Abstract
Studies of lipids in CKD, including ESRD, have been limited to measures of conventional lipid profiles. We aimed to systematically identify 17 different lipid classes and associate the abundance thereof with alterations in acylcarnitines, a metric of β-oxidation, across stages of CKD. From the Clinical Phenotyping Resource and Biobank Core (CPROBE) cohort of 1235 adults, we selected a panel of 214 participants: 36 with stage 1 or 2 CKD, 99 with stage 3 CKD, 61 with stage 4 CKD, and 18 with stage 5 CKD. Among participants, 110 were men (51.4%), 64 were black (29.9%), and 150 were white (70.1%), and the mean (SD) age was 60 (16) years old. We measured plasma lipids and acylcarnitines using liquid chromatography-mass spectrometry. Overall, we identified 330 different lipids across 17 different classes. Compared with earlier stages, stage 5 CKD associated with a higher abundance of saturated C16–C20 free fatty acids (FFAs) and long polyunsaturated complex lipids. Long-chain–to–intermediate-chain acylcarnitine ratio, a marker of efficiency of β-oxidation, exhibited a graded decrease from stage 2 to 5 CKD (P<0.001). Additionally, multiple linear regression revealed that the long-chain–to–intermediate-chain acylcarnitine ratio inversely associated with polyunsaturated long complex lipid subclasses and the C16–C20 FFAs but directly associated with short complex lipids with fewer double bonds. We conclude that increased abundance of saturated C16–C20 FFAs coupled with impaired β-oxidation of FFAs and inverse partitioning into complex lipids may be mechanisms underpinning lipid metabolism changes that typify advancing CKD.
Keywords: lipids, chronic kidney disease, Acylcarnitines, Free fatty acids, complex lipids
Dyslipidemia in CKD is characterized by increased small dense LDL cholesterol, VLDL cholesterol, and triglycerides, with decreased HDL cholesterol1,2 in a prevalence ranging from 30% to over 50% at various stages of CKD.3–5 Lipids are the most abundant molecules in plasma with substantial complexity and diversity, constituting integral components of cell structure and contributing to the pathophysiology of a number of diseases through alterations in metabolism and function.6 Derangement in metabolism across stages of CKD is highly linked to cardiovascular morbidity and mortality.7,8 Despite the diversity of human plasma lipidome, studies in CKD have been limited to only the measurement of total cholesterol, triglycerides, and lipoproteins among various stages.9–11 However, the technological advances in mass spectrometry–based lipidomic platforms not only provide the opportunity for identification and measurement of a large array of human lipids12–16 and their links with obesity,14 diabetes,15 hypertension,13 atherosclerosis,16 and HDL alterations12 but also, disclose the relation of acyl-chain length and saturation status with risk of disease, such as diabetes.15 These studies revealed important insights into disease mechanisms. However, these approaches are rarely undertaken in a CKD population.17 Fatty acid metabolism consists of β-oxidation, a catabolic process that generates energy after translocation of fatty acids from cytosolic compartment into mitochondria, and anabolic processes that create biologically important complex lipids in cytosol.18 We hypothesize that there is a dynamic change in abundance of plasma lipids within each CKD stage across the different stages of CKD and that the lipid alterations are partially explained by impairment of β-oxidation at more severe stages of CKD. In this study, we aimed to (1) systematically identify and quantify the human plasma lipidome for 17 different classes of lipids from stage 2 to stage 5 CKD, (2) explore the alteration in abundance of free fatty acids (FFAs) and complex lipids by carbon number and number of double bonds at various stages of CKD, (3) compare long-to-intermediate acylcarnitine ratio as a surrogate for the efficiency of β-oxidation by stages of CKD, and (4) explore the relationship between plasma complex lipids with acylcarnitine levels.
Results
Baseline
Overall, 214 patients were included in this study, with a mean age of 60 years old (SD=16). There were 110 men (51.4%), 64 blacks (29.9%), and 150 whites (70.1%). The etiology of CKD was diabetic nephropathy in 47 patients (22.0%), arteriolar nephrosclerosis in 44 (20.6%), glomerular disease in 73 (34.1%), tubulointerstitial disease in seven (3.3%), and other etiologies in 43 patients (20.1%). Table 1 reveals the baseline characteristics of the patients. Accordingly, there were 36, 99, 61, and 18 patients with stages 2–5 CKD, respectively. Patients in stages 1 and 2 CKD were significantly younger compared with those in the other stages (P<0.001) and had a lower body mass index compared with those in stage 3 CKD (P<0.01). There was a significant, linearly increasing trend of hypertension (P=0.04), diabetes (P=0.001), and urine protein-to-creatinine ratio (P<0.001) when comparing stage 2 CKD with stage 5 CKD. There were no significant statistical differences in mean or distribution for any other variables by CKD stage.
Table 1.
Distribution of patients’ characteristics by stage of CKD at the time of sampling
| Baseline Variables | GFR≥60 (Stages 1 and 2) | GFR 30–59 (Stage 3) | GFR 15–29 (Stage 4) | GFR<15 (Stage 5) |
|---|---|---|---|---|
| N | 36 | 99 | 61 | 18 |
| Age, yr | 46±15a | 62±14 | 62±14 | 66±18 |
| Men, % | 17 (47.2) | 49 (49.5) | 36 (59.0) | 8 (44.4) |
| White race, % | 27 (75.0) | 68 (68.7) | 43 (70.5) | 12 (66.7) |
| SBP, mmHg | 132±21 | 133±21 | 139±21 | 146±26 |
| DBP, mmHg | 77±10 | 72±10 | 76±13 | 78±13 |
| Height, m | 1.7±0.1 | 1.7±0.1 | 1.7±0.2 | 1.7±0.1 |
| Weight, kg | 83±19 | 93±23 | 91±20 | 84±16 |
| BMI, kg/m2 | 28.1±5.5b | 32.5±7.3 | 30.9±6.9 | 29.6±4.2 |
| Comorbidities, % | ||||
| Hypertensionc | 27 (75.0) | 79 (79.8) | 53 (86.9) | 17 (94.0) |
| Diabetesd | 5 (13.9) | 41 (41.4) | 35 (57.4) | 8 (44.4) |
| CAD | 8 (22.2) | 42 (42.4) | 26 (42.6) | 5 (27.8) |
| Heart failure | 1 (2.8) | 16 (16.2) | 12 (19.7) | 2 (11.1) |
| PVD | 1 (2.8) | 14 (14.1) | 12 (19.7) | 2 (11.1) |
| Nephrotic syndrome | 1 (2.8) | 11 (11.1) | 4 (6.6) | 1 (5.6) |
| Medications, % | ||||
| Statins | 14 (38.9) | 54 (54.5) | 36 (59.0) | 10 (55.6) |
| Fibrates | 0 (0) | 10 (10.1) | 8 (13.1) | 0 (0) |
| Niacin | 1 (2.8) | 5 (5.1) | 1 (1.6) | 0 (0) |
| Steroids | 9 (25.0) | 14 (14.1) | 5 (8.2) | 2 (11.1) |
| Mycophenolate mofetil | 6 (16.7) | 8 (8.1) | 3 (4.9) | 1 (5.6) |
| Albumin, g/dl | 4.1±0.5 | 4.1±0.5 | 4.0±0.4 | 3.9±0.3 |
| Cholesterol, mg/dl | 170±38 | 173±49 | 170±65 | 146±38 |
| LDL, mg/dl | 84±36 | 88±40 | 88±45 | 71±21 |
| HDL, mg/dl | 41±16 | 39±17 | 37±19 | 42±22 |
| Triglycerides, mg/dl | 146±88 | 171±120 | 169±104 | 130±111 |
| UPCRd,e | 0.5 [0.2–1.6] | 0.4 [0.1–1.7] | 0.8 [0.2–3.0] | 1.4 [0.3–3.3] |
| WBC, 1000/μl | 6.8±2.5 | 7.4±3.1 | 7.1±3.2 | 6.5±1.8 |
| Creatinine, mg/dl | 1.0±0.2 | 1.7±0.3 | 2.8±0.6 | 4.7±2.0 |
| eGFR, ml/min | 82±15 | 41±8 | 23±4 | 14±10 |
Continuous variables are shown as mean±SD, and categorical variables are shown as frequency (percentage). Hypertension was on the basis of prior diagnosis. SBP, systolic BP; DBP, diastolic BP; BMI, body mass index; CAD, coronary artery disease; PVD, peripheral vascular disease; UPCR, urine protein-to-creatinine ratio; WBC, white blood cell.
