Abstract
Objective:
The objective of this study is to determine the prevalence of elevated high-sensitivity C-reactive protein (hs-CRP) in normal pregnancy and gestational diabetes mellitus (GDM) and to compare the prevalence in both groups.
Materials and Methods:
We conducted a case–control study between April 2015 and April 2016. Statistical analysis was performed by the SPSS program version 17. Categorical variables were analyzed using either the Chi-square test or Fisher's exact test. Pregnant women who underwent oral glucose tolerance test at 24–28 weeks of gestation were enrolled. We took 25 women with GDM as cases and 25 normal pregnant as control. These patients were analyzed for the level of hs-CRP.
Results:
The prevalence of elevated hs-CRP in controls was 28% whereas in cases was 76%, with a P = 0.002 which is significant in this study. When compared to elevated hs-CRP in GDM with body mass index and gravid state a P = 0.430, 0.378 was obtained respectively which was not significant whereas age had a P = 0.049 which was significantly associated with elevated hs-CRP (≥3 mg/L).
Conclusion:
Our result shows an increased level of hs-CRP in GDM as compared to normal pregnant subjects. In this study, we also observed an association of increasing age with elevated level of hs-CRP in GDM. Therefore, hs-CRP can be used as a screening tool for early detection and risk assessment of GDM.
Keywords: Gestational diabetes mellitus, high-sensitivity C-reactive protein, normal pregnancy, oral glucose tolerance test, prevalence
Introduction
The consequences of pregnancy and childbirth are still the leading cause of disease, disability, and death among women of reproductive age group in developing countries.
Pregnancies are identified as “low” or “high” risk based on the chances of adverse maternal or neonatal outcome.[1] Pregnancy is a condition associated with profound inflammatory changes.[2] Therefore, it is important to have an integrated patient assessment during antenatal visits using maternal history and characteristics, and biochemical tests which can better define risk for pregnancy complications including fetal abnormalities, miscarriage, stillbirth, preeclampsia, preterm birth, gestational diabetes mellitus (GDM), intrauterine growth restriction (IUGR), and macrosomia.[3] This would define patient-specific risk,[3] and would allow early commencement of preventive therapies, institution of appropriate models of antenatal care and optimal level of surveillance.[1,3] There will be a better recruitment of high-risk populations to trails of interventions to develop better strategies for prevention of pregnancy complications and for improvement of maternal and fetal outcomes.[1,3] Infective or inflammatory conditions such as obesity, GDM, and other conditions of insulin resistance, when superimposed on pregnancy, may change this balance and compromise normal development.[4,5]
There is insulin resistance during normal pregnancy which is further enhanced in pregnancy complications such as GDM, disturbed placental function such as preeclampsia and IUGR.[6] There has been an association between abnormal high-sensitivity C-reactive protein (hs-CRP) and pregnancy specific complications.[7] The importance of measuring maternal circulating hs-CRP in diagnosing subclinical infection and/or inflammation in women with preterm rupture of membrane,[8] in predicting the risk of preterm delivery[9] and preeclampsia[10] has been shown by some studies. As most pregnancy complications appear in later part of pregnancy but underlying pathophysiology starts early in pregnancy therefore, an early hs-CRP determination may help in the prediction of adverse pregnancy outcome. The level of hs-CRP provides a better sensitivity in establishing inflammation than levels of C-reactive protein (CRP).[11]
Pregnancy is hyperglycemic period of life and is associated increasing insulin resistance starting at midgestation.[12] In GDM, an increased severity of insulin resistance can disrupt the intrauterine milieu, leading to abnormal fetal growth.[13] When increased insulin secretion cannot compensate for the pregnancy-induced insulin resistance then GDM results.[14]
The present study was conducted to examine the prevalence of elevated hs-CRP, an oxidative stress biomarker and acute phase reactant in normal pregnancy and GDM. In this study, IADPSG criteria were taken for diagnosis of GDM.
Materials and Methods
Study design
Case–control study.
Study population
The study population included pregnant women between 24 and 28 weeks of gestation presenting at the outpatient of Obstetrics and Gynaecology Department who consent to participate in the study and fulfilled the inclusion criteria.
Study period
April 2015 to April 2016.
