Abstract
Introduction:
In Nigeria, anemia in pregnancy is one of the leading causes of poor pregnancy outcomes. This study, therefore, determined the prevalence of anemia and its associated factors, among pregnant primary care clients in Sagamu, Nigeria.
Materials and Methods:
A cross-sectional descriptive study was carried out among 400 pregnant, primary care clients in Sagamu, selected through multi-stage sampling. Data were collected with the aid of an interviewer-administered, semi-structured questionnaire, a stadiometer, measuring tape, and a hemoglobinometer. Data were analyzed using SPSS version 17.00. Relevant descriptive and inferential statistics were calculated. Participation was fully voluntary.
Results:
The mean age of respondents was 25.4 ± 4.2 years. Most respondents (51.8%) were traders. About a third (32.5%) of respondents were anemic; of these, 72.1% were mildly anemic, while 27.1% were moderately anemic. Anemia was associated with household food security (P = 0.044) and level of food insecurity (P = 0.001) but not with age, occupation, educational status, household size, number of previous pregnancies, body mass index, mid-upper arm circumference, snacking, vegetable intake, and food avoidance (P > 0.05).
Conclusion:
Anemia in pregnancy is still high among respondents and associated with household food insecurity. Interventions targeted at improving household food security, dietary intake, and socioeconomic conditions will significantly reduce the prevalence and severity of anemia in pregnancy.
Keywords: Anemia, factors, pregnant, Sagamu, women
Introduction
Anemia in pregnancy remains an issue of public health importance worldwide, but more importantly in the developing countries of Sub-Saharan Africa and Southeast Asia.[1] It is a major contributor to negative pregnancy outcomes, including maternal morbidity and mortality. Anemia in pregnancy predisposes women to postpartum hemorrhage, pregnancy-induced hypertension, postnatal sepsis, a higher risk of preterm delivery, small-for-gestational age and low birth weight babies, stillbirth, and other negative perinatal outcomes.[2,3] The economic burden of anemia in pregnancy, along with its consequences, is enormous for developing countries with their fragile health systems, including those of Sub-Saharan Africa and Southeast Asia. This situation has persisted for several years despite the availability of low-cost, but highly effective interventions, like iron-folate supplementation in pregnancy.[4] The World Health Organization estimates that in the year 2011, about 38% of pregnant women globally, were anemic.[5] Anemia in pregnancy has been studied by several researchers worldwide, with diverse findings. A study of anemia among pregnant women in Kathmandu, Nepal found 42.6% of the women to be anemic. Most (90.8%) of these had a mild form of anemia, 7.1% had moderate anemia, while 2.7% had a severe form of anemia.[6] Researchers in urban Pakistan, while studying the prevalence and risk factors for anemia in pregnancy, found 90.5% of the study participants to be anemic. Most (75%) of these had mild anemia, 14.8% had moderate anemia, while others had the severe form.[7] An institution-based cross-sectional study of the prevalence and predictors of maternal anemia in Northwest Ethiopia reported a prevalence of 16.6% for anemia in pregnancy, of which 64% was of the mild type.[8] In Southern Ethiopia, researchers, in a study of anemia in pregnancy and its associated factors, found 39.9% of study participants to be anemic. Moderate anemia was present in 60% of the anemic women, 30.3% had mild anemia, while 9.7% were severely anemic.[9] An evaluation of anemia among booked antenatal clients in Puducherry, India, found 83% of the pregnant women being anemic.[10] A study of anemia among pregnant women in Northern Tanzania reported a prevalence of 47.4%, with 74.5% of these being mild anemia, 20.9% being moderate anemia, and 4.5% being of the severe form of anemia.[11] The prevalence of 51.8% was reported for anemia among pregnant women in Gombe, North-Eastern Nigeria. Mild anemia was found in 67.4% of respondents; moderate anemia in 30.5% and severe anemia in 2.1% of study participants.[12] A study of anemia and iron deficiency among pregnant women in Zaria found 12.2% of the women studied to be anemic.[13] A retrospective study among pregnant women accessing antenatal care in a mission hospital in South-South Nigeria reported a prevalence of 32.3% for anemia in pregnancy at booking.