Abstract
Objective
The effectiveness of Haemophilus influenzae type b (Hib) vaccine in preventing severe pneumonia in Asian children has been questioned, and many large Asian countries yet to introduce Hib conjugate vaccine in immunization programs. The primary objective of this study was to assess Hib conjugate vaccine effectiveness (VE) on radiologically-confirmed pneumonia in children born after introduction of Hib conjugate vaccine in Pakistan.
Study design
A matched case-control study enrolled cases of radiologically-confirmed pneumonia in several hospitals serving low-income populations during 2009–2011. Cases were matched by age and season with 3 hospital and 5 neighborhood controls. Pneumonia was diagnosed using standardized World Health Organization criteria for chest radiograph interpretation. Matched OR were estimated for VE.
Results
A total of 1027 children with radiologically-confirmed pneumonia were enrolled; 975 cases, 2925 hospital controls, and 4875 neighborhood controls were analyzed. The coverage for 3 doses of diphtheria-tetanus-pertussis-hepatitis B-Hib conjugate vaccine was 13.7%, 18%, and 22.7% in cases, hospital controls and neighborhood controls, respectively. Estimated Hib VE for radiologically-confirmed pneumonia was 62% with 3 doses of vaccine using hospital controls and 70% using neighborhood controls.
Conclusions
Hib conjugate vaccine prevented a significant fraction of radiologically-confirmed pneumonia in children in Pakistan. Maximizing impact on child survival needs improved immunization coverage.
Childhood pneumonia remains the leading cause of child mortality in developing countries, including Pakistan.1–3 It is estimated that of all cases of pneumonia, 7%–13% are severe, requiring hospitalization and may lead to death in the absence of appropriate management.4,5 Two major etiologic agents of severe pneumonia in children are Haemophilus influenzae type b (Hib) and Streptococcus pneumoniae, both effectively preventable through vaccines.6,7 However, determining bacterial pneumonia etiology by traditional microbiology has been challenging because of insensitive methods, resulting in a serious underestimation of disease burden, particularly in Asia where high antimicrobial use prior to tertiary care health facility presentation is frequently noted, rendering cultures sterile.8 The combination of insensitive surveillance methods for invasive Hib disease and the equivocal findings from a large cluster-randomized Hib conjugate vaccine-probe study in Indonesia9 has resulted in large Asian countries including China and India delaying the introduction of Hib conjugate vaccine in routine childhood immunization programs.8
Carefully conducted acute bacterial meningitis surveillance studies using nonculture methods for etiologic diagnosis documented a substantial burden of invasive Hib and pneumococcal disease in Pakistan,10–12 and it is likely that Hib, therefore, also causes a substantial burden of pneumonia in Pakistan.6 In 2000, Pakistan was estimated to have 410 478 cases of Hib pneumonia in children aged <5 years.7 The availability of financing by the GAVI Alliance led to a decision by the Government of Pakistan to introduce the pentavalent (diphtheria-tetanus-pertussis-hepatitis B-Hib [DTP-Hep B-Hib]) vaccine in the country’s Expanded Program on Immunization (EPI) in 2009. Pentavalent vaccine in routine EPI is scheduled for children at the age of 6, 10, and 14 weeks, respectively. The primary objective of this study was to assess the impact of introduction of Hib conjugate vaccine in Pakistan by measuring vaccine effectiveness (VE) against prevention of radiologically-confirmed pneumonia in children born after the introduction of Pentavalent vaccine in the country’s EPI. We also aimed to identify risk factors for radiologically-confirmed pneumonia in children in Pakistan.
Methods
A matched case-control study began immediately following the introduction of pentavalent vaccine in the Pakistan national EPI in January 2009. Sentinel surveillance was established at several public and private secondary and tertiary level health facilities serving low-income populations in 3 districts (Matiari-Sindh, Hyderabad-Sindh, and Jhelum-Punjab) and in the mega-city of Karachi, in Sindh in southern Pakistan. A total of 22 health facilities (9 public and 13 private) were included in this study based on: (1) parental/caregivers’ health seeking preferences/utilization assessed on baseline cross-sectional survey in the study areas; (2) more than 20 pediatric admissions weekly at the hospital; (3) availability of chest radiography (either digital or manual with at least a 100 mA machine); and (4) willingness of hospital administration to participate in the proposed evaluation. Screening for eligible cases was conducted in outpatient and inpatient areas at each sentinel site for children aged <5 years by a research officer. Children presenting with signs of acute respiratory infection were classified as suspected pneumonia if they presented with fast respiratory rate (>50/minutes) and fever (>101°F).13,14 Among these, parents/guardians of children who had chest indrawing as a sign of severe pneumonia,14 who were age-eligible to have received at least 1 dose of pentavalent vaccine, and were resident in the predefined catchment population were invited to participate in the study by a study physician. Children were excluded if they were previously enrolled as a case, if there was parental refusal, or if they lived outside the catchment area from which neighborhood controls could be enrolled. Death of the child did not exclude a case. A case report form recording baseline characteristics was completed if the parent/guardian provided informed consent. Chest radiographs of study-eligible children were obtained within 24 hours of hospital admission, and interpreted at first by the onsite study physician. Digitized images of chest radiographs were made using a VIDAR scanner15 (VI-DAR Systems Corporation, 365 Herndon Parkway Herndon, VA 20170) for all radiographs read as pneumonia, and every tenth radiograph (sequentially) read by the study physician as “no pneumonia.” The digitized images were separately sent to 2 pediatric radiologists who were trained in the interpretation of chest radiographs with a diagnosis of radiologically-confirmed pneumonia according to World Health Organization case definition of presence of substantial alveolar consolidation16,17 for second level interpretation at Aga Khan University Hospital (AKUH), in Karachi, Pakistan, on the same day. These 2 radiologists were not given any clinical information about the child and were blinded to each other’s interpretations. A senior pediatric radiologist (also trained in World Health Organization standardized interpretation of chest radiographs)16 served as arbiter for discordant chest radiograph interpretations. A case of radiologically-confirmed pneumonia was diagnosed based on the agreement of the 2 blinded radiologists at AKUH, or diagnosis by the senior radiologist if the AKUH radiologists were discordant.
