Ca2+-dependent facilitation is a positive feedback mechanism that regulates Cav2.1 P/Q-type channels but not closely related Cav2.2 N-type channels. Thomas et al. identify the molecular determinants that distinguish the ability of Cav2.1 and Cav2.2 to undergo Ca2+-dependent facilitation.
Abstract
Voltage-gated Cav2.1 (P/Q-type) Ca2+ channels undergo Ca2+-dependent inactivation (CDI) and facilitation (CDF), both of which contribute to short-term synaptic plasticity. Both CDI and CDF are mediated by calmodulin (CaM) binding to sites in the C-terminal domain of the Cav2.1 α1 subunit, most notably to a consensus CaM-binding IQ-like (IQ) domain. Closely related Cav2.2 (N-type) channels display CDI but not CDF, despite overall conservation of the IQ and additional sites (pre-IQ, EF-hand–like [EF] domain, and CaM-binding domain) that regulate CDF of Cav2.1. Here we investigate the molecular determinants that prevent Cav2.2 channels from undergoing CDF. Although alternative splicing of C-terminal exons regulates CDF of Cav2.1, the splicing of analogous exons in Cav2.2 does not reveal CDF. Transfer of sequences encoding the Cav2.1 EF, pre-IQ, and IQ together (EF-pre-IQ-IQ), but not individually, are sufficient to support CDF in chimeric Cav2.2 channels; Cav2.1 chimeras containing the corresponding domains of Cav2.2, either alone or together, fail to undergo CDF. In contrast to the weak binding of CaM to just the pre-IQ and IQ of Cav2.2, CaM binds to the EF-pre-IQ-IQ of Cav2.2 as well as to the corresponding domains of Cav2.1. Therefore, the lack of CDF in Cav2.2 likely arises from an inability of its EF-pre-IQ-IQ to transduce the effects of CaM rather than weak binding to CaM per se. Our results reveal a functional divergence in the CDF regulatory domains of Cav2 channels, which may help to diversify the modes by which Cav2.1 and Cav2.2 can modify synaptic transmission.
Introduction
Voltage-gated Cav Ca2+ channels are multi-subunit complexes that regulate a variety of biological activities such as gene expression, muscle contraction, and neurotransmitter release. Cav channels consist of an α1 subunit, which forms the pore, and two auxiliary subunits, β and α2δ (Simms and Zamponi, 2014). Of the multiple Cav channels that have been characterized (Cav1.x–Cav3.x), Cav2.1 (P/Q-type) and Cav2.2 (N-type) channels play prominent presynaptic roles in regulating neurotransmitter release (Dunlap et al., 1995). Cav2.1 Ca2+ signals promote exocytosis at most synapses, including CA3-CA1 hippocampal synapses (Wheeler et al., 1994), the calyx of Held auditory brainstem synapse (Forsythe et al., 1998; Inchauspe et al., 2004), and the parallel fiber–Purkinje cell synapse in the cerebellum (Mintz et al., 1995). Although Cav2.2 plays a secondary role to Cav2.1 at many central synapses, Cav2.2 is the major Cav channel regulating neurotransmitter release from terminals of spinal nociceptive neurons (Hatakeyama et al., 2001) and superior cervical ganglion neurons (Boland et al., 1994). Genetic inactivation of Cav2.2 in mice causes no overt phenotypes except for higher pain thresholds (Hatakeyama et al., 2001). In contrast, knockout of Cav2.1 causes ataxia, seizures, and premature death (Jun et al., 1999).
Perhaps to support their distinct physiological roles, Cav2.1 and Cav2.2 channels are differentially modulated by a variety of factors, including the Ca2+ ions that pass through the pore. Like other high voltage–activated Cav channels (Liang et al., 2003), Cav2.1 and Cav2.2 undergo Ca2+-dependent inactivation (CDI) mediated by calmodulin (CaM) binding to sites in the intracellular C-terminal domain (CTD) of the α1 subunit (Lee et al., 1999; DeMaria et al., 2001). These include a consensus IQ-like domain for binding CaM (IQ) as well as a CaM-binding domain (CBD; Fig. 1). During a train of depolarizations, the amplitude of Cav2.1 Ca2+ currents increases and then declines because of the onset of CDI. The initial increase is caused by Ca2+-dependent facilitation (CDF), which also requires CaM (Lee et al., 1999; DeMaria et al., 2001) and potentially other Ca2+ sensor proteins in neurons (Tsujimoto et al., 2002). CDF and CDI of Cav2.1 currents contribute to the facilitation and depression, respectively, of synaptic transmission at the calyx of Held (Cuttle et al., 1998; Forsythe et al., 1998; Tsujimoto et al., 2002) and other brain synapses (reviewed in Catterall et al., 2013).
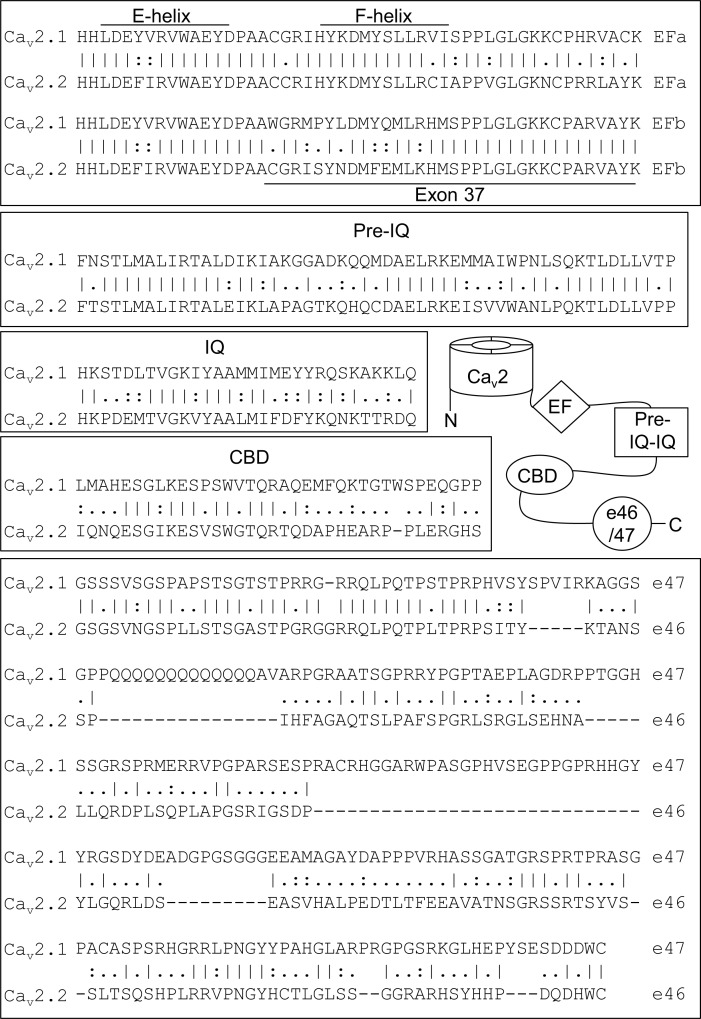
Figure 1.
CDF modulatory domains in the CTD of Cav2.1 and sequence alignment with analogous regions of Cav2.2. Vertical bars (|), identical residues; colons (:), conservative substitutions; periods (.), nonconservative substitutions. Alignment is with human Cav2.1 and 2.2 sequences (GenBank NM_023035.2, NM_001127222.1, NM_000718.3, and CM000671.2).
Despite the physiological importance of CDF of Cav2.1 in short-term synaptic plasticity (Nanou et al., 2016), there is little evidence that Cav2.2 channels are similarly regulated. In a heterologous expression system, CDF is not observed for Cav2.2 under conditions that evoke robust CDF of Cav2.1 (Liang et al., 2003). At the calyx of Held of mice lacking Cav2.1, Cav2.2 channels compensate for the loss of Cav2.1, but the resulting Ca2+ currents do not facilitate or support short-term plasticity (Inchauspe et al., 2004). Although a form of CDF has been reported for Cav2.2 channels in dorsal root ganglion neurons, the mechanism relies on CaM-dependent protein kinase II and is distinct from CaM-dependent CDF of Cav2.1 channels (Tang et al., 2012).
What prevents Cav2.2 from undergoing CDF is unknown but may involve unique sequence elements in the CTD of the α1 subunit based on analyses of Cav2.1 splice variants. Alternative splicing of exons in the proximal or distal CTD of the Cav2.1 α1 subunit (exons 37 and 47, respectively; Fig. 1) gives rise to channels with altered CDF (Chaudhuri et al., 2004). Notably, the corresponding exons of Cav2.2 also undergo alternative splicing with effects on Cav2.2 current density, modulation by G-proteins, and synaptic trafficking in neurons (Maximov and Bezprozvanny, 2002; Bell et al., 2004; Lipscombe et al., 2013). The potential of these alternatively spliced exons to regulate CDF of Cav2.2 has not been investigated.
