Steffen and Koehler preview work from the Chakrabarti et al. presenting new ways in which actin polymerization at the endoplasmic reticulum influences mitochondrial division.
Abstract
The formin-like protein INF2 is an important player in the polymerization of actin filaments. In this issue, Chakrabarti et al. (2018. J. Cell Biol. https://doi.org/10.1083/jcb.201709111) demonstrate that INF2 mediates actin polymerization at the endoplasmic reticulum (ER), resulting in increased ER–mitochondria contacts, calcium uptake by mitochondria, and mitochondrial division.
Mitochondria are surrounded by two membranes, an inner membrane (IM) and outer membrane (OM), and are responsible for metabolism and signaling within the eukaryotic cell. Mitochondria undergo regulated fusion and fission events to maintain a dynamic network. Disturbances in this equilibrium cause numerous diseases, including cancer and neurodegeneration. The dynamin-like protein DRP1 is recruited from the cytosol to mitochondria upon the initiation of fission to orchestrate fission of the OM. There, DRP1 oligomerizes around the mitochondria, which leads to constriction and scission of mitochondria. Close contacts between ER and mitochondria, referred to as the ER–mitochondria encounter structure (ERMES) in yeast, specialize in lipid transfer and calcium trafficking (Kornmann et al., 2009). Recent research shows that the ER wraps around the mitochondria and initiates a mitochondrial constriction at the contact sites before DRP1 is recruited to trigger mitochondrial fission (Friedman et al., 2011). Thus, the ER may play an active role in the early stages of mitochondrial fission, defining the division sites. Earlier work by Korobova et al. (2013) supports a similar role for the ER in mitochondrial division and establishes that the ER-bound protein inverted formin 2 (INF2) promotes mitochondrial fission by inducing constrictions before DRP1 is recruited to the mitochondria (Korobova et al., 2013). INF2 is a formin-like protein that controls actin assembly and leads to the rapid polymerization of actin at the ER upon calcium influx into the cell. Interestingly, INF2 interacts with the calcium binding protein calmodulin, which eventually controls the sensitivity of INF2 to changing calcium concentrations within the cell (Wales et al., 2016). In this study, Chakrabarti et al. show that INF2-mediated actin polymerization on the ER stimulates mitochondrial division through a second independent mechanism in which actin polymerization–triggered mitochondrial calcium uptake from the ER leads to IM constriction.
Chakrabarti et al. (2018) start by showing that the treatment of U2OS cells with ionomycin and histamine leads to a rapid increase of cytosolic calcium within 4.5 or 3.4 s, respectively. Whereas ionomycin leads to an influx of calcium from outside the cell, histamine increases cytosolic calcium by releasing calcium from intracellular stores. In response to the increased cytosolic calcium, actin polymerizes immediately, with kinetics of 8.3 s for ionomycin and 3.8 s for histamine. Chakrabarti et al. (2018) observe a subsequent spike in mitochondrial calcium after the actin polymerization burst occurred. All responses were short lived, lasting for ∼200 s. Finally, the calcium concentration in the cytosol, mitochondria, and actin polymerization returned to baseline.
Because mitochondrial calcium responses usually occur at ER–mitochondria contacts, Chakrabarti et al. (2018) measured ER calcium release upon stimulation with histamine or ionomycin. Histamine treatment lead to a rapid ER calcium release after 1.3 s, before the increase of cytosolic calcium and the polymerization of actin. Ionomycin treatment released calcium from the ER after 9.6 s, which occurred after the actin polymerization and indicated that calcium release from the ER also contributes to the increase of cytosolic calcium, implying a calcium-mediated calcium release.
To test for the direct influence of the ER-released calcium on the increase of mitochondrial calcium, Chakrabarti et al. (2018) prereleased ER calcium by thapsigargin addition. The pretreatment with thapsigargin blocked the increase of mitochondrial calcium and reduced the increase of cytoplasmic calcium and actin polymerization upon ionomycin stimulation. For histamine, calcium increase and actin polymerization were not detected.
Because the actin burst precedes mitochondrial calcium increase, Chakrabarti et al. (2018) tested whether actin polymerization is necessary for the increase of mitochondrial calcium. The treatment with latrunculin A, a chemical that prevents actin polymerization, strongly inhibited the mitochondrial calcium spike upon ionomycin or histamine treatment, indicating that the actin polymerization burst is essential for calcium entry into mitochondria. This is in strong agreement with previously published data that show that ER-mediated calcium release results in actin polymerization in neurons (Wang et al., 2002). Because actin polymerization enhances calcium release from the ER, this might provide a mechanism for a local amplification of the calcium signal.