Compared with all other groups: ANOVA P<0.001.
Compared with stage 3 CKD: ANOVA P<0.01.
Linear trend P<0.05.
Linear trend P=0.001.
Values are median [interquartile range].
Measured Lipids
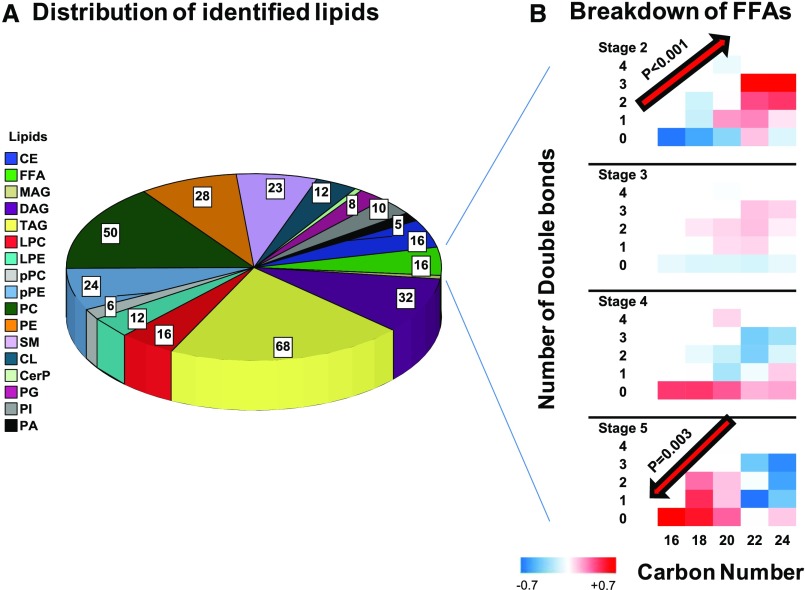
In total, 527 lipids, including different adducts of the same lipid features, were detected in positive and negative ion modes (Supplemental Table 1). After deleting duplicates and combining different adducts of the same feature, 330 unique lipids from 17 distinct classes entered the final analysis. These classes included triacylglycerols (TAGs), diacylglycerols (DAGs), monoacylglycerols, phosphatidylcholines, phosphatidylethanolamines (PEs), plasmenyl-phosphatidylcholines, plasmenyl-phosphatidylethanolamines, lysophosphatidylcholines, lysophosphatidylethanolamines, FFA, cholesteryl-esters, cardiolipins, phosphatidic acid, phosphatidylinositol (PI), phosphatidylglycerol, sphingomyelin (SM), and ceramide-phosphate (Figure 1A). Accordingly, glycerolipids accounted for 30.9% of identified lipids (n=102), phospholipids accounted for 41.2% (n=136), sphingolipids accounted for 7.0% (n=23), cholesterol esters (CEs) accounted for 4.8% (n=16), FFAs accounted for 4.8%, (n=16), and the rest of the identified lipids accounted 11.3% (n=37).
Figure 1.
Various lipids identified by the LC/MS-based lipidomics platform. (A) Distribution of 17 classes of identified lipids in the cohort. (B) Alteration of relative mean values of FFAs by acyl chain and number of double bonds from stage 2 to 5 CKD. CerP, ceramide-phosphates; CL, cardiolipin; LPC, lysophosphatidylcholine; LPE, lysophosphatidylethanolamine; MAG, monoacylglycerol; PC, phosphatidylcholine; PG, phosphatidylglycerol; PI, phosphatidylinositol; pPC, plasmenyl-phosphatidylcholine; pPE, plasmenyl-phosphatidylethanolamine.
Distribution of FFAs by CKD Stage
High double-bond C22 and C24 FFAs have a relatively higher abundance compared with C16–C20 FFAs in stage 2 CKD (P trend <0.001), a trend that is reversed in stage 5 CKD (Figure 1B), so that the low double-bond C16–C20 FFAs have the highest abundance compared with C22 and C24 FFAs in stage 5 CKD (P trend =0.003). Accordingly, palmitate (C16:0) had the highest relative concentration at stage 5 CKD but the lowest relative concentration in stage 2 CKD compared with other FFAs at the corresponding CKD stages.
Distribution of Complex Lipids by CKD Stage
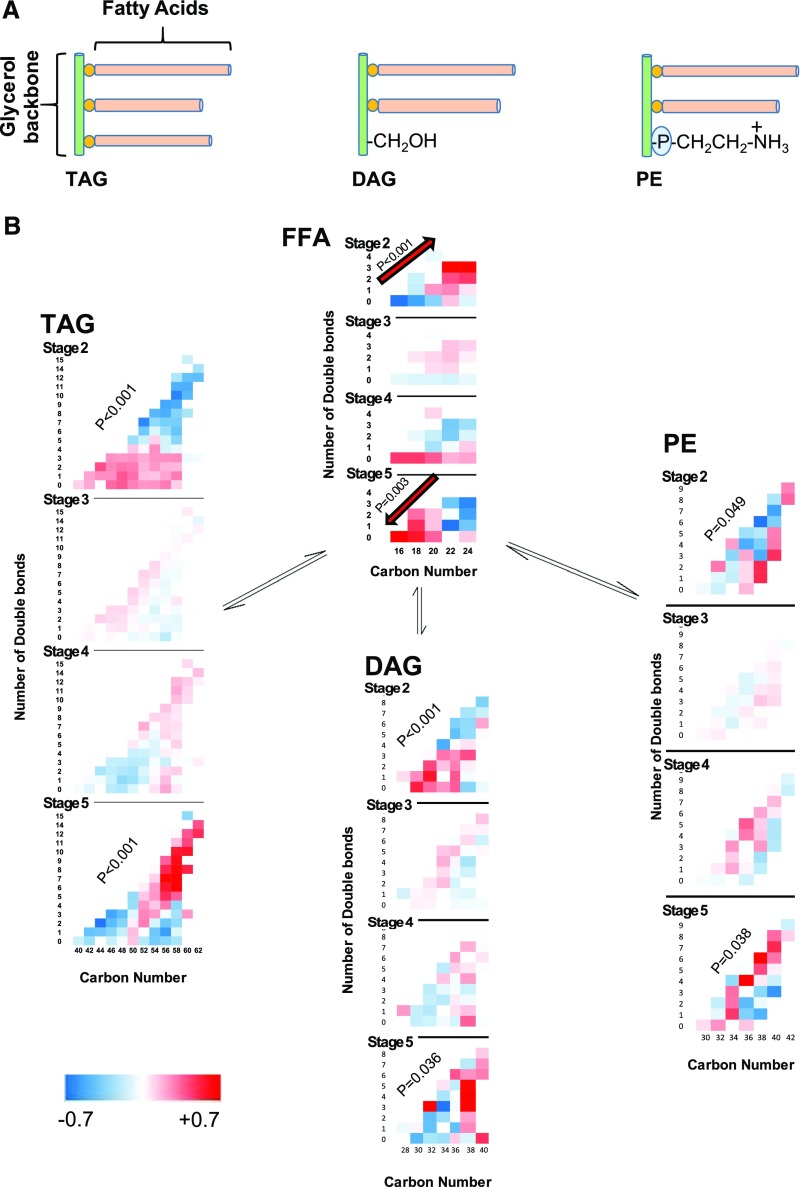
TAGs consist of three fatty acid acyl chains, but DAGs and PEs consist of only two (Figure 2A). Therefore, the numbers of carbons and double bonds in their acyl chains are the sum of the corresponding numbers in their constituent fatty acids. The composition of complex lipids by their carbon number and number of double bonds, including TAGs, DAGs, and PEs, within each stage as well as across different stages of CKD is depicted in Figure 2B. Accordingly, the high double-bond longer complex lipids have a relatively lower abundance compared with shorter complex lipids in stage 2 CKD (P trend ≤0.05), a trend that is reversed in stage 5 CKD, so that the low double-bond shorter complex lipids have the lowest abundance compared with longer complex lipids in stage 5 CKD (P trend ≤0.04). These trends are inverse to alterations of FFAs by CKD stage, namely that low double-bond shorter FFAs were more abundant as CKD stage progressed. A similar increase in the abundance of polyunsaturated longer complex lipids in stage 5 CKD is also observed in phosphatidylcholine, plasmenyl-phosphatidylcholine, PE, lysophosphatidylethanolamine, ceramide-phosphate, SM, CE, cardiolipin, phosphatidic acid, and PI (Supplemental Figure 1).