In this study, we tested whether sequences encoded by exons 37 and 46, as well as other regions of the CTD, underlie the absence of CDF in Cav2.2. We find that although splice variation of exons 37 and 46 was inconsequential, the transfer of the key CDF regulatory sites in Cav2.1 to Cav2.2 unmasked strong CDF in the chimeric channels. However, transfer of any of these sites alone was ineffective. Our results reveal an unexpected variance in the molecular determinants controlling CaM regulation of Cav2.1 and Cav2.2, which may shape the distinct coupling of these channels to vesicle release at the synapse.
Materials and methods
cDNAs and molecular biology
The following cDNAs were used: Cav2.1 (NM_001127221), Cav2.2 e37a (AF055477), Cav2.2 e37b (NM_147141), β2A (NM_053851), and α2δ-1 (NM_000722.3). The plasmid for β2a-CaM was a gift from I. Dick (University of Maryland, Baltimore, MD). Chimeras were constructed using NEBuilder HiFi DNA Assembly Cloning System (New England Biolabs) and Cav2.1 and Cav2.2 e37a as templates. The following constructs were generated by swapping the amino acids indicated in parentheses: Cav2.2-CT2.1, Cav2.1-CT2.2 (1,681–2,334 of Cav2.2, 1,786–2,261 of Cav2.1); Cav2.2-EF2.1, Cav2.1-EF2.2 (1,681–1,788 of Cav2.2, 1,786–1,892 of Cav2.1); Cav2.2-pre-IQ-IQ2.1, Cav2.1-pre-IQ-IQ2.2 (1,789–1,875 of Cav2.2, 1,893–1,985 of Cav2.1); and Cav2.2-CBD2.1, Cav2.1-CBD2.2 (1,912–1,990 of Cav2.2, 2,009–2,084 of Cav2.1). Additional chimeric channels containing subsets of the EF-hand, pre-IQ, IQ, and CBD were generated using the residues indicated above. For Cav2.2 Δe46, the sequence encoding exons 42–45 of Cav2.2 (1,927–2,162) followed by a stop codon was amplified by PCR and cloned into the corresponding site of Cav2.2 as an XbaI fragment. All chimeras and Cav2.2 Δe46 constructs were cloned into the pcDNA6V5His vector. For generating glutathione S-transferase (GST) fusion proteins, sequences corresponding to theaforementioned Cav2 domains were amplified by PCR and cloned into BamHI and XhoI sites of the pGEX-4T-1 vector.
Cell culture and transfection
Human embryonic kidney 293 cells transformed with the SV40 T-antigen (HEK 293T, CRL-3216, RRID:CVCL_0063; ATCC) were maintained in Dulbecco’s modified Eagle’s medium with 10% FBS at 37°C in a humidified atmosphere with 5% CO2. Cells were grown to 80% confluence and transfected using FuGene 6 (Promega) according to the manufacturer’s protocol. Cells were plated in 35-mm dishes and transfected with cDNAs encoding Cav channel subunits (for Cav2.1 and chimeras with Cav2.2 CTDs: 1.0 µg α1, 0.5 µg β2A, and 0.5 µg α2δ1; for Cav2.2 and chimeras with Cav2.1 CTDs: 1.8 µg α1, 0.6 µg β2A, and 0.6 µg α2δ1). Cotransfection with cDNA encoding enhanced green fluorescent protein (pEGFP, 50 ng) allowed visualization of transfected cells.
Electrophysiological recordings
Whole-cell patch recordings were performed 24–72 h after transfection with a EPC-8 patch clamp amplifier and PatchMaster software (HEKA Elektronik). External recoding solution contained (mM) 150 Tris, 1 MgCl2, and 5 CaCl2 or BaCl2. Intracellular solution contained (mM) 140 N-methyl-d-glucamine, 10 Hepes, 10 or 0.5 EGTA, 2 MgCl2, and 2 Mg-ATP. The pH of both solutions was adjusted to 7.3 using methanesulfonic acid. Electrode resistances were 4–6 MΩ in the bath solution. Series resistance was compensated 60–70%. Leak currents were subtracted using a P/−4 protocol. Data were analyzed using Igor Pro software (WaveMetrics). Averaged data represent mean ± SEM and results from at least three independent transfections.
Pull-down binding assays
The cDNA encoding full-length rat CaM (rCaM1–148 [Pedigo and Shea, 1995], provided by M. Shea) was expressed in BL21 DE3 Escherichia coli bacteria and purified as described previously (Theoharis et al., 2008). Purified CaM (1–10 µg) was added to GST or GST-tagged Cav2.1 or Cav2.2 proteins (5 µg) immobilized on glutathione Sepharose beads (GE Healthcare Life Sciences). The reaction was brought to a total volume of 750 µl with binding buffer (20 mM Tris-HCl, pH 7.3, 2 mM CaCl2, ± 150 mM NaCl; results were similar with or without the added NaCl and so were combined). Binding reactions were incubated at 4°C, rotating for 1 h. The beads were washed three times with 1 ml ice-cold binding buffer, and bound proteins were eluted, resolved by SDS-PAGE, and transferred to nitrocellulose. To detect the GST-proteins, the nitrocellulose was first stained with Ponceau S. Bound CaM was then detected by Western blot with rabbit polyclonal antibodies against CaM (1:1,000, 301 003, RRID:AB_2620046; Synaptic Systems). Blots were processed with HRP-conjugated secondary antibodies (anti–rabbit IgG, 1:4,000, I5006, RRID: AB_1163659; Sigma-Aldrich) and reagents for enhanced chemiluminescent detection (Thermo Fisher Scientific) before autoradiography.
For quantitative analysis, densitometry was performed using a Canon LIDE 200 scanner and ImageJ (NIH) software. The Western blot signal for CaM was normalized to the signal corresponding to the Ponceau-stained GST fusion proteins. Results from at least three independent experiments were pooled for statistical analysis.
Data presentation and statistical analysis
Data were incorporated into figures using SigmaPlot (Systat Software) and Adobe Illustrator software. Statistical analysis was performed with SigmaPlot or GraphPad Prism software. The data were first analyzed for normality using the Shapiro–Wilk test. For parametric data, significant differences were determined by Student’s t test or ANOVA with post hoc Dunnett or Tukey test. For nonparametric data, Kruskal–Wallis and post hoc Dunn’s tests were used.
Online supplemental material
Effects of varying EGTA concentration in the intracellular recording solution are presented in Fig. S1. Fig. S2 shows that enrichment of local CaM does not produce CDF of Cav2.1-EF-pre-IQ-IQ2.2 or Cav2.2 e37a.
Results
Effects of alternative splicing on CDF of Cav2.2
In both Cav2.1 and Cav2.2, exon 37 encodes a portion of an EF-hand-like (EF) domains similar to those found in a variety of Ca2+ binding proteins (Kawasaki and Kretsinger, 1995). Conserved in the proximal CTD of all Cav1 and Cav2 channels, the EF domain has been implicated in the regulation of CDI and Mg2+-dependent inhibition of Cav1.2 channels (Peterson et al., 2000; Kim et al., 2004; Brunet et al., 2005). Alternative splicing of exon 37 gives rise to two Cav2.1 variants with distinct EF domains (Fig. 1), but only channels containing one of the exons (exon 37a) exhibits strong CDF (Chaudhuri et al., 2004). The alternatively spliced exons 37a and 37b in Cav2.2 (Bell et al., 2004) are similar in sequence to the corresponding exons in Cav2.1 (Fig. 1). Notably, previous analysis of CDF in Cav2.2 used the variant containing exon 37b (Liang et al., 2003); the corresponding exon in long variants of Cav2.1 prevents CDF (Chaudhuri et al., 2004). Therefore, CDF may have been missed in the previous study (Liang et al., 2003) if exon 37a is required for CDF of Cav2.2.
