Abstract
Background
It has been suggested that vitamin D might protect from breast cancer, although studies on levels of vitamin D in association with breast cancer have been inconsistent. Genome-wide association studies (GWASs) have identified several single-nucleotide polymorphisms (SNPs) to be associated with vitamin D. The aim of this study was to investigate such vitamin D-SNP associations in relation to subsequent breast cancer risk. A first step included verification of these SNPs as determinants of vitamin D levels.
Methods
The Malmö Diet and Cancer Study included 17,035 women in a prospective cohort. Genotyping was performed and was successful in 4058 nonrelated women from this cohort in which 865 were diagnosed with breast cancer. Levels of vitamin D (25-hydroxyvitamin D) were available for 700 of the breast cancer cases and 643 of unaffected control subjects. SNPs previously associated with vitamin D in GWASs were identified. Logistic regression analyses yielding ORs with 95% CIs were performed to investigate selected SNPs in relation to low levels of vitamin D (below median) as well as to the risk of breast cancer.
Results
The majority of SNPs previously associated with levels of vitamin D showed a statistically significant association with circulating vitamin D levels. Heterozygotes of one SNP (rs12239582) were found to have a statistically significant association with a low risk of breast cancer (OR 0.82, 95% CI 0.68–0.99), and minor homozygotes of the same SNP were found to have a tendency towards a low risk of being in the group with low vitamin D levels (OR 0.72, 95% CI 0.52–1.00). Results from stratified analyses showed diverse associations with breast cancer risk for a few of the tested SNPs, depending on whether vitamin D level was high or low.
Conclusions
SNPs associated with vitamin D may also be associated with the risk of breast cancer. Even if such a risk is small, the allele frequency of the SNP variants is high, and therefore the population attributable risk could be substantial. It is also possible that vitamin D levels may interact with genomic traits with regard to breast cancer risk.
Electronic supplementary material
The online version of this article (doi:10.1186/s13058-017-0925-3) contains supplementary material, which is available to authorized users.
Keywords: Vitamin D, Breast cancer, Polymorphism, SNP
Background
About 5–10% of breast cancers are considered hereditary, from which the known breast cancer genes account for 3–4% [1]. Apart from known breast cancer genes, genome-wide association studies (GWASs) have previously identified more than 170 genetic polymorphisms associated with the risk of breast cancer [2]. It has been suggested that single-nucleotide polymorphisms (SNPs) may add up to 14% of heredity of breast cancer [1].
Ecological and epidemiological studies have suggested a beneficial effect of relatively high vitamin D levels, owing to solar exposure, on breast cancer risk and survival [3–6]. There are several prospective epidemiological studies on the relationship between serum levels of vitamin D and breast cancer incidence. The results have been conflicting [7–10], although authors of a meta-analysis concluded that there is an inverse relationship between levels of vitamin D and breast cancer risk [11]. Diverse results may be a result of misclassification of vitamin D status, and a better marker of stable and unconfounded vitamin D status over time may be found in the genotype, as studied using a Mendelian randomization approach [12].
Researchers investigating vitamin D-related SNPs and breast cancer risk have focused mainly on SNPs located in the vitamin D receptor (VDR) gene. Results derived from these studies have also been conflicting [13–16]. SNPs of the VDR have not been found to be associated with vitamin D in previous GWASs [2]. The aim of the present study was to investigate breast cancer risk in relation to SNPs previously identified in GWASs on vitamin D levels and related phenotypes. The present study was a prospective, nested case-control study with information on SNPs, vitamin D levels and subsequent breast cancer.
Methods
Malmö diet and cancer study
Between 1991 and 1996, all residents of Malmö, Sweden, born between 1923 and 1950 were invited to participate in a prospective cohort study. Among invited women, 43% participated, resulting in a female cohort of 17,035 women [17]. At inclusion, written informed consent was obtained from all participants. Baseline examinations included anthropometric measurements by a trained nurse who also drew blood samples. Subjects were included evenly over the calendar year, although there was less recruitment in December and June and none in July. Participants also provided information on reproductive factors and lifestyle via a self-administered questionnaire. Information on previous gynaecological surgery was retrieved from medical records, and menopausal status was defined using these data together with information provided in the questionnaire [18]. The Malmö Diet and Cancer Study (MDCS) (LU 51-90) and the present study (Dnr 153/2004 and Dnr 682/2009) were approved by the regional ethics committee in southern Sweden.
Study population
In a previous case-control study, women diagnosed with breast cancer in the MDCS until December 31, 2006 (n = 764), were matched on age and date of inclusion with control subjects (n = 764), based on 1482 individuals (some were included twice because incidence density matching was applied) [8]. One breast cancer case was classified to be without disease in a later follow-up. Until December 31, 2009, an additional 183 women were diagnosed with breast cancer, leading to inclusion of 946 women with breast cancer in the MDCS cohort. The control group was expanded to include an additional 2658 randomly selected women from the MDCS cohort without a breast cancer diagnosis. These women were also part of the MDCS cardiovascular cohort, a randomized subsample of MDCS [19]. Together with the 704 control subjects from the previous case-control study who had not developed breast cancer, potential control subjects added up to 3362. Material was accessible for genotyping from 901 of the breast cancer cases and 3335 of the control subjects (Fig. 1).
Fig. 1.
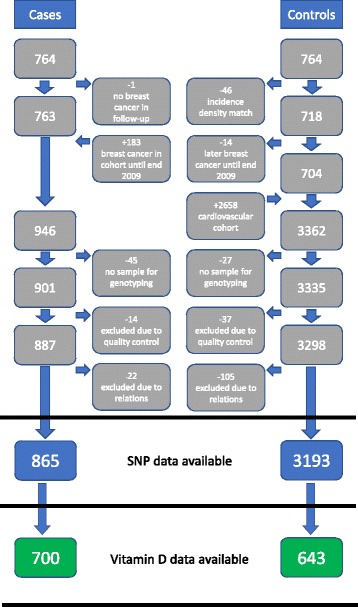
Flowchart of cases and control subjects. SNP Single-nucleotide polymorphism
Genotyping was performed using the HumanOmniExpressExome BeadChip and iScan System (Illumina, San Diego, CA, USA) during 2012–2013 at the Broad Institute of MIT and Harvard University (Cambridge, MA, USA) (n = 4058) and at the Clinical Research Centre, Skåne University Hospital, Malmö, Sweden (n = 178). Sample-based quality control (QC) included controls for call rate, > 95% of SNPs per individual, and control for excess heterozygosity, which led to exclusion of 51 individuals. Owing to first- and second-degree relationships between individuals, a further 127 individuals were excluded, and the relative with the highest call rate was kept in the study population. After QC and exclusion of relatives, 865 patients with breast cancer and 3193 control subjects were available for statistical analysis (Fig. 1). PLINK version 1.07 software was used for QC and exclusion of relatives.
Levels of vitamin D
In the previous case-control study of the MDCS cohort mentioned above, researchers investigated, among other things, serum levels of vitamin D in relation to breast cancer risk [8]. For this study, high-pressure liquid chromatography was used to analyse 25-hydroxyvitamin D [25(OH)D] levels, and laboratory analysis was successful in 700 breast cancer cases and 643 control subjects in the present study population (Fig. 1). Amongst subjects with data on SNPs and vitamin D level (1343 in total), 320 women (165 cases, 155 control subjects) were also subjects in the cardiovascular cohort. There is no consensus regarding adequate levels of vitamin D, and the levels vary substantially over the year; thus it is not possible to clinically define a high or low vitamin D level on a single measurement [20]. In this study, the median level of vitamin D of each calendar month of sampling was used as a cut-off between low and high levels of vitamin D.
