Abstract
BK virus–associated nephropathy (BKVAN) causes renal allograft dysfunction. The current management of BKVAN relies on pre-emptive adaptation of immunosuppression according to viral load monitoring. However, this empiric strategy is not always successful. Therefore, pretransplant predictive markers are needed. In a prospective longitudinal study, we enrolled 168 kidney transplant recipients and 69 matched donors. To assess the value of BKV genotype–specific neutralizing antibody (NAb) titers as a predictive marker for BKV replication, we measured BKV DNA load and NAb titers at transplant and followed patients for 24 months. After transplant, 52 (31%) patients displayed BKV replication: 24 (46%) patients were viruric and 28 (54%) patients were viremic, including 13 with biopsy-confirmed BKVAN. At any time, patients with high NAb titers against the replicating strain had a lower risk of developing BKV viremia (hazard ratio [HR], 0.44; 95% confidence interval [95% CI], 0.26 to 0.73; P=0.002). Each log10 increase in NAb titer decreased the risk of developing viremia by 56%. Replicating strains were consistent with donor transmission in 95% of cases of early BKV replication. Genotype mismatch between recipients’ neutralization profiles before transplant and their subsequently replicating strain significantly increased the risk of developing viremia (HR, 2.27; 95% CI, 1.06 to 4.88; P=0.04). A NAb titer against the donor’s strain <4 log10 before transplant significantly associated with BKV replication after transplant (HR, 1.88; 95% CI, 1.06 to 3.45; P=0.03). BKV genotype–specific NAb titers may be a meaningful predictive marker that allows patient stratification by BKV disease risk before and after transplant.
Keywords: kidney transplantation, transplant outcomes, immunology, Viral infection, Predictive marker
The emergence of BK virus–associated nephropathy (BKVAN) is one of the major causes of graft dysfunction and loss in kidney transplant recipients. BKVAN essentially arises from the use of highly potent immunosuppressive drugs1–4 and is a growing clinical problem as the population of transplant recipients continues to increase. BKV replication occurs in 30%–50% of recipients with progression to BKVAN in 7%–8% of patients which ultimately leads to graft dysfunction and loss in a majority of BKVAN cases.5 Furthermore, early BKV replication after transplantation increases the risk of late acute rejection.6 At present, there are no BKV-specific antiviral therapies available. Current guidelines recommend regular screening to detect BKV viruria or viremia after renal transplantation.2,7 Patients exhibiting persistently high BKV DNA loads usually undergo immunosuppression reduction to reduce BKV replication and to prevent BKVAN occurrence. However, because of its delayed nature, this pre-emptive strategy does not fully eliminate the risk of BKVAN8,9 and can increase the risk of donor-specific antibodies (DSA), graft rejection, and death.6,10 Therefore, a predictive marker for BKV replication before transplantation is needed to identify patients at risk. Neutralizing antibodies (NAbs) have been shown to significantly influence the outcome of chronic viral infections in patients with impaired cellular immune responses such as hepatitis C virus–infected liver transplant recipients or patients with HIV.11–13 In this prospective longitudinal study, for the first time, we analyzed the effect of NAbs on the outcome of BKV infection after kidney transplantation over a period of 24 months and investigated neutralization genotype–specific titers as a predictive marker for BKV replication.
Results
Patients’ Characteristics
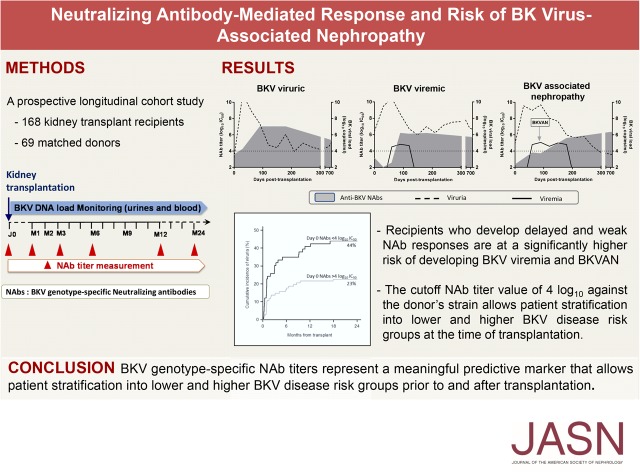
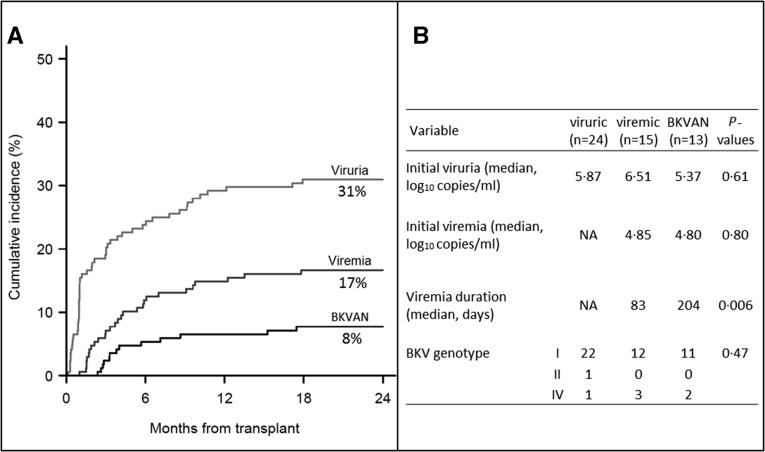
