Abstract
Objectives:
During the reproductive cycle, altered calcium homeostasis is observed due to variable demand for mineral requirements. This results in increased bone resorption during the time period leading up to parturition and subsequent lactation. During lactation, women will lose 1-3% of bone mineral density per month, which is comparable to the loss experienced on an annual basis post-menopausal. The purpose of this study was to determine the effect of parity on bone formation in middle-aged mice.
Methods:
Mice were mated and grouped by number of parity and compared with age matched nulliparous controls. Measurements were taken of femoral trabecular and cortical bone. Calcium, protein and alkaline phosphatase levels were also measured.
Results:
An increase in trabecular bone mineral density was observed when comparing mice that had undergone parity once to the nulliparous control. An overall decrease in trabecular bone mineral density was observed as parity increased from 1 to 5 pregnancies. No alteration was seen in cortical bone formation. No difference was observed when calcium, protein and alkaline phosphatase levels were assessed.
Conclusions:
This study demonstrates that number of parity has an impact on trabecular bone formation in middle-aged mice, with substantial changes in bone density seen among the parous groups.
Keywords: Multiparity, Trabecular Bone, Cortical Bone, Middle Age, Multiple Gestations
Introduction
During the reproductive cycle, from pregnancy to lactation, there is an increased demand for mineral requirements to help facilitate fetal development and milk production. In order to maintain appropriate mineral requirements, apart from exogenous intake, the maternal body exhibits an altered calcium homeostasis during this time. This increased demand can be partially met with alterations in intestinal calcium absorption and renal reabsorption[1]. These compensations alone, however, cannot meet fetal developmental requirements.
In order to meet metabolic demands, lactating humans exhibit higher serum levels of calcium and PTH during lactation compared to their non-lactating counterparts[1,2]. The subsequent increase in PTH results in a variety of effects. In particular, there is a significant increase in bone resorption during the time period leading up to parturition and subsequent lactation[1-6].
A variety of clinical studies have demonstrated a decrease in bone mineral density (BMD) during pregnancy, parturition and lactation[1,2,6-8]. However, this substantial decrease has been shown to be primarily transient, with no long-term adverse effects on bone health[9]. The only exception to this is when pregnancy-associated osteoporosis in humans occurs[1,2,6].
Bone loss during subsequent lactation has been documented in many other species, including sheep[10], dogs[11], pigs[12], monkeys[13,14] and humans[15,16]. Nursing humans typically produce 300 mg to 400 mg of calcium in milk on a daily basis[1,2,6]. During the nine-month period of lactation, humans exhibit a four-fold increase in loss of calcium greater than the metabolic requirement of a fetus during pregnancy[17]. As a result, women continue to lose BMD throughout lactation. In 2014, Tsvetov et al. described prolonged breast-feeding duration was significantly correlated to a low BMD[18]. In general, a lactating woman will lose 1-3 percent of her BMD per month. In comparison, a woman will lose a similar amount on a yearly basis following menopause[2].
Bone mineral density begins to increase immediately following lactation. In rodents, 20-30 percent of their skeletal mass is lost during pregnancy and lactation. That amount is regained within four weeks post-weaning[19-24]. Ardeshirpour et al. reported that at 28 days post-weaning, bone mineral density in mice had increased by 37 percent in the spine, 27 percent at the femur and 25 percent throughout the body[25].
Demirtaş et al. documented that grand multiparity had no effect on post-menopausal BMD[26]. Some additional studies in humans have supported no correlation between parity and post-menopausal BMD[27] but other studies have found both positive correlations[28,29] and negative correlations[30,31] between parity and BMD in humans. The overall effect of parity on post-menopausal BMD is unclear. One study has suggested an initial increase in post-menopausal hip BMD in humans, however, this difference quickly disappears following the menopausal transition[32]. Despite the contradiction within the literature, there is a wide consensus that depicts no long-term impact of parity on post-menopausal fracture incidence.
Given the lack of consensus regarding effect of multiple pregnancies on post-menopausal bone mineral structure, we investigated the effect of parity on bone formation in mice. In particular, we focused on BMD as well as the trabecular and cortical bone compartments in mice that have undergone parity 1-5 times. We found that number of parity had significant effect on bone formation in middle-aged mice with significant correlation between bone density and parity.
