Abstract
The role of immunosuppression in IgA nephropathy (IgAN) is controversial. In the Supportive Versus Immunosuppressive Therapy for the Treatment of Progressive IgA Nephropathy (STOP-IgAN) Trial, 162 patients with IgAN and proteinuria >0.75 g/d after 6 months of optimized supportive care were randomized into two groups: continued supportive care or additional immunosuppression (GFR≥60 ml/min per 1.73 m2: 6-month corticosteroid monotherapy; GFR=30–59 ml/min per 1.73 m2: cyclophosphamide for 3 months followed by azathioprine plus oral prednisolone). Coprimary end points were full clinical remission and GFR loss ≥15 ml/min per 1.73 m2 during the 3-year trial phase. In this secondary intention to treat analysis, we separately analyzed data from each immunosuppression subgroup and the corresponding patients on supportive care. Full clinical remission occurred in 11 (20%) patients receiving corticosteroid monotherapy and three (6%) patients on supportive care (odds ratio, 5.31; 95% confidence interval, 1.07 to 26.36; P=0.02), but the rate did not differ between patients receiving immunosuppressive combination and controls on supportive care (11% versus 4%, respectively; P=0.30). The end point of GFR loss ≥15 ml/min per 1.73 m2 did not differ between groups. Only corticosteroid monotherapy transiently reduced proteinuria at 12 months. Severe infections, impaired glucose tolerance, and/or weight gain in the first year were more frequent with either immunosuppressive regimen than with supportive care. In conclusion, only corticosteroid monotherapy induced disease remission in a minority of patients who had IgAN with relatively well preserved GFR and persistent proteinuria. Neither immunosuppressive regimen prevented GFR loss, and both associated with substantial adverse events.
Keywords: IgA nephropathy, glomerular disease, glomerulonephritis, immunosuppression
Risks and benefits of immunosuppressive therapies in IgA nephropathy (IgAN) currently remain uncertain. For patients with IgAN at risk of progressive disease, namely those with proteinuria above 0.5–1 g/d, hypertension, or reduced GFR, a number of supportive measures are recommended as a general approach for every patient. Those measures include control of BP, control of proteinuria, and use of renin-angiotensin system–blocking agents as well as dietary restriction of sodium and protein intake, weight normalization, smoking cessation, and avoidance of nephrotoxins (www.kdigo.org).1,2
If proteinuria persists above 1 g/d, despite supportive therapy for 3–6 months, the Kidney Disease Improving Global Outcomes (KDIGO) guidelines suggest a 6-month high-dose corticosteroid course in patients with IgAN with a GFR>50 ml/min per 1.73 m2, albeit at a low level of evidence.1 The efficacy of corticosteroids in patients with IgAN with persisting proteinuria was described in several randomized, controlled trials (RCTs).3–6 However, supportive measures, in particular renin-angiotensin system blockade, were used inconsistently or temporarily withheld before inclusion in many of these trials. Because proteinuric patients with IgAN with a GFR<50 ml/min per 1.73 m2 had been excluded in virtually all trials, the KDIGO guidelines do not give any recommendations for such patients with low GFR. However, a recently published retrospective analysis of a large European cohort suggested particular renal benefits from corticosteroids for patients with IgAN with a baseline GFR below 50 ml/min per 1.73 m2 as well as those with proteinuria above 3 g/d.7
Beyond corticosteroids, immunosuppressive combination strategies were evaluated in patients with IgAN in patient-control studies and small RCTs.8–12 For example, in patients with IgAN with a progressive decline in renal function, Ballardie and Roberts9 showed beneficial effects of a combined therapy of corticosteroids plus oral cyclophosphamide for 3 months and subsequent oral azathioprine for at least 2 years on proteinuria and renal function. In contrast, combination therapies with corticosteroids plus azathioprine or corticosteroids plus mycophenolate mofetil were not superior to corticosteroid monotherapy.8,13 Other immunosuppressant monotherapies using mycophenolate mofetil or rituximab yielded divergent results in patients with IgAN.14–19
In 2015, we reported the results of the Supportive Versus Immunosuppressive Therapy for the Treatment of Progressive IgA Nephropathy (STOP-IgAN) Trial, in which we enrolled 379 patients with IgAN for a 6-month run-in phase of optimizing supportive care and selected those with persistent proteinuria above 0.75 g/d as a homogenous high-risk group.20 One hundred sixty-two of such high-risk patients were then randomized to either continue on supportive care or receive additional immunosuppression. On the basis of the evidence of IgAN therapy at initiation of the STOP-IgAN Trial, immunosuppression was stratified according to the baseline GFR: either corticosteroid monotherapy for 6 months following the so-called Pozzi protocol3,4 in patients with a baseline GFR ≥60 ml/min per 1.73 m2 or the so-called Ballardie combination of corticosteroids, cyclophosphamide, and azathioprine9 (see above) in those with a baseline GFR between 30 and 59 ml/min per 1.73 m2. During the 3-year trial phase, patients under additional immunosuppression were more likely to reach full clinical remission but had a comparable annual loss of renal function (approximately 1.5 ml/min per 1.73 m2) and significantly more adverse effects compared with those under supportive care only. The objective of this secondary investigation was to analyze renal end points separately in patients in the two immunosuppressive regimens in comparison with corresponding patients with a similar GFR on supportive care only.