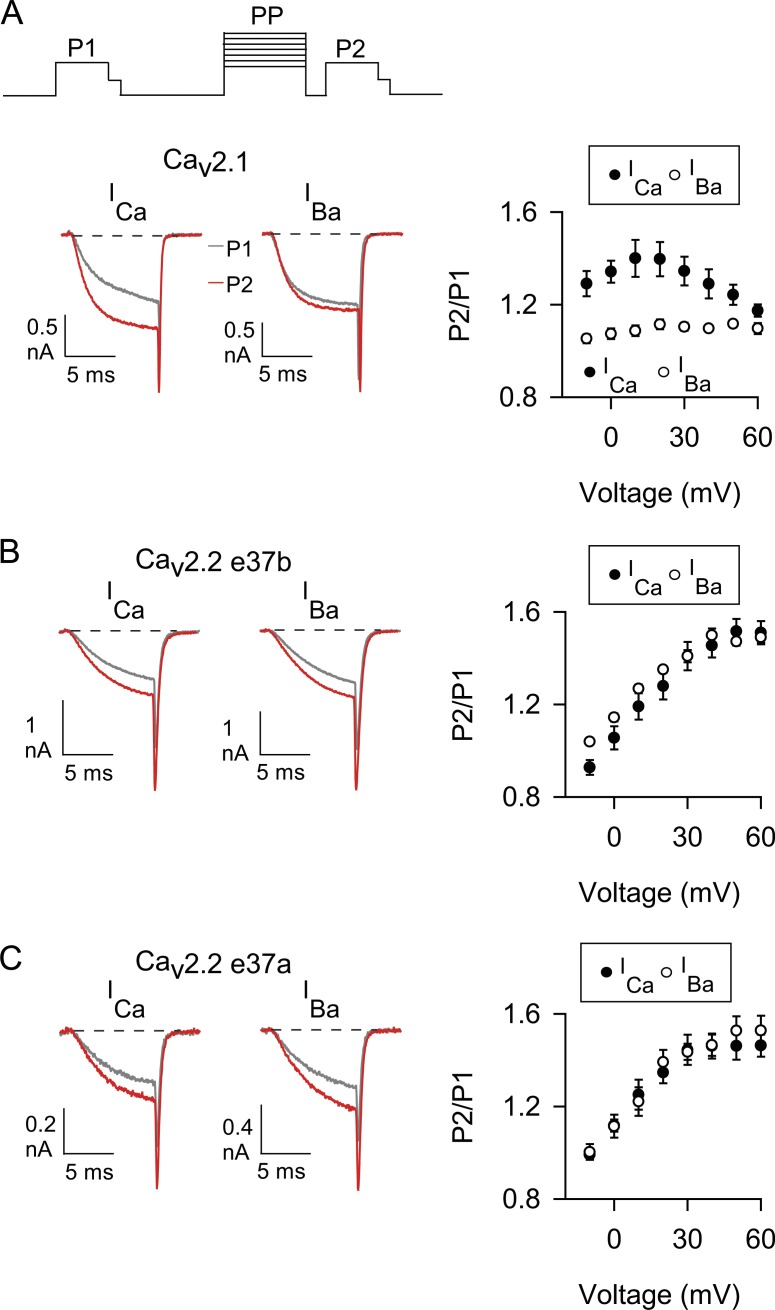
We tested this possibility in whole-cell patch clamp recordings of transfected HEK 293T cells. To analyze CDF, we used a classic voltage protocol in which the amplitudes of currents evoked before (P1) and after (P2) a conditioning prepulse are compared (Thomas and Lee, 2016). The extracellular solution contained either Ca2+ or Ba2+, and the intracellular recording solution contained a high concentration of EGTA (10 mM), which blocks CDI while sparing CDF of Cav2 channels (Lee et al., 2000; Liang et al., 2003). With this protocol, Cav2.1 (containing exon 37a) exhibited the hallmarks of CDF: the ratio of P2 to P1 was greater for Ca2+ currents (ICa) than for Ba2+ currents (IBa) for most prepulse voltages (Fig. 2 A). Consistent with a role for Ca2+ influx during the prepulse in promoting CDF (Lee et al., 2000), the difference between P2/P1 for ICa and IBa was greatest at prepulse voltages evoking the peak inward ICa (20 mV) and was used as a metric for CDF (FCDF). Similar to previous findings (Liang et al., 2003), Cav2.2 e37b did not undergo CDF, in that P2/P1 was similar for ICa and IBa across all prepulse voltages (Fig. 2 B) and FCDF was nominal (Table 1). The P2/P1 ratio for both ICa and IBa increased monotonically with prepulse voltage (Fig. 2 B), likely because of voltage-dependent removal of basal G-protein inhibition (Li et al., 2004). FCDF was not significantly different for Cav2.2 containing exons 37a or 37b (Fig. 2 C; and Table 1), which argued against this exon being permissive for CDF.
Figure 2.
The absence of CDF in Cav2.2 is not affected by alternative splicing of exon 37. (A–C) Left, representative ICa and IBa evoked before (P1, gray trace) and after (P2, red trace) a prepulse to 20 mV for Cav2.1 (A) and Cav2.2 variants with exon 37b (B) or exon 37a (C). Current traces were overlaid for comparison. Voltage protocol is shown above. P1 and P2 pulses were 10-ms steps from −80 mV to −5 mV (for ICa) or −10 mV (for IBa) 1 s before and 5 ms after, respectively, a 50-ms prepulse to various voltages. For P2 and P1, tail currents were resolved by repolarization to −60 mV for 2 ms before stepping to −80 mV. Right, the ratio of P2 and P1 tail currents is plotted against prepulse voltages for ICa and IBa. Numbers of cells for ICa and IBa are indicated in Table 1. Data represent mean ± SEM.
Table 1. FCDF and P2/P1 for ICa and IBa from double-pulse protocol (20-mV prepulse).
| Construct | P2/P1 for ICa | P2/P1 for IBa | P-value, ICa vs. IBaa | FCDF | P-value vs. Cav2.1b | P-value vs. Cav2.2ab |
|---|---|---|---|---|---|---|
| Cav2.1 | 1.40 ± 0.07 (5) | 1.11 ± 0.02 (7) | 0.002 | 0.28 ± 0.07 (5) | 0.032 | |
| Cav2.2 e37b | 1.28 ± 0.06 (10) | 1.35 ± 0.01 (5) | 0.437 | −0.07 ± 0.06 (10) | 0.015 | 1.000 |
| Cav2.2 e37a | 1.39 ± 0.06 (8) | 1.38 ± 0.04 (6) | 0.232 | 0.01 ± 0.06 (7) | 0.032 | |
| Cav2.2-CT2.1 | 1.65 ± 0.10 (13) | 1.32 ± 0.04 (11) | 0.009 | 0.33 ± 0.10 (13) | 1.000 | 0.019 |
| Cav2.1-CT2.2 | 1.16 ± 0.10 (4) | 1.16 ± 0.02 (5) | 0.717 | 0.00 ± 0.10 (4) | 0.035 | 1.000 |
FCDF and P2/P1 (mean ± SEM) were determined as indicated in the text. Number of cells in parentheses.
Determined by Student’s t test.
Determined by Kruskal–Wallis test and post-hoc Dunn’s test.
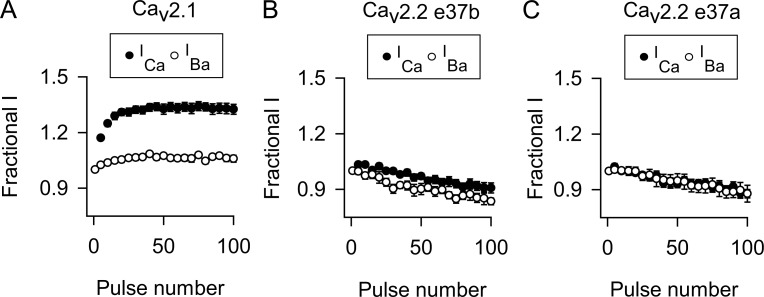
To determine whether CDF of the Cav2.2 splice variants might be revealed with more physiological stimuli, we analyzed ICa and IBa evoked by trains of depolarizations at 100 Hz. The amplitude of each current was normalized to that of the first pulse (Fractional I) and plotted against pulse number. As shown previously (Lee et al., 2000), ICa mediated by Cav2.1 undergoes a robust and sustained increase, whereas IBa undergoes relatively modest voltage-dependent facilitation during the train (Fig. 3 A). The mean of the last five pulses (F96–100) was significantly greater for ICa than for IBa (∼25%; Table 2), indicative of CDF. In contrast, there was no difference in F96–100 for ICa and IBa mediated by Cav2.2 e37a or e37b (Fig. 3, B and C; and Table 2). These results confirm that inclusion of exon 37a is insufficient to confer Cav2.2 channels with an ability to undergo CDF, in contrast to the role of the analogous exon in Cav2.1 (Chaudhuri et al., 2004).
Figure 3.
Repetitive depolarizations cause CDF for Cav2.1 but not Cav2.2. (A–C) ICa or IBa were evoked by 2-ms steps from −80 mV to 0 mV for ICa or −10 mV for IBa at 100 Hz in cells transfected with Cav2.1 (A) or Cav2.2 containing exon 37b (B) or exon 37a (C). The amplitude of each current was normalized to the first current of the train and plotted against pulse number. For clarity, every fifth point is plotted. Numbers of cells for ICa and IBa are indicated in Table 2. Data represent mean ± SEM.