Selecting SNPs
To find SNPs previously associated with vitamin D, a search in a GWAS catalogue using the search string “vitamin D” identified 20 SNPs [2, 21]. The researchers in these studies had evaluated associations between SNPs and levels of vitamin D (15 SNPs) [22–25], vitamin D insufficiency (9 SNPs) [26] and levels of vitamin D-binding protein (DBP) (3 SNPs) [27]. Nine of these SNPs were directly genotyped on the BeadChip used in our study. Using the SNAP web-based tool, we identified proxies on the basis of linkage disequilibrium (LD) and physical distance to selected SNPs for an additional eight SNPs [28]. When several proxies were found, the two with the highest LD and closest proximity to the selected SNP were used. Altogether, 20 SNPs from 10 different loci in the genome were tested for associations with vitamin D level and breast cancer risk: 2 from ST6GALNAC3 in chromosome 1, 4 from group-specific component (GC) intron in chromosome 4, 1 from chromosome 6, 2 upstream of NPY in chromosome 7, 1 from proximity to MGMT in chromosome 10, 2 from unidentified genes, 2 from PDE3B intron, 2 from CYP2R1, 3 from NADSYN1 in chromosome 11 and 1 from MTMR4 in chromosome 17. A description of tested SNPs and their original GWAS proxy, as well as previous association with vitamin D, is provided in Additional file 1.
During QC, monomorphic SNPs due to lack of variation in a European population were excluded, as were SNPs deviating from Hardy-Weinberg equilibrium (p < 10−6) or if the variant call rate was < 95% in all samples. PLINK version 1.07 software was used for QC and identification of selected SNPs [29].
Statistical methods
Cases and control subjects were compared in two different sets with regard to established and potential risk factors. The first set contained all cases and control subjects with available SNP data; the other set included only cases and control subjects in whom vitamin D had been analysed in the previous study [8]. Control groups and groups with and without SNPs and vitamin D data were also compared with regard to risk factors in order to investigate the risk of potential selection bias.
Unconditional binary logistic regression analyses were performed to investigate associations between SNPs and low levels of vitamin D (below median), as well as between selected SNPs and risk of breast cancer. ORs with 95% CIs were calculated using the homozygote for the major allele as the reference group. Stratification by low versus high levels of vitamin D was added to see if there were any differences in breast cancer risk between these groups based on genotypes. This analysis was tested for interaction of low versus high vitamin D level. All analyses were also adjusted for year of inclusion, for age at baseline and for established or potential risk factors for breast cancer, including level of education, type of occupation, age at menarche, age at first childbirth, exposure to oral contraceptives, exposure to hormone replacement therapy, height, body mass index (BMI), alcohol consumption and smoking. Missing values for adjustment factors were coded in a separate category and included in multivariable analyses.
Results
Potential confounders in cases and control subjects
Women with incident breast cancer were more often than control subjects to be non-manual labour workers (Table 1). This was applicable in both sets of study populations, although the difference between groups was smaller in the study population in which vitamin D levels were analysed. Another observation was that women with incident breast cancer in both study populations were more likely to be users of hormone replacement therapy and were also more likely to have been exposed to oral contraceptives (Table 1). In the complete study population, we also saw that women with incident breast cancer were more often included in the MDCS during the summer, had a higher level of education and were peri- or pre-menopausal to a greater extent at inclusion than women in the control group. These differences were not seen in the study population with analysed vitamin D levels, presumably owing to previous age and inclusion date matching of control subjects (Table 1).
Table 1.
Baseline characteristics of breast cancer cases and control subjects
| Factor | Category | Study population with SNP data (%) | Study population with data on vitamin D (%) | ||
|---|---|---|---|---|---|
| Control subjects (n = 3193) |
Cases (n = 865) |
Control subjects (n = 643) |
Cases (n = 700) |
||
| Age, years | Mean ± SD | 57.4 ± 6.3 | 56.6 ± 7.3 | 57.0 ± 7.3 | 56.9 ± 7.2 |
| Season of inclusion in MDCS | January–March | 33.1 | 28.8 | 29.9 | 29.9 |
| April–June | 19.0 | 24.7 | 24.0 | 23.9 | |
| July–September | 18.0 | 22.8 | 21.3 | 21.9 | |
| October–December | 29.9 | 23.7 | 24.9 | 24.4 | |
| Education | O-level college (7–9 years) | 73.6 | 66.5 | 68.0 | 67.7 |
| A-level college (11–12 years) | 6.8 | 7.1 | 7.3 | 7.3 | |
| University | 19.4 | 26.1 | 24.7 | 24.7 | |
| Type of occupation | Manual labour worker | 39.6 | 32.7 | 38.7 | 34.4 |
| Non-manual labour worker | 52.5 | 60.6 | 53.8 | 58.6 | |
| Employer/self-employed | 6.9 | 5.7 | 7.5 | 5.7 | |
| Missing | 0.9 | 1.0 | 0.0 | 1.3 | |
| Married/co-habitating | No | 31.0 | 33.5 | 32.0 | 34.0 |
| Yes | 69.0 | 66.5 | 68.0 | 66.0 | |
| Age at menarche, years | < 12 | 6.2 | 7.6 | 4.5 | 7.3 |
| 12–15 | 67.2 | 67.3 | 68.9 | 67.1 | |
| > 15 | 25.6 | 23.9 | 26.0 | 24.4 | |
| Missing | 0.9 | 1.2 | 0.6 | 1.1 | |
| Parity | Nulliparous | 12.0 | 14.1 | 11.2 | 13.6 |
| One child | 21.4 | 19.5 | 22.4 | 19.3 | |
| Two children | 40.6 | 44.7 | 40.9 | 44.1 | |
| Three children or more | 22.8 | 19.5 | 22.7 | 20.6 | |
| Missing | 3.1 | 2.1 | 2.8 | 2.4 | |
| Age at first birth, years | Nulliparous | 12.0 | 14.1 | 11.2 | 13.6 |
| ≤ 20 | 16.5 | 14.9 | 17.9 | 13.6 | |
| 21–24 | 28.2 | 27.3 | 27.7 | 27.7 | |
| 25–29 | 29.1 | 28.7 | 28.6 | 30.1 | |
| ≥ 30 | 11.1 | 12.9 | 11.8 | 12.6 | |
| Missing | 3.1 | 2.1 | 2.8 | 2.4 | |
| Age at menopause, years | Pre-/peri-menopause | 27.2 | 35.6 | 32.5 | 33.1 |
| < 45 | 9.8 | 9.0 | 8.6 | 9.4 | |
| 45–53 | 45.3 | 39.5 | 42.6 | 41.6 | |
| > 53 | 16.0 | 13.8 | 14.9 | 13.7 | |
| Missing | 1.7 | 2.1 | 1.4 | 2.1 | |
| Exposure to oral contraceptives | No | 54.3 | 45.9 | 50.1 | 48.6 |
| Yes | 45.7 | 54.0 | 49.9 | 51.3 | |
| Exposure to hormone replacement therapy (HRT) | No (pre-menopausal) | 22.0 | 28.4 | 26.3 | 25.7 |
| No (post-menopausal) | 59.8 | 44.5 | 53.3 | 45.7 | |
| Oestrogen only | 7.6 | 7.4 | 9.2 | 7.6 | |
| Progesterone only | 0.6 | 1.2 | 0.9 | 1.3 | |
| Combined HRT | 9.4 | 18.3 | 9.8 | 19.4 | |
| Height, m | ≤ 1.59 | 24.6 | 20.6 | 23.2 | 20.3 |
| 1.60–1.69 | 58.1 | 61.4 | 59.1 | 61.6 | |
| ≥ 1.70 | 17.2 | 18.0 | 17.7 | 18.1 | |
| Body mass index, kg/m2 | < 25 | 53.3 | 50.1 | 51.8 | 50.6 |
| ≥ 25 to < 30 | 33.6 | 34.5 | 36.1 | 33.3 | |
| ≥ 30 | 13.0 | 15.5 | 12.1 | 16.1 | |
| Alcohol consumption | Not in last year | 11.4 | 9.7 | 11.4 | 10.4 |
| Some in last year | 12.7 | 11.2 | 12.4 | 11.4 | |
| Some in last month | 75.6 | 78.8 | 75.9 | 78.0 | |
| Smoking | Never | 46.8 | 43.0 | 42.5 | 44.0 |
| Current | 26.7 | 26.7 | 27.8 | 26.6 | |
| Ex-smoker | 26.4 | 30.3 | 29.7 | 29.4 | |
MDCS Malmö Diet and Cancer Study, SNP Single-nucleotide polymorphism
Separate missing categories are given only if missing > 1%, Mean ± SD in italics
When comparing control subjects from the cardiovascular cohort with control subjects used in the previous case-control study, risk factors were similarly distributed, although small differences in a higher level of education and a slightly higher proportion of oral contraceptive users and smokers were seen amongst the previously matched control subjects (Additional file 2). Women whose data were excluded owing to QC of SNPs or relationships with other women in the cohort were less likely to have used oral contraceptives. They also had a lower BMI and had not used tobacco to the same extent as women with included data (Additional file 2). There also were small differences between women with or without available data on vitamin D level. Women with no data on vitamin D used oral contraceptives and hormone replacement therapy less and were also slightly older and less likely to smoke (Additional file 2).
