Abstract
Background:
Despite widespread clinical use of weight bearing exercises to manage low bone mineral density (BMD) in children and adolescents with cerebral palsy (CP), previous studies have reported heterogeneous results on the effect of weight bearing exercise on BMD.
Purpose:
We performed the current meta-analysis to assess the effects of weight bearing exercise on increasing BMD in children who have CP with low BMD.
Materials and methods:
We searched PubMed, Cochrane, and Embase from inception through to October 2016 for studies that aims to investigate the effect of weight bearing exercise on BMD in children with CP. Following the searching result, the 118 relevant studies were reviewed and undergone selection process. Standardized mean difference (SMD), 95% confidence intervals (CIs) and p-values were calculated for analysis.
Results:
Three studies were ultimately included in the meta-analysis: one randomized-controlled study and two case-controlled studies. No significant difference was observed in the BMD of the lumbar spine between before treatment and after treatment (SMD,0.341; 95% CI,-0.647-1.330; p=0.449) but the BMD of the femur significantly improved after applying weight bearing exercise compared to pre-treatment values (SMD, 0.916; 95% CI, 0.382-1.114; p<0.001).
Conclusions:
Weight bearing exercise has a significant effect on improving BMD of the femur in children with CP.
Keywords: Cerebral Palsy, Osteoporosis, Weight-bearing, Exercise, Bone Density
Introduction
Children and adolescents with cerebral palsy (CP) are at risk of secondary osteoporosis because of reduced mobility[1,2]. Immobilization predisposes patients to bone resorption, which can reduce bone mineral density (BMD), and lead to an increased risk of fractures[2,3]. Other risk factors of low BMD in children with CP are inadequate nutrition, and the use of anticonvulsants which may have adverse effects on bone mineralization[4]. Limited outdoor activities also cause a lack of sunlight exposure which leads to low serum 25-hydroxy vitamin D levels. In children with CP, the annual fracture rate is 5%, which is twice the fracture rate seen in typically developing children. The shaft or supracondylar region of the femur is the most common site of fractures in children with CP[5,6]. Significantly decreased bone density is prevalent in non-ambulatory children with moderate - severe CP after 10 years old. However, predicting which children will have a fracture and when to start treatment continues to be a challenge[7].
The current definition of osteoporosis in children includes a significant history of fracture as well as a BMD Z-score <-2, whereas osteoporosis in adults is diagnosed with a BMD T-score < -2.5 only[8,9]. Fractures, especially in the lower extremities, worsen the degree of immobilization, which can induce lower BMD with further increases in fracture risk[10]. Thus, it is inappropriate wait until after a diagnosis of osteoporosis to start treatment in children with CP.
Pharmacological and other conservative approaches have been clinically used to improve low BMD in children with CP. There is no consensus guideline in applying pharmacologic agents in children, despite their evidences in improving BMD[11]. Other conservative approaches including increasing sunshine exposure time, and taking dietary calcium and vitamin D supplements are also known to be helpful in improving low BMD in children with CP[4]. Weight bearing exercise is also widely used to improve BMD because mechanical stress is an important factor in maintaining bone health. Research has been performed to investigate the effects of weight bearing exercise, but the results of previous studies are too heterogeneous to draw a conclusion about the efficacy of weight bearing exercise on increasing BMD in CP. Thus, we searched for studies published before October 2016 and analyzed the efficacy of weight bearing exercise in children with CP.
Materials and methods
This review was conducted based on PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) checklist[12].