[14] Researchers found the prevalence of anemia in pregnancy, at booking, to be 40.4% in Enugu, Southeastern Nigeria. Anemia was of the mild form in 90.7% of these women while moderate anemia accounted for 9.3% of cases. No case of severe anemia was reported.[15] A cross-sectional study of hematocrit and arm preference for blood collection among gravid women in Southeastern Nigeria, found 28% of the women to be anemic, of which 94.6% were mildly anemic.[16] In Ilesha, Southwest Nigeria, a cross-sectional descriptive study, reported 62.2% of the women studied to be anemic, 2% of whom had severe anemia.[17] In a study of the sociodemographic determinants of anemia in pregnancy in Oyo State, Southwestern Nigeria, researchers reported a prevalence of 32.8% for anemia in pregnancy.[18] Researchers in Ibadan, Southwest Nigeria, found 30% of their study participants – pregnant women – to be anemic.[19] A survey of pregnant women in Abeokuta, Southwest Nigeria, reported 76.5% of the study participants to be anemic, of whom 40.3% had mild anemia; 57.8% had moderate anemia, while only 1.9% were severely anemic.[20] A cross-sectional study reported a prevalence of 35.3% for anemia in pregnancy at booking in a tertiary center in Lagos, Southwest Nigeria.[21]
Several factors have been associated with anemia in pregnancy as documented in medical and allied literature. Sociodemographic variables such as age, parity and gravidity, personal and household income, household size, level of education, a low socioeconomic status, short birth intervals, and higher gestational age at booking, particularly booking in the third trimester are some of the known factors associated with anemia.[8,11,16,18,19,20] Maternal and fetal anthropometry have also been associated with anemia among pregnant women. Researchers in Ethiopia, found low body mass index (BMI) to be an independent predictor of anemia in pregnancy. The height, weight, and BMI of nonanemic women were significantly higher than those of their anemic counterparts in a study conducted among pregnant urban women in Pakistan. Dietary factors including consumption of fruit, red meat, eggs, tea before and during pregnancy as well as consumption of nonfood materials such as clay, dirt, and so on (pica), were associated with anemia in pregnancy.[7] Anemia has also been associated with anthropometry, diet and socioeconomic status among nongravid women of reproductive age.[22,23] In China, women belonging to high and middle-income groups were more likely to be anemic than their counterparts with low socioeconomic status. The relationship between household food security and various nutritional parameters in women of reproductive age, including anemia, dietary intake, and anthropometry, has also been the subject of several research works.[24,25,26]
The primary health-care centers (PHCs) are the first contact for most patients, with the formalized health system in Ogun State, Nigeria. The PHCs provide a wide array of primary care services, including health promotion, disease prevention, and curative services. Antenatal care is a major component of the maternal and child health services provided in Sagamu. This study, therefore, determined the prevalence of anemia and its associated factors, among pregnant primary care clients in Sagamu, Southwest Nigeria.
Materials and Methods
The study was carried out in Sagamu, one of the twenty local government areas (LGA) in Ogun State, Southwest Nigeria. It is an urban LGA with a few rural settlements. It is located within the defunct Remo division, a part of the Ogun-East senatorial zone. It is bounded in the East by Ikenne LGA, in the North by Remo-North LGA, in the West by Obafemi-Owode LGA, and the South by Ikorodu LGA of Lagos State. Sagamu LGA is divided into 15 geo-political wards, with a diverse population, though mainly people of Yoruba extraction. It is home to several industries, including manufacturing, fast moving consumer goods, and the extractive industries. There are several health facilities in Sagamu, ranging from private hospitals to public health facilities such as the PHCs, General Hospital, and Olabisi Onabanjo University Teaching Hospital.
Women of reproductive age accessing ante-natal care services at selected urban PHCs, between January and April 2013, were recruited into the study. Only those with singleton pregnancies were eligible for recruitment into the study. Women with medical complications, including bleeding disorders, were excluded from the study.
