Keyes and Fuchs discuss the decline in stem cell renewal and function with aging and the ensuing consequences on tissue homeostasis and regeneration.
Abstract
Stem cells are imbued with unique qualities. They have the capacity to propagate themselves through symmetric divisions and to divide asymmetrically to engender new cells that can progress to differentiate into tissue-specific, terminal cell types. Armed with these qualities, stem cells in adult tissues are tasked with replacing decaying cells and regenerating tissue after injury to maintain optimal tissue function. With increasing age, stem cell functional abilities decline, resulting in reduced organ function and delays in tissue repair. Here, we review the effect of aging in five well-studied adult murine stem cell populations and explore age-related declines in stem cell function and their consequences for stem cell self-renewal, tissue homeostasis, and regeneration. Finally, we examine transcriptional changes that have been documented in aged stem cell populations and discuss new questions and future directions that this collection of data has uncovered.
Properties of stem cells
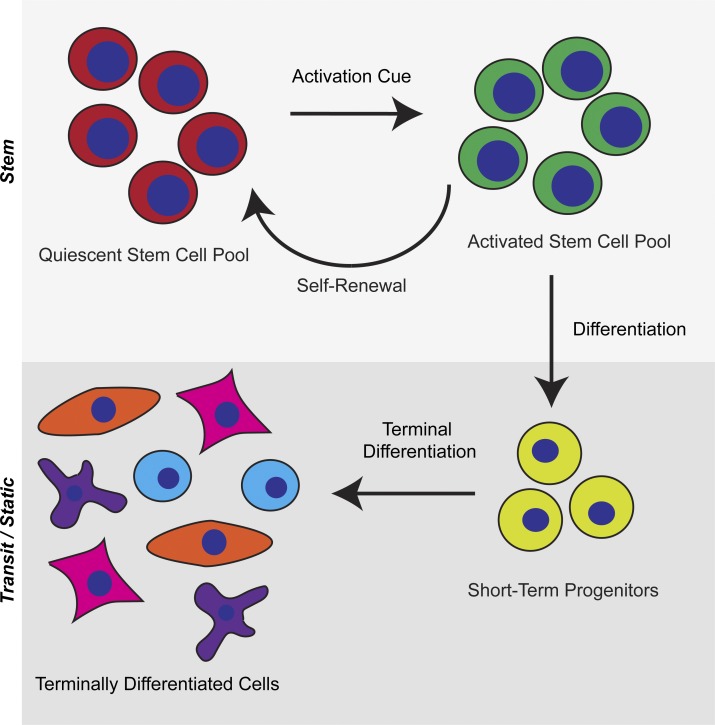
Somatic tissues of multicellular organisms have historically been compartmentalized into three general classes of cells: static, transit, and stem (Lajtha, 1979). Static cells are defined by their long persistence in the body; they decay over time and are not renewed. Transit cells are derived from a progenitor pool and have limited life spans and proliferative capacities. Stem cells have exceptional tissue regenerating capacity, can replenish themselves, and are long lived. These classifications of cell types still hold to this day, but our understanding of their dynamics in tissues has been refined. Transit cells within mammals have a wide diversity of life spans. Human adipose, skeletal cells, and cardiac muscle cells have life spans on the order of years, whereas some neurons can survive for the entire life span of the organism (Spalding et al., 2005). In humans, transit cells are replaced on the scale of months for skin, smooth muscle, salivary gland, and bladder cells, whereas cells from the colon, esophagus, blood, and spleen are replaced within days (Seim et al., 2016). At the top of this paradigm sits a population of long-lived cells in adult somatic tissues that is responsible for maintaining tissue integrity and function. These specialized tissue-resident stem cells are characterized by their core abilities of self-renewal, thereby maintaining their numbers within a tissue, and multipotency, generating cells that will progress to differentiate and become tissue-specific transit cells (Fig. 1).
Figure 1.
Stem cell properties. Adult tissues host a pool of quiescent stem cells that maintain their numbers throughout the life of the organism. When activated by extrinsic signals, stem cells can self-renew or differentiate to produce committed short-term progenitors that proliferate and terminally differentiate into tissue-specific, terminal cell types.
Tissue stem cells arise during embryogenesis and contribute to the formation and growth of their resident organ within the developing animal (Slack, 2008). In the adult, tissue stem cells reside in niches where they regulate cellular turnover by replacing cells lost to normal biological activity and repair tissue in response to acute injury. Resident tissue stem cells must self-renew to maintain stem cell pools to support normal homeostasis and tissue regeneration long term. Adult stem cells, such as those of the hematopoietic system, hair follicles, and muscles, experience extended periods of quiescence (G0 cell-cycle state), which has facilitated their identification in somatic tissues by their ability to retain label when pulsed with a marked nucleotide or fluorescent histones. Quiescence is tempered by the ability of those dormant stem cells to be activated in response to natural activation cues and after tissue damage to produce transient progenitors that differentiate into effector cell types. Perturbations in the balance of quiescence and activation can lead to the formation of hypoproliferative (degenerative diseases) and hyperproliferative (cancers) disorders; of which, age is the greatest risk factor (Rossi et al., 2008).
Stem cell aging
How do stem cells change with age, and what happens to their core stem cell properties of self-renewal and multipotency? Stem cell aging is perhaps best viewed through the lens of tissue homeostasis. Aged tissues show declines in their functional abilities, declines that can, in part, be traced back to failures of stem cell function. Aging can impinge on stem cell fitness at all levels: their capacity to self-renew, their activation and proliferative performance, and their production of downstream effector cells (Table 1). Ultimately, declines in stem cell function result in changes in tissue physiology that have an impact on the health of the organism and its viability. Mechanisms that underlie cellular aging can take the form of intrinsic alterations, such as telomere attrition, changes in proteostasis, shifts in the epigenetic landscape, DNA damage, mutational burden, and mitochondrial dysfunction. Additionally, extrinsic alterations can range from local niche/macroenvironmental changes to systemic level alterations to higher level environmental insults, such as irradiation, pathogen, and reactive oxygen exposure. The effects these changes have, the mechanisms by which they occur, and their roles in aging biology have been reviewed extensively elsewhere (Conboy and Rando, 2005; Brunet and Rando, 2007; Sharpless and DePinho, 2007; Liu and Rando, 2011; López-Otín et al., 2013; Signer and Morrison, 2013; Oh et al., 2014). However, the relative contributions of intrinsic versus extrinsic factors on aging in each adult stem cell population are still unclear, and few studies have systematically addressed the role each has in individual stem cell populations.
Table 1. Changes in stem cell properties with age.
| Population | Self-renewal (number of stem cells in aged tissue) | Proliferative activity | Differentiation potential | Regeneration and repair capacity |
|---|---|---|---|---|
| HSCs | Increased approximately two- to sixfold increased | Increased | Increased myeloid cell production | Decreased engraftment potential, immune response |
| HFSCs | Maintained at equivalent levels | Reduced | Maintained at equivalent levels | Deceased hair cycling and delayed wound healing |
| ISCs | Maintained at equivalent levels | Reduced | Increased secretary lineage cells | Reduced generation after UV exposure, delayed response |
| MuSCs | Decreased approximately twofold | Reduced | Increased fibrosis after injury | Decreased engraftment potential, myofiber regeneration |
| NSCs | Decreased approximately twofold | Reduced | Maintained in vitro | Not known |
DNA damage has emerged as a particularly relevant, intrinsic alteration in adult stem cell pools and has been linked to stem cell functional decline with age. Hematopoietic stem cells (HSCs), muscle stem cells (MuSCs), and hair follicle stem cells (HFSCs) have all been shown to accumulate DNA damage with age, as judged by an increase of γH2AX foci (histone variant H2A.X is phosphorylated in areas proximal to DNA damage) in cells as well as in assays that directly measure double strand breaks in DNA (Rossi et al., 2005; Beerman et al., 2014; Sinha et al., 2014; Matsumura et al., 2016). Along with an accumulation of DNA damage in aged HSCs, there is also a reduction in the DNA damage response (DDR; Rossi et al., 2007b; Beerman et al., 2014). Accumulation of DNA damage in aged HSCs has been linked to replicative stress (Rossi et al., 2007b; Flach et al., 2014), and it has been documented that aged HSCs that enter the cell cycle can repair DNA lesions by up-regulation of attenuated DDR pathways (Beerman et al., 2014). These rising levels of DNA damage may promote genomic instability and lead to permanent HSC dysfunction (Rossi et al., 2007a). Persistent, unrepaired DNA damage can lead to sustained DDR responses that compromise genomic integrity, resulting in mutational accumulation and genomic instability, which impairs stem cell function (Vijg and Suh, 2013).
An accumulation of DNA damage in stem cells and their long life span make them prime candidates for oncogenic transformation. Recently, the number of adult stem cell divisions has been linked to the frequency of cancer occurrence, with 17 types of cancers showing a strong correlation with the number of stem cell divisions and cancer development (Tomasetti and Vogelstein, 2015; Tomasetti et al., 2017). Tissues in which stem cells divide more frequently (e.g., epithelial tissues) have increased cancer incidence compared with tissues with slower dividing stem cell populations (e.g., brain). Adult stem cells are estimated to acquire three mutations per division, and as stem cells divide over time, the chance for developing cancer-driving mutations increases (Lynch, 2010; Tomasetti et al., 2013). Further investigation will be needed to establish whether transformation of aged stem cell populations is the source of cancer and at the root of the observed increased cancer incidence with age.
Changes in the systemic environment have also been shown to have a dramatic effect on adult stem cell behavior. The use of parabiosis (the joining of the circulatory systems of two individual animals) allows the exchange of blood-borne factors between two animals and has been widely used to investigate the effect of the systemic environment on aged stem cell function. Heterochronic parabiosis (the pairing of an old and young mouse together) is believed to restore the balance of key signaling factors important for stem cell function, which is altered in the blood or local environment in aged animals. Parabiosis has been shown to improve the functions of MuSCs, neural stem cells (NSCs), HFSCs, and hepatic progenitor-cell functions in aged animals (Conboy et al., 2005; Villeda et al., 2011; Keyes et al., 2013). Effects of parabiosis are most dramatic in the muscle, in which myofiber regeneration after injury in aged animals exposed to a young environment reach near-youthful levels (Conboy et al., 2005). Parabiosis restores the balance in Notch–Delta signaling, which, when activated, promotes the regenerative activity of MuSCs (Conboy et al., 2003). Intriguingly, parabiosis has also been shown to reduce the levels of DNA damage in aged MuSCs as well (Sinha et al., 2014). In the nervous system, parabiosis improves neurogenesis, synaptic plasticity, and cognitive function in aged animals (Villeda et al., 2011, 2014). Together, these studies show that, although some stem cell populations may become less responsive over time, stem cells in aged tissues do have the potential to be stimulated back into action and raises the possibility that aged stem cells could still be harnessed for therapeutic applications (Neves et al., 2017).
Aged stem cells in homeostasis and self-renewal
Invariably, there is a decline in tissue function with age. Stem cell self-renewal ensures maintenance of the stem cell reservoir within a tissue, whereas its multipotency allows continuous renewal of cells that preserve steady-state tissue function (homeostasis). Here, we focus on five different populations of murine adult stem cells and discuss how those populations are altered with age and the functional consequences of impaired stem cell function in their respective tissues.