Methods
Mice breeding colony and husbandry
C57BL/6J mice were bred and maintained in the Animal Facility, Indiana University School of Medicine, Indianapolis, IN. Six-week old (±3 days) C57BL/6J mice were obtained from Jackson Laboratory (Bar Harbor, ME) and acclimatized for at least one week, then breeding pairs were established to obtain timed pregnancies. The females were estrus synchronized by introducing male bedding materials for 24 hours, paired with males (1 male: 1 female mating) for three days, separated from males, and caged individually to determine the exact date of birth of the pups. The non-pregnant females were identified and mated with different males or group housed up to five females/cage. Young mice were weaned at three weeks of age. Some first generation siblings from the colony were used for subsequent breeding. All activities of the breeding colongy were carefully recorded to evaluate reproductive performance of females. Females were retired from breeding when litter size decreased, which was approximately at nine to 12 months of age, and then enrolled in the current study. All mice received commercial extruded lab rodent chow (Harlan 2018SX, Harlan Laboratories Inc., Indianapolis, IN) ad libitum in cage hoppers and automated reverse osmosis water. Animal rooms were maintained on a 12-h light/dark cycle were maintained at 21±3°C with 30-80% relative humidity and at least 10 air changes per hour of 100% conditioned fresh air. All studies were approved by the Indiana University School of Medicine Institutional Animal Care and Use Committee.
Analysis of femurs
Mice were euthanized and the right distal femurs were analyzed by standard micro-computed tomography (microCT, SkyScan 1172, Bruker-microCT, Kontich, Belvium) utilizing the procedures and nomenclature recommended by Bouxsein and colleagues[33]. For each femur, the trabecular bone compartment was sliced into 50 segments from the cortical shell in a region approximately 0.5 mm above the most proximal portion of the growth plate. The X-ray source was set at 60 keV and 167 µA over an angular range of 360 degrees with a 6-µm pixel size, and projection images were reconstructed using Skyscan and Nrecon. Images were binarized, with a threshold of 70 on a 0 to 255 scale. The following three-dimensional bone volume parameters were calculated: - trabecular bone volume fraction (BV/TV), trabecular thickness (Tb.Th), trabecular number (Tb.N) and trabecular separation (Tb.Sp). To convert grayscale values to density (mg/cm3), standard CTan software was used. Two densities were assessed, a 0.5 mm section of the trabecular bone compartment and the cortical bone itself.
Cell isolation and culture
Long bones were isolated from mice and crushed in phosphate buffered saline using a sterile mortar and pestle to remove bone marrow. Bone fragments were then transferred to collagenase and digested twice at 37°C, once for 15 minutes and once for 30 minutes. Digested cells were counted and seeded at 1x105 cells/ml for alkaline phosphatase and calcium deposition assays as detailed below.
Calcium deposition
Calcium deposition was assessed by eluting Alizarin Red S (Sigma-Aldrich, St. Louis, MO) from cell monolayers[34]. Monolayers were washed twice with PBS and fixed in ice cold 70% (vol/vol) ethanol for 1 hour. Monolayers were washed twice with water and stained with 40 mM Alizarin Red S (pH 4.2) for 10 minutes. Samples were washed with water to remove unbound dye five times and once with PBS. Bound dye was eluted by incubating monolayers with 1% (vol/vol) cetylpyridinium chloride in 10mM sodium phosphate (pH 7.0) for 15 minutes. Absorbance from aliquots was measured at 562 nm (GENios Plus; Tecan, Männedorf, Switzerland), and Alizarin Red Concentrations were calculated from a measured standard curve (Ca/mol of dye in solution).
Alkaline phosphatase assay
Alkaline phosphatase activity was determined by the colorimetric conversion of p-nitrophenol (Sigma-Aldrich) and normalized to total protein (bicinchoninic acid or BCA, Pierce Chemical. Rockford, IL)[34]. Cells were washed two times with PBS. They were then lysed with 0.1% (vol/vol) Triton X-100 supplemented with a cocktail of broad-range protease inhibitors (Pierce Chemical), frozen and thawed twice and cleared via centrifugation. Lysates were incubated with 3 mg/mL p-nitrophenol phosphate in an alkaline buffer (pH 8.0) for 30 minutes at 37°C. The reaction was stopped by the addition of 20 mM NaOH and read at 405 nM (GENios Plus; Tecan). Alkaline phosphatase activity was determined by comparison with known p-nitrophenol standards.
