Abstract
Preconditioning with a low dose of endotoxin confers unparalleled protection against otherwise lethal models of sepsis. The mechanisms of preconditioning have been investigated extensively in isolated immune cells such as macrophages. However, the role of tissue in mediating the protective response generated by preconditioning remains unknown. Here, using the kidney as a model organ, we investigated cell type–specific responses to preconditioning. Compared with preadministration of vehicle, endotoxin preconditioning in the cecal ligation and puncture mouse model of sepsis led to significantly enhanced survival and reduced bacterial load in several organs. Furthermore, endotoxin preconditioning reduced serum levels of proinflammatory cytokines, upregulated molecular pathways involved in phagocytosis, and prevented the renal function decline and injury induced in mice by a toxic dose of endotoxin. The protective phenotype involved the clustering of macrophages around S1 segments of proximal tubules, and full renal protection required both macrophages and renal tubular cells. Using unbiased S1 transcriptomic and tissue metabolomic approaches, we identified multiple protective molecules that were operative in preconditioned animals, including molecules involved in antibacterial defense, redox balance, and tissue healing. We conclude that preconditioning reprograms macrophages and tubules to generate a protective environment, in which tissue health is preserved and immunity is controlled yet effective. Endotoxin preconditioning can thus be used as a discovery platform, and understanding the role and participation of both tissue and macrophages will help refine targeted therapies for sepsis.
Keywords: sepsis, innate immunity, tlr4, metabolomics, transcriptomics
Systemic Gram-negative infections often result in sepsis, a condition of global organ failure and hemodynamic collapse. Despite decades of efforts at the bench and bedside, sepsis continues to be associated with unacceptably high mortality and morbidity.1,2 Amid numerous unsuccessful therapeutic approaches, supportive therapy with fluids and antibiotics remain the mainstay of treatment. Recent advances in our understanding of the role of endotoxin (LPS) and its receptor, Toll-like receptor 4 (TLR4), in the pathophysiology of sepsis have rekindled the hope for a more targeted therapy for this grave condition.3 Unfortunately, approaches that antagonize the endotoxin–TLR4 axis have not proven successful.4–6 The inhibition of TLR4, despite desensitizing the organism against endotoxin, can result in widespread bacterial growth and increased susceptibility to overwhelming infection.7 This indicates that interactions between endotoxin and TLR4 are not merely fueling the disease, but are also needed to fight the infection.
One example of the role of careful and selective TLR4 activation by endotoxin is the old phenomenon of preconditioning.8–10 Indeed, it is known that pre-exposure to low-dose endotoxin can result in complete protection against otherwise lethal endotoxin challenges.11,12 However, whether preconditioning is also protective against actual bacterial invasion or is rather a state of anergy and immunosuppression remains somewhat controversial.13–16 In addition, the target cells that mediate and propagate the effects of endotoxin preconditioning remain unknown.17 To date, the macrophage has been implicated as the most likely mediator of endotoxin preconditioning. In fact, we have also shown in an in vivo model of sepsis that macrophage subsets are indeed essential for successful preconditioning.18 However, in sepsis, renal toxicity is mediated primarily through TLR4-dependent, S1 tubule endotoxin uptake and is independent of macrophages.19 This raises the possibility that, in addition to macrophages, an important part of protective preconditioning may be mediated by the tissue itself.
In this study, we show that both renal tubules and macrophages participate in mediating the protective effects of preconditioning and creating an environment in which tissue health is preserved along with effective yet controlled immunity. Combined S1 transcriptomics and tissue metabolomics revealed several downstream molecules that are likely mediators of tissue protection. Therefore, preconditioning is a powerful discovery platform that can be used to investigate the organ’s inherent defenses against infection. The identified protective cellular, molecular, and metabolic pathways are promising tools for the prevention and treatment for sepsis.
Results
Endotoxin Preconditioning Confers Tissue Protection and Improves Survival in Models of Sepsis
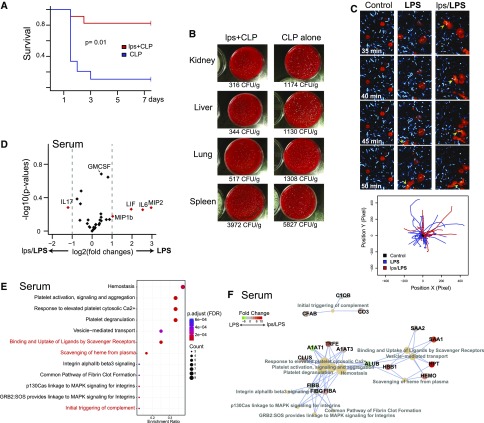
Cecal ligation and puncture (CLP) is a widely used model of sepsis, with mortality approaching 100% by 7 days.20 Endotoxin preconditioning dramatically increased survival to about 80% over the same time period (Figure 1A). The improved survival correlated with significantly reduced bacterial counts in various organs, indicating intact phagocytic function and antibacterial defense (Figure 1B). In fact, we have previously shown that active M2 macrophages are absolutely necessary for the full development of protective preconditioning.18 Here, we show that these macrophages, when isolated from preconditioned animals, responded normally to Escherichia coli stimulation by exhibiting active mobility and phagocytosis (Figure 1C). Furthermore, despite the increased and highly efficient phagocytic activity, preconditioned animals did not mount a high inflammatory response as evidenced by serum cytokine measurements (Figure 1D). In addition, serum proteomics revealed upregulation of global protective pathways including those involved in phagocytosis, such as complements and scavenging proteins (Figure 1, E and F, Supplemental Figure 1). Collectively, these data indicate that endotoxin preconditioning results in increased survival along with a controlled, yet effective immune response.
Figure 1.
Endotoxin preconditioning improves survival, reduces inflammation, and maintains scavenging ability. (A) Male C57BL/6 mice were subjected to CLP alone (blue; nonpreconditioned), or to 0.25 mg/kg endotoxin (E. coli serotype 0111:B4) followed 24 hours later by CLP (red; preconditioned), and survival was determined at indicated time points (n=8 per group). The preconditioning dose of endotoxin is denoted as lps in order to distinguish it from high-dose endotoxin (LPS, 5 mg/kg), which is used in subsequent experiments. The CLP alone group received an equivalent dose of sterile normal saline vehicle. P=0.01 by Cox proportional hazards model likelihood ratio. (B) Indicated organs were harvested 24 hours after CLP and bacterial count, estimated from CFU was adjusted for each organ’s weight (n=2 per group with triplicates for each dish). (C) Peritoneal macrophages (red; CellTracker) were incubated with live E. coli (cyan; CFP), and time-lapse images were obtained to assess phagocytic activities. These macrophages were harvested from C57BL/6 mice 12 hours after saline vehicle (control), LPS 5 mg/kg (LPS), or lps 0.25 mg/kg followed 24 hours later by 5 mg/kg administered intraperitoneally (lps/LPS). Arrowheads follow specific mobile macrophages with phagocytized E. coli (yellow) over time. Tracking of cells was performed using a custom plugin for ImageJ.18 Although macrophages from unstimulated mice showed minimal activity, macrophages from LPS and lps/LPS groups exhibited high mobility and phagocytosis. Original magnification ×60. (D) Volcano plot of serum cytokine/chemokine multiplex panel. The serum cytokine/chemokine levels were measured 4 hours after 5 mg/kg LPS administered intraperitoneally. Positive-value log2 fold changes on the x-axis indicate LPS (nonpreconditioned) > lps/LPS (preconditioned) (n=3 per group). (E and F) Differentially expressed serum proteins and their associated pathways are shown (LPS alone versus lps/LPS; Reactome pathway analysis37). Proteins were identified using two-dimensional difference gel electrophoresis in tandem with mass spectrometry (Supplemental Figure 1A). Protein extracts from serum were collected 4 hours after the 5 mg/kg LPS dose for both nonpreconditioned and preconditioned animals. Three samples were pooled for each experimental condition.