HSCs
HSCs persist for the lifetime of the organism, producing all the cells of the blood system of both myeloid and lymphoid lineages. Blood cells turn over within 1–2 d, giving those cells the highest rate of turnover in the mammalian body. It is estimated that HSCs divide approximately once a month to support that level of cellular turnover (Cheshier et al., 1999). With advanced age, the hematopoietic system undergoes many well-described changes. The adaptive immune response is decreased (Linton and Dorshkind, 2004), risk for anemia development increases (Beghé et al., 2004), lymphoid cell numbers are decreased, and myeloid cell numbers are increased (Sudo et al., 2000). This lineage skewing toward myeloid cells may potentially underlie the increase in myeloid proliferative diseases seen with age (Ramos-Casals et al., 2003). Activation of noncanonical WNT signaling in aged HSCs has been linked to loss of cell polarity and elevated activity of actin remodeler CDC42. Inhibition of noncanonical WNT signaling or CDC42 activity restores polarity in aged HSCs and corrects lineage skewing by reducing myeloid cell production, along with improvements in engraftment of aged HSCs (Florian et al., 2012, 2013).
When HSCs are examined in aged mice, although their numbers are increased, their per-cell functional abilities in blood-forming reconstitution assays are decreased (Morrison et al., 1996; de Haan et al., 1997; Dykstra et al., 2011). With advanced age, there is clonal selection of HSCs, which reduces the overall functional diversity of HSCs and their output and increases the risk for hematologic cancers and other adverse outcomes (Cho et al., 2008; Beerman et al., 2010; Crews et al., 2016). Additionally, within the bone marrow of aged animals, adipocyte progenitors expand, and there is an increased accumulation of adipocytes with age, which has been linked to impairment of bone regeneration and altered hematopoiesis with age (Naveiras et al., 2009; Ambrosi et al., 2017). Recent studies show that adipocyte progenitors secrete stem cell factor and, thus, have a crucial role in the HSC bone marrow niche of young mice (Zhou et al., 2017). It will be interesting in the future to see whether the expansion of these progenitors in aging is a reflection of a rescue effort by the tissue or whether the aged adipocyte progenitors have declined in their ability to fuel HSCs.
Intestinal stem cells (ISCs)
Epithelial tissues also have high rates of turnover. The intestinal epithelium is one of the most proliferative, which turns over every 3 to 5 d in the mouse (Creamer et al., 1961). It has long been proposed that a population of stem cells located at the base of each crypt fuels intestinal turnover (Cheng and Leblond, 1974; Potten and Loeffler, 1990; Barker et al., 2007). Studies using lineage-tracing experiments have shown that LGR5, a transcription factor enriched in intestinal stem cells (ISCs), marks a proliferative population of crypt cells, which are bona fide stem cells, dividing on average once daily (Barker et al., 2007).
With age, the intestine shows architectural and functional changes. The number of crypts decreases and crypt area increases in aged animals (Martin et al., 1998a; Kozar et al., 2013; Nalapareddy et al., 2017). ISC proliferation decreases in aged (20–23 mo) compared with young (2–3 mo) animals. ISC numbers are, however, maintained at comparable levels in aged and young mice (Kozar et al., 2013; Nalapareddy et al., 2017). In vitro, ISCs from aged mice have less organoid-forming potential upon long-term passage. Canonical WNT signaling, which has an important role in ISC maintenance, can restore organoid formation efficiency to youthful levels, suggesting that the ISC niche may decline in its potency with age (Nalapareddy et al., 2017).
HFSCs
Several of the most striking and observable phenotypes associated with aging occur in another epithelial tissue, the skin. Increased wrinkling, loss of dermal elasticity, increased susceptibility to infection, thinning of the epidermis and dermis, and hair loss are all age-associated declines in skin function. Within murine skin lies a reservoir of infrequently cycling HFSCs that reside in a niche known as the bulge, located at the base of the noncycling portion of the hair follicle. When activated, HFSCs at the base of the bulge (hair germ) proliferate (a mean of four divisions per cycle) and produce short-lived, “transit-amplifying cells” that proliferate briefly and then progress to differentiate and drive hair production (Waghmare et al., 2008; Greco et al., 2009; Hsu et al., 2011; Yang et al., 2017). Aged hair shafts are morphologically normal, suggesting that the differentiation process remains intact for aged HFSCs (Keyes et al., 2013). With age, HFSCs are maintained at equivalent numbers compared with their younger counterparts (Giangreco et al., 2008; Doles et al., 2012; Keyes et al., 2013; Chen et al., 2014). However, in areas of aged skin in which severe hair loss is seen, HFSCs are reduced in numbers (Matsumura et al., 2016). Aged mouse HFSCs exhibit reduced colony formation and proliferation in vitro (Giangreco et al., 2008; Doles et al., 2012; Keyes et al., 2013) and are more resistant to activation in vivo (Keyes et al., 2013). Similar results have been observed in human skin cells, where keratinocytes from older individuals form fewer colonies and proliferate less when cultured (Barrandon and Green, 1987).
Melanocyte stem cells (McSCs) coreside with HFSCs in the bulge of hair follicles (Nishimura et al., 2002). Interestingly, HFSCs and McSCs undergo cross talk and respond similarly to niche cues so that they are coactivated as the hair cycle progresses to transfer pigment from differentiated melanocytes to the differentiating hair cells (Rabbani et al., 2011; Chang et al., 2013). Like HFSCs, McSC activity declines with age, resulting in fewer melanin deposits into the hair shaft and the increasing appearance of gray hairs (Nishimura et al., 2005). The increased appearance of gray hairs has been linked to DNA damage in McSCs (Inomata et al., 2009).
MuSCs
With age, muscle mass and function declines, resulting in a general decrease in mobility and loss of independence. In adult muscle tissue, Pax7 (a transcription factor enriched in MuSCs) expressing MuSCs (also known as satellite cells) reside at the interface of the basal lamina and the myofiber (Mauro, 1961; Snow, 1978). Activity of MuSCs is limited mainly to repair of muscle tissue because cellular turnover in muscle tissue is low, with the cellular life span of muscle fibers averaging >5,000 d in humans. MuSCs numbers decrease modestly in aged muscle tissue (approximately twofold; Bernet et al., 2014; Cosgrove et al., 2014; Sousa-Victor et al., 2014; Schwörer et al., 2016), and they have proliferative defects (Cosgrove et al., 2014), decreased colony formation (Schwörer et al., 2016) and proliferation in vitro and ex vivo (Sousa-Victor et al., 2014). In an in vivo label-retaining experiment, aged MuSCs were found to cycle more frequently. Loss of quiescence was driven by increased Fgf2 expression in the aged MuSC niche, promoting increased levels of proliferation in MuSCs, resulting in diminished self-renewal and depletion of MuSC pools (Chakkalakal et al., 2012). In transplantation assays, when MuSCs are purified and then engrafted into host muscle tissue, aged MuSCs show reduced levels of self-renewal and muscle fiber differentiation. Serial transplantation assays also reveal lower levels of self-renewal and proliferation in aged MuSCs and suggest the presence of cell intrinsic defects in MuSCs (Cosgrove et al., 2014). Reduced levels of intracellular nicotinamide adenine dinucleotide have a role in age-related changes in MuSC behavior because nicotinamide riboside treatment increases Pax7-expressing MuSCs numbers and decreases signs of DNA damage (Zhang et al., 2016).
NSCs
Neurons are one of the longest-lived cell types in the mammalian body, most lasting the entire life span of the organism. Neurodegenerative conditions, dementia, Alzheimer’s disease, and impaired learning, memory, and other cognitive functions are associated with advancing age (Wyss-Coray, 2016). Non-disease–associated structural changes occur in the nervous system with age, such as the loss of neurons and total brain volume reduction (Svennerholm et al., 1997; Andrews-Hanna et al., 2007). Previously believed to be completely static as far as new cellularity, it is now well established that neurogenesis occurs in the adult mammalian brain (Altman and Das, 1965; Palmer et al., 1997; Eriksson et al., 1998; Gonçalves et al., 2016). Within the subventricular zone of the lateral ventricles and subgranular zone of the hippocampus, a population of NSCs is responsible for adult neurogenesis, which produces new glial cells and neurons (Doetsch et al., 1999; van Praag et al., 2002; Spalding et al., 2013). In aged mice, NSCs decrease in number, and in turn, neurogenesis is diminished (Kuhn et al., 1996; Maslov et al., 2004). NSCs from aged mice have an approximately threefold reduction in proliferation rate (Molofsky et al., 2006). NSCs isolated from aged (26 mo old) animals have a twofold reduced neurosphere production in vitro compared with young (4.5 mo old) animals and a twofold reduction in serial passage ability, a measurement of self-renewal potential (Maslov et al., 2004). Aged NSC differentiation potential in vitro remains largely similar to young NSCs, suggesting that cell extrinsic factors have a large part in their altered behavior (Ahlenius et al., 2009; Silva-Vargas et al., 2016).
Aged stem cells in repair and regeneration
In the absence of injury, tissue-resident stem cells have their natural rates of proliferation to support tissue homeostasis. In response to injury; however, a more-robust response of stem cells is needed. After acute injury, stem cells must both self-renew to reestablish their numbers and differentiate to regenerate tissue by replacing lost cells. Age-dependent declines in repair and regeneration responses have been linked to decreased stem cell activation, declines in their numbers are linked to dysfunction in their lineage potential and altered signaling in the niche/microenvironment.
HSCs
The regenerative potential of HSCs can be measured using bone marrow transplantation assays, testing both their self-renewal and differentiation capacity. Recipient mice whose bone marrow has been lethally irradiated are used as a site for engraftment of HSCs from a donor mouse. This assay challenges transplanted HSCs to completely reconstitute the hematopoietic blood lineages and reestablish their numbers in the bone marrow and mimics a wound-healing scenario, rather than a normal, homeostatic response (Till and McCulloch, 1961; Iscove and Nawa, 1997).
HSCs from aged animals have been tested in these assays relative to their younger counterparts. On an individual cell basis, aged HSCs underperform when compared with young HSCs, with decreased engraftment and reconstitution potential in transplantation assays (Morrison et al., 1996; Sudo et al., 2000; Rossi et al., 2005). Serial transplantation further tests HSC functions by pushing their long-term, multilineage potential by using successive transplantations into irradiated hosts. Aged HSCs exhibit lower capacity in long-term, multilineage reconstitution assays and produced altered ratios of downstream, committed progenitors, making more granulocyte macrophage progenitor cells and fewer common lymphoid progenitor cells, hinting that intrinsic alterations at the gene-expression level drive lineage skewing in aged HSCs (Rossi et al., 2005). Although aged HSCs have worse performance than young HSCs in these long-term assays, their regenerative potential still outlasts the life span of the organism.
HSCs, as well as mesenchymal stem cells, endothelial progenitor cells, and very small embryonic-like cells from bone marrow, migrate to sites of injury, including skin burns, muscle, bone, liver, and stroke in the brain (Orlic et al., 2001; Balsam et al., 2004; Rennert et al., 2012). The details of whether and how these cells participate in, and contribute to, the repair process in these tissues are still being investigated.
HFSCs
Aged skin shows a pronounced decline in regenerative potential, with aged HFSCs less likely to respond to activation cues for hair growth cycles. Each hair follicle undergoes naturally occurring cycles of regeneration and hair growth that are fueled by HFSC activity (Chase and Eaton, 1959; Cotsarelis et al., 1990; Tumbar et al., 2004). During each hair cycle, the lower two thirds of the hair follicle is regenerated by short-term progenitors from HFSCs that rapidly divide and differentiate into the layers of the mature hair follicle. This active phase of hair production lasts ∼3 wk in mice; after which, the bottom portion of the hair follicle enters a destructive phase, sparing only the HFSCs (Fuchs, 2007).