Selection of Hospitals and Neighborhood Controls
For each case of radiologically-confirmed pneumonia, 3 age-matched hospital controls were enrolled from the same hospital, and 5 neighborhood controls were enrolled from the community in which the case child lived. Controls were eligible if the child was age matched (±4 weeks for case children <12 months; and ±8 weeks of age for case children ≥12 months), age eligible (at least 6 weeks) to receive the first dose of pentavalent vaccine, and a resident of study catchment areas. Children (controls) were excluded if child had been previously enrolled as a case of severe pneumonia or meningitis in the previous 8 weeks, was a sibling of a previously recruited case/control who lived in the same household, or had a current severe illness possibly due to Hib. For identification of hospital controls, the study staff generated a list of all eligible children at the hospital from patient triage records (−7 to 30 days) based on the date of admission of case child, and 3 hospital controls were randomly selected and recruited after obtaining written parental consent. For identification of neighborhood controls, the project research assistants and community health workers visited the geographic area of the case child. A random direction was established by means of rotating a bottle, and the first household was approached to determine child’s eligibility. Upon identification of the first household with an age eligible child, study staff invited the parents to participate in the study and obtained written parental consent. Subsequently, staff approached households using a systematic sampling technique with a skip pattern of 3 households after each eligible household, and continued in this fashion until 5 matched neighborhood controls were enrolled. The recruitment of hospital or neighborhood controls was made within 30 days from the hospital admission date of the case to account for seasonality of exposure.
Sample Size
The sample size was calculated using the NCSS PASS v. 11 software18 (Dr. Jerry L. Hintze and NCSS, East Kaysville, Utah) for matched case-control study design. This study was designed to detect a VE of 20% for radiologically-confirmed pneumonia with 2 or more doses of Hib conjugate vaccine.19,20 Using alpha error of 0.05, power of 80%, 50% pentavalent vaccine coverage, and correlation coefficient for vaccination between matched cases and controls of 0.20, we estimated needing 960 cases with 3 hospital controls and 839 cases with 5 neighborhood controls. The target enrollment was 960 cases.
Ascertainment of Vaccination Exposure, Sociodemographic Information, and Anthropometric Assessment
Parents or primary caregivers of children (both cases and controls) were interviewed about the child’s past and current illnesses, family’s sociodemographic information, and child’s vaccination history. Each child’s height and weight measurement were also recorded. The vaccination history was verified with the child’s vaccination card when available. In addition, a computerized vaccination registry was maintained for all children in the selected rural districts through monthly visits to all local EPI facilities (Matiari and Jhelum), from where vaccination status for recruited cases and controls could be documented when the vaccination card was not available. In the urban areas (Hyderabad and Karachi) where the immunization registry was not available, in the event of a caretaker reporting receipt of vaccine that could not be verified by card, project staff visited the nearest vaccination facility from where parents reported to have their child vaccinated to obtain the relevant record. Only verbal reports were used from parents/guardians who reported that their child was never vaccinated.
Vaccine Coverage Surveys (Baseline and End-line)
Cross-sectional vaccine coverage surveys for routine immunizations were conducted first at baseline (April–June 2008) and second at end of case-control study enrollment (October–December 2011) at the selected study sites in Pakistan. The estimated sample size was 3850 households for the baseline survey and 3508 households for the end-line survey, assuming 50% vaccine coverage, level of significance 95%, and level of absolute precision 3%.
We used the multiple indicator cluster survey technique,21 and households with age eligible children (12–23 months) were randomly selected from each Union Council (subdistrict administrative unit for population of 25 000–30 000 thousands) based on the population proportional to size. Parents of age eligible children were invited to participate in the survey, and written consent was obtained. Child’s vaccination status (verbal history) was ascertained and verified with vaccination card (when available).