Results
Patients and GFR Strata
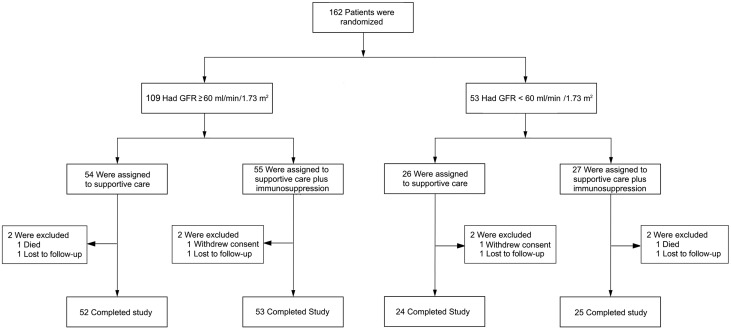
At baseline (i.e., at the end of the 6-month run-in phase), 162 patients were randomized in the 3-year trial phase. Of the 162 randomized patients, 109 (67%) had a GFR≥60 ml/min per 1.73 m2 (“high-GFR arm”) and 53 (33%) had a GFR between 30 and 59 ml/min per 1.73 m2 (“low-GFR arm”) (Figure 1) at baseline. In the high-GFR arm, 54 patients were assigned to supportive care, and 55 patients were assigned to corticosteroid monotherapy. Baseline mean GFRs in these two arms were 88.2 and 94.2 ml/min per 1.73 m2, respectively. In the low-GFR arm, 26 patients were assigned to supportive care, and 27 patients were assigned to immunosuppressive combination. Baseline mean GFRs were 49.9 and 42.4 ml/min per 1.73 m2, respectively. A complete list of baseline characteristics for all four subgroups is given in Table 1. Overall, there were no substantial differences between the two arms in each stratum (Table 1). In each of the four arms, two patients did not complete the trial (lost to follow-up, consent withdrawal, or death). Thus, 95% of randomized patients completed the trial.
Figure 1.
A total of 162 patients who had completed the 6-mo run-in phase of STOP-IgAN participated in the subsequent 3-yr trial phase. One hundred and nine of them had a GFR of at least 60 ml/min/1.73 m2 and 53 of them had a GFR between 30 and 59 ml/min/1.73 m2. These patients were randomly assigned to either continue supportive care or receive additional immunosuppression.
Table 1.
Baseline characteristics of randomized patients in the two GFR arms
| Characteristic | High-GFR Arm | Low-GFR Arm | ||
|---|---|---|---|---|
| Supportive Care, n=54 | Corticosteroid Monotherapy, n=55 | Supportive Care, n=26 | Immunsuppressive Combination, n=27 | |
| Women, % | 13 | 24 | 31 | 26 |
| Smoker, % | 19 | 20 | 12 | 11 |
| Age, yr | 45.6±11.9 | 41.7±13.3 | 46.0±14.0 | 45.1±12.8 |
| Body mass index, kg/m2 | 28.0±4.7 | 27.3±5.0 | 29.9±6.2 | 26.6±5.2 |
| BP, mm Hg | ||||
| Systolic | 127±8.4 | 124±10.2 | 125±8.7 | 126±8.7 |
| Diastolic | 79±7.7 | 77±6.7 | 78±5.3 | 78±7.6 |
| Serum creatinine, mg/dl | 1.4±0.5 | 1.3±0.4 | 2.0±0.6 | 2.2±0.7 |
| GFR, ml/min per 1.73 m2 | 88.2±28.6 | 94.2±32.2 | 49.9±17.5 | 42.4±11.4 |
| Daily urinary protein excretion, g/d | 1.6±0.7 | 1.6±0.8 | 1.7±0.7 | 2.0±0.8 |
| Urinary protein-to-creatinine ratio, g/g | 0.9±0.5 | 0.9±0.5 | 1.1±0.6 | 1.5±0.7 |
| Cholesterol, mg/dl | 196±46 | 194±42 | 183±25 | 193±53 |
Primary End Points
Similar to the original publication,20 we evaluated two scenarios in two intention to treat approaches. All randomized patients were considered (full analysis set), with the assumption of treatment failure in patients with missing end point information. An available patient analysis served as a sensitivity analysis. Further sensitivity analyses included baseline GFR in the models. The latter confirmed the primary results for all end points, thus excluding that baseline GFR influenced the observed results.