The sample size for the study was calculated using the formula for descriptive studies, N = Z2 pq/d2. The calculated sample size was 339, using a prevalence of 32.8% for anemia in pregnancy, from a previous study carried out in Southwest Nigeria.[18] Assuming a nonresponse rate of 20%, taking into consideration incomplete questionnaires and other challenges with data collection, the sample size was rounded up to 400. Therefore, 400 pregnant women, utilizing PHCs for antenatal care, were recruited into the study. Selection of study participants was carried out through multi-stage sampling technique. The first stage involved selection of four wards from the 15 existing wards in Sagamu LGA by simple random sampling. The second stage involved selection of one PHC in each of the preselected wards by simple random sampling technique. The final stage involved selection of study participants at each of the four PHCs using systematic sampling technique. A sampling interval of three was calculated, therefore, every third eligible client was recruited into the study.
Data collection was carried out with the aid of a validated semi-structured, interviewer-administered questionnaire, an adult weighing scale, stadiometer, measuring tape, hemoglobinometer. The questionnaire obtained information on sociodemographics, household food security and related data. Household food security was assessed with the aid of the 6-item questionnaire and classified as food secure, food insecure without hunger, and food insecure with hunger. Hemoglobin concentration was measured using a portable hemoglobinometer, as described in a previous study.[27] Anemia was defined as a hemoglobin concentration <11 g/dl. Mild anemia was defined as hemoglobin concentration between 10.0 and 10.9 g/dl, moderate anemia as hemoglobin concentration between 7.0 and 9.9 g/dl, while severe anemia was defined as a hemoglobin concentration below 7 g/dl. Respondents' weight was measured with the aid of an adult weighing scale, to the nearest 0.1 kg, while height was measured using a stadiometer. BMI (kg/m2) was calculated from measurements of participants' weight (kg) and height (m) as described in literature.[28,29] Mid-upper arm circumference was assessed using the measuring tape in centimeters (cm) and classified as normal (for readings above 23 cm), mild to moderate malnutrition (for reading between 21 and 23 cm) and severe malnutrition (for readings below 21 cm), as documented in literature.[30,31] Data collected daily were checked for completeness and accuracy, before entry. Data analysis was carried out, using SPSS (Statistical Package for the Social Sciences) version 17 (SPSS Inc. Released 2008. SPSS Statistics for Windows, Chicago). Frequencies, proportions, and means were calculated and presented in prose and tables. Chi-square test was used to test for association between categorical variables, with the level of significance set at ≤0.05.
Ethical approval was obtained from the Ogun State Primary Health Care Board, Abeokuta, as well as the Health Research and Ethics Committee of Olabisi Onabanjo University Teaching Hospital, Sagamu. Permission was also obtained from the Local Government Authority, through the Primary Health Care Department. Participants' written informed consent was obtained before commencement of the study. Strict confidentiality was ensured throughout the study and participation was fully voluntary.
Results
The mean age of respondents was 25.4 ± 4.2 years. The modal age of respondents was between 21 and 30 years of age, with 64.5% of respondents falling into this category; only 3.7% of study participants were aged between 41 and 50 years. About 98% of respondents were married, with 51.8% being traders; more than 50% had secondary education, while only about 5% had no formal education. About 80% of respondents were from polygamous families. There was no significant difference (P > 0.05) between the age distribution, occupation, educational status, partner's occupation and family type of anemic and nonanemic respondents [Table 1].
Table 1.
