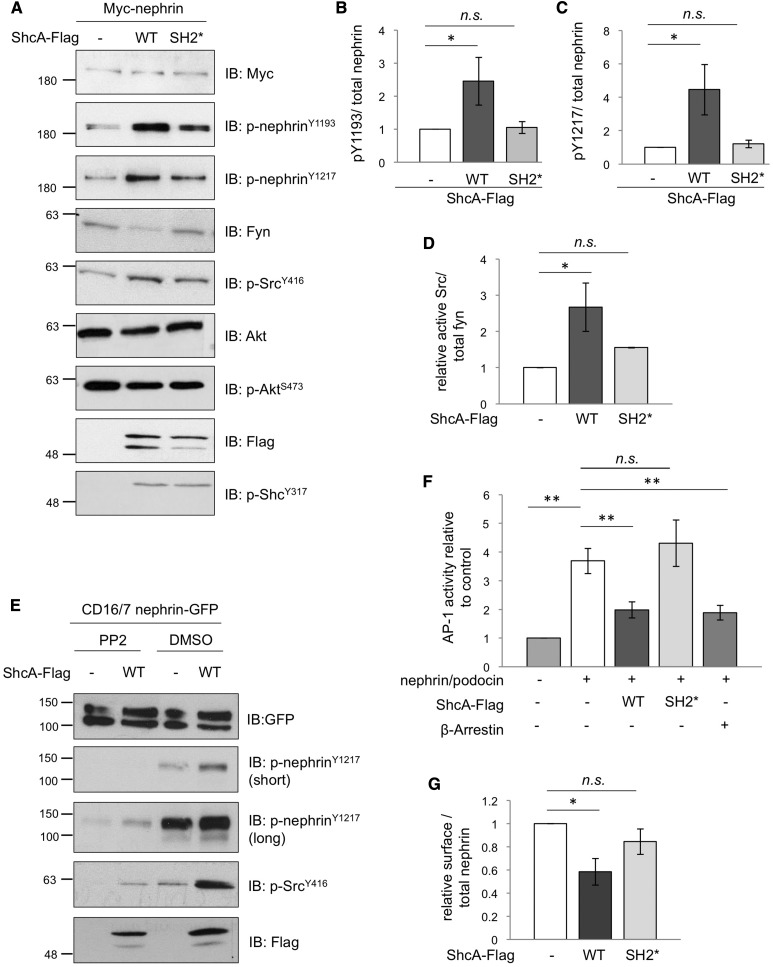
We next sought to determine the functional consequences of the nephrin-ShcA interaction on downstream signaling. Flag-tagged p46/52 ShcA was coexpressed with nephrin in HEK293T cells, and phosphorylation of several cellular targets was monitored. Intriguingly, nephrin tyrosine phosphorylation on Y1193 and Y1217 was enhanced in the presence of WT ShcA, and this effect was not seen with the ShcA SH2* mutation, which cannot bind nephrin (Figure 3, A–C). The increase in nephrin tyrosine phosphorylation was accompanied by an increase in SFK activation (Figure 3, A and D). Of note, however, phosphorylation of ShcA as well as Akt downstream of nephrin remained unchanged (Figure 3A). We confirmed this effect on nephrin hyperphosphorylation within our documented inducible setting of nephrin activation,19 where ShcA was coexpressed with CD16-nephrin-GFP, and cells were stimulated with CD16 to promote nephrin clustering, phosphorylation, and ShcA recruitment (Figure 3E, Supplemental Figure 1B). Inclusion of the SFK inhibitor PP2 but not vehicle (DMSO) alone suppressed the ShcA-mediated increase in nephrin tyrosine phosphorylation (Figure 3E). Nephrin signaling converges on the AP-1 transcription factor,8,20 thus we investigated whether coexpression of ShcA with nephrin could affect nephrin-mediated AP-1 transactivation using a previously established luciferase reporter assay. We found that WT ShcA significantly suppressed AP-1 activity compared with cells without excess ShcA, and this effect was abrogated with the ShcA SH2* mutation (Figure 3F). Further, the effect of WT ShcA was comparable to that of β-arrestin (Figure 3F), which has been shown to attenuate nephrin signaling via induction of nephrin endocytosis.8 Accordingly, biotinylation assays revealed a significant reduction in nephrin cell surface expression with WT ShcA but not the ShcA SH2* mutant (Figure 3G). We conclude that Fyn-mediated hyperphosphorylation of nephrin induced by high levels of ShcA promotes nephrin endocytosis, and thereby suppresses nephrin signaling.
Figure 3.
ShcA promotes tyrosine phosphorylation and endocytosis of nephrin. (A) Lysates from HEK293T cells transiently coexpressing Myc-nephrin with WT p46/52 ShcA-Flag or an SH2* variant that cannot bind nephrin were immunoblotted (IB) as indicated. Changes in phosphorylation in nephrin and Src but not ShcA or Akt are detected. (B–D) Densitometric quantitation of p-nephrinY1193 (n=3), p-nephrinY1217 (n=3), and p-SrcY416 (n=4) from (A), respectively. (E) Lysates from HEK293T cells transiently coexpressing CD16/7-nephrin-GFP with or without ShcA-Flag were stimulated with anti-CD16 antibody in the presence or absence of the SFK inhibitor PP2 or vehicle alone (DMSO), and IB as indicated. PP2 suppresses the ShcA-induced increase in nephrin and Src phosphorylation. (F) HEK293T cells transiently coexpressing ShcA-Flag or β-arrestin with Myc-nephrin, podocin, AP1-firefly luciferase, and renilla luciferase were subject to a dual reporter assay. ShcA WT but not SH2* suppressed the nephrin/podocin-induced activation of AP-1, similar to β-arrestin (n=4). (G) HEK293T cells transiently coexpressing WT or SH2* ShcA-Flag with Myc-nephrin were subject to surface biotinylation. Densitometric comparison of streptavidin-precipitated biotinylated nephrin (surface) to total nephrin (input) indicates a reduction in surface nephrin with WT ShcA but not SH2* (n=3). All values were made relative to biotinylation result of cells transfected with Myc-nephrin alone. *P<0.05; **P<0.01, two-tailed t test.
