Abstract
Plasminogen activator inhibitor type 1 (PAI-1), a key negative regulator of fibrinolysis, has been investigated to be one of the potential mechanisms of the development of impaired insulin sensitivity, insulin resistance, and diabetes mellitus. Because chronically stable HIV-infected individuals frequently develop abnormal glucose metabolism, including insulin resistance and diabetes mellitus, we postulated that PAI-1 could be one of the multifactorial pathogenic roles in the development of impaired insulin sensitivity and insulin resistance among chronic HIV-infected individuals. From our longitudinal cohort study, we selectively recruited chronically stable HIV-infected individuals without diagnosis of diabetes mellitus at baseline (N = 62) to analyze the correlation of baseline inflammatory cytokines, including PAI-1 and whole-body insulin sensitivity, with 2-year follow-up, as measured by Matsuda Index. We found a negative correlation between baseline PAI-1 and Matsuda Index (r = −0.435, p = .001) and a negative correlation between baseline PAI-1 and Matsuda Index at 2 years (r = −0.377, p = .005). In a linear regression model that included age, total body fat mass percentage, serum amyloid A, and family history of diabetes mellitus, PAI-1 still remained significantly associated with Matsuda Index at 2-year follow-up (β = −.397, p = .002). Our longitudinal study suggests that PAI-1 is an independent predictor of impaired insulin sensitivity among chronic HIV-infected individuals.
Keyword: : plasminogen activator inhibitor-1, insulin sensitivity, chronic HIV infection
Introduction
Since the introduction of antiretroviral therapy (ART), HIV-associated mortality and morbidity has remarkably declined and the life expectancy of HIV-infected individuals is close to the general population.1 However, adverse metabolic effects such as insulin resistance, diabetes mellitus, and hyperlipidemia have been more frequently found in the chronically stable HIV-infected population than the general population.1–3 To date, the underlying pathophysiologic mechanism of impaired insulin sensitivity, insulin resistance, and diabetes mellitus in treated HIV-infected individuals is still not well understood.
Plasminogen activator inhibitor type 1 (PAI-1), a crucial inhibitor of fibrinolysis in the body, may play an important role in the development of impaired insulin sensitivity, insulin resistance, and diabetes mellitus.4–7 Although the clear explanation of the relationship between increased PAI-1 levels and impaired insulin sensitivity and insulin resistance remains complicated, in vitro studies suggested that increased PAI-1 level may interfere with the insulin signaling through complex signaling molecules, including αvβ3 integrin and the insulin receptor substrate. Hence, increased PAI-1 can potentially inhibit the full mechanism of action of insulin. PAI-1 concentration among HIV-infected patients who have HIV-associated lipodystrophy syndrome and obesity has been found to be elevated compared with HIV-negative controls.8,9 There are currently no reported studies of using inflammatory cytokines, including PAI-1, to predict impaired insulin sensitivity in patients with chronic HIV infection. The primary rationale of our study was to investigate the potential predictor of the development of impaired insulin sensitivity in patients with chronic HIV infection.
We included 62 chronically stable HIV-infected individuals and over time, we investigated the correlation between soluble biomarkers of inflammation, including PAI-1, and whole-body insulin sensitivity measured by the Matsuda Index. We hypothesized that PAI-1 would be closely associated with impaired insulin sensitivity in chronically HIV-infected patients on stable therapy.
Materials and Methods
Study design
This was a longitudinal study using baseline and 2-year follow-up data from participants enrolled into the Hawaii Aging with HIV-Cardiovascular cohort (HAHC-CVD). Complete details of the cohort study design were previously published.10 In brief, criteria for entry into the initial cohort were documented HIV infection, 40 years of age or older, and on stable ART for >3 months. The study was approved by the Committee on Human Subjects of the University of Hawaii, and written informed consent was obtained from all participants.