SNPs and risk of low vitamin D level
There were statistically significant associations between a majority of tested SNPs and levels of vitamin D (Table 2). An increased risk of being in the group with lower vitamin D was seen for minor homozygotes of rs705117 (OR 3.38, 95% CI 1.20–9.49), rs7041 (OR 2.12, 95% CI 1.51–2.99) and rs2282679 (OR 2.51, 95% CI 1.56–4.05), all of which are located in the intron of the GC gene on chromosome 4 (Table 2). For rs7041 and rs2282679, the association was seen also for heterozygotes (respectively, OR 1.39, 95% CI 1.09–1.77; OR 1.42, 95% CI 1.13–1.80) (Table 2). Similarly, higher risk of low vitamin D was associated with minor homozygotes of rs12295888 (OR 1.80, 95% CI 1.24–2.63), rs1007392 (OR 1.78, 95% CI 1.26–2.52), rs7944926 (OR 2.39, 95% CI 1.59–3.59) and rs3829251 (OR 3.26, 95% CI 1.72–6.17) on chromosome 11 (Table 2). A statistically significant decreased risk of low vitamin D was seen with heterozygotes (OR 0.78, 95% CI 0.61–1.00) and minor homozygotes (OR 0.58, 95% CI 0.42–0.81) of rs2060793 also located on chromosome 11 but in the CYP2R1 gene (Table 2).
Table 2.
Selected single-nucleotide polymorphisms in relation to vitamin D level and breast cancer
| SNP (GWAS SNP) | Risk of low vitamin D | Risk of breast cancer | ||||
|---|---|---|---|---|---|---|
| Allele | Frequency of low/high vitamin Da (n = 1343) | Crude OR of low vitamin D (95% CI) | Adjusted ORb of low vitamin D (95% CI) | Cases/control subjects (n = 4058) | Crude OR (95% CI) | Adjusted ORb (95% CI) |
| rs12239582 (rs12144344) | ||||||
| CC | 224/188 | 1.00 | 1.00 | 290/986 | 1.00 | 1.00 |
| CA | 344/336 | 0.86 (0.67–1.10) | 0.87 (0.67–1.12) | 412/1604 | 0.87 (0.74–1.04) | 0.82 (0.68–0.99) |
| AA | 116/135 | 0.72 (0.53–0.99) | 0.72 (0.52–1.00) | 163/600 | 0.92 (0.74–1.15) | 0.93 (0.73–1.17) |
| Missing | – | – | – | 0/3 | – | – |
| rs705117 | ||||||
| CC | 504/486 | 1.00 | 1.00 | 657/2376 | 1.00 | 1.00 |
| CT | 164/168 | 0.94 (0.73–1.21) | 0.94 (0.73–1.22) | 194/762 | 0.92 (0.77–1.10) | 0.86 (0.71–1.05) |
| TT | 16/5 | 3.06 (1.11–8.44) | 3.38 (1.20–9.49) | 14/55 | 0.92 (0.51–1.67) | 0.94 (0.49–1.81) |
| rs7041 | ||||||
| AA | 213/270 | 1.00 | 1.00 | 332/1149 | 1.00 | 1.00 |
| AC | 341/308 | 1.40 (1.11–1.78) | 1.39 (1.09–1.77) | 397/1521 | 0.90 (0.77–1.07) | 0.88 (0.74–1.05) |
| CC | 130/81 | 2.03 (1.46–2.83) | 2.12 (1.51–2.99) | 136/522 | 0.90 (0.72–1.13) | 0.89 (0.70–1.13) |
| Missing | – | – | – | 0/1 | – | – |
| rs2282679 (rs17467825) | ||||||
| GG | 342/397 | 1.00 | 1.00 | 480/1734 | 1.00 | 1.00 |
| GT | 278/230 | 1.40 (1.12–1.76) | 1.42 (1.13–1.80) | 326/1222 | 0.96 (0.82–1.13) | 0.98 (0.83–1.16) |
| TT | 62/29 | 2.45 (1.56–3.95) | 2.51 (1.56–4.05) | 57/229 | 0.90 (0.66–1.22) | 0.91 (0.66–1.27) |
| Missing | 2/3 | – | – | 2/8 | – | – |
| rs10485165 | ||||||
| TT | 515/501 | 1.00 | 1.00 | 650/2385 | 1.00 | 1.00 |
| TC | 153/144 | 1.04 (0.80–1.35) | 1.03 (0.79–1.34) | 194/735 | 0.97 (0.81–1.16) | 0.99 (0.82–1.20) |
| CC | 16/14 | 1.10 (0.53–2.29) | 1.04 (0.49–2.21) | 21/73 | 1.06 (0.65–1.73) | 1.00 (0.60–1.74) |
| rs198300 (rs156299) | ||||||
| GG | 218/229 | 1.00 | 1.00 | 295/1134 | 1.00 | 1.00 |
| GA | 344/319 | 1.13 (0.89–1.44) | 1.08 (0.84–1.38) | 430/1513 | 1.09 (0.92–1.29) | 1.10 (0.91–1.30) |
| AA | 122/111 | 1.16 (0.84–1.59) | 1.09 (0.79–1.52) | 140/545 | 0.99 (0.79–1.24) | 0.96 (0.75–1.22) |
| Missing | – | – | – | 0/1 | – | – |
| rs4751058 | ||||||
| AA | 496/460 | 1.00 | 1.00 | 614/2286 | 1.00 | 1.00 |
| AG | 174/183 | 0.88 (0.69–1.13) | 0.88 (0.68–1.13) | 234/828 | 1.05 (0.89–1.25) | 1.05 (0.88–1.27) |
| GG | 14/16 | 0.81 (0.39–1.68) | 0.77 (0.36–1.63) | 17/79 | 0.80 (0.47–1.36) | 0.66 (0.38–1.17) |
| rs12295888 (rs12287212) | ||||||
| CC | 270/296 | 1.00 | 1.00 | 367/1384 | 1.00 | 1.00 |
| CT | 321/302 | 1.16 (0.93–1.46) | 1.22 (0.96–1.54) | 402/1435 | 1.06 (0.90–1.24) | 1.05 (0.88–1.24) |
| TT | 93/61 | 1.67 (1.16–2.40) | 1.80 (1.24–2.63) | 96/373 | 0.97 (0.75–1.25) | 0.93 (0.71–1.23) |
| Missing | – | – | – | 0/1 | – | – |
| rs1007392 | ||||||