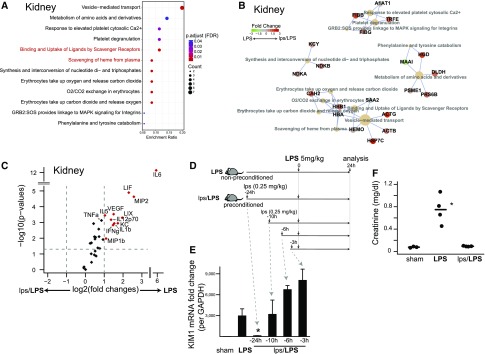
AKI is a common complication of sepsis that further increases mortality and morbidity.2,21 We have previously shown that preconditioning reduces tubular oxidative stress, prevents peroxisomal damage, and increases protective tubular proteins such as heme oxygenase 1.18 Here, and in parallel to the protected systemic phenotype (Figure 1), we show that preconditioning preserved kidney function as measured by serum creatinine, upregulated scavenging proteins, and reduced tubular injury (kidney injury molecule-1) and inflammatory markers (Figure 2, A–F). In addition, we determined that a lead time (24 hours) is required for effective preconditioning and this regimen is used throughout the rest of experiments (Figure 2D). In summary, we have established a robust model of preconditioning that provides a solid foundation for the investigation of in vivo protective mechanisms.
Figure 2.
Endotoxin preconditioning confers renal tissue protection. (A and B) Differentially expressed kidney proteins and their associated pathways are shown (LPS alone versus lps/LPS). (C) Volcano plot of kidney cytokine/chemokine multiplex panel. Proteins were extracted from whole kidney tissues 4 hours after the 5 mg/kg LPS dose. (D and E) Preconditioning dose endotoxin (0.25 mg/kg lps) was administered to mice at indicated time points followed by toxic-dose LPS (5 mg/kg; n=4 per group). Renal tissue kidney injury molecule-1 (KIM1) levels were determined using quantitative PCR 24 hours after 5 mg/kg intraperitoneal LPS, and normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Note that KIM1 is an established renal proximal tubule injury marker whose level correlates well with other kidney injury and functional markers, such as BUN, in models of sepsis.18 *P<0.01 versus nonpreconditioned LPS 5 mg/kg. Error bars are SD. (F) Serum creatinine level was measured 24 hours after the LPS 5 mg/kg dose.
Macrophages Are Necessary but Not Sufficient for Renal Protection
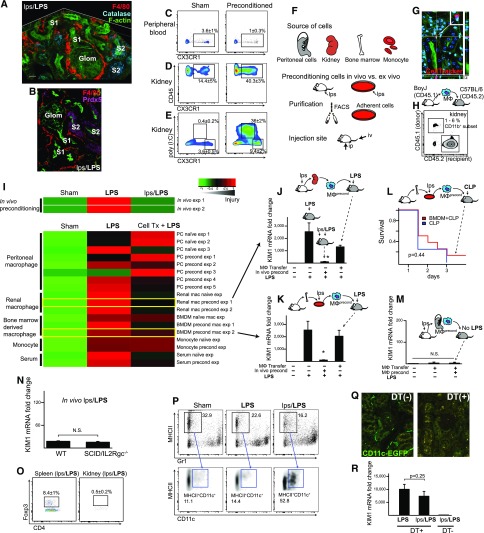
Previously, intravital imaging of the protected kidney in preconditioned mice revealed highly increased number of trafficking macrophages around renal tubules.18 This macrophage activity culminated in their clustering, specifically around S1 proximal tubular segments18 (Figure 3, A and B). These S1 proximal tubules are the site of TLR4-mediated, renal endotoxin uptake.19 Because the increased macrophage activity and clustering was not observed in the nonpreconditioned animals,18 we hypothesized that macrophage clustering is an essential component of preconditioning-induced renal protection, possibly through macrophage–tubule crosstalk. Furthermore, because these protective macrophages were likely derived from extrarenal sources (Figure 3, C–E), we tested whether infused preconditioned macrophages could be used for the treatment of sepsis and kidney injury. Here, we show that preconditioned macrophages resulted, at most, in partial protection when infused into septic animals. This was despite a number of optimization including the source and number of macrophages, as well as the route and timing of infusion (Figure 3, F–M, Supplemental Table 1). We further noted that only about 5% of total renal macrophages could be replaced by the infused cells. Therefore, the failure of protection could be due to (1) insufficient macrophage homing to the kidney, (2) the requirement for other immune cells, and (3) a necessary participation of renal tissue, such as the S1 tubular epithelial cell.
Figure 3.
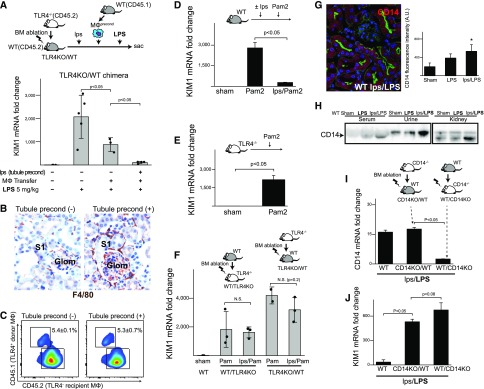
Adoptive macrophage transfer confers partial protection. (A and B) Endotoxin preconditioning results in macrophage clustering around S1 segments. Representative fluorescence microscopy of fixed kidney sections from male C57BL/6 mice treated with lps/LPS. The tissues were immunostained for F4/80 (red), F-actin (green), and catalase (a marker of S2/S3 segments; blue) in Figure 2A (z-stack reconstruct), or Prdx5 (peroxiredoxin 5, a marker of S2/S3; purple) in Figure 2B. Note that male mice have extension of S1 segment into the Bowman’s space in the glomerulus. Original magnification, ×60. (C and D) Flow cytometric analysis of peripheral leukocytes and renal myeloid cells isolated from CX3CR1-EGFP mice treated with vehicle control (sham) or 0.25 mg/kg lps for 24 hours (preconditioned). Note the decrease in the number of peripheral CX3Cr1+ cells and concurrent increase in the number of kidney CX3CR1+ cells. (E) Poly (I:C), a TLR3 ligand, was administered intravenously 20 minutes before harvesting the kidneys to label preconditioned mobile macrophages, as previously reported.18 n=2 per group. (F) Schematic of adoptive macrophage transfer optimization procedure. (G) Approximately 1×106 preconditioned macrophages (0.25 mg/kg intraperitoneal lps at 24 hours) were labeled with CellTracker (red) and administered via tail vein (blue, DAPI; green, F-actin). Representative microscopy of kidney tissue is shown. Original magnification ×60. (H) To quantify renal homing, macrophages from donor mice (BoyJ; CD45.1+) were infused into C57BL/6 recipients (CD45.2+) and kidney myeloid cells were analyzed with flow cytometry. Under various adoptive transfer experimental conditions (Supplemental Table 1), donor cells constituted only 1%–6% of renal macrophages. (I) Summary of adoptive cell transfer experiments is shown (details in Supplemental Table 1). The degree of renal protection was determined with KIM1 mRNA levels of kidney tissues after the 5 mg/kg LPS dose. Injury levels are normalized to GAPDH and scaled for each row with indicated color gradient. n=3 per group per experiment. (J and K) The results of two specific experimental designs are shown. (L) In a model of CLP, preconditioned macrophage transfer did not result in significant survival improvement. P=0.44 by Cox proportional hazards model likelihood ratio (n=8 per group). (M) Macrophage transfer itself did not cause kidney injury. (N) SCID/IL2Rgc−/− mice lack mature T cells, B cells, and natural killer cells. These mice mounted successful preconditioning as indicated by low KIM1 levels comparable with those of C57BL/6 WT mice after lps/LPS treatment (n=3). (O) Although measurable in the spleen, the number of regulatory T cells (CD4+CD25+Foxp3+) in the kidneys was minimal in all conditions (sham, LPS, lps/LPS). (P) The number of MHCII+CD11c+ macrophage and/or dendritic cell subsets was increased in the preconditioned kidney. (Q and R) CD11c-diphtheriae toxin receptor mice were injected with C. diphtheriae toxin (DT+; 5 ng/g body wt) or its vehicle (DT-) to deplete CD11c+ cells. DT depletion persisted for at least for 4 days in mice that received lps/LPS. In these mice with depleted CD11c+ cells, preconditioning failed to protect the kidney as shown by KIM1 levels. Original magnification ×60. *P<0.01 versus nonpreconditioned LPS 5 mg/kg. Error bars are SD.