Respondents’ sociodemographic characteristics
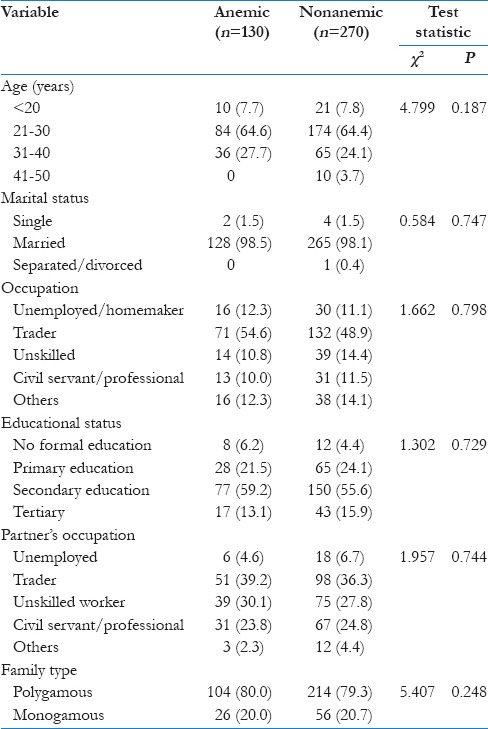
Most respondents (51.6% of anemic and 61.6% of nonanemic) had water carriage systems as their sewage disposal method; about 90% of respondents had access to pipe-borne water; 45.4% of anemic women and 41.9% of nonanemic pregnant women lived in single room accommodations. Most respondents (about 55%) had a household size between 1 and 3, while <1% had a household size of ten persons and above. About a quarter (24.9%) of respondents were primigravidae. There was no significant difference between the sewage disposal methods (P = 0.106), source of drinking water (P = 0.165), household size (P = 0.411), number of previous pregnancies (P = 0.645), and type of housing (P = 0.272) of anemic and nonanemic women [Table 2].
Table 2.
Household characteristics and living conditions of respondents
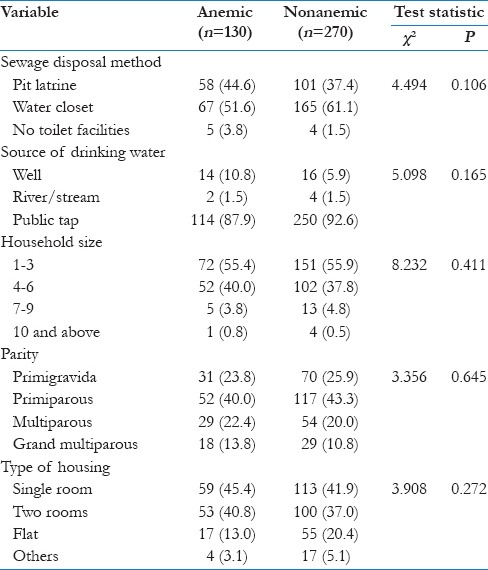
One hundred and thirty (32.5%) respondents were anemic; of these, 94 (72.1%) were mildly anemic, while 36 (27.9%) were moderately anemic. No case of severe anemia was found among study participants.
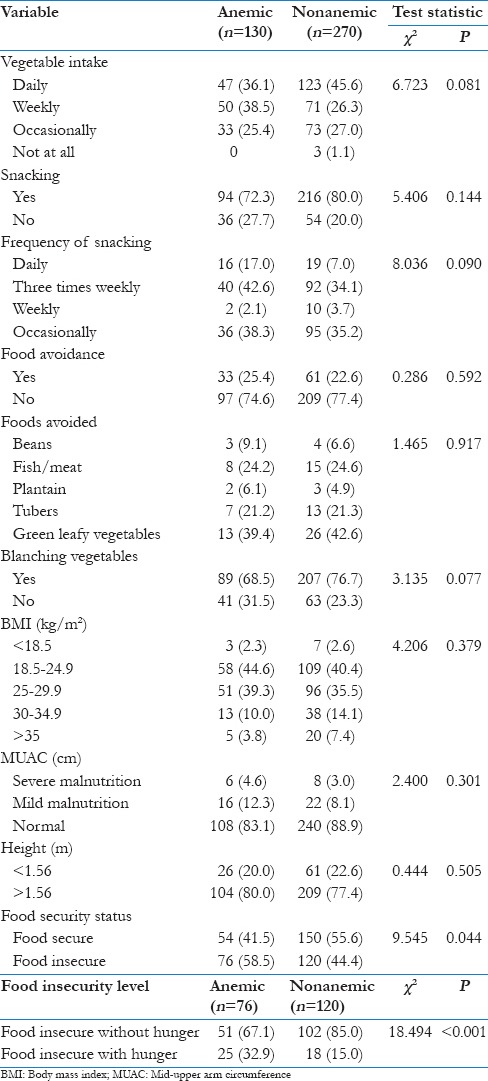
Daily vegetable intake was reported by 36.1% of anemic respondents compared with 45.6% of nonanemic women; only 25.4% and 27.0% of anemic and nonanemic respondents consumed vegetables occasionally. There was no significant difference (P = 0.081) between the vegetable consumption patterns of anemic and nonanemic women. There was no significant difference in the prevalence (P = 0.144) and frequency (P = 0.090) of snacking between anemic and nonanemic respondents. Food avoidance was higher (25.4%) among anemic than nonanemic women (22.6%). There was no significant difference in the prevalence of food avoidance (P = 0.592) and type of food avoided (P = 0.917). Only 2.3% of anemic women had evidence of undernutrition (BMI <18.5 kg/m2) compared with 2.6% of the nonanemic respondents. There was no significant difference between the BMI (P = 0.379) and mid upper arm circumference (P = 0.301) of anemic and nonanemic respondents. The prevalence of household food security among anemic women was 41.5% while that among the nonanemic respondents was 56.6%. There was a significant difference between the household food security status of anemic and nonanemic respondents (P = 0.044) as well as the level of food insecurity (P ≤ 0.001) [Table 3].