Statistics
Unless otherwise stated, data obtained are presented as mean plus or minus standard deviation. Pearson correlation coefficients (bivariate correlation) were used to determine R2 values. Linear regressions using an analysis of variance model were performed to compare groups. All statistical analyses were performed with Statistical Package for Social Sciences (SPSS 21; Norusis/SPSS Inc) software and were 2-tailed or ANOVA with Tukey’s test with a level of significance set at 0.05.
Results
Analysis of reproductive performance
In this study 63 female mice were used. Numbers of mice in each group is as follows: no litters-3mice, 1 litter-6 mice, 2 litters-5 mice, 3 litters-29 mice, 4 litters-13 mice, 5 litters-2 mice. Age, mice delivered in each litter, and time between litters were tracked for all mice. Averages can be found in Table 1. Specifically, the average age of these mice range from approximately 9 months to 1 year. The average age of the mice in this study ranged from 287±27 days for the mice with one litter to 355±35 days for mice with 5 litters. The average number of mice per litter was not correlated to number of litters and ranges from 6.5±3.5 to 8.0±0.3 pups per litter. Time from birth to first litter decreased with number of litters (137±0 days for 1 litter to 74±6 days for 5 litters). Average time from birth to last litter increased with number of litters (137±0 days for 1 litter to 283±24 days for 5 litters.
Table 1.
Number of total litters, average age, mice per litter, age at birth of first litter, and age at birth of last litter.
| Number of Litters | Number of mice | avg age (days) | avg litter size (pups.litter) | avg time to first litter (days) | avg time to last litter (days) |
|---|---|---|---|---|---|
| 1 | 6 | 283 ± 27 | 6.5 ± 3.5 | 137 ± 0 | 137 ± 0 |
| 2 | 5 | 295 ± 22 | 7.8 ± 1.3 | 114 ± 34 | 155 ± 35 |
| 3 | 29 | 218 ± 23 | 7.6 ± 2.0 | 94 ± 29 | 219 ± 44 |
| 4 | 13 | 339 ± 31 | 6.3 ± 1.3 | 89 ± 22 | 272 ± 39 |
| 5 | 2 | 355 ± 35 | 8.0 ± 0.3 | 74 ± 6 | 283 ± 24 |
*avg=average.
Analysis of trabecular and cortical bone
The trabecular bone from the distal end of a femur for each mouse was analyzed and bone volume/tissue volume (BV/TV), trabecular thickness (Tb.Th), trabecular number (Tb.N), and trabecular separation (Tb.Sp) were determined. BV/TV, decreased as pregnancies increased from 1-5 liters (2.05±0.37% for 1 litter to 0.43±0.47 for 5 litters). Interestingly, mice with no pregnancies had a smaller trabecular bone BV/TV than mice with 1 pregnancy (1.12±0.77% for no pregnancies vs. 2.05±0.37% for 1 litter). All groups showed statistically significant differences from the mice with 1 litter (p<0.05) (Figure 1a).
Figure 1.