Subjects who had baseline and 2-year follow-up measures of the Matsuda Index and baseline characteristics, including monocyte subset and inflammatory biomarkers, were included in the analyses. Subjects diagnosed with diabetes mellitus, at baseline as determined by the American Diabetic Association guidelines, as well as those on diabetic medications, including Biguanide, insulin secretagogues, and insulin, were excluded.11
Clinical assessment
Blood pressure, height, weight, and waist and hip measurement were obtained at study entry. Total fat mass percentage was used as an indicator of body fat mass instead of body mass index (BMI) because it provides a better measurement of body fat composition, which was measured by a dual-energy X-ray absorptiometry scan. BMI number generally provides a simple measurement of weight status, which was derived from the individual's mass (weight of fat, muscles, and tissues) and height. Fasting laboratory measurements, including complete blood count (CBC), CD4 cell count, plasma HIV RNA, glucose, insulin, total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, and triglycerides, were also obtained. Clinical history included age, gender, current medications, past medical and family histories, CD4 cell count nadir, and tobacco use. Metabolic syndrome status was determined by using the National Cholesterol Education Program's Adult Treatment Panel III report.12
Matsuda Index was used to measure whole-body insulin sensitivity and was calculated as 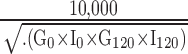 , using mean plasma glucose and insulin concentration from the oral glucose tolerance test (OGTT). Because this method reflects how well both hepatic and peripheral tissues respond to glucose, the Matsuda Index provides more precise whole-body insulin sensitivity than HOMA2, which uses only fasting glucose and insulin concentrations.13
, using mean plasma glucose and insulin concentration from the oral glucose tolerance test (OGTT). Because this method reflects how well both hepatic and peripheral tissues respond to glucose, the Matsuda Index provides more precise whole-body insulin sensitivity than HOMA2, which uses only fasting glucose and insulin concentrations.13
Plasma-soluble biomarkers
Plasminogen activator inhibitor (PAI-1) along with other soluble biomarkers, including soluble E-selectin (sE-selectin), soluble vascular cell adhesion molecule-1 (sVCAM-1), soluble intercellular adhesion molecule-1 (sICAM-1), matrix metalloproteinase (MMP)-9, myeloperoxidase (MPO), C-reactive protein (CRP), serum amyloid A (SAA), serum amyloid P (SAP), IL-1b, IL-6, IL-8, IL-10, TNF-α, MCP-1, vascular endothelial growth factor, MCP-1, and interferon (IFN)-γ, were assessed at baseline. The Milliplex Human Cardiovascular Disease panels (EMD Millipore) was used to assay plasma-soluble biomarkers as previously described.10 We did not obtain blood samples for the soluble biomarkers at the 2 year follow-up.
Statistical analysis
Demographic and clinical information was summarized by median (quartile 1, quartile 3) for continuous variables, and frequency (percentage) for categorical variables. Inflammatory cytokines were log-10 transformed to adjust for normality, where necessary. Pearson correlations were used to assess the correlation between variables. The associations of baseline and 2-year Matsuda Index with inflammatory cytokines were assessed by separate multivariable linear regression models, adjusting for insulin resistance-associated risk factors such as age, body fat percentage, and family history of diabetes mellitus. The outcome variable was the 2-year follow-up Matsuda Index. The two-year follow-up Matsuda Index was subsequently adjusted for the insulin resistance-associated risk factors. This approach, rather than using change in the Matsuda Index over 2 years, would provide better precision and power in small sample size.14,15 Stepwise backward elimination regression was performed to determine the predictors associated with whole-body insulin sensitivity, by using a selection criterion of p < .05 to remain in the model. All statistical analyses were conducted in SPSS (IBM, Version 23). A p-value of < .05 was regarded as statistically significant.
Results
Patient characteristics
Of the 160 subjects enrolled in the HAHC-CVD cohort, 62 had an available Matsuda Index at baseline and 2-year follow-up. Of the 62 subjects, soluble inflammatory cytokines and monocyte subsets at baseline were available in 54 subjects. The demographic, clinical, and immunologic characteristics of the patients are summarized in Table 1. The patients were predominately Caucasian (61.3%) and of male gender (90.3%). The median age was 51 years. Study enrollment inclusion criteria required patients to be on stable ART for at least 3 months, and 85% had suppressed viral load that was defined as HIV RNA <50 copies/ml. The median CD4 cell count was 513 cells/μl, and the median CD4 cell count nadir was 160 cells/μl. ART consisted of nucleoside reverse transcriptase inhibitors, non-nucleoside reverse transcriptase inhibitors, and protease inhibitors (PIs) at entry by 98%, 50%, 48% of patients, respectively. None of the subjects were on integrase inhibitors.
Table 1.