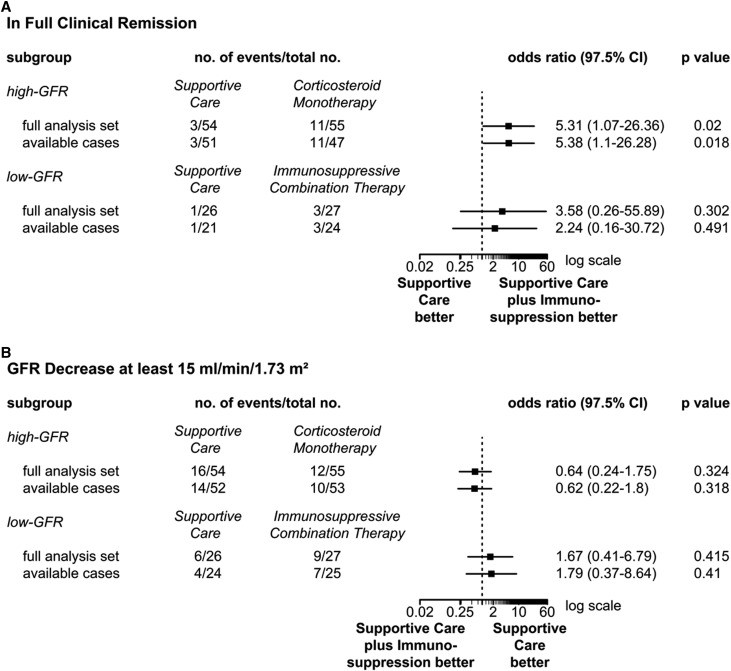
In the high-GFR arm, the first coprimary end point (i.e., full clinical remission) occurred in three out of 54 (5.5%) patients with supportive care (full analysis set) (Figure 2A) and 11 out of 55 (20.0%) patients with corticosteroids (P=0.02). The available patient analysis yielded similar results (Figure 2A). In the low-GFR arm, full remission rates were not significantly different between the two groups: one out of 26 (3.8%) with supportive care versus three out of 27 (11.1%) with immunosuppressive combination (P=0.30; full analysis set).
Figure 2.
Primary end points in the two GFR subgroups of STOP-IgAN. Panel A shows the first primary end point: full clinical remission at the end of the 3-year trial phase (i.e. urinary protein-to-creatinine ratio <0.2 g/g and a GFR-loss <5 ml/min/1.73 m2). Panel B shows the second primary end point: a GFR decrease of at least 15 ml/min/1.73 m2 during the trial phase. Analyses were performed for both end points with the use of a full analysis set and an available-case analysis set. 97.5% CI, 97.5% confidence interval.
The second coprimary end point, GFR decline of ≥15 ml/min per 1.73 m2 over the 3 years, was not different with supportive care versus immunosuppression in the high- and low-GFR arms (Figure 2B) (full analysis set). In the high-GFR arm, 16 out of 54 (29.6%) in the supportive care arm and 12 out of 55 patients with corticosteroid monotherapy (21.8%) reached this end point (P=0.32). Within the low-GFR arm, six of 26 (23.1%) under supportive therapy and nine of 27 (30.0%) under immunosuppressive combination therapy lost at least 15 ml/min per 1.73 m2 of GFR during the 3-year trial phase (P=0.42). The available patient analysis confirmed these results (Figure 2B).
Secondary End Points
At the end of the trial, we did not observe significant differences in either GFR strata between the immunosuppression group and the corresponding supportive care group with respect to the numbers of patients losing at least 30 ml/min per 1.73 m2 of GFR during the trial phase, the number of patients who developed ESRD, the mean absolute change in GFR, and the mean annual change in the slope of the reciprocal of serum creatinine concentration (Tables 2 and 3).
Table 2.
Secondary end points on the basis of the available patients at the end of the trial phase: high-GFR arm
| End Point | Supportive Care, n=54 | Corticosteroid Monotherapy, n=55 | P Value | ||
|---|---|---|---|---|---|
| Patients with Available Data, No. | End Point Value, Mean±SD or No. (%) | Patients with Available Data, No. | End Point Value, Mean±SD or No. (%) | ||
| GFR decrease ≥30 ml/min per 1.73 m2 | 52 | 6 (12) | 53 | 5 (9) | 0.68 |
| Onset of ESRD | 52 | 5 (10) | 53 | 1 (2) | 0.16 |
| Disappearance of microhematuria | 38a | 7 (18) | 40a | 19 (48) | 0.02 |
| Absolute GFR changes at month 36, ml/min per 1.73 m2 | 48 | −3.78±13.41 | 52 | −4.07±15.66 | 0.98 |
| Mean annual change in the slope of the reciprocal of serum creatinine concentration, mg/dl | 53 | −0.02±0.06 | 52 | −0.01±0.06 | 0.35 |
| Urinary protein-to-creatinine ratio at month 12, g/g | 47 | 0.79±0.74 | 41 | 0.5±0.52 | 0.01 |
| Urinary protein-to-creatinine ratio at month 36, g/g | 46 | 0.80±0.64 | 43 | 0.57±0.53 | 0.26 |
A total of 45 patients in the supportive care group and 50 patients in the corticosteroid monotherapy group had microhematuria at baseline.
Table 3.