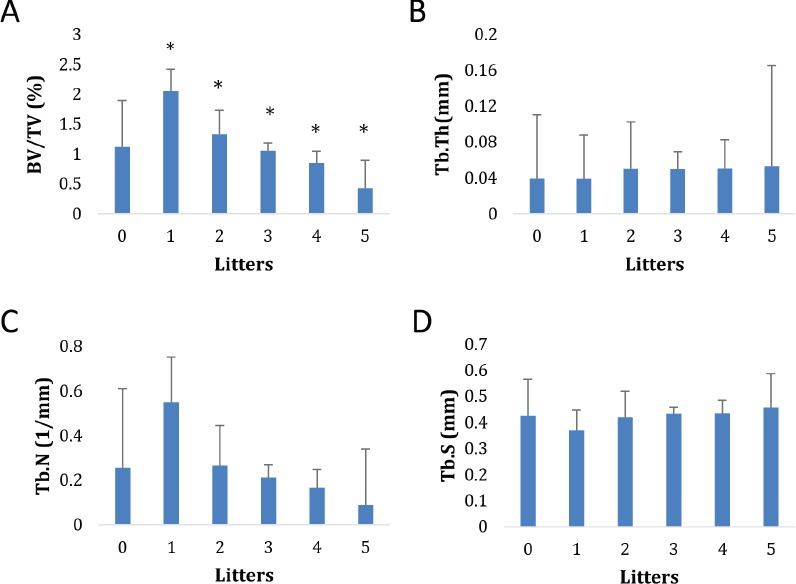
Effect of number of litters on trabecular BV/TV (A), Trabecular thickness (B), Trabecular Number (C), and Trabecular Separation (D). Bars represents mean±standard deviation * Indicates statistically significant difference (p<0.05) compared with 1 litter.
Trabecular number decreased as number of litters increased (from 1 to 5 litters). Values ranged from 0.55±0.20/mm to 0.09±0.25/mm (Figure 1c). Mice that had 1 litter had significantly higher Tb.N than all other groups, including mice with no pregnancies (p<0.05). Analysis of trabecular thickness and spacing, as well as tissue mineral density, revealed no significant difference with respect to number of pregnancies (Figure 1 b,d).
Mid-shafts of extracted femurs were analyzed. No differences were found in BV/TV or in cortical bone area (Figure 2 a,b). Observed BV/TV values with respect to age also did not indicate significant difference in values. In addition, no differences were observed in cortical bone porosity.
Figure 2.
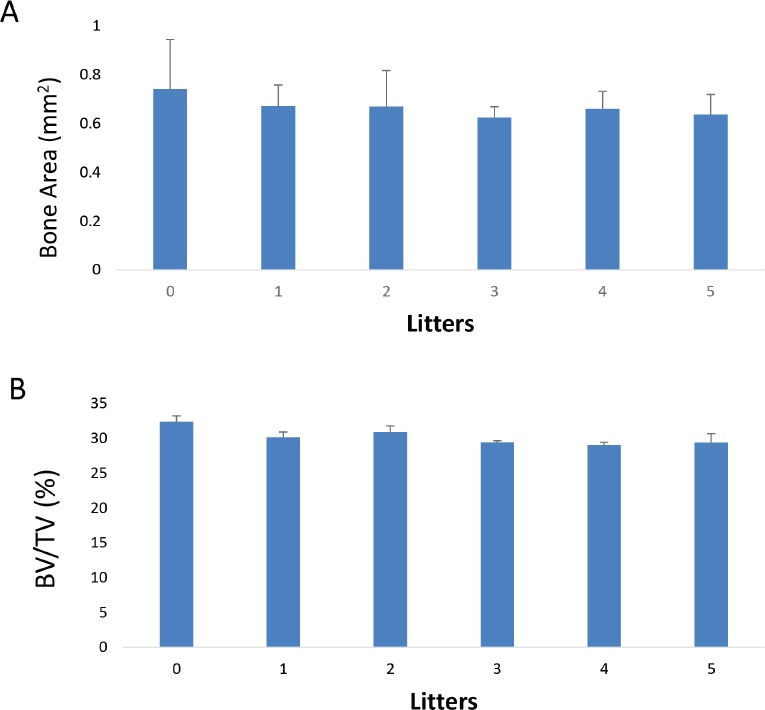
Effect of number of litters on cortical bone area (A), and cortical BV/TV (B), Bars represents mean±standard deviation.
Cell function
Osteoblast lineage cells were generated from individual mice and were assessed in terms of their functionality by determining alkaline phosphatase activity and calcium deposition (Figure 3 a-c). Alkaline phosphatase is the major enzymatic activity of osteoblasts and calcium deposition serves as a surrogate for mineralization. The highest alkaline phosphatase level was observed in mice with 4 litters while the lowest level recorded was at those with 1 litter. In general, alkaline phosphatase levels gradually increased as litters increased from 1 to 5 (Figure 3 a,b).
Figure 3.