Baseline Characteristics of the Subjects
| Baseline characteristics | |
|---|---|
| N | 62 |
| Age, year | 51 [47–56] |
| Male, n (%) | 56 (90.3%) |
| Caucasian race | 38 (61.3%) |
| BMI, kg/m2 | 26.07 [24.48–27.79] |
| Framingham risk score | 0.06 [0.03–0.11] |
| History of clinical CVD events, n (%) | |
| Hypertension | 20 (32.26) |
| Stroke | 2 (3.2) |
| History of myocardial infarction | 3 (4.8) |
| Family history of diabetes, n (%) | 29 (46.77) |
| Smoking history, n (%) | 37 (59.7) |
| Undetectable HIV RNA, n (%) | 53 (85) |
| Current antiretroviral medications, n (%) | |
| Nucleoside reverse transcriptase inhibitor | 61 (98.4) |
| Non-nucleoside reverse transcriptase inhibitor | 31 (50) |
| Protease inhibitor | 30 (48.4) |
| Metabolic syndrome, n (%) | 16 (25.8) |
| Fasting glucose, mg/dl | 87.5 [81.0–93.0] |
| Matsuda Index | 4.91 [3.42–9.89] |
| Mean glucose during OGTT, mg/dl | 117.13 [101.5–139.62] |
| Mean insulin during OGTT, mg/dl | 50.86 [28.27–74.85] |
| Soluble markers | |
| sE-Selectin ng/ml | 36 [19–46] |
| sICAM 1, ng/ml | 136 [109–158] |
| sVCAM 1, ng/ml | 1174 [936–1343] |
| MMP9, ng/ml | 55 [45–92] |
| MPO, ng/ml | 16.1 [12.0–21.2] |
| SAA, ng/ml | 10270 [3188–43826] |
| CRP, ng/ml | 8186 [3081–26244] |
| IL-10, pg/ml | 2.18 [0.93–4.65] |
| IFN-y, pg/ml | 0.66 [0.37–1.30] |
| PAI-1, pg/ml | 88 [68–115] |
| IL-6, pg/ml | 1.44 [1.02–2.42] |
| IL-8, pg/ml | 3.38 [2.66–4.23] |
| MCP-1, pg/ml | 146 [111–186] |
| VEGF, pg/ml | 33 [14–55] |
| TNF-a, pg/ml | 3.21 [1.54–4.34] |
| Year 2 data | |
| Fasting glucose, mg/dl | 87.50 [83.00–96.00] |
| Matsuda Index | 4.38 [2.82–7.62] |
| Mean glucose during OGTT, mg/dl | 117.81 [99.75–140.00] |
| Mean insulin during OGTT, mg/dl | 53.77 [39.15–71.12] |
CVD, cardiovascular cohort; PAI-1, plasminogen activator inhibitor type 1; BMI, body mass index; VEGF, vascular endothelial growth factor; OGTT, oral glucose tolerance test.
Associations between Matsuda Index and soluble biomarkers
Baseline levels of measured soluble biomarkers are shown in Table 1.
At baseline, the median Matsuda Index was 4.91 and there were significant negative associations between baseline Matsuda Index and biomarkers, including PAI-1, CRP, IL-1b, MPO, SAP, SAA, and svCAM. At 2-year follow-up, the median Matsuda Index was 4.38 and only PAI-1 and SAA were associated with the 2-year follow-up Matsuda Index. Two of the 62 patients had developed type 2 diabetes mellitus by the second year of this study.
We found a negative correlation between baseline Matsuda Index and PAI-1 (r = −0.435 and p = .001) and a negative correlation between 2-year Matsuda Index and baseline PAI-1 (r = −0.376, p = .004). In addition, a negative correlation between baseline Matsuda Index and SAA (r = −0.323, p = .017) and a negative correlation between year 2 Matsuda Index and baseline SAA were found (r = −0.278, p = .042). Neither baseline nor year 2 Matsuda Index was significantly different between genders, Table 2.
Table 2.