Literature sources and study identification
We searched PubMed, Embase, and Cochrane from inception of the study through October 2016 to identify interventional studies that applied weight bearing exercise to improve BMD in children with CP. First, we searched PubMed records using the following criteria: (“Bone Diseases, Metabolic” [Mesh] OR osteoporosis OR osteopenia OR bone mineral OR bone density) AND (“Pediatrics” [Mesh] OR child OR children OR adolescent) AND (cerebral palsy) AND (intervention OR exercise OR rehabilitation OR physical therapy OR physiotherapy OR weight bearing OR standing). Second, we searched Embase using the following criteria: (‘osteoporosis’/exp OR ‘osteopenia’/exp OR ‘bone mineral’/exp OR ‘bone density’/exp) AND (‘child’/exp OR ‘pediatrics’/exp OR ‘adolescent’/exp) AND (‘cerebral palsy’/exp) AND (intervention OR exercise OR rehabilitation OR ‘physical therapy’ OR physiotherapy OR ‘weight bearing’ OR standing). Third, we searched Cochrane using following criteria: (osteoporosis OR osteopenia) AND (child* OR pediatric* OR adolescent*) AND (cerebral palsy) AND (intervention OR exercise OR rehabilitation OR physical therapy OR physiotherapy OR weight bearing OR standing). One hundred and eighteen studies retrieved. Two physicians screened all the titles and abstracts independently for candidate studies and excluded studies that applied physical intervention without weight bearing exercise, studies that contained other osteoporosis treatment, and studies involving other pediatric diseases. The full texts of the screened studies were reviewed for eligibility and disagreements between the two reviewers were resolved by consensus. Only studies written in English were selected.
Study selection
All studies applying weight bearing exercise for the purpose of improving low BMD in children with CP were included. Studies involving other concomitant intervention procedures were excluded. We included studies that provided both pre-treatment and post-treatment BMD, which are expressed in grams per square centimeter in the lumbar spine or femur. BMD was measured by dual energy X-ray absorptiometry (DXA). Inclusion criteria were: (1) English-language full-test studies; (2) participants <18 years old; (3) participants with CP; (4) participants received physical intervention containing weight bearing exercise; (5) studies providing both pre-treatment and post-treatment BMD values; (6) outcome values included BMD (g/cm2).
Data abstraction
We extracted data regarding number of patients, treatment duration, pre- and post-treatment BMD values, and p-values for comparisons between time intervals. In the article by Gudjonsdottir et al.[3], within one week of the initial measurements, 4 children with CP stood for 30 min per day, five times a week for 8 weeks in a static or dynamic stander. The dynamic stander provided 2.0 cm of vertical movement distance. For statistical analysis, we regarded all participants as one group regardless of the stander type, because both standers contained a weight bearing component. In the article by Dalén el al.[2], 4 children with CP used a vibrating platform with standing shell for 8 or 9 months. Two of the children used the vibrating platform for 8 to 9 months, whilst the others were controls (period I). After 1 year, the groups were switched, with the former controls using the platform for 8-9 months (period II) over 1 year. No instruction was given on the frequency of use of the platform in period I, but during period II the study group was instructed to use the platform two to three times per week. In the article by Chen et al.[1], a home-based virtual cycling training (hVCT) program was applied for 40 min per day, three times a week, for 12 weeks to 13 children with CP. The hVCT included a loaded sit-to-stand exercise or 20 times. The load was adjusted by adding weight bags to a backpack worn by the participant, with weights ranging from 0.5 to 3 kg.
Statistical analysis
BMD values, expressed in grams per square centimeter, were analyzed statistically. Mean ± standard deviation or mean ± standard error values of the BMD of the lumbar spine and femur were obtained before and after treatment. Heterogeneity was calculated with the Cochrane Q statistic test and the I2 test. The I2 test describes the rate of variation across studies due to heterogeneity rather than chance and ranges from 0 (no heterogeneity) to 100 (maximum heterogeneity). All results are reported with 95% confidence intervals (CIs), and all p-values are two-tailed. The DerSimonian-Laird random-effects model was used when there was significant heterogeneity among the outcomes (I2>50). This model assumes that the true treatment effects in individual studies may be different from one another, and that these treatment effects are normally distributed. Outcomes that did not present with heterogeneity (I2<50) were analyzed with a fixed-effects model. The fixed-effects model uses the inverse variance approach and assumes that all studies come from a common population. Because of the small number of studies, we used a funnel plot, Begg’s test, and Egger’s test simultaneously to detect publication bias. Analyses were performed by using Comprehensive Meta-Analysis ver. 2.0 (Biostat, Englewood, NJ, USA).