ShcA Is Upregulated during PAN Nephrosis Coincident with Redistribution of Tyrosine Phosphorylated Nephrin into Early Endosomes
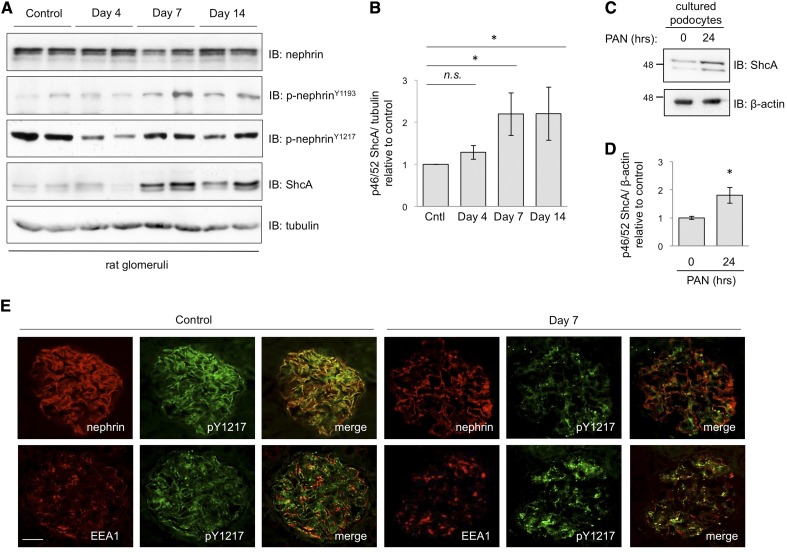
We and others have previously demonstrated that nephrin tyrosine phosphorylation is perturbed in the puromycin aminonucleoside (PAN) nephrosis rodent model of human minimal change disease (MCD),21,22 thus we next examined whether expression of ShcA might also be altered during the injury time course. Glomerular extracts were prepared from PBS-injected control rats and PAN-injected rats at the onset (day 4) and peak (days 7 and 14) of proteinuria (Supplemental Figure 2A). Immunoblotting for ShcA revealed a rise in p46/52 ShcA expression, which reached a maximum on days 7 and 14 (Figure 4, A and B). Upregulation of ShcA was similarly seen in PAN-treated cultured podocytes (Figure 4, C and D). Intriguingly, the increase in ShcA expression in vivo correlated with a modest rise in nephrin phosphorylation on Y1193 and Y1217 at later points during the time course, after the decrease (in Y1217) observed on day 4 (Figure 4A). Immunostaining of glomeruli on day 7 revealed a change in phospho-nephrin distribution compared with the control, appearing focal and punctate during injury, with areas of intense staining showing colocalization with the early endosome marker EEA1 (Figure 4E). Podocin and EEA1 colocalization was also enhanced at this time point (Supplemental Figure 2B).
Figure 4.
PAN nephrosis induces upregulation of ShcA and nephrin endocytosis. (A) Glomerular lysates from PAN-injected rats and controls were immunoblotted as indicated. Nephrin tyrosine phosphorylation on Y1217 decreases on day 4 of the injury time course, and then both Y1193 and Y1217 increase on day 7, coincident with ShcA expression. (B) Densitometric quantitation of p46/52 ShcA levels from (A), showing a significant increase in ShcA levels on days 7 and 14 (n=6). *P<0.05 by two-tailed t test. (C) Lysates from differentiated human podocytes treated with PAN (10 mg/ml) or vehicle control for 24 hours were immunoblotted for ShcA or β-actin loading control. (D) Densitometric quantitation of p46/52 ShcA levels from (C), showing a significant increase in ShcA after PAN exposure (n=4). *P<0.05 by two-tailed t test. (E) Dual immunofluorescence staining for p-nephrinY1217 (green) and nephrin (red) or EEA1 (red) on kidney sections of control and PAN-injected rats on day 7. Phosphorylated nephrin is decreased and relocalized on day 7. Regions of intense p-nephrin signal show colocalization with the early endosome marker EEA1. Scale bar, 20 μm.
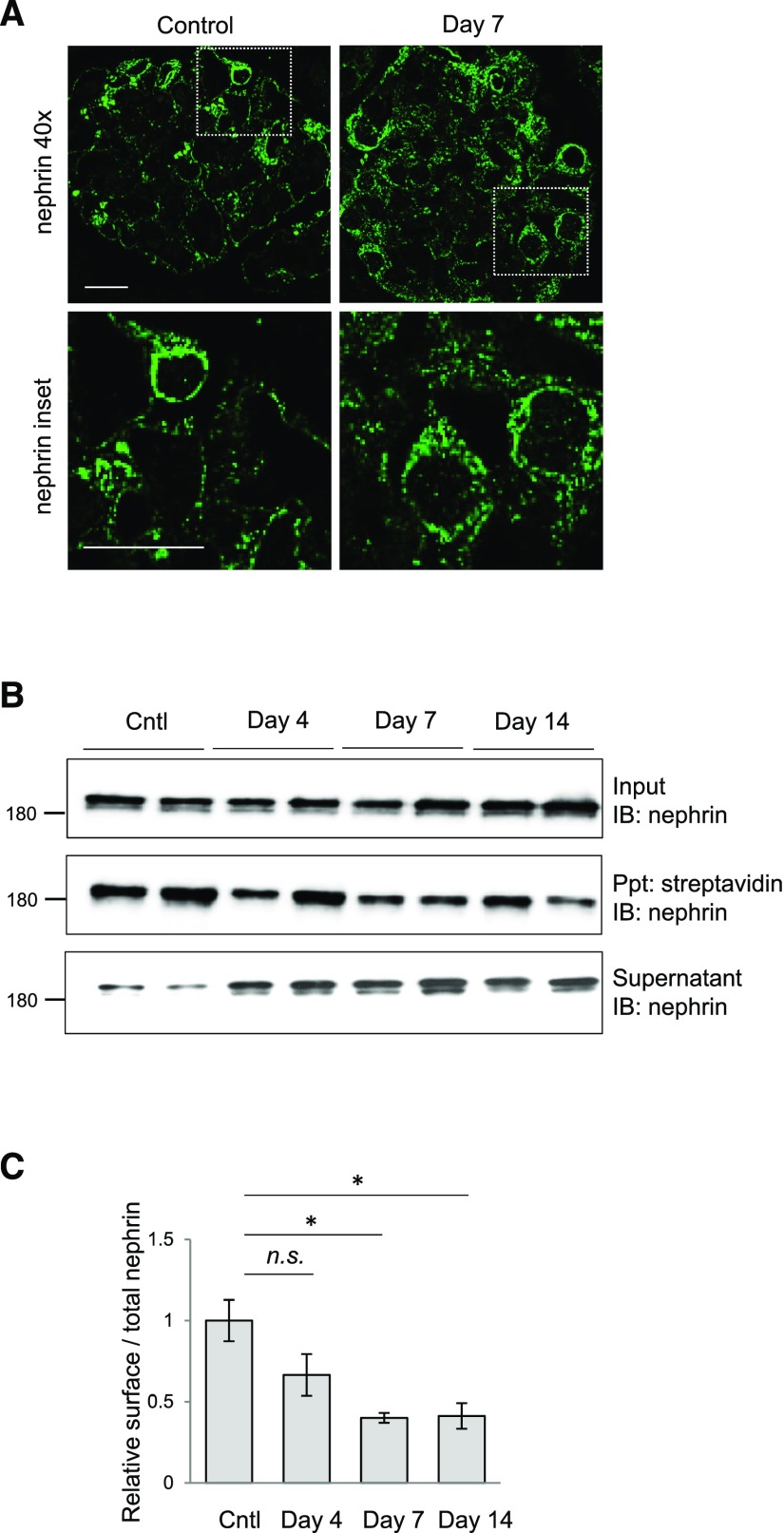
Nephrin Is Mislocalized from the Membrane to the Cytosol in Response to Podocyte Injury
To further explore the nature of nephrin redistribution during PAN injury, we utilized super-resolution structured illumination microscopy to image nephrin-immunostained kidney sections prepared from PAN-injected rats and controls. Continuous linear staining of nephrin can be observed along the capillary wall in control animals, whereas PAN injection leads to a punctate appearance, suggesting cytosolic accumulation of nephrin with injury (Figure 5A). To investigate this in more detail, we performed biotinylation assays on intact glomeruli isolated from PAN-injected rats and controls, followed by immunoblotting of enriched and lysate fractions to monitor changes in nephrin cellular partitioning (Figure 5B). Quantified streptavidin precipitation reveals a significant steady decrease in nephrin surface expression during the injury time course (Figure 5, B and C), and a corresponding increase in cytosolic nephrin can be seen in the remaining supernatant (Figure 5B). This redistribution from the membrane to the cytoplasm correlates with the rise in ShcA expression shown in Figure 4. A transient decline in podocin surface expression was also observed (Supplemental Figure 2, C and D), consistent with previous findings,23 and no surface labeling of the podocyte nuclear/cytosolic WT-1 protein was detected, verifying the specificity of this approach (Supplemental Figure 2E). Together, these results support the hypothesis that upregulation of ShcA triggers nephrin endocytosis and filtration barrier demise.
