Abstract
An increasing proportion of US smokers smoke ≤10 cigarettes per day (CPD) or do not smoke every day, yet the health effects of low-intensity smoking are poorly understood. We identified lifelong smokers of <1 or 1–10 CPD and evaluated risk of incident cancer among 238,525 cancer-free adults, aged 59–82, in the NIH-AARP Diet and Health Study. A questionnaire administered in 2004–2005 assessed CPD during nine age-periods (<15 to ≥70). We estimated hazard ratios (HR) and 95% confidence intervals (CI) using multivariable-adjusted Cox proportional hazards regression with age as the underlying time metric. Of the 18,233 current smokers (7.6%), 137 and 1,243 reported consistently smoking <1 CPD and 1–10 CPD, respectively. Relative to never smokers, current smokers who reported consistently smoking 1–10 CPD over their lifetime were 2.34 (95% CI=1.86–2.93) times more likely to develop smoking-related cancer. Current lifetime smokers of <1 CPD were 1.89 (95% CI=0.90–3.96) times more likely to develop tobacco-related cancer, although the association did not reach statistical significance. Associations were observed for lifelong smoking of ≤10 CPD with lung cancer (HR=9.65, 95% CI=6.93–13.43); bladder cancer (HR=2.22, 95% CI=1.22–4.05); and pancreatic cancer (HR=2.03, 95%CI: 1.05–3.95). Among lifelong ≤10 CPD smokers, former smokers had lower risks of smoking-related cancer with longer time since cessation and longer smoking duration. Lifelong <1 and 1–10 CPD smokers are at increased risk of incident cancer relative to never smokers and would benefit from cessation, providing further evidence that even even low-levels of cigarette smoking cause cancer.
Keywords: cigarette, low-intensity smoking, lifetime smoking, incident cancer
INTRODUCTION
Tobacco use continues to cause about a third of all cancer deaths in the United States.1 During 2009–2013, approximately 660,000 persons were diagnosed with one of the more than 20 tobacco-related cancer sites per year and 343,000 persons died from these cancers in the United States (US).2–5
Accurate measures of the health effects of low-intensity cigarette smoking are needed since proportionately more US smokers smoke at low-intensity. The recent National Health Interview Survey showed that the number of US adults who smoke on some days increased from 8.7 million (19%) to 8.9 million (24%) from 2005 to 2015, and the proportion of daily smokers who smoke 1–9 cigarettes per day (CPD) increased from 16% to 25%.6,7
The health risks of high-intensity smoking are large and well studied, but far less is known about the health risks of low-intensity smoking, such as smoking 10 or fewer CPD. A strong dose-response association has been established between duration and intensity of cigarette smoking and many cancer types.8–11 Furthermore, second-hand smoke exposure is causally related to elevated risks of lung cancer and other diseases12, suggesting that smoking regularly at low-intensity may also cause of disease.3 Nevertheless, many smokers, including youth, consider low-intensity smoking to be low-risk.13
Few studies have directly examined the health effects of long-term low-intensity smoking. Several studies have shown an increased risk of tobacco-related cancer, but these studies14,15 have largely been unable to distinguish those who smoked at low-intensity over their lifetime from those who smoked much more earlier in life.16,17
We recently found strong associations between lifelong low-intensity smoking (≤10 CPD) and total and cause-specific mortality in the National Institutes of Health (NIH)-AARP cohort.18 To provide further data on the health risks of low-intensity smoking, we now extend these findings to examine the incidence of smoking-related cancers among lifelong low-intensity smokers.
MATERIALS AND METHODS
The NIH-AARP Diet and Health Study has been previously described.19 In 1995–1996, an initial questionnaire regarding demographics, lifestyle, diet, and medical history was mailed to approximately 3.5 million AARP members, aged 50–71, in six US states (California, Florida, New Jersey, North Caroline, and Pennsylvania) and two metropolitan areas (Atlanta, Georgia and Detroit, Michigan); 566,398 men and women successfully completed the questionnaire. In 2004–2005, the follow-up questionnaire was mailed to the remaining cohort participants to update information on medical history and lifestyle, including comprehsenive assessment of lifetime cigarette smoking. The follow-up questionaire was completed by 313,363 participants and serves as baseline for the current analysis. After excluding proxy respondents (n=13,392), participants with a prior cancer diagnosis (n=36,507), a death only record for cancer (n=1,366), who moved out of the cancer registry catchment area prior to follow-up (n=16,093), and those reporting incomplete smoking information (n=7,480), our analytic cohort consisted of 238,525 individuals (134,802 men and 103,723 women). The NIH-AARP Diet and Health Study was approved by the Special Studies Institutional Review Board of the National Cancer Institute. Participants were informed in a letter accompanying the baseline questionnaire and consented by completion and return of questionnaires.
Cigarette Smoking and Covariates
In addition to ever and current cigarette smoking, the 2004–2005 questionnaire assessed their cigarette smoking during nine age-periods (<15, 15–19, 20–24, 25–29, 30–39, 40–49, 50–59, 60–69, ≥70 years) up to current age. Participants were asked to choose the category of CPD (none, <1, 1–10, 11–20, 21–30, 31–40, 41–60, and >60) which best described their smoking in each age-period. We determined age at smoking initiation and duration of smoking based on this information. We previously reported that 74% of participants who reported lifelong smoking of 10 or fewer CPD in the 2004–2005 questionnaire also reported currently smoking 10 or fewer CPD, and 24% reported smoking 11 to 20 CPD at the time of the 1995–1996 questionnaire.18
We collapsed reported CPD categories at each age-period into five categories (<1, 1–10, 11–20, 21–30, and >30 CPD) for the analysis. Because of small numbers of most cancer cases among consistent low-intensity smokers, low-intensity smokers were defined as ≤10 CPD combining <1 and 1–10 CPD categories for analyses of individual smoking-related cancer sites or groups. To assess smoking over the lifetime, we considered CPD from age 20 or age-period of smoking initiation, whichever occurred later. To identify smokers who reported consistently smoking the same CPD category or who varied their smoking intensity over the lifetime, we excluded individuals who had a missing value for CPD in one or more age-periods (6,029 former and 1,593 current smokers) or who started smoking at age 60 or older (n=438), resulting in 230,465 participants.
Additional factors including body mass index (BMI; computed using self-reported height and weight), physical activity, familial history of cancer, history of comorbid conditions (e.g. heart attack, stroke, diabetes, and chronic obstructive pulmonary disease), and perceived general health were also re-assessed in the 2004–2005 questionnaire. Gender, race/ethnicity, highest achieved education, alcohol intake, and ever use of cigar or pipe were assessed in the initial cohort questionnaire in 1995–1996.