| GG | 231/267 | 1.00 | 1.00 | 325/1184 | 1.00 | 1.00 |
| GA | 337/310 | 1.26 (0.99–1.59) | 1.31 (1.03–1.67) | 417/1543 | 0.99 (0.84–1.16) | 0.99 (0.83–1.18) |
| AA | 115/82 | 1.62 (1.16–2.26) | 1.78 (1.26–2.52) | 123/462 | 0.97 (0.77–1.23) | 0.91 (0.71–1.17) |
| Missing | 1/0 | – | – | 0/4 | – | – |
| rs2060793 (rs10741657) | ||||||
| AA | 246/194 | 1.00 | 1.00 | 283/1040 | 1.00 | 1.00 |
| AG | 328/323 | 0.80 (0.63–1.02) | 0.78 (0.61–1.00) | 418/1544 | 1.00 (0.84–1.18) | 1.10 (0.92–1.33) |
| GG | 110/142 | 0.61 (0.45–0.84) | 0.58 (0.42–0.81) | 164/606 | 1.00 (0.80–1.24) | 1.04 (0.82–1.32) |
| Missing | – | – | – | 0/3 | – | – |
| rs7944926 (rs12785878) | ||||||
| AA | 297/343 | 1.00 | 1.00 | 423/1436 | 1.00 | 1.00 |
| AG | 299/272 | 1.27 (1.01–1.59) | 1.30 (1.03–1.64) | 366/1400 | 0.89 (0.76–1.04) | 0.91 (0.77–1.08) |
| GG | 88/44 | 2.31 (1.56–3.43) | 2.39 (1.59–3.59) | 76/356 | 0.73 (0.55–0.95) | 0.77 (0.57–1.02) |
| Missing | – | – | – | 0/1 | – | – |
| rs3829251 | ||||||
| AA | 402/435 | 1.00 | 1.00 | 545/1954 | 1.00 | 1.00 |
| AG | 242/210 | 1.25 (0.99–1.57) | 1.30 (1.02–1.64) | 287/1079 | 0.95 (0.81–1.12) | 0.99 (0.83–1.18) |
| GG | 40/14 | 3.09 (1.65–5.77) | 3.26 (1.72–6.17) | 33/160 | 0.74(0.50–1.09) | 0.77 (0.51–1.16) |
| rs2302190 | ||||||
| CC | 451/466 | 1.00 | 1.00 | 566/2122 | 1.00 | 1.00 |
| CT | 211/176 | 1.24 (0.98–1.57) | 1.24 (0.97–1.58) | 268/955 | 1.05(0.89–1.24) | 1.09 (0.91–1.30) |
| TT | 20/13 | 1.59 (0.78–3.23) | 1.45 (0.69–3.02) | 28/109 | 0.96 (0.63–1.47) | 0.99 (0.62–1.58) |
| Missing | 2/4 | – | – | 3/7 | – | – |
GWAS Genome-wide association study, SNP Single-nucleotide polymorphism
aLow vs high vitamin D grouped by calendar month
bAdjusted for age at baseline, year of inclusion, level of education, type of occupation, age at menarche, age at first childbirth, exposure to oral contraceptives, exposure to hormone replacement therapy, height, body mass index, alcohol consumption and smoking
When several proxies were used for one GWAS SNP, the results in the analyses were very similar, but only the proxy with an R 2 value closest to 1 is presented in this report.
SNPs and overall breast cancer risk
The tested SNP proxy rs12239582 for the GWAS SNP rs12144344 (located in ST6GALNAC3 in chromosome 1) was found to be statistically significantly associated with a relatively low risk of breast cancer (OR 0.82, 95% CI 0.68–0.99) in the adjusted model when heterozygotes were compared with major homozygotes (Table 2). In the crude analysis, minor homozygotes of SNP proxy rs12791871 for GWAS SNP rs12785878 (located in NADSYN1 in chromosome 11) also had a statistically significant decreased risk of breast cancer (OR 0.73, 95% CI 0.55–0.95) compared with major homozygotes (Table 2). This result was similar in the adjusted analysis, although with only borderline statistical significance (OR 0.77, 95% CI 0.57–1.02) (Table 2).
SNPs and breast cancer risk in different strata of serum vitamin D
A test of interaction of high/low vitamin D levels and breast cancer risk was statistically significant at p < 0.01 for rs198300 (proxy for GWAS rs156299, located upstream of neuropeptide Y [NPY] gene in chromosome 7). Minor homozygotes of rs198300 who were within the group with low vitamin D levels had a statistically significant decrease in breast cancer risk compared with major homozygotes (OR 0.53, 95% CI 0.33–0.87) (Table 3). Looking at minor homozygotes of the same SNP (rs198300) within the group with high vitamin D levels, their risk of breast cancer seemed to be increased (OR 1.38, 95% CI 0.85–2.23) (Table 3). Similar but not statistically significant results were seen for rs2060793 (proxy for GWAS rs10741657 located in gene encoding cytochrome P450 [CYP2R1] in chromosome 11); compared with major homozygotes, heterozygotes in the group with lower levels of vitamin D had a slightly decreased risk of breast cancer (OR 0.95, 95% CI 0.67–1.35), but in the high vitamin D group, a reverse effect with increased breast cancer risk was seen (OR 1.35, 95% CI 0.93–1.97; p = 0.10 for interaction) (Table 3).
Table 3.