Sample size
There are two groups of patients in this study, namely, normal pregnancy as controls and pregnancy with GDM as cases. From the past record and discussion with consultants and research group, it was found that number of women reported with GDM were around 42 in our hospital from February 2013 to December 2014. Based on this estimate, we did a census study to cover all the women reported with GDM in 1 year time (April 2015 to April 2016). The sample size taken was 25 with normal pregnancy and 25 with GDM.
Inclusion criteria
Pregnant patients with age >20 years at 24–28 weeks of gestation.
Exclusion criteria
Patient with history of type 2 diabetes mellitus (DM), hypertension, coronary artery disease
History of smoking/alcohol abuse
History of acute/chronic liver diseases
History of chronic obstructive pulmonary disease/respiratory illness
History of chronic kidney disease/any renal illness
History of nonsteroidal anti-inflammatory drugs's ingestion in past 10 days
All other conditions which cause an increase in hs-CRP are to be excluded as patients with acute infections, connective tissue disorder or as arthritis, scleroderma, polymyositis, dermatomyositis, inflammatory bowel disease, and peripheral arterial disease.
Data collection technique and tools
Those women who underwent screening test with oral glucose tolerance test (OGTT) at 24–28 weeks of gestation were enrolled for the study. Full explanation was given to the study subjects in a language preferred by them. Those subjects who agreed to take part in the study gave a verbal and informed written consent. During the study, each participant's demographic data such as age, gender, address was noted in the pro forma. A detailed present, past, and obstetric history was taken followed by general and systemic examinations with relevant investigations.
After initial investigations, Women with impaired OGTT diagnosed as GDM. Then a baseline hs-CRP was done in both normal pregnancy and GDM which could be normal or elevated. We classified them into two groups (case and control) based on serum hs-CRP level. A reference value of hs-CRP concentration below 3 mg/L is considered normal for this study.[15]
Case-serum hs-CRP level above or equal to 3 mg/L
Control-serum hs-CRP level below 3 mg/L.
Parameters of patients including parity, period of gestation, vitals, height, weight, body mass index (BMI), and OGTT were recorded as observational data.
At the end prevalence of elevated hs-CRP was determined in both cases and control then prevalence of elevated hs-CRP was compared in both cases and control.
Statistical analysis
Statistical analysis was performed by Using the SPSS statistical package (version 17.0, SPSS Inc., Chicago, IL, USA). Continuous variables are presented as mean ± standard deviation (SD), and categorical variables are presented as absolute numbers and percentage. Data were checked for normality before statistical analysis. Normally distributed continuous variables were compared using the unpaired t-test, whereas the Mann–Whitney U-test was used for those variables that were not normally distributed. Categorical variables were analyzed using either the Chi-square test or Fisher's exact test. For all statistical tests, a P < 0.05 was taken to indicate a significant difference.
Observations and Results
Serum level of hs-CRP was done in all patients. Mean value of hs-CRP in control and cases were compared and a P = 0.001 which is significant in this study as shown in Table 1 and Figure 1.
Table 1.
Mean of high.sensitivity C-reactive protein in cases and controls

Figure 1.
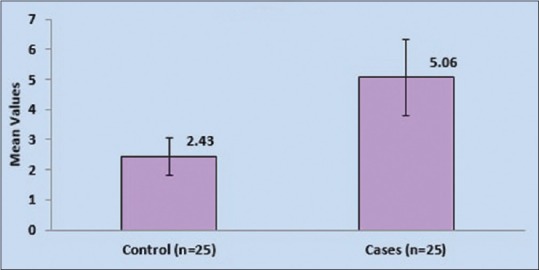
Comparison of mean of high-sensitivity C-reactive protein in cases and controls
The prevalence of elevated hs-CRP (≥3 mg/L) in controls was 28% whereas in cases is 76%, whereas hs-CRP (<3 md/L) was seen in 72% of controls and 24% of cases, with a P = 0.002 which is significant in this study.
Thus, it was observed that the prevalence of elevated hs-CRP (≥3 mg/L) was significantly high in cases as compared to controls (P = 0.002) as shown in Table 2 and Figure 2.
Table 2.
Prevalence of high-sensitivity C-reactive protein in cases and controls
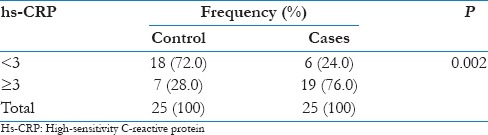
Figure 2.