One hundred sixty-eight patients were prospectively followed for a mean duration of 693±125 days. BKV load was measured in 2850 pairs of blood and urine samples. NAb titers were tested in a total of 773 blood samples. Patients’ characteristics are summarized in Table 1. Among the 168 patients, 116 (69%) showed no detectable levels of BKV viruria or viremia during the time of the study (BKV DNA–negative). Fifty-two (31%) patients displayed evidence of BKV replication (BKV DNA–positive). Of these, 24 (46%) remained only viruric (viruric group) whereas 28 (54%) developed viremia, including 13 with biopsy-confirmed nephropathy, 7 stage A, 6 stage B, and 0 stage C (BKVAN group) (Figure 1A). Initial viruria was similar for patients who were viruric, viremic, and those with BKVAN (medians 5.87, 6.51, and 5.37 log10 copies per milliliter, respectively; P=0.61). No significant difference was observed for initial viremia between patients who were viremic and those with BKVAN (medians 4.85 and 4.80 log10 copies per milliliter; P=0.80). Sequencing analysis of BKV VP1 identified genotype I, genotype IV, and genotype II in 45 (86%), six (12%), and one (2%) patients who were BKV DNA–positive, respectively (Figure 1B). No significant difference was observed in BKV genotype prevalence among viruric, viremic, and BKVAN groups (P=0.47). No significant difference relative to patients’ attributes or immunosuppressive treatment at transplantation was noted between the four groups, except for rituximab administration (Supplemental Table 1). Three out of five patients having received rituximab before transplantation developed BKVAN. Acute rejection occurred in 20% (23 of 116), 17% (4 of 24), 26% (4 of 15), and 46% (6 of 13) of the patients belonging to the BKV DNA–negative, viruric, viremic, and BKVAN groups, respectively. Of note, 10 of 14 (71%) of the rejection episodes occurred after the diagnosis of BKV infection (2 of 4 for patients who were viruric, 3 of 4 for patients who were viremic, and 5 of 6 for the BKVAN group). During follow-up, seven patients died, two of whom were BKV DNA–positive. Graft loss occurred in eight cases after antibody-mediated or cellular rejection in three and five cases, respectively. Among them, one patient had developed BKVAN.
Table 1.
Patients’ characteristics
| Variable | Patient Cohort (n=168) |
|---|---|
| Age, median (range) | 56.2 (17.7–75.7) |
| Male, n (%) | 101 (60.1) |
| First graft, n (%) | 128 (79.2) |
| Living donor, n (%) | 24 (14.3) |
| HLA mismatches, median (range) | 4.0 (0.0–6.0) |
| Cold ischemia time, min, median (range) | 841 (70–1861) |
| Thymoglobulin, n (%) | 82 (48.8) |
| Tacrolimus, n (%) | 91 (54.2) |
| Rituximab, n (%) | 5 (3.0) |
Figure 1.
Monitoring of BK virus (BKV) replication. Kaplan–Meier estimates of BKV viruria, viremia, and BKVAN in 168 kidney transplant recipients followed-up for 24 months post-transplant. BKVAN was diagnosed on the basis of histologic findings in allograft biopsy specimens. (A) Cumulative incidence values at month 24 are indicated. (B) Virologic characteristics of viruric, viremic, and BKVAN groups are shown. NA, not applicable.
BKV Genotype–Specific Seroconversion after Transplantation
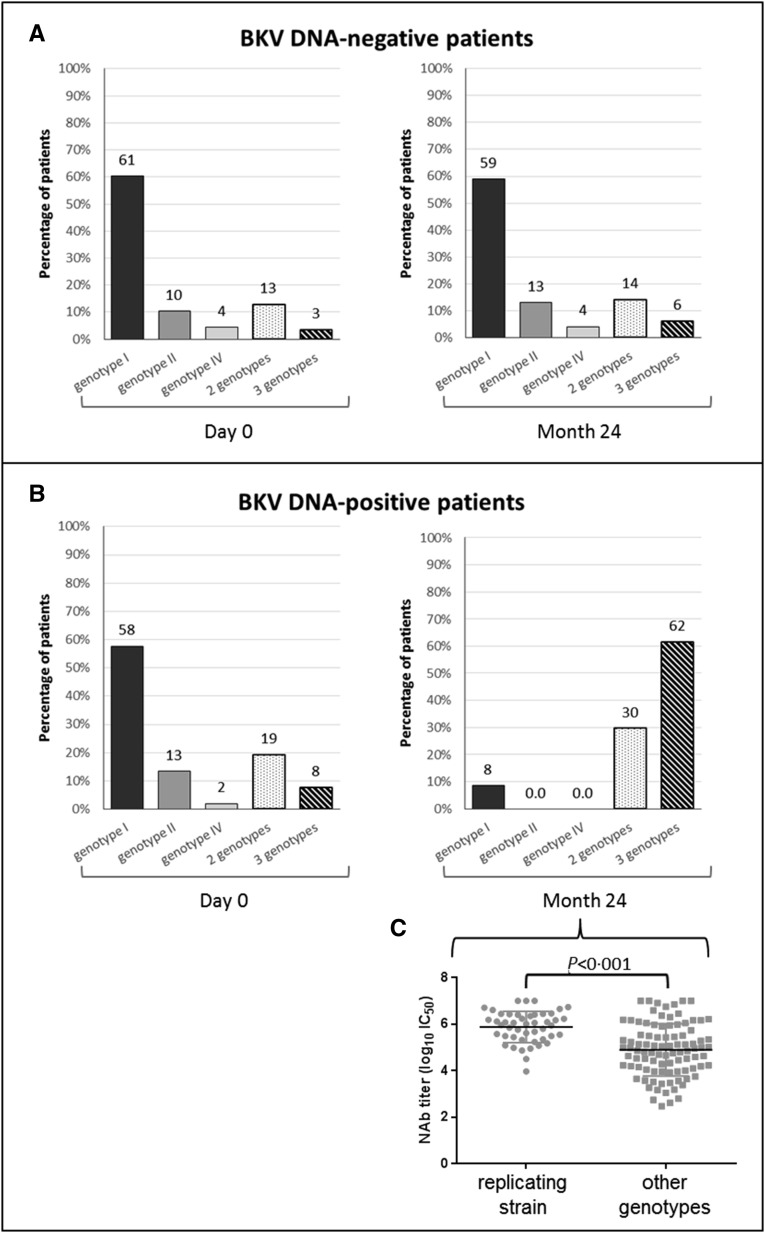
At the time of transplantation, 158 patients (94%) harbored NAbs against at least one BKV genotype; ten patients were BKV-seronegative. Regarding patients who were BKV DNA–negative (n=116), 70 (61%) harbored NAbs against BKV genotype I, 12 (10%) against genotype II, and five (4%) against genotype IV at the time of transplantation. Nineteen (16%) patients harbored NAbs against two or three BKV genotypes and ten (9%) were BKV-seronegative (Figure 2A). At month 24, BKV genotype neutralization profiles remained unchanged.