Figure 2.
Reciprocal significant alteration in abundance of FFAs and complex lipids by worsening stages of CKD: (A) TAGs carry three fatty acid acyl chains, whereas DAGs and PEs carry only two fatty acids. (B) Comparison of standardized mean values of TAGs, DAGs, PEs, and FFAs by carbon number and number of double bonds in stages 2–5 CKD. The significance of the interaction term of change in abundance of lipid by carbon number and number of double bonds from stage 2–5 CKD is shown by P values in the diagonal of each lipid class using mixed linear model.
Alteration of Acylcarnitines by CKD Stage
Unbound FFAs enter cells through transport proteins, after which the CoA-modified FFA uses the carnitine shuttle to traverse the mitochondrial membrane to participate in β-oxidation, a process in which fatty acids are broken down two carbons at a time for energy production (Figure 3A). Impaired β-oxidation manifests as diminished long (C16–C20)-to-intermediate (C5–C14) acylcarnitine ratio, because impaired oxidative efficiency leads to accumulation of intermediate-chain acylcarnitines.19 We found a lower relative concentration of intermediate acylcarnitines compared with long acylcarnitines in stage 2 CKD (Figure 3B) (P trend =0.01). At stage 5 CKD, this distribution was reversed, so that the relative concentration of intermediate acylcarnitines was higher than that of long-chain acylcarnitines (P trend =0.04). As such, the ratio of long to intermediate plasma acylcarnitines was significantly lower in stages 3–5 CKD compared with stages 1 and 2 CKD, a sign of impaired β-oxidation with advancing CKD stage (Figure 3C) (ANOVA; comparing all stages with stage 2 CKD: P<0.001). The ratio in stage 3 CKD was not significantly different from that in stage 4 or 5 CKD. In the literature, the ratio of acylcarnitine to l-carnitine is used as a prognostic biomarker.20 We also found that alterations of l-carnitine were comparable with intermediate acylcarnitine, and therefore, the trend of long-to-intermediate acylcarnitine ratio was similar to long-to–l-carnitine ratio by stage of CKD (Supplemental Figure 2).
Figure 3.
Impaired β-oxidation by advancing stages of CKD. (A) Illustration of the relationship between FFAs and acylcarnitines during mitochondrial β-oxidation pathway. (B) Comparing standardized mean values of plasma acylcarnitines by carbon number and number of double bonds by CKD stages. The P values refer to significance of alteration of plasma acylcarnitine level by carbon number and numbers of double-bond interaction terms at various stages of CKD. (C) Comparing the different length acylcarnitine ratios by stage of CKD. Values are shown as mean and SEMs.
Principal Component Analyses
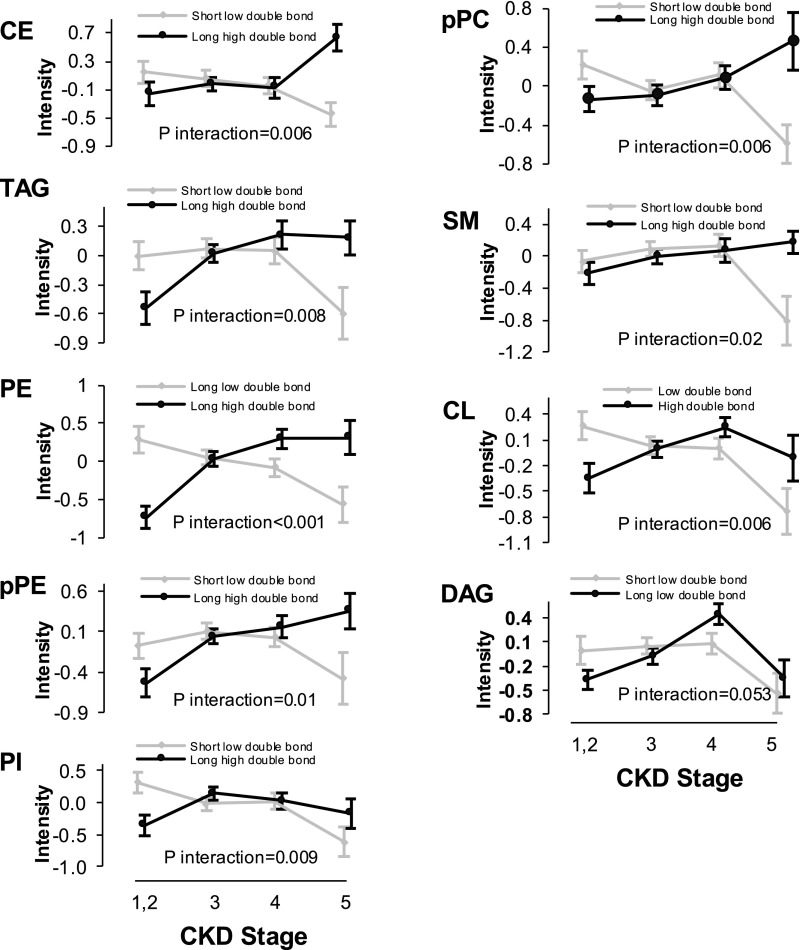
We applied principal component analysis (PCA) in major lipid classes with high intraclass diversity to reduce the lipid number in certain subgroups (Supplemental Table 2). Application of PCA, an unsupervised procedure, constructed at least two distinct subgroups per class that were characterized by the number of double bonds and the length of acyl chains. As shown in Figure 4, we observed a decreasing trend in the level of low double-bond shorter complex lipids from stage 2 to stage 5 CKD, a trend that was reciprocated by an increase in high double-bond longer complex lipids from stage 2 to stage 5 CKD. The interaction terms for all of the complex lipids are statistically significant (P≤0.02), except for the borderline significance for DAG class (P=0.05).
Figure 4.
Increased abundance of polyunsaturated complex lipids with higher number of double bonds by advancing CKD. Estimated subclasses of complex lipids are defined on the basis of application of PCA data reduction from the list of the entire complex lipids within each lipid class. The significance of the interaction term was estimated using mixed linear model. CL, cardiolipin; pPC, plasmenyl-phosphatidylcholine; pPE, plasmenyl-phosphatidylethanolamine.
Correlation of FFAs with Complex Lipid Subclasses Derived from PCA
There seems to be an inverse correlation between C16 and C20 FFAs and shorter low double-bond complex lipids (Figure 2B). Table 2 confirms the presence of an inverse correlation between C16 and C20 FFAs with the following complex lipids: low double-bond shorter TAGs, longer low double-bond TAGs, low double-bond shorter DAGs, and low double-bond intermediate PEs. It also shows a direct correlation between C22–C24 FFAs and longer low double-bond TAGs and longer PEs. Supplemental Table 3 presents similar associations between different length FFAs and other complex lipids.
Table 2.
FFA subtypes independently associated with complex lipid subclasses of TAGs, DAGs, and PEs using multiple linear regression models adjusted for age, sex, and race
| Complex Lipid Subclasses (Dependent Variable) | Independent FFA Associates | B | SEM | r | P Value |
|---|---|---|---|---|---|
| Short, low double-bond TAG | Length ≤ C-20 | −0.25 | 0.089 | −0.19 | <0.01 |
| Long, low double-bond TAG | Length ≤ C-20 | −0.754 | 0.128 | −0.58 | <0.001 |
| Long, low double-bond TAG | Length ≤ C-20 | 0.660 | 0.135 | 0.48 | <0.001 |
| Short, low double-bond DAG | Length ≤ C-20 | −0.221 | 0.089 | −0.17 | 0.01 |
| Intermediate-length, low double-bond PE | Length ≤ C-20 | −0.219 | 0.089 | −0.17 | 0.02 |
| Long PE | Length ≤ C-20 | 0.458 | 0.087 | 0.33 | <0.001 |
Short FFA is defined as ≤20 carbons, and long is defined as >20 carbons. Complex lipid subclasses as well as FFA cutoffs for acyl-chain length are defined by PCA. Acyl chain >20 carbons is indicated as >C-20, and acyl chain ≤20 is indicated as ≤C-20.