Data Quality and Statistical Analyses
The senior project staff at each site made random observations of parental interviews and conducted 10% of parental re-interviews at hospital and in community settings for ensuring data quality control. The case control registry forms were independently double-entered in real time by trained data input operators using software developed in Visual FoxPro v 6.0 (Microsoft Corporation, United States of America ) Data were analyzed in SAS 9.1.3 software (SAS Institute, Cary, North Carolina). Median age and proportion receiving pentavalent vaccine doses were calculated, and conditional multivariate logistic regression was applied to identify significant risk factors for radiologically-confirmed pneumonia. Hib VE, defined as (1 − aOR) × 100%, and the associated 95% CI were estimated for each dose compared with 0 dose; we also compared ≥1 dose and ≥2 doses with 0 dose. VE estimates were adjusted for confounders and risk factors (P value <.05). This study received ethical approval from Ethics Review Committee of Aga Khan University, Karachi, Pakistan.
Results
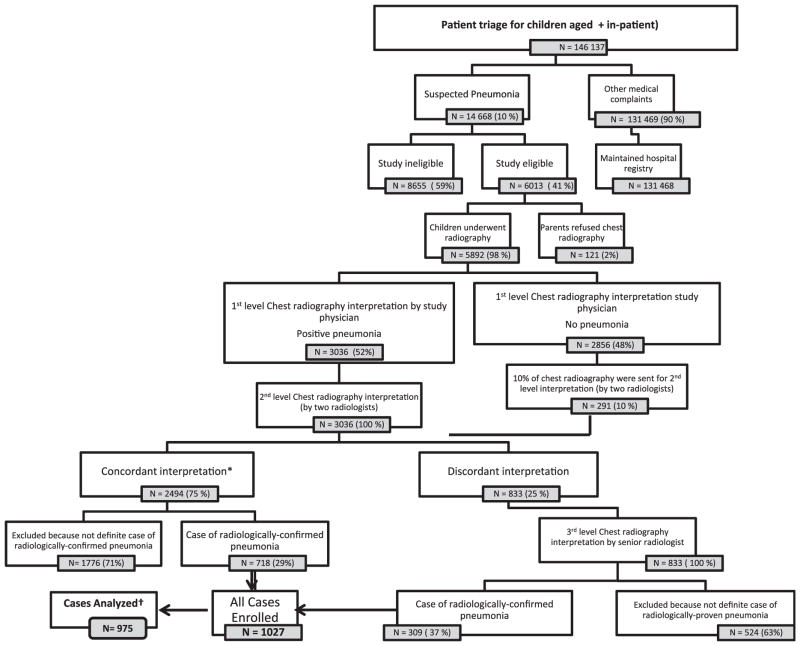
Over 3 years of surveillance (January 2009–October 2011), a total of 6013 eligible cases were identified (Figure). Ninety eight percent (5892) of eligible children underwent chest radiography, and of these radiographs, 1027 (17.4%) were interpreted as radiologically-confirmed pneumonia (Figure). Among these, 52 cases were excluded in the analysis phase because matched controls could not be identified in the stipulated time period, or inappropriate controls were selected.
Figure.
Levels of chest radiograph interpretation for radiologically-confirmed pneumonia. Suspected pneumonia = Fast respiratory rate (>50/min) and fever (>101°F). Study eligible = Lower chest indrawing, age eligible to receive first dose of pentavalent vaccine; and resident of study catchments. Definite radiologically-confirmed pneumonia = Alveolar consolidation observed in any of the lungs parenchyma. *Includes 291 cases where study physician read film negative. †52 cases dropped because appropriate controls could not be found in stipulated time period or controls were inappropriately selected.
A total of 975 cases and their associated controls were analyzed. Median age of cases was 5 months (minimum 1.5; maximum 31 months); 810 (83%) were <12 months of age. Of all cases, 678 (69.5%) were recruited from Karachi, 158 (16.2%) from Hyderabad, 104 (10.6%) from Matiari (Sindh), and 35 (3.6%) from Jhelum district in Punjab. A total of 2925 hospital controls and 4875 neighborhood controls were analyzed. Among neighborhood control children who had received 3 doses of pentavalent vaccine, the median age at time of pentavalent 3 vaccination was 17 weeks (minimum 10- maximum 69). The most common diagnosis for hospital controls was acute gastroenteritis (Table I).
Table I.