Outcome ascertainment
Participant addresses were updated annually in response to change of address requests and by matching cohort participants to the US Post Office National Change of Address database. Vital status was ascertained by linkage to the Social Security Administration Death Master File and response to mailings. Participants were followed from the date when their 2004–2005 questionnaire was returned and scanned until the date of first primary cancer diagnosis, death, movement out of the registry catchment area, or end of follow-up (December 31, 2011), whichever occurred first.
First incident primary cancers were identified via linkage to cancer registries of the eight baseline recruitment states and three additional states (Arizona, Texas, and Nevada) to which participants were the most likely to move during the follow-up period, an approach estimated to ascertain about 90% of incident cancers in the eight baseline recruitment states in a previous validation study.20 Smoking-related cancers included cancers reported to be associated with tobacco use with sufficient evidence by the IARC and US Surgeon General Report.3,4,21 These cancers defined using International Classification of Diseases for Oncology (ICD-O)-3 include cancers of head & neck [oral cavity and pharynx (C000-C148) and nose and nasal cavity (C300-C301 and C310-C329)], esophagus (C150-159), stomach (C160-C169), colon (CC180-C189 and C260), rectum (C199 and C209), liver (C220), pancreas (C250-C259), lung (C340-C349), uterine cervix (C530-C539), Ovary (mucinous; C569), urinary bladder (C670-679), kidney (C649 and C659), ureter (C669), and acute myeloid leukemia (histology code: 9840, 9861, 9865–9867, 9869, 9871–9874, 9895–9898, 9910–9911, and 9920) (Supplementary Table S1). Because of small case numbers, we combined cancers of oral cavity, pharynx, nose, and nasal cavity as head and neck cancer, and cancers of esophagus and stomach as upper gastrointestinal (UGI) cancer for the analyses of individual smoking-related cancers.
Statistical analysis
We estimated hazard ratios (HRs) and 95% confidence intervals (CIs) for all cancer, all smoking-related cancer, and individual smoking-related cancers using Cox proportional hazards regression with age as the underlying time metric. Never cigarette smokers served as the referent group in all analyses. Covariates in the final models were determined based on literature review and included gender, race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, Asian/pacific islander/native American), education (<high school, high school, post high school, some college, college/post graduate), alcohol intake (continuous), familial history of any cancer (yes/no), and ever regular use of pipe or cigar (yes/no). Missing values in covariates were categorized as “unknown”. Including other potential confounders, such as physical activity, had little change on the associations and thus were not included in the final models. A proportional hazards assumption was confirmed by including a cross-product of the exposure and follow-up time in the model (p = 0.28).
We performed a sensitivity analysis whereby we excluded those who reported ever regular use of pipe or cigar (16.7%). We also assessed average smoking intensity by dividing cumulative cigarette exposure by duration of smoking. For example, for a participant who reported smoking 1–10 CPD at age 30–39, we assigned 50 cigarette-years (5 CPD times 10 years) for that age-period, and summed cigarette-years from all the other age-periods that the participant reported smoking to compute the cumulative cigarette exposure. We then divided by smoking duration to create average CPD. All analytic tests were performed using SAS version 9.3 (Cary, CA) with statistical significance defined as p-values <0.05 (two-sided).
RESULTS
The average age of the 238,525 participants at baseline was 70.4 years old (range 59–82) with a majority in their 60s (44.7%) or 70s (54.1%). The cohort included 18,233 current smokers (7.6%), 127,121 former smokers (53.3%), and 93,171 never smokers (39.0%). Among ever smokers, 66.6% started smoking before 20 years and 30.0% started in their 20s, and only 3.4% started smoking in their 30s or older. Almost all (99%) current smokers reported smoking for over 30 years with a mean duration of 48 years. Most smokers who reported currently smoking <1 (85.6%) and 1–10 (67.4%) CPD at baseline had smoked more CPD earlier in their lives (Supplementary Table S2). However, 137 and 1,243 reported consistently using <1 and 1–10 CPD over their lifetime.
Lifelong consistent smokers of <1 and 1–10 CPD tended to have started smoking at an older age, were more likely to be female, non-Hispanic black, Hispanic, or Asian/pacific islander/native American, and were less likely to have comorbid conditions than other groups of current smokers (Table 1).
Table 1.
Demographic and lifestyle characteristics compared by baseline number of cigarettes smoked per day among current and former smokers reporting smoking consistent cigarettes per day (CPD) and current smokers reporting different CPD over the lifetime