Table 3.
Nutrition and related factors
Discussion
The mean age of respondents in this study is lower than findings from Enugu, where respondents' mean age was 30.2 ± 5.2 years.[15] The prevalence of anemia in this study is similar to findings from Southern Ethiopia,[9] Ibadan,[19] Lagos[21] South-South Nigeria,[14] and a multicenter study in rural and urban areas of Oyo State.[18] It was, however, higher than those reported by researchers in Enugu,[16] Zaria,[13] Gondar, Ethiopia,[8] and India.[25] The proportion of anemia in pregnancy in our study is very much lower than those found in Ilesha,[17] Abeokuta,[20] Gombe,[12] Pakistan,[7] and India.[10] It is a little lower than the proportions reported in Enugu,[15] Nepal,[6] and Tanzania.[11]
In this study, almost three-quarters (72.1%) of the anemic women were mildly anemic, a finding consistent with those from Pakistan,[7] similar to those from Gondar, Ethiopia,[8] Northern Tanzania[11] and Gombe, Nigeria.[12] It is, however, lower than the prevalence reported in Enugu, Nigeria[15,16] and in Kathmandu, Nepal.[6] Some other studies in Southern Ethiopia[9] and Abeokuta, Southwest Nigeria,[20] reported much lower proportions of mild anemia.
The proportion of women with moderate anemia (27.9%) is comparable to 30.5% reported in Gombe, Northeast Nigeria[12] and a little higher than that reported from Northern Tanzania[11] but much lower than findings from Sodo Town, Southern Ethiopia, where 60% of anemic women had moderate anemia[9] and Abeokuta, Southwest Nigeria, where 57.8% of anemia in pregnancy, was of the moderate form.[20] It is however much higher than findings from Nepal,[6] Pakistan,[7] and Enugu.[15,16] The absence of cases of severe anemia in this study is similar to the report from Enugu, Nigeria[15] where none of the pregnant women studied was severely anemic. This contrasts with several other studies, which reported varying proportions of severe anemia among the pregnant women studied.[6,7,11,12,17,20] This observation may be due to the fact that iron-folate supplementation was routinely practiced in all the PHCs, used for the study. The importance of adherence to supplements in pregnancy was stressed during the health education sessions, which took place at every antenatal visit.