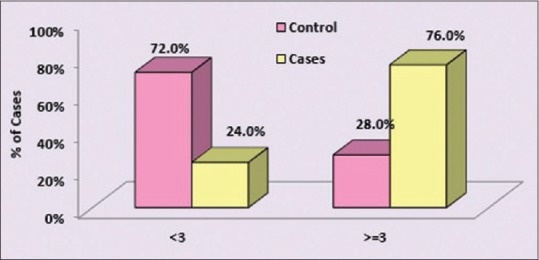
Comparison of high-sensitivity C-reactive protein distribution in cases and controls
In this study, it was observed that the level of hs-CRP (≥3 mg/L) when compared with age, BMI, and gravida in controls, a P = 0.490 for age, 0.156 for BMI, and 0.673 for gravida was obtained which was not significantly associated with age, BMI, and gravid state in controls as shown in Table 3 and Figure 3.
Table 3.
Association of high-sensitivity C-reactive protein with age, body mass index, and gravida in controls
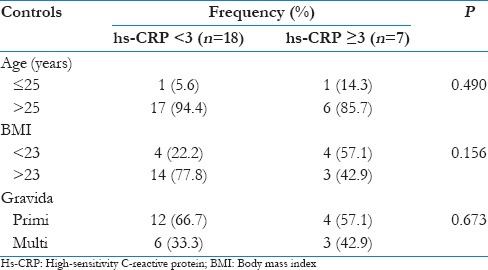
Figure 3.
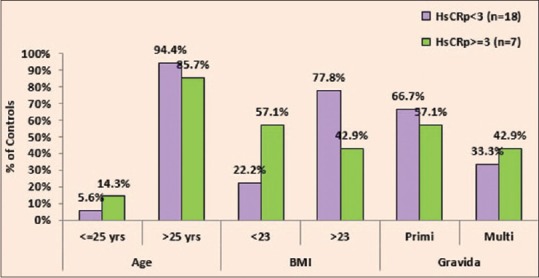
Association of high-sensitivity C-reactive protein with age, body mass index, and gravida in controls
The association of elevated hs-CRP (≥3 mg/L) in cases with age, BMI, and gravid state was observed and it was found that BMI and gravida had a P = 0.430, 0.378, respectively which was not significantly associated with the level of hs-CRP (≥3 mg/L) whereas Age had a P = 0.049 which was significantly associated with elevated hs-CRP as shown in Table 4 and Figure 4.
Table 4.
Association of high-sensitivity C-reactive protein with age, body mass index, and gravida in cases
Figure 4.
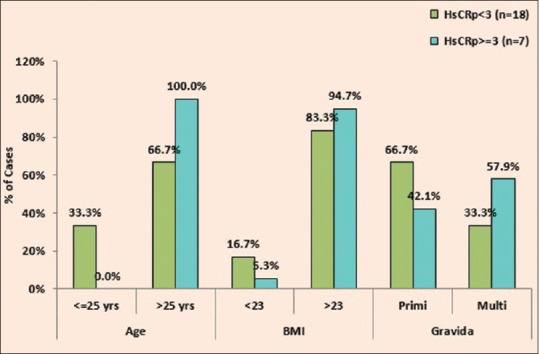
Association of high-sensitivity C-reactive protein with age, body mass index, and gravida in cases
The prevalence of patients with age ≤25 is only 8% in control and 12% in cases whereas age >25 is 92% in control and 88% in cases with P = 1.000 which is not significant in this study.
In this study, it was observed that prevalence of BMI <23 is 32% in controls and 8% in cases whereas BMI >23 is 68% in control and 92% in cases with a P = 0.044 which is significant.
In our study, mean gestational age is 26.44 ± 1.29 SD in control and 25.84 ± 1.55 SD in cases with a P = 0.143 which was not significant.
The prevalence of primigravida in control is 64% and 48% in cases whereas the prevalence of multigravida in control is 36% in control and 52% in cases with a P = 0.254 which shows that there is no significant difference in gravid state between cases and controls.