Using SCID/IL2Rgc−/− mice, we excluded possible involvement of T, B, and natural killer cells in successful preconditioning (Figure 3, N and O). Note that a possible involvement of renal dendritic cells could not be determined because of the significant overlap with macrophages, both phenotypically and in depletion models (Figure 3, P–R). In addition, the possibility of direct negative effect of diphtheria toxin could not be excluded in the dendritic cell depletion model. Indeed, this model resulted in high mortality after endotoxin treatment. In surviving animals, preconditioning failed to protect the kidney indicating a possible role for CD11c+ cells.
Therefore, we next investigated the role of the renal tubule as an essential participant in the generation of the protected phenotype.
Tubular Preconditioning Is Required for Full Protection
To test whether tubular preconditioning is indeed required for full protection, we made use of TLR4 knockout (TLR4KO) into wild-type (WT) bone marrow chimeras (TLR4KO/WT) in which nearly 100% of renal macrophages are replaced by TLR4KO donor cells.18 Endotoxin preconditioning in this chimera would precondition the tubules but not the endogenous TLR4KO macrophages, thus allowing the investigation of the combined roles of tubular preconditioning and infused preconditioned macrophages. Indeed, we show that the full protection was obtained in chimeric mice that underwent both tubular preconditioning and macrophage transfer, indicating that tubular preconditioning contributed significantly to full tissue protection (Figure 4A). Notably, macrophage clustering was observed only in the fully protected chimeras, confirming that macrophage clustering is a feature of successful, robust preconditioning (Figure 4B). Interestingly, tubular preconditioning did not cause preferential recruitment of adoptively transferred macrophages to the kidney. Indeed, despite full protection, donor macrophages still constituted only about 5% of total renal macrophages (Figure 4C). This indicates that the majority of clustered macrophages acquired a protective phenotype, possibly through direct or indirect interactions with donor macrophages and/or renal tubules.
Figure 4.
Tissue preconditioning is required for full renal protection. (A) To test the combined roles of tubular preconditioning and macrophage transfer, TLR4KO/WT mice (CD45.2+) were intraperitoneally injected with 0.25 mg/kg lps or vehicle followed 24 hours later by the intravenous infusion of ex vivo preconditioned CD45.1+ bone marrow–derived macrophages (lps 10 ng/ml for 24 hours) or an equivalent volume of saline vehicle. These mice were subsequently challenged with 5 mg/kg intraperitoneal LPS as shown in the diagram. The degree of tubular injury was determined with KIM1 mRNA levels of kidney tissues 24 hours after the 5 mg/kg LPS dose, normalized to GAPDH. (B) Representative images of F4/80 staining (brown) are shown for TLR4KO/WT mice with and without tubular preconditioning. Note the clustering of F4/80+ macrophages around the S1 proximal tubular segment with tubular preconditioning. Original magnification ×60. (C) The number of donor macrophages (CD45.1) that homed to the recipient kidneys (CD45.2; TLR4KO/WT chimeras) with and without tubular preconditioning. Representative flow cytometric data that specifically correspond to the F4/80 staining data are shown (i.e., matched contralateral kidneys; n=3 per group). (D) Lps 0.25 mg/kg preconditioning leads to crosstolerance against Pam2CSK4 (a ligand of TLR2/6; 2.5 mg/kg administered intraperitoneally). n=3 for each group. (E) The degree of Pam2CSK4-induced kidney injury in TLR4KO mice determined by KIM1 mRNA levels. n=3 for each condition. (F) WT/TLR4KO and TLR4KO/WT bone marrow chimeric mice were treated with 0.25 mg/kg intraperitoneal lps or vehicle followed 24 hours later by Pam2CSK4 2.5 mg/kg. Protective effect of endotoxin preconditioning was abrogated in both WT/TLR4KO and TLR4KO/WT. (G) CD14 is expressed in renal proximal tubular cells. Fluorescence microscopy of kidney section from preconditioned WT mice immunostained for CD14. Original magnification ×60. Quantitation of CD14 tubular fluorescence intensity for the indicated conditions is shown. (H) Western blot analysis of CD14 under indicated conditions. The high molecular weight CD14 observed in the urine is possibly due to the membrane-bound form. (I) CD14 knockout (CD14KO)/WT and WT/CD14KO bone marrow chimeric mice were generated and CD14 mRNA levels were determined after lps/LPS treatment. CD14 mRNA expression levels were significantly higher in CD14KO/WT mice, indicating that nonimmune cell–derived CD14 contributed significantly to the overall CD14 level in the kidney (n=3 per group). (J) CD14KO/WT and WT/CD14KO chimeras were subjected to lps/LPS and both chimeras failed to mount protection.
The reverse chimera, WT/TLR4KO, lacks TLR4 in renal tubules but not macrophages. This chimera would therefore be a good model to further investigate the role of tubular preconditioning without the inherent problems of adoptive macrophage transfer. This is because endotoxin is sensed exclusively by the endogenous macrophages and not the renal tubules in this chimera. However, as we have shown previously, this chimera is not susceptible to endotoxin toxicity, which is mediated by tubular TLR4,18,19 and therefore cannot be used to assess the necessary role of tubular preconditioning. To bypass this limitation, we exploited the phenomenon of crosstolerance in which preconditioning with the TLR4 agonist endotoxin is used to protect against toxicity from the TLR2 ligand, Pam2. Indeed, as shown in Figure 4D, endotoxin preconditioning effectively protects against Pam2 toxicity in the WT animal. Furthermore, we show that Pam2 is an effective toxin in TLR4KO mice that are resistant to endotoxin (Figure 4E). Using this model, we found that endotoxin-preconditioned WT/TLR4KO (where tubules could not sense endotoxin) failed to mount protection against Pam2, confirming that tubular preconditioning is necessary. The reverse chimera TLR4KO/WT (where macrophages could not sense endotoxin) also failed to mount protective preconditioning (Figure 4F). Finally, using chimeras between WT and CD14, a coreceptor of TLR4, we found that both macrophages and renal tubule CD14 is also required for full protection (Figure 4, G–J). Collectively, these findings indicate that full preconditioning requires concordant activation of macrophages and renal tubules.