With age, hair growth becomes more asynchronous, the domains are more fragmented, and the intervals between hair growth expand over time (Keyes et al., 2013). Serial activation of HFSCs in aged animals, by repeated depilation, causes HFSC numbers in the hair follicle to diminish, and hair growth to become sparse (Keyes et al., 2013). These results are mimicked by conditional ablation of Nfatc1 or Foxc1, where hair follicles spend almost no time in the resting phase between hair cycles (Lay et al., 2016; Wang et al., 2016). Collectively, these findings raise the tantalizing possibility that, when challenged constantly with hair production, HFSCs may not have an endless capacity.
MuSCs
MuSCs are almost exclusively dedicated to repair processes in muscle tissue. When activated by stress or damage, MuSCs exit G0 and proliferate to repair injured muscle. Injury models use injection of cardiotoxin or other chemical agents into the tibialis anterior muscle to produce a lesion that results in activation of MuSCs, which produce myogenic progenitors that, in turn, yield new myofibers. In aged muscle tissue, the capacity for injury repair is reduced, with less myofiber regeneration after injury (Conboy et al., 2005; Brack et al., 2007; Cosgrove et al., 2014) and aberrant differentiation into fibrotic tissue (Brack et al., 2007). The increase in fibrotic tissue formation after injury was linked to elevated levels of canonical WNT signaling, paralleling findings in aged HSCs with altered WNT signaling. In aged animals, MuSC activation after injury is diminished in vivo, as well as after transplantation into young hosts (Liu et al., 2013; Sousa-Victor et al., 2014). New muscle fibers that arise during repair after an injury in aged animals are also smaller, not achieving the same size found in young animals undergoing a similar injury (Chakkalakal et al., 2012).
Reduced performance of aged MuSCs in regeneration assays is not explained by a reduction in numbers alone. Two thirds of aged MuSCs on a per-cell basis have been shown to be incompetent for repair in regeneration assays (Cosgrove et al., 2014). A recent study has also found muscle tissue in aged animals fails to recruit sufficient muscle regulatory T cells, which also aid in the repair of damaged muscle tissue, to the site of injury (Kuswanto et al., 2016). Aged animals treated with nicotinamide riboside showed improved mitochondrial and MuSC function, leading to gains in both engraftment and repair of muscle (Zhang et al., 2016). Additionally, reduced repair in aged muscle can, in part, be a result of increased expression of Hoxa9 in aged MuSCs. Hoxa9, a transcription factor expressed during development, promotes WNT, TGF-β, and JAK-STAT signaling pathways in MuSCs, known inhibitors of muscle regeneration (Brack and Rando, 2012). Consistently, MuSCs from aged animals perform better in self-renewal and muscle regeneration assays after Hoxa9 knockdown (Schwörer et al., 2016).
ISCs and NSCs
The roles of aging ISCs and NSCs in repair processes have been studied less extensively. After exposure to low doses of irradiation, apoptosis in aged (29 mo old) intestine is increased twofold compared with young and middle-aged (5 mo and 15–18 mo) animals (Martin et al., 1998a,b). High levels of irradiation result in less crypt survival in aged animals. Crypts that do survive in aged animals show similar rates of cell replacement but, rather, are delayed in their response to injury, suggesting that their response to injury is altered (Nalapareddy et al., 2017). The mammalian central nervous system shows very limited ability to regenerate after injury. NSCs have been shown to proliferate in areas that suffer ischemia (Arvidsson et al., 2002) and traumatic injury (Chirumamilla et al., 2002). Neurodegenerative damage may also stimulate NSC proliferation (Donovan et al., 2006). However, how those responses to injury change with age has yet to be explored.
Transcriptional profiling of stem cell populations
What are the molecular underpinnings that enable adult stem cell populations to achieve their unique properties? With the identification of stem cells within adult tissues in vivo and subsequent development of purification strategies using cell-surface antigens, coupled with FACS, early studies of stem cells sought to understand the genetic programs that underlie how stem cells achieve self-renewal and multipotency at the transcriptional level. By focusing on genes expressed commonly among different stem cell populations, it was hypothesized that a universal genetic basis of “stemness” (self-renewal and lineage differentiation) of those cells would be elucidated on a molecular level.
To define the genetic program for stemness, multiple groups used microarrays to transcriptionally profile and compare mRNAs purified from mouse embryonic stem cells (ESCs), NSCs, and HSCs. Initially those mRNA transcripts were prefiltered to include only transcripts that were enriched in each stem cell population relative to their own downstream progeny. Once enriched, those genes were then compared with each other to identify shared genes or programs that could confer stemness. Three independent studies approached that question. One study found 283 transcripts commonly expressed in HSCs, ESCs, and NSCs (Ivanova et al., 2002), whereas another study found 216 transcripts were enriched in the same three populations of stem cells (Ramalho-Santos et al., 2002). These commonly shared genes have roles in transcriptional regulation, DNA repair, signaling, cell cycle regulation, translation, protein folding, ubiquitination, vesicle trafficking, and stress response. Finally, an additional study searching for transcripts enriched in ESCs, neural precursor cells (NPCs) and retinal progenitor/stem cells identified 385 commonly enriched transcripts (Fortunel et al., 2003a).
Unexpectedly, a cross comparison of those three studies revealed very little overlap among transcripts commonly expressed in stem cells. Although comparisons of identical stem cell population (i.e., NPCs versus NPCs and ESCs versus ESCs) profiles had significant overlap, comparisons of jointly expressed transcripts in NSCs and ESCs from all three studies yielded only nine overlapping genes. When taken together, the stem cell populations (ESCs, NPCs, HSCs, and retinal progenitor/stem cells) from each study, only 1 gene (Itga6) was found to be expressed in all stem cell populations examined (Fortunel et al., 2003a). Although genes that confer stemness may be transiently expressed or not captured under those conditions in those populations, those analyses of transcriptional profiles suggest that stem cells are defined by their functional abilities or heavily influenced by their microenvironment and, rather than a core set of expressed genes, use a genetic signature that is tissue specific. Indeed, increasing evidence suggests that the stem cell niche has a powerful role in dictating the molecular properties of stem cells (Xie and Spradling, 1998; Calvi et al., 2003; Adams et al., 2006; Morrison and Spradling, 2008; Hsu et al., 2011).
Transcriptional profiling of aged stem cells
Like the researchers who sought to identify the signature of stemness, researchers today are focusing on gaining a better understanding of the intrinsic molecular changes in stem cells in aged tissues. Furthermore, these signatures have shed light on the genetic programs adult stem cells use to achieve self-renewal and multipotency.
HSC profiling
Aged HSCs were the first to be profiled, and as isolation methods have been refined progressively toward purer populations of HSCs, our understanding of the changes that occur in HSCs over time has increased. Initial work profiling young and aged HSCs with microarrays identified ∼900 genes differentially expressed between young (2–3 mo old) and aged (22–24 mo old) mouse HSCs, with Gene Ontology (GO) terms enriched in signal transducer activity and receptor activity (Rossi et al., 2005). A second group isolated HSCs (using c-kit+, LIN−, Sca1+ [KLS]) from young (2 mo old) and aged (21 mo old) animals and reported ∼1,600 up-regulated and ∼1,500 down-regulated genes in aged HSCs (Chambers et al., 2007). Terms enriched in up-regulated genes were NO-mediated signaling transduction, stress response (protein folding), and inflammatory response. Terms enriched in down-regulated genes were genomic integrity, chromatin remodeling and chromatin repair. Isolation of HSCs from 6 and 12 mo old mice suggested that changes in NO-mediated signaling arising first, followed by stress response genes and then inflammation (Chambers et al., 2007).
A more-recent study using RNAseq to quantify age-related transcriptional changes used KLS and CD150+ markers to purify HSCs from young (4 mo old) and aged (24 mo old) animals. They found ∼1,340 genes up-regulated and ∼1,300 genes down-regulated in aged HSCs (Sun et al., 2014). Up-regulated genes had GO annotations involved in cell adhesion and glycoproteins, as well as in ribosomal function. Down-regulated genes had GO annotations involved in DNA repair, DNA replication, and cell cycle. Strikingly, the authors found genes known to have a role in HSC self-renewal were up-regulated, which may underlie the observed expansion of HSCs with age. Further analysis revealed that TGF-β1 targets were being significantly altered, suggesting TGF-β1 could be a potential upstream regulator of the changes observed with age. The authors also observed an expansion of histone H3 lysine 4 trimethylation in aged HSCs; those areas of expansion were correlated with transcripts involved in self-renewal, which reinforce phenotypic observations made in aged HSCs.
Additional changes in chromatin regulators have been observed in aged HSCs. Expression of the histone deacetylase Sirt7 is reduced in aged HSCs. Loss-of-function studies revealed increased proliferation in Sirt7−/− HSCs and reduced performance in long-term reconstitution assays; furthermore, restoration of youthful levels of Sirt7 expression in aged HSCs reversed their age-associated myeloid-bias in differentiation (Mohrin et al., 2015).
Single-cell RNAseq has also been used to profile aged HSCs. More than 400 individual HSCs from young (2–3 mo old) and aged (22 mo old) from C57BL6 animals were purified (KLS, CD150+, and CD48− SLAM markers) and used for single-cell RNAseq (Kowalczyk et al., 2015). Principal component analyses of expression profiles identified GO terms involved in cell cycle as the major feature of transcriptional variation between young and aged HSCs. Further analysis showed fewer aged HSCs are engaged in the G1/S phase of the cell cycle. These findings were replicated in DBA/2 mice, showing similar trends in transcriptional profiles in young and aged HSCs, with a twofold expansion of HSCs with age in the DBA/2 genetic background. In cells in which the cell cycle profile was not the dominant signature, an inverse relationship between aging and differentiation was uncovered. Young HSCs could be distinguished from aged HSCs by their more differentiated state, being more similar to aged HSC progenitors (Kowalczyk et al., 2015). Those data also revealed population-wide shifts in transcriptional state, with a large number of genes changing expression with age.
HFSC profiling
In aged skin, isolation of HFSCs from young (3 mo old) versus aged (18 mo old) from Krt15-GFP (to label basal layer keratinocytes) transgenic animals, in addition to selection for CD34+ integrin-α6+ (expression enriched in HFSCs) cells, found ∼2,500 transcripts differentially age-regulated in HFSCs (Doles et al., 2012). Transcripts were enriched in metabolic, protein modification, chromatin modification, and regulation of gene expression processes. Terms enriched in down-regulated genes included response to infection, response to biotic stimulus, keratinocyte differentiation, and regulation of lymphocyte activation. Following up on the presence of inflammation signatures, JAK-STAT and NOTCH signaling pathways were found to be elevated in aged HFSCs, along with increased expression of cytokines in the epidermis. Experiments using JAK and NOTCH pharmacological inhibitors were able to stimulate aged HFSC growth in vitro, enhancing aged HFSC proliferative activity (Doles et al., 2012). Elevated JAK-STAT signaling has also been observed in aged MuSCs, and treatment with inhibitors of JAK-STAT signaling improves aged MuSC self-renewal in in vitro engraftment and muscle repair (Price et al., 2014).
In another study, RNAseq of quiescent HFSCs using CD34+ integrin-α6+ and Sca1− markers from young (2–4 mo old) and aged (22–24 mo old) revealed 725 transcripts differentially regulated by age (Keyes et al., 2013). Up-regulated transcripts were enriched for keratinocyte differentiation, immune system function, response to hypoxia, epidermis development, and locomotory behavior. Down-regulated transcripts were enriched for processes in fatty acid biosynthetic process, response to peptide hormone, wound healing, extracellular matrix organization, and mitotic nuclear division.