| Never smoker | Current - Lifelonga | Former - Lifelongb | Current - Inconsistentc | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| < 1 CPD | 1–10 CPD | 11–20 CPD | > 20 CPD | < 1 CPD | 1–10 CPD | < 1 CPD | 1–10 CPD | ||
| N | 93,171 | 137 | 1,243 | 2,254 | 906 | 4,827 | 16,849 | 978 | 3,655 |
| Age started smoking (%d) | |||||||||
| < 15 | NA | 21.9 | 12.3 | 30.0 | 46.6 | 11.9 | 5.7 | 22.1 | 21.7 |
| 15 – 19 | NA | 30.7 | 38.1 | 46.1 | 36.6 | 34.8 | 41.7 | 41.9 | 43.5 |
| 20 – 24 | NA | 23.4 | 29.8 | 16.9 | 11.7 | 30.0 | 37.9 | 27.5 | 27.7 |
| 25 – 29 | NA | 11.0 | 7.9 | 3.1 | 2.0 | 9.7 | 7.8 | 4.2 | 4.4 |
| ≥ 30 | NA | 13.0 | 11.9 | 3.9 | 3.1 | 13.6 | 6.9 | 4.3 | 2.7 |
| Ever regular use of pipe or cigar (%) | 8.6 | 16.1 | 8.7 | 11.3 | 17.7 | 22.0 | 18.6 | 21.3 | 15.5 |
| Agee,f | 70.4 (0.0) | 68.7 (0.4) | 70.0 (0.2) | 69.0 (0.1) | 68.7 (0.2) | 70.2 (0.1) | 70.8 (0.0) | 69.3 (0.2) | 69.4 (0.1) |
| Genderd (%) | |||||||||
| Male | 47.5 | 54.7 | 30.6 | 49.4 | 70.8 | 58.4 | 47.1 | 54.0 | 48.2 |
| Female | 52.5 | 45.3 | 69.4 | 50.6 | 29.3 | 41.6 | 52.9 | 46.0 | 51.8 |
| Raceg (%) | |||||||||
| Non-Hispanic White | 91.4 | 82.5 | 80.7 | 93.6 | 96.4 | 88.9 | 89.1 | 89.0 | 90.9 |
| Non-Hispanic Black | 3.6 | 9.5 | 13.3 | 3.2 | 2.1 | 4.9 | 5.2 | 6.3 | 5.2 |
| Hispanic | 2.0 | 2.9 | 2.2 | 1.0 | 0.8 | 2.9 | 2.5 | 1.7 | 1.5 |
| Asian/Pacific Islander/Native American | 2.0 | 2.9 | 1.9 | 0.9 | 0.1 | 2.1 | 1.8 | 1.5 | 1.3 |
| Educationd (%) | |||||||||
| ≤ High school | 21.9 | 35.0 | 30.3 | 30.4 | 31.2 | 15.3 | 21.8 | 20.5 | 23.3 |
| Post-high school training/some college | 28.6 | 26.3 | 35.5 | 39.8 | 36.3 | 28.0 | 32.0 | 35.9 | 38.3 |
| ≥ College | 47.4 | 34.3 | 30.8 | 26.8 | 30.2 | 54.4 | 43.5 | 41.2 | 36.1 |
| Body mass indexe,f (kg/m2) | 26.9 (0.0) | 27.0 (0.5) | 25.5 (0.1) | 25.8 (0.1) | 26.8 (0.2) | 26.9 (0.1) | 26.8 (0.0) | 27.1 (0.2) | 26.0 (0.1) |
| Familial history of cancer (%) | 56.7 | 52.6 | 56.1 | 57.1 | 56.6 | 56.4 | 56.2 | 57.8 | 57.7 |
| Alcoholic beverage intakee,g (g/d) | 96.5 (1.3) | 134.6 (19.4) | 164.1 (14.8) | 270.8 (21.6) | 407.7 (41.0) | 125.2 (5.8) | 131.2 (3.8) | 234.8 (24.0) | 216.0 (12.8) |
| Physical activitye,f (MET-hrs/wk) | 26.1 (0.1) | 24.6 (3.4) | 21.7 (0.9) | 18.0 (0.6) | 17.2 (1.1) | 27.3 (0.5) | 28.3 (0.3) | 24.8 (1.0) | 21.3 (0.5) |
| Fair/poor self-reported healthf (%) | 9.7 | 11.7 | 11.0 | 16.4 | 25.9 | 9.6 | 10.1 | 14.8 | 16.8 |
| Comorbid conditione,h (%) | |||||||||
| Heart attack | 13.7 | 10.2 | 10.3 | 17.9 | 22.5 | 15.1 | 14.3 | 17.9 | 18.8 |
| Stroke | 2.9 | 2.9 | 3.6 | 4.6 | 5.6 | 3.2 | 2.9 | 4.6 | 4.4 |
| Diabetes | 13.6 | 16.8 | 11.2 | 13.2 | 18.9 | 14.3 | 13.6 | 14.0 | 11.8 |
| COPD | 3.9 | 4.4 | 8.2 | 19.7 | 25.5 | 4.2 | 5.1 | 12.5 | 19.1 |
Current smokes who reported consistent number of cigarettes smoked per day during the lifetime.
Former smokers who reported smoking consistent number of cigarettes per day when they smoked. We show only former smokers who consistently smoked <1 or 1–10 cigarettes per day.
Current smokers whose reported current number of cigarettes smoked per day was different from amounts earlier in their life. We show only those who reported currently smoking <1 or 1–10 cigarettes per day.
Percentages for a “unknown” category for categorical variables were considered in calculation but not shown.
Mean (standard error) for continuous variables
Assessed in the follow-up questionnaire in 2004–2005
Assessed in the cohort baseline questionnaire in 1995–1996
Self-reported ever diagnosis before the follow-up survey in 2004–2005
During a mean follow-up of 6.2 years, we identified 33,286 incident cancers including 12,330 that were smoking-related, such as lung (n=3,980), colorectal (n=2,515), bladder (n=2,098), upper gastrointestinal (n=1,311 including 366 gastric, 313 esophageal, and 632 head & neck), 929 kidney, and 900 pancreatic cancers.
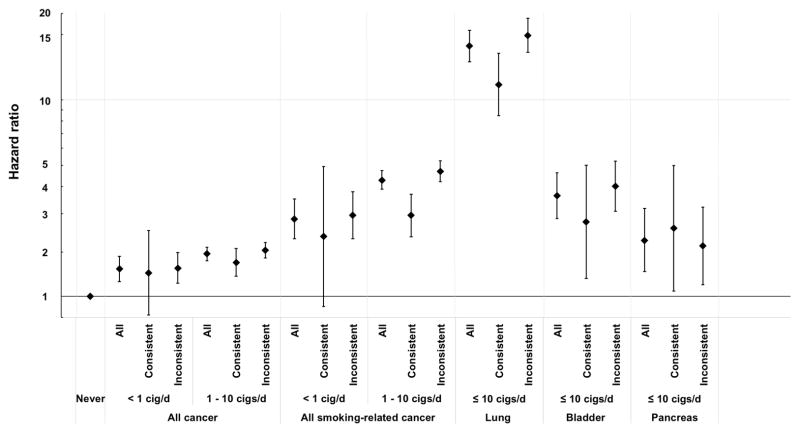
CPD among current smokers was positively associated with smoking-related cancer in a dose-dependent manner after adjusting for covariates (Table 2). Even smokers of <1 and 1–10 CPD at baseline were at increased risk of incident smoking-related cancer compared with never smokers. After stratification by consistent or varied CPD during the lifetime, consistent lifelong 1–10 CPD smokers had higher risk of smoking-related cancer (HR, 2.34; 95% CI, 1.86–2.93) than never smokers (Figure 1). Consistent lifelong smokers of <1 CPD were also at higher risk of smoking-related cancer (HR, 1.89; 95% CI, 0.90–3.96) than never smokers, although the association was not statistically significant. Higher HRs were observed among <1 (2.36, 95% CI, 1.84–3.03) and 1–10 CPD (3.77, 95% CI, 3.33–4.27) smokers at baseline who had varied their CPD over their lifetime. Similar patterns were also observed for overall cancer (Supplementary Table S3). We observed similar patterns in a sensitivity analysis where we assessed average smoking intensity: cancer risk was higher among current smokers with lifetime average intensity of 1–10 CPD (HR, 2.43, 95% CI, 2.11–2.80) or <1 CPD (HR, 1.87, 95% CI, 0.97–3.60) than never smokers, although the risk estimate for <1 CPD did not reach statistical significance (Supplementary Table S4).