Results
Search results and characteristics
The primary search for the effect of weight bearing exercise on low BMD in children with CP identified 154 relevant studies, among them 36 duplicated studies were discarded. One hundred and six studies were excluded after screening the abstract. Nine studies were additionally excluded: two studies used other measurements (cancellous bone density and bone quantitative ultrasound), three studies were measured in vTBMD with insufficient data for meta-analysis, and the other four studies showed only percentage change of BMD or correlation with other variables. Finally, three studies were included for meta-analysis. These three studies examined the BMD of the lumbar spine and femur (Table 1-1, Table 1-2, Figure 1). Two of the studies showed raw data of pre- and post-treatment BMD in both femurs, from which we could calculate the mean, standard deviation, and p-value for each side. The New Castle Ottawa scale was used to assess the quality of studies included in this meta-analysis (Table 2).
Table 1-1.
Studies to evaluate the effects of weight bearing exercise on BMD of lumbar spine.
| Study | Year | Design | Title | Intervention | Outcomes (BMD of Lumbar spine) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Before treatment | After treatment | P value | |||||||||
| n | mean | SD | n | mean | SD | ||||||
| Gudjonsdottir B | 2002 | Prospective case control | Effects of a dynamic versus a static prone stander on bone mineral density and behavior in four children with severe cerebral palsy. | 8-week standing (2 dynamic /2 conventional) | 4 | 0.458 | 0.057 | 4 | 0.462 | 0.062 | 0.759 |
| Dalén Y. | 2012 | Prospective case control | Observations of four children with severe cerebral palsy using a novel dynamic platform. A case report | 8-9month, dynamic platform with standing swell (vibration, jump, rotation) | 4 | 0.383 | 0.048 | 4 | 0.423 | 0.051 | 0.034 |
| C.-L. Chen | 2013 | Randomized controlled studies | Efficacy of home-based virtual cycling training on bone mineral density in ambulatory children with cerebral palsy | 12-week home-based virtual cycling training including standing | 13 | 0.584 | 0.140 | 13 | 0.583 | 0.136 | 0.357 |
Table 1-2.
Studies to evaluate the effects of weight bearing exercise on BMD of femur.
| Study | Year | Design | Title | Intervention | Outcomes (BMD of femur) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Before treatment | After treatment | P value | |||||||||
| n | mean | SD | n | mean | SD | ||||||
| Gudjonsdottir B | 2002 | Prospective case control | Effects of a dynamic versus a static prone stander on bone mineral density and behavior in four children with severe cerebral palsy. | 8-week standing (2 dynamic /2 conventional) | 8 | 0.418 | 0.112 | 8 | 0.484 | 0.095 | 0.022 |
| Dalén Y. | 2012 | Prospective case control | Observations of four children with severe cerebral palsy using a novel dynamic platform. A case report | 8-9 month, dynamic flatform with standing swell (vibration, jump, rotation) | 8 | 0.535 | 0.077 | 8 | 0.585 | 0.097 | 0.012 |
| C.-L. Chen | 2013 | Randomized, controlled studies | Efficacy of home-based virtual cycling training on bone mineral density in ambulatory children with cerebral palsy | 12-week home-based virtual cycling training including standing | 13 | 0.730 | 0.124 | 13 | 0.744 | 0.097 | 0.022 |
Figure 1.
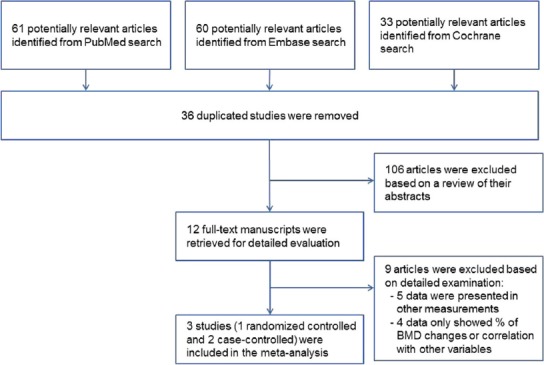
Flow diagram of the study selection process
Table 2.