RNAseq of young and aged HFSCs after activation of hair growth revealed more extensive changes in the transcriptional response, with aged HFSCs less transcriptionally dynamic. NFATc1, a regulator of HFSC quiescence, levels were elevated in aged hair follicles, keeping them dormant during periods in which young follicles were cycling (Keyes et al., 2013). Up-regulation of stem cell quiescence regulators explains, in part, the reduced activity of aged HFSCs in proliferative hair cycles. Inhibition of NFATc1 activity in aged skin led to higher levels of colony formation and proliferation in HFSCs isolated from treated animals (Keyes et al., 2013). Changes within the local environment also heavily influence HFSC quiescent behavior because activators of hair regeneration (e.g., follistatin) decrease with age and inhibitors of that process (e.g., Bmp2, Dkk1, and Sfrp4) increase in the surrounding tissue with age (Chen et al., 2014).
ISC profiling
In the intestine, purification of LGR5-GFP+ ISCs from aged (20–23 mo old) and young (2–3 mo old) mice has been profiled by RNAseq (Nalapareddy et al., 2017). Approximately 750 genes were found to be ±1.5-fold regulated in aged ISCs. GO term analysis of those genes found genes involved in immune response, exocytosis, and cell differentiation up-regulated in aged ISCs. Among down-regulated processes in aged ISCs were cell proliferation, extracellular matrix proteins, PPAR and SMAD signaling, and WNT signaling pathways. Critically, Ascl2, a downstream target of WNT signaling in ISCs, was found to be down-regulated in aged ISCs. Ascl2 regulates stem cell fate in ISCs, and its altered expression may account of the increased production of goblet cells seen in aged intestines (Schuijers et al., 2015; Yan and Kuo, 2015).
MuSC profiling
Transcriptional profiling of integrin-α7+ CD34+ MuSCs from muscles of young (2–3 mo old), aged (20–24 mo old), and geriatric (38–32 mo old) animals identified ∼530 differentially regulated transcripts (Sousa-Victor et al., 2014). Expression of polycomb complexes 1 and 2 was increased in geriatric MuSCs. Interestingly, the senescence-associated gene p16INK4A was found to be up-regulated in geriatric MuSCs, suggesting a move of quiescent G0 arrested MuSCs into a more-senescent state. However, that state was shown to be reversible because inhibition of p16 resulted in increased repair activity of geriatric MuSCs. Increased expression of p16INK4A and p21CIP1 in MuSCs was been observed in another independent study (Cosgrove et al., 2014).
Additional analysis of quiescent MuSCs from young (2–3 mo old) and aged (22–24 mo old) animals used VCAM+ cells to purify stem cells from muscle fibers (Liu et al., 2013). Among the ∼330 differentially expressed transcripts GO terms were enriched for cell division, cell cycle, notch signaling chemokine signaling, cytokine, chemotaxis, immune response, and cell migration. The authors also observed a broad increase of H3K27me3 across the genome in aged MuSCs. Another recent study profiling aged MuSCs used syndecan-4+ to isolated MuSCs from young (3–6 mo old) and aged (20–25 mo old) animals (Bernet et al., 2014). The study found ∼850 age-regulated transcripts, with GO terms associated with asymmetric cell division, cell growth, and cell differentiation, with reduced expression in aged MuSCs.
Asymmetric cell divisions, marked by phosphorylated-p38, in aged MuSCs were reduced by half. Furthermore, engraftment of aged MuSCs into donor muscle resulted in a reduction of their numbers 30 d after transplantation. Declines in self-renewal were attributed to alterations in fibroblast growth factor signaling via p38α and p38β MAPK signaling in aged MuSCs. Increased p38α/β MAPK signaling in aged MuSCs and impaired response to fibroblast growth factor ligands were also observed. Testing the functional consequences of those changes, inhibition of MAPK activity was able to rescue the self-renewal MuSCs from aged mice. Altered p38α/β MAPK signaling in aged MuSCs has also been implicated in the poor ability to repair myofibers after muscle injury (Cosgrove et al., 2014).
Stem cell aging signature
Is there a common age-related signature that can be identified from these profiling studies? Although transcriptional profiling methodologies have identified numerous genes with altered expression in the course of stem cell aging, identifying overarching trends remains a challenge. Differences in the ages of animals, the purification strategies, and the analysis used for all those transcriptional profiling studies, as well as recognized batch effects, may have contributed to differences seen in age-regulated genes (Table 2; Leek et al., 2010). Comparison of the GO terms enriched in those data sets provides a broader view of the molecular changes occurring in aged stem cells.
Table 2. Isolation of aged stem cells and transcriptional profile analysis.
| Population | Age groups | Genetic background | Tissue processing | Purification/selection scheme | Platform | Analysis | Differential expression cutoff,change | Accession no. | Study |
|---|---|---|---|---|---|---|---|---|---|
| mo | |||||||||
| HSCs | Young: 2–3; Aged: 22–24 | C57BL/6 | Whole bone marrow; Dynabeads for lineage depilation; depleted cells were enriched for c-Kit by magnetic bead purification; followed by FACS, double-sorted | KLS; Flk2−; CD34−; LIN− = D3, CD4, CD5, CD8, IL7R, B220, Ter119, Gr1, Mac1 | Affymetrix Mouse Genome 430, 2.0 Array | dCHIP software for normalization; PM/MM model for calculating of expression values. Expression data were analyzed by using microarray software for statistical analysis | ±1.3-fold; FDR ∼10% | GSE4332 | Rossi et al., 2005 |
| Young: 2; Intermediate: 6; Intermediate: 12; Aged: 21 | C57BL/6 males | Whole bone marrow; Sca-1 enrichment with Miltenyi beads; followed by FACS | KLS + Hoechst 33342 efflux LIN− = Mac-1,Gr-1, CD4, CD8, B220, Ter119 | Affymetrix Mouse Expression 430A Array | GC-RMA LIMMA linear contrast modeling to determine differentially expressed genes | ±2-fold; P < 0.05 | GSE6503 | Chambers et al., 2007 | |
| Young: 4; Aged: 24 | C57BL/6 males | Whole bone marrow cells were isolated from femurs, tibias, pelvis, and humerus; cells were magnetically enriched for c-Kit expression, antibiotin microbeads for lineage depletion, followed by FACS | SP-KLS + Hoechst 33342 efflux, CD150+ LIN− = Mac1, Gr1, CD4, CD8, CD3, B220, Ter119 | Illumina HiSeq 2000; paired-end sequencing | Bowtie alignment of RNAseq reads, Blat for additional read mapping. DESeq for differential expression analysis. The alignment was performed using RUM-UCSC known genes | ±1.2-fold; Q < 0.05 | GSE47819 | Sun et al., 2014 | |
| HFSCs | Young: 3; Aged: 18 | B6SJL/F1 | Dorsal back skin; trypsinized and epidermal cells were removed and made into single-cell suspension; FACS-sorted cells were collected in keratinocyte medium supplemented to 50% FBS. | CD34+ integrin-α6+ Krt15-GFP+ viability (DAPI) | Illumina HiSeq 2000 | TopHat alignment of RNAseq reads. Cuffdiff tool from the Cufflinks package for differential expression analysis. Final transcript levels of all mouse Ensembl-known genes | ±2-fold; Q < 0.05 | N/A | Doles et al., 2012 |
| Young: 2–4; Aged: 22–24 | C57BL/6 males | Dorsal back skin; trypsinized and epidermal cells were removed and made into single-cell suspension; FACS-sorted cells were collected in TRIzol | CD34+ integrin-α6+ Sca1− viability (DAPI) | Illumina HiSeq 2000 | TopHat alignment of RNAseq reads. Cufflinks package for differential expression analysis. RefSeq known genes guided assembly. Analysis was performed using cummeRbund software | ±1.5-fold; Q < 0.05 | GSE74283 | Keyes et al., 2013 | |
| ISCs | Young: 2–3; Aged: 22–23 | Lgr5-eGFP-IRES-CreERT2 C57BL6/SV129 males | Proximal small-intestine crypts; 4% TrypLE treatment to make single-cell suspension; followed by FACS | LGR5-GFP+ | Illumina HiSeq 2500; paired-end reads | Strand NGS (Agilent Technologies) DESeq for differential expression analysis using UCSC annotation | ±1.5-fold; P < 0.05 | GSE84061 | Nalapareddy et al., 2017 |
| MuSCs | Young: 2–3; Aged: 20–24; Geriatric: 32–38 | C57BL/6 males | Resting mouse muscle tissue; mechanical disaggregation, collagenase, and trypsin treatment, followed by FACS | Integrin-α7+ CD34+ LIN− = CD31−, CD45−, Sca1−, Mac1− | Agilent SurePrint G3 Mouse GE 8 × 60K high density microarray slides | AFM 4.0 software; Genesis software for cluster analysis of microarray data | ±1.5-fold; adjusted, P < 0.05 | GSE53728 | Sousa-Victor et al., 2014 |
| Young: 2–3; Aged: 22–24 | C57BL/6 males | Hind limb muscle; minced, collagenase II and dispase treated; cells passed though 20-gauge needle to make single-cell suspension, followed by FACS | VCAM+, CD31−, CD45−, Sca1− | Affymetrix Mouse Gene 1.0 ST arrays | Data were analyzed with the Affymetrix Expression Console software using Affymetrix default RMA analysis settings | ±2-fold; adjusted, P < 0.05 | GSE47177 | Liu et al., 2013 | |
| Young: 3–6; Aged: 20–25 | C57BL/6 females | Mice hind limb muscle; minced, collagenase treated, centrifuged to remove undigested tissue, followed by FACS | Syndecan-4+ | Affymetrix Mouse Genome 430, 2.0 Array | GeneSpring 12.5 (Agilent Technologies) software | ±2-fold; P < 0.05 | GSE47104 | Bernet et al., 2014 |
AFM, Array File Maker; Blat, BLAST-Like Alignment Tool; dCHIP, DNA–Chip Analyzer; FDR, false-discovery rate; MM, mismatch; N/A, not available; NGS, next-generation sequencing; PM, perfect match; RMA, Robust Multiarray Average; RUM, RNA-Seq Unified Mapper; UCSC, University of California, Santa Cruz. CummeRbund, DESeq, LIMMA, and GC-RMA are packages for Bioconductor software.