In previous work, Korobova et al. (2013) showed that the ER-bound isoform of INF2, INF2-CAAX, plays a role in mitochondrial fission and that its knockdown prevents the ionomycin-induced actin burst. In their study, Chakrabarti et al. (2018) analyzed whether INF2-CAAX is also necessary for the mitochondrial calcium spike. With INF2-knockout U2OS cells, they observed that deletion of INF2 eliminated the actin polymerization and calcium spike in mitochondria upon ionomycin or histamine treatment. However, the cytoplasmic calcium increase still occurred. Conversely, expression of INF2-CAAX rescued the actin burst and influx of calcium into mitochondria. In addition, the actin polymerization mediated by INF2 increased ER–mitochondria contact sites, as assessed by electron microscopy. Ionomycin-mediated calcium release from the ER increased the local concentration of calcium at these contact sites, leading to an influx of calcium into mitochondria. Manipulations to increase tethering of the ER to mitochondria by overexpressing tether proteins in INF2-knockout cells yielded similar results. These intricate studies support a model in which the INF2-mediated actin polymerization and the subsequent increase of ER–mitochondria contact sites are necessary for the calcium release from the ER. The release amplifies the local calcium concentrations at the contact sites, which leads to calcium uptake by mitochondria.
Calcium can freely pass through the OM via voltage-dependent anion-selective channels (VDACs). However, the IM is impermeable to calcium, and the mitochondrial calcium uniporter (MCU) is the main channel for calcium transport across the IM (Baughman et al., 2011; De Stefani et al., 2011). Chakrabarti et al. (2018) silenced MCU expression in U2OS cells and observed that the ionomycin-induced calcium spike in mitochondria was blocked but that cytosolic calcium increased and actin polymerization still proceeded. Surprisingly, MCU knockdown resulted in a mitochondrial network that was more fused between the hyperfused phenotype of DRP1 knockdown and the typical network morphology of WT cells. Chakrabarti et al. (2018) also noted a 2.5-fold decrease in fission events in cells lacking MCU. In contrast, ionomycin treatment lead to a threefold increase in mitochondrial fission, which was attenuated with MCU knocked down, indicating that mitochondrial calcium entry plays a role in fission. Still, the calcium influx is seemingly necessary but not sufficient because a mitochondrial calcium increase does not necessarily lead to mitochondrial division in neurons (Cho et al., 2017).
It had been shown that ionomycin leads to the recruitment of DRP1 and finally to mitochondrial fission (Ji et al., 2015). However, whether MCU-mediated calcium transfer into the mitochondrial matrix is necessary for DRP1 recruitment to mitochondria was not known. Chakrabarti et al. (2018) established that MCU knockdown does not alter DRP1 recruitment to mitochondria. Interestingly, knockdown of DRP1 caused an increased expression of MCU and vice versa. The knockdown of INF2 also elevated the expression of both DRP1 and MCU. Thus, cells have a compensatory mechanism to maintain fission upon depletion of any of these key proteins.
In unstimulated U2OS cells, mitochondria occasionally constrict along their length and rarely undergo a full scission. These constrictions are mostly accompanied with a transient rise in mitochondrial matrix calcium. When treated with ionomycin, Chakrabarti et al. (2018) observed a sevenfold increase in mitochondrial constrictions with a similar time frame for calcium influx into mitochondria, and 90% of the constriction sites also colocalized to ER–mitochondria contact sites. The constrictions were not mediated by DRP1 recruitment and oligomerization. Conversely, knockdown of MCU resulted in reduced constriction sites for both unstimulated and ionomycin-treated cells. Thus, the constrictions seemingly were caused by an influx of mitochondrial calcium rather than DRP1 activity, and the constrictions were more likely at the IM than OM. Using superresolution microscopy, Chakrabarti et al. (2018) observed a stage in which the matrix was divided while the OM was still connected, suggesting that IM fission occurs before and independently from OM fission.
The protein optic atrophy 1 (OPA1) is a member of the GTPase family and has been described as the major regulator for IM fusion. OPA1 consists of approximately eight mRNA OPA1 isoforms in humans (Delettre et al., 2001). IM-bound long L-OPA1 forms can be processed by proteolytic cleavage to generate short S-OPA1. L-OPA1 has been associated with mitochondrial fusion, whereas S-OPA1 is thought to play a role in mitochondrial fission (Anand et al., 2014). L-OPA1 is processed to S-OPA1 by the metalloprotease OMA1 upon mitochondrial depolarization, a process that leads to mitochondria fission. Although ionomycin treatment results in constriction and fission of the IM, Chakrabarti et al. (2018) did not detect changes in OPA1 processing or oligomerization. Furthermore, OMA1 knockdown did not prevent the appearance of IM constrictions, suggesting that OPA1 processing by OMA1 was not required. In contrast, OMA1-dependent processing of OPA1 has been reported in neurons (Cho et al., 2017). In future work, it would be interesting to test the influence of the other OPA1-processing protease YME1L on the effect of calcium-mediated IM constriction, because YME1L processes L-OPA1 to S-OPA1 under nonstress conditions (Anand et al., 2014).