Selected single-nucleotide polymorphisms in relation to risk of breast cancer, stratified by vitamin D level
| SNP (proxy for GWAS) | Low vitamin Da | High vitamin Da | |||
|---|---|---|---|---|---|
| Allele | Frequency in cases/control subjects (n = 684) | ORb of breast cancer (95% CI) | p Value for interaction | Frequency in cases/control subjects (n = 659) | ORb of breast cancer (95% CI) |
| rs12239582 (rs12144344) | |||||
| CC | 127/97 | 1.00 | 99/89 | 1.00 | |
| CA | 176/168 | 0.85 (0.59–1.21) | 0.73 | 164/172 | 0.91 (0.62–1.33) |
| AA | 60/56 | 0.83 (0.52–1.34) | 0.30 | 74/61 | 1.16 (0.72–1.87) |
| rs705117 | |||||
| CC | 278/226 | 1.00 | 251/235 | 1.00 | |
| CT | 76/88 | 0.65 (0.45–0.95) | 0.28 | 84/84 | 0.87 (0.60–1.27) |
| TT | 9/7 | 1.14 (0.41–3.22) | 0.36 | 2/3 | 0.45 (0.66–3.11) |
| rs7041 | |||||
| AA | 120/93 | 1.00 | 141/129 | 1.00 | |
| AC | 169/172 | 0.70 (0.48–1.00) | 0.14 | 161/147 | 1.05 (0.74–1.47) |
| CC | 74/56 | 1.11 (0.69–1.76) | 0.15 | 35/46 | 0.65 (0.38–1.11) |
| rs2282679 (rs17467825) | |||||
| GG | 174/168 | 1.00 | 209/188 | 1.00 | |
| GT | 155/123 | 1.25 (0.89–1.75) | 0.16 | 113/117 | 0.91 (0.65–1.29) |
| TT | 33/29 | 1.21 (0.68–2.16) | 0.69 | 14/15 | 0.92 (0.42–2.04) |
| Missing | 1/1 | – | – | 1/2 | – |
| rs10485165 | |||||
| TT | 267/248 | 1.00 | 262/239 | 1.00 | |
| TC | 88/65 | 1.34 (0.91–1.98) | 0.08 | 67/77 | 0.83 (0.56–1.22) |
| CC | 8/8 | 0.94 (0.32–2.78) | 0.79 | 8/6 | 1.11 (0.36–3.40) |
| rs198300 (rs156299) | |||||
| GG | 126/92 | 1.00 | 110/119 | 1.00 | |
| GA | 184/160 | 0.82 (0.57–1.18) | 0.25 | 165/154 | 1.06 (0.74–1.52) |
| AA | 53/69 | 0.53 (0.33–0.87) | <0.01 | 62/49 | 1.38 (0.85–2.23) |
| rs4751058 | |||||
| AA | 267/229 | 1.00 | 235/225 | 1.00 | |
| AG | 89/85 | 0.91 (0.63–1.329 | 0.37 | 96/87 | 1.06 (0.74–1.52) |
| GG | 7/7 | 1.00 (0.33–3.03) | 0.42 | 6/10 | 0.47 (0.16–1.38) |
| rs12295888 (rs12287212) | |||||
| CC | 146/124 | 1.00 | 157/139 | 1.00 | |
| CT | 169/152 | 0.94 (0.66–1.33) | 0.88 | 154/148 | 0.86 (0.61–1.20) |
| TT | 48/45 | 1.01 (0.60–1.67) | 0.33 | 26/35 | 0.65 (0.36–1.17) |
| rs1007392 | |||||
| GG | 129/102 | 1.00 | 140/127 | 1.00 | |
| GA | 177/160 | 0.87 (0.61–1.25) | 0.71 | 159/151 | 0.91 (0.64–1.29) |
| AA | 57/58 | 0.81 (0.50–1.30) | 0.98 | 38/44 | 0.79 (0.47-1.34) |
| Missing | 0/1 | – | – | – | |
| rs2060793 (rs10741657) | |||||
| AA | 134/112 | 1.00 | 89/105 | 1.00 | |
| AG | 168/160 | 0.95 (0.67–1.35) | 0.10 | 174/149 | 1.35 (0.93–1.97) |
| GG | 61/49 | 0.99 (0.61–1.60) | 0.44 | 74/68 | 1.31 (0.83–2.08) |
| rs7944926 (rs12785878) | |||||
| AA | 167/130 | 1.00 | 180/163 | 1.00 | |
| AG | 158/141 | 0.88 (0.62–1.24) | 0.75 | 137/135 | 0.93 (0.66–1.29) |
| GG | 38/50 | 0.58 (0.35–0.98) | 0.49 | 20/24 | 0.78 (0.40–1.51) |
| rs3829251 | |||||
| AA | 218/184 | 1.00 | 222/213 | 1.00 | |
| AG | 126/116 | 0.95 (0.68–1.34) | 0.56 | 110/100 | 1.07 (0.76–1.51) |
| GG | 19/21 | 0.74 (0.37–1.49) | 0.62 | 5/9 | 0.57 (0.18–1.79) |
| rs2302190 | |||||
| CC | 236/215 | 1.00 | 229/237 | 1.00 | |
| CT | 114/97 | 1.03 (0.73–1.47) | 0.49 | 97/79 | 1.27 (0.88–1.84) |
| TT | 12/8 | 1.36 (0.51–3.60) | 0.52 | 9/4 | 2.11 (0.61–7.30) |
| Missing | 1/1 | – | – | 2/2 | – |
GWAS Genome-wide association study, SNP Single-nucleotide polymorphism
aLow vs high vitamin D grouped by calendar month
bAdjusted for age at baseline, year of inclusion, level of education, type of occupation, age at menarche, age at first childbirth, exposure to oral contraceptives, exposure to hormone replacement therapy, height, body mass index, alcohol consumption and smoking
Adverse associations of breast cancer risk dependent on vitamin D levels were seen for heterozygotes of rs10485165 (in chromosome 6). Heterozygotes with low levels of vitamin D were associated with an increased risk of breast cancer (OR 1.34, 95% CI 0.91–1.98), whereas heterozygotes with higher vitamin D levels had a decreased risk of breast cancer (OR 0.83, 95% CI 0.56–1.22), with a p value for interaction of 0.08 (Table 3).
Discussion
In the present study, a majority of the selected SNPs were associated with vitamin D levels that were in agreement with those reported in previous studies. As for breast cancer risk, a statistically significant association was observed with one of the tested loci (rs12239582 and rs2209458; proxies for GWAS SNP rs12144344). Another locus (rs12791871 and rs7944926; proxies for GWAS SNP rs12785878) showed a tendency towards an association with breast cancer risk. The analyses stratified for level of vitamin D showed diverse associations with breast cancer risk for three of the tested loci: rs156299 (proxies used, rs198300 and rs13245518), rs10741657 (proxies used, rs2060793 and rs1993116) and rs10485165, depending on whether vitamin D level was high or low.
SNPs and risk of low vitamin D
There is no current consensus regarding which level of vitamin D should be considered insufficient; in addition, the level varies over the year owing to sun exposure. Grouping individuals by high or low vitamin D level by calendar month was considered pragmatic to calculate differences in vitamin D level owing to allele variants. SNPs located in the intron of the GC gene in chromosome 4 have previously been associated in several studies with levels of vitamin D and/or vitamin D insufficiency [22, 23, 25–27]. Results derived from the present analyses are consistent with previous results because minor alleles of all tested SNPs located in the intron of the GC gene (rs705117, rs7041, rs4588 and rs2282679) were statistically significantly associated with a low level of vitamin D. Equally confirming results were seen for several other SNPs of other genome sites, such as PDE3B intron, CYP2R1, and NADSYN1 in chromosome 11. Borderline statistically significant associations were seen for further SNPs on ST6GALNAC3 in chromosome 1 and on MTMR4 in chromosome 17. SNPs in chromosome 6, chromosome 7 and chromosome 10 did not show any association with low vitamin D, in contrast to previous studies of children in Western Australia [23, 24]. This might depend on the different genetic backgrounds of the two populations, limited statistical power due to small groups with vitamin D levels in our material, or different vitamin D exposures because it is expected that children in Western Australia are likely to have different exposure to sun than middle-aged women in the south of Sweden.
If there is a true association between low levels of vitamin D and an increased risk of breast cancer, a Mendelian randomization study would show that SNPs associated with low vitamin D levels would also be associated with an increased risk of breast cancer [12]. This is somewhat in line with the present results derived from analysing rs12239582 and rs2209458 in ST6GALNAC3, in chromosome 1, where minor homozygotes had a borderline statistically significant association with a decreased risk of low vitamin D level and heterozygotes with a statistically significant decreased risk of breast cancer (Table 2). Contradictory to this, SNPs whose minor alleles showed an association with an increased risk of low vitamin D—rs12791871, rs7944926 and rs3829251—of the NADSYN1 in chromosome 11 were, if anything, associated with a decreased risk of breast cancer. Also, for most SNPs with an association with vitamin D level, no association with breast cancer risk could be found. This might be explained either by no such association being able to be found or by such an association being too small to be observed in our study. The clinical relevance of such small associations is questionable.
SNPs and risk of breast cancer
The association found in our study between rs12144344 (proxies, rs12239582 and rs2209458) in chromosome 1 and breast cancer risk has not been reported previously. This SNP is positioned in ST6GALNAC3, a gene encoding a sialyltransferase which might affect DBP synthesis, concentration and function and that has previously been associated with levels of DBP [27]. No statistically significant association with vitamin D level was seen in our study, although minor homozygotes had a borderline statistically significant association with low levels of vitamin D. Previous associations between rs12144344 and vitamin D levels have not been reported.