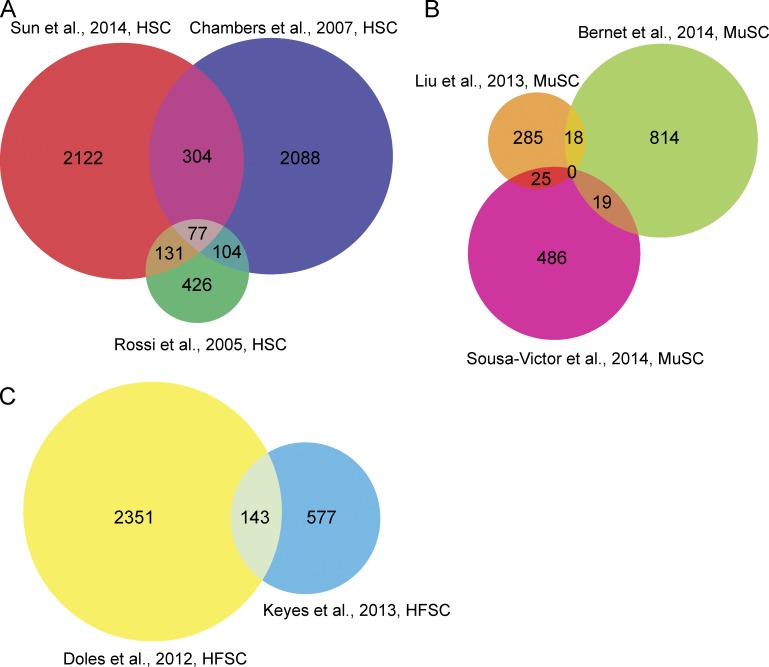
Comparison of three profiling experiments with aged HSCs yielded 77 transcripts that were shared among all three studies (Fig. 2 A). Those genes were enriched positive regulation of inflammatory response (Cd47, S100a8, S100a9, Ccl4, and Ccl6), TGF-β receptor signaling pathway (Ptprk, Itgb5, Tgfb1, and Fos), blood vessel development (Mef2c, Bmp4, Hif1a, Foxo1, and Tbx1), integrin-mediated signaling pathway (Fgr, ItgB2-6, and Lama5), and cell migration (Ptprk, Itga4, Mmp14, Cd151, Mmp2, TgfB1, Hes1, and Adam9). When comparing GO terms identified in each profiling experiment, 28 terms were shared among all three studies. In addition to those processes, genes involved in protein phosphorylation, apoptosis, regulation of transcription, cell proliferation, cell adhesion, response to hypoxia, and immune system processes were also enriched. Several of those 77 genes already have established functions in HSC biology. Mef2c, a transcription factor, regulates the suppressor of cytokine signaling 2 (Socs2), which is necessary to promote lymphoid cell development and suppress myeloid lineage differentiation (Vitali et al., 2015). Jam2 is a junctional adhesion molecule that regulates the interaction of HSCs and bone marrow stromal cells; loss of those interactions results in a decrease in quiescence in HSCs (Arcangeli et al., 2011). Expression of Alcam, which has a role in HSC differentiation and self-renewal was also found to be altered, with increased Alcam expression resulting in reduced long-term passaging in vitro and a decreased engraftment potential (Jeannet et al., 2013). Finally, Pbx1 expression was altered in aged HSCs; loss of Pbx1 function in HSCs leads to reduced quiescence and self-renewal in transplantation assays (Ficara et al., 2008).
Figure 2.
Comparison of differentially expressed genes in aged stem cell populations. Venn diagrams of age-regulated genes from studies indicated for HSCs (A), MuSCs (B), and HFSCs (C). Age-regulated genes (∼2 mo old vs. ∼24 mo old) from profiles of quiescent stem cells were used in these comparisons.
Many (20%) of the transcripts found to be differentially regulated in aged HFSCs were shared in the two studies (Fig. 2 B). They are enriched in processes involved in keratinocyte differentiation or keratinization (late cornified envelope genes Lce1g, Lce1h, Lcef, Lce1a1, Lce1b, Lce1m, Lce1a2, Lce1l, and Sprr1b), epidermal development (Lor and Cnfn), and cell differentiation (Bmp3, Eya2, Sfrp2, Npnt, and Dlk1), suggesting that HFSCs may be differentiating with age, as suggested by Matsumura et al. (2016). GO terms for cell cycle (mitotic nuclear division, negative regulation of cell growth, chromosome segregation), apoptosis, antigen processing, metabolism (fatty acid and lipid), response to hypoxia, angiogenesis, response to virus, and the oxidation-reduction process were also found to be enriched. Several of the genes differentially regulated in aged HFSCs have described roles in HFSC function. Tcf7l1 (encoding TCF3, a DNA binding partner of WNT/β-catenin signaling) has been directly implicated in the maintenance of HFSCs and repression of WNT-regulated lineage genes. Loss of Tcf7l1 promotes proliferation of HFSCs and their commitment to differentiate, whereas elevated TCF3 represses hair follicle differentiation (Lien et al., 2014). Fgf18, a key signaling gene also found to be age-regulated, contributes to the timing of quiescence in HFSCs. Foxp1, an up-stream regulator of Fgf18 expression and cyclin-dependent kinase inhibitor p57KIP2 (Cdkn1c; Leishman et al., 2013), were also age-regulated in both studies.
Surprisingly, comparison of aged MuSC profiles did not reveal any genes in common in all three studies (Fig. 2 C). However, several transcripts, albeit a small proportion, were common in at least two studies. That is most likely a reflection of the different markers used to isolate MuSCs in each study and the different ages of young and aged mice used (Table 2). The common genes, taken together, were enriched for GO terms in cartilage development (Prrx1, Zbtb16, Bmpr1b), response to peptide hormone (Serpina3n and Atp2a1), and muscle contraction (Anxa1, Scn7a, and Actn3). Genes differentially regulated in MuSCs share functions in the regulation of apoptosis, oxidation reduction, neutrophil chemotaxis, wound healing, and protein localization to the nucleus. Given that MuSCs primary defects are in injury repair, genes with functions in apoptosis and wound healing are particularly relevant. Anxa1 has been described as having a role in myoblast differentiation. Its inhibition results in decreased myotube formation upon injury in muscle tissue (Bizzarro et al., 2010). Additionally, both age-regulated Mest and Bex1 have roles in regulating muscle regeneration after injury (Koo et al., 2007; Hiramuki et al., 2015).
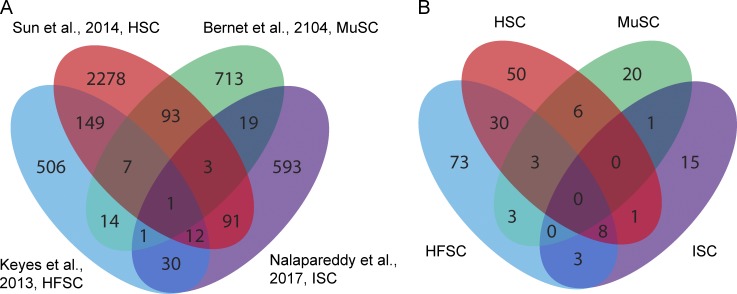
Cross-comparison of studies using RNAseq to profile aged HSCs, HFSCs, and ISCs with MuSCs uncovered varying levels of overlapping transcripts that were age regulated (Fig. 3 A) among those stem cells. The largest pairwise overlap was observed between HSCs and HFSCs, sharing 169 genes in common. Overlap of gene signatures between HFSCs–HSCs and HFSCs–ISCs were statistically significant, but when data from all four aging populations were compared, the level of overlap dropped to below statistical significance. Only one transcript was found to be shared among those four studies (Mef2c). Given that young stem cell profiles also shared little to no overlap in gene expression (Fortunel et al., 2003b), the lack of commonality in the aging stem cell signatures is, perhaps, not surprising.
Figure 3.
Comparison of differentially expressed genes in various aged stem cell populations. (A) Venn diagram showing overlap of age-regulated transcripts in HSCs (red) MuSCs (green), HFSCs (blue), and ISCs (purple) from the studies indicated. HFSC–HSC gene set overlap, P < 0.0001; HFSC-ISC overlap, P = 0.003 by χ2 test. (B) Venn diagram showing overlap in GO terms enriched in gene sets from A.
GO terms enriched in those populations (Fig. 3 B) showed similar trends, with no single ontology term shared among all four studies. However, HSCs and MuSCs shared terms enriched in cell division, phosphorylation, intracellular signal transduction, and transcription regulation, whereas oxidation reduction, fatty acid metabolism, and metabolic processes were commonly found among genes in HFSCs and MuSCs. In addition, HSCs and HFSCs shared several processes in cell proliferation, immune response, angiogenesis, cell migration (chemotaxis, axon guidance, cell–cell adhesion), cell differentiation, regulation of cell shape, developmental processes, apoptotic process, extracellular matrix organization, and response to TNF.
Many of the unique GO processes are indicative of the tissue type the stem cell population came from because keratinocyte and pigmentation process are found only in aged HFSCs, muscle-contraction processes in aged MuSCs, and, interestingly, many terms associated with DDR in aged HSCs. Although there was no specific gene or process uncovered from those comparisons, there were GO terms associated with immune response, hypoxia, cell proliferation, and apoptosis that repeatedly appeared in those profiling studies, suggesting that they may be core processes altered in aged stem cells. Determining which expression changes occur first in those stem cell populations may lead to a better understanding of events that drive the stem cell aging process.
Prospectus
Given their long life span and their position at the top of the tissue hierarchy, stem cells are prime targets for the aging process. Stem cells can behave as integration sites for many of the mechanisms that drive aging. Problems in proteostasis, mitochondrial dysfunction, and DNA damage can be passed down to progeny. Those changes can alter tissue physiology, resulting in age-related phenotypes.
Identifying root causes of stem cell dysfunction with age still remains the most challenging hurdle to overcome. Much like driver mutations in cancer cells, identification of transcriptional changes in aged stem cells that drive phenotypic changes will be of critical importance. Whether transcriptional changes are a result of altered signaling within the niche or of intrinsic changes will be equally difficult to distinguish. Realistically, a combination of both of those processes seems most likely. Surprisingly, only in MuSCs was the up-regulation of p16INK4A observed by transcriptional profiling. Although p16INK4A has been commonly used as a marker for aging, it may not have a major role in stem cell aging. Comparison of aged stem cell transcriptome profiles of the same cell type yielded several genes with known roles in self-renewal and differentiation properties for that cell type. It will be intriguing to investigate whether aging studies may be an inroad to finding genes that are critical for stem cell function.
Acknowledgments
We would like to thank M. Mohrin, C.-Y. Chang, K. Lay, Y. Ge, S. Liu, R. Adam, and D. Gottschling for critical reading of the manuscript and helpful discussions.
E. Fuchs is an investigator of the Howard Hughes Medical Institute and was the recipient of an Ellison Senior Scholar award on aging. Her research on stem cells and aging has also been supported by the National Institutes of Health (grant R01-AR050452).
The authors declare no competing financial interests.