S1 Generates Local Chemokine Gradient
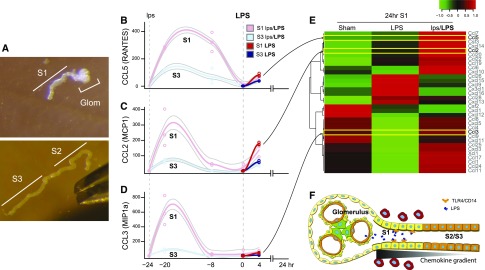
We next asked what mediates the preferential local clustering of macrophages around S1 as opposed to the more downstream S2/S3 segments in the preconditioned animal. We hypothesized that, despite the overall downregulation of chemokines/cytokines in the total kidney, their cell type–specific expression levels could be significantly different, thereby generating local chemokine gradient and promoting macrophage clustering around S1. Indeed, cell type–specific analysis revealed that a set of S1-derived chemokines had robust responses to preconditioning, and were not induced in the downstream S3 segments (Figure 5, A–E). This local chemokine/cytokine gradient can therefore explain the clustering of macrophages around S1 (Figure 5F). Such a microenvironmental signature could not be detected in the whole kidney homogenate analysis (Figure 2C).
Figure 5.
A set of chemokines/cytokines are increased in preconditioned S1 tubules. (A) Mouse tubule manual microdissection was performed to isolate S1 and S3 proximal tubule subsegments. Dumont Forceps #5 super thin tips are also shown in the bottom picture. Original magnification ×5. (B–E) Select chemokine levels for S1 and S3 segments were determined by quantitative PCR at −24, −20, and −8 hours before LPS, as well as 0 and 4 hours post LPS. Locally weighted regression curve fitting was applied for generating the trajectories and error lines. n=3 for each time point per group. The 24-hour time point data are derived from transcriptomics of laser microdissected S1 as described in Figure 6. (F) Model of local chemokine gradient in the kidney that explains macrophage clustering around S1.
Protective Pathways Operative in Preconditioning
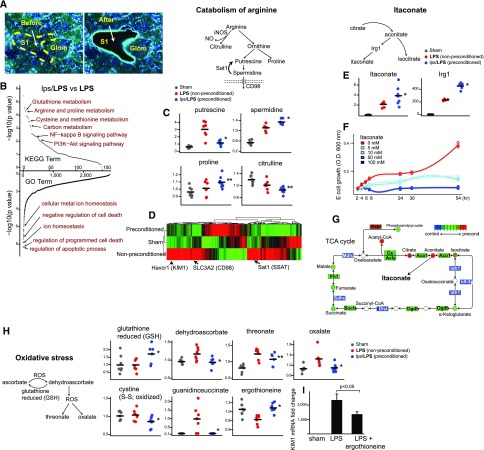
We next sought to identify protective pathways that may arise from the macrophage–S1 tubule microenvironment. S1-specific transcriptomics analysis revealed a number of pathways that could be important for mediating tissue protection (Figure 6, A and B, Supplemental Figures 2 and 3). To reinforce confidence and to filter out less relevant biologic changes, we also performed metabolomics analysis and integrated these two omics data sets (Supplemental Figure 4). We found that arginine catabolism was skewed globally toward proline and spermidine,22 and away from citrulline in the preconditioned kidneys, consistent with protective M2 polarization (Figure 6C). Conversely, nonpreconditioned, endotoxin-treated kidneys had lower levels of spermidine and instead, had accumulation of its precursor putrescine. This is likely due, in part, to significantly increased spermidine/spermine N1-acetyltransferase 1 (Sat1), which contributes to depletion of spermidine (Figure 6D). It has been shown that Sat1-knockout mice are less susceptible to endotoxin-induced kidney injury.23 Therefore, spermidine, discovered by an unbiased omics approach, is likely an important intermediary of protective preconditioning.
Figure 6.
Renal transcriptomics and metabolomics reveal the signature of preconditioning. (A) An example of S1 segment collected by laser microdissection. Original magnification ×60. (B) Transcriptomics pathway enrichment analysis comparing LPS and lps/LPS S1 samples (n=4 for LPS, n=5 for lps/LPS). Approximately 160 Kyoto Encyclopedia of Genes and Genomics (KEGG) metabolic pathways and >2000 Gene Ontology terms are aligned in the order of statistical significance. (C) Key metabolite levels involved in arginine and proline metabolism pathway are shown. Tissue repair molecules spermidine and proline were elevated in the preconditioned kidneys, whereas cytotoxic putrescine was elevated in nonpreconditioned kidneys. Metabolites were measured with ultrahigh performance liquid chromatography-tandem mass spectroscopy performed on kidney tissues under the indicated conditions (n=8 per group). (D) The heat map generated from top 500 genes by ANOVA is shown to highlight unique gene expression changes in S1 under the indicated conditions and significant increases in the expression levels of Sat1 and CD98, both of which can contribute to depletion of spermidine in the nonpreconditioned mice. KIM1 location is also shown as reference. (E) Antimicrobial molecule itaconate and its activating enzyme Irg1 (also known as Acod1) levels in preconditioned kidneys. Itaconate arises from TCA cycle intermediate aconitate as shown in the diagram. Irg1 levels were measured by quantitative PCR 4 hours after LPS 5 mg/kg. (F) Dose-dependent effect of itaconate on E. coli growth. To determine specifically the inhibitory effect of itaconate on isocitrate lyase, glucose depleted media with sodium acetate supplementation was used to induce glyoxylate shunt in bacteria. (G) Coordinated global TCA gene and metabolite level changes favoring increase in itaconate in preconditioned animals are summarized. (H) Metabolites involved in redox regulation. Ascorbate pathway is shown to illustrate the deranged redox balance in nonpreconditioned, LPS-treated kidneys. Guanidinosuccinate is a uremic toxin and emerges from oxidation of argininosuccinic acid by reactive oxygen species (ROS). Protective antioxidant ergothioneine and reduced glutathione levels were preserved in preconditioned mice. (I) Mice were intraperitoneally subjected to vehicle (PBS) or ergothioneine (50 mg/g) 1 hour after LPS and injury levels were determined by KIM1. n=3 per group. *P<0.05 versus LPS; **P>0.05 versus LPS.
In addition to arginine catabolism, we also found that the Irg1/Acod1 gene and its product itaconate levels are significantly increased in the preconditioned kidneys (Figure 6E). Itaconate is a molecule recently found to have important antibacterial and anti-inflammatory properties.24–26 It arises from the TCA intermediate aconitate and exerts its bactericidal effect through inhibition of isocitrate lyase,24 an enzyme essential for bacterial survival (Figure 6F). Itaconate also exerts its anti-inflammatory effect by inhibiting succinate dehydrogenase-mediated oxidation.27,28 Indeed, our data demonstrate that endotoxin preconditioning induced a large-scale reprogramming in the TCA cycle, giving rise to effective generation of itaconate (Figure 6G). Together with the recent strong in vivo evidence,27 the upregulation of the Irg1–itaconate axis is likely an important component of protective preconditioning.