Independent Correlates of Complex Lipid Subclasses with Long-to-Intermediate Acylcarnitine Ratio
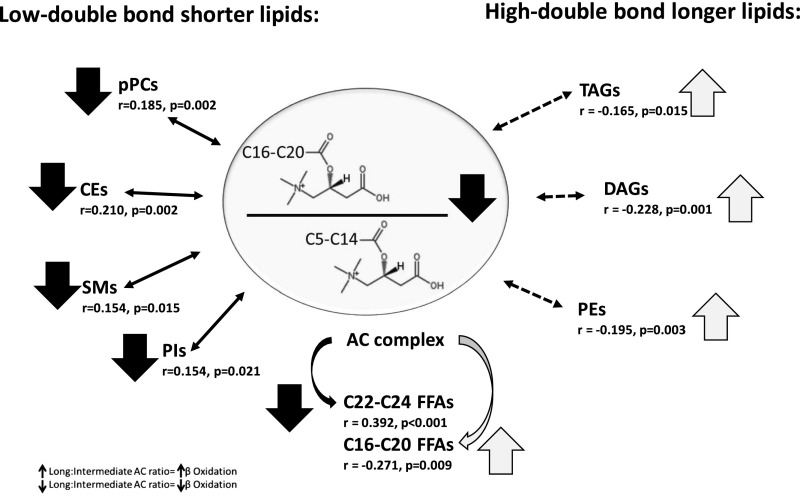
We found a significant inverse association between the long-to-intermediate chain acylcarnitine ratio and the C16–C20 FFA as well as the high double-bond longer-chain complex lipids within TAG, DAG, and PE (Figure 5). We also observed a significant direct independent correlation between the long-to-intermediate chain acylcarnitine ratio and the low double-bond shorter-chain complex lipids within plasmenyl-phosphatidylethanolamine, SM, CE, and PI. There were no significant correlations between the long-to-intermediate chain acylcarnitine ratio and the other lipid subclasses.
Figure 5.
Impaired β-oxidation is associated with higher abundance of polyunsaturated complex lipids with higher number of double bonds in TAG, DAG, and PE class; but is associated with a lower abundance of shorter complex lipids with lower number of double bonds in pPC, CE, SM, and PI class. Independent associates of long (C16–C20)-to-intermediate (C5–C14) acylcarnitine ratio among the high double-bond long and low double-bond short lipids. The subgroups of long and short complex lipids were defined according to unsupervised PCA. Multiple linear regression models showing the partial correlation coefficients (r) adjusted for age, sex, race, short-chain FFAs, and long-chain FFAs were used for the estimations. Decrease and increase in abundance are shown with the down and up arrows, respectively. AC, acylcarnitine; pPC, plasmenyl-phosphatidylcholine.
Discussion
This study highlights significant alterations in the human plasma lipidome with advancing CKD. Key alterations include higher abundance of saturated C16–C20 FFAs coupled with a significantly lower long-to-intermediate acylcarnitine ratio (a marker of impaired β-oxidation) and a significant increase in longer polyunsaturated complex lipids in stage 5 CKD compared with stage 2 CKD. Impaired β-oxidation of fatty acids causes intracellular accumulation of toxic lipids, creating a vicious cycle that leads to further cellular dysfunction and death, which contributes to CKD, irrespective of the underlying etiology.9,21
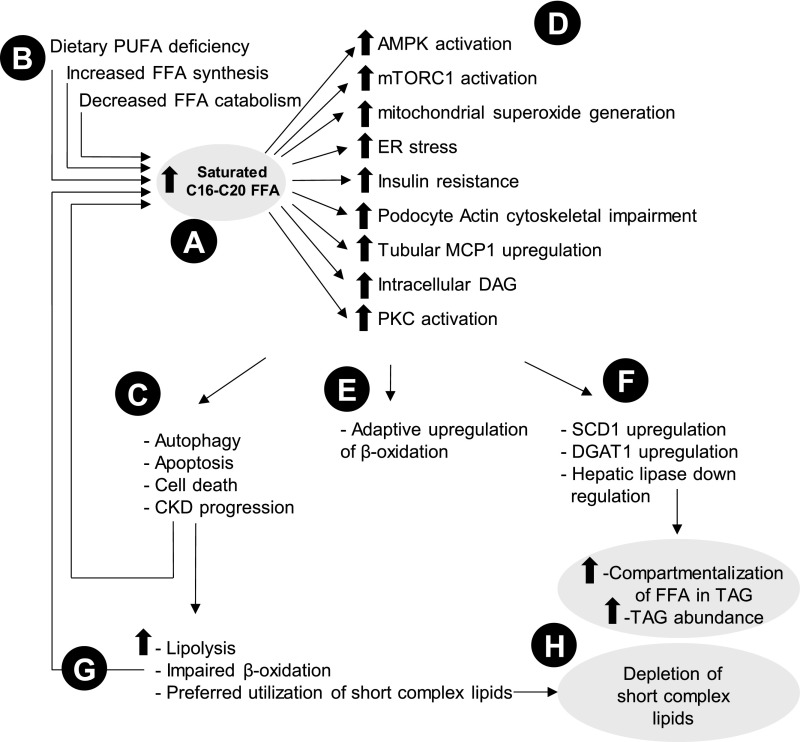
A number of mechanisms are proposed to explain the detrimental contribution of FFAs to lipotoxicity (Figure 6).22–30 Palmitate (C16:0) promotes podocyte injury by activation of AMP-activated protein kinase and mammalian target of rapamycin complex-1 signaling pathways, induces mitochondrial superoxide generation and endoplasmic reticulum stress, promotes autophagy, and eventually, triggers apoptosis and cell death, effects that are inhibited by palmitoleate (C16:1), oleate (C18:1), and eicosapentaenoate (C20:5).22–24,27,29,30 Palmitate also induces insulin resistance and actin cytoskeletal impairment in podocytes.25,26 It has also been shown to upregulate the renal tubular monocyte chemoattractant protein-1, leading to intracellular activation of DAG and subsequent activation of protein kinase C, altogether contributing to renal tubular injury.28 Several factors may contribute to the higher abundance of saturated FFAs in advanced CKD. These include low intake of a polyunsaturated fatty acid diet in advanced CKD,31,32 increased synthesis of FFAs evidenced by upregulation of carbohydrate-responsive element binding protein, and their decreased catabolism evidenced by downregulation of peroxisome proliferator–activated receptor-α noticed in a 5/6th nephrectomy model.33 In line with our findings, Chen et al.34 compared 180 patients at stages 4 and 5 CKD with 120 healthy individuals and similarly found a higher abundance of saturated fatty acids in sera of patients with advanced CKD compared with the control group, supporting the overall change in FFA architecture with advancing CKD.
Figure 6.
Summary of proposed mechanisms of lipid alterations observed in CKD. Advanced CKD is characterized by (A) increased saturated FFA partially due to (B) poor dietary intake of the polyunsaturated fatty acid (PUFA) diet and increased synthesis and decreased catabolism of FFAs. Increased FFA leads to a flurry of adverse outcomes, including (C) autophagy, apoptosis, cell death, and eventually, CKD progression via (D) intermediary mechanisms, including endoplasmic reticulum (ER) stress. (E) Early in the course of disease, adaptive upregulation of β-oxidation tries to ameliorate the adverse effects by metabolizing FFAs faster. (F) Furthermore, mechanisms for utilization and compartmentalization of FFAs into complex lipids activate, which include stearoyl-CoA desaturase 1 (SCD1) and diacylglycerol O-acyltransferase 1 (DGAT1) upregulation, leading to TAG synthesis. (G) With further progression of CKD, impairment of β-oxidation prevails, which leads to further intracellular accumulation of FFAs, creating a vicious cycle for further injury. (H) Short complex lipids consisting of shorter FFAs with less dependence on mitochondrial function become preferred lipids for catabolism, leading to their low abundance in plasma. AMPK, adenosine monophosphate–activated protein kinase; MCP1, monocyte chemotactic protein 1; mTORC1, mammalian target of rapamycin complex 1; PKC, protein kinase C.