Table 2. FCDF calculated from F96–100 for ICa and IBa from 100-Hz protocol.
| Construct | F96-100 for ICa | F96-100 for IBa | P-value, ICa vs. IBaa | FCDF | P-value vs. Cav2.1b | P-value vs. Cav2.2ab |
|---|---|---|---|---|---|---|
| Cav2.1 | 1.33 ± 0.03 (10) | 1.06 ± 0.02 (12) | <0.001 | 0.27 ± 0.03 (10) | <0.001 | |
| Cav2.2 e37b | 0.91 ± 0.03 (10) | 0.83 ± 0.02 (11) | 0.067 | 0.07 ± 0.03 (10) | <0.001 | 0.360 |
| Cav2.2 e37a | 0.89 ± 0.02 (10) | 0.88 ± 0.04 (10) | 0.970 | −0.01 ± 0.02 (10) | <0.001 | |
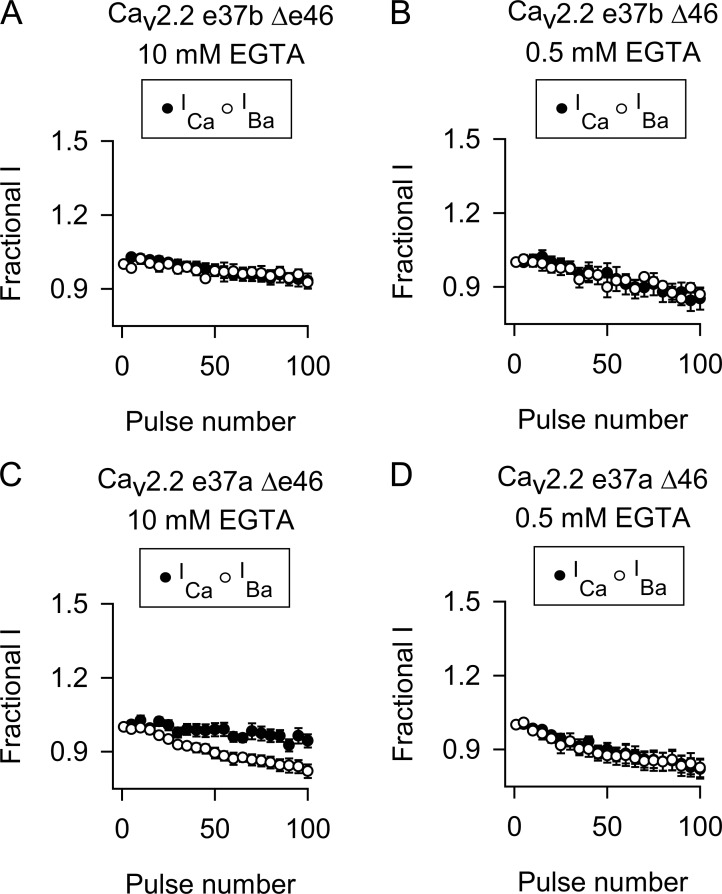
| Cav2.2e37a Δ46 (10 mM) | 0.95 ± 0.03 (12) | 0.83 ± 0.03 (10) | 0.005 | 0.12 ± 0.03 (12) | 0.002 | 0.028 |
| Cav2.2e37a Δ46 (0.5 mM) | 0.82 ± 0.04 (10) | 0.82 ± 0.03 (10) | 0.968 | 0.00 ± 0.04 (10) | <0.001 | 1.000 |
| Cav2.2e37b Δ46 (10 mM) | 0.94 ± 0.03 (8) | 0.94 ± 0.02 (4) | 0.985 | 0.00 ± 0.03 (8) | <0.001 | 1.000 |
| Cav2.2e37b Δ46 (0.5 mM) | 0.86 ± 0.04 (5) | 0.88 ± 0.02 (5) | 0.646 | −0.02 ± 0.04 (5) | <0.001 | 0.985 |
FCDF and F96-100 (mean ± SEM) were determined as indicated in the text. Number of cells in parentheses.
Determined by Student’s t test.
Determined by one-way ANOVA test and post-hoc Dunnett's test.
For Cav2.1 channels, the insensitivity of CDF to high intracellular Ca2+ buffering arises from its dependence on local Ca2+ signals detected by the C-terminal lobe of CaM (Chaudhuri et al., 2007). In the context of exon 37b, deletion of exon 47 from Cav2.1 (Cav2.1 e37b Δe47) converts CDF to a reliance on global elevations in Ca2+, which are sensed by the N-terminal lobe of CaM and can be blunted by a high intracellular concentration of Ca2+ chelator (Chaudhuri et al., 2004). Therefore, we tested whether deletion of the analogous exon 46 of Cav2.2 e37b (Cav2.2e37b Δex46) might reveal CDF under conditions of limited Ca2+ buffering (0.5 mM EGTA). With this approach, there was no significant difference in ICa and IBa evoked by the 100-Hz protocol in cells transfected with Cav2.2e37b Δex46 with either 10 or 0.5 mM EGTA (Fig. 4, A and B; and Table 2). With 10 mM EGTA, deletion of exon 46 from Cav2.2e37a led to a small increase in the F96–100 for ICa at the end of the train compared with IBa, but FCDF was nominal and significantly weaker than that for Cav2.1 (Fig. 4, C and D; and Table 2). Our strategy of manipulating the Ca2+-dependent effects of CaM was effective in that strong inactivation of ICa caused by CDI with 0.5 mM EGTA was significantly reduced with 10 mM EGTA in the intracellular recording solution (Fig. S1). Collectively, our results show that alternative splicing of exons in the proximal and distal CTD do not account for the lack of CDF of Cav2.2.
Figure 4.
Deletion of exon 46 does not influence the absence of CDF in Cav2.2. (A–D) As in Fig. 3 except cells transfected with Cav2.2 e37b (A and B) or Cav2.2 e37a (C and D) without exon 46. The intracellular recording solution contained 10 or 0.5 mM EGTA as indicated. Data represent mean ± SEM.
Role of CaM-regulatory regions in CDF of Cav2 channels
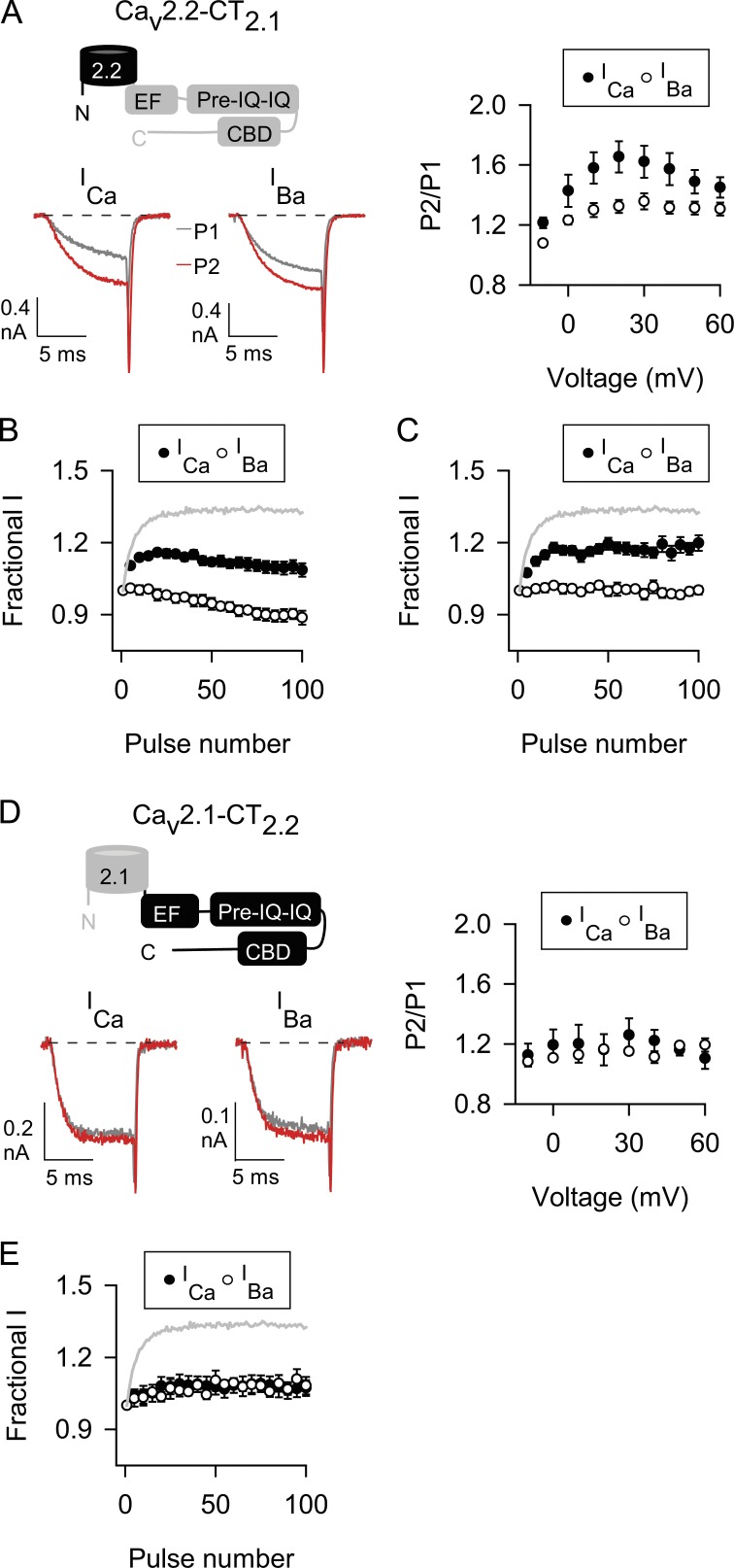
Mutations of the IQ-like domain that inhibit CaM binding abolish CDF (DeMaria et al., 2001; Lee et al., 2003), whereas deletion of the CBD diminishes CDF and CDI (Lee et al., 1999, 2000, 2003). Although its role in CDF of Cav2.1 is not established, the pre-IQ domain upstream of the IQ domain also interacts with CaM and regulates CDI and CDF of Cav1.2 channels (Pitt et al., 2001; Kim et al., 2004, 2010). Each of these domains is conserved in Cav2.1 and Cav2.2 (Fig. 1), but key differences in their amino acid sequences may allow CDF of Cav2.1 but not Cav2.2. If so, then CDF should be conferred to Cav2.2 upon transfer of the corresponding domains from Cav2.1. Consistent with this prediction, chimeric Cav2.2 channels containing the CTD of Cav2.1 (Cav2.2-CT2.1; Cav2.2a variant was used for all Cav2.2 chimeras) exhibited robust CDF with the double-pulse protocol, with FCDF not significantly different from that of Cav2.1 channels (Fig. 5 A and Table 1).