Secondary end points on the basis of the available patients at the end of the trial phase: low-GFR arm
| End Point | Supportive Care, n=26 | Immunosuppressive Combination, n=27 | P Value | ||
|---|---|---|---|---|---|
| Patients with Available Data, No. | End Point Value, Mean±SD or No. (%) | Patients with Available Data, No. | End Point Value, Mean±SD or No. (%) | ||
| GFR decrease ≥30 ml/min per 1.73 m2 | 24 | 1 (4) | 25 | 5 (20) | 0.18 |
| Onset of ESRD | 24 | 1 (4) | 25 | 5 (20) | 0.18 |
| Disappearance of microhematuria | 17a | 2 (12) | 17a | 5 (29) | 0.28 |
| Absolute GFR changes at month 36, ml/min per 1.73 m2 | 23 | −5.49±8.63 | 20 | −4.64±9.02 | 0.90 |
| Mean annual change in the slope of the reciprocal of serum creatinine concentration, mg/dl | 24 | −0.02±0.03 | 22 | −0.03±0.05 | 0.58 |
| Urinary protein-to-creatinine ratio at month 12, g/g | 20 | 0.80±0.49 | 18 | 0.74±0.52 | 0.37 |
| Urinary protein-to-creatinine ratio at month 36, g/g | 18 | 0.98±0.71 | 16 | 1.27±1.4 | 0.35 |
A total of 22 patients in the supportive care group and 24 patients in the immunnosuppressive combination therapy group had microhematuria at baseline.
In the high-GFR arm, proteinuria was lower with corticosteroid monotherapy compared with supportive care but only at 12 months (0.50±0.52 versus 0.79±0.74 g/g; P=0.01) (Tables 2 and 3) and not at the end of the trial (0.57±0.53 versus 0.80±0.64 g/g; P=0.26). Microhematuria, when present at randomization, disappeared in a significantly higher proportion of patients with corticosteroid monotherapy (47.5% versus 18.4% in the supportive care group; P<0.01). Proteinuria and microhematuria were not different between the two treatment groups of the low GFR stratum (Tables 2 and 3).
Adverse Events
The number of patients who experienced at least one serious adverse event (SAE), the total number of SAEs, and the total number of infectious SAEs were higher among patients under immunosuppressive combination compared with those under supportive care (Table 4). One patient died in the supportive care group of the high-GFR arm (motor vehicle accident), and one patient died in the immunosuppressive combination arm (sepsis). We did not detect any differences with respect to elevated liver enzymes or blood count, including leukopenia. Two malignancies occurred with immunosuppressive combination. Impaired glucose tolerance occurred in more patients with corticosteroid monotherapy compared with those under supportive care. Compared with the size of each treatment group, more patients under immunosuppression increased their body weight >5 kg within the first year compared with the appropriate supportive care group (in both GFR arms). However, over the entire 3 years, body weight gain rates were comparable between the corresponding arms of either GFR strata.
Table 4.
Adverse events during the trial
| Variable | High-GFR Arm | Low-GFR Arm | ||
|---|---|---|---|---|
| Supportive Care, n=54 | Corticosteroid Monotherapy, n=55 | Supportive Care, n=26 | Immunosuppressive Combination, n=27 | |
| Patients with at least one SAE | 14 | 12 | 6 | 17 |
| Total no. of SAEs | 19 | 14 | 9 | 19 |
| Total no. of infectious events | 69 | 115 | 48 | 59 |
| Total no. of infectious SAEs | 2 | 4 | 1 | 4 |
| Death | 1a | 0 | 0 | 1b |
| Additional side effects of interestc | ||||
| Increase of liver enzymes (i.e., ALT>50 IU/ml) | 10 | 8 | 2 | 5 |
| Leukopenia (i.e., leukocyte count <4000/ml) | 3 | 1 | 0 | 1 |
| Malignancy | 0 | 0 | 0 | 2 |
| Impaired glucose tolerance/diabetes mellitus | 1 | 9 | 1 | 1 |
| Gastrointestinal bleeding | 0 | 0 | 0 | 0 |
| Fracture | 0 | 1 | 0 | 0 |
| Osteonecrosis | 0 | 0 | 0 | 0 |
| Body weight gain (≥5 kg within the first year) | 3 | 9 | 2 | 5 |
| Body weight gain (≥5 kg during the trial phase) | 10 | 13 | 5 | 6 |
| Body weight gain (≥10 kg during the trial phase) | 2 | 3 | 3 | 2 |
ALT, alanine transaminase.
One patient died in a motor vehicle accident.
One patient died of pneumogenic sepsis.
Number of patients with at least one event.