Previous studies on vitamin D-associated SNPs and breast cancer risk have been focused mainly on genetic variants of the vitamin D receptor, and a few studies have been done on GC, which is the gene encoding DBP [30]. The present study was focused on SNPs previously associated with vitamin D in GWASs, and because no association was previously found for VDR SNPs, no VDR SNPs were included. Of the SNPs analysed in this study, only rs7041 and rs4588, both located in the GC gene, were previously studied in association with breast cancer risk [31–35]. Our results showing a tendency towards a protective effect of one or two minor alleles of rs7041 and no association of rs4588 are consistent with those of one previous study in which researchers showed a similar statistically significant association [32], as well as one other study in which researchers combined variants of rs7041 and rs4588 and found a protective effect of some combinations for post-menopausal breast cancer [36]. However, other studies have shown no association [30, 33, 35].
In the present study, the common homozygote was used as a reference, and relative risks were calculated individually for heterozygotes and less common homozygotes. Others have used other approaches, calculating on the basis of risk alleles and/or grouping several genotypes together. Sometimes it is not clear which allele was used as a reference, which means that comparing results between studies is difficult.
SNPs and breast cancer risk in different strata of serum vitamin D
When the association of analysed SNPs with breast cancer risk was stratified by level of vitamin D, some results differed depending on vitamin D level. This association was found for rs198300 and rs13245518, both proxies for rs156299 located on chromosome 7 upstream of gene encoding neuropeptide Y, a neurotransmitter involved in mediating physiological processes, including food intake and bone homeostasis, previously associated with levels of vitamin D [23]. In non-stratified analyses, no association with either breast cancer risk or vitamin D level was observed. Similar results were found for rs2060793 and rs1993116, proxies for GWAS rs10741657 located in CYP2R1, which encodes cytochrome P450, an enzyme that converts vitamin D to the circulating form 25(OH)D. rs10741657 was previously associated with vitamin D levels and insufficiency [22, 23, 25, 26]. Also, rs10485165, a proxy for rs7763511 (in chromosome 6) previously associated with vitamin D level but with unknown function [24], showed diverse ORs for breast cancer risk dependent on high versus low vitamin D level.
We found no previous reports of associations of this kind. Because this was an exploratory part of the present study, and because vitamin D level was available for only a smaller fraction of cases and control subjects, we suggest that these findings ought to be regarded with caution. The findings must be replicated, but at the same time they indicate that vitamin D level may interact with genetic traits in breast cancer risk.
Methodological issues
All patients diagnosed with cancer within the boundaries of Sweden are reported to the Swedish Cancer Registry, which is regarded as a highly validated source of information [37]. Thanks to the unique civil registration number given to all Swedes at birth, it is possible to link individuals included in the cohort to this registry and retrieve complete and correct information on breast cancer diagnosis. Women who have emigrated and received their diagnosis elsewhere are not included in this registry, which is a limitation of the present study; hence a few cancer cases might be lost, but emigration is not likely to be linked to risk of breast cancer or genetic traits.
An individual’s vitamin D level is influenced by several factors, not only sun exposure. It has also been shown that levels decrease with increasing age and increasing BMI [38–40], which was taken into consideration when analyses were adjusted. Physical activity has been shown to influence both level of vitamin D and breast cancer incidence [41, 42], but because the validity of physical activity data in the MDCS has been questioned, this was not included in the statistical model. Moreover, the effect of physical activity on vitamin D levels seems to be limited to outdoor activity, and hence physical activity may be a marker of sun exposure [43]. Vitamin D levels were analysed in only one blood sample per individual, which might be considered insufficient, though it has been shown previously that 25(OH)D levels analysed twice several years apart are individually very highly correlated [44, 45].
Differences noted when study populations were compared with regard to risk (Additional file 2: Table S1) were small. Those differences might depend on the fact that the previously matched control subjects had more in common with breast cancer cases. Considering differences between subjects with or without data regarding vitamin D level, there are of course a larger proportion of breast cancer cases amongst women with available vitamin D levels, which explains some of the differences. Also, because we adjusted analyses for risk factors in which subjects differ, we consider the risk of potential selection affecting our results to be small.
It is not plausible that any confounder included in this study affect an individual’s genetic composition. It might therefore be debatable whether adjustments should be added to the analyses. Because there is still a possibility of a chance association between single SNPs and established and potential risk factors for breast cancer, we chose to include such factors in our statistical model. A limitation of the present study is that there were no data accessible regarding family history of breast cancer or prior benign breast disease; hence those known risk factors were not included when adjustments were made.
In total, 20 SNPs from 10 different loci were tested for associations with breast cancer risk and risk of low vitamin D level in the present study. For each one of these, we used the same assumption: If there is an association with vitamin D, there might also be one with risk of breast cancer. We therefore decided not to increase the CI. With so many tested SNPs, one might suspect associations to be chance findings as a type I error, but when such associations were found, we also saw the same association for proxy SNPs, which strengthens the findings.
Individual SNPs change the risk of getting breast cancer to a very small extent, and large groups are a must in order to find statistically significant associations. Even the present population with 865 cases and 3193 control subjects may be too small to identify all associations. In the population with analysed levels of vitamin D, groups were even smaller, and therefore we expect a higher risk of a type II error in these analyses.
Conclusions
Many previous findings of SNPs associated with vitamin D levels were reassuringly replicated in this study. One SNP (rs12239582) previously associated with levels of DBP was associated with a slightly decreased risk of breast cancer, as was a tendency towards a decreased risk of low vitamin D level, in the present study. The allele frequency of the SNP variants is high, and therefore even a small increase in risk per individual may be a substantial population attributable risk. Further results of the present study show vitamin D level to be an effect modifier of the risk of breast cancer associated with certain SNPs. This suggests that an individual’s composition of SNPs may affect the extent to which levels of vitamin D are associated with breast cancer risk.
Additional files
Appendix. Description of GWAS SNPs associated with vitamin D, SNP proxies and SNPs analysed. (DOCX 27 kb)
Baseline characteristics of control subjects and subjects with and without data on SNPs/vitamin D. (DOCX 24 kb)
Acknowledgements
The authors thank the data manager and registered nurse, Anna Hwasser, who kindly prepared original files and assisted in data management.
Funding
The present study was supported by The Einar and Inga Nilsson Foundation, The Gunnar Nilsson Cancer Foundation, The Ernhold Lundström Foundation, The Swedish Cancer Society, The Swedish Research Council (VR), The Malmö University Hospital Cancer Research Fund, The Skåne University Hospital Funds and Donations, The Breast Cancer network at Lund University (BCLU), and The Region Skåne (ALF).
Availability of data and materials
The authors have full access to the data, and the dataset used for the present study is kept by the corresponding author.
Abbreviations
- BMI
Body mass index
- DBP
Vitamin D-binding protein
- GC
Group-specific component
- GWAS
Genome-wide association study
- HRT
Hormone replacement therapy
- LD
Linkage disequilibrium
- MDCS
Malmö Diet and Cancer Study
- QC
Quality control
- SNP
Single-nucleotide polymorphism
- VDR
Vitamin D receptor
Authors’ contributions
LH selected SNPs to analyse, extracted information of such SNPs from a PLINK file and did all statistical analyses using SPSS software. LH was the main author of the manuscript and constructed the tables and figures. STB and JM supervised the project. PA performed quality control of SNPs and exclusion of relatives and supervised PLINK software use. SB and JB revised and added material to the manuscript. AF supervised selection of SNPs and provided information on handling SNP data. AF revised the manuscript. OM performed SNP analyses. All authors read and approved the final manuscript.
Authors’ information
LH is a general surgeon in the Department of Surgery at Skane University Hospital and is also a doctoral student.
Ethics approval and consent to participate
The MDCS and the present study were approved by the ethics committee in Lund, Sweden (LU 51-90, Dnr 153/2004 and Dnr 682/2009). At the time of inclusion in MDCS, written informed consent was obtained from all participants. Follow-up studies have been advertised in local media with an option to withdraw participation.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1186/s13058-017-0925-3) contains supplementary material, which is available to authorized users.