Pearson's Correlation of Baseline Cytokines and Matsuda Index at Baseline and at 2-Year Follow-up
| Matsuda Index at baseline | Matsuda Index at 2-year follow-up | |||
|---|---|---|---|---|
| Correlation coefficient, r | p | Correlation coefficient, r | p | |
| Total monocyte counts at baseline | −0.262* | .040 | −.069 | .594 |
| CRPa | −0.310* | .022 | −0.261 | .057 |
| IFNya | −0.072 | .606 | 0.080 | .567 |
| IL10a | −0.177 | .201 | −0.261 | .056 |
| IL1ba | −0.309* | .025 | −0.070 | .619 |
| IL6a | −0.158 | .253 | −0.151 | .277 |
| IL8a | 0.044 | .754 | −0.151 | .276 |
| MCP1a | 0.114 | .412 | 0.033 | .815 |
| MPOa | −0.334* | .014 | −0.206 | .136 |
| MMP9a | −0.206 | .136 | −0.255 | .062 |
| SAAa | −0.323* | .017 | −0.278* | .042 |
| Selectina | −0.181 | .191 | −0.235 | .086 |
| SAPa | −0.333* | .014 | −0.196 | .155 |
| SICAMa | −0.109 | .431 | −0.252 | .066 |
| SVCAMa | −0.341* | .012 | −0.237 | .084 |
| TNFaa | 0.076 | .585 | 0.008 | .953 |
| PAI-1a | −0.435** | .001 | −0.377** | .005 |
| VEGFa | −0.050 | .718 | 0.068 | .626 |
Variable was log-10 transformed.
Significant at p < .05.
Significant at p < .01.
Bold, statistically significant correlation with Matsuda Index.
The relationship between 2-year Matsuda Index and the inflammatory cytokines, including PAI-1 and SAA, was examined further in a multivariable regression model with the Matsuda Index at 2-year follow-up as the dependent variable, Table 3. After adjusting for traditional risk factors of insulin resistance at baseline (age, total body fat percentage, family history of diabetes mellitus), baseline PAI remained significantly associated with the 2-year Matsuda Index after backward selection was performed (β = −.397, p = .002). Backward selection also determined that age was the most important risk factor for the 2-year Matsuda Index (p = .014). SAA, family history of diabetes mellitus, and total body fat percentage were no longer significantly associated with the Matsuda Index at 2-year follow-up after the backward elimination regression approach. Nine of the 62 subjects (14.5%) had changes in their ART regimen during the 2 year period. Changes to their ART regimens are as follows: Three had changed from non-nucleoside reverse transcriptase inhibitors (NNRTIs) to Integrase Strand Transfer Inhibitors (INSTIs); three had changed from PIs to INSTIs; one had addition of PI to an NNRTI-based regimen; and two had intensification of their ART regimens with Maraviroc. No obvious pattern in the Matsuda Index was seen with subjects who had changes in their ART regimen compared with those who had no change in ART. Removing the individuals who had changes in ART from the analysis did not change the results.
Table 3.
Multivariable Linear Regression of Matsuda Index at Two-Year Follow-up
| Model | Independent variable | Unstandardized coefficients beta | Standardized coefficients beta | Sig. |
|---|---|---|---|---|
| 1 | Age | −0.115 | −0.274 | 0.033 |
| Total body fat percentage | −0.019 | −0.057 | 0.672 | |
| SAAa | −0.447 | −0.130 | 0.324 | |
| PAI-1a | −4.87 | −0.365 | 0.008 | |
| Family history of DM | −0.444 | −0.193 | 0.119 | |
| 2 | Age | −0.114 | −0.271 | 0.033 |
| SAAa | −0.492 | −0.143 | 0.260 | |
| PAI-1a | −5.11 | −0.383 | 0.003 | |
| Family history of DM | −0.456 | −0.199 | 0.105 | |
| 3 | Age | −0.128 | −0.303 | 0.015 |
| PAI-1a | −5.55 | −0.416 | 0.001 | |
| Family history of DM | −0.453 | −0.197 | 0.107 | |
| 4 | Age | −0.130 | −0.310 | 0.014 |
| PAI-1a | −5.30 | −0.397 | 0.002 |
Dependent variable: Matsuda Index at 2-year follow-up.
Models represent stages of backward selection.
Variable was log-10 transformed.