Finally, Chakrabarti et al. (2018) analyzed the influence of the electron transport chain (ETC) on the formation of IM constrictions. Treatment with complex I or complex III inhibitors decreased the ionomycin-induced IM constriction without changing the actin polymerization burst or the mitochondrial calcium influx. This suggests that the calcium-mediated increase in ETC activity is ultimately required for IM constrictions.
Overall, this comprehensive study describing the interplay of calcium trafficking between the ER and mitochondria, actin polymerization, and IM constrictions indicates that mitochondrial fission is more complex than simply DRP1-mediated scission and that IM fission can precede OM fission (Fig. 1). Moreover, the ER and actin polymerization play a central role in mitochondrial fission. Chakrabarti et al. (2018) also show that the influx of ER calcium into mitochondria is needed for activation. These findings raise the question of how fission occurs at the IM, because the processing of L-OPA1 to S-OPA1 does not seem to be essential for IM fission even though it had been previously reported (Anand et al., 2014; Cho et al., 2017). The IM may have a fission machinery that is independent of the OM, or OPA1 processing may be bypassed. In yeast, the protein Mdm33 has been suggested to be important for IM fission (Messerschmitt et al., 2003). The study by Chakrabarti et al. (2018) nicely expands our understanding of mitochondrial fission and presents a complex model to serve as a platform for future studies of the mechanics and mechanisms by which the ER exerts control over mitochondrial dynamics.
Figure 1.
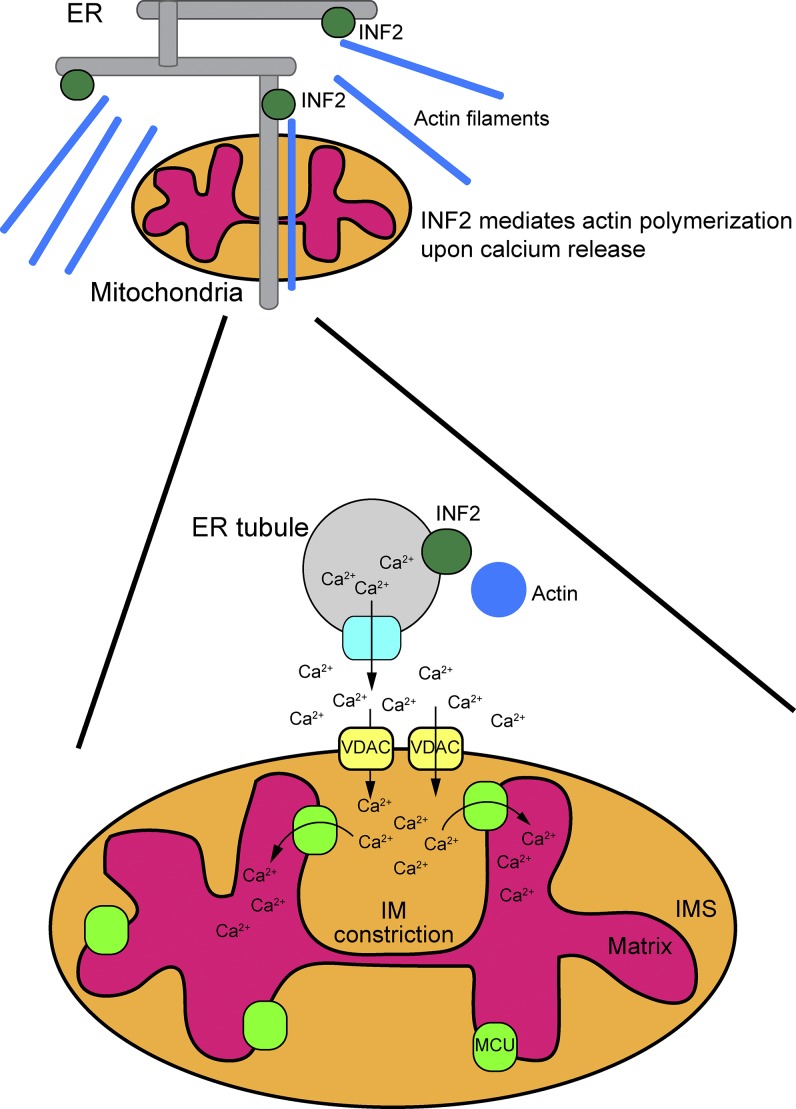
Schematic showing interactions between the ER and mitochondria in mitochondrial division. ER tubules are intimately associated with the mitochondria at contact sites. Calcium release from the ER induces INF2-mediated actin polymerization, leading to increased contact between the ER and mitochondria. Subsequently, mitochondrial calcium uptake by VDAC pores spikes, and calcium is transported into the matrix via the MCU. Finally, mitochondrial IM constriction is induced, followed by OM constriction. This complex model serves as a starting point for directed studies to dissect key events and components. IMS, intermembrane space.