Quality assessments using New Castle Ottawa scale.
| Published Year | Author | New Castle Ottawa Scale | ||
|---|---|---|---|---|
| Selection | Comparability | Exposure | ||
| 2002 | Gudjonsdottir et al. | **** | * | *** |
| 2012 | Dalén et al. | **** | * | ** |
| 2013 | Chen et al. | *** | ** | *** |
Outcomes
Three studies assessed the effects of weight bearing exercise on improving BMD after treatment. Lumbar spine BMD did not improve significantly after weight-bearing exercise compared to the baseline values (standardized mean difference [SMD], 0.341; 95% CI, -0.647-1.330; p=0.499) (Figure 2A). Significant inter-study heterogeneity was found (χ2=6.059, P=0.048, I2=66.989%). Begg’s test (p=0.30) and Egger’s test (p=0.18) did not indicate publication bias. A funnel plot analysis is also provided (Figure 3A). We conducted a sensitivity analysis, which shows the statistics with the removed study, and it also showed no significant improvements in BMD (Figure 4A).
Figure 2.
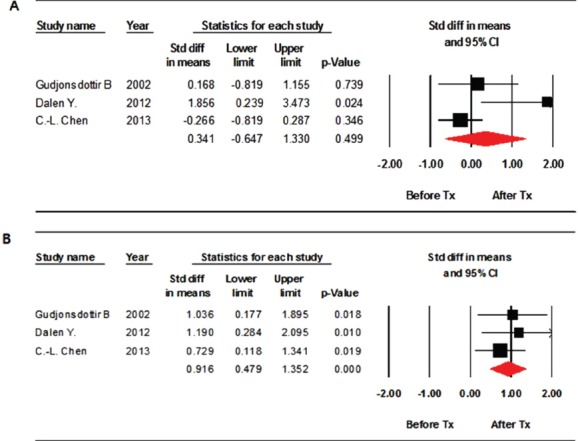
Forest plot of the effects on the lumbar spine (A) and femur (B) assessed after weight bearing exercise. Std diff = standard difference; CI = confidence interval.
Figure 3.
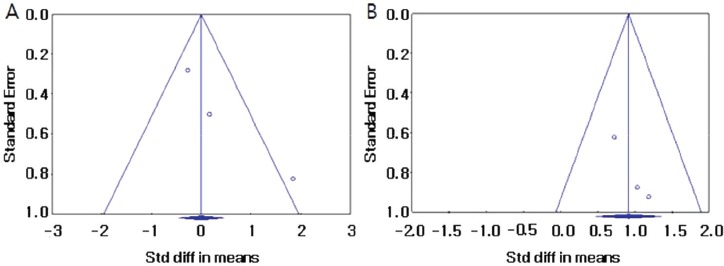
Funnel plots to assess publication bias in the lumbar spine (A) and in the femur (B). Std diff = standard difference.
Figure 4.
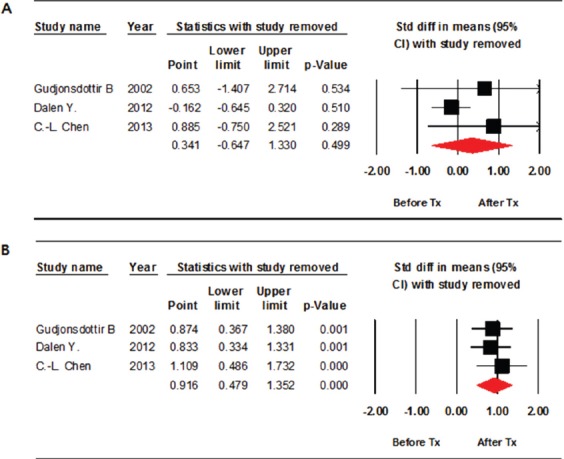
Forest plot of the sensitivity analysis. Assessed bone mineral density (BMD) in the lumbar spine (A) and in the femur (B). Std diff = standard difference; CI = confidence interval.