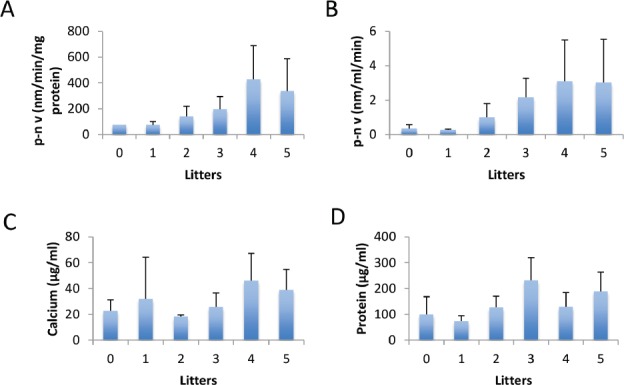
Effect of number of litters on alkaline phosphatase (A,B), calcium deposition (C), and protein (D). Bars represents mean±standard deviation.
Calcium deposition was highest among mice that had 4 litters, and lowest in nulliparous mice (Figure 3c). There was no significant correlation observed between calcium deposition and litter number.
Next, a BCA assay was utilized to analyze the overall protein concentration in cultures (Figure 3d). The highest protein concentration noted was in mice with 3 litters. The lowest value noted was in mice with 1 litter. There was no significant correlation between protein concentration and litter number.
Discussion
During pregnancy, parturition and subsequent lactation, numerous species, including humans, mice and rats, undergo significant alterations in calcium homeostasis[35-39]. This results in varying bone densities at different stages. While it has been shown that multiparity does not affect fracture incidence in humans[9], its effect on various aspects of bone mineral density, specifically cortical or trabecular bone, has not been well studied.
When analyzing the trabecular bone of mice in this study, a correlation was observed between parity and bone density. As the number of litters increased (from 1 to 5 litters), measurements of trabecular bone in BV/TV and Tb.N both decreased. These results may suggest that more trabecular bone is broken down as number of pregnancies increases. However, while Tb.N decreased, there was no correlation seen with regard to Tb.Th and Tb.Sp. This indicates that no differences exist in the width or spacing of the rods and plates that make up trabecular bone. There were also no significant differences in tissue mineral density or cortical bone parameters. Taken together these data suggest that parity in mice affects trabecular bone, but does not impact cortical bone or the total density of the bone. These observations are similar to findings from a study conducted by MJ de Bakker et al.[9] where they observed a decrease in trabecular bone following multiple gestations when measurements were taken following a 6-week post-weaning phase[9]. With respect to cortical bone analysis, no significant change in cortical bone measurements was observed with respect to number of litters. Of note, Bakker et al[9] reported an increase in cortical bone following multiple gestations.
In our study, we noted a similar trend in trabecular bone with respect to parity to Bakker et al[9]. However, we did not see any difference in cortical bone. Despite this, our results support Bakker et al claim of a decrease in trabecular bone with increasing parity. In order to further elucidate the presence of cortical bone compensation hypothesized by Bakker et al, additional studies are required[9].
Interestingly, trabecular bone volume fraction was significantly increased in mice having undergone parity once as compared to nulliparous mice. Bone density was also observed at its highest level for mice that had undergone parity once. This appears to indicate that undergoing pregnancy once may positively impact trabecular bone volume and bone density later in life for mice, but whether this is true for humans remains to be determined.
Protein concentration and functional analysis of effects of parity on osteoblast cultures generated from these mice demonstrated that as parity increases, alkaline phosphatase enzymatic activity increases, with no change in calcium deposition or total protein concentration. However, these data must be interpreted cautiously as the sample size was smaller for certain parity groups.
There are several limitations with the present study. First, while alterations in mouse bones have been shown to be indicative of changes seen in human bone, there are innate differences between the two species that cannot be accounted for. The observations in this study require additional confirmation in a clinical study. Notably, the altered pattern of trabecular vs. cortical bone requires further investigation. Secondly, when observing trabecular bone, a decrease was observed with respect to number of litters as well as age. The latter has been well documented in the literature, with progression of age resulting in a decrease in trabecular bone at a post-menopausal phase. However, there seems to be a greater decrease in trabecular bone exhibited in our study than what would ordinarily be accounted for by age alone (as observed in nulliparous mice). Additionally, this study primarily focused on analysis at one skeletal site, the distal femur. While this location has been frequently utilized in prior bone studies, location-specific effects must be taken into consideration when applied to other skeletal areas.