Figure 2.
BK virus (BKV) genotype–specific seroconversion after transplantation. Genotype specificity was determined for each patient on the basis of NAb titers. Percentages of patients who were (A) BKV DNA–negative and (B) BKV DNA–positive displaying NAbs against genotype I, II, IV, or multiple genotypes at the time of transplantation and 24 months post-transplantation are shown. (C) NAb titers against the replicative strain and the other genotypes are depicted at month 24 for patients who were BKV DNA–positive.
Patients who were BKV DNA–positive (n=52) showed genotype-specific NAb prevalence rates similar to patients who were BKV DNA–negative at the time of transplantation, with 58%, 13%, and 2% of the patients harboring NAbs against genotypes I, II, or IV, respectively. Fourteen (27%) patients harbored NAbs against two or three genotypes. An important change in BKV genotype–specific NAb distribution was observed for patients who were BKV DNA–positive at month 24, among which 92% displayed NAbs against two or three genotypes, with higher titers directed against the replicating strain as compared with other strains (P<0.001) (Figure, 2B and C). These patients did not display PCR nor sequencing data suggestive of mixed infection (data not shown). Sixty out of the 69 (87%) analyzed donors harbored NAbs against at least one BKV genotype (62% genotype I, 6% genotype II, 4% genotype IV, 9% two genotypes, and 6% three genotypes; Supplemental Figure 1).
A Delayed and Weak NAb Response Is Associated with Severe BKV Infection Outcome
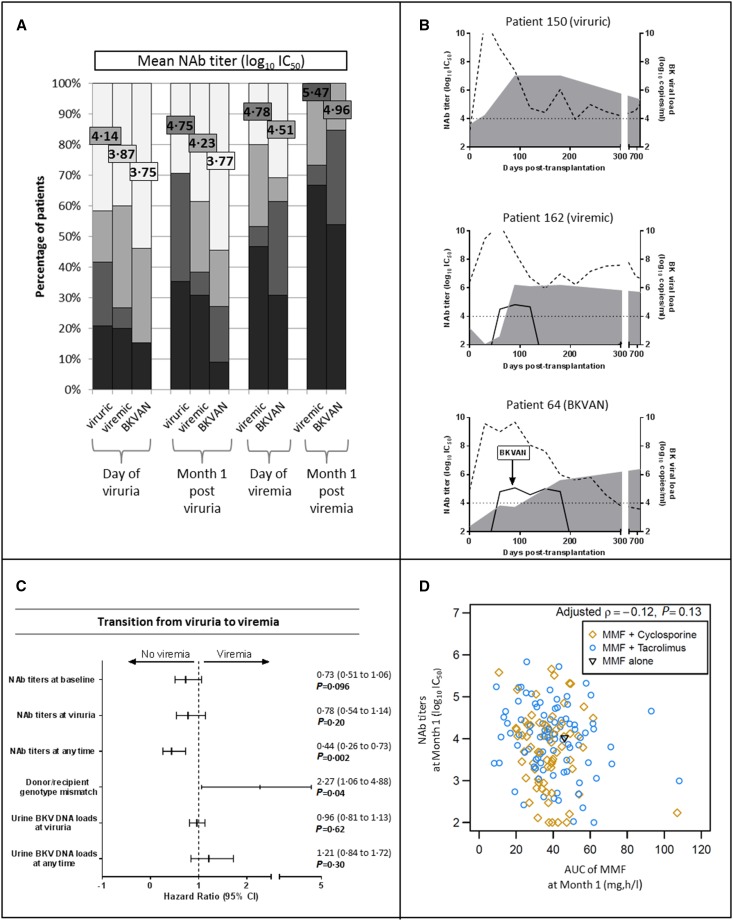
Longitudinal measures of NAb titers directed against the replicating BKV genotype were performed in patients who were BKV DNA–positive. Four out of 24 (17%) patients who were viruric, 5 of 15 (30%) who were viremic, and 7 of 13 (54%) patients with BKVAN displayed a genotype mismatch for their replicating strain. An increase in NAb titers was observed as early as 1 month after viruria; however, titers and kinetics of the neutralizing response differed among patients (Figure 3, A and B and a detailed description of BKV DNA load and NAb titer kinetics for each patient is provided in the Supplemental Figure 2). Rising NAb titers coincided with falling viral load in urine for 19 of 24 patients who were viruric, 11 of 15 who were viremic, and 11 of 13 patients with BKVAN; for 9 of 15 patients who were viremic and 9 of 13 patients with BKVAN, a falling viral load was also observed in blood (Supplemental Figure 2). These kinetics could not be assessed for patients 92 and 132 (missing time points due to sample unavailability or death). Joint models showed that at any time, patients with high NAb titers against the replicating strain were at a lower risk of BKV viremia (hazard ratio [HR], 0.44; 95% confidence interval [95% CI], 0.26 to 0.73; P=0.002; Figure 3C, Supplemental Material). Each 1 log10 increase in NAb titer decreased the risk of developing viremia by 56%. Recipients displaying no or low NAb titer against the genotype for which their donor was seropositive were at a significantly higher risk of developing viremia (HR, 2.27; 95% CI, 1.06 to 4.88; P=0.04; Figure 3C). Surprisingly, the joint model analysis of urine BKV DNA load at the onset of viruria or at any time before viremia onset failed to show such a strong relationship, with an HR per log10 increase of 1.21 (0.84 to 1.72) (Figure 3C, Supplemental Table 2). We observed no association between NAb titers and the AUC of mycophenolate mofetil measured at month 1 (r=−0.12, P=0.13; Figure 3D). Four out of five patients who received rituximab became viremic and showed a delayed increase in NAb titers during follow-up, and three of them developed BKVAN.