Femur BMD improved significantly compared with those at baseline (SMD, 0.916; 95% CI, 0.382-1.114; p<0.001) (Figure 2B). No significant inter-study heterogeneity was found (χ2=0.784, P=0.676, I2=0.000%). Begg’s test (p=0.30) and Egger’s test (p=0.10) did not indicate publication bias. A funnel plot analysis was also provided (Figure 3B). A sensitivity analysis with the removed study also showed significant improvements in BMD (Figure 4B).
Discussion
This meta-analysis indicates that weight bearing exercise significantly improved the BMD in femurs compared with pretreatment values, but had no effect on the BMD of the lumbar spine. Mechanical stress on bone is a determinant of bone morphology, BMD, and bone strength[13]. In children with CP who have difficulties in maintaining an upright position, less mechanical force is transmitted to the femurs than the lumbar spine because mechanical stress can be imposed on the lumbar spine in a seated position. Henderson et al. reported a 2-year longitudinal assessment of bone density in children and adolescents with moderate to severe CP. Bone mineral density increased an average of 2~5% per year in the distal femur and lumbar spine. A large variability in the changes of bone density was found ranging from +42% per year to -31% per year[14]. In spite of increases in BMD, distal femur BMD Z-scores decreased with age in this population[15]. These also can be supported by the fact that distal femur is the most common location of fractures in children with CP[5,6]. These previous research results are supported by this meta-analysis.
Previously, a wide variety of weight-bearing interventions has been evaluated. However, conflicting results were observed in physiotherapy programs focused on weight bearing exercise and no clear consensus has yet been reached[11]. This study can be meaningful to draw a conclusion although a small number of studies included.
The diagnostic criteria of osteoporosis in children contains occurrence of fracture[8,9]. However, having a fracture impairs functional mobility which aggravates complications in children with CP. Thus, it is important to prevent fractures before they occur. Among the methods of reducing fracture risk, pharmacological approaches to osteoporosis in children have been effective in previous researches, but there is no consensus on appropriate doses, optimal time to start treatment, duration, or risk of uses[11]. Therefore, other conservative managements such as weight bearing exercises may be safer in trying to increase BMD to prevent fractures.
There are several limitations to this study. First, a small number of studies were included because of the heterogeneity of physical intervention and measurements used in previous researches. Various physical interventions without weight bearing exercise were used in some of the previous studies. Different methods to assess bone health have been used in researches, and different measurements such as bone mineral content and volumetric BMD have also been used. In some studies, results were expressed only in median, changes of BMD, or percentages, rather than mean and p-value. Some studies were excluded because BMD was measured in different parts of the body. It is also difficult to measure BMD in children with CP accurately. In children with moderate to severe CP, joint contracture, spinal deformity, metal implants, positioning restrictions, and uncontrolled movements limit BMD measurement. If researches use with standardized measurement methods, more intensive analysis can be done with larger number of studies. Second, although this study shows the effectiveness of weight bearing exercises, it is difficult to suggest optimal methods of weight bearing exercise to increase BMD. Further research is needed for this. Also, bias of individual studies can exist because of small number of participants of included studies although it satisfies many items of each category of New Castle Ottawa Quality Assessment Scale.
Conclusion
Weight bearing exercise has a significant effect on improving BMD of the femurs in children with CP. Further analysis of randomized-controlled studies with more participants and researches on the standardization of protocols of weight bearing exercise are needed.
Footnotes
The authors have no conflict of interest.