In this study, we also found a graded decrease in long-to-intermediate acylcarnitine ratio by stage of CKD. In a recent work, Overmyer et al.19 showed that the long-to-intermediate acylcarnitine ratio may serve as a marker of increased cellular import of fatty acids and their efficient β-oxidation, because impaired β-oxidation was associated with accumulation of intermediate-chain acylcarnitines. In our study, the decline in the long-to-intermediate acylcarnitine ratio was present as early as stage 3 CKD and therefore, unlikely to reflect decreased clearance of long-chain acylcarnitines, which if true, would be more pronounced in stages 4 and 5 CKD. In rodent models of diabetic kidney disease, diabetic tubular mitochondria oxidize fatty acid substrates at a higher rate than the controls,35,36 although it was unclear whether the increased β-oxidation was an early compensatory mechanism. However, enhancement of fatty acid β-oxidation is shown to be protective against palmitate-induced podocyte endoplasmic reticulum stress and cell death.37 In a genome-wide transcriptomic study of normal and fibrotic tubules in humans, it was shown that presence of tubulointerstitial fibrosis was associated with lower expression of enzymes involved in fatty acid oxidation.38 Collectively, these studies suggest that increased fatty acid oxidation early in the course of disease may serve as an adaptive phenomenon in response to increased saturated fatty acids, but with progression of CKD, impairment of β-oxidation prevails21,36,37 and creates a vicious cycle, in which impaired oxidation leads to further intracellular accumulation of fatty acids and thus, further mitochondrial damage. The higher likelihood of mitochondrial DNA damage in patients on dialysis,39–41 low carnitine levels, and elevated plasma malonate, methylmalonate, and malate42 suggests that mitochondrial dysfunction is likely a common prevalent metabolic abnormality in ESRD.
Cellular accumulation of fatty acids after impaired β-oxidation also promotes synthesis and cytosolic accumulation of long-chain lipids.43–45 This mechanism might be responsible for the inverse association between long-to-intermediate acylcarnitine ratio and the higher abundance of the polyunsaturated long complex lipids in advanced stages of CKD as observed in this study. This process may be facilitated in part by upregulation of stearoyl-CoA desaturases 1 and 246 as well as acyl-CoA:DAG-acyltransferase 1,46 with the net effect of utilization and compartmentalization of FFAs into TAG. Therefore, the higher abundance of unsaturated complex lipids at advanced CKD may reflect an adaptive mechanism aimed at detoxification of toxic saturated fatty acids.46 Another potential mechanism for the increased abundance of TAG in CKD is downregulation of hepatic lipase, which is responsible for TAG catabolism.47–49 However, we observed lower abundance of complex lipids with lower carbon number and lower number of double bonds in stage 5 CKD, findings that are in agreement with the report of Rhee et al.42 on comparison of 44 patients on hemodialysis with ten healthy controls. One explanation could be that the short complex lipids may be composed of relatively shorter FFAs (<12 carbons), which are less dependent on carnitine for mitochondrial transport.50 Therefore, they become a preferred substrate for catabolic processes42 and along with accelerated lipolysis from uremia,51–53 contribute to the lower abundance of short saturated complex lipids in stage 5 CKD.
This study has several strengths. Our lipidomic platform provided a robust dataset with excellent reproducibility, reasonably low coefficients of variations for the measured lipids, and no drift in measurements over time, with extremely low batch to batch variability (Supplemental Figures 3 and 4). In addition, multiple levels of quality control measures, including reference samples, pooled samples, authentic lipid standards, and continuous internal calibration, were used to achieve high levels of confidence in the results. Our unique analytic approaches incorporated acyl chain and number of double bonds, which not only validated a previous similar observation in TAGs42 but also, revealed similar alterations with other complex lipids, such as phospholipids and SMs, suggestive of similar underlying mechanisms. Incorporation of acylcarnitine measurements into the analyses provided a mechanistic link to complex lipid alterations associating advanced CKD with impaired β-oxidation. All patients at stage 5 CKD were sampled before ever being exposed to dialysis treatment, thereby eliminating the effect of dialysis on these plasma markers. Additionally, multivariable analysis corrected for observed background covariates. This study also has limitations. Unlike the quantification of acylcarnitines, which was on the basis of a targeted metabolomic platform, our lipidomics approach was on the basis of untargeted data collection, and therefore, the abundances of the lipids are given as relative, normalized amounts. However, we believe that similarity of our results with recent reports34,42 further argues for the validity of our platform. Theoretically, any complex lipid may include several isomers at various acyl chains and saturation status, and therefore, a variety of several different isomers can be present for any given lipid feature. In this study, our platform did not distinguish various isomers from each other, and rather, we reported them combined as one lipid feature. Separation of the theoretical isomers would require application of Sequential Windowed Acquisition of All Theoretical Ion Mass Spectra, an approach that, in our assessment, might not have added further to our conclusions. The observational nature of this study does not allow for inferring causal effect, and the cross-sectional design does not allow for capturing the longitudinal outcomes. Samples size is relatively small, although large enough to allow for capturing the variabilities and the associations. Obtaining the samples from biobank, resulting in an additional freeze and thaw cycle, might have contributed to loss of unstable compounds, despite standardized research protocol. Results reflect the Clinical Phenotyping Resource and Biobank Core (CPROBE) samples and may not be extrapolated to other CKD cohorts or patients with type 1 diabetes. We also did not have a separate dietary assessment at the time of sample collection. However, the pattern of lipid alterations was universally observed across almost all lipid classes, including those that are not directly influenced by diet, making it less likely that the observed effects were substantially influenced by diet alone.
A number of clinical implications are worth noting. Cardiorespiratory fitness is defined by the ability of the circulatory, respiratory, and muscular systems to supply oxygen to skeletal muscle during sustained physical activity.54 A high cardiorespiratory fitness is protective against several metabolic diseases and associated with lower all-cause mortality.55–57 It is widely believed that cardiorespiratory fitness as a manifest of mitochondrial function is determined mostly by genetic factors.58 However, the notion of impaired β-oxidation by worsening stages of CKD is suggestive of acquired processes of mitochondrial dysfunction and highlights a pathway amenable to interventions. Moreover, recent randomized, controlled clinical trials failed to show any beneficial effect of statins on major cardiovascular outcomes in patients on dialysis.59–61 The proposed mechanisms of lipid alterations question whether statins cover the entire spectrum of lipid abnormalities and if 3-hydroxy-3-methyl-glutaryl-CoA reductase is a relevant target in stage 5 CKD. Last but not least, the reversal of the detrimental effects of saturated fatty acids on podocytes by dietary interventions31,32 underscores the importance of dietary modification in advanced CKD. The high level of saturated FFAs, impaired β-oxidation, and increased lipolysis and the higher level of polyunsaturated complex lipids in advanced CKD highlight the potential mechanisms driving lipid alterations in advanced CKD, and their modification may be key to improved clinical outcomes. Figure 6 summarizes the proposed pathophysiology underpinning the lipid alterations in CKD.
In conclusion, increased abundance of saturated C16–C20 FFAs coupled with impaired β-oxidation are noted in advanced stages of CKD, which may contribute to further accumulation of saturated fatty acids, promotion of cell dysfunction and cell death, and further progression of CKD. Higher abundance of polyunsaturated long complex lipids may be a manifestation of an adaptive altered metabolism aimed at shifting toxic saturated fatty acids toward synthesis of less toxic complex lipids. However, depletion of short complex lipids may signify their preferred utilization in catabolism, because they are less dependent on pristine mitochondrial function in the absence of efficient β-oxidation.
Concise Methods
Patients
This is a cross-sectional study. Established under the auspices of the George O’Brien Kidney Center at the University of Michigan, the CPROBE is a multicenter cohort of 1235 adult individuals with CKD. High-quality biologic specimens and clinical data have been collected to conduct translational research. The target population was the first 771 patients enrolled in the study between January of 2009 and July of 2012 whose data and samples were available to us by the time of this study. After our sample selection, another 464 adult patients (up to 1235) were continuously recruited in the parent cohort through an ongoing recruitment effort. On the basis of our prior work,62 at least 30 patients within each stage of CKD provide a sufficiently large sample size to assess the distribution of lipids within each stage, suggesting a total sample size of 120 from stage 2 to stage 5 CKD. We finally selected 214 individuals (28%) to provide additional power and decrease the margin of error in case of technical loss. The inclusion criteria were patients from all stages of CKD balanced by sex and race across all stages of CKD. We selected all 18 patients at stage 5 CKD available to us, which made us oversample from other stages to compensate for the difference (up to 214). Selection of patients from other stages was on the basis of frequency match by sex and race of patients at stage 5 CKD. Baseline clinical and laboratory data as well as stored plasma samples were gathered cross-sectionally at the time of enrollment. We used the CKD Epidemiology Collaboration equation for eGFR calculation. We defined the acylcarnitine ratio as the ratio of long (C16–C20) to intermediate (C5–C14) plasma acylcarnitines. One sample per patient was used for the cross-sectional analysis. The baseline clinical characteristics of the selected patients are compared with those of the rest of the unselected cohort showing lack of any clinical or statistically significant differences (Supplemental Table 4), suggesting that the selected patients are an unbiased representative of the entire cohort.