Figure 3.
A delayed and weak NAb response is associated with severe BK virus (BKV) infection outcome. (A) The evolution of NAb titers directed against the replicating strain in patients who were viruric, viremic, and those with BKVAN. NAb titer distribution is depicted in colored bars and mean NAb titer values (log10 IC50) for each group are represented by colored squares. (B) The detailed evolution of three representative patients of viruric, viremic, and BKVAN groups. Dotted and solid lines represent urine and blood viral loads, respectively. NAb titers are depicted by gray areas. (C) The association of NAb titers, donor/recipient genotype mismatch, and urine BKV DNA loads with the risk of transition from viruria to viremia state. Continuous or joint models were used. HR (95% CI) and P values were calculated using two-fold crossvalidation after section of the optimal cutoff at 4 log10 IC50 NAb titer. (D) Spearman’s rank correlation between NAb titers and mycophenolic acid (MMF) area under the concentration time curve (AUC), alone or associated to cyclosporine or tacrolimus. Spearman’s coefficient and associated P value are indicated.
Neutralizing Titers before Transplantation Predict the Development and Outcome of BKV Infection after Transplantation
To investigate whether pretransplantation NAb titers could predict the occurrence of BKV replication, we compared NAb titers measured at day 0 in patients who were BKV DNA–negative versus those who were BKV DNA–positive. NAbs directed against the replicating BKV genotype were considered for patients who were BKV DNA–positive. A significant difference was observed between the two groups, with median NAb titers of 4.40 log10 IC50 and 3.92 log10 IC50 (P=0.04) in patients who were BKV DNA–negative and BKV DNA–positive, respectively. This difference suggests that viral replication correlates with absent or insufficient NAbs against the donor strain. On the other hand, it appears that patients who were BKV DNA–negative either had sufficient NAb titers to control the replication of any BKV strain “hitchhiking” in the donated organ or that their donors were BKV-seronegative.
We addressed this hypothesis by analyzing sera from 69 donor/recipient pairs. The analysis of 46 matched sera of donor/BKV DNA–negative recipient pairs at day 0 showed that eight (17%) donors were BKV-seronegative or equivocal, 24 (52%) recipients harbored high titers of NAbs against the BKV genotype for which the donor was neutralization seropositive, whereas 14 (31%) recipients showed low NAb titers (Supplemental Figure 3).
The analysis of 23 matched sera from donor/BKV DNA–positive recipient pairs at day 0 showed that one donor was seronegative. Seven (31%) BKV DNA–positive recipients harbored high NAb titers against the BKV genotype for which the donor was neutralization seropositive. None of these recipients progressed to BKVAN and only three became viremic with a noticeably short duration of viremia (37, 62, and 82 days). Fifteen (65%) recipients showed low neutralizing titers against the BKV genotype for which the donor was neutralization seropositive (Supplemental Figure 3). Moreover, when considering the first 10 months post-transplantation, 19 of 20 (95%) of the pairs showed a concordance between the donor’s genotype-specific neutralization profile and the genotype found replicating in the recipient; one recipient whose donor was BKV-seronegative developed BKV viruria at 102 days post-transplantation. The three remaining recipients developed delayed viruria at 292, 369, and 556 days after transplantation, suggesting an exogenous BKV reinfection. Interestingly, NAb titers were higher for donors of BKV DNA–positive recipients than those for donors of BKV DNA–negative recipients (5.07 versus 3.96, P<0.001). However, donor NAb titers were similar for the three BKV DNA–positive groups (viruric, viremic, and BKVAN) (P=0.45).