Discussion
Common pathologic state like GDM had increased the incidence of complications both in the mother and the fetus.[16] In addition, GDM and pregnancy related dysregulation of blood glucose levels expose the women affected to higher risk for subsequent development of type 2 DM and cardiovascular disease later in their lives,[17] the risk being proportional to the degree of the dysregulation.
The present study primarily examined the prevalence of elevated hs-CRP an oxidative stress indicator with GDM in pregnant women at 24–28 weeks of gestation. As reduced ability to compensate for oxidative stress found among these women was associated with increased insulin resistance which might be important factors in GDM.
In this case–control study, we calculated the prevalence of elevated hs-CRP in normal pregnant women and GDM. We found that the prevalence of elevated hs-CRP is 28% in normal pregnant women (controls) and 76% in GDM when values of elevated hs-CRP compared in both normal pregnancy and GDM, it was significantly (P = 0.002) associated with GDM. In a study conducted by Maged et al., in which a comparative study done between different biomarkers for early prediction GDM which showed the hs-CRP sensitivity 89% and specificity 55%.[18] Therefore, making hs-CRP an important marker for early prediction for GDM.
In another case–control study conducted by Zhu et al., in 2015 which showed the association of elevated hs-CRP with GDM in pregnant women.[19]
A well-known fact is that inflammatory and stress responses mediate insulin resistance,[20] and inflammatory mediators play an important role in the development and progression of GDM. CRP, is a sensitive marker of the inflammation and a classical acute phase reactant, in numerous pathologic conditions, and elevated CRP levels have been associated with abnormal metabolic conditions such as insulin resistance, hyperglycemia, and type 2 DM.[21]
A study conducted by Bo et al., on CRP and tumor necrosis factor-α in gestational hyperglycemia which showed that during pregnancy, increased CRP levels are associated with insulin resistance, maternal dysglycemia, and GDM.[22] As Asians have a higher percentage of body fat than Caucasian people of the same age, sex, and BMI. The occurrence of type 2 diabetes is more in lower BMI than the WHO cut-off limit of 25 kg/m2. Therefore, WHO has recommended that for many Asians the limits of BMI should be 23 kg/m2. Suggested categories for Asians are: <18.5 kg/m2 (underweight); 18.5–23 kg/m2 (normal); 23–27.5 kg/m2 (overweight); and 27.5 kg/m2 or higher (obesity).[23]
In this study, we observe that mean BMI of cases (GDM) was higher when compared to controls which was significantly associated with GDM (P = 0.008), and this was similar to the study conducted by Makgoba et al., in 2012[24] which states that there is strong link between GDM and BMI in all the racial groups.
In the present study, there was no association between age, BMI, and gravida with the prevalence of elevated hs-CRP(≥3 mg/L) in controls whereas when compared to cases (GDM) there was a significant correlation between increasing age and elevated hs-CRP with a P value of 0.049. As no direct study has shown the association of elevated hs-CRP with increasing age in GDM, therefore on the basis of study and reference mentioned below we have tried to explain the outcome of this study result.
A study conducted by Lao et al., in 2003, in which their findings indicates that the risk of GDM becomes significantly and progressively increased from 25 years onward. This supports the American Diabetes Association recommendation on the use of age ≥25 years as the cutoff for screening and the observation that maternal age ≥25 years is the factor most predictive of GDM[25,26] and as elevated hs-CRP is significantly related to GDM in this study (P = 0.002) as well as a case–control study conducted by Zhu et al., in 2015 which showed the association of elevated hs-CRP with GDM in pregnant women.[19] Hence, we can indirectly relate the association of elevated hs-CRP with increasing age in GDM.
Conclusion
Induction of an inflammatory state in pregnancy, and worsening of insulin resistance is further exacerbated by obesity, gestational weight gain, and may result in GDM. GDM is now common these days and had an adverse impact on short and long-term maternal and child health.
Our result shows an increased level of hs-CRP (an inflammatory marker) in GDM as compared to normal pregnant subjects. In this study, we also observed an association of increasing age with elevated level of hs-CRP in GDM. These results suggest that there is decreased ability of pregnant women with GDM to compensate for oxidative stress which manifested as increased insulin resistance, reduced insulin sensitivity, and β-cell dysfunction, all of which may play important roles in GDM.