During lactation, humans exhibit higher calcium and PTH levels in order to meet the newly established homeostasis[1,2]. While a significant portion is compensated by increased intestinal absorption and renal reabsorption, bone resorption is still required in order to meet fetal developmental requirements. Our observations suggest parity affects certain aspects of skeletal homeostasis. In particular, trabecular bone and osteoblast enzymatic activity seem to be most impacted. Furthermore, it seems increasingly likely that there are additional compensatory mechanisms at play in order to preserve bone health. The increased production of cortical bone during post-weaning periods could suggest compensation for decreased trabecular bone seen with multiparity.
Acknowledgements
This project has been funded in whole or in part with federal funds from the National Institute (NIH) of Aging R01 AG046246. Opinions, interpretations, conclusions, and recommendations are those of the author(s) and are not necessarily endorsed by the NIH.
Footnotes
The authors have no conflict of interest.
Edited by: F. Rauch
References
- 1.Kovacs CS. Maternal Mineral and Bone Metabolism During Pregnancy, Lactation, and Post-Weaning Recovery. Physiol Rev. 2016;96:449–547. doi: 10.1152/physrev.00027.2015. [DOI] [PubMed] [Google Scholar]
- 2.Kirby BJ, Ardeshirpour L, Woodrow JP, Wysolmerski JJ, Sims NA, Karaplis AC, et al. Skeletal recovery after weaning does not require PTHrP. J Bone Miner Res. 2011;26:1242–51. doi: 10.1002/jbmr.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salari P, Abdollahi M. The influence of pregnancy and lactation on maternal bone health: a systematic review. J Family Reprod Health. 2014;8:135–48. [PMC free article] [PubMed] [Google Scholar]
- 4.Wysolmerski JJ. The evolutionary origins of maternal calcium and bone metabolism during lactation. J Mammary Gland Biol Neoplasia. 2002;7:267–276. doi: 10.1023/a:1022800716196. [DOI] [PubMed] [Google Scholar]
- 5.Lanske B, Razzaque MS. Vitamin D and aging: old concepts and new insights. J Nutr Biochem. 2007;18:771–7. doi: 10.1016/j.jnutbio.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ohata Y, Ozono K, Michigami T. Current concepts in perinatal mineral metabolism. Clin Pediatr Endocrinol. 2016;25:9–17. doi: 10.1297/cpe.25.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim HJ, Kwon H, Oh SW, Lee CM, Joh HK, Kim Y, et al. Breast feeding is associated with postmenopausal bone loss: findings from the korea national health and nutrition examination survey. Korean J Fam Med. 2015;36:216–20. doi: 10.4082/kjfm.2015.36.5.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Costa ML, Krupa FG, Rehder PM, Sousa MH, Costa-Paiva L, Cecatti JG. Forearm bone mineral density changes during postpartum and the effects of breastfeeding, amenorrhea, body mass index and contraceptive use. Osteoporosis Int. 2012;23:1691–8. doi: 10.1007/s00198-011-1767-y. [DOI] [PubMed] [Google Scholar]
- 9.de Bakker MJC, Altman-Singles AR, Li Y, Tseng W, Li C, Liu XS. Adaptations in the Microarchitecture and Load Distribution of Maternal Cortical and Trabecular Bone in Response to Multiple Reproductive Cycles in Rats. JBMR. 2017 doi: 10.1002/jbmr.3084. ahead of eprint. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benzie D, Boyne AD, Dalgarno AC, Duckworth J, Hill R, Walker DM. Studies of the skeleton of the sheep. I. The effect of different levels of dietary calcium during pregnancy and lactation on individual bones. J Argic Sci. 1955;46:425–444. [Google Scholar]
- 11.Miller MA, Omura TH, Miller SC. Increased cancellous bone remodeling during lactation in beagles. Bone. 1989;10:279–285. doi: 10.1016/8756-3282(89)90065-3. [DOI] [PubMed] [Google Scholar]