Finally, as shown in Figure 6H, deregulated redox balance is a prominent feature of endotoxin-induced kidney injury. When compared with the preconditioned kidney tissues, nonpreconditioned kidneys had significantly increased levels of oxidized metabolites and decreased levels of free radical, scavenging species such as ergothioneine (Figure 6H). Ergothioneine is a powerful scavenger of hydroxyl radicals, and its transporter (Slc22a4) is predominantly expressed in the kidney and CD14+ monocytes.29 Therefore, we hypothesized that ergothioneine is a particularly relevant scavenging metabolite involved in renal protection. Indeed, we found that supplementation of ergothioneine reduced sepsis-induced kidney injury, underscoring the importance of restoring redox balance (Figure 6I). Note, however, that the protection with ergothioneine, although significant, was not complete as would be the case with endotoxin preconditioning. In fact, itaconate and spermidine also resulted in marginal protection in various sepsis models (Supplemental Figure 5). We reasoned that the lack of complete resolution of injury after treatment with these metabolites is because of the requirement for multiple, equally important pathways in sepsis and preconditioning. It is therefore very likely that successful sepsis therapy will require a carefully timed and dosed combination of multiple metabolites. It is beyond the scope of this article to attempt all possible combinations of metabolite-based therapy. However, we list several additional potential therapeutic targets in Supplemental Table 2 (see Supplemental Material for references) and provide annotated transcriptomics and metabolomics data sets (Supplemental Figures 3 and 4). We note that a number of genes activated by endotoxin are located in the vicinity of heterochromatin (Supplemental Figure 4), implying that epigenetic regulation is possibly involved in the preconditioning response. The provided data can be used by the scientific community as a platform for discovery.
Discussion
In models of sepsis, endotoxin preconditioning conveys global protection that, to our knowledge, is not matched by any other therapeutic intervention. In this article, we show that the protective pathways activated by preconditioning require the participation of a set of macrophages, as well as a tissue response that is likely unique to each individual organ. This crosstalk between macrophages and tissue ultimately generates the protective environment that maintains effective immunity without collateral damage. To our knowledge, this is the first demonstration of the essential role of specific renal epithelial cells in mounting protective preconditioning.
In the kidney, this tissue response to preconditioning is centered around S1 proximal tubular segments. Indeed, the S1 segment occupies an upstream position that makes it uniquely poised to function as the primary sensor of the glomerular filtrate.30,31 As such, the S1 segment relays systemic signals found in the filtrate to the rest of the nephron, thus orchestrating the overall response of the kidney. This function of S1 is served by a robust endocytic machinery including TLR4/CD14-mediated endocytosis, as well as multiple autoprotective mechanisms that we have previously described.19
Here, we show that protective preconditioning is mediated by a functional unit composed of macrophages clustered around S1 tubules. Although both macrophages and S1 epithelial cells are essential components of this unit, the exact mechanisms operative in their crosstalk remain to be investigated. This functional unit activates multiple protective pathways involved in phagocytosis, redox balance, and tissue healing. It is also likely that protective preconditioning involves broader epigenetic regulation.10
Our combined use of S1 transcriptomics and tissue metabolomics resolved protective pathways with high accuracy by reducing noise inherent to any single omics approach. Although transcriptomics alone identified several potential target proteins, the translational potential is challenging. This is because sepsis is a rapidly evolving condition in which targeting multiple upstream proteins and pathways is difficult because of drug delivery problems, efficiency, and cost. In contrast, unbiased metabolomics identified several downstream products that are the propagators of tissue protection. We believe that many of these protective metabolites are also operative in other organs. Metabolites can be relatively safe, inexpensive, and can provide widespread tissue protection that is not limited to one particular organ. Although we discussed three such metabolites, our study identified several others that warrant further investigation. Ultimately, we believe that one form of sepsis therapy would involve the judicious use of metabolite combinations given at specific points in the sepsis timeline.
Concise Methods
Animals
All animal protocols were approved by Indiana University Institutional Animal Care Committee and conform to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Male mouse strains C57BL/6J, B6.SJL-PtpcraPepcb/BoyJ (BoyJ), B6.129S-Cd14tm1Frm/J (CD14−/−), B6.B10ScN-Tlr4lps-del/JthJ (TLR4−/−), B6.FVB-TG(Itgax-DTR/EGFP)57Lan/J (CD11c-DTR), and heterozygous B6.129P-Cx3cr1tm1Litt/J (CX3CR1EGFP/WT) were obtained from the Jackson Laboratory. Nonobese/severe combined immunodeficient mice (SCID/IL2Rgc−/−) were maintained under specific pathogen-free conditions at the Indiana University Laboratory Animal Resource Center. Mice were 8–12 weeks of age and weighed 20–30 g.
Endotoxin Preconditioning
Nonpreconditioned animals were subjected to a single-dose, 5 mg/kg lipopolysaccharide (LPS) intraperitoneal injection. Preconditioned animals were subjected to 0.25 mg/kg lipopolysaccharide (lps) intraperitoneal injection followed 24 hours later by 5 mg/kg LPS intraperitoneal injection. Untreated mice received an equivalent volume of sterile, normal saline vehicle. LPS from E. coli serotype 0111:B4 (smooth strain, L2630; Sigma) was used in all experiments except for CD14−/− mouse experiments, in which Salmonella enterica serovar Minnesota Re 595 (rough strain, L9764; Sigma) was used. This is because smooth LPS (but not rough LPS) is unable to engage in TLR4-MyD88 signaling in the absence of CD14.32 To deplete CD11c+ cells, CD11c-DTR mice were intraperitoneally injected with Corynebacterium diphtheriae (5 ng/g body wt; D0564; Sigma). Preconditioning dose lps was administered intraperitoneally at 24 hours after diphtheria toxin injection, and toxic-dose LPS was administered 48 hours after diphtheria toxin injection. Pam2CSK4 (tlrl-pm2s-1, TLR2/TLR6 ligand; InvivoGen) was resuspended with endotoxin-free water and administered intraperitoneally (2.5 mg/kg). Measurement of serum creatinine concentrations was performed at the University of Texas Southwestern O’Brien Kidney Research Core Center, using capillary electrophoresis. Ergothioneine (50 mg/kg weight; Cayman Chemical) was dissolved in 100 μl PBS and administered intraperitoneally 1 hour after 5 mg/kg LPS injection. Membrane-permeant dimethyl itaconate (160 mg/kg; Sigma) was dissolved in 300 μl normal saline and administered intraperitoneally 1 hour before live E. coli intravenous injection (3.2×106 CFU). N1-acetylspermidine (20 mg/kg, hydrochloride; Cayman Chemical), spermine (20 mg/kg; Sigma), and spermidine (20 mg/kg; Sigma) were dissolved in normal saline and administered intraperitoneally (100 μl) or via gavage (300 μl) daily for 3 days before LPS injection. GC7 (deoxyhypusine synthase inhibitor; BioSearch) was dissolved in 10 mM acetic acid (10 mg/ml), then diluted 20× with normal saline before use (4 mg/kg administered intraperitoneally, daily for 3 days). In all experiments, an equal volume of vehicle was administered into control animals.