Attributes of cases and controls enrolled in the study
| Variables | Cases by study site
|
Cases (all sites) N = 975 |
Hospital controls (all sites) N = 2925 |
Neighborhood controls (all sites) N= 4875 |
|||
|---|---|---|---|---|---|---|---|
| Karachi (n = 678) | Hyderabad (n = 158) | Matiari (n = 104) | Jhelum (n = 35) | ||||
| Ages of children enrolled in the study | |||||||
| Median age in mo (minimum-maximum) of children at the time of enrollment | 5 (1.5–31) | 5 (1.5–29) | 4.5 (1.5–30) | 3 (1.5–15) | 5 (1.5–31) | 5 (1.5–33) | 5 (1.5–33) |
| Median age in wk (minimum-maximum) of children at the time of pentavalent vaccine 3 | 17 (11–39) | 17 (14–36) | 17 (14–37) | 18 (13–29) | 17 (11–39) | 17 (12–67) | 17 (10–69) |
| Children never vaccinated with any vaccine (%) | 291 (43) | 37 (23.4) | 24 (23.1) | 6 (17.1) | 358 (36.7) | 1259 (43) | 1510 (31) |
| Methods of vaccination verification (those ever vaccinated) n (%) | |||||||
| Verified through child’s vaccination card available at the time of interview | 201 (52) | 72 (59) | 54 (68) | 16 (55) | 343 (56) | 1266 (76) | 2726 (81) |
| Verified through computerized immunization registry | NR | NR | 18 (22) | 11 (38) | 29 (4) | 133 (8) | 471 (14) |
| Verified through EPI center | 186 (48) | 49 (41) | 8 (10) | 2 (7) | 245 (40) | 267 (16) | 168 (5) |
| Hospital admission diagnosis (%) | |||||||
| Radiologically-confirmed pneumonia | 100% | 100% | 100% | 100% | 100% | - | NA |
| Acute gastroenteritis | - | - | - | - | - | 39% | NA |
| Fever without focus/presumed enteric fever | - | - | - | - | - | 22% | NA |
| Skin allergies | - | - | - | - | - | 7% | NA |
| Daycare surgeries (fracture/circumcision) | - | - | - | - | - | 8% | NA |
| Upper respiratory tract infection | - | - | - | - | - | 7% | NA |
| Acute urinary tract infection | 3% | NA | |||||
| Others | 14% | NA | |||||
NA, not applicable; NR, no registry (Computerized vaccination registry was not possible given the large birth cohort and catchment size in urban cities). Never vaccinated in urban areas is based on parental report.
On multivariate analysis, a number of risk factors were found to be significantly associated with radiologically-confirmed pneumonia. Lack of paternal education, use of biomass/charcoal/wood for heating, at least 1 other child aged <5 years (other than the case/control child) in the same household, wasting, and underweight were significant risk factors for radiologically-confirmed pneumonia compared with hospital and neighborhood controls (Table II).
Table II.
Risk factors for radiologically-confirmed pneumonia and matched controls, Pakistan 2009–2011
| Characteristics | Number Of cases (%) n = 975 |
Number of hospital controls (%) n = 2925 |
Number neighborhood controls (%) n = 4875 |
Matched OR hospital controls (95% CI or P value) | P value | Matched OR, neighborhood controls (95% CI or P value) | P value |
|---|---|---|---|---|---|---|---|
| Sex: male | 566 (58) | 1673 (57) | 2474 (51) | 0.98 (0.8–1.2) | .86 | 1.1 (0.96–1.4) | .122 |
| No maternal education | 749 (77) | 2120 (73) | 3392 (70) | 1.0 (0.7–1.3) | .93 | 1.1 (0.85–1.3) | .488 |
| No paternal education | 649 (67) | 1804 (62) | 2605 (54) | 1.3 (1.1–1.7) | .01 | 1.8 (1.4–2.2) | <.0001 |
| Person smoking cigarettes in the house | 310 (33) | 1118 (38) | 1618 (33) | 0.7 (0.5–0.8) | .0002 | 0.96 (0.78–1.12 | .56 |
| Use of biomass/wood/paper/crop residue for cooking | 193 (20) | 437 (15) | 965 (20) | 1.95 (1.4–2.7) | .0001 | 1.04 (0.7–1.6) | .86 |
| Use of biomass/charcoal/wood for heating | 78 (8) | 175 (6) | 288 (6) | 1.3 (0.8–2.1) | .27 | 1.9 (1.3–2.8) | .0016 |
| Exposure to cooking smoke <2 m from place of baby sleeping | 179 (18) | 458 (16) | 1376 (28) | 1.2 (0.9–1.6) | .15 | 0.47 (0.4–0.6) | <.0001 |
| At least 1 other child <5 y in house | 772 (79) | 2151 (74) | 3571 (73) | 1.5 (1.2–1.9) | .0008 | 1.8 (1.4–2.2) | <.0001 |
| Large family size (≥7 family members) | 606 (62) | 1476 (51) | 3126 (64) | 1.5 (1.2–1.8) | .0003 | 0.7 (0.6–0.86) | .0009 |
| Crowding >2 people sleeping in the same room as of the child | 622 (64) | 1950 (66) | 3412 (70) | 0.7 (0.6–0.9) | .03 | 0.6 (0.5–0.8) | <.0001 |
| Never vaccinated for any vaccine | 358 (37) | 1259 (43) | 1509 (31) | 0.6 (0.5–0.8) | <.001 | 1.2 (0.98–1.5) | .063 |
| Wasting (weight-to height Z-score <−2) | 283 (34) | 723 (28) | 709 (15) | 1.4 (1.1–1.8) | .004 | 2.4 (1.9–3.0) | <.0001 |
| Underweight (weight-to-age Z-score <−2) | 533 (57) | 1313 (46) | 1113 (23) | 1.3 (1.1–1.7) | .014 | 2.9 (2.3–3.6) | <.0001 |
| Stunting (height-to-age Z-score <−2) | 387 (45) | 1107 (42) | 1712 (35) | 1.2 (0.92–1.5) | .20 | 1.2 (0.93–1.4) | .17 |
The coverage for 3 doses of pentavalent vaccine (DTP-Hep B-Hib) was 13.7%, 18%, and 22.7% in children enrolled as cases, hospital controls, and neighborhood controls, respectively. Hib conjugate VE for radiologically-confirmed pneumonia was 62% (95% CI 47%–73%), with 3 doses of pentavalent vaccine using hospital controls, and 70% (95% CI 59%–78%) using neighborhood controls (Table III). The results of the baseline and end-line vaccine coverage survey are presented in Table IV.