Discussion
In the original analysis plan of the STOP-IgAN Trial, supportive care was compared with immunosuppression. The latter consisted of either corticosteroid monotherapy (when baseline GFR was high) or corticosteroids with cyclophosphamide induction and azathioprine maintenance (when GFR was low). In this secondary analysis, we separately compared renal end points between supportive care and corticosteroid monotherapy as well as between supportive care and immunosuppressive combination. Indeed, the first primary end point, full clinical remission, occurred more frequently under corticosteroids but not under immunosuppressive combination compared with the corresponding supportive care arms. In accordance with the main trial, differences in the achievement of clinical remission were mainly driven by the proteinuria criterion of this end point (i.e., <0.2 g/g at the end of the trial phase), because numbers of patients with a GFR loss <5 ml/min per 1.73 m2 did not differ significantly between the corresponding groups in either strata. This was mirrored by a secondary end point, disappearance of microhematuria, which also was more frequent in the corticosteroid monotherapy group. In contrast to a recently published trial,21 microhematuria was mostly (>90% of the patients) locally assessed by dipstick and was not assessed as a quantitative measure in patients in the STOP-IgAN Trial. Thus, unfortunately, “disappearance of microhematuria” in the STOP-IgAN Trial is a relatively crude measure. However, the second primary outcome, change in GFR, was not affected by either immunosuppressive treatment. Also, all other renal end points, particularly those reflecting loss of renal function and ESRD development, did not differ significantly between the two treatment groups and their corresponding supportive care arms. Thus, the few benefits from immunosuppression described in the primary publication20 were exclusively driven by the corticosteroid arm of the trial. Notably, findings from this secondary analysis uncouple traditional surrogate parameters, such as early remission of proteinuria, from a beneficial treatment effect and a good long-term prognosis.22 Certainly, the inter-relationship between proteinuria and microhematuria remission and GFR outcome needs more investigation.
As expected, infectious adverse events, glucose metabolism disturbances, and body weight increases were more frequent in patients in the STOP-IgAN Trial under corticosteroid therapy compared with their controls. Our STOP-IgAN Trial experience is notably different from the RCT data of Pozzi et al.,3 where an identical corticosteroid regimen did not lead to an increase in body weight and where only a single patient developed diabetes mellitus 2 years after the treatment. Similarly, two further RCTs using a high-dose oral corticosteroid regimen in IgAN noted only minor adverse events.5,23 However, in another placebo-controlled trial that compared the efficacy of an oral prednisolone regimen over 2 years with that of fish oil intake, Hogg et al.24 reported increased appetite and weight gain in the steroid arm. A meta-analysis noted particularly that steroid-related adverse events, such as impaired glucose metabolism or weight increase, had only been reported very inconsistently in previous trials.6 Reasons for these differences in adverse events remain speculative. However, the STOP-IgAN Trial is entirely consistent with more recent RCTs in IgAN using high-dose oral corticosteroid monotherapy, in which adverse events were systematically captured; they also showed that infections, weight gain, and diabetes induction were common,13,25 and in one case, they even led to the early termination of the trial.25 Particularly, we recorded weight gain rates over the 3-year trial phase more strictly than many previous RCTs. After 1 year, 16% of patients in the STOP-IgAN Trial under the Pozzi protocol, which involved an average cumulative corticosteroid dosage of 12.4 g during the first 6 months, gained ≥5 kg compared with corresponding patients under supportive measures (6%). However, this difference almost dissolved at the end of the trial phase. In contrast, weight gain differences in the low-GFR arm with slightly lower cumulative corticosteroid doses under the Ballardie protocol (i.e., 10.4 g extended over the entire 3 years) were less obvious after both 12 and 36 months.
In the low-GFR arm of the STOP-IgAN Trial, 53 patients (i.e., 33% of randomized patients) were randomized to either continue on supportive care or be treated with more intensive immunosuppression as proposed by Ballardie and Roberts.9 The latter protocol is on the basis of a single-center RCT in 38 patients with IgAN with a >15% decline in renal function over 1 year before enrollment. The study noted prominent benefits from an immunosuppressive combination protocol using corticosteroids, cyclophosphamide, and azathioprine.9 Different from the STOP-IgAN Trial, only 26% of patients received angiotensin-converting enzyme inhibitors, and BP was not sufficiently controlled by today’s standards. In contrast, using the same regimen, the STOP-IgAN Trial found no benefit of the immunosuppressive combination therapy with respect to the course of renal function or proteinuria compared with administration of supportive care alone. In striking contrast to the original trial of Ballardie and Roberts,9 which showed a 5-year ESRD rate of 8% in the immunosuppressive combination therapy arm compared with 95% in the control arm, in our study, five (20%) patients developed ESRD with immunosuppression as proposed by Ballardie and Roberts,9 and only one (4%) patient developed ESRD with supportive care. Once again, this suggests that our comprehensive supportive care approach reduced progression of renal disease so much that potential additive effects of immunosuppression became nondetectable in the STOP-IgAN Trial.
In the original trial of Ballardie and Roberts,9 one patient in the immunosuppressive treatment group developed pulmonary tuberculosis, and two withdrew from the trial due to azathioprine-induced bone marrow suppression and secondary diabetes mellitus. Once again, adverse events were more frequent in our patients in the STOP-IgAN Trial under immunosuppressive combination therapy, and one patient even died from pneumogenic sepsis.
Our secondary analysis from the main STOP-IgAN Trial has several limitations, first of all relating to its post hoc character. Nota bene, the GFR strata had been prespecified when the trial was planned and were also included into the randomization algorithm. When the STOP-IgAN Trial was designed, we deliberately decided to also include patients with reduced renal function, because this group had been largely under-represented in previous trials. Notably, study power was not calculated to analyze significant treatment effects in the subgroups. That said, the statistical power of this subgroup analysis was particularly low in those with a low GFR, where immunosuppressive combination was examined. Nonetheless, in the low-GFR stratum, we assessed a larger cohort treated with the Ballardie protocol than in the original publication that had established this protocol.9 Another limitation of both the main STOP-IgAN Trial and this secondary analysis is the relatively short follow-up period. Given the slow disease course in most patients with IgAN, ESRD develops over decades rather than years. Because the STOP-IgAN Trial was performed in a white European population, it remains speculative whether our findings can be extended to other ethnicities.