Dietary practices and anthropometric indices were not associated with anemia, in this study. This contrasts with findings from Ethiopia and Pakistan, where body mass, height, and weight were associated with anemia.[7,9] In Andhra Pradesh, India, anemia was found to have an inverse relationship with BMI among the women studied,[23] just as it showed a significant decreasing trend with increasing BMI among Chinese women.[22] Sociodemographic variables showed no association with anemia, in contrast to other studies. Maternal age had no association with anemia, in agreement with findings from Enugu.[15] This however contrasts with findings from Oyo State, Nigeria[18,19] and Puducherry, India.[10] Educational status was not associated with anemia, in contrast with findings from India.[10] Several studies found an association between parity and anemia[9,16,17,19] in contrast with this study as well as others conducted in Eastern Nigeria[15] and Gombe.[12] In contrast to some other studies,[8,21,23] characteristics depicting socioeconomic status, were not associated with anemia.
In contrast to the findings from a study of the impact of household food security on maternal profile, anemia was associated with household food security status as well as the level of food insecurity in this study.[25] A study among Mexican women, associated household food insecurity with anemia. It reported that women with anemia were less likely to be food secure. It also found anemia to be associated with educational status, age group, number of previous pregnancies as well as BMI.[26]
Anemia is associated with sub-optimal work performance, reduced mental functioning, and productivity among adults.[32] This implies reduced earning capacity for the concerned individual and often times, the entire household. When pregnant women are anemic, in a resource-constrained environment as Nigeria, the effect on the individual and household economy can be enormous. Pregnant anemic women are more likely to produce anemic offspring, with its attendant effects of reduced cognitive capacity, psychomotor challenges, and behavioral anomalies, which are sometimes lifelong.[26] This exerts additional strain on the household and community resources, available for childcare. Due to its adverse health and associated consequences, particularly increased infant and maternal morbidity and mortality, maternal anemia should be controlled through intersectoral action.[4] The poor socioeconomic conditions and health-related factors, predisposing to anemia and iron deficiency in women and children, are underlying issues that need to be considered, if sustainable progress will be made in reducing maternal and child morbidity. Food security aids in the realization of dietary diversification, at the individual and household levels.
The importance of dietary diversity and nutrient adequacy in pregnancy need to be emphasized in program planning, implementation and evaluation, especially as it relates to community-level programs, which have a potential to reach all pregnant and lactating women, in order to optimize their health and wellbeing, as well as those of their babies in utero. A limitation of this study is that it recruited only primary care clients, who may not adequately represent the entire population of gravid women in Sagamu, having left out clients, accessing antenatal care in secondary and tertiary centers. It also did not follow-up clients to delivery, to assess any association between anemia in pregnancy and delivery outcome. This is a subject for further research.
Conclusion and Recommendation
The prevalence of anemia among pregnant primary care clients in Sagamu was fairly high, mostly of the mild form. Household food security and level of food insecurity were the only factors associated with anemia in pregnancy.
Dietary intake of nutritious foods, as well as supplementation with iron and folate, in addition to improvement of the socioeconomic conditions of pregnant women, will greatly improve their health status, including hemoglobin concentration. Primary Health Care is an effective approach to meeting the health and health-related needs of any community. Therefore, proper implementation of maternal and child health strategies, at the PHC level, has great prospects for a significant reduction in maternal and infant morbidity and mortality.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors express our profound gratitude to Dr. O. A. Jeminusi, Professor Olayinka Abosede, Dr. Adetola Osinubi and Mrs. R. Ademola-Raheem, for their immense contributions toward data collection and manuscript preparation.