Table III.
Pentavalent vaccine coverage and VE (95% CI)* for radiologically-confirmed pneumonia, Pakistan 2009–2011
| Vaccination status | Cases N = 975 |
Hospital controls N = 2925 |
Neighborhood controls N = 4875 |
||
|---|---|---|---|---|---|
| n (%) | n (%) | VE (1 − OR) × 100% (95% CI) | n (%) | % of VE (1 − OR) × 100% (95% CI) | |
| Zero dose of pentavalent | 662 (68) | 1826 (62) | Reference | 2602 (53.4) | Reference |
| 3 doses | 134 (13.7) | 523 (18) | 62† (47, 73) | 1107 (22.7) | 70† (59, 78) |
| 2 doses | 57 (5.9) | 211 (7.2) | 60† (41, 74) | 451 (9.3) | 66† (51, 77) |
| 1 dose | 122 (12.5) | 365 (12.5) | 37† (14, 54) | 715 (14.8) | 46† (29, 60) |
| ≥1doses | 313 (32) | 1099 (37.6) | 52† (37, 63) | 2273 (46.6) | 60† (50, 68) |
| ≥2 doses | 191 (20) | 734 (25) | 61† (48, 71) | 1558 (32) | 68† (59, 76) |
Adjusted for no paternal education, cigarette smoking, exposure to smoke, use of biomass/word/charcoal for cooking/heating, number of <5 years aged children at household, large family size, no Bacillus Calmette–Guérin vaccine and malnutrition.
Significant difference (P value of <.05).
Table IV.
Vaccine coverage surveys (baseline and end-line)
| Variables | Baseline survey (sites)
|
End-line survey (sites)
|
||||||
|---|---|---|---|---|---|---|---|---|
| Karachi n = 850 |
Matiari n = 1400 |
Jhelum n = 1600 |
Total N = 3850 |
Hyderabad n = 427 |
Matiari n = 1473 |
Jhelum n = 1608 |
Total N = 3508 |
|
| Vaccination coverage for age eligible children at the time of survey (%) | ||||||||
| Fully vaccinated with 3 doses of tetravalent/pentavalent vaccine (card verified plus parental recall) | 35% | 75% | 98% | 69% | 83% | 77% | 97% | 86% |
| Child vaccination card available at the household and verified at the time of survey (those ever vaccinated) (%) | 27% [163/603] | 10% [128/1288] | 41% [650/1584] | 27% [941/3475] | 60% [235/391] | 33% [855/1425] | 71% [1121/1579] | 65% [2211/3395] |
Tetravalent vaccine = DTP-Hep B was available in national EPI until December 2009 in Pakistan.
Pentavalent vaccine = DTP-Hep B-Hib replaced tetravalent vaccine and was introduced from January 2009 in Pakistan.
Discussion
Our study provides evidence that a significant fraction of radiologically-confirmed pneumonia can be prevented by Hib conjugate vaccine in Pakistani children. The point estimate for effectiveness of 3 doses of Hib conjugate vaccine ranged from 62%–70%, depending on the type of control used. These results are not that dissimilar from what has been seen in other Hib VE studies.22 Compared with other Asian countries where Hib VE studies against radiologically-confirmed pneumonia have been done, our findings confirm earlier observations from Bangladesh based on a smaller case-control study and are contrary to the lack of Hib conjugate vaccine efficacy reported from Indonesia.9,19 Interpretation of Hib conjugate vaccine trial results in Indonesia may have been affected by the development of strong herd immunity in the neighboring populations, if there was unrecognized population mingling, thus, underestimating vaccine efficacy, as has been shown in cholera vaccine trials.23
Several reasons may underlie the finding of much higher than expected point estimates of VE in our study compared with The Gambian trial findings where Hib VE was found to be 21% (95% CI 5%–35%). One major characteristic for which our cases were different from the control population selected is that cases were significantly more malnourished than controls (Table II). Malnutrition increases the risk of developing severe, radiologically-confirmed pneumonia, the end-point used in this study. If the unvaccinated population is more likely to be exposed to the disease than the vaccinated, this can result in an overestimation of the true VE.24 It is also possible that in children with severe malnutrition, Hib is responsible for a larger fraction of severe, radiologically proven pneumonia that can be prevented by vaccine than in well-nourished children, especially in the very young child. It is worth noting that the median age of cases enrolled in this study was only 5 months, much lower than other published studies.