The STOP-IgAN Trial is the first trial in patients with IgAN with a consistent, intense, and comprehensive optimization of supportive care in accordance with the current KDIGO guidelines. The sequential study design allowed us to evaluate the effects of additional immunosuppression in relatively homogeneous groups, because supportive measures had been used in the preceding run-in phase. Consistent with the primary study report, this secondary analysis failed to show a clear benefit for GFR preservation in either GFR strata using different immunosuppressive regimens. Thus, we conclude that, at both higher and lower baseline GFRs, intense supportive care apparently blunts benefits from immunosuppression in IgAN and mainly increases the risk of adverse events, in particular SAEs. The latter were particularly of concern with the protocol of Ballardie and Roberts.9 Despite the limited statistical power of this latter subgroup, we failed to detect even trends for any significant effect on proteinuria, decline in renal function, or ESRD development with this therapeutic approach and thus, suggest that it should no longer be used in IgAN. Whether the regimen of Ballardie and Roberts9 is useful in the very rare patients with IgAN with a very rapid decline in renal function, like in patients with vasculitic IgAN, is also uncertain. Observational data in full-blown, rapidly progressive crescentic IgAN suggest disappointing outcomes, even with aggressive immunosuppression in these patients.26
In contrast to immunosuppressive combination therapy, a potential benefit from corticosteroid monotherapy in patients with IgAN is still possible. Thus, we cannot exclude that the higher rate of remission induction and disappearance of microhematuria21 in patients with IgAN with higher GFRs translate into better long-term outcomes, an issue that we are currently studying in the STOP-IgAN Trial population. Also, whether corticosteroid therapy is more beneficial in patients with IgAN with nephrotic-range proteinuria, as suggested by secondary analyses of the Validation of the Oxford Classification of IgA Nephropathy cohort,7 cannot be answered on the basis of the STOP-IgAN Trial data, because such patients were not eligible for randomization. Of note, however, such patients were very rare in our total cohort of 309 patients completing the run-in phase and only made up 7% of this population. However, potential benefits from corticosteroid monotherapy in IgAN have to be balanced against the significant increase in adverse events, which stresses that novel, alternative therapeutic approaches to IgAN are urgently needed.
Concise Methods
Main Trial
The STOP-IgAN Trial was a prospective, open label, randomized, controlled clinical trial with a two-group, parallel, group-sequential design. The study protocol and results from the main trial have been published (ClinicalTrials.gov number NCT00554502).20,27 Briefly, 337 patients with biopsy-proven IgAN were enrolled in the trial and had their supportive treatment optimized for 6 months (run-in phase). Patients with persistent proteinuria >0.75 g/d but <3.5 g/d, despite optimized supportive care, were then randomized into the subsequent 3-year trial phase and assigned to either continue on supportive care alone or receive additional immunosuppression. On the basis of the knowledge at the time of the trial design, immunosuppressive regimens were chosen according to renal function at baseline (i.e., at the end of the 6-month run-in phase). Patients with a GFR≥60 ml/min per 1.73 m2 (high-GFR arm) received corticosteroid monotherapy for 6 months under the Pozzi protocol,3,4 whereas patients with a GFR between 30 and 59 ml/min per 1.73 m2 (low-GFR arm) received immunosuppressive combination therapy of oral prednisolone and oral cyclophosphamide for the first 3 months followed by azathioprine until month 36.9
The two coprimary end points were full clinical remission (defined as a urinary protein-to-creatinine ratio <0.2 g/g and a GFR loss <5 ml/min per 1.73 m2 at the end of the 3-year trial phase) and a GFR decrease ≥15 ml/min per 1.73 m2. Secondary end points were absolute GFR change, ESRD occurrence, and others.20
Statistical Methods
All end point data were collected and analyzed using the intention to treat principle. Subsequent analyses of patient subgroups were performed for prespecified GFR strata, namely patients with a GFR≥60 ml/min per 1.73 m2 and those with a GFR between 30 and 59 ml/min per 1.73 m2. Data are presented as means±SDs for continuous variables and counts, percentages, and odds ratios with 97.5% confidence intervals for categorical variables. The full analysis set was used for the primary end points, where patients with missing data were considered to have treatment failure (“worst case scenario”). Available patients are also described for all end points; here, multiple imputation was used for model calculation to account for missing values. Because the study combined two different immunosuppressive regimens according to the predefined GFR criteria, we assumed a hierarchical model structure, with treatment being nested within baseline GFR groups. A hierarchical logistic regression model that included baseline proteinuria and treatment nested within baseline GFR was fitted to the data of the categorical end points. A hierarchical covariance analysis model that included baseline proteinuria and treatment nested within baseline GFR was fitted to the data of the continuous end points. We adjusted the significance level in each model to account for multiple testing; P values <0.03 are considered to be significant. As a sensitivity analysis, we included the continuous baseline GFR as an additional covariate in all models. Statistical analyses were performed with SAS (version 9.4; SAS Institute Inc., Cary, NC).