Table 2.
Incident smoking-related cancera risk and current cigarettes smoked per day (CPD) among all participants and by reported consistent or different CPD over the lifetime
| N | Cases | Adjusted HR (95% CI)b | Excluding ever regular users of cigars/pipesc | |
|---|---|---|---|---|
| All participants | ||||
| Never | 93,171 | 2,748 | 1.00 | 1.00 |
| < 1 CPD | 1,466 | 93 | 2.29 (1.85 – 2.82) | 2.35 (1.84 – 2.99) |
| 1–10 CPD | 5,382 | 490 | 3.39 (3.08 – 3.74) | 3.46 (3.11 – 3.85) |
| 11–20 CPD | 6,355 | 736 | 4.43 (4.08 – 4.81) | 4.58 (4.19 – 5.01) |
| 21–30 CPD | 2,718 | 362 | 4.92 (4.40 – 5.50) | 5.24 (4.65 – 5.90) |
| > 30 CPD | 2,312 | 344 | 5.49 (4.90 – 6.15) | 5.50 (4.84 – 6.24) |
|
| ||||
| Participants who reported consistent CPD during the lifetime | ||||
| Never | 93,171 | 2,748 | 1.00 | 1.00 |
| < 1 CPD | 137 | 8 | 1.89 (0.90 – 3.96) | 2.75 (1.31 – 5.78) |
| 1–10 CPD | 1,243 | 78 | 2.34 (1.86 – 2.93) | 2.57 (2.03 – 3.24) |
| 11–20 CPD | 2,254 | 290 | 4.87 (4.31 – 5.50) | 5.13 (4.50 – 5.85) |
| 21–30 CPD | 551 | 78 | 5.01 (3.98 – 6.30) | 5.44 (4.22 – 7.02) |
| > 30 CPD | 355 | 56 | 5.43 (4.15 – 7.10) | 4.73 (3.42 – 6.52) |
|
| ||||
| Participants who reported different CPD during the lifetime | ||||
| Never | 93,171 | 2,748 | 1.00 | 1.00 |
| < 1 CPD | 978 | 65 | 2.36 (1.84 – 3.03) | 2.40 (1.79 – 3.22) |
| 1–10 CPD | 3,655 | 362 | 3.74 (3.34 – 4.17) | 3.77 (3.33 – 4.27) |
| 11–20 CPD | 3,623 | 402 | 4.32 (3.89 – 4.80) | 4.38 (3.90 – 4.91) |
| 21–30 CPD | 1,954 | 262 | 5.08 (4.47 – 5.78) | 5.38 (4.70 – 6.17) |
| > 30 CPD | 1,452 | 244 | 6.40 (5.60 – 7.31) | 6.49 (5.61 – 7.50) |
HR: hazard ratio, CI: confidence interval
Cancer of oral cavity, oropharynx, nasopharynx, hypopharynx, esophagus, stomach, colorectum, liver, pancreas, nasal cavity, paranasal sinuses, larynx, lung, uterine cervix, ovary (mucinous) urinary bladder, kidney, ureter, and acute myeloid leukemia.
Adjusted for gender, race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, Asian/pacific islander/native American, or unknown), education (< high school, high school, post high school, some college, college/post graduate, or unknown), alcohol intake (continuous), familial history of any cancer (yes, no, or unknown), and ever regular use of pipe or cigar (yes, no, or unknown). Age was used as the underlying time metric.
Excluding participants who reported ever using pipes or cigars regularly in the 1995–1996 initial cohort questionnaire.
Figure 1.
Hazard ratio (HR) and 95% confidence interval (CI) for incident cancer overall and smoking-related cancer by lifetime consistent and inconsistent smoking intensity among current smokers of < 1 or 1–10 cigarettes per day. The HRs and 95% CIs were adjusted for gender, race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, Asian/pacific islander/native American, or unknown), education (< high school, high school, post high school, some college, college/post graduate, or unknown), alcohol intake (continuous), familial history of any cancer (yes, no, or unknown), and ever regular use of pipe or cigar (yes, no, or unknown). Age was used as the underlying time metric. Smoking-related cancers include cancer of oral cavity, oropharynx, nasopharynx, hypopharynx, esophagus, stomach, colorectum, liver, pancreas, nasal cavity, paranasal sinuses, larynx, lung, uterine cervix, ovary (mucinous) urinary bladder, kidney, ureter, and acute myeloid leukemia.
Next, we examined smoking cessation. Former smokers who had consistently smoked 1–10 CPD during the years they smoked had higher risk of smoking-related cancer (HR, 1.15; 95% CI, 1.05–1.26) and overall cancer (HR, 1.07; 95% CI, 1.02–1.12) than never-smokers (Table 3 and Supplementary Table S5), however these HRs were weaker than those of current smokers who had continued to smoke at this level. HRs were lower among participants who quit long time ago. Among former consistent 1–10 CPD smokers, the HR for developing a smoking-related cancer was 1.04 (95% CI, 0.89–1.21) for those who quit 40 or more years ago and 2.28 (95% CI, 1.68–3.09) for those who quit less than 10 years ago. Similarly, we observed higher risks among former consistent 1–10 CPD smokers who had smoked for a longer period: HRs were 1.50 (95% CI, 1.17–1.92) for those who had smoked >30 years and 1.02 (95% CI, 0.90–1.15) for those who smoked ≤10 years. No clear pattern was observed among former consistant <1 CPD smokers, but our case numbers for these analyses were low. Associations persisted when excluding ever-users of other tobacco products (Tables 2–3).
Table 3.