References
- 1.Milman N. Anemia – Still a major health problem in many parts of the world! Ann Hematol. 2011;90:369–77. doi: 10.1007/s00277-010-1144-5. [DOI] [PubMed] [Google Scholar]
- 2.Noronha JA, Khasawneb EA, Seshan V, Ramasubramaniam S, Raman S. Anemia in pregnancy – Consequences and challenges: A review of literature. J South Asian Fed Obstet Gynaecol. 2012;4:64–70. [Google Scholar]
- 3.Kozuki N, Lee AC, Katz J Child Health Epidemiology Reference Group. Moderate to severe, but not mild, maternal anemia is associated with increased risk of small-for-gestational-age outcomes. J Nutr. 2012;142:358–62. doi: 10.3945/jn.111.149237. [DOI] [PubMed] [Google Scholar]
- 4.Cambodia: Council for Agriculture and Rural Development, United Nations Children Fund, World Food Program; 2013. CARD/UNICEF/WFP. The Economic Consequences of Malnutrition in Cambodia. A Damage Assessment Report. [Google Scholar]
- 5.Geneva: World Health Organization; 2015. World Health Organization. The Global Prevalence of Anaemia in 2011. [Google Scholar]
- 6.Marahatta R. Study of anaemia in pregnancy and its outcome in Nepal Medical College Teaching Hospital, Kathmandu, Nepal. 2007;9:270–4. [PubMed] [Google Scholar]
- 7.Baig-Ansari N, Badruddin SH, Karmaliani R, Harris H, Jehan I, Pasha O, et al. Anemia prevalence and risk factors in pregnant women in an urban area of Pakistan. Food Nutr Bull. 2008;29:132–9. doi: 10.1177/156482650802900207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Melku M, Addis Z, Alem M, Enawgaw B. Prevalence and predictors of maternal anemia during pregnancy in Gondar, Northwest Ethiopia: An institutional based cross-sectional study. Anemia. 2014;2014:108593. doi: 10.1155/2014/108593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gedefaw L, Ayele A, Asres Y, Mossie A. Anemia and Associated Factors among pregnant women attending antenatal care clinic in Wolayita Sodo town, Southern Ethiopia. Ethiop J Health Sci. 2015;25:155–62. doi: 10.4314/ejhs.v25i2.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amel Ivan E, Mangaiarkkarasi A. Evaluation of anaemia in booked antenatal mothers during the last trimester. J Clin Diagn Res. 2013;7:2487–90. doi: 10.7860/JCDR/2013/6370.3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Msuya SE, Hussein TH, Uriyo J, Sam NE, Stray-Pedersen B. Anaemia among pregnant women in Northern Tanzania: Prevalence, risk factors and effect on perinatal outcomes. Tanzan J Health Res. 2011;13:33–9. doi: 10.4314/thrb.v13i1.60881. [DOI] [PubMed] [Google Scholar]
- 12.Bukar M, Audu BM, Yahaya UR, Melah GS. Anaemia in pregnancy at booking in Gombe, North-Eastern Nigeria. J Obstet Gynaecol. 2008;28:775–8. doi: 10.1080/01443610802463835. [DOI] [PubMed] [Google Scholar]
- 13.Hassan AA, Mamman AI, Adaji S, Musa B, Kene S. Anemia and iron deficiency in pregnant women in Zaria, Nigeria. Sub Saharan Afr J Med. 2014;1:36–9. [Google Scholar]
- 14.Ikeanyi EM, Ibrahim AI. Does antenatal care attendance prevent anemia in pregnancy at term? Niger J Clin Pract. 2015;18:323–7. doi: 10.4103/1119-3077.151730. [DOI] [PubMed] [Google Scholar]
- 15.Dim CC, Onah HE. The prevalence of anemia among pregnant women at booking in Enugu, South Eastern Nigeria. MedGenMed. 2007;9:11. [PMC free article] [PubMed] [Google Scholar]