Contributor Information
Linnea Huss, Email: linnea.huss@med.lu.se.
Salma Tunå Butt, Email: salma.butt@med.lu.se.
Peter Almgren, Email: peter.almgren@med.lu.se.
Signe Borgquist, Email: signe.borgquist@med.lu.se.
Jasmine Brandt, Email: jasmine.brandt@med.lu.se.
Asta Försti, Email: asta.forsti@med.lu.se.
Olle Melander, Email: olle.melander@med.lu.se.
Jonas Manjer, Email: jonas.manjer@med.lu.se.
References
- 1.Eccles SA, Aboagye EO, Ali S, Anderson AS, Armes J, Berditchevski F, Blaydes JP, Brennan K, Brown NJ, Bryant HE, et al. Critical research gaps and translational priorities for the successful prevention and treatment of breast cancer. Breast Cancer Res. 2013;15(5):R92. doi: 10.1186/bcr3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burdett T, Hall PN, Hastings E, Hindorff LA, Junkins HA, Klemm AK, MacArthur J, Manolio TA, Morales J, Parkinson H, Welter D. The NHGRI-EBI Catalog of published genome-wide association studies. www.ebi.ac.uk/gwas. Accessed 11 Apr 2016 and April 23, 2017, version 1.0.
- 3.Grant WB. An ecological study of cancer incidence and mortality rates in France with respect to latitude, an index for vitamin D production. Dermatoendocrinol. 2010;2(2):62–7. doi: 10.4161/derm.2.2.13624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Porojnicu A, Robsahm TE, Berg JP, Moan J. Season of diagnosis is a predictor of cancer survival. Sun-induced vitamin D may be involved: a possible role of sun-induced vitamin D. J Steroid Biochem Mol Biol. 2007;103(3-5):675–8. doi: 10.1016/j.jsbmb.2006.12.031. [DOI] [PubMed] [Google Scholar]
- 5.Robsahm TE, Tretli S, Dahlback A, Moan J. Vitamin D3 from sunlight may improve the prognosis of breast-, colon- and prostate cancer (Norway) Cancer Causes Control. 2004;15(2):149–58. doi: 10.1023/B:CACO.0000019494.34403.09. [DOI] [PubMed] [Google Scholar]
- 6.Rohan T. Epidemiological studies of vitamin D and breast cancer. Nutr Rev. 2007;65(Suppl 2):S80–3. doi: 10.1301/nr.2007.aug.S80-S83. [DOI] [PubMed] [Google Scholar]
- 7.Colston KW. Vitamin D, and breast cancer risk. Best Pract Res Clin Endocrinol Metab. 2008;22(4):587–99. doi: 10.1016/j.beem.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 8.Almquist M, Bondeson AG, Bondeson L, Malm J, Manjer J. Serum levels of vitamin D, PTH and calcium and breast cancer risk-a prospective nested case-control study. Int J Cancer. 2010;127(9):2159–68. doi: 10.1002/ijc.25215. [DOI] [PubMed] [Google Scholar]
- 9.Gorham ED, Mohr SB, Garland FC, Garland CF. Vitamin D for cancer prevention and survival. Clin Rev Bone Miner Metab. 2009;7(2):159–75. doi: 10.1007/s12018-009-9028-8. [DOI] [Google Scholar]
- 10.Shao T, Klein P, Grossbard ML. Vitamin D and breast cancer. Oncologist. 2012;17(1):36–45. doi: 10.1634/theoncologist.2011-0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang D, Velez de-la-Paz OI, Zhai JX, Liu DW. Serum 25-hydroxyvitamin D and breast cancer risk: a meta-analysis of prospective studies. Tumour Biol. 2013;34(6):3509–17. doi: 10.1007/s13277-013-0929-2. [DOI] [PubMed] [Google Scholar]
- 12.Ong JS, Cuellar-Partida G, Lu Y, Fasching PA, Hein A, Burghaus S, Beckmann MW, Lambrechts D, Van Nieuwenhuysen E, Vergote I, et al. Association of vitamin D levels and risk of ovarian cancer: a Mendelian randomization study. Int J Epidemiol. 2016;45(5):1619–30. doi: 10.1093/ije/dyw207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khan MI, Bielecka ZF, Najm MZ, Bartnik E, Czarnecki JS, Czarnecka AM, Szczylik C. Vitamin D receptor gene polymorphisms in breast and renal cancer: current state and future approaches. Int J Oncol. 2014;44(2):349–63. doi: 10.3892/ijo.2013.2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shan JL, Dai N, Yang XQ, Qian CY, Yang ZZ, Jin F, Li M, Wang D. FokI polymorphism in vitamin D receptor gene and risk of breast cancer among Caucasian women. Tumour Biol. 2014;35(4):3503–8. doi: 10.1007/s13277-013-1462-z. [DOI] [PubMed] [Google Scholar]
- 15.Xu Y, He B, Pan Y, Deng Q, Sun H, Li R, Gao T, Song G, Wang S. Systematic review and meta-analysis on vitamin D receptor polymorphisms and cancer risk. Tumour Biol. 2014;35(5):4153–69. doi: 10.1007/s13277-013-1544-y. [DOI] [PubMed] [Google Scholar]
- 16.Zhang K, Song L. Association between vitamin D receptor gene polymorphisms and breast cancer risk: a meta-analysis of 39 studies. PLoS One. 2014;9(4):e96125. doi: 10.1371/journal.pone.0096125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manjer J, Carlsson S, Elmstahl S, Gullberg B, Janzon L, Lindstrom M, Mattisson I, Berglund G. The Malmo diet and cancer study: representativity, cancer incidence and mortality in participants and non-participants. Eur J Cancer Prev. 2001;10(6):489–99. doi: 10.1097/00008469-200112000-00003. [DOI] [PubMed] [Google Scholar]
- 18.Manjer J, Elmståhl S, Janzon L, Berglund G. Invitation to a population-based cohort study: differences between subjects recruited using various strategies. Scand J Public Health. 2002;30(2):103–12. doi: 10.1177/14034948020300020401. [DOI] [PubMed] [Google Scholar]
- 19.Fernandez C, Sandin M, Sampaio JL, Almgren P, Narkiewicz K, Hoffmann M, Hedner T, Wahlstrand B, Simons K, Shevchenko A, et al. Plasma lipid composition and risk of developing cardiovascular disease. PLoS One. 2013;8(8):e71846. doi: 10.1371/journal.pone.0071846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lucas RM, Gorman S, Black L, Neale RE. Clinical, research, and public health implications of poor measurement of vitamin D status. J AOAC Int. 2017;100(5):1225–9. doi: 10.5740/jaoacint.17-0082. [DOI] [PubMed] [Google Scholar]
- 21.Welter D, MacArthur J, Morales J, Burdett T, Hall P, Junkins H, Klemm A, Flicek P, Manolio T, Hindorff L, et al. The NHGRI GWAS catalog, a curated resource of SNP-trait associations. Nucleic Acids Res. 2014;42(Database issue):D1001–6. doi: 10.1093/nar/gkt1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahn J, Yu K, Stolzenberg-Solomon R, Simon KC, McCullough ML, Gallicchio L, Jacobs EJ, Ascherio A, Helzlsouer K, Jacobs KB, et al. Genome-wide association study of circulating vitamin D levels. Hum Mol Genet. 2010;19(13):2739–45. doi: 10.1093/hmg/ddq155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anderson D, Holt BJ, Pennell CE, Holt PG, Hart PH, Blackwell JM. Genome-wide association study of vitamin D levels in children: replication in the Western Australian pregnancy cohort (raine) study. Genes Immun. 2014;15(8):578–83. doi: 10.1038/gene.2014.52. [DOI] [PubMed] [Google Scholar]