Discussion
In this prospective cohort study, we chronically analyzed stable HIV-infected patients to investigate the correlation between inflammatory cytokines and changes in whole-body physiological insulin sensitivity. Our results found that elevated PAI-1 levels were associated with a decreased Matsuda Index, suggesting that PAI-1 could independently predict future negative alterations in whole-body insulin sensitivity in patients with chronic HIV infection. This finding was consistent with our previous cross-sectional study, which found a correlation between PAI-1 and HOMA-IR.10
PAI-1 is a key regulator of fibrinolysis, which has been found to be associated with coronary artery disease, thrombosis, insulin resistance, and diabetes mellitus.4,5,8 PAI-1 concentration in chronically stable HIV individuals appears to be higher compared with HIV-negative individuals because of the increased incidence of abnormalities of body fat redistribution and the effect of ART.8,16,17 One article from the Official Journal of The International AIDS Society reported that ART could influence the endothelial activation by direct or indirect effects, which contribute to increased secretion of PAI-1 from the endothelial cells.16 Lipodystrophy and abnormal fat redistribution are often found in chronic HIV infection, especially in those who are on ART. An increase in PAI-1 gene expression was found in HIV-positive patients with lipodystrophy, thereby increasing PAI-1 concentration.8,18 Lipodystrophy is associated with increased liver fat content and the liver usually clears PAI-1, so PAI-1 concentration can be increased by clearance defect from fat accumulation in the liver.8,19
Because of the enigmatic mechanism of the development of impaired insulin sensitivity and insulin resistance by enhanced PAI-1 levels, there have been numerous studies that attempted to elucidate the precise underlying mechanism.4,5,20 One hypothesis was the interference of insulin signaling by PAI-1. The complete insulin signaling required full activation of several signaling molecules such as αvβ3 integrin and insulin receptor substrate.20 PAI-1 is capable of competing with αvβ3 integrin, which could potentially reduce tyrosine phosphorylation of the insulin receptor substrate. Several longitudinal studies in the general population found that subjects who developed type 2 diabetes mellitus had higher levels of PAI-1 at baseline compared with subjects who did not develop type 2 diabetes mellitus.21
Our study has some limitations that should be acknowledged. First and most notably, the inflammatory cytokines were not available for the 2-year follow-up. It would be helpful to have the inflammatory cytokines at 2-year follow-up to examine the changes of the inflammatory cytokines over time in terms of responsiveness of PAI-1 level among chronically HIV-infected individuals on ART, not to examine the causality of diabetes mellitus in chronic HIV infection. In the absence of inflammatory cytokine data at 2-year follow-up, we believe that using baseline inflammatory cytokines to predict the 2-year follow-up Matsuda Index provides a more precise and intuitive analysis compared with using the 2-year change in the Matsuda Index. Second, our study had no control group or HIV-negative individuals and this analysis was retrospective in nature. Hence, we could not account for unmeasured confounders and interpret the result as a causal factor of diabetes mellitus. Despite these limitations, the strength of this study was the robust association between the Matsuda Index and PAI-1 in this well-characterized cohort independently of potential confounding factors such as age, total body fat percentage, and family history of diabetes mellitus.
This is the first study to investigate the potential predictor of the development of impaired insulin sensitivity among patients with chronic HIV infection. Our results indicated that an elevation of PAI-1 concentrations at baseline in chronically stable HIV infection was associated with negative alterations in whole-body insulin sensitivity over time. This could suggest that PAI-1 is a potentially independent predictor of impaired insulin sensitivity among chronically stable HIV-infected individuals. Exploring the underlying mechanism of PAI-1 production may offer insights into insulin resistance and insulin sensitivity in HIV-infected individuals, and it may be a novel therapeutic target for insulin resistance and diabetes mellitus in the future.
Acknowledgments
The authors thank the clinical and laboratory staff of the Hawaii Center for AIDS, University of Hawaii, and the patients of the Hawaii Aging with HIV Cardiovascular cohort study who made this study possible.
Funding
National Heart, Lung, and Blood Institute [Grant No. R01HL095135]; National Institute on Minority Health and Health Disparities [Grant No. U54MD007584].