Figure 5.
The CTDs of Cav2.1 and Cav2.2 distinguish their abilities to undergo CDF. (A–E) As in Fig. 2 (double-pulse protocol) and Fig. 3 (100-Hz protocol) except cells transfected with Cav2.2 channels with the CTD of Cav2.1 (A–C) or Cav2.1 channels with the CTD of Cav2.2 (D and E). In C, interpulse voltage was −140 mV. Gray line representing strong CDF of Cav2.1 ICa (from Fig. 3 A) is overlaid for comparison. Data represent mean ± SEM.
With the 100-Hz protocol, F96–100 for Cav2.2-CT2.1 ICa was not as great as that for Cav2.1 (Fig. 5 B and Tables 2 and 3) perhaps because of closed-state inactivation, which is prominent for Cav2.2 during repetitive depolarizations and relieved by hyperpolarized interpulse voltages (Patil et al., 1998). Cav2.2 inactivation (ICa and IBa) was stronger than that for Cav2.1 during 100-Hz trains (Fig. 3) and could partially occlude facilitation of Cav2.2-CT2.1 ICa. Changing the interpulse voltage from −80 to −140 mV increased F96–100 for ICa (1.19 ± 0.03 for −140 mV, n = 10, vs. 1.09 ± 0.03 for −80 mV, n = 15, P = 0.013 by t test) to a similar extent as for IBa (F96–100 = 0.99 ± 0.02 for −140 mV, n = 10, vs. 0.89 ± 0.03 for −80 mV, n = 15, P = 0.014 by t test; Fig. 5 C), such that FCDF was similar regardless of interpulse voltage (0.21 ± 0.03 for −140 mV, n = 10, vs. 0.20 ± 0.03 for −80 mV, n = 15, P = 0.926 by t test; Fig. 5 C). Thus, although closed-state inactivation does indeed underlie the smaller F96–100 for Cav2.2-CT2.1 ICa compared with Cav2.1 ICa, it does not affect the magnitude of CDF. In fact, FCDF of Cav2.2-CT2.1 was not significantly different from that for Cav2.1 (Table 3). Collectively, our results indicate that molecular determinants within the CTD of Cav2.1 are sufficient to enable Cav2.2-CT2.1 to undergo CDF.
Table 3. FCDF calculated from F96–100 for ICa and IBa from 100-Hz protocol.
| Construct | F96-100 for ICa | F96-100 for IBa | P-value, ICa vs. IBaa | FCDF | P-value vs. Cav2.1b | P-value vs. Cav2.2ab |
|---|---|---|---|---|---|---|
| Cav2.2-CT2.1 | 1.09 ± 0.03 (15) | 0.89 ± 0.03 (15) | <0.001 | 0.20 ± 0.03 (15) | 1.000 | |
| Cav2.2-pCT2.1 | 1.07 ± 0.02 (13) | 0.93 ± 0.02 (11) | <0.001 | 0.14 ± 0.02 (13) | 0.370 | |
| Cav2.2-dCT2.1 | 0.90 ± 0.03 (8) | 0.92 ± 0.02 (10) | 0.914 | 0.02 ± 0.03 (8) | <0.001 | |
| Cav2.2-EF2.1 | 0.85 ± 0.03 (10) | 0.88 ± 0.03 (10) | 0.520 | −0.03 ± 0.03 (10) | <0.001 | |
| Cav2.2-pre-IQ-IQ2.1 | 0.90 ± 0.03 (10) | 0.92 ± 0.02 (12) | 0.583 | −0.02 ± 0.03 (10) | <0.001 | |
| Cav2.2-CBD2.1 | 0.99 ± 0.03 (10) | 0.93 ± 0.02 (11) | 0.149 | 0.06 ± 0.03 (10) | 0.006 | |
| Cav2.2-pre-IQ-IQ-CBD2.1 | 0.95 ± 0.02 (11) | 0.95 ± 0.04 (6) | 0.689 | 0.00 ± 0.02 (11) | <0.001 | |
| Cav2.2-EF&CBD2.1 | 0.89 ± 0.03 (12) | 0.86 ± 0.02 (10) | 0.508 | 0.03 ± 0.03 (12) | 0.001 | |
| Cav2.2-EF-pre-IQ-IQ2.1 | 1.07 ± 0.03 (18) | 0.94 ± 0.03 (13) | 0.004 | 0.13 ± 0.03 (18) | 0.851 | |
| Cav2.1-CT2.2 | 1.08 ± 0.04 (10) | 1.08 ± 0.02 (5) | 0.966 | 0.00 ± 0.04 (10) | 1.000 | |
| Cav2.1-pCT2.2 | 1.05 ± 0.02 (5) | 1.01 ± 0.02 (7) | 0.073 | 0.04 ± 0.02 (5) | 1.000 | |
| Cav2.1-dCT2.2 | 1.24 ± 0.03 (4) | 1.02 ± 0.02 (3) | 0.024 | 0.22 ± 0.03 (4) | 0.054 | |
| Cav2.1-EF2.2 | 1.08 ± 0.03 (4) | 1.02 ± 0.02 (6) | 0.257 | 0.06 ± 0.03 (4) | 1.000 | |
| Cav2.1-pre-IQ-IQ2.2 | 1.03 ± 0.01 (8) | 1.03 ± 0.02 (6) | 0.831 | 0.00 ± 0.02 (8) | 1.000 | |
| Cav2.1-CBD2.2 | 1.27 ± 0.02 (8) | 1.04 ± 0.04 (10) | <0.001 | 0.23 ± 0.02 (8) | 0.005 | |
| Cav2.1-EF-pre-IQ-IQ2.2 | 1.05 ± 0.01 (11) | 1.06 ± 0.02 (11) | 0.645 | −0.01 ± 0.00 (11) | 1.000 |
FCDF and F96-100 (mean ± SEM) were determined as indicated in the text. Number of cells in parentheses.
Determined by Student’s t test.
Determined by Kruskal–Wallis test and post-hoc Dunn's test.
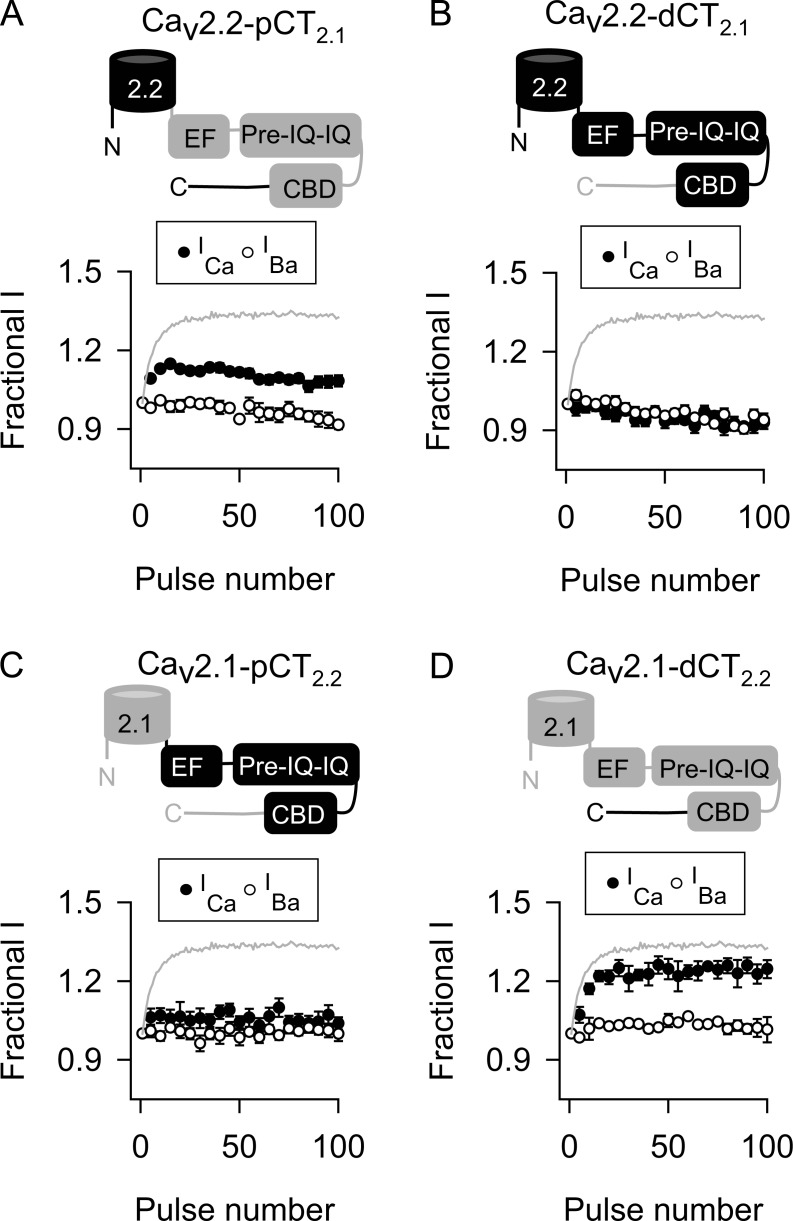
We next tested the converse prediction that transfer of the Cav2.2 CTD to Cav2.1 should blunt CDF. In contrast to the wild-type Cav2.1, ICa and IBa behaved similarly in double-pulse and 100-Hz protocols in cells transfected with the chimeric Cav2.1-CT2.1 channels (Fig. 5, D and E; and Tables 1 and 3). To further refine the molecular determinants in the CTD responsible for “turning off” Cav2.2 CDF, we analyzed additional chimeric channels. For these studies, data are shown only for the 100-Hz protocol because similar results were obtained with double-pulse protocols. If the CDF-regulatory domains of Cav2.1 and Cav2.2 distinguish their abilities to undergo CDF, Cav2.2 channels containing the proximal CTD (Cav2.2-pCT2.1) but not the distal CTD (Cav2.2-dCT2.1) should exhibit CDF. As expected, Cav2.2-pCT2.1 underwent CDF (Fig. 6 A and Table 3). In contrast, Cav2.2-dCT2.1 was similar to wild-type Cav2.2 in that there was no difference in F96–100 for ICa and IBa (Fig. 6 B and Table 3). Consistent with these findings, transfer of the proximal CTD but not the distal CTD of Cav2.2 to Cav2.1 resulted in chimeric channels that did not undergo CDF (Fig. 6, C and D; and Table 3). Therefore, the proximal CTD contains the sequence elements that distinguish the ability of Cav2.1 and Cav2.2 to undergo CDF.