- 24.Benjamin EJ, Dupuis J, Larson MG, Lunetta KL, Booth SL, Govindaraju DR, Kathiresan S, Keaney JF, Jr, Keyes MJ, Lin JP, et al. Genome-wide association with select biomarker traits in the Framingham heart study. BMC Med Genet. 2007;8(Suppl 1):S11. doi: 10.1186/1471-2350-8-S1-S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jorde R, Schirmer H, Wilsgaard T, Joakimsen RM, Mathiesen EB, Njølstad I, Løchen ML, Figenschau Y, Berg JP, Svartberg J, et al. Polymorphisms related to the serum 25-hydroxyvitamin D level and risk of myocardial infarction, diabetes, cancer and mortality: the Tromsø study. PLoS One. 2012;7(5):e37295. doi: 10.1371/journal.pone.0037295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang TJ, Zhang F, Richards JB, Kestenbaum B, van Meurs JB, Berry D, Kiel DP, Streeten EA, Ohlsson C, Koller DL, et al. Common genetic determinants of vitamin D insufficiency: a genome-wide association study. Lancet. 2010;376(9736):180–8. doi: 10.1016/S0140-6736(10)60588-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moy KA, Mondul AM, Zhang H, Weinstein SJ, Wheeler W, Chung CC, Mannisto S, Yu K, Chanock SJ, Albanes D. Genome-wide association study of circulating vitamin D-binding protein. Am J Clin Nutr. 2014;99(6):1424–31. doi: 10.3945/ajcn.113.080309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson AD, Handsaker RE, Pulit SL, Nizzari MM, O’Donnell CJ, de Bakker PI. SNAP: a web-based tool for identification and annotation of proxy SNPs using HapMap. Bioinformatics. 2008;24(24):2938–9. doi: 10.1093/bioinformatics/btn564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MAR, Bender D, Maller J, Sklar P, de Bakker PIW, Daly MJ, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81(3):559–75. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tagliabue E, Raimondi S, Gandini S. Meta-analysis of vitamin D-binding protein and cancer risk. Cancer Epidemiol Biomarkers Prev. 2015;24(11):1758–65. doi: 10.1158/1055-9965.EPI-15-0262. [DOI] [PubMed] [Google Scholar]
- 31.Abbas S, Linseisen J, Slanger T, Kropp S, Mutschelknauss EJ, Flesch-Janys D, Chang-Claude J. The Gc2 allele of the vitamin D binding protein is associated with a decreased postmenopausal breast cancer risk, independent of the vitamin D status. Cancer Epidemiol Biomarkers Prev. 2008;17(6):1339–43. doi: 10.1158/1055-9965.EPI-08-0162. [DOI] [PubMed] [Google Scholar]
- 32.Anderson LN, Cotterchio M, Cole DE, Knight JA. Vitamin D-related genetic variants, interactions with vitamin D exposure, and breast cancer risk among Caucasian women in Ontario. Cancer Epidemiol Biomarkers Prev. 2011;20(8):1708–17. doi: 10.1158/1055-9965.EPI-11-0300. [DOI] [PubMed] [Google Scholar]
- 33.Jorde R, Schirmer H, Wilsgaard T, Bøgeberg Mathiesen E, Njølstad I, Lochen ML, Joakimsen RM, Grimnes G. The DBP phenotype Gc-1f/Gc-1f is associated with reduced risk of cancer: the Tromsø Study. PLoS One. 2015;10(5):e0126359. doi: 10.1371/journal.pone.0126359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maneechay W, Boonpipattanapong T, Kanngurn S, Puttawibul P, Geater SL, Sangkhathat S. Single nucleotide polymorphisms in the Gc gene for vitamin D binding protein in common cancers in Thailand. Asian Pac J Cancer Prev. 2015;16(8):3339–44. doi: 10.7314/APJCP.2015.16.8.3339. [DOI] [PubMed] [Google Scholar]
- 35.Shi J, Grundy A, Richardson H, Burstyn I, Schuetz JM, Lohrisch CA, SenGupta SK, Lai AS, Brooks-Wilson A, Spinelli JJ, et al. Genetic variation in vitamin D-related genes and risk of breast cancer among women of European and East Asian descent. Tumour Biol. 2016;37(5):6379–87. doi: 10.1007/s13277-015-4417-8. [DOI] [PubMed] [Google Scholar]
- 36.Abbas S, Nieters A, Linseisen J, Slanger T, Kropp S, Mutschelknauss EJ, Flesch-Janys D, Chang-Claude J. Vitamin D receptor gene polymorphisms and haplotypes and postmenopausal breast cancer risk. Breast Cancer Res. 2008;10(2):R31. doi: 10.1186/bcr1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barlow L, Westergren K, Holmberg L, Talbäck M. The completeness of the Swedish cancer register: a sample survey for year 1998. Acta Oncol. 2009;48(1):27–33. doi: 10.1080/02841860802247664. [DOI] [PubMed] [Google Scholar]
- 38.Brock K, Huang WY, Fraser DR, Ke L, Tseng M, Stolzenberg-Solomon R, Peters U, Ahn J, Purdue M, Mason RS, et al. Low vitamin D status is associated with physical inactivity, obesity and low vitamin D intake in a large US sample of healthy middle-aged men and women. J Steroid Biochem Mol Biol. 2010;121(1-2):462–6. doi: 10.1016/j.jsbmb.2010.03.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lips P. Vitamin D, physiology. Prog Biophys Mol Biol. 2006;92(1):4–8. doi: 10.1016/j.pbiomolbio.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 40.Need AG, O’Loughlin PD, Morris HA, Horowitz M, Nordin BE. The effects of age and other variables on serum parathyroid hormone in postmenopausal women attending an osteoporosis center. J Clin Endocrinol Metab. 2004;89(4):1646–9. doi: 10.1210/jc.2003-031539. [DOI] [PubMed] [Google Scholar]
- 41.Romieu II, Amadou A, Chajes V. The role of diet, physical activity, body fatness, and breastfeeding in breast cancer in young women: epidemiological evidence. Rev Invest Clin. 2017;69(4):193–203. doi: 10.24875/ric.17002263. [DOI] [PubMed] [Google Scholar]
- 42.Scragg R, Camargo CA., Jr Frequency of leisure-time physical activity and serum 25-hydroxyvitamin D levels in the US population: results from the third national health and nutrition examination survey. Am J Epidemiol. 2008;168(6):577–91. doi: 10.1093/aje/kwn163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pilch W, Tota L, Sadowska-Krepa E, Piotrowska A, Kepinska M, Palka T, Maszczyk A. The effect of a 12-week health training program on selected anthropometric and biochemical variables in middle-aged women. Biomed Res Int. 2017;2017:9569513. doi: 10.1155/2017/9569513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meng JE, Hovey KM, Wactawski-Wende J, Andrews CA, Lamonte MJ, Horst RL, Genco RJ, Millen AE. Intraindividual variation in plasma 25-hydroxyvitamin D measures 5 years apart among postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2012;21(6):916–24. doi: 10.1158/1055-9965.EPI-12-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Platz EA, Leitzmann MF, Hollis BW, Willett WC, Giovannucci E. Plasma 1,25-dihydroxy- and 25-hydroxyvitamin D and subsequent risk of prostate cancer. Cancer Causes Control. 2004;15(3):255–65. doi: 10.1023/B:CACO.0000024245.24880.8a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix. Description of GWAS SNPs associated with vitamin D, SNP proxies and SNPs analysed. (DOCX 27 kb)
Baseline characteristics of control subjects and subjects with and without data on SNPs/vitamin D. (DOCX 24 kb)
Data Availability Statement
The authors have full access to the data, and the dataset used for the present study is kept by the corresponding author.