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Samji H, Cescon A, Hogg RS, et al. : Closing the gap: Increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS One 2013;8:e81355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown TT, Cole SR, Li X, et al. : Antiretroviral therapy and the prevalence and incidence of diabetes mellitus in the multicenter AIDS cohort study. Arch Intern Med 2005;165:1179–1184 [DOI] [PubMed] [Google Scholar]
- 3.Gutierrez AD, Balasubramanyam A: Dysregulation of glucose metabolism in HIV patients: Epidemiology, mechanisms, and management. Endocrine 2012;41:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brazionis L, Rowley K, Jenkins A, Itsiopoulos C, O'Dea K: Plasminogen activator inhibitor-1 activity in type 2 diabetes: A different relationship with coronary heart disease and diabetic retinopathy. Arterioscler Thromb Vasc Biol 2008;28:786–791 [DOI] [PubMed] [Google Scholar]
- 5.Schneider DJ, Sobel BE: PAI-1 and diabetes: A journey from the bench to the bedside. Diabetes Care 2012;35:1961–1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eddy AA, Fogo AB: Plasminogen activator inhibitor-1 in chronic kidney disease: Evidence and mechanisms of action. J Am Soc Nephrol 2006;17:2999–3012 [DOI] [PubMed] [Google Scholar]
- 7.Byberg L, Siegbahn A, Berglund L, McKeigue P, Reneland R, Lithell H: Plasminogen activator inhibitor-1 activity is independently related to both insulin sensitivity and serum triglycerides in 70-year-old men. Arterioscler Thromb Vasc Biol 1998;18:258–264 [DOI] [PubMed] [Google Scholar]
- 8.Yki-Jarvinen H, Sutinen J, Silveira A, et al. : Regulation of plasma PAI-1 concentrations in HAART-associated lipodystrophy during rosiglitazone therapy. Arterioscler Thromb Vasc Biol 2003;23:688–694 [DOI] [PubMed] [Google Scholar]
- 9.He G, Andersen O, Haugaard SB, et al. : Plasminogen activator inhibitor type 1 (PAI-1) in plasma and adipose tissue in HIV-associated lipodystrophy syndrome. Implications of adipokines. Eur J Clin Invest 2005;35:583–590 [DOI] [PubMed] [Google Scholar]
- 10.Shikuma CM, Chow DC, Gangcuangco LM, et al. : Monocytes expand with immune dysregulation and is associated with insulin resistance in older individuals with chronic HIV. PLoS One 2014;9:e90330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Executive summary: Standards of medical care in diabetes—2013. Diabetes Care 2013;36 Suppl 1:S4–S10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grundy SM, Brewer HB, Jr., Cleeman JI, Smith SC, Jr., Lenfant C: Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Arterioscler Thromb Vasc Biol 2004;24:e13–e8 [DOI] [PubMed] [Google Scholar]
- 13.Gutch M, Kumar S, Razi SM, Gupta KK, Gupta A: Assessment of insulin sensitivity/resistance. Indian journal of endocrinology and metabolism 2015;19:160–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tu YK: Testing the relation between percentage change and baseline value. Sci Rep 2016;6:23247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.How Should Change be Measured?. Vanderbilt University, 2017. Available at http://biostat.mc.vanderbilt.edu/wiki/Main/MeasureChange, accessed March, 2017 [Google Scholar]
- 16.de Gaetano Donati K, Rabagliati R, Tumbarello M, et al. : Increased soluble markers of endothelial dysfunction in HIV-positive patients under highly active antiretroviral therapy. AIDS 2003;17:765–768 [DOI] [PubMed] [Google Scholar]
- 17.Schulte-Hermann K, Schalk H, Haider B, et al. : Impaired lipid profile and insulin resistance in a cohort of Austrian HIV patients. J Infect Chemother 2016;22:248–253 [DOI] [PubMed] [Google Scholar]
- 18.Freitas P, Carvalho D, Santos AC, et al. : Adipokines, hormones related to body composition, and insulin resistance in HIV fat redistribution syndrome. BMC Infect Dis 2014;14:347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Safar Zadeh E, Lungu AO, Cochran EK, et al. : The liver diseases of lipodystrophy: The long-term effect of leptin treatment. J Hepatol 2013;59:131–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lopez-Alemany R, Redondo JM, Nagamine Y, Munoz-Canoves P: Plasminogen activator inhibitor type-1 inhibits insulin signaling by competing with alphavbeta3 integrin for vitronectin binding. Eur J Biochem 2003;270:814–821 [DOI] [PubMed] [Google Scholar]
- 21.Festa A, D'Agostino R, Jr., Tracy RP, Haffner SM: Elevated levels of acute-phase proteins and plasminogen activator inhibitor-1 predict the development of type 2 diabetes: The insulin resistance atherosclerosis study. Diabetes 2002;51:1131–1137 [DOI] [PubMed] [Google Scholar]