Therefore, hs-CRP can be used as a screening tool for early detection and risk assessment of GDM, and this would allow early commencement of preventive therapies, institution of appropriate models of antenatal care in pregnant women with GDM to prevent maternal and fetal complications related to GDM.
Recommendations/suggestions
Risk prediction in GDM is mostly based on maternal history and clinical risk factors and may not optimally identify high-risk pregnancies. Hence, screening with hs-CRP is widely recommended
We suggest that there is a clear need for additional studies on markers that can be used to identify and monitor GDM
Our data were collected at 24–28 weeks of gestation, methods for prevention of GDM may have been more effective if employed before this unstable period (24–28 weeks) of pregnancy, and further research is needed to identify women at this stage of disease development
Another important limitation is small number of patients which may have affected the study result. Despite the relatively small sample size, the validity of our results are strengthened by close matching of control subjects against GDM subjects
It is recommended that women who develop GDM and are predispose to develop type 2 DM later in life, they should adopt a lifestyle modifications, dietary habits, primary health-care strategies to prevent or delay the appearance of glucose intolerance states.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Kane SC, Costa Fda S, Brennecke S. First trimester biomarkers in the prediction of later pregnancy complications. Biomed Res Int. 2014;2014:807196. doi: 10.1155/2014/807196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hwang HS, Kwon JY, Kim MA, Park YW, Kim YH. Maternal serum highly sensitive C-reactive protein in normal pregnancy and pre-eclampsia. Int J Gynaecol Obstet. 2007;98:105–9. doi: 10.1016/j.ijgo.2007.03.050. [DOI] [PubMed] [Google Scholar]
- 3.Nicolaides KH. Turning the pyramid of prenatal care. Fetal Diagn Ther. 2011;29:183–96. doi: 10.1159/000324320. [DOI] [PubMed] [Google Scholar]
- 4.Challis JR, Lockwood CJ, Myatt L, Norman JE, Strauss JF, 3rd, Petraglia F. Inflammation and pregnancy. Reprod Sci. 2009;16:206–15. doi: 10.1177/1933719108329095. [DOI] [PubMed] [Google Scholar]
- 5.Gomes CP, Torloni MR, Gueuvoghlanian-Silva BY, Alexandre SM, Mattar R, Daher S. Cytokine levels in gestational diabetes mellitus: A systematic review of the literature. Am J Reprod Immunol. 2013;69:545–57. doi: 10.1111/aji.12088. [DOI] [PubMed] [Google Scholar]
- 6.Briana DD, Malamitsi-Puchner A. Reviews: Adipocytokines in normal and complicated pregnancies. Reprod Sci. 2009;16:921–37. doi: 10.1177/1933719109336614. [DOI] [PubMed] [Google Scholar]
- 7.Retnakaran R, Hanley AJ, Raif N, Connelly PW, Sermer M, Zinman B. C-reactive protein and gestational diabetes: The central role of maternal obesity. J Clin Endocrinol Metab. 2003;88:3507–12. doi: 10.1210/jc.2003-030186. [DOI] [PubMed] [Google Scholar]
- 8.Loukovaara MJ, Alfthan HV, Kurki MT, Hiilesmaa VK, Andersson SH. Serum highly sensitive C-reactive protein in preterm premature rupture of membranes. Eur J Obstet Gynecol Reprod Biol. 2003;110:26–8. doi: 10.1016/s0301-2115(03)00084-8. [DOI] [PubMed] [Google Scholar]
- 9.Hvilsom GB, Thorsen P, Jeune B, Bakketeig LS. C-reactive protein: A serological marker for preterm delivery? Acta Obstet Gynecol Scand. 2002;81:424–9. doi: 10.1034/j.1600-0412.2002.810509.x. [DOI] [PubMed] [Google Scholar]
- 10.Djurovic S, Clausen T, Wergeland R, Brosstad F, Berg K, Henriksen T. Absence of enhanced systemic inflammatory response at 18 weeks of gestation in women with subsequent pre-eclampsia. BJOG. 2002;109:759–64. doi: 10.1111/j.1471-0528.2002.01330.x. [DOI] [PubMed] [Google Scholar]