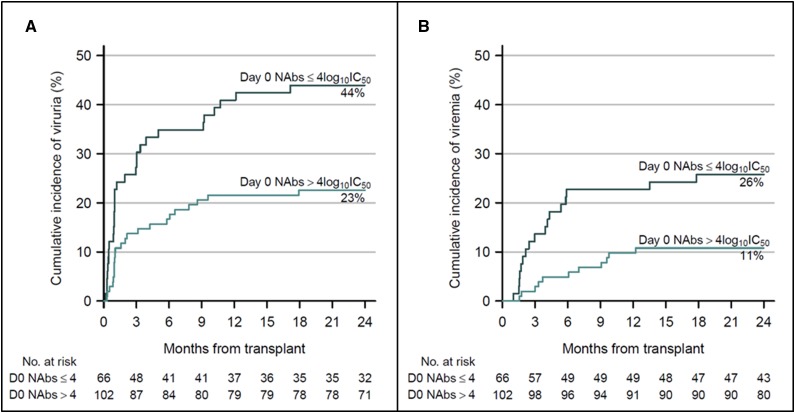
Using two-fold crossvalidation, we determined a NAb titer value of 4 log10 IC50 as the optimal cutoff for predicting the transition from a negative to a viruric, viremic, or BKVAN state with a C-index of 0.603, 0.635, and 0.658 for viruria, viremia, and BKVAN occurrence, respectively (Figure 4A). Indeed, a NAb titer <4 log10 IC50 on the day of transplantation was significantly associated with BKV replication after transplantation (HR, 1.88; 95% CI, 1.06 to 3.45; P=0.03). There was a trend toward a higher probability of developing viremia for patients harboring low NAb titers before transplantation (HR, 1.37; 95% CI, 0.94 to 1.96; P<0.10; Figure 4B). Eleven patients were BKV viremic (with or without BKVAN) despite displaying a baseline NAb titer >4 log10. However, nine of them showed decreasing NAb titers (<4 log10 for five patients) between M1 and M3 after transplantation, whereas two patients—one of whom received rituximab—showed an increase in NAb titers during follow-up without decreasing viral load, suggesting a possible immune escape mechanism.
Figure 4.
Neutralizing titers before transplantation predict the development and outcome of BK virus (BKV) infection after transplantation. Kaplan–Meier curves represent (A) BKV viruria and (B) viremia cumulative incidence according to NAb titer (log10 IC50) at the time of transplantation (day 0 NAbs). Black curves indicate NAb titers ≤4log10 IC50 and green curves indicate NAb titers >4log10 IC50. Numbers at risk are indicated at each timepoint. Cumulative incidence values at month 24 are indicated.
Discussion
In this study involving 168 kidney transplant recipients prospectively followed over 24 months and 69 donors, for the first time, we demonstrate that (1) recipients who develop delayed and weak NAb responses are at a significantly higher risk of developing BKV viremia and BKVAN; (2) on the basis of the neutralization profile, genotype mismatch between the recipients’ and donors’ strains significantly increases the risk of developing viremia; and (3) the cutoff NAb titer value of 4 log10 IC50 against the donor’s strain is a robust marker that allows patient stratification into lower and higher BKV disease risk groups at the time of transplantation. Altogether, these results indicate that the amount and kinetics of genotype-specific NAb titers influence BKV disease severity after transplantation. Although the BKV-specific T cell immune response has been thoroughly investigated and appears essential in controlling BKV replication,14–16 this is the first study that reveals a key role of the anti-BKV antibody–mediated neutralizing response in protecting against BKV infection and associated diseases.
Analysis of the NAb titers at the time of transplantation showed a similar BKV genotype distribution in BKV DNA–negative and BKV DNA–positive recipients and donors. It should be noted that genotype II was more prevalent than genotype IV, whereas only 2% of viruses were genotype II by sequencing samples of DNA-positive BKV recipients. Similar findings were reported by Pastrana et al.,17 where genotype II seroprevalence was about 20-fold higher than prior PCR-based prevalence. This cannot be explained by PCR assay performance because our PCR assay is able to detect and adequately quantify all BKV genotypes.18,19 On the other hand, this result raises the hypothesis that BKV genotype II may be more easily cleared compared with genotype I and IV. Further investigations are needed to address this question.
In patients positive for BKV DNA after transplantation, we observed a dramatic change in BKV genotype specificity of NAbs over time with an increase of NAb titers directed specifically against the replicating strain but also against the other genotypes. Similar findings were reported in mice after monovalent prime-boost vaccination with VLPs from individual BKV subtypes that led not only to a significant increase of cognate neutralizing responses, but also to the development of crossneutralizing NAbs.17 The possible explanation could be that high BKV replication stimulates a broader range of existing memory B cells able to neutralize different BKV genotypes and which become antibody-secreting plasma cells as reported for other viruses.20 The change of BKV genotype specificity over time suggests an occurrence of a de novo (mainly of donor’s origin)21 BKV infection after transplantation. These data also confirm that patients under T cell immunosuppression can still develop efficient humoral responses.17 However, the kinetics of NAb responses differed among individuals, because patients developing nephropathy had a slower increase in BKV genotype–specific NAb titers. Fifty four percent of patients with BKVAN displayed a genotype mismatch for their replicating strain (which is assumed to be of donor origin),21 indicating that recipients had no NAbs or low titers against the donor’s genotype, as opposed to only 17% of patients who were viruric. This finding suggests a model in which for patients displaying past immunity against a BKV genotype similar to the one found in their matched donor, BKV replication in the graft may act as a “booster vaccination” eliciting a strong neutralizing response. Thus, NAbs appear to efficiently control BKV replication in the graft and prevent transition to a viremic state. In patients who fail to rapidly mount a NAb response against new donor-derived BKV genotypes, the risk of viremia is higher. Interestingly, analysis of donor/recipient HLA I mismatches showed no difference between patients who were BKV DNA-negative, viruric, viremic, and those with BKVAN (Supplemental Table 1). One can assume that both cellular and humoral immune responses protect against BKV infection. However, during transplantation, when a functional cellular reaction is impaired by immunosuppression, the neutralizing humoral response may gain importance.