Figure 5.
PAN injury results in internalization of surface nephrin and podocin. (A) Super-resolution structured illumination microscopy for nephrin (green) on kidney sections of control and PAN-injected rats on day 7 postinjection. Scale bar, 20 μm. (B) Isolated glomeruli from PAN-injected rats and controls were subject to surface biotinylation, followed by lysis, streptavidin agarose precipitation (Ppt), and nephrin immunoblotting (IB). A portion of the initial lysate was saved to represent the total input, and the supernatant postprecipitation contains nonbiotinylated (i.e., cytosolic) proteins. (C) Densitometric quantitation of streptavidin-precipitated biotinylated nephrin (surface) compared with total nephrin (input) from (B), showing a stepwise decrease in surface nephrin levels. All values were made relative to surface nephrin levels in control samples. *P<0.05 by two-tailed t test.
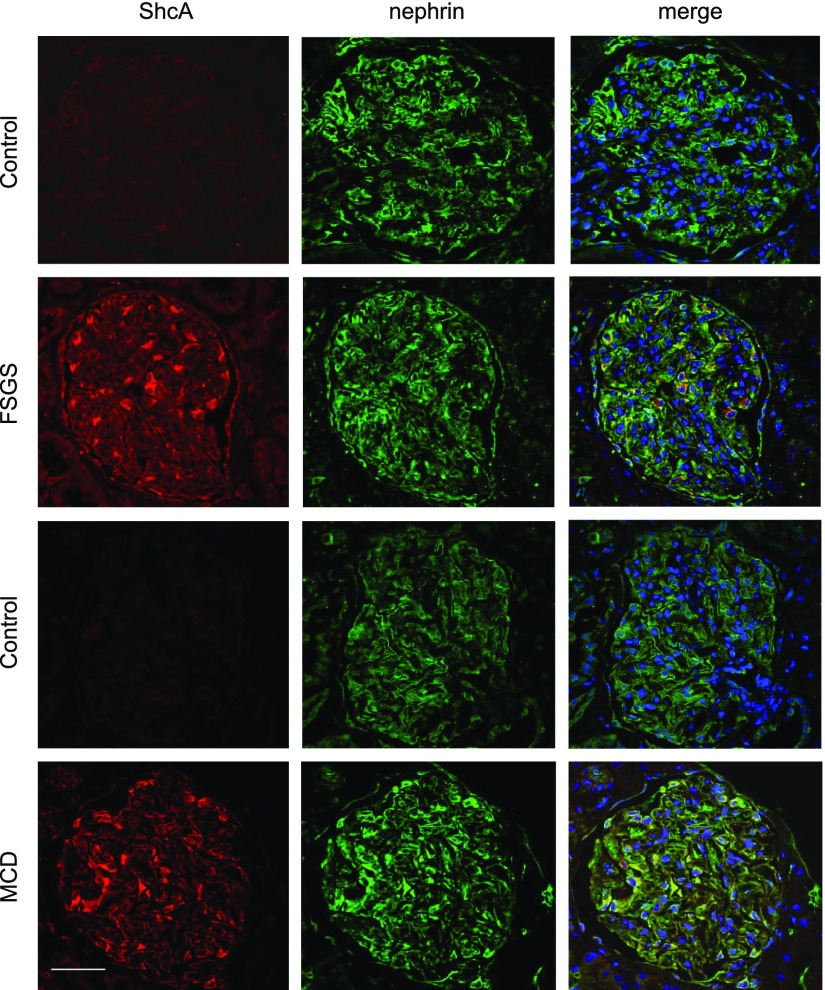
ShcA Is Overexpressed in Human Glomerulopathies Associated with Proteinuria
To determine whether the increase in ShcA expression within the rodent model of podocyte injury might be similarly observed in human glomerulopathies, we performed dual immunostaining of ShcA and nephrin on biopsies from patients with MCD or FSGS, or healthy donor controls (Figure 6). In healthy adult controls, ShcA is expressed at low levels, similar to our findings in mature versus neonatal mice (Figure 1B). However, a striking increase in ShcA was reproducibly observed in both patients with MCD and patients with FSGS, wherein it displayed robust colocalization with nephrin. Analysis of the open-source Nephroseq database (nephroseq.org) similarly shows upregulation of ShcA expression in FSGS and MCD, in addition to IgA nephropathy (IgAN) (Table 1). Of note, ShcA is among the top 2%–4% of overexpressed glomerular genes in several of the data sets analyzed. These findings strongly imply that aberrant ShcA signaling is induced in human proteinuric glomerular diseases.
Figure 6.
ShcA is upregulated in human proteinuric kidney diseases. Representative confocal images after dual staining for ShcA (red) and nephrin (green) on kidney biopsies from control individuals (n=3) or patients with FSGS (n=4) or MCD (n=3). ShcA is prominently upregulated in FSGS and MCD compared with control biopsies, and is colocalized with nephrin. Biopsies shown were immunostained in parallel, and imaged at the same gain and exposure. Scale bar, 20 μm.