Figure 6.
The proximal CTD containing CDF-regulatory domains in Cav2.1 is not functionally conserved in Cav2.2. (A–D) As in Fig. 5 B except cells transfected with Cav2.2 channels containing proximal (A) or distal (B) CTD or Cav2.1 channels containing proximal (C) or distal (D) CTD of Cav2.2. Data represent mean ± SEM.
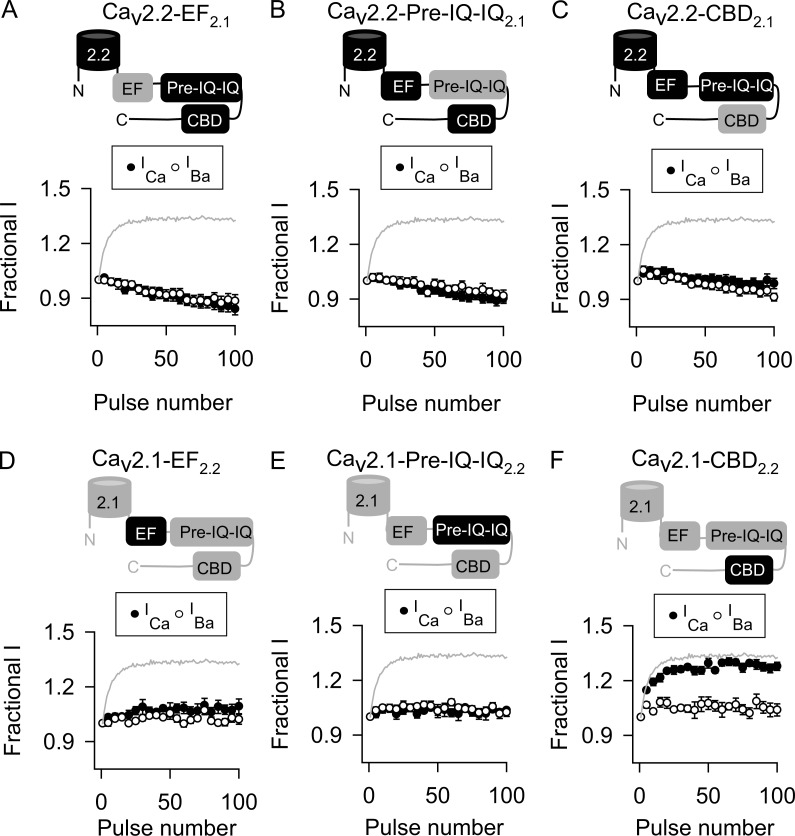
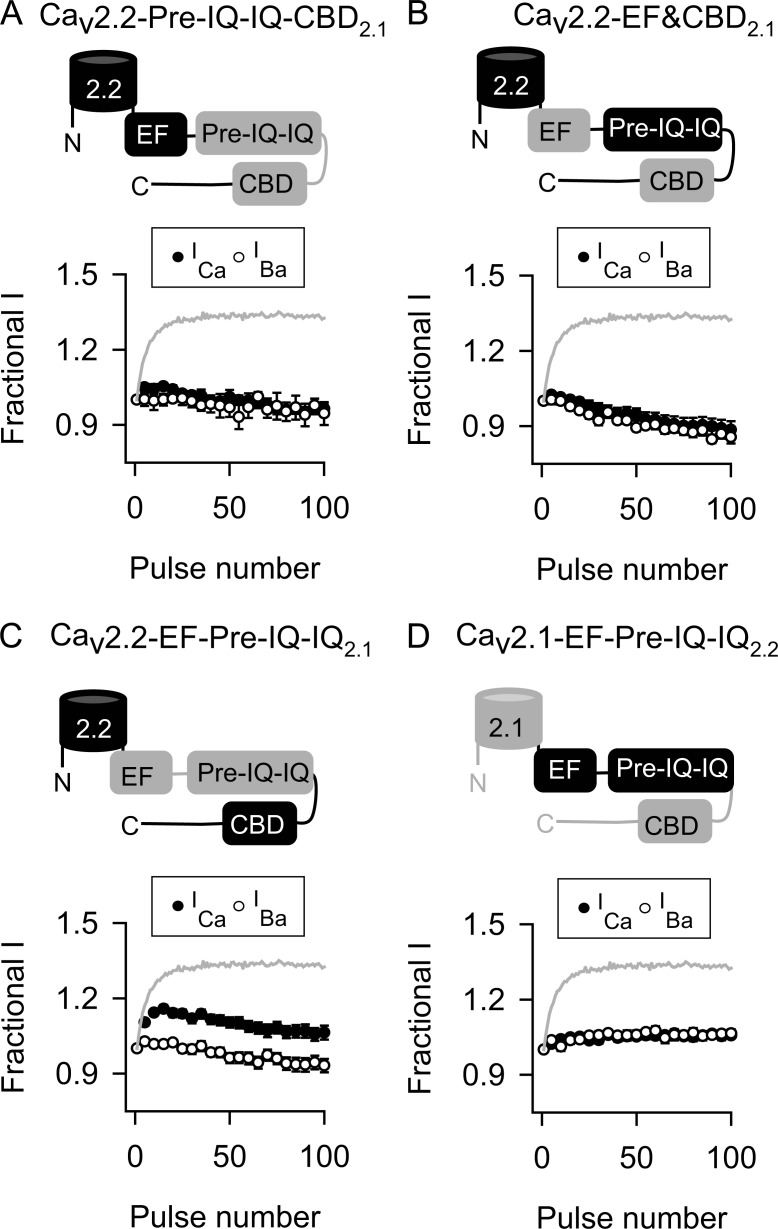
We next determined the relative contributions of the EF, pre-IQ, IQ, and CBDs in disabling CDF in Cav2.2 channels. In these experiments, the pre-IQ and IQ sequences were transferred together because they work in concert to transduce effects of CaM in Cav1.2 (Pitt et al., 2001; Kim et al., 2004). None of the Cav2.2 chimeras containing these domains from Cav2.1 exhibited CDF (Fig. 7, A–C; and Table 3), indicating that the individual CDF-regulatory sites in Cav2.1 are dysfunctional within the context of the Cav2.2 proximal CTD. At the same time, substitution of the pre-IQ-IQ or EF-hand domain, but not the CBD, of Cav2.2 into Cav2.1 abolished CDF normally observed for the wild-type Cav2.1 (Fig. 7, D–F; and Table 3). Collectively, these results suggested that functional differences primarily in the EF-hand and pre-IQ-IQ domain of Cav2.2 prevent CDF. To test this, we analyzed chimeric Cav2.2 channels containing subsets of the CDF-regulatory sites in Cav2.1. Of these, only the Cav2.2 chimera containing the EF-hand and pre-IQ-IQ domain (EF-pre-IQ-IQ) of Cav2.1 exhibited CDF (Fig. 8, A–C; and Table 3). Conversely, CDF was abolished in Cav2.1 channels containing the Cav2.2 EF-pre-IQ-IQ domain (Fig. 8 D and Table 3).