In our cohort, we observed a BKVAN incidence of 8% at 2 years despite tapering of immunosuppressive therapy according to international guidelines, suggesting incomplete success of this pre-emptive intervention due to its delayed nature.8 Here, we demonstrate that patients displaying titers of NAbs directed against the donor strain lower than the cutoff value 4 log10 IC50 before transplantation have a significantly increased risk of BKV replication compared with patients harboring higher anti-BKV NAb titers. In a retrospective study, Abend et al.22 reported that donor neutralizing serostatus correlates with incidence of post-transplant BKV viremia. In our cohort, donors whose recipients became BKV DNA–positive exhibited elevated NAb titers. However, donor titers were similar for viruric, viremic, and BKVAN groups, suggesting that BKV disease outcome after reactivation depends on recipient NAb kinetics after transplantation, rather than donor characteristics. A limitation of our study is the small number of donor/recipient pairs investigated. Nevertheless, our findings point to a key role of recipients’ NAbs specific to the donor strain and demonstrate that it could be possible for patients to be further assigned to lower and higher BKV disease risk groups before transplantation. This early stratification could be useful for transplant physicians, potentially allowing adaptation of the immunosuppressive strategy and optimal patient monitoring on the basis of individual BKV infection risks. Furthermore, in patients who develop BKV infection, monitoring of NAb titers directed against the replicating strain could serve as a valuable disease progression marker in conjunction with viral DNA load measurement and prompt early clinical management. Finally, our findings support the potential benefit of administering broadly NAbs as a preventive or therapeutic strategy against BKV infection.
Concise Methods
Study Design
The study was approved by the institutional review boards of the Strasbourg University Hospitals (Clinical Trials.gov identifier: NCT02826811). All of the subjects enrolled in this study completed a written informed consent. No commercial sponsor was involved in the study.
Study Population
Adults (≥18 years of age) who underwent kidney transplantation in Strasbourg University Hospitals between July 2012 and July 2014 were enrolled in this study. Serum samples from 69 matched donors were available for the study. The following two exclusion criteria were used: (1) retransplantation due to BKVAN, and (2) no blood sample available at transplantation. On the basis of these criteria, 168 kidney transplant recipients were included and 12 were excluded.
Immunosuppression and Management of BKV Infection
Before transplantation, patients with preformed DSA or those undergoing ABO-incompatible transplantation were desensitized using rituximab and plasmapheresis. After transplantation, immunosuppression consisted of induction therapy with either basiliximab or thymoglobulins, calcineurin inhibitors (CNI, cyclosporine, or tacrolimus), mycophenolate mofetil, and steroids. Steroids were initially given at a dose of 1 mg/kg per day and were then progressively tapered off during the first 4 months post-transplant (for details refer to Supplemental Material).
BKV replication was monitored using BKV quantitative real-time PCR (BK Virus R-Gene kit, bioMérieux, Marcy l’Etoile, France)19 in urine and blood samples harvested at the time of transplantation (day 0), monthly for the first 6 months, and then every 3 months until 2 years after transplantation. The 95% limit of detection of the PCR assay was 2.4 log10 copies per milliliter.19 In case of a BKV load >8 log10 copies per milliliter in urine, mycophenolate mofetil was reduced by 50%. Detection of BKV viremia at ≥4 log10 copies per milliliter prompted adjustment of the immunosuppressive regimen with a 50% reduction of calcineurin inhibitors, a 50% reduction or cessation of mycophenolate mofetil, and introduction of leflunomide.
Allograft biopsies were performed systematically at month 3 since first January 2013 (141 recipients) and during 2 years of follow-up in case of graft dysfunction, DSA occurrence, or sustained BKV viremia (>4 log10 copies per milliliter in two subsequent blood specimens). Kidney biopsy specimens were graded according to the severity of viral cytopathic changes, inflammatory infiltrates, tubular atrophy, and fibrosis.23 BKVAN was diagnosed by immunohistochemistry with crossreacting antibodies against the large T antigen of the related simian polyomavirus (SV40 antibody, Calbiochem, San Diego, CA).
BKV genotyping was performed for all BKV DNA–positive recipients at different time points as previously described.19 the basis of the results of BKV DNA loads in urine and blood, patients were classified as negative (no detectable BKV replication during follow-up), viruric (positive viruria in at least two consecutive samplings and negative viremia), viremic (positive viruria and positive viremia), or biopsy-confirmed BKVAN.24
Neutralization Assay
NAb titers were measured at the following time points: time of transplantation (day 0); months 1, 3, 6, 12, and 24; onset of viruria and viremia; 1 month postviruria and postviremia; and viremia clearance. Neutralization assays were performed using a BKV pseudovirion system expressing the capsid proteins of BKV genotypes I, II, or IV as previously described17,25 (and Supplemental Appendix). This assay enables the quantification of antibody titers that functionally neutralize the infectivity of the different BKV genotypes, each of which is known to be a distinct neutralization serotype.17 BKV genotype III is not represented in our study because none of our patients were infected by BKV genotype III (genotype III represents <1% of BKV isolates in Europe and several studies suggest that BKV genotype II and III may belong to the same genotype).18,26 The neutralization titer was defined as the sample dilution that yielded 50% inhibition of pseudovirion infectivity (IC50) and was expressed as the log10 of the IC50. Sera were considered non-neutralizing if the 1:100 dilution (2.0 log10) did not mediate at least a 50% luminometric signal reduction relative to the control condition without serum or with negative control (i.e., 50% neutralization of the reporter vector). A neutralization titer of 2.5 log10 IC50 was set as the threshold of antibody-mediated neutralization quantification.