Incident smoking-related cancera risk by age at cessation and duration of smoking among former smokers who reported consistent < 1 or 1–10 cigarettes per day (CPD) over the time they smoked
| N | Cases | Adjusted HR (95% CI)b | Excluding ever regular users of cigars/pipesc | |
|---|---|---|---|---|
| Never smoker | 93,171 | 2,748 | 1.00 | 1.00 |
|
| ||||
| Former consistent < 1 CPD | ||||
| All | 4,827 | 156 | 1.09 (0.93 – 1.28) | 1.11 (0.92 – 1.34) |
| Years since cessation | ||||
| < 10 y | 104 | 3 | 1.05 (0.34 – 3.27) | 1.32 (0.33 – 5.26) |
| 10 – 19 y | 573 | 17 | 1.14 (0.71 – 1.83) | 0.98 (0.54 – 1.77) |
| 20 – 29 y | 655 | 28 | 1.47 (1.01 – 2.13) | 1.49 (0.95 – 2.34) |
| 30 – 39 y | 1,416 | 42 | 1.17 (0.86 – 1.59) | 1.02 (0.69 – 1.51) |
| ≥ 40 y | 2,079 | 66 | 0.92 (0.72 – 1.18) | 1.07 (0.82 – 1.42) |
| Duration of smoking | ||||
| ≤ 10 y | 3,596 | 110 | 0.99 (0.82 – 1.21) | 1.07 (0.86 – 1.34) |
| 11 – 20 y | 673 | 25 | 1.21 (0.82 – 1.80) | 1.18 (0.72 – 1.94) |
| 21 – 30 y | 213 | 13 | 1.36 (0.79 – 2.35) | 1.35 (0.68 – 2.71) |
| > 30 y | 179 | 6 | 1.09 (0.49 – 2.43) | 0.89 (0.29 – 2.77) |
|
| ||||
| Former consistent 1–10 CPD | ||||
| All | 16,849 | 574 | 1.15 (1.05 – 1.26) | 1.15 (1.04 – 1.27) |
| Years since cessation | ||||
| < 10 y | 696 | 42 | 2.28 (1.68 – 3.09) | 2.35 (1.66 – 3.33) |
| 10 – 19 y | 2,139 | 70 | 1.18 (0.93 – 1.50) | 1.14 (0.87 – 1.49) |
| 20 – 29 y | 3,228 | 116 | 1.29 (1.07 – 1.55) | 1.30 (1.05 – 1.61) |
| 30 – 39 y | 5,799 | 164 | 1.03 (0.88 – 1.21) | 1.04 (0.86 – 1.25) |
| ≥ 40 y | 4,987 | 182 | 1.04 (0.89 – 1.21) | 1.05 (0.88 – 1.25) |
| Duration of smoking | ||||
| ≤ 10 y | 9,235 | 279 | 1.02 (0.90 – 1.15) | 1.03 (0.89 – 1.19) |
| 11 – 20 y | 3,854 | 139 | 1.17 (0.98 – 1.39) | 1.17 (0.95 – 1.43) |
| 21 – 30 y | 2,335 | 91 | 1.28 (1.04 – 1.58) | 1.34 (1.06 – 1.69) |
| > 30 y | 1,381 | 64 | 1.50 (1.17 – 1.92) | 1.45 (1.10 – 1.92) |
HR: hazard ratio, CI: confidence interval
Cancer of oral cavity, oropharynx, nasopharynx, hypopharynx, esophagus, stomach, colorectum, liver, pancreas, nasal cavity, paranasal sinuses, larynx, lung, uterine cervix, ovary (mucinous) urinary bladder, kidney, ureter, and acute myeloid leukemia.
Adjusted for gender, race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, Asian/pacific islander/native American, or unknown), education (< high school, high school, post high school, some college, college/post graduate, or unknown), alcohol intake (continuous), familial history of any cancer (yes, no, or unknown), and ever regular use of pipe or cigar (yes, no, or unknown). Age was used as the underlying time metric.
Excluding participants who reported ever using pipes or cigars regularly in the 1995–1996 initial cohort questionnaire.
We also examined associations with specific cancer types. For these analyses, we examined <1 and 1–10 CPD together as a single category of low-intensity smokers (≤ 10 CPD) to maximize case numbers. Even among this combined category, numbers of cases for each cancer type or group were low. Current consistent smokers of ≤10 CPD had higher risks of lung (HR, 9.65, 95% CI, 6.93–13.43), bladder (HR, 2.22, 95% CI, 1.22–4.05), and pancreatic cancers (HR, 2.03, 95% CI, 1.05–3.95) than never smokers (Table 4). Risks of lung and bladder cancers among former consistent ≤10 CPD smokers generally fell with longer time since cessation and longer duration of smoking. For example, the HR for lung cancer was 6.04 (95% CI, 3.53–10.32) for those who quit less than 10 years ago and 1.50 (95% CI, 1.08–2.10) for those who quit 40 or more years ago. Despites small number of cases, longer duration of smoking was also associated with higher risks of head and neck cancer and UGI cancer.
Table 4.
Incident smoking-related cancer risk among former smokers reporting consistent smoking of ≤ 10 cigarettes per day (CPD)a by time since cessation
| Lung | Bladder | Kidney | Head & Neck | UGIc | Colon & Rectum | Pancreas | |
|---|---|---|---|---|---|---|---|
| Never smoker | |||||||
| Cases | 299 | 439 | 316 | 142 | 160 | 969 | 337 |
| HR | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
|
| |||||||
| Current consistent smokers of ≤ 10 CPD | |||||||
| Cases | 40 | 11 | 5 | 3 | 1 | 11 | 9 |
| HR (95% CI)b | 9.65 (6.93 – 13.43) | 2.22 (1.22 – 4.05) | 1.24 (0.51 – 3.00) | 1.66 (0.53 – 5.21) | 0.48 (0.07–3.42) | 0.81 (0.43 – 1.51) | 2.03 (1.05 – 3.95) |
|
| |||||||
| Former consistent smokers of ≤ 10 CPD | |||||||
|
| |||||||
| Yeas since cessation | |||||||
|
| |||||||
| < 10 y | |||||||
| Cases | 14 | 10 | 1 | 2 | 1 | 12 | 2 |
| HR (95% CI) | 6.04 (3.53 – 10.32) | 3.20 (1.71 – 6.00) | 0.42 (0.06 – 2.96) | 1.80 (0.45 – 7.29) | 0.78 (0.11 – 5.55) | 1.71 (0.97 – 3.02) | 0.78 (0.19 – 3.12) |
| 10 – 19 y | |||||||
| Cases | 21 | 12 | 7 | 11 | 13 | 12 | 8 |
| HR (95% CI) | 2.57 (1.65 – 4.00) | 1.10 (0.62 – 1.96) | 0.84 (0.40 – 1.78) | 2.90 (1.57 – 5.37) | 3.00 (1.70 – 5.29) | 0.49 (0.28 – 0.87) | 0.88 (0.44 – 1.77) |
| 20 – 29 y | |||||||
| Cases | 31 | 28 | 15 | 5 | 7 | 35 | 16 |
| HR (95% CI) | 2.61 (1.80 – 3.78) | 1.67 (1.14 – 2.45) | 1.39 (0.85 – 2.26) | 0.88 (0.36 – 2.14) | 1.07 (0.50 – 2.29) | 1.00 (0.71 – 1.40) | 1.19 (0.72 – 1.96) |