Sample Preparation
Details of sample preparation and lipid identification for lipidomics analysis have been previously published.62 In brief, we extracted lipids from 50 μl human plasma by a modified Bligh and Dyer method63 for the lipidomics platform and another 25 μl for measurement of acylcarnitines. For lipidomics analysis, water:methanol:dichloromethane at room temperature with 2:2:2 volume ratio was used after spiking internal standards (lysophosphatidylcholine 17:0/0:0, phosphatidylglycerol 17:0/17:0, PE 17:0/17:0, phosphatidylcholine 17:0/17:0, TAG 17:0/17:0/17:0, SM 18:1/17:0, monoacylglycerol 17:0, DAG 16:0/18:1, cholesteryl-ester 17:0, ceramide d 18:1/17:0, phosphatidic acid 17:0, PI 17:0/20:4, and phosphatidylserine 17:0/17:0). The organic layer was collected, and the extract was completely dried under nitrogen. The dry extract was resuspended in 100 μl (10:5:85 acetonitrile:water:isopropyl alcohol) followed by 10 mM NH4OAc. We used a Shimadzu CTO-20A Nexera ×2 UHPLC with water acquity UPLC HSS T3 1.8-μm column (Waters, Milford, MA) and buffers A and B for mobile phase using details published before.62 We applied liquid chromatography-mass spectrometry ABSciex quadrupole time of flight–5600 equipped with a Turbo V ion source (AB Sciex, Concord, Canada). Acylcarnitines were measured by liquid chromatography-mass spectrometry as described previously36 with an Agilent 6410 Triple quadrupole tandem mass spectrometer (Agilent, Santa Clara, CA).
Marker Identification, Mass Spectrometry
We used a Shimadzu CTO-20A Nexera ×2 UHPLC system for chromatographic separation. The data acquisition was performed in both positive and negative ionization modes. The instrument was set to perform one time of flight mass spectrometry survey scan (150 milliseconds) and 15 mass spectrometry/mass spectrometry scans controlled by a data-dependent acquisition function with a total duty cycle time of 2.4 seconds. The mass range in both modes was 50–1200 m/z. We used proteoWizard software to convert the raw data to mgf data format64 and the NIST MS PepSearch Program to search the converted files against LipidBlast libraries.65,66 The m/z width for chromatogram extraction was determined by the mass accuracy of internal standards and set to 0.001 D for positive mode and 0.005 D for negative mode, with overall mass error of <2 ppm. We used mass spectrometry/mass spectrometry data to help with identification and mass spectrometry 1 for quantification. For relative quantification, the peak areas of extracted ion chromatograms were normalized to the peak area of the lipid standard and compared across samples. The lipids, identified in both positive and negative ion modes, were initially analyzed separately for their relationship with outcome to ensure persistent results. For lipid features with multiple adducts, the sum of spectral peaks from different adducts was used for the corresponding lipid.
Quality Control
A pool of study samples was injected at the beginning and after every ten mass spectrometry runs to assess the stability of the measures over time as well as the batch effects (Supplemental Figures 3 and 4).
Statistical Analyses
Mean±SD and count (%) were used for description of continuous and categorical variables, respectively. For description of skewed variables, median and interquartile range were applied. We used the chi-squared test to test the relationship of categorical variables across stages of CKD. ANOVA with Bonferroni post hoc analysis to correct for multiple comparisons was applied to compare the mean of background continuous variables by stages of CKD. Acquired data from mass spectrometry underwent k nearest neighbors imputation67 followed by normalization by internal standards, log2 transformation, and z-score standardization. Percentage contribution of each lipid within the corresponding lipid class was calculated by logit transformation of the ratio of abundance of each lipid over sum of the lipids within the corresponding class followed by z-score standardization. Analysis of covariance adjusting for background covariates, including age, sex, race, diabetes, urine protein-to-creatinine ratio, and use of statins with backward removal of nonsignificant covariates, was applied to compare the mean value of lipid classes by CKD stages. Mixed linear models were applied to explore the significance of association of carbon number and number of double bonds with lipid abundance by CKD stage. z-Score standardized percentage contribution of each lipid was applied for analysis of individual lipid levels in mixed linear models. PCA with Varimax orthogonal transformation was applied to generate principle components representative of various lipids for lipid classes with >16 intraclass lipids. Short versus long acyl chain as well as low double bond versus high double bond for each lipid class are similarly defined by application of PCA (Supplemental Table 2). The secondary generated principle components were applied in the construct of adjusted general linear models to compare changes of lipids by CKD stage. Multiple linear regression analysis was applied to explore independent precursors of the lipid subclasses as well as the long-to-intermediate acylcarnitine ratio. Pearson correlation coefficient was applied to test the associations between various lipids.
Disclosures
None.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health grants K08DK106523 (to F.A.), P30DK089503, DK082841, P30DK081943, P30DK020572, and DK097153 (to S.P.) and grant UL1TR000433 from the Michigan Institute for Clinical & Health Research (to S.W. and K.M.S.).
The Michigan Kidney Translational Core Clinical Phenotyping Resource and Biobank Core Investigator Group includes Matthias Kretzler and Debbie Gipson (University of Michigan), Keith Bellovich (St. Clair Nephrology Research, Detroit), Zeenat Bhat (Wayne State University), Crystal Gadegbeku (Temple University Health System), Susan Massengill (Levin Children’s Hospital), and Kalyani Perumal, JH Stroger Hospital.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2017030350/-/DCSupplemental.
Contributor Information
Collaborators: Matthias Kretzler, Zeenat Bhat, Crystal Gadegbeku, Susan Massengill, and Kalyani Perumal
References
- 1.Attman PO, Alaupovic P: Lipid abnormalities in chronic renal insufficiency. Kidney Int Suppl 31: S16–S23, 1991 [PubMed] [Google Scholar]
- 2.Trevisan R, Dodesini AR, Lepore G: Lipids and renal disease. J Am Soc Nephrol 17[Suppl 2]: S145–S147, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Kuznik A, Mardekian J, Tarasenko L: Evaluation of cardiovascular disease burden and therapeutic goal attainment in US adults with chronic kidney disease: An analysis of national health and nutritional examination survey data, 2001-2010. BMC Nephrol 14: 132, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Longenecker JC, Coresh J, Powe NR, Levey AS, Fink NE, Martin A, Klag MJ: Traditional cardiovascular disease risk factors in dialysis patients compared with the general population: The CHOICE Study. J Am Soc Nephrol 13: 1918–1927, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Pennell P, Leclercq B, Delahunty MI, Walters BA: The utility of non-HDL in managing dyslipidemia of stage 5 chronic kidney disease. Clin Nephrol 66: 336–347, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Quehenberger O, Dennis EA: The human plasma lipidome. N Engl J Med 365: 1812–1823, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heymann EP, Kassimatis TI, Goldsmith DJ: Dyslipidemia, statins, and CKD patients’ outcomes - review of the evidence in the post-sharp era. J Nephrol 25: 460–472, 2012 [DOI] [PubMed] [Google Scholar]
- 8.Sun W, Liu D, Gong P, Shi X, Wang Y, Wang P, Gong W: Predicting cardiovascular mortality in chronic kidney disease (CKD) patients. Ann Transplant 19: 513–518, 2014 [DOI] [PubMed] [Google Scholar]