- 12.Spencer GR. Pregnancy and lactational osteoporosis. Animal model: porcine lactational osteoporosis. Am J Pathol. 1979;95:277–280. [PMC free article] [PubMed] [Google Scholar]
- 13.Hiyaoka A, Yoshida T, Cho F, Yoshikawa Y. Changes in bone mineral density of lumbar vertebrae after parturition in African green monkeys (Cercopithecus aethiops) Exp Anim. 1996;45:257–259. doi: 10.1538/expanim.45.257. [DOI] [PubMed] [Google Scholar]
- 14.Lees CJ, Jerome CP, Register TC, Carson CS. Changes in bone mass and bone biomarkers of cynomolgus monkeys during pregnancy and lactation. J Clin Endocrinol Metab. 1998;83:4298–4302. doi: 10.1210/jcem.83.12.5344. [DOI] [PubMed] [Google Scholar]
- 15.Kovacs CS. Calcium and bone metabolism disorders during pregnancy and lactation. Endocrinol Metab Clin North Am. 2011;40:795–826. doi: 10.1016/j.ecl.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 16.Hwang IR, Choi YK, Lee WK, Kim JG, Lee IK, Kim SW, et al. Association between prolonged breastfeeding and bone mineral density and osteoporosis in postmenopausal women: KNHANES 2010-2011. Osteoporosis Int. 2016;27:257–65. doi: 10.1007/s00198-015-3292-x. [DOI] [PubMed] [Google Scholar]
- 17.O'Brien KP, Donangelo CM, Ritchie LD, Gildengorin G, Abrams S, King JC. Serum 1,25-dihydroxyvitamin D and calcium intake affect rates of bone calcium deposition during pregnancy and the early postpartum period. Am J Clin Nutr. 2012;96:64–72. doi: 10.3945/ajcn.111.029231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsvetov G, Levy S, Benbassat C, Shraga-Slutzky I, Hirsch D. Influence of number of deliveries and total-breast-feeding time on bone mineral density in premenopausal and young postmenopausal women. Maturitas. 2014;77:249–54. doi: 10.1016/j.maturitas.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 19.VanHouten JN, Wysolmerski JJ. Low estrogen and high parathyroid hormone relate peptide levels contrinute to accelerated bone resorption and bone loss in lactating mice. Endocrinology. 2003;144:5521–9. doi: 10.1210/en.2003-0892. [DOI] [PubMed] [Google Scholar]
- 20.VanHouten JN, Dann P, Stewart AF, Watson CJ, Pollak M, Karaplis AC, et al. Mammary-specific deletion of parathyroid hormone-related protein preserves bone mass during lactation. J Clin Invest. 2003;112:1429–36. doi: 10.1172/JCI19504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ardeshirpour L, Brian S, Dann P, VanHouten J, Wysolmerski J. Increased PTHrP and decreased estrogens alter bone turnover but do not reproduce the effects of lactation on the skeleton. Endocrinology. 2010;151:5591–601. doi: 10.1210/en.2010-0566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hood WR, Hobensack M. The effect of locomotion on the mobilization of minerals from the maternal skeleton. PLoS One. 2015;10:e0122702. doi: 10.1371/journal.pone.0122702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Honda A, Kurabayashi T, Yahata T, Tomita M, Matsushita H, Takakuwa K, et al. Effects of pregnancy and lactation on trabecular bone and marrow adipocytes in rats. Calcif Tissue Int. 2000;67:367–72. doi: 10.1007/s002230001145. [DOI] [PubMed] [Google Scholar]
- 24.Gonen E, Sahin P, Ozbek M, Kovalak E, Yologlu S, Ates Y. Effects of pregnancy and lactation on bone mineral density, and their relation to the serum calcium, phosphorus, calcitonin and parathyroid hormone levels in rats. J Endocrinol Invest. 2005;28:322–6. doi: 10.1007/BF03347197. [DOI] [PubMed] [Google Scholar]