Statistical Analyses
The distributions of continuous data were compared using nonparametric Mann–Whitney and Kruskal–Wallis tests when comparing different groups of patients and Wilcoxon signed-rank tests for paired comparisons. The distribution of categoric variables was compared using chi-squared or Fisher exact tests. When more than two tests were performed for the same question, Holm’s correction for multiplicity was applied. The association between plasma concentrations of immunosuppressive drugs and NAb titers was assessed using Spearman’s rank correlation. Multistate models were used to estimate the transition probabilities into each state of interest (viruria, viremia, and BKVAN), and Cox proportional hazard models to assess the association of relevant variables (NAb titers, donor/recipient genotype mismatch, and urine BKV DNA load) with these transitions. Markov or semi-Markov formulations were adopted depending on the aims of the analyses. In addition to the association between NAb titer and BKV DNA load measured at baseline and at each state entry, the association between the hazards of events and NAb titers or BKV DNA loads measured over time was analyzed using joint models for the longitudinal process (NAb titers or BKV DNA load over time being alternatively considered the longitudinal processes) and the event process. NAb titers and BKV DNA loads were first analyzed as continuous variables in the models, with a nonlinear effect estimated nonparametrically by splines. If linearity could be assumed, then the HR associated with the increase of one unit of the variable was presented. The optimal NAb titer cutoff at baseline for the hazard of viruria was determined using two-fold crossvalidation to limit bias and over-optimism associated with cutoff selection.27 The capacity of the different predictors to discriminate between patients developing the different outcomes was assessed using the concordance index.28 All analyses were performed using the R statistical software version 3.2.0 (The R Foundation for Statistical Computing, Vienna, Austria).
Disclosures
None.
Supplementary Material
Acknowledgments
We thank Christopher B. Buck for expression plasmids for VP1, VP2, and VP3 and the National Cancer Institute Tumor Repository for 293TT cells. We thank Rachel Freitag for her excellent technical assistance.
This work was supported by grants from the Hôpitaux Universitaires de Strasbourg (Recherche Non-Interventionnelle [RNI] 2016 – N° 6368), the Agence Nationale de la Recherche (ANR), Laboratoire d'Excellence TRANSPLANTEX (ANR-11-LABX-0070_TRANSPLANTEX), and Institut National de la Santé et de la Recherche Médicale (UMR_S 1109).
This work was presented in abstract form on September 15, 2016 at the European Society for Clinical Virology in Lisbon, Portugal and on December 8, 2016 at the Société Francophone de Transplantation in Liege, Belgium.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2017050532/-/DCSupplemental.
References
- 1.Hirsch HH, Knowles W, Dickenmann M, Passweg J, Klimkait T, Mihatsch MJ, Steiger J: Prospective study of polyomavirus type BK replication and nephropathy in renal-transplant recipients. N Engl J Med 347: 488–496, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Hirsch HH, Randhawa P; AST Infectious Diseases Community of Practice : BK polyomavirus in solid organ transplantation. Am J Transplant 13[Suppl 4]: 179–188, 2013 [DOI] [PubMed] [Google Scholar]
- 3.Hirsch HH, Vincenti F, Friman S, Tuncer M, Citterio F, Wiecek A, Scheuermann EH, Klinger M, Russ G, Pescovitz MD, Prestele H: Polyomavirus BK replication in de novo kidney transplant patients receiving tacrolimus or cyclosporine: A prospective, randomized, multicenter study. Am J Transplant 13: 136–145, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Binet I, Nickeleit V, Hirsch HH, Prince O, Dalquen P, Gudat F, Mihatsch MJ, Thiel G: Polyomavirus disease under new immunosuppressive drugs: A cause of renal graft dysfunction and graft loss. Transplantation 67: 918–922, 1999 [DOI] [PubMed] [Google Scholar]
- 5.Kuypers DR: Management of polyomavirus-associated nephropathy in renal transplant recipients. Nat Rev Nephrol 8: 390–402, 2012 [DOI] [PubMed] [Google Scholar]
- 6.Seifert ME, Gunasekaran M, Horwedel TA, Daloul R, Storch GA, Mohanakumar T, Brennan DC: Polyomavirus reactivation and immune responses to kidney-specific self-antigens in transplantation. J Am Soc Nephrol 28: 1314–1325, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hirsch HH, Babel N, Comoli P, Friman V, Ginevri F, Jardine A, Lautenschlager I, Legendre C, Midtvedt K, Munoz P, Randhawa P, Rinaldo CH, Wieszek A: European perspective on human polyomavirus infection, replication and disease in solid organ transplantation. Clinl Microbiol Infect 20[Suppl 7]: 74–88, 2014 [DOI] [PubMed] [Google Scholar]
- 8.Knight RJ, Gaber LW, Patel SJ, DeVos JM, Moore LW, Gaber AO: Screening for BK viremia reduces but does not eliminate the risk of BK nephropathy: A single-center retrospective analysis. Transplantation 95: 949–954, 2013 [DOI] [PubMed] [Google Scholar]
- 9.Wright AJ, Gill JS: Strategies to prevent BK virus infection in kidney transplant recipients. Curr Opin Infect Dis 29: 353–358, 2016 [DOI] [PubMed] [Google Scholar]