Table 1.
Fold change and gene rank expression data for ShcA in control individuals (n=21, n=31, n=9) and patients with FSGS (n=25, n=17), IgAN (n=27, n=25) or MCD (n=14, n=14, n=7)
| Disease Group | Data Set | Probe Identification | Tissue Subset | % Top OE | Gene Rank/Total Genes | Fold Change | P Value |
|---|---|---|---|---|---|---|---|
| FSGS | Ju CKD | 6464 | Glom | 3 | 392/17,379 | 2.286 | <0.001 |
| Ju CKD | TubInt | 9 | 1491/17,379 | 2.147 | 0.01 | ||
| IgAN | Ju CKD | 6464 | Glom | 4 | 546/17,379 | 2.333 | <0.001 |
| Ju CKD | TubInt | 12 | 1979/17,379 | 2.116 | 0.03 | ||
| MCD | Ju CKD | 6464 | Glom | 16 | 2634/17,379 | 2.120 | <0.08 |
| Ju CKD | TubInt | 29 | 4975/17,379 | 2.021 | <0.35 | ||
| Hodgin FSGS | Hs.81972.0.A | Glom | 2 | 268/19,139 | 2.570 | 0.01 | |
| 1_3p_a_at |
Dataset obtained from Nephroseq.org. ShcA overexpression is observed in both glomerular (Glom) and tubulointerstitial (TubInt) regions, but is greater in glomerular regions. OE, overexpressed.
Discussion
Here, we have identified the ShcA adaptor protein as a putative novel regulator of nephrin surface expression and filtration barrier integrity. ShcA associates with tyrosine phosphorylated nephrin, and in the mature glomerulus, we propose that low levels of ShcA signaling contribute to a steady-state pool of phosphorylated nephrin within the slit diaphragm and aid in nephrin recycling. On injury, ShcA expression increases, triggering a pathogenic positive feedback loop of nephrin hyperphosphorylation and enhanced endocytosis. Concurrent loss of nephrin and podocin from the podocyte surface destabilizes the slit diaphragm, leading to proteinuria. ShcA signaling provides a mechanism to control turnover and stabilization of this dynamic cell junction.
Shc proteins possess a unique domain architecture that allows them to simultaneously engage multiple signaling pathways.11 The PTB domain is the most common mediator of these interactions; however, we have determined that recruitment of ShcA to phosphorylated nephrin is via its SH2 domain. The ShcA SH2 domain-binding consensus is pY-ϕ-X-ϕ24, which is in line with sequences surrounding nephrin Y1176, Y1193, and Y1217. Notably, these tyrosines were predicted by the DomPep method18 to bind the ShcA SH2 domain (Supplemental Table 1), and ShcA was previously reported as an interaction partner of recombinant phospho-nephrin.25 The function of the ShcA PTB domain in podocytes remains to be determined, although an intriguing target is IQGAP1,26 which can indirectly bind nephrin and regulate podocyte actin dynamics.27 Alternately, the ShcA PTB domain can bind acidic phospholipids, which may stabilize ShcA localization at the slit diaphragm.28,29The central Grb2-binding phosphotyrosine residues on ShcA appear to be dispensable in nephrin signaling, consistent with reports that Grb2 does not bind nephrin and is not required for podocyte function.30,31 These findings also support a noncanonical role for ShcA in nephrin signaling through additional elements, such as the AP-2 α-adaptin–binding motif. Accordingly, our studies have revealed that ShcA promotes nephrin phosphorylation in a Fyn-mediated fashion, leading to nephrin endocytosis. ShcA may enhance activation of Src or Fyn directly32 or indirectly via its interaction with the Shp2 phosphatase, which in turn activates Fyn.33 Of note, Shp2 overexpression was recently shown to enhance nephrin phosphorylation, and similar to ShcA, Shp2 is overexpressed in a subset of human glomerular diseases.34
Binding of ShcA to multiple phosphorylated tyrosine residues on nephrin appears to overlap with that of Nck,19 and both of these proteins can promote nephrin hyperphosphorylation.35 However, the downstream consequences of nephrin hyperphosphorylation are distinct between ShcA and Nck,35 and these findings implicate dual roles for nephrin tyrosine phosphorylation in healthy versus injured podocytes. ShcA and Nck can both associate with Y1176, Y1193, and Y1217; however, ShcA shows preferential binding to Y1193 and Nck has the highest affinity for Y1217.19 We have recently established that phosphorylation on these tyrosine residues is required throughout life to maintain podocyte function.10 Further, we and others have shown transient changes in their phosphorylation patterns in PAN nephrosis, as well as in the protamine sulfate (PS) model of rapid reversible foot process effacement,10 a podocyte injury model associated with both cytoskeletal remodeling and nephrin endocytosis.4 Here, Y1217 phosphorylation decreases in conjunction with loss of nephrin-Nck binding during PS-induced damage, whereas nephrin phosphorylation and Nck binding are restored during foot process recovery.10 Loss of Y1217 phosphorylation (and thus nephrin-Nck binding) was recently reported to induce β-arrestin binding to nephrin and subsequent endocytosis.36 By contrast, phosphorylation of Y1193 increases during PS-induced damage and declines again during recovery.10,34 It is therefore tempting to speculate that in the prolonged PAN model, the rise in Y1193 (and Y1217) phosphorylation could promote recruitment of ShcA, and trigger a parallel switch-like mechanism to sustain clathrin-dependent removal of nephrin from the cell surface.
Consistent with this notion, we have shown that glomerular ShcA expression is induced in rodents exposed to PAN and humans with FSGS and MCD, coincident with nephrin mislocalization and proteinuria. Such atypical localization of nephrin is similarly seen in early stages of membranous nephropathy.37 We further demonstrate prominent internalization of phosphorylated nephrin, overlapping with EEA1 and podocin in the PAN model, as well as reduced surface expression of nephrin and podocin. Our findings provide compelling evidence to suggest that excessive nephrin endocytosis underlies the disease process. Moreover, the overall decrease in total nephrin phosphorylation reported previously in PAN, MCD, and membranous nephropathy21,22,37 implies that internalized nephrin is subject to dephosphorylation. Intriguingly, nephrin dephosphorylation is accompanied by an increase in tyrosine phosphatase PTP1B expression in PAN.38 PTP1B is anchored in the membrane of the endoplasmic reticulum, and it has been characterized to dephosphorylate the EGF receptor in endosomes and instruct receptor fate inside the cell.39 We postulate a similar mechanism within podocytes, whereby ShcA upregulation leads to nephrin hyperphosphorylation and endocytosis, with a corresponding upregulation of PTP1B leading to dephosphorylation of nephrin in endosomes. This may prolong disruption of the filtration barrier and traffic internalized nephrin into recycling or destruction pathways.40
Surface expression and intracellular trafficking of nephrin and podocin are intimately linked, as we observed throughout the PAN injury time course. Of note, missense mutations in podocin associated with steroid-resistant nephrotic syndrome cause retention of both podocin and nephrin in the cytosol.41,42 Further, consistent with our findings, others have demonstrated mislocalization of podocin from the podocyte cell surface to the cytoplasm in PAN, and this report also identified podocin internalization in patients with IgAN with poor prognosis.23 ShcA is similarly upregulated in IgAN, as well as in other proteinuric kidney diseases including FSGS and MCD, and we propose that aberrant ShcA expression disrupts steady-state recycling of nephrin and podocin, leading to destabilization of the slit diaphragm. Further studies will reveal the potential for ShcA to serve as a biomarker for podocyte injury.