Footnotes
Abbreviations used:
- DDR
- DNA damage response
- ESC
- embryonic stem cell
- GO
- Gene Ontology
- HFSC
- hair follicle stem cell
- HSC
- hematopoietic stem cell
- ISC
- intestinal stem cell
- McSc
- melanocyte stem cell
- MuSC
- muscle stem cell
- NPC
- neural precursor cell
- NSC
- neural stem cell
References
- Adams G.B., Chabner K.T., Alley I.R., Olson D.P., Szczepiorkowski Z.M., Poznansky M.C., Kos C.H., Pollak M.R., Brown E.M., and Scadden D.T.. 2006. Stem cell engraftment at the endosteal niche is specified by the calcium-sensing receptor. Nature. 439:599–603. 10.1038/nature04247 [DOI] [PubMed] [Google Scholar]
- Ahlenius H., Visan V., Kokaia M., Lindvall O., and Kokaia Z.. 2009. Neural stem and progenitor cells retain their potential for proliferation and differentiation into functional neurons despite lower number in aged brain. J. Neurosci. 29:4408–4419. 10.1523/JNEUROSCI.6003-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman J., and Das G.D.. 1965. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J. Comp. Neurol. 124:319–335. 10.1002/cne.901240303 [DOI] [PubMed] [Google Scholar]
- Ambrosi T.H., Scialdone A., Graja A., Gohlke S., Jank A.-M., Bocian C., Woelk L., Fan H., Logan D.W., Schürmann A., et al. 2017. Adipocyte Accumulation in the Bone Marrow during Obesity and Aging Impairs Stem Cell-Based Hematopoietic and Bone Regeneration. Cell Stem Cell. 20:771.e6–784.e6. 10.1016/j.stem.2017.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna J.R., Snyder A.Z., Vincent J.L., Lustig C., Head D., Raichle M.E., and Buckner R.L.. 2007. Disruption of large-scale brain systems in advanced aging. Neuron. 56:924–935. 10.1016/j.neuron.2007.10.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcangeli M.-L., Frontera V., Bardin F., Obrados E., Adams S., Chabannon C., Schiff C., Mancini S.J.C., Adams R.H., and Aurrand-Lions M.. 2011. JAM-B regulates maintenance of hematopoietic stem cells in the bone marrow. Blood. 118:4609–4619. 10.1182/blood-2010-12-323972 [DOI] [PubMed] [Google Scholar]
- Arvidsson A., Collin T., Kirik D., Kokaia Z., and Lindvall O.. 2002. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat. Med. 8:963–970. 10.1038/nm747 [DOI] [PubMed] [Google Scholar]
- Balsam L.B., Wagers A.J., Christensen J.L., Kofidis T., Weissman I.L., and Robbins R.C.. 2004. Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature. 428:668–673. 10.1038/nature02460 [DOI] [PubMed] [Google Scholar]
- Barker N., van Es J.H., Kuipers J., Kujala P., van den Born M., Cozijnsen M., Haegebarth A., Korving J., Begthel H., Peters P.J., and Clevers H.. 2007. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 449:1003–1007. 10.1038/nature06196 [DOI] [PubMed] [Google Scholar]
- Barrandon Y., and Green H.. 1987. Three clonal types of keratinocyte with different capacities for multiplication. Proc. Natl. Acad. Sci. USA. 84:2302–2306. 10.1073/pnas.84.8.2302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beerman I., Bhattacharya D., Zandi S., Sigvardsson M., Weissman I.L., Bryder D., and Rossi D.J.. 2010. Functionally distinct hematopoietic stem cells modulate hematopoietic lineage potential during aging by a mechanism of clonal expansion. Proc. Natl. Acad. Sci. USA. 107:5465–5470. 10.1073/pnas.1000834107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beerman I., Seita J., Inlay M.A., Weissman I.L., and Rossi D.J.. 2014. Quiescent hematopoietic stem cells accumulate DNA damage during aging that is repaired upon entry into cell cycle. Cell Stem Cell. 15:37–50. 10.1016/j.stem.2014.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beghé C., Wilson A., and Ershler W.B.. 2004. Prevalence and outcomes of anemia in geriatrics: a systematic review of the literature. Am. J. Med. 116:3S–10S. 10.1016/j.amjmed.2003.12.009 [DOI] [PubMed] [Google Scholar]
- Bernet J.D., Doles J.D., Hall J.K., Kelly Tanaka K., Carter T.A., and Olwin B.B.. 2014. p38 MAPK signaling underlies a cell-autonomous loss of stem cell self-renewal in skeletal muscle of aged mice. Nat. Med. 20:265–271. 10.1038/nm.3465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizzarro V., Fontanella B., Franceschelli S., Pirozzi M., Christian H., Parente L., and Petrella A.. 2010. Role of Annexin A1 in mouse myoblast cell differentiation. J. Cell. Physiol. 224:757–765. 10.1002/jcp.22178 [DOI] [PubMed] [Google Scholar]
- Brack A.S., and Rando T.A.. 2012. Tissue-specific stem cells: lessons from the skeletal muscle satellite cell. Cell Stem Cell. 10:504–514. 10.1016/j.stem.2012.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brack A.S., Conboy M.J., Roy S., Lee M., Kuo C.J., Keller C., and Rando T.A.. 2007. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science. 317:807–810. 10.1126/science.1144090 [DOI] [PubMed] [Google Scholar]
- Brunet A., and Rando T.A.. 2007. Ageing: from stem to stern. Nature. 449:288–291. 10.1038/449288a [DOI] [PubMed] [Google Scholar]
- Calvi L.M., Adams G.B., Weibrecht K.W., Weber J.M., Olson D.P., Knight M.C., Martin R.P., Schipani E., Divieti P., Bringhurst F.R., et al. 2003. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 425:841–846. 10.1038/nature02040 [DOI] [PubMed] [Google Scholar]
- Chakkalakal J.V., Jones K.M., Basson M.A., and Brack A.S.. 2012. The aged niche disrupts muscle stem cell quiescence. Nature. 490:355–360. 10.1038/nature11438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers S.M., Shaw C.A., Gatza C., Fisk C.J., Donehower L.A., and Goodell M.A.. 2007. Aging hematopoietic stem cells decline in function and exhibit epigenetic dysregulation. PLoS Biol. 5:e201 10.1371/journal.pbio.0050201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C.-Y., Pasolli H.A., Giannopoulou E.G., Guasch G., Gronostajski R.M., Elemento O., and Fuchs E.. 2013. NFIB is a governor of epithelial-melanocyte stem cell behaviour in a shared niche. Nature. 495:98–102. 10.1038/nature11847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase H.B., and Eaton G.J.. 1959. The growth of hair follicles in waves. Ann. N. Y. Acad. Sci. 83:365–368. 10.1111/j.1749-6632.1960.tb40912.x [DOI] [PubMed] [Google Scholar]
- Chen C.-C., Murray P.J., Jiang T.-X., Plikus M.V., Chang Y.-T., Lee O.K., Widelitz R.B., and Chuong C.-M.. 2014. Regenerative hair waves in aging mice and extra-follicular modulators follistatin, Dkk1 and Sfrp4. J. Invest. Dermatol. 134:2086–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H., and Leblond C.P.. 1974. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. V. Unitarian Theory of the origin of the four epithelial cell types. Am. J. Anat. 141:537–561. 10.1002/aja.1001410407 [DOI] [PubMed] [Google Scholar]
- Cheshier S.H., Morrison S.J., Liao X., and Weissman I.L.. 1999. In vivo proliferation and cell cycle kinetics of long-term self-renewing hematopoietic stem cells. Proc. Natl. Acad. Sci. USA. 96:3120–3125. 10.1073/pnas.96.6.3120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirumamilla S., Sun D., Bullock M.R., and Colello R.J.. 2002. Traumatic brain injury induced cell proliferation in the adult mammalian central nervous system. J. Neurotrauma. 19:693–703. 10.1089/08977150260139084 [DOI] [PubMed] [Google Scholar]
- Cho R.H., Sieburg H.B., and Muller-Sieburg C.E.. 2008. A new mechanism for the aging of hematopoietic stem cells: aging changes the clonal composition of the stem cell compartment but not individual stem cells. Blood. 111:5553–5561. 10.1182/blood-2007-11-123547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conboy I.M., and Rando T.A.. 2005. Aging, stem cells and tissue regeneration: lessons from muscle. Cell Cycle. 4:407–410. 10.4161/cc.4.3.1518 [DOI] [PubMed] [Google Scholar]
- Conboy I.M., Conboy M.J., Smythe G.M., and Rando T.A.. 2003. Notch-mediated restoration of regenerative potential to aged muscle. Science. 302:1575–1577. 10.1126/science.1087573 [DOI] [PubMed] [Google Scholar]
- Conboy I.M., Conboy M.J., Wagers A.J., Girma E.R., Weissman I.L., and Rando T.A.. 2005. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 433:760–764. 10.1038/nature03260 [DOI] [PubMed] [Google Scholar]
- Cosgrove B.D., Gilbert P.M., Porpiglia E., Mourkioti F., Lee S.P., Corbel S.Y., Llewellyn M.E., Delp S.L., and Blau H.M.. 2014. Rejuvenation of the muscle stem cell population restores strength to injured aged muscles. Nat. Med. 20:255–264. 10.1038/nm.3464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotsarelis G., Sun T.T., and Lavker R.M.. 1990. Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell. 61:1329–1337. 10.1016/0092-8674(90)90696-C [DOI] [PubMed] [Google Scholar]
- Creamer B., Shorter R.G., and Bamforth J.. 1961. The turnover and shedding of epithelial cells. I. The turnover in the gastro-intestinal tract. Gut. 2:110–116. 10.1136/gut.2.2.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews L.A., Balaian L., Delos Santos N.P., Leu H.S., Court A.C., Lazzari E., Sadarangani A., Zipeto M.A., La Clair J.J., Villa R., et al. 2016. RNA Splicing Modulation Selectively Impairs Leukemia Stem Cell Maintenance in Secondary Human AML. Cell Stem Cell. 19:599–612. 10.1016/j.stem.2016.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Haan G., Nijhof W., and Van Zant G.. 1997. Mouse strain-dependent changes in frequency and proliferation of hematopoietic stem cells during aging: correlation between lifespan and cycling activity. Blood. 89:1543–1550. [PubMed] [Google Scholar]
- Doetsch F., Caillé I., Lim D.A., García-Verdugo J.M., and Alvarez-Buylla A.. 1999. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 97:703–716. 10.1016/S0092-8674(00)80783-7 [DOI] [PubMed] [Google Scholar]
- Doles J., Storer M., Cozzuto L., Roma G., and Keyes W.M.. 2012. Age-associated inflammation inhibits epidermal stem cell function. Genes Dev. 26:2144–2153. 10.1101/gad.192294.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan M.H., Yazdani U., Norris R.D., Games D., German D.C., and Eisch A.J.. 2006. Decreased adult hippocampal neurogenesis in the PDAPP mouse model of Alzheimer’s disease. J. Comp. Neurol. 495:70–83. 10.1002/cne.20840 [DOI] [PubMed] [Google Scholar]
- Dykstra B., Olthof S., Schreuder J., Ritsema M., and de Haan G.. 2011. Clonal analysis reveals multiple functional defects of aged murine hematopoietic stem cells. J. Exp. Med. 208:2691–2703. 10.1084/jem.20111490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson P.S., Perfilieva E., Björk-Eriksson T., Alborn A.M., Nordborg C., Peterson D.A., and Gage F.H.. 1998. Neurogenesis in the adult human hippocampus. Nat. Med. 4:1313–1317. 10.1038/3305 [DOI] [PubMed] [Google Scholar]