- 9.Wahl P, Ducasa GM, Fornoni A: Systemic and renal lipids in kidney disease development and progression. Am J Physiol Renal Physiol 310: F433–F445, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reiss AB, Voloshyna I, De Leon J, Miyawaki N, Mattana J: Cholesterol metabolism in CKD. Am J Kidney Dis 66: 1071–1082, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsuruya K, Yoshida H, Nagata M, Kitazono T, Iseki K, Iseki C, Fujimoto S, Konta T, Moriyama T, Yamagata K, Narita I, Kimura K, Kondo M, Asahi K, Kurahashi I, Ohashi Y, Watanabe T: Impact of the triglycerides to high-density lipoprotein cholesterol ratio on the incidence and progression of CKD: A longitudinal study in a large Japanese population. Am J Kidney Dis 66: 972–983, 2015 [DOI] [PubMed] [Google Scholar]
- 12.Kontush A, Lhomme M, Chapman MJ: Unraveling the complexities of the HDL lipidome. J Lipid Res 54: 2950–2963, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Graessler J, Schwudke D, Schwarz PE, Herzog R, Shevchenko A, Bornstein SR: Top-down lipidomics reveals ether lipid deficiency in blood plasma of hypertensive patients. PLoS One 4: e6261, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pietiläinen KH, Sysi-Aho M, Rissanen A, Seppänen-Laakso T, Yki-Järvinen H, Kaprio J, Oresic M: Acquired obesity is associated with changes in the serum lipidomic profile independent of genetic effects--a monozygotic twin study. PLoS One 2: e218, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rhee EP, Cheng S, Larson MG, Walford GA, Lewis GD, McCabe E, Yang E, Farrell L, Fox CS, O’Donnell CJ, Carr SA, Vasan RS, Florez JC, Clish CB, Wang TJ, Gerszten RE: Lipid profiling identifies a triacylglycerol signature of insulin resistance and improves diabetes prediction in humans. J Clin Invest 121: 1402–1411, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stegemann C, Drozdov I, Shalhoub J, Humphries J, Ladroue C, Didangelos A, Baumert M, Allen M, Davies AH, Monaco C, Smith A, Xu Q, Mayr M: Comparative lipidomics profiling of human atherosclerotic plaques. Circ Cardiovasc Genet 4: 232–242, 2011 [DOI] [PubMed] [Google Scholar]
- 17.Reis A, Rudnitskaya A, Chariyavilaskul P, Dhaun N, Melville V, Goddard J, Webb DJ, Pitt AR, Spickett CM: Top-down lipidomics of low density lipoprotein reveal altered lipid profiles in advanced chronic kidney disease. J Lipid Res 56: 413–422, 2015 [DOI] [PMC free article] [PubMed]
- 18.Houten SM, Wanders RJ: A general introduction to the biochemistry of mitochondrial fatty acid β-oxidation. J Inherit Metab Dis 33: 469–477, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Overmyer KA, Evans CR, Qi NR, Minogue CE, Carson JJ, Chermside-Scabbo CJ, Koch LG, Britton SL, Pagliarini DJ, Coon JJ, Burant CF: Maximal oxidative capacity during exercise is associated with skeletal muscle fuel selection and dynamic changes in mitochondrial protein acetylation. Cell Metab 21: 468–478, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoshihisa A, Watanabe S, Yokokawa T, Misaka T, Sato T, Suzuki S, Oikawa M, Kobayashi A, Takeishi Y: Associations between acylcarnitine to free carnitine ratio and adverse prognosis in heart failure patients with reduced or preserved ejection fraction. ESC Heart Fail 4: 360–364, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sieber J, Jehle AW: Free fatty acids and their metabolism affect function and survival of podocytes. Front Endocrinol (Lausanne) 5: 186, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang XS, Chen XM, Wan JM, Gui HB, Ruan XZ, Du XG: Autophagy protects against palmitic acid-induced apoptosis in podocytes in vitro. Sci Rep 7: 42764, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karaskov E, Scott C, Zhang L, Teodoro T, Ravazzola M, Volchuk A: Chronic palmitate but not oleate exposure induces endoplasmic reticulum stress, which may contribute to INS-1 pancreatic beta-cell apoptosis. Endocrinology 147: 3398–3407, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Lee E, Choi J, Lee HS: Palmitate induces mitochondrial superoxide generation and activates AMPK in podocytes. J Cell Physiol 232: 3209–3217, 2017 [DOI] [PubMed] [Google Scholar]
- 25.Lennon R, Pons D, Sabin MA, Wei C, Shield JP, Coward RJ, Tavaré JM, Mathieson PW, Saleem MA, Welsh GI: Saturated fatty acids induce insulin resistance in human podocytes: Implications for diabetic nephropathy. Nephrol Dial Transplant 24: 3288–3296, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martínez-García C, Izquierdo-Lahuerta A, Vivas Y, Velasco I, Yeo TK, Chen S, Medina-Gomez G: Renal lipotoxicity-associated inflammation and insulin resistance affects actin cytoskeleton organization in podocytes. PLoS One 10: e0142291, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sieber J, Lindenmeyer MT, Kampe K, Campbell KN, Cohen CD, Hopfer H, Mundel P, Jehle AW: Regulation of podocyte survival and endoplasmic reticulum stress by fatty acids. Am J Physiol Renal Physiol 299: F821–F829, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soumura M, Kume S, Isshiki K, Takeda N, Araki S, Tanaka Y, Sugimoto T, Chin-Kanasaki M, Nishio Y, Haneda M, Koya D, Kashiwagi A, Maegawa H, Uzu T: Oleate and eicosapentaenoic acid attenuate palmitate-induced inflammation and apoptosis in renal proximal tubular cell. Biochem Biophys Res Commun 402: 265–271, 2010 [DOI] [PubMed] [Google Scholar]
- 29.Xu S, Nam SM, Kim JH, Das R, Choi SK, Nguyen TT, Quan X, Choi SJ, Chung CH, Lee EY, Lee IK, Wiederkehr A, Wollheim CB, Cha SK, Park KS: Palmitate induces ER calcium depletion and apoptosis in mouse podocytes subsequent to mitochondrial oxidative stress. Cell Death Dis 6: e1976, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yasuda M, Tanaka Y, Kume S, Morita Y, Chin-Kanasaki M, Araki H, Isshiki K, Araki S, Koya D, Haneda M, Kashiwagi A, Maegawa H, Uzu T: Fatty acids are novel nutrient factors to regulate mTORC1 lysosomal localization and apoptosis in podocytes. Biochim Biophys Acta 1842: 1097–1108, 2014 [DOI] [PubMed] [Google Scholar]
- 31.Friedman AN, Moe SM, Perkins SM, Li Y, Watkins BA: Fish consumption and omega-3 fatty acid status and determinants in long-term hemodialysis. Am J Kidney Dis 47: 1064–1071, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Saifullah A, Watkins BA, Saha C, Li Y, Moe SM, Friedman AN: Oral fish oil supplementation raises blood omega-3 levels and lowers C-reactive protein in haemodialysis patients--a pilot study. Nephrol Dial Transplant 22: 3561–3567, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Kim HJ, Moradi H, Yuan J, Norris K, Vaziri ND: Renal mass reduction results in accumulation of lipids and dysregulation of lipid regulatory proteins in the remnant kidney. Am J Physiol Renal Physiol 296: F1297–F1306, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen H, Chen L, Liu D, Chen DQ, Vaziri ND, Yu XY, Zhang L, Su W, Bai X, Zhao YY: Combined clinical phenotype and lipidomic analysis reveals the impact of chronic kidney disease on lipid metabolism. J Proteome Res 16: 1566–1578, 2017 [DOI] [PubMed] [Google Scholar]
- 35.Rosca MG, Vazquez EJ, Chen Q, Kerner J, Kern TS, Hoppel CL: Oxidation of fatty acids is the source of increased mitochondrial reactive oxygen species production in kidney cortical tubules in early diabetes. Diabetes 61: 2074–2083, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sas KM, Kayampilly P, Byun J, Nair V, Hinder LM, Hur J, Zhang H, Lin C, Qi NR, Michailidis G, Groop PH, Nelson RG, Darshi M, Sharma K, Schelling JR, Sedor JR, Pop-Busui R, Weinberg JM, Soleimanpour SA, Abcouwer SF, Gardner TW, Burant CF, Feldman EL, Kretzler M, Brosius FC 3rd, Pennathur S: Tissue-specific metabolic reprogramming drives nutrient flux in diabetic complications. JCI Insight 1: e86976, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kampe K, Sieber J, Orellana JM, Mundel P, Jehle AW: Susceptibility of podocytes to palmitic acid is regulated by fatty acid oxidation and inversely depends on acetyl-CoA carboxylases 1 and 2. Am J Physiol Renal Physiol 306: F401–F409, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kang HM, Ahn SH, Choi P, Ko YA, Han SH, Chinga F, Park AS, Tao J, Sharma K, Pullman J, Bottinger EP, Goldberg IJ, Susztak K: Defective fatty acid oxidation in renal tubular epithelial cells has a key role in kidney fibrosis development. Nat Med 21: 37–46, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lim PS, Ma YS, Cheng YM, Chai H, Lee CF, Chen TL, Wei YH: Mitochondrial DNA mutations and oxidative damage in skeletal muscle of patients with chronic uremia. J Biomed Sci 9: 549–560, 2002 [DOI] [PubMed] [Google Scholar]