Concise Methods
Plasmids
Constructs encoding human, Myc-tagged, full-length and CD16/7-fused nephrin and mutants have been described previously.19,43 Plasmids for Flag-tagged ShcA variants in pcDNA3 were generated previously,44 and used as templates to construct GST-fused ShcA SH2 and PTB domains in pGEX-4T-1. Plasmids for WT and constitutively active Fyn, podocin, and AP-1 luciferase were described previously.45 Plasmid for Flag-tagged β-arrestin was provided by Dr. Stephen Ferguson (Western University, Ontario, Canada), and plasmid for renilla luciferase was provided by Dr. Ray Lu (University of Guelph, Ontario, Canada).
Antibodies
Commercial antibodies used were as follows: mouse anti-ShcA (sc-967; Santa Cruz Biotechnology), guinea pig anti-nephrin (20R-NP002; Fitzgerald Inc.), mouse anti-phosphotyrosine 4G10 (16–101; Upstate Biotechnology Inc.), rabbit anti-Fyn (sc-16; Santa Cruz Biotechnology), rabbit anti-podocin (P0372; Sigma-Aldrich), rabbit anti-ShcA (Ab-24787; Abcam), rabbit anti-pY416 (active) Src (2101; Cell Signaling), mouse anti-CD16 (sc-19620; Santa Cruz Biotechnology), mouse anti-GST (sc-138; Santa Cruz Biotechnology), mouse anti-EEA1 (610456; BD Biosciences), mouse anti-Flag M2 (F3165; Sigma-Aldrich), mouse anti-Myc 9B11 (2276; Cell Signaling), rabbit anti-GFP (290; Abcam), mouse anti-GAPDH (clone 1D4, G041; Applied Biologic Materials Inc.), mouse anti-tubulin (T5168; Sigma-Aldrich), and mouse anti–β-actin (A1978; Sigma-Aldrich). Rabbit anti-nephrin was described previously.45 Phospho-specific anti-nephrin antibodies were generated and validated previously.21 Secondary horseradish peroxidase-conjugated goat anti-mouse (170–6516) and goat anti-rabbit (170–6515) secondary antibodies (BioRad) were used for immunoblot detection. Secondary antibodies for immunofluorescence (all from Invitrogen) were goat anti-rabbit Alexa Fluor 488 (A11008) and 594 (A11037), goat anti-mouse Alexa Fluor 488 (A11001) and 594 (A11005), and goat anti-guinea pig Alexa Fluor 594 (A11076).
Cell Culture
HEK293T cells were grown in DMEM supplemented with 10% FBS (both from HyClone), 200 units/ml penicillin, and 200 μg/ml streptomycin (Invitrogen), and maintained at 37°C with 5% CO2. Transient transfection was performed using polyethyleneimine for 48 hours. For CD16 experiments, transfected cells were serum-starved for 12 hours before stimulation with 1 μg/ml anti-CD16 antibody for 15 minutes at 37°C. For inhibition of SFKs, cells were treated with 10 μM PP2 (Sigma-Aldrich) for 4 hours before CD16 stimulation. Podocytes were grown in RPMI with 10% FBS, at 33°C and 5% CO2, and thermoswitched to 37°C with 2% FBS for 7–14 days to induce differentiation.
Cell Lysis, Immunoprecipitation, and GST Pulldown
Cells were lysed in Phospholipase C (PLC) lysis buffer (50 mM Hepes [pH 7.5], 150 mM NaCl, 10% glycerol, 1% Triton X-100, 15 mM MgCl2, 1 mM EGTA, 10 mM NaPPi, and 100 mM NaF) supplemented with fresh protease and phosphatase inhibitors (1 mM PMSF, 1 mM sodium orthovanadate, 10 μg/ml aprotinin, and 10 μg/ml leupeptin) by vortexing and sonicating on ice. Glomeruli from mice and rats were prepared using differential sieving as described previously,10,45 and processed as above. Protein concentrations were determined using a BCA protein assay (Pierce). Immunoprecipitation was performed overnight at 4°C with rotation, followed by washing three times with PLC buffer. Protein complexes were eluted from the beads in 2 × SDS loading buffer by boiling at 100°C for 2 minutes. For immunoprecipitation of endogenous ShcA from glomerular lysates, protein A beads (Invitrogen) were first conjugated to ShcA antibodies (Abcam) to avoid interference from IgG heavy chain during immunoblotting, as described elsewhere.46 A total of 100 µl conjugated α-ShcA beads were incubated with 2 mg glomerular lysate for 5 hours at 4°C. For GST pulldowns, 5 μg GST-fused bait proteins purified from Escherichia coli were incubated with lysate for 3 hours at 4°C with rotation.
Immunoblotting and Spot Peptide Arrays
Proteins from total lysates (10–100 µg) and immunoprecipitants were resolved on 8%–12% SDS-PAGE gels and transferred to PVDF membrane (Millipore). Spot peptide arrays were constructed as described previously,19 and incubated with 1 μM eluted GST fusion proteins before Far Western analysis. Membranes were blocked in TBST containing 5% nonfat milk powder or BSA, and incubated overnight at 4°C with primary antibody. After washing, membranes were incubated with horseradish peroxidase-conjugated secondary antibody diluted at 1:10,000 in TBST for 1 hour at room temperature. Signals were detected using ECL Western blotting substrate (Pierce) or Luminata Crescendo (Millipore), and membranes were exposed to film (Pierce) or imaged using a ChemiDoc XRS+ (BioRad). Densitometry was performed using ImageLab v2.0 analysis software (BioRad).
AP-1 Luciferase Assay
HEK293T cells were transfected with 1 μg AP-1 firefly luciferase, 80 ng renilla luciferase, and additional vectors as indicated, to a total DNA amount of 2 μg per 35-mm dish. Serum-starved cells were harvested on ice in 1 ml PBS, spun at 4000 rpm at 4°C for 2 minutes, lysed in 100 μl of 1× passive lysis buffer (Promega), and spun again at 13,000 rpm at 4°C for 2 minutes to remove insoluble material. Luciferase activity was determined using a commercial dual luciferase assay system (Promega) on a POLARstar Omega microplate reader (BMG Labtech) and normalized to renilla activity to correct for transfection efficiency. Protein expression was confirmed by immunoblot.