It is also possible that some biases existed in control selection and their vaccine exposure ascertainment. For example, results might have been biased if, despite thorough training in enrolling all eligible controls, some research staff selectively dropped controls with no vaccination card and replaced them with other controls who had vaccination cards. This could have resulted in overestimating vaccine coverage in neighborhood controls, in turn resulting in an overestimated VE. There may also have been unidentified and unmeasured potential confounders that we were unable to control for. Despite these potential biases, there is internal validity for the VE estimates because the VE rises with increasing doses of Hib conjugate vaccine (Table III).
This study, as has been reported elsewhere, highlights the effect of lack of paternal education, use of biomass/charcoal/wood for heating, number of children <5 years of age in the household, and malnutrition as significant risk factors for severe pneumonia.25,26 More crowding was identified in the neighborhood controls, and it was most likely due to inaccurate reporting by case families. Cases were interviewed in hospital compared with direct observation in control neighborhood homes.
The strengths of our study are the large sample size, rigorously standardized radiograph interpretation throughout the study using 3 blinded radiologists, and the extensive efforts made for accurate vaccine exposure ascertainment. For enhancing card retention among rural populations, where our baseline vaccine coverage survey had shown poor retention, we distributed plastic pouches to all vaccinators at local EPI facilities to be distributed to families for card retention. This had a marked effect on increasing vaccine card retention by 23%–30% as observed on end-line vaccine coverage survey in districts Matiari and Jhelum (Table IV). In addition, a prospective registry of all children coming to each local EPI facility in the rural areas was also maintained by entering the data of each child into an electronic database through monthly visits to the centers. In this way, for over 95% of the recruited cases and controls from rural areas, vaccination history could be cross-verified with our computerized registry. Accurate exposure ascertainment remained a challenge in urban areas among those reporting no vaccine receipt, where parental report of no vaccination ever had to be taken as evidence of receipt of no vaccine (Table I).
Operational challenges included frequent lack of electricity in rural areas delaying radiographs and digitization and the difficult security situation in some areas of Karachi where cases came from and controls had to be recruited, sometimes resulting in delays in control enrollment.
Only 23% of community controls and 18% of hospital controls had received 3 doses of pentavalent vaccine. The findings of low and delayed vaccination uptake among the study population underscore the fact that vaccines in high child mortality countries such as Pakistan do not reach those most in need.27–29 Before pentavalent vaccine was introduced in national EPI in Pakistan, the coverage for 3 doses of tetravalent vaccine (DTP-Hep B) on our baseline population-representative survey in the year 2008 was 69% (Table IV). Comparing with baseline, the overall coverage for 3 doses of pentavalent vaccine (DTP-Hep B-Hib) on the end-line survey in the year 2011 had considerably improved to 86% (Karachi was not surveyed again as these data were available from other work). These estimates reflect vaccine coverage in general in the community of the study sites. However, our study population of cases and controls were overwhelmingly poor, lacking the most basic of amenities, and had lower vaccination rates (39%, 44%, and 53% in cases, hospital controls, and neighborhood controls aged 12–23 months, respectively). They had high rates of malnutrition even among community controls (15% with moderate to severe wasting, 23% underweight, and 35% stunted). To maximize the child survival benefit of these Global Alliance for Vaccines and Immunization-supported vaccines, it is imperative to remove inequities in vaccine access. Preventing childhood pneumonia is important for reaching Millennium Development Goal 4 to reduce child mortality, and this study supports the importance of Hib conjugate vaccine to reach those goals.
Acknowledgments
Supported by the GAVI Hib Initiative, and funded by the GAVI Alliance to Johns Hopkins University, with a sub-grant to Aga Khan University, Karachi Pakistan. A.R.K. received partial training support from the Fogarty International Center, National Institutes of Health, USA (ID43 TW0075 85-01). The findings and conclusions in this article are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
The authors are grateful to all study staff with involvement throughout Karachi, Hyderabad, Matiari, and Jhelum sites. They are also grateful to medical superintendents, radiology technicians of participating health facilities, and district health management teams at Jhelum, Matiari, Hyderabad, Karachi, Federal and Provincial EPI Pakistan, Federal and Provincial Lady Health Workers Program Pakistan, Data Management Unit of Department of Pediatrics, Aga Khan University, Infectious Disease Research Laboratory of Department of Pediatrics, Aga Khan University, and Department of Radiology, Aga Khan University.