- 11.Yu H, Rifai N. High-sensitivity C-reactive protein and atherosclerosis: From theory to therapy. Clin Biochem. 2000;33:601–10. doi: 10.1016/s0009-9120(00)00186-7. [DOI] [PubMed] [Google Scholar]
- 12.Catalano PM, Tyzbir ED, Roman NM, Amini SB, Sims EA. Longitudinal changes in insulin release and insulin resistance in nonobese pregnant women. Am J Obstet Gynecol. 1991;165(6 Pt 1):1667–72. doi: 10.1016/0002-9378(91)90012-g. [DOI] [PubMed] [Google Scholar]
- 13.Radaelli T, Varastehpour A, Catalano P, Hauguel-de Mouzon S. Gestational diabetes induces placental genes for chronic stress and inflammatory pathways. Diabetes. 2003;52:2951–8. doi: 10.2337/diabetes.52.12.2951. [DOI] [PubMed] [Google Scholar]
- 14.Buchanan TA, Xiang AH. Gestational diabetes mellitus. J Clin Invest. 2005;115:485–91. doi: 10.1172/JCI24531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dhok AJ, Daf S, Mohod K, Kumar S. Role of early second trimester high sensitivity C-reactive protein for prediction of adverse pregnancy outcomes. JK Sci. 2011:13. [Google Scholar]
- 16.Vitoratos N, Vrachnis N, Valsamakis G, Panoulis K, Creatsas G. Perinatal mortality in diabetic pregnancy. Ann N Y Acad Sci. 2010;1205:94–8. doi: 10.1111/j.1749-6632.2010.05670.x. [DOI] [PubMed] [Google Scholar]
- 17.Retnakaran R, Qi Y, Sermer M, Connelly PW, Hanley AJ, Zinman B. Glucose intolerance in pregnancy and future risk of pre-diabetes or diabetes. Diabetes Care. 2008;31:2026–31. doi: 10.2337/dc08-0972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maged AM, Moety GA, Mostafa WA, Hamed DA. Comparative study between different biomarkers for early prediction of gestational diabetes mellitus. J Matern Fetal Neonatal Med. 2014;27:1108–12. doi: 10.3109/14767058.2013.850489. [DOI] [PubMed] [Google Scholar]
- 19.Zhu C, Yang H, Geng Q, Ma Q, Long Y, Zhou C, et al. Association of oxidative stress biomarkers with gestational diabetes mellitus in pregnant women: A case-control study. PLoS One. 2015;10:e0126490. doi: 10.1371/journal.pone.0126490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005;115:1111–9. doi: 10.1172/JCI25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pickup JC. Inflammation and activated innate immunity in the pathogenesis of type 2 diabetes. Diabetes Care. 2004;27:813–23. doi: 10.2337/diacare.27.3.813. [DOI] [PubMed] [Google Scholar]
- 22.Bo S, Signorile A, Menato G, Gambino R, Bardelli C, Gallo ML, et al. C-reactive protein and tumor necrosis factor-alpha in gestational hyperglycemia. J Endocrinol Invest. 2005;28:779–86. doi: 10.1007/BF03347566. [DOI] [PubMed] [Google Scholar]
- 23.Stegenga H, Haines A, Jones K, Wilding J. Guideline Development Group. Identification, Assessment and Management of Overweight and Obesity: Summary of Updated NICE Guidance. BMJ. 2014;349:g6608. doi: 10.1136/bmj.g6608. [DOI] [PubMed] [Google Scholar]
- 24.Makgoba M, Savvidou MD, Steer PJ. An analysis of the interrelationship between maternal age, body mass index and racial origin in the development of gestational diabetes mellitus. BJOG. 2012;119:276–82. doi: 10.1111/j.1471-0528.2011.03156.x. [DOI] [PubMed] [Google Scholar]
- 25.Lao TT, Ho LF, Chan BC, Leung WC. Maternal age and prevalence of gestational diabetes mellitus. Diabetes Care. 2006;29:948–9. doi: 10.2337/diacare.29.04.06.dc05-2568. [DOI] [PubMed] [Google Scholar]
- 26.Danilenko-Dixon DR, Van Winter JT, Nelson RL, Ogburn PL., Jr Universal versus selective gestational diabetes screening: Application of 1997 American Diabetes Association recommendations. Am J Obstet Gynecol. 1999;181:798–802. doi: 10.1016/s0002-9378(99)70304-2. [DOI] [PubMed] [Google Scholar]