Biotinylation
Isolated glomeruli or adherent cells were incubated with 5 ml of 1 mg/ml EZ-Link Biotin-X-NHS dissolved in borate buffer (10 mM boric acid, 150 mM NaCl [pH 8.0]) for 45 minutes under gentle agitation at 4°C. Coupling was terminated by washing two times (2 minutes each) with 15 mM glycine in PBS at 4°C. After a final wash in PBS, glomeruli or cells were lysed in PLC+ buffer, and sonicated for 10 seconds. Lysates were then subjected to streptavidin agarose precipitation overnight at 4°C or resuspended in two times SDS loading buffer to act as the input loading control. After overnight precipitation, beads were washed three times successively in PLC+, and spun at 3000 × g for 1 minute between washes before finally being resuspended and boiled in 50 μl two times SDS loading buffer. Surface nephrin or podocin in each sample was determined by dividing the density of band observed in immunoblotting of the precipitated sample by the density of band observed in the input sample. The ratio of this value in test samples to the value obtained in control samples was calculated and represents the amount of surface nephrin in indicated test groups relative to control samples.
PAN Nephrosis
For in vivo experiments, male Sprague–Dawley rats (250–300 g) were obtained from Charles River (St. Constant, Quebec, Canada). One injection of PAN (50 mg/kg body wt) was used to induce podocyte injury, as described previously.47 Rats were placed in metabolic cages for 24 hours and allowed to urinate freely. Urinary protein was quantified by the modified Bradford assay.48 For induction in cell culture, human podocyte cells differentiated for 7 days were treated with 10 mg/ml PAN for 24 hours before further processing.
Indirect Immunofluorescence of Kidney Tissue Sections
Kidneys were either snap frozen in cryomatrix (Thermo Fisher) or fixed and embedded in paraffin before sectioning and immunostaining. Cryosections (6 µm) were dried at room temperature for 10 minutes, then fixed and permeabilized in acetone at −20°C for 10 minutes before immunostaining. For paraffin embedding, kidneys were first fixed in fresh 4% PFA (#28908; Thermo Fisher) for 30 minutes at room temperature and washed three times for 30 minutes in PBS, then stored in 70% ethanol until embedding. Paraffin sections (4 µm) were deparaffinized and rehydrated using standard protocols. After antigen retrieval with heated 10 mM trisodium citrate dihydrate buffer, immunostaining was performed as with frozen kidneys. All subsequent steps were carried out at room temperature. When using the phospho-nephrin antibodies, phosSTOP tablets (Roche) were added to all solutions. Slides were blocked for 1 hour in 10% goat serum, then incubated with primary antibodies for 1 hour (guinea pig anti-nephrin [1:100], rabbit anti-nephrin [1:50], rabbit anti–pY1217-nephrin [1:50], rabbit anti-podocin [1:100], mouse anti-ShcA [1:50], or mouse anti-EEA1 [1:100]). After three washes in PBS, slides were incubated with secondary antibodies (1:400) for 1 hour. Slides were washed, then mounted using Prolong Gold antifade mounting medium (Invitrogen). Epifluorescence images were obtained from cryosections using Volocity software version 5.3.2 (Improvision) on a DMIRE2 microscope (Leica) using a 40× objective. Stacks were captured at 0.2 µm z-intervals, then deconvolved using an iterative restoration function (95% confidence with 15 iterations) in Volocity. Super-resolution structured illumination microscopy images were gathered from rat PAN paraffin-embedded sections using an Elyra LSM 880 microscope (Zeiss) and processed with ZEN software. Human sections were paraffin embedded and processed as described above, and imaged using ZEISS LSM780 confocal microscope.
Nephroseq Analysis
Human ShcA expression data were downloaded from Ju et al.49,50 and Hodgin et al.51 data sets using the Nephroseq data mining platform (www.nephroseq.org, 2017; University of Michigan, Ann Arbor, MI).
Statistical Analyses
Values are presented as mean±SEM. Differences between two groups were analyzed via t test. P<0.05 was considered statistically significant.
Study Approval
Animal studies were carried out in accordance with Canadian Council on Animal Care protocols. Formalin-fixed, paraffin-embedded samples from freshly curated human biopsy material were obtained from the McGill University Health Centre Kidney Disease Biorepository. Informed consent was obtained to use kidney biopsy samples left unused after clinically indicated biopsies. Patient characteristics are provided in Supplemental Table 2. This study was approved by the Research Ethics Board of the McGill University Health Centre.
Disclosures
None.
Supplementary Material
Acknowledgments
We gratefully acknowledge Dr. Laura New, Dr. Melanie Wills, Steve Hawley, Lauren Hampton, and Peihua Lu (all University of Guelph) for providing reagents, advice, and technical support. This work was supported by grants from Canadian Institutes of Health Research (CIHR) and the Kidney Foundation of Canada (to N.J. and T.T.), and CIHR/Sick Kids Foundation (to N.J.). N.J. holds a Tier 2 Canada Research Chair in Eukaryotic Cellular Signalling and was the recipient of a New Investigator Award from the Kidney Research Scientist Core Education and National Training Program. C.E.M. is supported by an Natural Sciences and Engineering Research Council of Canada Alexander Graham Bell Canada Graduate Doctoral Scholarship and by an Ontario Graduate Scholarship. K.A.P. was supported by a CIHR Canada Graduate Masters Scholarship. M.T. is supported by a University of Guelph College of Biological Sciences Studentship.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2017030285/-/DCSupplemental.