CLP
Under isoflurane anesthesia, 75% of the mouse cecum was ligated and punctured twice with a 27-gauge needle. Animals were resuscitated with 500 μl of normal saline subcutaneously. No antibiotics were used. Preconditioned animals were subjected to 0.25 mg/kg lps administered intraperitoneally 24 hours before CLP. To study survival, animals were monitored twice a day for 7 days after CLP. To assess bacterial load, kidney, liver, spleen, and lungs were harvested and weighed 24 hours after CLP. These organs were homogenized in 2 ml cold sterile PBS, diluted at 1:1000, and incubated on Trypticase Soy Agar with 5% sheep blood (TSA II, BD 221239; Becton Dickinson).
Generation of Chimeric Mice
The procedure was performed at the Herman B Wells Cancer Center at Indiana University. Recipient mice were irradiated via a 139-Cs source with 1100 cGy total given in two divided doses. Approximately 10 million bone marrow cells obtained from the long bones of donor mice were transplanted via the lateral tail vein, as previously described.18 Only animals with chimerism exceeding 97% after 8 weeks were used.
Adoptive Cell Transfer and Flow Cytometry
Bone marrow–derived macrophages were generated using 10 ng/ml macrophage colony-stimulating factor (Life Technologies) and were preconditioned in vitro with LPS, before harvesting as indicated in Supplemental Table 1. Peritoneal cells were collected with 10 ml cold sterile PBS. To exclude nonmacrophage cells (mostly B cells) from peritoneal space, peritoneal cells were negatively selected by sorting (FACS Aria; CD3−CD19−PI−). In some experiments, we also isolated macrophages on the basis of forward and side scatterplots alone to avoid potential confounding effects from antibody incubation. In separate experiments, peritoneal macrophages were enriched by short incubation (4 hours), which eliminates nonadherent B cells. To obtain renal macrophages, heterozygous CX3CR1EGFP/WT kidneys were harvested, homogenized, and incubated with collagenase type IA (Sigma) in HBSS with calcium and magnesium. Note that we have previously found that CX3CR1EGFP/WT can mount protective preconditioning comparable to C57BL/6 and BoyJ mice.18 A density separation medium, Lympholyte-M (Cedarlane), was used to eliminate erythrocytes, and then cells were sorted by FACS. An equal number of cells were injected (either intraperitoneally or intravenously) into each animal (the number ranged from 0.5×106 to 2×106, depending on particular sets of experiments). Flow cytometric analyses were performed on an LSR II (Becton Dickinson) using a combination of antibodies (eBioscience, unless specified otherwise), including anti-mouse F4/80 (BM8), CD11b (M1/70), CD11c (N418), MHC class II (I-A/I-E, M5/114.15.2), B220 (RA3–6B2), CD3e (145–2c11), CD4 (RM 4–5), CD19 (1D3), Gr1 (RB6–8C5; BD Pharmingen), Foxp3 (FJK-16s), CD45 (30F-11), CD45.1 (A20; BD Pharmingen), and CD45.2 (104; BD Pharmingen). These antibodies were conjugated with eFluor 450, FITC, PE, PE-Cy7, Alexa 647, and APC-Cy7 in various combinations, and their concentrations were titrated before use. To identify a subset of renal macrophages, rhodamine-conjugated poly (I:C) (InvivoGen) was used as previously described.18 Flow cytometric data were analyzed using Flow Jo software (Tree Star Inc.).
Live E. coli and Macrophage Imaging
Approximately 4×105 peritoneal macrophages were placed in each well (Greiner Bio One SensoPlate, 24-well glass-bottom plates). These macrophages were freshly isolated from animals that had received intraperitoneal LPS or vehicle in vivo. CellTracker Orange CMRA (Life Technologies) was used to label macrophages. E. coli were transformed with cyan fluorescence protein plasmid (BL21 Star DE3 and pRSET/CFP; Life Technologies) and incubated in Luria broth media until OD600=0.5 was achieved. Approximately 4×106 CFU of E. coli were added to each well. Time-lapse images were obtained with a custom spinning-disk microscope built around a CSU-10 Confocal head (Yokogawa), an 897 Xion electron multiplying charge coupled device camera (Andor), a four-line monolithic laser launch (Agilent), and a TiE inverted microscope stand (Nikon Instruments) equipped with a stage top incubator that regulates carbon dioxide and temperature. In separate experiments, E. coli BL21 were incubated in M9 minimal media with 0.27% sodium acetate and dimethyl itaconate (0, 5, 10, 50, and 100 mM). Growth of E. coli was measured as OD600 at indicated time points.
Real-Time Quantitative PCR
RNA extraction from snap-frozen kidneys was performed using TRIzol and the extracted RNA was reverse-transcribed using High Capacity cDNA Reverse Transcription Kit (Life Technologies). TaqMan gene expression assays used include kidney injury molecule-1 (Mm00506686_m1), CD14 (Mm00438094_g1), Irg1 (Mm01224532_m1), CCL5 (Mm01302427_m1), CCL2 (Mm00441242_m1), and CCL3 (Mm00441259_g1). Real-time quantitative PCR amplifications were performed for 40 cycles using 7500 Real-Time PCR systems (Life Technologies). The ΔΔCt method was used to analyze the relative changes in gene expression. Glyceraldehyde-3-phosphate dehydrogenase was used as an endogenous control for normalization.
Laser Microdissection and Microarray Analysis
Kidney sections (10-µm thick) were placed on polyphenylene sulfate membrane slides (Leica). Immunofluorescence staining was performed in the following order: 100% alcohol (EtOH) for 30 seconds ×2, 95% EtOH for 20 seconds ×2, 75% EtOH for 20 seconds ×2, 50% EtOH for 20 seconds ×2, water for 30 seconds ×2, water for 30 seconds ×2, staining with FITC-phalloidin and DAPI (Molecular Probes) in PBS with 2% BSA for 3 minutes, and phosphate buffer water (pH 7.4) wash for 30 seconds ×3. Sections were air dried and dissection was performed on a Leica LMD6000 microscope.33 RNA was extracted using PicoPure RNA kit (Life Technologies) and further concentrated using isopropanol precipitation. On average, 200,000 µm2 of S1 segments were dissected from each section. S1 segments were recognized as they extended from Bowman’s space. Three consecutive sections (from the same kidney tissue) were pooled to increase the amount of RNA. Total RNA was analyzed using the Agilent Bioanalyzer to assess the quality of the RNA. The average RNA integrity number obtained was 6.2 (Supplemental Figure 2A). Two nanograms of total RNA for each sample was labeled using the standard protocol for the Affymetrix WT Pico kit. Individually labeled samples were hybridized to the Mouse Gene 2.0 ST GeneChips for 17 hours, then washed, stained, and scanned with the standard protocol, using Affymetrix GeneChip Command Console Software to generate data (CEL files). Arrays were visually scanned for abnormalities or defects; none were found except that the arrays were not as bright as those using the standard protocol with 100 ng of RNA. All samples were processed in one batch. CEL files were imported into Partek Genomics Suite 6.6 for analysis. Robust multichip average (RMA) expression levels were generated for all probe sets using the RMA background correction, quantile normalization, and summarization by Median Polish. Summarized signals for each probe set were log2 transformed. These log-transformed signals were used for principal components analysis (Supplemental Figure 2B), hierarchical clustering, and signal histograms to determine if there were any outlier arrays. No outliers were found. All contrasts were performed and fold changes were calculated using the untransformed RMA signals. Probe sets with log2 expression levels <3.0 are below background. Probe sets (transcripts) with average expression levels <3.0 for all experimental groups (7529 probe sets) and Affymetrix control probe sets (6955 probe sets) were removed34 before the false discovery rate (FDR) was calculated using the Storey q-value method.35
Manual Microdissection of Mouse Proximal Tubule Subsegments
Mouse kidneys were sliced and tubular microdissection was performed in ice-cold dissection solution (135 mM NaCl, 1 mM Na2HPO4, 1.2 mM Na2SO4, 1.2 mM MgSO4, 5 mM KCl, 2 mM CaCl2, 5.5 mM glucose, 5 mM HEPES, 0.1% BSA; pH 7.4) using a Leica MZ 12.5 stereomicroscope equipped with Leica MC 170 HD digital microscope camera and ThermaZone cooling device. Microdissected tubules were transferred to another dish for washing and elimination of contaminated cells.36 The average RNA integrity number of manually microdissected tubules was 8.7. RNA was extracted using PicoPure RNA kit.