Glossary
- AKUH
Aga Khan University Hospital
- DTP
Diphtheria-tetanus-pertussis
- EPI
Expanded Program on Immunization
- Hep B
Hepatitis B
- Hib
Haemophilus influenzae type b
- VE
Vaccine effectiveness
Appendix
Members of the Pakistan Hib Vaccine Study Group Include: Abdul Rehman Siyal (Civil Hospital Hyderabad-Sindh), Azra Jamal (Sindh Government Qatar Hospital-Karachi-Sindh), Khalid Iqbal (Kharadar General Hospital-Karachi-Sindh), Mehmood Suleiman (Kharadar General Hospital-Karachi-Sindh), Mazhar Khamisani (Project Director Expanded Program of Immunization-Sindh), M. Hassan Memon (Isra University-Hyderabad-Sindh), M. Shahid Ghaffar (Chinniot Hospital-Karachi-Sindh), Naeem H. Gardaizi (District Headquarter Hospital-Jhelum-Punjab), Rao Saleemuddin Aziz (Chinniot Hospital-Karachi-Sindh), Shaheen Ahmed (Combined Military Hospital-Jhelum-Punjab), and Usha Khatri (Chinniot Hospital-Karachi-Sindh), Afroze Ramzan (National Institute of Child Health, Karachi-Sindh), Asad Hafeez (National Lady Health Workers Program Pakistan), Atta ur Rehman (Theseel Headquarter Hospital-Pind Dadan Khan-Punjab), Arshad I Daar (PD Expanded Program of Immunization-Punjab) Abdul G. Nagi (National Institute of Child Health, Karachi-Sindh), Abdul H. Ansari (Taluqa Headquarter Hospital-Hala-Sindh), Aftab Ahmed (Al-Karam Hospital-Jhelum-Punjab, Abid Raza (Tehseel Headquarter Hospital-Sohawa-Punjab), Feroz Memon (National Lady Health Workers Program Sindh), Girdari Lal (General Practitioner Clinic–Matiari-Sindh), Jennifer Verani (Centers for Disease Control and Prevention, Atlanta, GA), Jennifer Loo (Centers for Disease Control and Prevention, Atlanta, GA), and Tanveer Ahmed (National Lady Health Workers Program Punjab).
Footnotes
Author Disclosures
The authors declare no conflicts of interest, real or perceived.
References
- 1.Liu L, Johnson HL, Cousens S, Perin J, Scott S, Lawn JE, Rudan I, et al. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet. 2012;379:2151–61. doi: 10.1016/S0140-6736(12)60560-1. [DOI] [PubMed] [Google Scholar]
- 2.Greenwood BM, Weber MW, Mulholland K. Childhood pneumonia-preventing the world’s biggest killer of children. Bull World Health Organ. 2007;85:502–3. doi: 10.2471/BLT.07.044032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hazir T. Pneumonia: no. 1 killer of Pakistan’s children. Bull World Health Organ. 2008;86:321–416. doi: 10.2471/BLT.08.040508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.D’Souza RM. Role of health seeking behavior in child mortality in the slums of Karachi, Pakistan. J Biosoc Sci. 2003;35:131–44. [PubMed] [Google Scholar]
- 5.Rudan I, Boschi-Pinto C, Biloglav Z, Mulholland K, Campbell H. Epidemiology and etiology of childhood pneumonia. Bull World Health Organ. 2008;86:408–16. doi: 10.2471/BLT.07.048769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watt JP, Wolfson LJ, O’Brien KL, Henkle E, Deloria-Knoll M, McCall N, et al. Burden of disease caused by Haemophilus influenzae type b in children younger than 5 years: global estimates. Lancet. 2009;374:903–11. doi: 10.1016/S0140-6736(09)61203-4. [DOI] [PubMed] [Google Scholar]
- 7.O’Brien KL, Wolfson LJ, Watt JP, Henkle E, Deloria-Knoll M, McCall Natalie, et al. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet. 2009;374:893–902. doi: 10.1016/S0140-6736(09)61204-6. [DOI] [PubMed] [Google Scholar]
- 8.Shetty S, Cohen AL, Edmond K, Ojo L, Loo J, O’Loughlin R, et al. A systematic review and critical evaluation of invasive Haemophilus influenzae type B disease burden studies in Asia from the last decade: lessons learned for invasive bacterial disease surveillance. Pediatr Infect Dis J. 2010;29:656–61. doi: 10.1097/INF.0b013e3181d3ce19. [DOI] [PubMed] [Google Scholar]
- 9.Gessner BD, Sutanto A, Linehan M, Djelantik GG, Fletcher T, Gerudug IK, et al. Incidences of vaccine-preventable Haemophilus influenza type b pneumonia and meningitis in Indonesian children: hamlet-randomized vaccine-probe trial. Lancet. 2005;356:43–52. doi: 10.1016/s0140-6736(04)17664-2. [DOI] [PubMed] [Google Scholar]
- 10.Owais A, Tikmani SS, Sultana S, Zaman U, Ahmed I, Allana S, et al. Incidence of pneumonia, bacteremia, and invasive pneumococcal disease in Pakistani children. Trop Med Int Health. 2010;15:1029–36. doi: 10.1111/j.1365-3156.2010.02591.x. [DOI] [PubMed] [Google Scholar]
- 11.Zaidi AKM, Lasi R, Mahesar W the Sindh Meningitis group. Surveillance of pneumococcal meningitis among children in Sindh, Southern Pakistan. Clin Infect Dis. 2009;48:129–35. doi: 10.1086/596491. [DOI] [PubMed] [Google Scholar]
- 12.Zaidi AKM, Khan H, Sherali AR, Lasi R the Sindh Meningitis group. Burden of Haemophilus influenzae type b disease in Pakistani children. East Mediterr Health J. 2010;16:460–4. [PubMed] [Google Scholar]
- 13.Management of the child with a serious infection or severe malnutrition. World Health Organization; Geneva, Switzerland: 2000. WHO Guidelines for care at first-referral level in developing countries. [Google Scholar]
- 14.Scott JAG, Wonodi C, Moisi JC, Knoll MD, DeLuca AN, Karrow RA, et al. The definition of pneumonia, the assessment of severity, and clinical standardization in the pneumonia etiology research for child health study. Clin Infect Dis. 2012;54:S109–16. doi: 10.1093/cid/cir1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. [Accessed August 28, 2012];VIDAR Digitizers Support World Health Organization Childhood Pneumonia Studies. Available at: http://www.vidar.com/film/images/stories/PDFs/studies/pdf/casestudies/who.pdf.