Edited by: F. Rauch
References
- 1.Chen CL, Chen CY, Liaw MY, Chung CY, Wang CJ, Hong WH. Efficacy of home-based virtual cycling training on bone mineral density in ambulatory children with cerebral palsy. Osteoporos Int. 2013;24:1399–406. doi: 10.1007/s00198-012-2137-0. [DOI] [PubMed] [Google Scholar]
- 2.Dalén Y, Sääf M, Nyrén S, Mattson E, Haglund Å, kerlind Y, Klefbeck B. Observations of four children with severe cerebral palsy using a novel dynamic platform. A case report. Adv Physiother. 2012;14:132–9. [Google Scholar]
- 3.Gudjonsdottir B, Mercer VS. Effects of a dyanmic versus a static prone stander on bone mineral density and behavior in four children with severe cerebral palsy. Pediatr Phys Ther. 2002;14:38–46. [PubMed] [Google Scholar]
- 4.Houlihan CM. Bone health in cerebral palsy: Who's at risk and what to do about it? J Pediatr Rehabil Med. 2014;7:143–53. doi: 10.3233/PRM-140283. [DOI] [PubMed] [Google Scholar]
- 5.Muuns CFJ, Cowell PT. Prevention and treatmet of osteoporosis in chronically ill children. J Musculoskelet Neuronal Interact. 2005;5:262–72. [PubMed] [Google Scholar]
- 6.Henderson R. Low doses of panmidronate for the treatment of osteopenia in non-ambulatory children. Dev Med Child Neurol. 2006;48:708. doi: 10.1017/S0012162206001514. [DOI] [PubMed] [Google Scholar]
- 7.Henderson RC, Lark RK, Gurka MJ, Worley G, Fung EB, Conaway M, et al. Bone density and metabolism in children and adolescents with moderate to severe cerebral palsy. [[cited 2015 Sep 23]];Pediatrics [Internet] 2002 Jul;110(1):e5. doi: 10.1542/peds.110.1.e5. Available from: http://www.pediatrics.org/cgi/content/full/110/1/e5 . [DOI] [PubMed] [Google Scholar]
- 8.Fehilings D, Switzer L, Agarwal P, Wong C, Sochett E, Stevenson R, et al. Informing evidence-based clinical practice guidelines for children with cerebral palsy at risk of osteoporosis: a systematic review. Dev Med Child Neurol. 2012;54:106–16. doi: 10.1111/j.1469-8749.2011.04091.x. [DOI] [PubMed] [Google Scholar]
- 9.Lewiecki EM, Gordon CM, Biam S, Leonard MB, Bishop NJ, Bianchi ML, et al. International society for clinical densitometry 2007 adult and pediatric official positions. Bone. 2008;43:1115–21. doi: 10.1016/j.bone.2008.08.106. [DOI] [PubMed] [Google Scholar]
- 10.Chen CL, Ke JY, Lin KC, Wang CJ, Wu CY, Liu WY, et al. Anthropometric and fitness variables associated with bone mineral density ad broadband ultrasound attenuation in ambulatory children with cerebral palsy. J Child Neurol. 2011;26:552–9. doi: 10.1177/0883073810385235. [DOI] [PubMed] [Google Scholar]
- 11.Kim MJ, Kim SN, Lee IS, Chung S, Lee J, Yang Y, et al. Effects of bisphosphonates to treat osteoporosis in children with cerebral palsy: a meta-analysis. J Petiatr Endocrinol Metab. 2015;28:1343–50. doi: 10.1515/jpem-2014-0527. [DOI] [PubMed] [Google Scholar]
- 12.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takata S, Yasui N. Disuse osteoporosis. J Med Invest. 2001;48:147–56. [PubMed] [Google Scholar]
- 14.Palisano R, Rosenbaum P, Walter S, Russell D, Wood E, Galuppi B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol. 1997;39:214–23. doi: 10.1111/j.1469-8749.1997.tb07414.x. [DOI] [PubMed] [Google Scholar]
- 15.Henderson RC, Kairalla JA, Barrington JW, Abbas A, Stevenson RD. Longitudinal changes in bone mineral density in children and adolesencts with moderate to severe cerebral palsy. J Pediatr. 2005;146:769–75. doi: 10.1016/j.jpeds.2005.02.024. [DOI] [PubMed] [Google Scholar]