Disclosures
J.F. has received consultant honoraria from Pharmalink (Sweden) and Omeros (the United States) and investigator fees from Anthera Pharmaceuticals Inc. (the United States). The other authors declare no competing financial interests.
Supplementary Material
Acknowledgments
We are grateful to all study participants and trials centers for their contribution to this work and Natalie Luetzeler for data monitoring.
The Supportive Versus Immunosuppressive Therapy for the Treatment of Progressive IgA Nephropathy Trial was funded through German Federal Ministry of Education and Research grant GFVT01044604.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.KDIGO: KDIGO clinical practice guideline for glomerulonephritis. Kidney Int Suppl (2011) 2: 139–274, 2012
- 2.Rauen T, Floege J: Inflammation in IgA nephropathy [published online ahead of print March 14, 2017]. Pediatr Nephrol 10.1007/s00467-017-3628-1 [DOI] [PubMed] [Google Scholar]
- 3.Pozzi C, Bolasco PG, Fogazzi GB, Andrulli S, Altieri P, Ponticelli C, Locatelli F: Corticosteroids in IgA nephropathy: A randomised controlled trial. Lancet 353: 883–887, 1999 [DOI] [PubMed] [Google Scholar]
- 4.Pozzi C, Andrulli S, Del Vecchio L, Melis P, Fogazzi GB, Altieri P, Ponticelli C, Locatelli F: Corticosteroid effectiveness in IgA nephropathy: Long-term results of a randomized, controlled trial. J Am Soc Nephrol 15: 157–163, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Manno C, Torres DD, Rossini M, Pesce F, Schena FP: Randomized controlled clinical trial of corticosteroids plus ACE-inhibitors with long-term follow-up in proteinuric IgA nephropathy. Nephrol Dial Transplant 24: 3694–3701, 2009 [DOI] [PubMed] [Google Scholar]
- 6.Lv J, Xu D, Perkovic V, Ma X, Johnson DW, Woodward M, Levin A, Zhang H, Wang H; TESTING Study Group : Corticosteroid therapy in IgA nephropathy. J Am Soc Nephrol 23: 1108–1116, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tesar V, Troyanov S, Bellur S, Verhave JC, Cook HT, Feehally J, Roberts IS, Cattran D, Coppo R; VALIGA study of the ERA-EDTA Immunonephrology Working Group : Corticosteroids in IgA nephropathy: A retrospective analysis from the VALIGA study. J Am Soc Nephrol 26: 2248–2258, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pozzi C, Andrulli S, Pani A, Scaini P, Del Vecchio L, Fogazzi G, Vogt B, De Cristofaro V, Allegri L, Cirami L, Procaccini AD, Locatelli F: Addition of azathioprine to corticosteroids does not benefit patients with IgA nephropathy. J Am Soc Nephrol 21: 1783–1790, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ballardie FW, Roberts IS: Controlled prospective trial of prednisolone and cytotoxics in progressive IgA nephropathy. J Am Soc Nephrol 13: 142–148, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Liu H, Xu X, Fang Y, Ji J, Zhang X, Yuan M, Liu C, Ding X: Comparison of glucocorticoids alone and combined with cyclosporine a in patients with IgA nephropathy: A prospective randomized controlled trial. Intern Med 53: 675–681, 2014 [DOI] [PubMed] [Google Scholar]
- 11.Floege J, Rauen T: Immunosuppression in IgA nephropathy: how certain are we? Kidney Int 89: 9–11, 2016 [DOI] [PubMed] [Google Scholar]
- 12.Min L, Wang Q, Cao L, Zhou W, Yuan J, Zhang M, Che X, Mou S, Fang W, Gu L, Zhu M, Wang L, Yu Z, Qian J, Ni Z: Comparison of combined leflunomide and low-dose corticosteroid therapy with full-dose corticosteroid monotherapy for progressive IgA nephropathy. Oncotarget 8: 48375–48384, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hou JH, Le WB, Chen N, Wang WM, Liu ZS, Liu D, Chen JH, Tian J, Fu P, Hu ZX, Zeng CH, Liang SS, Zhou ML, Zhang HT, Liu ZH: Mycophenolate mofetil combined with prednisone versus full-dose prednisone in IgA nephropathy with active proliferative lesions: A randomized controlled trial. Am J Kidney Dis 69: 788–795, 2017 [DOI] [PubMed] [Google Scholar]
- 14.Hogg RJ, Bay RC, Jennette JC, Sibley R, Kumar S, Fervenza FC, Appel G, Cattran D, Fischer D, Hurley RM, Cerda J, Carter B, Jung B, Hernandez G, Gipson D, Wyatt RJ: Randomized controlled trial of mycophenolate mofetil in children, adolescents, and adults with IgA nephropathy. Am J Kidney Dis 66: 783–791, 2015 [DOI] [PubMed] [Google Scholar]