Figure 7.
EF-hand and pre-IQ-IQ domains are the minimal determinants in the CTD that disable CDF in Cav2.2. (A–F) As in Fig. 5 B except cells transfected with Cav2.2 channels containing EF-hand, pre-IQ-IQ, or CBD of Cav2.1 (A–C) or Cav2.1 channels containing corresponding regions of Cav2.2 (D–F). Data represent mean ± SEM.
Figure 8.
Both EF-hand and pre-IQ-IQ domains of Cav2.1 are required to unmask CDF in Cav2.2. (A–D) As in Fig. 5 B except cells transfected with Cav2.2 channels containing pre-IQ-IQ and CBD (A), EF-hand and CBD (B), or EF-hand and pre-IQ-IQ (C) of Cav2.1 or Cav2.2 channels containing EF-hand and pre-IQ-IQ of Cav2.1 (D). Data represent mean ± SEM.
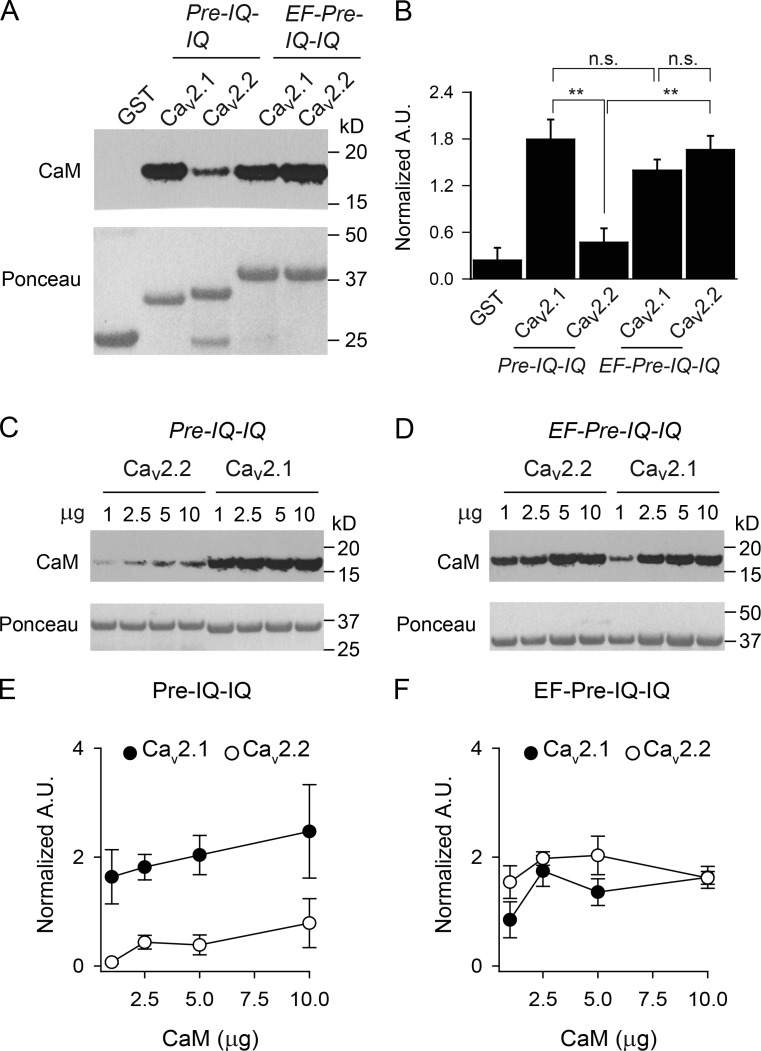
The inability of EF-pre-IQ-IQ to support CDF in Cav2.2 could be caused by weaker interactions with CaM compared with this region in Cav2.1. Indeed, past work suggests that CaM binds with lower affinity to the pre-IQ and IQ regions of Cav2.2 than of Cav2.1 (Peterson et al., 1999; Liang et al., 2003). To test whether this is the case in the context of EF-pre-IQ-IQ, we compared binding to GST-tagged Cav2.1 or Cav2.2 fusion proteins in pull-down assays. Consistent with previous results (Liang et al., 2003), CaM binding was significantly stronger to the pre-IQ-IQ of Cav2.1 than to this region of Cav2.2 or the GST control (Fig. 9, A and B). Remarkably, addition of the EF-hand to the pre-IQ-IQ of Cav2.2 greatly enhanced the interaction with CaM such that there was no significant difference in CaM binding to the EF-pre-IQ-IQ domain of Cav2.2 and Cav2.1 (Fig. 9, A and B). In contrast, CaM bound equally well to the pre-IQ-IQ and EF-pre-IQ-IQ domains of Cav2.1 (Fig. 9, A and B). The impact of the EF-hand on CaM binding to the Cav2.2 pre-IQ-IQ was particularly apparent with increasing amounts of CaM added to the binding reactions. For all concentrations of CaM tested, the amount of CaM bound to the Cav2.2 pre-IQ-IQ was only ∼20% of that to the Cav2.1 pre-IQ-IQ (Fig. 9, C and D) whereas there was no difference in CaM binding to the EF-pre-IQ-IQ of the two channels (Fig. 9, E and F).
Figure 9.
CaM differentially interacts with pre-IQ-IQ and EF-pre-IQ-IQ of Cav2.1 and Cav2.2 in pull-down assays. (A and B) GST or GST-tagged Cav2 proteins were incubated with CaM (2.5 µg), and bound CaM was detected by Western blotting. Ponceau staining indicated the amount of each GST protein in the reactions. In B, the signal intensity corresponding to CaM was normalized to that for the GST-protein. A.U., arbitrary units; n.s., not significant; **, P < 0.001, one-way ANOVA and post hoc Tukey test. Data are representative of four independent experiments. (C–F) As in A and B except that variable amounts of CaM (1–10 µg) were used in the assay. In E and F, data were analyzed by two-way ANOVA. There was a significant difference in results obtained for pre-IQ-IQ (P < 0.01) but not EF-pre-IQ-IQ (P = 0.75) of Cav2.1 and Cav2.2. Data are representative of three independent experiments. Data represent mean ± SEM.
The similar CaM binding abilities of the EF-pre-IQ-IQ domain of Cav2.1 and Cav2.2 suggested that the lack of CDF in Cav2.2 channels does not simply result from reduced affinity for CaM. If so, then increasing the concentration of CaM to overcome any such differences in CaM binding affinity between Cav2.1 and Cav2.2 should not uncover CDF. To test this prediction, we used a strategy to enrich the local concentration of CaM near Cav channels in which CaM is tethered to the auxiliary Cavβ2a subunit (β2a-CaM; Sang et al., 2016). Using β2a as a control, we analyzed the effects of β2a-CaM on the amplitude of ICa evoked by 100-Hz stimuli in cells cotransfected with Cav2.2 or Cav2.1 chimeras containing the EF-pre-IQ-IQ domain of Cav2.2 (Cav2.1-EF-pre-IQ-IQ2.2). Coexpression of β2a-CaM (verified by Western blots) had no effect on ICa: CDF was not rescued in Cav2.1-EF-pre-IQ-IQ2.2, nor was it uncovered in Cav2.2 (Fig. S2). We conclude that the lack of CDF shown by Cav2.2 channels does not arise from weaker binding of CaM, but likely through an inability of the EF-pre-IQ-IQ domain to convert CaM binding into channel conformations that support CDF.
Discussion
In this study, we uncovered new insights into the molecular determinants regulating CDF of Cav2 channels. First, we discounted a role for alternatively spliced C-terminal exons 37 and 46. Inclusion of exon 37a, which is permissive for CDF in Cav2.1 (Chaudhuri et al., 2004), did not reveal CDF in Cav2.2 (Figs. 2 and 3), nor did deletion of exon 46 (Fig. 4), which influences the Ca2+ dependence of Cav2.1 CDF (Chaudhuri et al., 2004). Second, we identified the EF-hand and pre-IQ-IQ domains as the critical determinants distinguishing the abilities of Cav2.1 and Cav2.2 to undergo CDF. These domains in the proximal CTD of Cav2.2 functionally diverge from those in Cav2.1 because their transfer to Cav2.1 prevented CDF (Fig. 6 C). Third, we discovered an unexpected role for the EF-hand domain in strengthening the ability of the pre-IQ-IQ of Cav2.2 to bind CaM. Our results support a model in which CaM binds to the EF-pre-IQ-IQ of Cav2.2 in a way that is functionally uncoupled from CDF.