| 30 – 39 y | |||||||
| Cases | 35 | 39 | 17 | 12 | 17 | 56 | 15 |
| HR (95% CI) | 1.71 (1.20 – 2.44) | 1.24 (0.89 – 1.72) | 0.77 (0.48 – 1.24) | 1.05 (0.58 – 1.89) | 1.42 (0.86 – 2.35) | 0.91 (0.69 – 1.19) | 0.62 (0.37 – 1.04) |
| ≥ 40 y | |||||||
| Cases | 40 | 46 | 18 | 16 | 10 | 85 | 19 |
| HR (95% CI) | 1.50 (1.08 – 2.10) | 1.01 (0.74 – 1.38) | 0.91 (0.60 – 1.38) | 1.28 (0.769 – 2.14) | 0.65 (0.34 – 1.23) | 1.17 (0.93 – 1.46) | 0.65 (0.41 – 1.04) |
|
| |||||||
| Duration of smoking | |||||||
|
| |||||||
| ≤ 10 y | |||||||
| Cases | 63 | 69 | 38 | 23 | 17 | 127 | 33 |
| HR (95% CI) | 1.61 (1.22 – 2.12) | 1.05 (0.81 – 1.36) | 0.86 (0.61 – 1.21) | 1.04 (0.66 – 1.62) | 0.74 (0.44 – 1.22) | 1.08 (0.89 – 1.30) | 0.73 (0.51 – 1.04) |
| 11 – 20 y | |||||||
| Cases | 31 | 33 | 15 | 11 | 16 | 38 | 14 |
| HR (95% CI) | 1.99 (1.36 – 2.91) | 1.50 (1.05 – 2.15) | 1.99 (0.59 – 1.66) | 1.46 (0.78 – 2.71) | 1.93 (1.15 – 3.25) | 0.87 (0.63 – 1.20) | 0.85 (0.50 – 1.46) |
| 21 – 30 y | |||||||
| Cases | 25 | 21 | 13 | 6 | 8 | 20 | 7 |
| HR (95% CI) | 2.72 (1.80 – 4.10) | 1.70 (1.09 – 2.64) | 1.49 (0.85 – 2.59) | 1.46 (0.64 – 3.32) | 1.67 (0.82 – 3.42) | 0.77 (0.49 – 1.20) | 0.74 (0.35 – 1.56) |
| > 30 y | |||||||
| Cases | 22 | 11 | 0 | 6 | 7 | 15 | 6 |
| HR (95% CI) | 3.76 (2.43 – 5.82) | 1.56 (0.85 – 2.84) | 0 | 2.64 (1.16 – 6.01) | 2.49 (1.16 – 5.35) | 0.94 (0.56 – 1.57) | 1.06 (0.47 – 2.37) |
UGI: upper gastrointestine, HR: hazard ratio, CI: confidence interval
< 1 CPD and 1–10 CPD categories were combined due to small number of individual cancer cases.
Adjusted for gender, race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, Asian/pacific islander/native American, or unknown), education (< high school, high school, post high school, some college, college/post graduate, or unknown), alcohol intake (continuous), familial history of any cancer (yes, no, or unknown), and ever regular use of pipe or cigar (yes, no, or unknown). Age was used as the underlying time metric.
Esophageal and gastric cancers
DISCUSSION
In our study of over 230,000 older US adults, current lifelong <1 and 1–10 CPD smokers had higher risk of incident cancer than never-smokers, with associations noted for cancers of the lung, bladder, and pancreas. Associations were strongest among current lifelong <1 and 1–10 CPD smokers and attentuated among former smokers, particularly those who quit smoking long time ago.
Cigarette smoking is causally related to at least 20 types of cancer.3–5 Nevertheless, previous studies have focused mostly on participants who smoke 10 CPD or more. Just a few studies to date have examined associations of low-intensity smoking with cancer. Results from these studies are generally consistent with our findings, observing higher risk of cancers, such as lung and head & neck cancers, among those who smoked 1–10 CPD.9,14,15 Data on disease risks among non-daily smokers are even more limited. A previous analysis of the national survey data in Finland from 1978 to 1991 showed that occasional cigarette smoking was associated with 1.7 times (95% CI, 1.3–2.1) higher risk of lung cancer than never smoking in men.22 Another study in the European Prospective Investigation into Cancer and Nutrition (EPIC) indicates higher risks of smoking-related cancer (HR, 1.24; 95% CI, 0.80–1.94) and bladder cancer (HR, 1.92; 95% CI, 0.93–3.98) among occasional smokers than never smokers, although the associations were not statistically significant and were limited by small case numbers.23 It should be noted, however, that these previous studies largely assessed smoking at one point in time and did not assess changes in smoking intensity over the lifetime.
Although the previous literature on low-intensity smoking is relatively limited, several lines of evidence support our findings. Cigarette smoke contains more than 700 compounds, hundreds of which are known to be harmful (please add reference). A substantial body of literature has shown that second-hand smoke, containing all of the same carcinogens and toxicants as main-stream cigarette smoke, although at lower doses,24,25 is causally related to lung cancer and other diseases.12 For example, the US Surgeon General’s 2006 Report concluded that non-smokers who had smoking husbands had up to 29% higher risk than those with non-smoker husbands.12 Non-smokers exposed to workplace second-hand smoke were at 22% higher risk of lung cancer than those without workplace second-hand smoke exposure. These results together support that smoking at low-intensity over the lifetime is associated with higher cancer risk.
An important strength of the current study is detailed data on cigarette smoking intensity over the lifespan, which enabled us to distinguish consistent lifelong low-intensity smokers from low-intensity smokers at baseline who had smoked different amounts per day earlier in their life. A large sample size and a prospective study design were key strengths that allowed us to evaluate the risk of incident cancer among long-term consistent low-intensity smokers, although our case numbers in this group was relatively small.