References
- 1.Scott RP, Quaggin SE: Review series: The cell biology of renal filtration. J Cell Biol 209: 199–210, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tryggvason K, Patrakka J, Wartiovaara J: Hereditary proteinuria syndromes and mechanisms of proteinuria. N Engl J Med 354: 1387–1401, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Swiatecka-Urban A: Membrane trafficking in podocyte health and disease. Pediatr Nephrol 28: 1723–1737, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qin XS, Tsukaguchi H, Shono A, Yamamoto A, Kurihara H, Doi T: Phosphorylation of nephrin triggers its internalization by raft-mediated endocytosis. J Am Soc Nephrol 20: 2534–2545, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soda K, Balkin DM, Ferguson SM, Paradise S, Milosevic I, Giovedi S, Volpicelli-Daley L, Tian X, Wu Y, Ma H, Son SH, Zheng R, Moeckel G, Cremona O, Holzman LB, De Camilli P, Ishibe S: Role of dynamin, synaptojanin, and endophilin in podocyte foot processes. J Clin Invest 122: 4401–4411, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tossidou I, Teng B, Drobot L, Meyer-Schwesinger C, Worthmann K, Haller H, Schiffer M: CIN85/RukL is a novel binding partner of nephrin and podocin and mediates slit diaphragm turnover in podocytes. J Biol Chem 285: 25285–25295, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jeon J, Leibiger I, Moede T, Walter B, Faul C, Maiguel D, Villarreal R, Guzman J, Berggren PO, Mundel P, Ricordi C, Merscher-Gomez S, Fornoni A: Dynamin-mediated Nephrin phosphorylation regulates glucose-stimulated insulin release in pancreatic beta cells. J Biol Chem 287: 28932–28942, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quack I, Rump LC, Gerke P, Walther I, Vinke T, Vonend O, Grunwald T, Sellin L: beta-Arrestin2 mediates nephrin endocytosis and impairs slit diaphragm integrity. Proc Natl Acad Sci U S A 103: 14110–14115, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.New LA, Martin CE, Jones N: Advances in slit diaphragm signaling. Curr Opin Nephrol Hypertens 23: 420–430, 2014 [DOI] [PubMed] [Google Scholar]
- 10.New LA, Martin CE, Scott RP, Platt MJ, Keyvani Chahi A, Stringer CD, Lu P, Samborska B, Eremina V, Takano T, Simpson JA, Quaggin SE, Jones N: Nephrin tyrosine phosphorylation is required to stabilize and restore podocyte foot process architecture. J Am Soc Nephrol 27: 2422–2435, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wills MK, Jones N: Teaching an old dogma new tricks: Twenty years of Shc adaptor signalling. Biochem J 447: 1–16, 2012 [DOI] [PubMed] [Google Scholar]
- 12.Pelicci G, Lanfrancone L, Grignani F, McGlade J, Cavallo F, Forni G, Nicoletti I, Grignani F, Pawson T, Pelicci PG: A novel transforming protein (SHC) with an SH2 domain is implicated in mitogenic signal transduction. Cell 70: 93–104, 1992 [DOI] [PubMed] [Google Scholar]
- 13.Sakaguchi K, Okabayashi Y, Kasuga M: Shc mediates ligand-induced internalization of epidermal growth factor receptors. Biochem Biophys Res Commun 282: 1154–1160, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Migliaccio E, Giorgio M, Mele S, Pelicci G, Reboldi P, Pandolfi PP, Lanfrancone L, Pelicci PG: The p66shc adaptor protein controls oxidative stress response and life span in mammals. Nature 402: 309–313, 1999 [DOI] [PubMed] [Google Scholar]
- 15.Lai KM, Pawson T: The ShcA phosphotyrosine docking protein sensitizes cardiovascular signaling in the mouse embryo. Genes Dev 14: 1132–1145, 2000 [PMC free article] [PubMed] [Google Scholar]
- 16.Arany I, Faisal A, Nagamine Y, Safirstein RL: p66shc inhibits pro-survival epidermal growth factor receptor/ERK signaling during severe oxidative stress in mouse renal proximal tubule cells. J Biol Chem 283: 6110–6117, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Menini S, Amadio L, Oddi G, Ricci C, Pesce C, Pugliese F, Giorgio M, Migliaccio E, Pelicci P, Iacobini C, Pugliese G: Deletion of p66Shc longevity gene protects against experimental diabetic glomerulopathy by preventing diabetes-induced oxidative stress. Diabetes 55: 1642–1650, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Li L, Zhao B, Du J, Zhang K, Ling CX, Li SS: DomPep--a general method for predicting modular domain-mediated protein-protein interactions. PLoS One 6: e25528, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones N, Blasutig IM, Eremina V, Ruston JM, Bladt F, Li H, Huang H, Larose L, Li SS, Takano T, Quaggin SE, Pawson T: Nck adaptor proteins link nephrin to the actin cytoskeleton of kidney podocytes. Nature 440: 818–823, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Huber TB, Kottgen M, Schilling B, Walz G, Benzing T: Interaction with podocin facilitates nephrin signaling. J Biol Chem 276: 41543–41546, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Jones N, New LA, Fortino MA, Eremina V, Ruston J, Blasutig IM, Aoudjit L, Zou Y, Liu X, Yu GL, Takano T, Quaggin SE, Pawson T: Nck proteins maintain the adult glomerular filtration barrier. J Am Soc Nephrol 20: 1533–1543, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Uchida K, Suzuki K, Iwamoto M, Kawachi H, Ohno M, Horita S, Nitta K: Decreased tyrosine phosphorylation of nephrin in rat and human nephrosis. Kidney Int 73: 926–932, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Fukuda H, Hidaka T, Takagi-Akiba M, Ichimura K, Oliva Trejo JA, Sasaki Y, Wang J, Sakai T, Asanuma K, Tomino Y: Podocin is translocated to cytoplasm in puromycin aminonucleoside nephrosis rats and in poor-prognosis patients with IgA nephropathy. Cell Tissue Res 360: 391–400, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Songyang Z, Shoelson SE, McGlade J, Olivier P, Pawson T, Bustelo XR, Barbacid M, Sabe H, Hanafusa H, Yi T: Specific motifs recognized by the SH2 domains of Csk, 3BP2, fps/fes, GRB-2, HCP, SHC, Syk, and Vav. Mol Cell Biol 14: 2777–2785, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Verma R, Kovari I, Soofi A, Nihalani D, Patrie K, Holzman LB: Nephrin ectodomain engagement results in Src kinase activation, nephrin phosphorylation, Nck recruitment, and actin polymerization. J Clin Invest 116: 1346–1359, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith MJ, Hardy WR, Li GY, Goudreault M, Hersch S, Metalnikov P, Starostine A, Pawson T, Ikura M: The PTB domain of ShcA couples receptor activation to the cytoskeletal regulator IQGAP1. EMBO J 29: 884–896, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rigothier C, Auguste P, Welsh GI, Lepreux S, Deminière C, Mathieson PW, Saleem MA, Ripoche J, Combe C: IQGAP1 interacts with components of the slit diaphragm complex in podocytes and is involved in podocyte migration and permeability in vitro. PLoS One 7: e37695, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou MM, Ravichandran KS, Olejniczak EF, Petros AM, Meadows RP, Sattler M, Harlan JE, Wade WS, Burakoff SJ, Fesik SW: Structure and ligand recognition of the phosphotyrosine binding domain of Shc. Nature 378: 584–592, 1995 [DOI] [PubMed] [Google Scholar]