- Ficara F., Murphy M.J., Lin M., and Cleary M.L.. 2008. Pbx1 regulates self-renewal of long-term hematopoietic stem cells by maintaining their quiescence. Cell Stem Cell. 2:484–496. 10.1016/j.stem.2008.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flach J., Bakker S.T., Mohrin M., Conroy P.C., Pietras E.M., Reynaud D., Alvarez S., Diolaiti M.E., Ugarte F., Forsberg E.C., et al. 2014. Replication stress is a potent driver of functional decline in ageing haematopoietic stem cells. Nature. 512:198–202. 10.1038/nature13619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florian M.C., Dörr K., Niebel A., Daria D., Schrezenmeier H., Rojewski M., Filippi M.-D., Hasenberg A., Gunzer M., Scharffetter-Kochanek K., et al. 2012. Cdc42 activity regulates hematopoietic stem cell aging and rejuvenation. Cell Stem Cell. 10:520–530. 10.1016/j.stem.2012.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florian M.C., Nattamai K.J., Dörr K., Marka G., Uberle B., Vas V., Eckl C., Andrä I., Schiemann M., Oostendorp R.A.J., et al. 2013. A canonical to non-canonical Wnt signalling switch in haematopoietic stem-cell ageing. Nature. 503:392–396. 10.1038/nature12631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortunel N.O., Otu H.H., Ng H.-H., Chen J., Mu X., Chevassut T., Li X., Joseph M., Bailey C., Hatzfeld J.A., et al. 2003a Comment on “‘Stemness’: transcriptional profiling of embryonic and adult stem cells” and “a stem cell molecular signature.” Science. 302:393. [DOI] [PubMed] [Google Scholar]
- Fortunel N.O., Otu H.H., Ng H.-H., Chen J., Mu X., Chevassut T., Li X., Joseph M., Bailey C., Hatzfeld J.A., et al. 2003b Comment on “ ‘Stemness’: transcriptional profiling of embryonic and adult stem cells” and “a stem cell molecular signature”. Science. 302:393 10.1126/science.1086384 [DOI] [PubMed] [Google Scholar]
- Fuchs E. 2007. Scratching the surface of skin development. Nature. 445:834–842. 10.1038/nature05659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giangreco A., Qin M., Pintar J.E., and Watt F.M.. 2008. Epidermal stem cells are retained in vivo throughout skin aging. Aging Cell. 7:250–259. 10.1111/j.1474-9726.2008.00372.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonçalves J.T., Schafer S.T., and Gage F.H.. 2016. Adult Neurogenesis in the Hippocampus: From Stem Cells to Behavior. Cell. 167:897–914. 10.1016/j.cell.2016.10.021 [DOI] [PubMed] [Google Scholar]
- Greco V., Chen T., Rendl M., Schober M., Pasolli H.A., Stokes N., Dela Cruz-Racelis J., and Fuchs E.. 2009. A two-step mechanism for stem cell activation during hair regeneration. Cell Stem Cell. 4:155–169. 10.1016/j.stem.2008.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiramuki Y., Sato T., Furuta Y., Surani M.A., and Sehara-Fujisawa A.. 2015. Mest but Not MiR-335 Affects Skeletal Muscle Growth and Regeneration. PLoS One. 10:e0130436 10.1371/journal.pone.0130436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu Y.-C., Pasolli H.A., and Fuchs E.. 2011. Dynamics between stem cells, niche, and progeny in the hair follicle. Cell. 144:92–105. 10.1016/j.cell.2010.11.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inomata K., Aoto T., Binh N.T., Okamoto N., Tanimura S., Wakayama T., Iseki S., Hara E., Masunaga T., Shimizu H., and Nishimura E.K.. 2009. Genotoxic stress abrogates renewal of melanocyte stem cells by triggering their differentiation. Cell. 137:1088–1099. 10.1016/j.cell.2009.03.037 [DOI] [PubMed] [Google Scholar]
- Iscove N.N., and Nawa K.. 1997. Hematopoietic stem cells expand during serial transplantation in vivo without apparent exhaustion. Curr. Biol. 7:805–808. 10.1016/S0960-9822(06)00341-1 [DOI] [PubMed] [Google Scholar]
- Ivanova N.B., Dimos J.T., Schaniel C., Hackney J.A., Moore K.A., and Lemischka I.R.. 2002. A stem cell molecular signature. Science. 298:601–604. 10.1126/science.1073823 [DOI] [PubMed] [Google Scholar]
- Jeannet R., Cai Q., Liu H., Vu H., and Kuo Y.-H.. 2013. Alcam regulates long-term hematopoietic stem cell engraftment and self-renewal. Stem Cells. 31:560–571. 10.1002/stem.1309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyes B.E., Segal J.P., Heller E., Lien W.-H., Chang C.-Y., Guo X., Oristian D.S., Zheng D., and Fuchs E.. 2013. Nfatc1 orchestrates aging in hair follicle stem cells. Proc. Natl. Acad. Sci. USA. 110:E4950–E4959. 10.1073/pnas.1320301110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo J.H., Smiley M.A., Lovering R.M., and Margolis F.L.. 2007. Bex1 knock out mice show altered skeletal muscle regeneration. Biochem. Biophys. Res. Commun. 363:405–410. 10.1016/j.bbrc.2007.08.186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalczyk M.S., Tirosh I., Heckl D., Rao T.N., Dixit A., Haas B.J., Schneider R.K., Wagers A.J., Ebert B.L., and Regev A.. 2015. Single-cell RNA-seq reveals changes in cell cycle and differentiation programs upon aging of hematopoietic stem cells. Genome Res. 25:1860–1872. 10.1101/gr.192237.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozar S., Morrissey E., Nicholson A.M., van der Heijden M., Zecchini H.I., Kemp R., Tavaré S., Vermeulen L., and Winton D.J.. 2013. Continuous clonal labeling reveals small numbers of functional stem cells in intestinal crypts and adenomas. Cell Stem Cell. 13:626–633. 10.1016/j.stem.2013.08.001 [DOI] [PubMed] [Google Scholar]
- Kuhn H.G., Dickinson-Anson H., and Gage F.H.. 1996. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J. Neurosci. 16:2027–2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuswanto W., Burzyn D., Panduro M., Wang K.K., Jang Y.C., Wagers A.J., Benoist C., and Mathis D.. 2016. Poor Repair of Skeletal Muscle in Aging Mice Reflects a Defect in Local, Interleukin-33-Dependent Accumulation of Regulatory T Cells. Immunity. 44:355–367. 10.1016/j.immuni.2016.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lajtha L.G. 1979. Stem cell concepts. Differentiation. 14:23–33. 10.1111/j.1432-0436.1979.tb01007.x [DOI] [PubMed] [Google Scholar]
- Lay K., Kume T., and Fuchs E.. 2016. FOXC1 maintains the hair follicle stem cell niche and governs stem cell quiescence to preserve long-term tissue-regenerating potential. Proc. Natl. Acad. Sci. USA. 113:E1506–E1515. 10.1073/pnas.1601569113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leek J.T., Scharpf R.B., Bravo H.C., Simcha D., Langmead B., Johnson W.E., Geman D., Baggerly K., and Irizarry R.A.. 2010. Tackling the widespread and critical impact of batch effects in high-throughput data. Nat. Rev. Genet. 11:733–739. 10.1038/nrg2825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leishman E., Howard J.M., Garcia G.E., Miao Q., Ku A.T., Dekker J.D., Tucker H., and Nguyen H.. 2013. Foxp1 maintains hair follicle stem cell quiescence through regulation of Fgf18. Development. 140:3809–3818. 10.1242/dev.097477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lien W.-H., Polak L., Lin M., Lay K., Zheng D., and Fuchs E.. 2014. In vivo transcriptional governance of hair follicle stem cells by canonical Wnt regulators. Nat. Cell Biol. 16:179–190. 10.1038/ncb2903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linton P.J., and Dorshkind K.. 2004. Age-related changes in lymphocyte development and function. Nat. Immunol. 5:133–139. 10.1038/ni1033 [DOI] [PubMed] [Google Scholar]
- Liu L., and Rando T.A.. 2011. Manifestations and mechanisms of stem cell aging. J. Cell Biol. 193:257–266. 10.1083/jcb.201010131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Cheung T.H., Charville G.W., Hurgo B.M.C., Leavitt T., Shih J., Brunet A., and Rando T.A.. 2013. Chromatin modifications as determinants of muscle stem cell quiescence and chronological aging. Cell Reports. 4:189–204. 10.1016/j.celrep.2013.05.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Otín C., Blasco M.A., Partridge L., Serrano M., and Kroemer G.. 2013. The hallmarks of aging. Cell. 153:1194–1217. 10.1016/j.cell.2013.05.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch M. 2010. Rate, molecular spectrum, and consequences of human mutation. Proc. Natl. Acad. Sci. USA. 107:961–968. 10.1073/pnas.0912629107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin K., Kirkwood T.B., and Potten C.S.. 1998a Age changes in stem cells of murine small intestinal crypts. Exp. Cell Res. 241:316–323. 10.1006/excr.1998.4001 [DOI] [PubMed] [Google Scholar]
- Martin K., Potten C.S., Roberts S.A., and Kirkwood T.B.. 1998b Altered stem cell regeneration in irradiated intestinal crypts of senescent mice. J. Cell Sci. 111:2297–2303. [DOI] [PubMed] [Google Scholar]
- Maslov A.Y., Barone T.A., Plunkett R.J., and Pruitt S.C.. 2004. Neural stem cell detection, characterization, and age-related changes in the subventricular zone of mice. J. Neurosci. 24:1726–1733. 10.1523/JNEUROSCI.4608-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura H., Mohri Y., Binh N.T., Morinaga H., Fukuda M., Ito M., Kurata S., Hoeijmakers J., and Nishimura E.K.. 2016. Hair follicle aging is driven by transepidermal elimination of stem cells via COL17A1 proteolysis. Science. 351:aad4395–aad4395. 10.1126/science.aad4395 [DOI] [PubMed] [Google Scholar]
- Mauro A. 1961. Satellite cell of skeletal muscle fibers. J. Biophys. Biochem. Cytol. 9:493–495. 10.1083/jcb.9.2.493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohrin M., Shin J., Liu Y., Brown K., Luo H., Xi Y., Haynes C.M., and Chen D.. 2015. Stem cell aging. A mitochondrial UPR-mediated metabolic checkpoint regulates hematopoietic stem cell aging. Science. 347:1374–1377. 10.1126/science.aaa2361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molofsky A.V., Slutsky S.G., Joseph N.M., He S., Pardal R., Krishnamurthy J., Sharpless N.E., and Morrison S.J.. 2006. Increasing p16INK4a expression decreases forebrain progenitors and neurogenesis during ageing. Nature. 443:448–452. 10.1038/nature05091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison S.J., and Spradling A.C.. 2008. Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell. 132:598–611. 10.1016/j.cell.2008.01.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison S.J., Wandycz A.M., Akashi K., Globerson A., and Weissman I.L.. 1996. The aging of hematopoietic stem cells. Nat. Med. 2:1011–1016. 10.1038/nm0996-1011 [DOI] [PubMed] [Google Scholar]
- Nalapareddy K., Nattamai K.J., Kumar R.S., Karns R., Wikenheiser-Brokamp K.A., Sampson L.L., Mahe M.M., Sundaram N., Yacyshyn M.-B., Yacyshyn B., et al. 2017. Canonical Wnt Signaling Ameliorates Aging of Intestinal Stem Cells. Cell Reports. 18:2608–2621. 10.1016/j.celrep.2017.02.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naveiras O., Nardi V., Wenzel P.L., Hauschka P.V., Fahey F., and Daley G.Q.. 2009. Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment. Nature. 460:259–263. 10.1038/nature08099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neves J., Sousa-Victor P., and Jasper H.. 2017. Rejuvenating Strategies for Stem Cell-Based Therapies in Aging. Cell Stem Cell. 20:161–175. 10.1016/j.stem.2017.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura E.K., Jordan S.A., Oshima H., Yoshida H., Osawa M., Moriyama M., Jackson I.J., Barrandon Y., Miyachi Y., and Nishikawa S.. 2002. Dominant role of the niche in melanocyte stem-cell fate determination. Nature. 416:854–860. 10.1038/416854a [DOI] [PubMed] [Google Scholar]
- Nishimura E.K., Granter S.R., and Fisher D.E.. 2005. Mechanisms of hair graying: incomplete melanocyte stem cell maintenance in the niche. Science. 307:720–724. 10.1126/science.1099593 [DOI] [PubMed] [Google Scholar]