- 10.Sawinski D, Forde KA, Trofe-Clark J, Patel P, Olivera B, Goral S, Bloom RD: Persistent BK viremia does not increase intermediate-term graft loss but is associated with de novo donor-specific antibodies. J Am Soc Nephrol 26: 966–975, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fafi-Kremer S, Fofana I, Soulier E, Carolla P, Meuleman P, Leroux-Roels G, Patel AH, Cosset FL, Pessaux P, Doffoël M, Wolf P, Stoll-Keller F, Baumert TF: Viral entry and escape from antibody-mediated neutralization influence hepatitis C virus reinfection in liver transplantation. J Exp Med 207: 2019–2031, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walker LM, Huber M, Doores KJ, Falkowska E, Pejchal R, Julien JP, Wang SK, Ramos A, Chan-Hui PY, Moyle M, Mitcham JL, Hammond PW, Olsen OA, Phung P, Fling S, Wong CH, Phogat S, Wrin T, Simek MD, Koff WC, Wilson IA, Burton DR, Poignard P; Protocol G Principal Investigators : Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature 477: 466–470, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burton DR, Poignard P, Stanfield RL, Wilson IA: Broadly neutralizing antibodies present new prospects to counter highly antigenically diverse viruses. Science 337: 183–186, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ginevri F, Azzi A, Hirsch HH, Basso S, Fontana I, Cioni M, Bodaghi S, Salotti V, Rinieri A, Botti G, Perfumo F, Locatelli F, Comoli P: Prospective monitoring of polyomavirus BK replication and impact of pre-emptive intervention in pediatric kidney recipients. Am J Transplant 7: 2727–2735, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Mueller K, Schachtner T, Sattler A, Meier S, Friedrich P, Trydzenskaya H, Hinrichs C, Trappe R, Thiel A, Reinke P, Babel N: BK-VP3 as a new target of cellular immunity in BK virus infection. Transplantation 91: 100–107, 2011 [DOI] [PubMed] [Google Scholar]
- 16.Schachtner T, Stein M, Babel N, Reinke P: The loss of BKV-specific immunity from pretransplantation to posttransplantation identifies kidney transplant recipients at increased risk of BKV replication. Am J Transplant 15: 2159–2169, 2015 [DOI] [PubMed] [Google Scholar]
- 17.Pastrana DV, Ray U, Magaldi TG, Schowalter RM, Çuburu N, Buck CB: BK polyomavirus genotypes represent distinct serotypes with distinct entry tropism. J Virol 87: 10105–10113, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Solis M, Meddeb M, Sueur C, Domingo-Calap P, Soulier E, Chabaud A, Perrin P, Moulin B, Bahram S, Stoll-Keller F, Caillard S, Barth H, Fafi-Kremer S; French BKV Study Group : Sequence variation in amplification target genes and standards influences interlaboratory comparison of BK virus DNA load measurement. J Clin Microbiol 53: 3842–3852, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sueur C, Solis M, Meddeb M, Soulier E, Domingo-Calap P, Lepiller Q, Freitag R, Bahram S, Caillard S, Barth H, Stoll-Keller F, Fafi-Kremer S: Toward standardization of BK virus monitoring: Evaluation of the BK virus R-gene kit for quantification of BK viral load in urine, whole-blood, and plasma specimens. J Clin Microbiol 52: 4298–4304, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Purtha WE, Tedder TF, Johnson S, Bhattacharya D, Diamond MS: Memory B cells, but not long-lived plasma cells, possess antigen specificities for viral escape mutants. J Exp Med 208: 2599–2606, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmitt C, Raggub L, Linnenweber-Held S, Adams O, Schwarz A, Heim A: Donor origin of BKV replication after kidney transplantation. J Clin Virol 59: 120–125, 2014 [DOI] [PubMed] [Google Scholar]
- 22.Abend JR, Changala M, Sathe A, Casey F, Kistler A, Chandran S, Howard A, Wojciechowski D: Correlation of BK virus neutralizing serostatus with the incidence of BK viremia in kidney transplant recipients. Transplantation 101:1495–1505, 2017 [DOI] [PubMed] [Google Scholar]
- 23.Drachenberg CB, Papadimitriou JC, Hirsch HH, Wali R, Crowder C, Nogueira J, Cangro CB, Mendley S, Mian A, Ramos E: Histological patterns of polyomavirus nephropathy: Correlation with graft outcome and viral load. Am J Transplant 4: 2082–2092, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Masutani K, Shapiro R, Basu A, Tan H, Wijkstrom M, Randhawa P: The Banff 2009 Working Proposal for polyomavirus nephropathy: A critical evaluation of its utility as a determinant of clinical outcome. Am J Transplant 12: 907–918, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pastrana DV, Brennan DC, Cuburu N, Storch GA, Viscidi RP, Randhawa PS, Buck CB: Neutralization serotyping of BK polyomavirus infection in kidney transplant recipients. PLoS Pathog 8: e1002650, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luo C, Bueno M, Kant J, Randhawa P: Biologic diversity of polyomavirus BK genomic sequences: Implications for molecular diagnostic laboratories. J Med Virol 80: 1850–1857, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Faraggi D, Simon R: A simulation study of cross-validation for selecting an optimal cutpoint in univariate survival analysis. Stat Med 15: 2203–2213, 1996 [DOI] [PubMed] [Google Scholar]
- 28.Wolbers M, Blanche P, Koller MT, Witteman JC, Gerds TA: Concordance for prognostic models with competing risks. Biostatistics 15: 526–539, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.