- 29.Ravichandran KS, Zhou MM, Pratt JC, Harlan JE, Walk SF, Fesik SW, Burakoff SJ: Evidence for a requirement for both phospholipid and phosphotyrosine binding via the Shc phosphotyrosine-binding domain in vivo. Mol Cell Biol 17: 5540–5549, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bisson N, Ruston J, Jeansson M, Vanderlaan R, Hardy WR, Du J, Hussein SM, Coward RJ, Quaggin SE, Pawson T: The adaptor protein Grb2 is not essential for the establishment of the glomerular filtration barrier. PLoS One 7: e50996, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garg P, Verma R, Nihalani D, Johnstone DB, Holzman LB: Neph1 cooperates with nephrin to transduce a signal that induces actin polymerization. Mol Cell Biol 27: 8698–8712, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sato K, Nagao T, Kakumoto M, Kimoto M, Otsuki T, Iwasaki T, Tokmakov AA, Owada K, Fukami Y: Adaptor protein Shc is an isoform-specific direct activator of the tyrosine kinase c-Src. J Biol Chem 277: 29568–29576, 2002 [DOI] [PubMed] [Google Scholar]
- 33.Fang X, Lang Y, Wang Y, Mo W, Wei H, Xie J, Yu M: Shp2 activates Fyn and Ras to regulate RBL-2H3 mast cell activation following FcεRI aggregation. PLoS One 7: e40566, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Verma R, Venkatareddy M, Kalinowski A, Patel SR, Salant DJ, Garg P: Shp2 associates with and enhances nephrin tyrosine phosphorylation and is necessary for foot process spreading in mouse models of podocyte injury. Mol Cell Biol 36: 596–614, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.New LA, Keyvani Chahi A, Jones N: Direct regulation of nephrin tyrosine phosphorylation by Nck adaptor proteins. J Biol Chem 288: 1500–1510, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Königshausen E, Zierhut UM, Ruetze M, Potthoff SA, Stegbauer J, Woznowski M, Quack I, Rump LC, Sellin L: Angiotensin II increases glomerular permeability by β-arrestin mediated nephrin endocytosis. Sci Rep 6: 39513, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ohashi T, Uchida K, Asamiya Y, Tsuruta Y, Ohno M, Horita S, Nitta K: Phosphorylation status of nephrin in human membranous nephropathy. Clin Exp Nephrol 14: 51–55, 2010 [DOI] [PubMed] [Google Scholar]
- 38.Aoudjit L, Jiang R, Lee TH, New LA, Jones N, Takano T: Podocyte protein, nephrin, is a substrate of protein tyrosine phosphatase 1B. J Signal Transduct 2011: 1–10, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baumdick M, Brüggemann Y, Schmick M, Xouri G, Sabet O, Davis L, Chin JW, Bastiaens PI: EGF-dependent re-routing of vesicular recycling switches spontaneous phosphorylation suppression to EGFR signaling. eLife 4: e12223, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shono A, Tsukaguchi H, Kitamura A, Hiramoto R, Qin XS, Doi T, Iijima K: Predisposition to relapsing nephrotic syndrome by a nephrin mutation that interferes with assembly of functioning microdomains. Hum Mol Genet 18: 2943–2956, 2009 [DOI] [PubMed] [Google Scholar]
- 41.Nishibori Y, Liu L, Hosoyamada M, Endou H, Kudo A, Takenaka H, Higashihara E, Bessho F, Takahashi S, Kershaw D, Ruotsalainen V, Tryggvason K, Khoshnoodi J, Yan K: Disease-causing missense mutations in NPHS2 gene alter normal nephrin trafficking to the plasma membrane. Kidney Int 66: 1755–1765, 2004 [DOI] [PubMed] [Google Scholar]
- 42.Philippe A, Weber S, Esquivel EL, Houbron C, Hamard G, Ratelade J, Kriz W, Schaefer F, Gubler MC, Antignac C: A missense mutation in podocin leads to early and severe renal disease in mice. Kidney Int 73: 1038–1047, 2008 [DOI] [PubMed] [Google Scholar]
- 43.Blasutig IM, New LA, Thanabalasuriar A, Dayarathna TK, Goudreault M, Quaggin SE, Li SS, Gruenheid S, Jones N, Pawson T: Phosphorylated YDXV motifs and Nck SH2/SH3 adaptors act cooperatively to induce actin reorganization. Mol Cell Biol 28: 2035–2046, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hardy WR, Li L, Wang Z, Sedy J, Fawcett J, Frank E, Kucera J, Pawson T: Combinatorial ShcA docking interactions support diversity in tissue morphogenesis. Science 317: 251–256, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li H, Lemay S, Aoudjit L, Kawachi H, Takano T: SRC-family kinase Fyn phosphorylates the cytoplasmic domain of nephrin and modulates its interaction with podocin. J Am Soc Nephrol 15: 3006–3015, 2004 [DOI] [PubMed] [Google Scholar]
- 46.Qoronfleh MW, Ren L, Emery D, Perr M, Kaboord B: Use of immunomatrix methods to improve protein-protein interaction detection. J Biomed Biotechnol 2003: 291–298, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhu J, Sun N, Aoudjit L, Li H, Kawachi H, Lemay S, Takano T: Nephrin mediates actin reorganization via phosphoinositide 3-kinase in podocytes. Kidney Int 73: 556–566, 2008 [DOI] [PubMed] [Google Scholar]
- 48.Zor T, Selinger Z: Linearization of the Bradford protein assay increases its sensitivity: Theoretical and experimental studies. Anal Biochem 236: 302–308, 1996 [DOI] [PubMed] [Google Scholar]
- 49.Ju W, Nair V, Smith S, Zhu L, Shedden K, Song PXK, Mariani LH, Eichinger FH, Berthier CC, Randolph A, Lai JY, Zhou Y, Hawkins JJ, Bitzer M, Sampson MG, Thier M, Solier C, Duran-Pacheco GC, Duchateau-Nguyen G, Essioux L, Schott B, Formentini I, Magnone MC, Bobadilla M, Cohen CD, Bagnasco SM, Barisoni L, Lv J, Zhang H, Wang HY, Brosius FC, Gadegbeku CA, Kretzler M, Ercb CPN; ERCB, C-PROBE, NEPTUNE, and PKU-IgAN Consortium : Tissue transcriptome-driven identification of epidermal growth factor as a chronic kidney disease biomarker. Sci Transl Med 7: 316ra193, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ju W, Greene CS, Eichinger F, Nair V, Hodgin JB, Bitzer M, Lee YS, Zhu Q, Kehata M, Li M, Jiang S, Rastaldi MP, Cohen CD, Troyanskaya OG, Kretzler M: Defining cell-type specificity at the transcriptional level in human disease. Genome Res 23: 1862–1873, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hodgin JB, Borczuk AC, Nasr SH, Markowitz GS, Nair V, Martini S, Eichinger F, Vining C, Berthier CC, Kretzler M, D’Agati VD: A molecular profile of focal segmental glomerulosclerosis from formalin-fixed, paraffin-embedded tissue. Am J Pathol 177: 1674–1686, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.