Our study also had several limitations. We relied on participants’ recalling their smoking intensity retrospectively; therefore, participants may have underestimated or overestimated their smoking intensity. Yet, self-reported smoking has been shown to have good correlation with biomarkers, such as nicotine and its metabolites, in blood or urine.26,27 Methodologic studies have shown high reliability of retrospective assessment of lifetime smoking, such as age when they started smoking and minimum, maximum, and average CPD.28,29 Short-term recall of smoking is generally more reliable than long-term recall. However, a previous longitudinal study showed that people reported past CPD with good validity for 20 years earlier (kappa = 0.63) and fair validity for 32 years earlier (kappa = 0.36).30 In the NIH-AARP cohort, we also observed good concordance of recalled smoking after 10 years in the present study (74% concordance among lifelong consistent smokers of 10 or fewer CPD).18 Despite our large sample size, we were underpowered to estimate associations between lifelong low-intensity smoking and individual cancers. Although results were similar when we examined average smoking over the lifetime. We also lacked detailed information on non-cigarette tobacco use. We performed a sensitivity analysis excluding individuals who reported ever use of cigar or pipe in the initial cohort questionnaires (16.7%) and the results did not change considerably. Nonetheless, we lacked detailed information on other tobacco products, and were unable to examine disease risks of low-intensity cigarette smoking in combination with other tobacco products. Future studies are needed to provide these important risk estimates. Our study participants were also mostly white and older. Future studies are needed in different populations, especially among younger age groups as well as racial/ethnic minorities, as low-intensity smoking has been historically more common among racial/ethnic minorities.31,32
In conclusion, in our study of older US adults, participants who consistently smoked 10 or fewer CPD over the lifetime had higher risk of developing cancer than never smokers. Furthermore, risks were lower among former smokers of this level, particularly those who quit at a younger age. These findings provide further evidence that even low levels of cigarette smoking cause cancer. All smokers should quit smoking, regardless of how few cigarettes they smoke per day.
Supplementary Material
Novelty and Impact.
In a large cohort of older US adults, current smokers of <1 and 1–10 cigarettes per day were at increased risk of incident cancer relative to never smokers, with elevated but attenuated risks among those who had quit smoking. Our findings provide further evidence that even low-levels of cigarette smoking cause cancer and that all smokers would benefit from cessation regardless of how few cigarettes they smoke.
Acknowledgments
All authors had full access to all of the data (including statistical analysis results and tables) in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis. We thank the participants in the NIH-AARP Diet and Health Study for their cooperation, and David Campbell and Jane Wang at Information management Services (Sipver Spring, MD) for data support.
Grant Sponsor: This work was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Division of Cancer Epidemiology & Genetics. The sponsor reviewed and approved final submission but had no role in the design and conduct of the study; the collection, management, analysis, and interpretation of the data; the preparation of the manuscript; and decision to submit the manuscript for publication.
Abbreviations
- US
United States
- CPD
cigarettes per day
- NIH
National Institutes of Health
- BMI
body mass index
- ICD-O
International Classification of Diseases for Oncology
- UGI
upper gastrointestinal
- HR
hazard ratio
- CI
confidence interval
- SD
standard deviation
- EPIC
European Prospective Investigation into Cancer and Nutrition
Footnotes
Disclosure: None of the authors have conflict of interest to disclose.
References
- 1.American Cancer Society. Cancer Facts & Figures 2016. Atlanta: American Cancer Society; 2016. [Google Scholar]
- 2.Henley SJ, Thomas CC, Sharapova SR, Momin B, Massetti GM, Winn DM, Armour BS, Richardson LC. Vital Signs: Disparities in Tobacco-Related Cancer Incidence and Mortality - United States, 2004–2013. MMWR Morb Mortal Wkly Rep. 2016;65(44):1212–1218. doi: 10.15585/mmwr.mm6544a3. [DOI] [PubMed] [Google Scholar]
- 3.IARC. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, vol. 100E. Personal Habits and Indoor Combustions. Lyon, France: International Agency for Research on Cancer; 2012. [PMC free article] [PubMed] [Google Scholar]
- 4.The health consequences of smoking - 50 years of progress: a report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. [Google Scholar]
- 5.Freedman ND, Abnet CC, Caporaso NE, Fraumeni JF, Jr, Murphy G, Hartge P, Hollenbeck AR, Park Y, Shiels MS, Silverman DT. Impact of changing US cigarette smoking patterns on incident cancer: risks of 20 smoking-related cancers among the women and men of the NIH-AARP cohort. Int J Epidemiol. 2016;45(3):846–856. doi: 10.1093/ije/dyv175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morbidity and Mortality Weekly Report (MMWR) Centers for Disease Control and Prevention; Nov 13, 2015. Current cigarette smoking among adults - United States, 2005–2014. [Google Scholar]
- 7.Jamal A, King BA, Neff LJ, Whitmill J, Babb SD, Graffunder CM. Current Cigarette Smoking Among Adults - United States, 2005–2015. MMWR Morb Mortal Wkly Rep. 2016;65(44):1205–1211. doi: 10.15585/mmwr.mm6544a2. [DOI] [PubMed] [Google Scholar]
- 8.International Agency for Research on Cancer. Tobacco Smoke and Involuntary Smoking. Lyon, France: IARC; 2004. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, vol. 83. [PMC free article] [PubMed] [Google Scholar]
- 9.Doll R, Peto R. Cigarette smoking and bronchial carcinoma: dose and time relationships among regular smokers and lifelong non-smokers. J Epidemiol Community Health. 1978;32(4):303–313. doi: 10.1136/jech.32.4.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flanders WD, Lally CA, Zhu BP, Henley SJ, Thun MJ. Lung cancer mortality in relation to age, duration of smoking, and daily cigarette consumption: results from Cancer Prevention Study II. Cancer Res. 2003;63(19):6556–6562. [PubMed] [Google Scholar]
- 11.Lubin JH, Caporaso NE. Cigarette smoking and lung cancer: modeling total exposure and intensity. Cancer Epidemiol Biomarkers Prev. 2006;15(3):517–523. doi: 10.1158/1055-9965.EPI-05-0863. [DOI] [PubMed] [Google Scholar]