Acknowledgments
Work in the authors' laboratory is supported by the National Institutes of Health grant GM61721 and the California Stem Cell Agency grant RT307678 to C.M. Koehler and the Deutsche Forschungsgemeinschaft grant STE 2045/1-1 to J. Steffen.
The authors declare no competing financial interests.
References
- Anand R., Wai T., Baker M.J., Kladt N., Schauss A.C., Rugarli E., and Langer T.. 2014. The i-AAA protease YME1L and OMA1 cleave OPA1 to balance mitochondrial fusion and fission. J. Cell Biol. 204:919–929. 10.1083/jcb.201308006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baughman J.M., Perocchi F., Girgis H.S., Plovanich M., Belcher-Timme C.A., Sancak Y., Bao X.R., Strittmatter L., Goldberger O., Bogorad R.L., et al. . 2011. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature. 476:341–345. 10.1038/nature10234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti R., Ji W.-K., Stan R.V., de Juan Sanz J., Ryan T.A., and Higgs H.N.. 2018. INF2-mediated actin polymerization at the ER stimulates mitochondrial calcium uptake, inner membrane constriction, and division. J. Cell Biol. 10.1083/jcb.20170911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho B., Cho H.M., Jo Y., Kim H.D., Song M., Moon C., Kim H., Kim K., Sesaki H., Rhyu I.J., et al. . 2017. Constriction of the mitochondrial inner compartment is a priming event for mitochondrial division. Nat. Commun. 8:15754 10.1038/ncomms15754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delettre C., Griffoin J.M., Kaplan J., Dollfus H., Lorenz B., Faivre L., Lenaers G., Belenguer P., and Hamel C.P.. 2001. Mutation spectrum and splicing variants in the OPA1 gene. Hum. Genet. 109:584–591. 10.1007/s00439-001-0633-y [DOI] [PubMed] [Google Scholar]
- De Stefani D., Raffaello A., Teardo E., Szabò I., and Rizzuto R.. 2011. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature. 476:336–340. 10.1038/nature10230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman J.R., Lackner L.L., West M., DiBenedetto J.R., Nunnari J., and Voeltz G.K.. 2011. ER tubules mark sites of mitochondrial division. Science. 334:358–362. 10.1126/science.1207385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji W.K., Hatch A.L., Merrill R.A., Strack S., and Higgs H.N.. 2015. Actin filaments target the oligomeric maturation of the dynamin GTPase Drp1 to mitochondrial fission sites. eLife. 4:e11553 10.7554/eLife.11553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornmann B., Currie E., Collins S.R., Schuldiner M., Nunnari J., Weissman J.S., and Walter P.. 2009. An ER-mitochondria tethering complex revealed by a synthetic biology screen. Science. 325:477–481. 10.1126/science.1175088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korobova F., Ramabhadran V., and Higgs H.N.. 2013. An actin-dependent step in mitochondrial fission mediated by the ER-associated formin INF2. Science. 339:464–467. 10.1126/science.1228360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messerschmitt M., Jakobs S., Vogel F., Fritz S., Dimmer K.S., Neupert W., and Westermann B.. 2003. The inner membrane protein Mdm33 controls mitochondrial morphology in yeast. J. Cell Biol. 160:553–564. 10.1083/jcb.200211113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wales P., Schuberth C.E., Aufschnaiter R., Fels J., García-Aguilar I., Janning A., Dlugos C.P., Schäfer-Herte M., Klingner C., Wälte M., et al. . 2016. Calcium-mediated actin reset (CaAR) mediates acute cell adaptations. eLife. 5:e19850 10.7554/eLife.19850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Mattson M.P., and Furukawa K.. 2002. Endoplasmic reticulum calcium release is modulated by actin polymerization. J. Neurochem. 82:945–952. 10.1046/j.1471-4159.2002.01059.x [DOI] [PubMed] [Google Scholar]