- 16.Standardization of interpretation of chest radiographs for the diagnosis of pneumonia in children. World Health Organization Pneumonia Vaccine Trial Investigators’ Group. World Health Organization. Department of Vaccines and Biologicals; Geneva: 2001. [Accessed on August 28, 201]. Available at: http://www.who.int/vaccines-documents/DocsPDF01/www616.pdf. [Google Scholar]
- 17.O’Grady KAF, Thomson DMT, Chang AB, Torzillo PJ, Morris PS, Mackenzie GA, et al. Rates of radiologically confirmed pneumonia as defined by the World Health Organization in Northern Territory indigenous children. Med J Aust. 2010;192:592–5. doi: 10.5694/j.1326-5377.2010.tb03644.x. [DOI] [PubMed] [Google Scholar]
- 18. [Accessed on September 25, 2012];NCSS Power Analysis and Sample Size (PASS) 11. Available at: http://www.ncss.com/pass.html.
- 19.Baqui AH, Arifeen SE, Saha SK, Persson L, Zaman K, Gessner BD, et al. Effectiveness of Haemophilus influenzae type B conjugate vaccine on prevention of pneumonia and meningitis in Bangladeshi children: a case-control study. Pediatr Infect Dis J. 2007;26:565–71. doi: 10.1097/INF.0b013e31806166a0. [DOI] [PubMed] [Google Scholar]
- 20.De-La-Hoz F, Higuera AB, Di Fabio JL, Luna M, Naranjo AG, Valencia dlL, et al. Effectiveness of Haemophilus influenzae type b vaccination against bacterial pneumonia in Colombia. Vaccine. 2004;23:36–42. doi: 10.1016/j.vaccine.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 21.Multiple Indicator Cluster Survey Manual 2005: monitoring the situation of children and women. United Nations Children’s Fund: Division of Policy and Planning; 2006. [Google Scholar]
- 22.O’Loughlin RE, Edmond K, Mangtani P, Cohen AL, Shetty S, Hajjeh R, et al. Methodology and measurement of the effectiveness of Haemophilus influenzae type b vaccine: systematic review. Vaccine. 2010;28:6128–36. doi: 10.1016/j.vaccine.2010.06.107. [DOI] [PubMed] [Google Scholar]
- 23.Emch M, Ali M, Park JK, Yunus M, Sack DA, Clemens JD. Relationship between neighborhood-level killed oral cholera vaccine coverage and protective efficacy: evidence for herd immunity. Int J Epidemiol. 2006;35:1044–50. doi: 10.1093/ije/dyl100. [DOI] [PubMed] [Google Scholar]
- 24.Orenstein WA, Bernier RH, Hinman AR. Assessing vaccine efficacy in the field: further observations. Epidemiol Rev. 1988;10:212–41. doi: 10.1093/oxfordjournals.epirev.a036023. [DOI] [PubMed] [Google Scholar]
- 25.Almirall J, Bar B, Prat-Serra M, Roig J, Hospital I, Carandell E, et al. New evidence of risk factors for community acquired pneumonia: a population-based study. Eur Respir J. 2008;31:1274–84. doi: 10.1183/09031936.00095807. [DOI] [PubMed] [Google Scholar]
- 26.Broor S, Panday RM, Ghosh M, Maitry RS, Lodha R, Singhal T, et al. Risk factors for severe acute lower respiratory tract infection in under-five children. Indian Pediatr. 2001;38:1361–9. [PubMed] [Google Scholar]
- 27.Masud T, Navaratne KV. The Expanded Program on Immunization in Pakistan. Recommendations for improving performance. [Accessed on April 19, 2013];World Bank: HNP Discussion Paper. 2012 :1–42. Available at: http://reliefweb.int/report/pakistan/expanded-program-immunization-pakistan-recommendations-improving-performance.
- 28.Roy AMS, Mhatre SL. The fallacy of coverage: uncovering disparities to improve immunization rates through evidence. Results from the Canadian International Immunization Initiative Phase 2—operational research grant. BMC Int Health Hum Rights. 2009;9:S1. doi: 10.1186/1472-698X-9-S1-S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khowaja AR, Zaman U, Feroze A, Rizvi A, Zaidi AKM. Routine EPI coverage: subdistrict inequalities and reasons for immunization failure at rural setting in Pakistan. Asia Pacific J Public Health. 2011 doi: 10.1177/1010539511430850. Epub-ahead of print. [DOI] [PubMed] [Google Scholar]