- 16.Dim C, Ugwu E, Dim N, Anyaehie U. Hematocrit, anemia, and arm preference for blood sample collection: A cross-sectional study of pregnant women in Enugu, South-Eastern, Nigeria. Ann Med Health Sci Res. 2015;5:36–41. doi: 10.4103/2141-9248.149783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Komolafe JO, Kuti O, Oni O, Egbewale BE. Sociodemographic characteristics of anaemic gravidae at booking: A preliminary study at Llesha, Western Nigeria. Niger J Med. 2005;14:151–4. doi: 10.4314/njm.v14i2.37172. [DOI] [PubMed] [Google Scholar]
- 18.Dairo MD, Lawoyin TO. Socio-demographic determinants of anaemia in pregnancy at primary care level: A study in urban and rural Oyo State, Nigeria. Afr J Med Med Sci. 2004;33:213–7. [PubMed] [Google Scholar]
- 19.Olubukola A, Odunayo A, Adesina O. Anemia in pregnancy at two levels of health care in Ibadan, South West Nigeria. Ann Afr Med. 2011;10:272–7. doi: 10.4103/1596-3519.87042. [DOI] [PubMed] [Google Scholar]
- 20.Idowu OA, Mafiana CF, Dapo S. Anaemia in pregnancy: A survey of pregnant women in Abeokuta, Nigeria. Afr Health Sci. 2005;5:295–9. doi: 10.5555/afhs.2005.5.4.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anorlu RI, Oluwole AA, Abudu OO. Sociodemographic factors in anaemia in pregnancy at booking in Lagos, Nigeria. J Obstet Gynaecol. 2006;26:773–6. doi: 10.1080/01443610600963846. [DOI] [PubMed] [Google Scholar]
- 22.Qin Y, Melse-Boonstra A, Pan X, Yuan B, Dai Y, Zhao J, et al. Anemia in relation to body mass index and waist circumference among Chinese women. Nutr J. 2013;12:10. doi: 10.1186/1475-2891-12-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hemamalini J. Anemia in relation to body mass index and waist circumference among Andhra Pradesh women. J Obes Weight Loss Ther. 2013;3:173. [Google Scholar]
- 24.Laraia BA, Siega-Riz AM, Gundersen C. Household food insecurity is associated with self-reported pregravid weight status, gestational weight gain, and pregnancy complications. J Am Diet Assoc. 2010;110:692–701. doi: 10.1016/j.jada.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jose S, Subhasree S. Impact of household food security on maternal nutritional profile. Int J Sci Res. 2015;4:105–8. [Google Scholar]
- 26.Fischer NC, Shamah-Levy T, Mundo-Rosas V, Méndez-Gómez-Humarán I, Pérez-Escamilla R. Household food insecurity is associated with anemia in adult Mexican women of reproductive age. J Nutr. 2014;144:2066–72. doi: 10.3945/jn.114.197095. [DOI] [PubMed] [Google Scholar]
- 27.Sholeye OO, Jeminusi OA, Shorunmu TO, Salako AA, Ademola-Raheem R. A comparative study of anemia among pregnant women in rural and urban areas of Ogun State, Southwest Nigeria. Health Care. 2015;3:21–6. [Google Scholar]
- 28.Saxena V, Srivastava VK, Idris MZ, Mahan U, Bhushan V. Nutritional status of rural pregnant women. Indian J Community Med. 2000;25:104–7. [Google Scholar]
- 29.Okwu GN, Ukoha AI, Nwachukwu N, Agha NC. Studies on the predisposing factors to protein energy malnutrition among pregnant women in Nigerian community. Online J Health Allied Sci. 2007;6:1–9. [Google Scholar]
- 30.New York: UNICEF; 2009. [Last accessed on 2013 Nov 29]. United Nations Children's Fund. Mid-upper Arm Circumference (MUAC) Measuring Tapes. Technical Bulletin No. 13; p. 3. Available from: http://www.supply.unicef.org/unicef-620/mimes . [Google Scholar]
- 31.Jeminusi OA, Sholeye OO, Abosede OA. Maternal anthropometry in rural and urban areas of Ogun-East senatorial district, Nigeria: A comparative study. Int J Nutr Metab. 2015;7:39–45. [Google Scholar]
- 32.Haas JD, Brownlie T., 4th Iron deficiency and reduced work capacity: A critical review of the research to determine a causal relationship. J Nutr. 2001;131:676S–88S. doi: 10.1093/jn/131.2.676S. [DOI] [PubMed] [Google Scholar]