- 12.The health consequences of involuntary exposure to tobacco smoke : a report of the Surgeon General. U.S. Dept. of Health and Human Services, Centers for Disease Control and Prevention, Coordinating Center for Health Promotion, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2006. [PubMed] [Google Scholar]
- 13.Amrock SM, Weitzman M. Adolescents’ perceptions of light and intermittent smoking in the United States. Pediatrics. 2015;135(2):246–254. doi: 10.1542/peds.2014-2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garfinkel L, Stellman SD. Smoking and lung cancer in women: findings in a prospective study. Cancer Res. 1988;48(23):6951–6955. [PubMed] [Google Scholar]
- 15.Berthiller J, Straif K, Agudo A, Ahrens W, Bezerra Dos Santos A, Boccia S, Cadoni G, Canova C, Castellsague X, Chen C, Conway D, Curado MP, Dal Maso L, Daudt AW, Fabianova E, Fernandez L, Franceschi S, Fukuyama EE, Hayes RB, Healy C, Herrero R, Holcatova I, Kelsey K, Kjaerheim K, Koifman S, Lagiou P, La Vecchia C, Lazarus P, Levi F, Lissowska J, Macfarlane T, Mates D, McClean M, Menezes A, Merletti F, Morgenstern H, Muscat J, Olshan AF, Purdue M, Ramroth H, Rudnai P, Schwartz SM, Serraino D, Shangina O, Smith E, Sturgis EM, Szeszenia-Dabrowska N, Thomson P, Vaughan TL, Vilensky M, Wei Q, Winn DM, Wunsch-Filho V, Zhang ZF, Znaor A, Ferro G, Brennan P, Boffetta P, Hashibe M, Lee YA. Low frequency of cigarette smoking and the risk of head and neck cancer in the INHANCE consortium pooled analysis. Int J Epidemiol. 2015 doi: 10.1093/ije/dyv146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu SH, Sun J, Hawkins S, Pierce J, Cummins S. A population study of low-rate smokers: quitting history and instability over time. Health Psychol. 2003;22(3):245–252. doi: 10.1037/0278-6133.22.3.245. [DOI] [PubMed] [Google Scholar]
- 17.Holford TR, Levy DT, McKay LA, Clarke L, Racine B, Meza R, Land S, Jeon J, Feuer EJ. Patterns of birth cohort-specific smoking histories, 1965–2009. Am J Prev Med. 2014;46(2):e31–37. doi: 10.1016/j.amepre.2013.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inoue-Choi M, Liao LM, Reyes-Guzman C, Hartge P, Caporaso N, Freedman ND. Association of Long-term, Low-Intensity Smoking With All-Cause and Cause-Specific Mortality in the National Institutes of Health-AARP Diet and Health Study. JAMA Intern Med. 2017;177(1):87–95. doi: 10.1001/jamainternmed.2016.7511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schatzkin A, Subar AF, Thompson FE, Harlan LC, Tangrea J, Hollenbeck AR, Hurwitz PE, Coyle L, Schussler N, Michaud DS, Freedman LS, Brown CC, Midthune D, Kipnis V. Design and serendipity in establishing a large cohort with wide dietary intake distributions : the National Institutes of Health-American Association of Retired Persons Diet and Health Study. Am J Epidemiol. 2001;154(12):1119–1125. doi: 10.1093/aje/154.12.1119. [DOI] [PubMed] [Google Scholar]
- 20.Michaud DS, Midthune D, Hermansen S, Leitzmann M, Harlan LC, Kipnis V, Schatzkin A. Comparison of cancer registry case ascertainment with SEER estimates and self-reporting in a subset of the NIH-AARP Diet and Health Study. Journal of Registry Management. 2005;32(2):70–75. [Google Scholar]
- 21.Secretan B, Straif K, Baan R, Grosse Y, El Ghissassi F, Bouvard V, Benbrahim-Tallaa L, Guha N, Freeman C, Galichet L, Cogliano V Group WHOIAfRoCMW. A review of human carcinogens--Part E: tobacco, areca nut, alcohol, coal smoke, and salted fish. Lancet Oncol. 2009;10(11):1033–1034. doi: 10.1016/s1470-2045(09)70326-2. [DOI] [PubMed] [Google Scholar]
- 22.Luoto R, Uutela A, Puska P. Occasional smoking increases total and cardiovascular mortality among men. Nicotine Tob Res. 2000;2(2):133–139. doi: 10.1080/713688127. [DOI] [PubMed] [Google Scholar]
- 23.Bjerregaard BK, Raaschou-Nielsen O, Sorensen M, Frederiksen K, Tjonneland A, Rohrmann S, Linseisen J, Bergman MM, Boeing H, Sieri S, Palli D, Tumino R, Sacerdote C, Bueno-de-Mesquita HB, Buchner FL, Gram IT, Braaten T, Lund E, Hallmans G, Agren A, Riboli E. The effect of occasional smoking on smoking-related cancers: in the European Prospective Investigation into Cancer and Nutrition (EPIC) Cancer Causes Control. 2006;17(10):1305–1309. doi: 10.1007/s10552-006-0068-9. [DOI] [PubMed] [Google Scholar]
- 24.Pirkle JL, Bernert JT, Caudill SP, Sosnoff CS, Pechacek TF. Trends in the exposure of nonsmokers in the U.S. population to secondhand smoke: 1988–2002. Environ Health Perspect. 2006;114(6):853–858. doi: 10.1289/ehp.8850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suwan-ampai P, Navas-Acien A, Strickland PT, Agnew J. Involuntary tobacco smoke exposure and urinary levels of polycyclic aromatic hydrocarbons in the United States, 1999 to 2002. Cancer Epidemiol Biomarkers Prev. 2009;18(3):884–893. doi: 10.1158/1055-9965.EPI-08-0939. [DOI] [PubMed] [Google Scholar]
- 26.Caraballo RS, Giovino GA, Pechacek TF, Mowery PD. Factors associated with discrepancies between self-reports on cigarette smoking and measured serum cotinine levels among persons aged 17 years or older: Third National Health and Nutrition Examination Survey, 1988–1994. Am J Epidemiol. 2001;153(8):807–814. doi: 10.1093/aje/153.8.807. [DOI] [PubMed] [Google Scholar]
- 27.Shiffman S, Dunbar MS, Benowitz NL. A comparison of nicotine biomarkers and smoking patterns in daily and nondaily smokers. Cancer Epidemiol Biomarkers Prev. 2014;23(7):1264–1272. doi: 10.1158/1055-9965.EPI-13-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brigham J, Lessov-Schlaggar CN, Javitz HS, McElroy M, Krasnow R, Swan GE. Reliability of adult retrospective recall of lifetime tobacco use. Nicotine Tob Res. 2008;10(2):287–299. doi: 10.1080/14622200701825718. [DOI] [PubMed] [Google Scholar]
- 29.Colby SM, Clark MA, Rogers ML, Ramsey S, Graham AL, Boergers J, Kahler CW, Papandonatos GD, Buka SL, Niaura RS, Abrams DB. Development and reliability of the lifetime interview on smoking trajectories. Nicotine Tob Res. 2012;14(3):290–298. doi: 10.1093/ntr/ntr212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krall EA, Valadian I, Dwyer JT, Gardner J. Accuracy of recalled smoking data. Am J Public Health. 1989;79(2):200–202. doi: 10.2105/ajph.79.2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tobacco use among U.S. racial/ethinic minority groups. Atlanta, GA: U.S. Department of Health and Human Services, Center for Disease Control; 1998. [Google Scholar]
- 32.Reyes-Guzman CM, Pfeiffer RM, Lubin J, Freedman ND, Cleary SD, Levine PH, Caporaso NE. Determinants of Light and Intermittent Smoking in the United States: Results from Three Pooled National Health Surveys. Cancer Epidemiol Biomarkers Prev. 2017;26(2):228–239. doi: 10.1158/1055-9965.EPI-16-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.