Metabolomic Profiling
Metabolomic analysis was performed at Metabolon Inc. Snap-frozen kidney tissues were processed following the Metabolon standard extraction method (60% methanol). The extracts were analyzed by gas chromatography-mass spectroscopy and ultrahigh performance liquid chromatography-tandem mass spectroscopy platforms.
Proteomic Analysis
Two-dimensional difference gel electrophoresis was performed at Applied Biomics (Hayward, CA). Equal amounts of protein extracts from each kidney tissue and sera (sham, nonpreconditioned, and preconditioned; three samples were pooled for each experimental condition) were labeled with Cy2, Cy3, or Cy5, then mixed and separated on a two-dimensional gel. In-gel data analysis was performed using DeCyder software.
A fold change >2.0 in at least one direct comparison was used as a cutoff in the DeCyder analysis, with which approximately 60 (kidney) and 100 (sera) spots were identified. These protein spots were digested and extracted from the gel and identified by mass spectrometry (matrix-assisted laser desorption/ionization-time of flight). Protein identification was performed using peptide fingerprint mass mapping (using mass spectrometry data) and peptide fragmentation mapping (using mass spectrometry/mass spectrometry data). The MASCOT search engine was used to identify proteins from primary sequence databases.
Data Analyses
Data were analyzed for statistical significance and visualization with R software 3.3.2. The following packages were used: gplots, heatmap3 (Figures 3I, 5E, and 6D), and loess (Figures 5B–D and 6F; error lines were generated using the predict$se.fit function), survival (Figures 1A and 3L; the survival curves were generated using survfit and P values were generated using coxph), Bioconductor STRINGdb (Figure 6B; get_enrichment function with FDR method), Pathview (Figure 6G), OmicCircos (Supplemental Figure 3; P values were adjusted using p.adjust with FDR method), Circlize (Supplemental Figure 4; the scaled metabolite levels were generated using colorRamp2; hierarchical clustering was generated with hclust and Euclidean dist function; P values were adjusted using p.adjust with FDR method), and ReactomePA (Figure 1, E–F and Figure 2, A–B; proteins with fold changes >±1.4 were included for enrichPathway and cnetplot analyses; FDR-adjusted P values were calculated on the basis of the hypergeometric model).
Data Availability
The transcriptomics dataset has been deposited to the National Center for Biotechnology Information Gene Expression Omnibus database (accession number GSE102453). In addition, annotated transcriptomics and metabolomics data sets are available in Supplemental Figures 3 and 4.
Immunohistochemical and Immunofluorescence Microscopy
Kidneys were fixed with 4% paraformaldehyde and subsequently processed for standard histochemistry or immunofluorescence staining (50-μm vibratome sections permeabilized with 0.1% Triton X-100). For CD14 staining, 10 μm frozen sections with antigen retrieval (1% SDS for 3 minutes) was applied. The following antibodies were used: CD14 (M305; Santa Cruz Biotechnology), F4/80 (Cl:A3–1; AbD Serotec), catalase (ab1877; Abcam), and Prdx5 (abc281; Millipore). Sections were counterstained with labeled secondary antibodies and FITC-phalloidin, and imaged with Olympus FV1000-MPE confocal/multiphoton microscope.
Western Blotting
Kidney proteins were extracted with 1% SDS. Total protein levels were determined using the bicinchoninic acid assay (Pierce). Equal amounts of kidney proteins (30 µg) and equal volumes of serum (1:20 dilution) and urine (1:2 dilution) were separated by electrophoreses on 4%–12% Tris-Glycine gels (Life Technologies) and transferred to PVDF membranes. PVDF membranes were blocked with 10% newborn calf serum, incubated with CD14 antibody (sc9150; Santa Cruz Biotechnology) and visualized by chemiluminescence.
Quantification of Cytokines and Chemokines
Analysis of serum and kidney homogenate cytokines/chemokines was performed using Milliplex MAP Mouse Cytokine/Chemokine Magnetic Bead Panel–Premixed 32 Plex (Millipore).
Disclosures
None.
Supplementary Material
Acknowledgments
We thank Seth Winfree for cell tracking analysis. The labeling, hybridization, scanning of arrays, and statistical analyses were performed by the Center for Medical Genomics at the Indiana University School of Medicine.
This work was supported by National Institutes of Health (NIH) grant R01-DK107623 and Veteran’s Affairs Merit (1I01BX002901) to P.C.D, a Paul Teschan research grant (Dialysis Clinics Inc.), an Indiana Clinical and Translational Sciences Institute grant (KL2TR001106), and NIH grant K08-DK113223 to T.H. This work was also supported by the Indiana University O’Brien Center grant P30-DK079312.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2017060624/-/DCSupplemental.