- Oh J., Lee Y.D., and Wagers A.J.. 2014. Stem cell aging: mechanisms, regulators and therapeutic opportunities. Nat. Med. 20:870–880. 10.1038/nm.3651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlic D., Kajstura J., Chimenti S., Jakoniuk I., Anderson S.M., Li B., Pickel J., McKay R., Nadal-Ginard B., Bodine D.M., et al. 2001. Bone marrow cells regenerate infarcted myocardium. Nature. 410:701–705. 10.1038/35070587 [DOI] [PubMed] [Google Scholar]
- Palmer T.D., Takahashi J., and Gage F.H.. 1997. The adult rat hippocampus contains primordial neural stem cells. Mol. Cell. Neurosci. 8:389–404. 10.1006/mcne.1996.0595 [DOI] [PubMed] [Google Scholar]
- Potten C.S., and Loeffler M.. 1990. Stem cells: attributes, cycles, spirals, pitfalls and uncertainties. Lessons for and from the crypt. Development. 110:1001–1020. [DOI] [PubMed] [Google Scholar]
- Price F.D., von Maltzahn J., Bentzinger C.F., Dumont N.A., Yin H., Chang N.C., Wilson D.H., Frenette J., and Rudnicki M.A.. 2014. Inhibition of JAK-STAT signaling stimulates adult satellite cell function. Nat. Med. 20:1174–1181. 10.1038/nm.3655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabbani P., Takeo M., Chou W., Myung P., Bosenberg M., Chin L., Taketo M.M., and Ito M.. 2011. Coordinated activation of Wnt in epithelial and melanocyte stem cells initiates pigmented hair regeneration. Cell. 145:941–955. 10.1016/j.cell.2011.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramalho-Santos M., Yoon S., Matsuzaki Y., Mulligan R.C., and Melton D.A.. 2002. “Stemness”: transcriptional profiling of embryonic and adult stem cells. Science. 298:597–600. 10.1126/science.1072530 [DOI] [PubMed] [Google Scholar]
- Ramos-Casals M., García-Carrasco M., Brito M.P., López-Soto A., and Font J.. 2003. Autoimmunity and geriatrics: clinical significance of autoimmune manifestations in the elderly. Lupus. 12:341–355. 10.1191/0961203303lu383ed [DOI] [PubMed] [Google Scholar]
- Rennert R.C., Sorkin M., Garg R.K., and Gurtner G.C.. 2012. Stem cell recruitment after injury: lessons for regenerative medicine. Regen. Med. 7:833–850. 10.2217/rme.12.82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi D.J., Bryder D., Zahn J.M., Ahlenius H., Sonu R., Wagers A.J., and Weissman I.L.. 2005. Cell intrinsic alterations underlie hematopoietic stem cell aging. Proc. Natl. Acad. Sci. USA. 102:9194–9199. 10.1073/pnas.0503280102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi D.J., Bryder D., Seita J., Nussenzweig A., Hoeijmakers J., and Weissman I.L.. 2007a Deficiencies in DNA damage repair limit the function of haematopoietic stem cells with age. Nature. 447:725–729. 10.1038/nature05862 [DOI] [PubMed] [Google Scholar]
- Rossi D.J., Seita J., Czechowicz A., Bhattacharya D., Bryder D., and Weissman I.L.. 2007b Hematopoietic stem cell quiescence attenuates DNA damage response and permits DNA damage accumulation during aging. Cell Cycle. 6:2371–2376. 10.4161/cc.6.19.4759 [DOI] [PubMed] [Google Scholar]
- Rossi D.J., Jamieson C.H.M., and Weissman I.L.. 2008. Stems cells and the pathways to aging and cancer. Cell. 132:681–696. 10.1016/j.cell.2008.01.036 [DOI] [PubMed] [Google Scholar]
- Schuijers J., Junker J.P., Mokry M., Hatzis P., Koo B.-K., Sasselli V., van der Flier L.G., Cuppen E., van Oudenaarden A., and Clevers H.. 2015. Ascl2 acts as an R-spondin/Wnt-responsive switch to control stemness in intestinal crypts. Cell Stem Cell. 16:158–170. 10.1016/j.stem.2014.12.006 [DOI] [PubMed] [Google Scholar]
- Schwörer S., Becker F., Feller C., Baig A.H., Köber U., Henze H., Kraus J.M., Xin B., Lechel A., Lipka D.B., et al. 2016. Epigenetic stress responses induce muscle stem-cell ageing by Hoxa9 developmental signals. Nature. 540:428–432. 10.1038/nature20603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seim I., Ma S., and Gladyshev V.N.. 2016. Gene expression signatures of human cell and tissue longevity. NPJ Aging Mech. Dis. 2:783–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpless N.E., and DePinho R.A.. 2007. How stem cells age and why this makes us grow old. Nat. Rev. Mol. Cell Biol. 8:703–713. 10.1038/nrm2241 [DOI] [PubMed] [Google Scholar]
- Signer R.A.J., and Morrison S.J.. 2013. Mechanisms that regulate stem cell aging and life span. Cell Stem Cell. 12:152–165. 10.1016/j.stem.2013.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva-Vargas V., Maldonado-Soto A.R., Mizrak D., Codega P., and Doetsch F.. 2016. Age-Dependent Niche Signals from the Choroid Plexus Regulate Adult Neural Stem Cells. Cell Stem Cell. 19:643–652. 10.1016/j.stem.2016.06.013 [DOI] [PubMed] [Google Scholar]
- Sinha M., Jang Y.C., Oh J., Khong D., Wu E.Y., Manohar R., Miller C., Regalado S.G., Loffredo F.S., Pancoast J.R., et al. 2014. Restoring systemic GDF11 levels reverses age-related dysfunction in mouse skeletal muscle. Science. 344:649–652. 10.1126/science.1251152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack J.M.W. 2008. Origin of stem cells in organogenesis. Science. 322:1498–1501. 10.1126/science.1162782 [DOI] [PubMed] [Google Scholar]
- Snow M.H. 1978. An autoradiographic study of satellite cell differentiation into regenerating myotubes following transplantation of muscles in young rats. Cell Tissue Res. 186:535–540. 10.1007/BF00224941 [DOI] [PubMed] [Google Scholar]
- Sousa-Victor P., Gutarra S., García-Prat L., Rodriguez-Ubreva J., Ortet L., Ruiz-Bonilla V., Jardí M., Ballestar E., González S., Serrano A.L., et al. 2014. Geriatric muscle stem cells switch reversible quiescence into senescence. Nature. 506:316–321. 10.1038/nature13013 [DOI] [PubMed] [Google Scholar]
- Spalding K.L., Bhardwaj R.D., Buchholz B.A., Druid H., and Frisén J.. 2005. Retrospective birth dating of cells in humans. Cell. 122:133–143. 10.1016/j.cell.2005.04.028 [DOI] [PubMed] [Google Scholar]
- Spalding K.L., Bergmann O., Alkass K., Bernard S., Salehpour M., Huttner H.B., Boström E., Westerlund I., Vial C., Buchholz B.A., et al. 2013. Dynamics of hippocampal neurogenesis in adult humans. Cell. 153:1219–1227. 10.1016/j.cell.2013.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudo K., Ema H., Morita Y., and Nakauchi H.. 2000. Age-associated characteristics of murine hematopoietic stem cells. J. Exp. Med. 192:1273–1280. 10.1084/jem.192.9.1273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D., Luo M., Jeong M., Rodriguez B., Xia Z., Hannah R., Wang H., Le T., Faull K.F., Chen R., et al. 2014. Epigenomic profiling of young and aged HSCs reveals concerted changes during aging that reinforce self-renewal. Cell Stem Cell. 14:673–688. 10.1016/j.stem.2014.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svennerholm L., Boström K., and Jungbjer B.. 1997. Changes in weight and compositions of major membrane components of human brain during the span of adult human life of Swedes. Acta Neuropathol. 94:345–352. 10.1007/s004010050717 [DOI] [PubMed] [Google Scholar]
- Till J.E., and McCulloch E.A.. 1961. A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiat. Res. 14:213–222. 10.2307/3570892 [DOI] [PubMed] [Google Scholar]
- Tomasetti C., and Vogelstein B.. 2015. Cancer etiology. Variation in cancer risk among tissues can be explained by the number of stem cell divisions. Science. 347:78–81. 10.1126/science.1260825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasetti C., Vogelstein B., and Parmigiani G.. 2013. Half or more of the somatic mutations in cancers of self-renewing tissues originate prior to tumor initiation. Proc. Natl. Acad. Sci. USA. 110:1999–2004. 10.1073/pnas.1221068110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasetti C., Li L., and Vogelstein B.. 2017. Stem cell divisions, somatic mutations, cancer etiology, and cancer prevention. Science. 355:1330–1334. 10.1126/science.aaf9011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumbar T., Guasch G., Greco V., Blanpain C., Lowry W.E., Rendl M., and Fuchs E.. 2004. Defining the epithelial stem cell niche in skin. Science. 303:359–363. 10.1126/science.1092436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H., Schinder A.F., Christie B.R., Toni N., Palmer T.D., and Gage F.H.. 2002. Functional neurogenesis in the adult hippocampus. Nature. 415:1030–1034. 10.1038/4151030a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijg J., and Suh Y.. 2013. Genome instability and aging. Annu. Rev. Physiol. 75:645–668. 10.1146/annurev-physiol-030212-183715 [DOI] [PubMed] [Google Scholar]
- Villeda S.A., Luo J., Mosher K.I., Zou B., Britschgi M., Bieri G., Stan T.M., Fainberg N., Ding Z., Eggel A., et al. 2011. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature. 477:90–94. 10.1038/nature10357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeda S.A., Plambeck K.E., Middeldorp J., Castellano J.M., Mosher K.I., Luo J., Smith L.K., Bieri G., Lin K., Berdnik D., et al. 2014. Young blood reverses age-related impairments in cognitive function and synaptic plasticity in mice. Nat. Med. 20:659–663. 10.1038/nm.3569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitali C., Bassani C., Chiodoni C., Fellini E., Guarnotta C., Miotti S., Sangaletti S., Fuligni F., De Cecco L., Piccaluga P.P., et al. 2015. SOCS2 Controls Proliferation and Stemness of Hematopoietic Cells under Stress Conditions and Its Deregulation Marks Unfavorable Acute Leukemias. Cancer Res. 75:2387–2399. 10.1158/0008-5472.CAN-14-3625 [DOI] [PubMed] [Google Scholar]
- Waghmare S.K., Bansal R., Lee J., Zhang Y.V., Mcdermitt D.J., and Tumbar T.. 2008. Quantitative proliferation dynamics and random chromosome segregation of hair follicle stem cells. EMBO J. 27:1309–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Siegenthaler J.A., Dowell R.D., and Yi R.. 2016. Foxc1 reinforces quiescence in self-renewing hair follicle stem cells. Science. 351:613–617. 10.1126/science.aad5440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyss-Coray T. 2016. Ageing, neurodegeneration and brain rejuvenation. Nature. 539:180–186. 10.1038/nature20411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie T., and Spradling A.C.. 1998. decapentaplegic is essential for the maintenance and division of germline stem cells in the Drosophila ovary. Cell. 94:251–260. 10.1016/S0092-8674(00)81424-5 [DOI] [PubMed] [Google Scholar]
- Yan K.S., and Kuo C.J.. 2015. Ascl2 reinforces intestinal stem cell identity. Cell Stem Cell. 16:105–106. 10.1016/j.stem.2015.01.014 [DOI] [PubMed] [Google Scholar]
- Yang H., Adam R.C., Ge Y., Hua Z.L., and Fuchs E.. 2017. Epithelial-Mesenchymal Micro-niches Govern Stem Cell Lineage Choices. Cell. 169:483.e13–496.e13. 10.1016/j.cell.2017.03.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Ryu D., Wu Y., Gariani K., Wang X., Luan P., D’Amico D., Ropelle E.R., Lutolf M.P., Aebersold R., et al. 2016. NAD+ repletion improves mitochondrial and stem cell function and enhances life span in mice. Science. 352:1436–1443. 10.1126/science.aaf2693 [DOI] [PubMed] [Google Scholar]
- Zhou B.O., Yu H., Yue R., Zhao Z., Rios J.J., Naveiras O., and Morrison S.J.. 2017. Bone marrow adipocytes promote the regeneration of stem cells and haematopoiesis by secreting SCF. Nat. Cell Biol. 19:891–903. 10.1038/ncb3570 [DOI] [PMC free article] [PubMed] [Google Scholar]