- 40.Liu CS, Ko LY, Lim PS, Kao SH, Wei YH: Biomarkers of DNA damage in patients with end-stage renal disease: Mitochondrial DNA mutation in hair follicles. Nephrol Dial Transplant 16: 561–565, 2001 [DOI] [PubMed] [Google Scholar]
- 41.Rossato LB, Nunes AC, Pereira ML, de Souza CF, Dummer C, Milani V, Porsch DB, de Mattos CB, Barros EJ: Prevalence of 4977bp deletion in mitochondrial DNA from patients with chronic kidney disease receiving conservative treatment or hemodialysis in southern Brazil. Ren Fail 30: 9–14, 2008 [DOI] [PubMed] [Google Scholar]
- 42.Rhee EP, Souza A, Farrell L, Pollak MR, Lewis GD, Steele DJ, Thadhani R, Clish CB, Greka A, Gerszten RE: Metabolite profiling identifies markers of uremia. J Am Soc Nephrol 21: 1041–1051, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morino K, Petersen KF, Shulman GI: Molecular mechanisms of insulin resistance in humans and their potential links with mitochondrial dysfunction. Diabetes 55[Suppl 2]: S9–S15, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Muoio DM, Neufer PD: Lipid-induced mitochondrial stress and insulin action in muscle. Cell Metab 15: 595–605, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Petersen KF, Dufour S, Befroy D, Garcia R, Shulman GI: Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N Engl J Med 350: 664–671, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sieber J, Weins A, Kampe K, Gruber S, Lindenmeyer MT, Cohen CD, Orellana JM, Mundel P, Jehle AW: Susceptibility of podocytes to palmitic acid is regulated by stearoyl-CoA desaturases 1 and 2. Am J Pathol 183: 735–744, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sato T, Liang K, Vaziri ND: Protein restriction and AST-120 improve lipoprotein lipase and VLDL receptor in focal glomerulosclerosis. Kidney Int 64: 1780–1786, 2003 [DOI] [PubMed] [Google Scholar]
- 48.Vaziri ND: Molecular mechanisms of lipid disorders in nephrotic syndrome. Kidney Int 63: 1964–1976, 2003 [DOI] [PubMed] [Google Scholar]
- 49.Klin M, Smogorzewski M, Ni Z, Zhang G, Massry SG: Abnormalities in hepatic lipase in chronic renal failure: Role of excess parathyroid hormone. J Clin Invest 97: 2167–2173, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Harvey RA, Champe PC: Biochemistry, Baltimore, MD, Lippincott Williams & Wilkins, 2005 [Google Scholar]
- 51.Axelsson J, Aström G, Sjölin E, Qureshi AR, Lorente-Cebrián S, Stenvinkel P, Rydén M: Uraemic sera stimulate lipolysis in human adipocytes: Role of perilipin. Nephrol Dial Transplant 26: 2485–2491, 2011 [DOI] [PubMed] [Google Scholar]
- 52.Hoppel C: The role of carnitine in normal and altered fatty acid metabolism. Am J Kidney Dis 41[Suppl 4]: S4–S12, 2003 [DOI] [PubMed] [Google Scholar]
- 53.Zhu Y, Chen YL, Li C, Ding XY, Xu GY, Hu LL, Hou FF, Zhou QG: The effect of inhibition of endoplasmic reticulum stress on lipolysis in white adipose tissue in a rat model of chronic kidney disease. Acta Pharmacol Sin 35: 356–362, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.World Health Organization : Global Recommendations on Physical Activity for Health, Geneva, Switzerland, WHO, 2010 [PubMed] [Google Scholar]
- 55.Erikssen G, Liestøl K, Bjørnholt J, Thaulow E, Sandvik L, Erikssen J: Changes in physical fitness and changes in mortality. Lancet 352: 759–762, 1998 [DOI] [PubMed] [Google Scholar]
- 56.LaMonte MJ, Barlow CE, Jurca R, Kampert JB, Church TS, Blair SN: Cardiorespiratory fitness is inversely associated with the incidence of metabolic syndrome: A prospective study of men and women. Circulation 112: 505–512, 2005 [DOI] [PubMed] [Google Scholar]
- 57.McAuley PA, Sui X, Church TS, Hardin JW, Myers JN, Blair SN: The joint effects of cardiorespiratory fitness and adiposity on mortality risk in men with hypertension. Am J Hypertens 22: 1062–1069, 2009 [DOI] [PubMed] [Google Scholar]
- 58.Schutte NM, Nederend I, Hudziak JJ, Bartels M, de Geus EJ: Twin-sibling study and meta-analysis on the heritability of maximal oxygen consumption. Physiol Genomics 48: 210–219, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Baigent C, Landray MJ, Reith C, Emberson J, Wheeler DC, Tomson C, Wanner C, Krane V, Cass A, Craig J, Neal B, Jiang L, Hooi LS, Levin A, Agodoa L, Gaziano M, Kasiske B, Walker R, Massy ZA, Feldt-Rasmussen B, Krairittichai U, Ophascharoensuk V, Fellström B, Holdaas H, Tesar V, Wiecek A, Grobbee D, de Zeeuw D, Grönhagen-Riska C, Dasgupta T, Lewis D, Herrington W, Mafham M, Majoni W, Wallendszus K, Grimm R, Pedersen T, Tobert J, Armitage J, Baxter A, Bray C, Chen Y, Chen Z, Hill M, Knott C, Parish S, Simpson D, Sleight P, Young A, Collins R; SHARP Investigators : The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): A randomised placebo-controlled trial. Lancet 377: 2181–2192, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fellström BC, Jardine AG, Schmieder RE, Holdaas H, Bannister K, Beutler J, Chae DW, Chevaile A, Cobbe SM, Grönhagen-Riska C, De Lima JJ, Lins R, Mayer G, McMahon AW, Parving HH, Remuzzi G, Samuelsson O, Sonkodi S, Sci D, Süleymanlar G, Tsakiris D, Tesar V, Todorov V, Wiecek A, Wüthrich RP, Gottlow M, Johnsson E, Zannad F; AURORA Study Group : Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N Engl J Med 360: 1395–1407, 2009 [DOI] [PubMed] [Google Scholar]
- 61.Wanner C, Krane V, März W, Olschewski M, Mann JF, Ruf G, Ritz E; German Diabetes and Dialysis Study Investigators : Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med 353: 238–248, 2005 [DOI] [PubMed] [Google Scholar]
- 62.Afshinnia F, Rajendiran TM, Karnovsky A, Soni T, Wang X, Xie D, Yang W, Shafi T, Weir MR, He J, Brecklin CS, Rhee EP, Schelling JR, Ojo A, Feldman H, Michailidis G, Pennathur S: Lipidomic signature of progression of chronic kidney disease in the chronic renal insufficiency cohort. Kidney Int Rep 1: 256–268, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bligh EG, Dyer WJ: A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37: 911–917, 1959 [DOI] [PubMed] [Google Scholar]
- 64.Chambers MC, Maclean B, Burke R, Amodei D, Ruderman DL, Neumann S, Gatto L, Fischer B, Pratt B, Egertson J, Hoff K, Kessner D, Tasman N, Shulman N, Frewen B, Baker TA, Brusniak MY, Paulse C, Creasy D, Flashner L, Kani K, Moulding C, Seymour SL, Nuwaysir LM, Lefebvre B, Kuhlmann F, Roark J, Rainer P, Detlev S, Hemenway T, Huhmer A, Langridge J, Connolly B, Chadick T, Holly K, Eckels J, Deutsch EW, Moritz RL, Katz JE, Agus DB, MacCoss M, Tabb DL, Mallick P: A cross-platform toolkit for mass spectrometry and proteomics. Nat Biotechnol 30: 918–920, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kind T, Meissen JK, Yang D, Nocito F, Vaniya A, Cheng YS, Vandergheynst JS, Fiehn O: Qualitative analysis of algal secretions with multiple mass spectrometric platforms. J Chromatogr A 1244: 139–147, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Meissen JK, Yuen BT, Kind T, Riggs JW, Barupal DK, Knoepfler PS, Fiehn O: Induced pluripotent stem cells show metabolomic differences to embryonic stem cells in polyunsaturated phosphatidylcholines and primary metabolism. PLoS One 7: e46770, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Altman NS: An introduction to kernel and nearest-neighbor nonparametric regression. Am Stat 46: 175–185, 1992 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.