References
- 1.Hsu RK, McCulloch CE, Dudley RA, Lo LJ, Hsu CY: Temporal changes in incidence of dialysis-requiring AKI. J Am Soc Nephrol 24: 37–42, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bagshaw SM, George C, Bellomo R; ANZICS Database Management Committee : Early acute kidney injury and sepsis: A multicentre evaluation. Crit Care 12: R47, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hotchkiss RS, Monneret G, Payen D: Sepsis-induced immunosuppression: From cellular dysfunctions to immunotherapy. Nat Rev Immunol 13: 862–874, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rice TW, Wheeler AP, Bernard GR, Vincent JL, Angus DC, Aikawa N, Demeyer I, Sainati S, Amlot N, Cao C, Ii M, Matsuda H, Mouri K, Cohen J: A randomized, double-blind, placebo-controlled trial of TAK-242 for the treatment of severe sepsis. Crit Care Med 38: 1685–1694, 2010 [DOI] [PubMed] [Google Scholar]
- 5.Tidswell M, Tillis W, Larosa SP, Lynn M, Wittek AE, Kao R, Wheeler J, Gogate J, Opal SM; Eritoran Sepsis Study Group : Phase 2 trial of eritoran tetrasodium (E5564), a toll-like receptor 4 antagonist, in patients with severe sepsis. Crit Care Med 38: 72–83, 2010 [DOI] [PubMed] [Google Scholar]
- 6.Opal SM, Laterre PF, Francois B, LaRosa SP, Angus DC, Mira JP, Wittebole X, Dugernier T, Perrotin D, Tidswell M, Jauregui L, Krell K, Pachl J, Takahashi T, Peckelsen C, Cordasco E, Chang CS, Oeyen S, Aikawa N, Maruyama T, Schein R, Kalil AC, Van Nuffelen M, Lynn M, Rossignol DP, Gogate J, Roberts MB, Wheeler JL, Vincent JL; ACCESS Study Group : Effect of eritoran, an antagonist of MD2-TLR4, on mortality in patients with severe sepsis: The ACCESS randomized trial. JAMA 309: 1154–1162, 2013 [DOI] [PubMed] [Google Scholar]
- 7.Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B: Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: Mutations in Tlr4 gene. Science 282: 2085–2088, 1998 [DOI] [PubMed] [Google Scholar]
- 8.López-Collazo E, del Fresno C: Pathophysiology of endotoxin tolerance: Mechanisms and clinical consequences. Crit Care 17: 242, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pena OM, Pistolic J, Raj D, Fjell CD, Hancock RE: Endotoxin tolerance represents a distinctive state of alternative polarization (M2) in human mononuclear cells. J Immunol 186: 7243–7254, 2011 [DOI] [PubMed] [Google Scholar]
- 10.Foster SL, Hargreaves DC, Medzhitov R: Gene-specific control of inflammation by TLR-induced chromatin modifications. Nature 447: 972–978, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Lehner MD, Ittner J, Bundschuh DS, van Rooijen N, Wendel A, Hartung T: Improved innate immunity of endotoxin-tolerant mice increases resistance to Salmonella enterica serovar typhimurium infection despite attenuated cytokine response. Infect Immun 69: 463–471, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wheeler DS, Lahni PM, Denenberg AG, Poynter SE, Wong HR, Cook JA, Zingarelli B: Induction of endotoxin tolerance enhances bacterial clearance and survival in murine polymicrobial sepsis. Shock 30: 267–273, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Biswas SK, Lopez-Collazo E: Endotoxin tolerance: New mechanisms, molecules and clinical significance. Trends Immunol 30: 475–487, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Gómez H, Kellum JA, Ronco C: Metabolic reprogramming and tolerance during sepsis-induced AKI. Nat Rev Nephrol 13: 143–151, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schneider DS, Ayres JS: Two ways to survive infection: What resistance and tolerance can teach us about treating infectious diseases. Nat Rev Immunol 8: 889–895, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Medzhitov R, Schneider DS, Soares MP: Disease tolerance as a defense strategy. Science 335: 936–941, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matzinger P, Kamala T: Tissue-based class control: The other side of tolerance. Nat Rev Immunol 11: 221–230, 2011 [DOI] [PubMed] [Google Scholar]
- 18.Hato T, Winfree S, Kalakeche R, Dube S, Kumar R, Yoshimoto M, Plotkin Z, Dagher PC: The macrophage mediates the renoprotective effects of endotoxin preconditioning. J Am Soc Nephrol 26: 1347–1362, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kalakeche R, Hato T, Rhodes G, Dunn KW, El-Achkar TM, Plotkin Z, Sandoval RM, Dagher PC: Endotoxin uptake by S1 proximal tubular segment causes oxidative stress in the downstream S2 segment. J Am Soc Nephrol 22: 1505–1516, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doi K, Leelahavanichkul A, Yuen PS, Star RA: Animal models of sepsis and sepsis-induced kidney injury. J Clin Invest 119: 2868–2878, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zarjou A, Agarwal A: Sepsis and acute kidney injury. J Am Soc Nephrol 22: 999–1006, 2011 [DOI] [PubMed] [Google Scholar]
- 22.Madeo F, Eisenberg T, Büttner S, Ruckenstuhl C, Kroemer G: Spermidine: A novel autophagy inducer and longevity elixir. Autophagy 6: 160–162, 2010 [DOI] [PubMed] [Google Scholar]
- 23.Zahedi K, Barone S, Kramer DL, Amlal H, Alhonen L, Jänne J, Porter CW, Soleimani M: The role of spermidine/spermine N1-acetyltransferase in endotoxin-induced acute kidney injury. Am J Physiol Cell Physiol 299: C164–C174, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Michelucci A, Cordes T, Ghelfi J, Pailot A, Reiling N, Goldmann O, Binz T, Wegner A, Tallam A, Rausell A, Buttini M, Linster CL, Medina E, Balling R, Hiller K: Immune-responsive gene 1 protein links metabolism to immunity by catalyzing itaconic acid production. Proc Natl Acad Sci U S A 110: 7820–7825, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jamal Uddin M, Joe Y, Kim SK, Oh Jeong S, Ryter SW, Pae HO, Chung HT: IRG1 induced by heme oxygenase-1/carbon monoxide inhibits LPS-mediated sepsis and pro-inflammatory cytokine production. Cell Mol Immunol 13: 170–179, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Y, Zhang P, Wang C, Han C, Meng J, Liu X, Xu S, Li N, Wang Q, Shi X, Cao X: Immune responsive gene 1 (IRG1) promotes endotoxin tolerance by increasing A20 expression in macrophages through reactive oxygen species. J Biol Chem 288: 16225–16234, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lampropoulou V, Sergushichev A, Bambouskova M, Nair S, Vincent EE, Loginicheva E, Cervantes-Barragan L, Ma X, Huang SC, Griss T, Weinheimer CJ, Khader S, Randolph GJ, Pearce EJ, Jones RG, Diwan A, Diamond MS, Artyomov MN: Itaconate links inhibition of succinate dehydrogenase with macrophage metabolic remodeling and regulation of inflammation. Cell Metab 24: 158–166, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cordes T, Wallace M, Michelucci A, Divakaruni AS, Sapcariu SC, Sousa C, Koseki H, Cabrales P, Murphy AN, Hiller K, Metallo CM: Immunoresponsive gene 1 and itaconate inhibit succinate dehydrogenase to modulate intracellular succinate levels. J Biol Chem 291: 14274–14284, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gründemann D, Harlfinger S, Golz S, Geerts A, Lazar A, Berkels R, Jung N, Rubbert A, Schömig E: Discovery of the ergothioneine transporter. Proc Natl Acad Sci U S A 102: 5256–5261, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hato T, El-Achkar TM, Dagher PC: Sisters in arms: Myeloid and tubular epithelial cells shape renal innate immunity. Am J Physiol Renal Physiol 304: F1243–F1251, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hato T, Dagher PC: How the innate immune system senses trouble and causes trouble. Clin J Am Soc Nephrol 10: 1459–1469, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang DL, Zheng HM, Yu BJ, Jiang ZW, Li JS: Association of polymorphisms of IL and CD14 genes with acute severe pancreatitis and septic shock. World J Gastroenterol 11: 4409–4413, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Micanovic R, Khan S, El-Achkar TM: Immunofluorescence laser micro-dissection of specific nephron segments in the mouse kidney allows targeted downstream proteomic analysis. Physiol Rep 3: e12306, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McClintick JN, Edenberg HJ: Effects of filtering by present call on analysis of microarray experiments. BMC Bioinformatics 7: 49, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Storey JD, Tibshirani R: Statistical significance for genomewide studies. Proc Natl Acad Sci U S A 100: 9440–9445, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee JW, Chou CL, Knepper MA: Deep sequencing in microdissected renal tubules identifies nephron segment-specific transcriptomes. J Am Soc Nephrol 26: 2669–2677, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yu G, He QY: ReactomePA: An R/Bioconductor package for reactome pathway analysis and visualization. Mol Biosyst 12: 477–479, 2016 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The transcriptomics dataset has been deposited to the National Center for Biotechnology Information Gene Expression Omnibus database (accession number GSE102453). In addition, annotated transcriptomics and metabolomics data sets are available in Supplemental Figures 3 and 4.