- 15.Tang S, Leung JC, Chan LY, Lui YH, Tang CS, Kan CH, Ho YW, Lai KN: Mycophenolate mofetil alleviates persistent proteinuria in IgA nephropathy. Kidney Int 68: 802–812, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Tang SC, Tang AW, Wong SS, Leung JC, Ho YW, Lai KN: Long-term study of mycophenolate mofetil treatment in IgA nephropathy. Kidney Int 77: 543–549, 2010 [DOI] [PubMed] [Google Scholar]
- 17.Maes BD, Oyen R, Claes K, Evenepoel P, Kuypers D, Vanwalleghem J, Van Damme B, Vanrenterghem YF: Mycophenolate mofetil in IgA nephropathy: Results of a 3-year prospective placebo-controlled randomized study. Kidney Int 65: 1842–1849, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Frisch G, Lin J, Rosenstock J, Markowitz G, D’Agati V, Radhakrishnan J, Preddie D, Crew J, Valeri A, Appel G: Mycophenolate mofetil (MMF) vs placebo in patients with moderately advanced IgA nephropathy: A double-blind randomized controlled trial. Nephrol Dial Transplant 20: 2139–2145, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Lafayette RA, Canetta PA, Rovin BH, Appel GB, Novak J, Nath KA, Sethi S, Tumlin JA, Mehta K, Hogan M, Erickson S, Julian BA, Leung N, Enders FT, Brown R, Knoppova B, Hall S, Fervenza FC: A randomized, controlled trial of rituximab in IgA nephropathy with proteinuria and renal dysfunction. J Am Soc Nephrol 28: 1306–1313, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rauen T, Eitner F, Fitzner C, Sommerer C, Zeier M, Otte B, Panzer U, Peters H, Benck U, Mertens PR, Kuhlmann U, Witzke O, Gross O, Vielhauer V, Mann JF, Hilgers RD, Floege J; STOP-IgAN Investigators : Intensive supportive care plus immunosuppression in IgA nephropathy. N Engl J Med 373: 2225–2236, 2015 [DOI] [PubMed] [Google Scholar]
- 21.Sevillano AM, Gutiérrez E, Yuste C, Cavero T, Mérida E, Rodríguez P, García A, Morales E, Fernández C, Martínez MA, Moreno JA, Praga M: Remission of hematuria improves renal survival in IgA nephropathy [published online ahead of print June 7, 2017]. J Am Soc Nephrol doi: 10.1681/ASN.2017010108 [DOI] [PMC free article] [PubMed]
- 22.Inker LA, Mondal H, Greene T, Masaschi T, Locatelli F, Schena FP, Katafuchi R, Appel GB, Maes BD, Li PK, Praga M, Del Vecchio L, Andrulli S, Manno C, Gutierrez E, Mercer A, Carroll KJ, Schmid CH, Levey AS: Early change in urine protein as a surrogate end point in studies of IgA nephropathy: An individual-patient meta-analysis. Am J Kidney Dis 68: 392–401, 2016 [DOI] [PubMed] [Google Scholar]
- 23.Lv J, Zhang H, Chen Y, Li G, Jiang L, Singh AK, Wang H: Combination therapy of prednisone and ACE inhibitor versus ACE-inhibitor therapy alone in patients with IgA nephropathy: A randomized controlled trial. Am J Kidney Dis 53: 26–32, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Hogg RJ, Lee J, Nardelli N, Julian BA, Cattran D, Waldo B, Wyatt R, Jennette JC, Sibley R, Hyland K, Fitzgibbons L, Hirschman G, Donadio JV Jr., Holub BJ; Southwest Pediatric Nephrology Study Group : Clinical trial to evaluate omega-3 fatty acids and alternate day prednisone in patients with IgA nephropathy: Report from the Southwest Pediatric Nephrology Study Group. Clin J Am Soc Nephrol 1: 467–474, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Lv J, Zhang H, Wong MG, Jardine MJ, Hladunewich M, Jha V, Monaghan H, Zhao M, Barbour S, Reich H, Cattran D, Glassock R, Levin A, Wheeler D, Woodward M, Billot L, Chan TM, Liu ZH, Johnson DW, Cass A, Feehally J, Floege J, Remuzzi G, Wu Y, Agarwal R, Wang HY, Perkovic V; TESTING Study Group : Effect of oral methylprednisolone on clinical outcomes in patients with IgA nephropathy: The TESTING randomized clinical trial. JAMA 318: 432–442, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lv J, Yang Y, Zhang H, Chen W, Pan X, Guo Z, Wang C, Li S, Zhang J, Zhang J, Liu L, Shi S, Wang S, Chen M, Cui Z, Chen N, Yu X, Zhao M, Wang H: Prediction of outcomes in crescentic IgA nephropathy in a multicenter cohort study. J Am Soc Nephrol 24: 2118–2125, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eitner F, Ackermann D, Hilgers RD, Floege J: Supportive versus immunosuppressive therapy of progressive IgA nephropathy (STOP) IgAN trial: Rationale and study protocol. J Nephrol 21: 284–289, 2008 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.