- 25.Ardeshirpour L, Dann P, Adams DJ, Nelson T, VanHouten J, Horowitz MC, et al. Weaning triggers a decrease in receptor activator of nuclear factor-kappaB ligand expression, widespread osteoclast apoptosis, and rapid recovery of bone mass after lactation in mice. Endocrinology. 2007;148:3875–86. doi: 10.1210/en.2006-1467. [DOI] [PubMed] [Google Scholar]
- 26.Demirtaş Ö, Demirtaş G, Hurşitoğlu BS, Terzi H, Şekerci Z, Ök N. Is grand multiparity a risk factor for osteoporosis in postmenopausal women of lower socioeconomic status? Eur Rev Med Pharmacol Sci. 2014;18:2709–14. [PubMed] [Google Scholar]
- 27.Kojima N, Douchi T, Kosha S, Nagata Y. Cross-sectional Study of the effects of parturition and lactation on bone mineral density later in life. Maturitas. 2002;41:203–209. doi: 10.1016/s0378-5122(01)00296-1. [DOI] [PubMed] [Google Scholar]
- 28.Cure-Cure C, Cure-Ramirez P, Teran E, Lopez-Jaramillo P. Bone-mass peak in multiparity and reduced risk of bone fractures in menopause. Int J Gynaecol Obstet. 2002;76:285–291. doi: 10.1016/s0020-7292(01)00583-5. [DOI] [PubMed] [Google Scholar]
- 29.Fox KM, Magaziner J, Sherwin R, Scott JC, Plato CC, Nevitt M, et al. Reproductive correlates of bone mass in elderly women: The Study of Osteoporotic Fractures Research Group. J Bone Miner Res. 1993;8:901–908. doi: 10.1002/jbmr.5650080802. [DOI] [PubMed] [Google Scholar]
- 30.Gur A, Nas K, Cevik R, Sarac AJ, Ataoglu S, Karakoc M. Influence of number of pregnancies on bone mineral density in postmenopausal women of different age groups. J Bone Miner Metab. 2003;21:234–241. doi: 10.1007/s00774-003-0415-9. [DOI] [PubMed] [Google Scholar]
- 31.Allali F, Maaroufi H, Aichaoui SE, Khazani H, Saoud B, et al. Influence of parity on bone mineral density and peripheral fracture risk in Moroccan postmenopausal women. Maturitas. 2007;57:392–398. doi: 10.1016/j.maturitas.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 32.Streeten EA, Ryan KA, McBride DJ, Pollin TI, Shuldiner AI, Mitchell BD. The Relationship between Parity and Bone Mineral Density in Women Characterized by a homogenous Lifestyle and high parity. J Clin Endocrinol Metab. 2013;90:4536–41. doi: 10.1210/jc.2004-1924. [DOI] [PubMed] [Google Scholar]
- 33.Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, Müller R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. JBMR. 2010;27:1468–86. doi: 10.1002/jbmr.141. [DOI] [PubMed] [Google Scholar]
- 34.Ciovacco WA, Goldberg CG, Taylor AF, Lemieux JM, Horowitz MC, Donahue HJ, et al. The role of gap junctions in megakaryocyte-mediated osteoblast proliferation and differentiation. Bone. 2009;44:80–86. doi: 10.1016/j.bone.2008.08.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vanhouten JN, Wysolmerski JJ. The calcium-sensing receptor in the breast. Best Pract Res Clin Endocrinol Metab. 2013;27:403–14. doi: 10.1016/j.beem.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salari P, Abdollahi M. The influence of pregnancy and lactation on maternal bone health: a systematic review. J Family Reprod Health. 2014;8:135–48. [PMC free article] [PubMed] [Google Scholar]
- 37.Vajda EG, Bowman BM, Miller SC. Cancellous and cortical bone mechanical properties and tissue dynamics during pregnancy, lactation and postlactation in the rat. Biol Reprod. 2001;65:689–695. doi: 10.1095/biolreprod65.3.689. [DOI] [PubMed] [Google Scholar]
- 38.Wysolmerski JJ. Conversations between breast and bone: physiological bone loss during lactation as evolutionary template for osteolysis in breast cancer and pathological bone loss after menopause. IBMS. 2007;4:209–225. [Google Scholar]
- 39.Clarke BL, Khosla S. Female reproductive system and bone. Arch Biochem Biophys. 2010;503:118–28. doi: 10.1016/j.abb.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]