The importance of the IQ domain for CDF is demonstrated by findings that mutation of the initial isoleucine and glutamine in the Cav2.1 IQ domain diminishes CaM binding and blunts CDF (DeMaria et al., 2001; Lee et al., 2003). Although the IQ domain is highly conserved in Cav1 and Cav2 channels, sequence alterations between Cav subtypes could underlie functional differences in channel regulation by CaM. By x-ray crystallography, Kim et al. (2008) found subtle differences in how CaM interacts with peptides corresponding to the IQ domain Cav2.2 and Cav2.1. These differences include less contact with the methionine at position −1 and greater interaction with phenylalanine at position 1 relative to the central isoleucine (position 0; Kim et al., 2008). These alterations may account for weaker CaM binding to the IQ and pre-IQ-IQ of Cav2.2 compared with Cav2.1 (Fig. 9; DeMaria et al., 2001; Liang et al., 2003). However, they are not sufficient to explain the absence of CDF in Cav2.2 channels because transfer of the Cav2.1 pre-IQ-IQ region alone to Cav2.2 did not reverse the inability of Cav2.2 to undergo CDF (Fig. 7 B). Moreover, Cav2.3 does not undergo CDF, and yet the crystal structures of CaM bound to the Cav2.1 and Cav2.3 IQ domains are nearly identical (Kim et al., 2008; Mori et al., 2008).
In this context, the crystal structure presented by Kim et al. (2010) of CaM in complex with the Cav1.2 pre-IQ-IQ may be informative. The structure indicates a 2:1 stoichiometry with one Ca2+/CaM bound to the IQ domain and a second to a lower-affinity site in the pre-IQ region. A key tryptophan residue in the Cav1.2 pre-IQ region was identified as an anchoring site for the C-terminal lobe of CaM, and the mutation of this residue disrupted CDF when the initial isoleucine in the IQ-domain was also mutated so as to disrupt CDI (Kim et al., 2010). This tryptophan is conserved among all Cav1 and Cav2 channels and therefore may serve as an analogous region for binding Ca2+/CaM in Cav2 channels. Differences between the pre-IQ region of Cav2.1 and Cav2.2 include residues at positions −3, −4, and −12 from this tryptophan, which are all methionines in Cav2.1. Such differences could prevent the ability of CaM bound to the pre-IQ to produce CDF in Cav2.2, which would explain the absence of CDF in any of the Cav2.1 chimeras containing the Cav2.2 pre-IQ-IQ (Fig. 5, D and E; Fig. 6 C; and Fig. 7 E).
Considering the weak binding of CaM to the pre-IQ-IQ of Cav2.2 (Fig. 9; DeMaria et al., 2001; Liang et al., 2003), the equivalence of CaM binding of the EF-pre-IQ-IQ of Cav2.2 and Cav2.1 (Fig. 9) suggests that the EF-hand domain differentially regulates interactions with CaM in the two channels. This is surprising given the strong sequence conservation in the EF-hand domains of Cav2.1 and Cav2.2 (Fig. 1). The divergent residues in the Cav2.2 EF-hand may be significant enough to facilitate interaction of CaM with the pre-IQ-IQ in ways that are unnecessary for Cav2.1. The Cav2.2 EF-hand might reposition CaM bound to the pre-IQ-IQ so as to prevent CDF, which could explain the absence of CDF in the Cav2.1 chimera containing the Cav2.2 EF-hand (Fig. 7 D). Alternatively, interactions of the EF-pre-IQ-IQ with other parts of the channel such as the cytoplasmic loops linking domains I and II (Kim et al., 2004) and III and IV (Wu et al., 2016) may be unfavorable for entry of Cav2.2 into the facilitated state that is normally triggered by Ca2+/CaM in Cav2.1.
Although it binds CaM and regulates CDI of Cav2.1 (Lee et al., 1999, 2000), the CBD plays a more modulatory role and works with the IQ domain to promote CDF (Lee et al., 2003). This is supported by our findings that Cav2.2 channels containing only the Cav2.1 CBD were unable to undergo CDF (Fig. 7 C). Only when cotransferred with the Cav2.1 EF-hand and pre-IQ-IQ domain was the Cav2.1 CBD effective in producing CDF in Cav2.2 (Figs. 5 A and 6 D). The CBD may be functionally redundant in Cav2.1 and Cav2.2, because CDF in Cav2.1 channels containing the Cav2.2 CBD was comparable to that in WT Cav2.1 channels (Fig. 7 F). Considering that CDF was slightly weaker in Cav2.2-pCT2.1 than Cav2.2-CT2.1 (Table 3), it may be that the CBD requires the distal CTD of Cav2.1 to fully promote CDF. An understanding of how the EF-hand, pre-IQ-IQ, and CBD domains coordinately regulate CDF is an important challenge for future studies.
The neurophysiological importance of disabling CDF in Cav2.2 channels is not entirely clear but may relate to the major roles of these channels in the peripheral nervous system (Hirning et al., 1988). Localized in the presynaptic terminals of small-diameter nociceptive neurons, Cav2.2 channels mediate the release of neuropeptides into the superficial layers of the spinal dorsal horn in response to painful stimuli (Holz et al., 1988; Maggi et al., 1990). Because the amount of neurotransmitter released is proportional to the third or fourth power of the presynaptic Ca2+ concentration (Dodge and Rahamimoff, 1967; Sakaba and Neher, 2001), the inability of Cav2.2 to undergo Ca2+/CaM-dependent CDF may have evolved to limit additive effects with other forms of Cav2.2 modulation that could collectively exacerbate transmission of painful stimuli. For example, Cav2.2 channel spinal nociceptive neurons undergo a CaMKII-dependent longer-term CDF that is eliminated with peripheral nerve injury (Tang et al., 2012). In sympathetic neurons, Cav2.2 channels are inhibited by a wide range of hormones and neurotransmitters acting via G protein–coupled receptors (Hille, 1994). If present in Cav2.2, CDF would oppose this inhibition, leading to improper neurohumoral control of sympathetic outflow.
Supplementary Material
Acknowledgments
We thank Dr. Madeline Shea for providing guidance on CaM binding assays and Dr. Ivy Dick for providing the β2a-CaM cDNA.
This work is supported by grants from the National Institutes of Health (NS084190, DC009433, and NS045549) and a Carver Research Program of Excellence Award.
The authors declare no competing financial interests.
Author contributions: J.R. Thomas, J. Hagen, and D. Soh conducted the research. All authors contributed to experimental design. J.R. Thomas and A. Lee wrote the paper.
Richard W. Aldrich served as editor.
References
- Bell T.J., Thaler C., Castiglioni A.J., Helton T.D., and Lipscombe D.. 2004. Cell-specific alternative splicing increases calcium channel current density in the pain pathway. Neuron. 41:127–138. 10.1016/S0896-6273(03)00801-8 [DOI] [PubMed] [Google Scholar]
- Boland L.M., Morrill J.A., and Bean B.P.. 1994. omega-Conotoxin block of N-type calcium channels in frog and rat sympathetic neurons. J. Neurosci. 14:5011–5027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet S., Scheuer T., Klevit R., and Catterall W.A.. 2005. Modulation of CaV1.2 channels by Mg2+ acting at an EF-hand motif in the COOH-terminal domain. J. Gen. Physiol. 126:311–323. 10.1085/jgp.200509333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall W.A., Leal K., and Nanou E.. 2013. Calcium channels and short-term synaptic plasticity. J. Biol. Chem. 288:10742–10749. 10.1074/jbc.R112.411645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri D., Chang S.Y., DeMaria C.D., Alvania R.S., Soong T.W., and Yue D.T.. 2004. Alternative splicing as a molecular switch for Ca2+/calmodulin-dependent facilitation of P/Q-type Ca2+ channels. J. Neurosci. 24:6334–6342. 10.1523/JNEUROSCI.1712-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri D., Issa J.B., and Yue D.T.. 2007. Elementary mechanisms producing facilitation of Cav2.1 (P/Q-type) channels. J. Gen. Physiol. 129:385–401. 10.1085/jgp.200709749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuttle M.F., Tsujimoto T., Forsythe I.D., and Takahashi T.. 1998. Facilitation of the presynaptic calcium current at an auditory synapse in rat brainstem. J. Physiol. 512:723–729. 10.1111/j.1469-7793.1998.723bd.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMaria C.D., Soong T.W., Alseikhan B.A., Alvania R.S., and Yue D.T.. 2001. Calmodulin bifurcates the local Ca2+ signal that modulates P/Q-type Ca2+ channels. Nature. 411:484–489. 10.1038/35078091 [DOI] [PubMed] [Google Scholar]
- Dodge F.A. Jr., and Rahamimoff R.. 1967. Co-operative action a calcium ions in transmitter release at the neuromuscular junction. J. Physiol. 193:419–432. 10.1113/jphysiol.1967.sp008367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlap K., Luebke J.I., and Turner T.J.. 1995. Exocytotic Ca2+ channels in mammalian central neurons. Trends Neurosci. 18:89–98. 10.1016/0166-2236(95)80030-6 [DOI] [PubMed] [Google Scholar]
- Forsythe I.D., Tsujimoto T., Barnes-Davies M., Cuttle M.F., and Takahashi T.. 1998. Inactivation of presynaptic calcium current contributes to synaptic depression at a fast central synapse. Neuron. 20:797–807. 10.1016/S0896-6273(00)81017-X [DOI] [PubMed] [Google Scholar]
- Hatakeyama S., Wakamori M., Ino M., Miyamoto N., Takahashi E., Yoshinaga T., Sawada K., Imoto K., Tanaka I., Yoshizawa T., et al. . 2001. Differential nociceptive responses in mice lacking the α1B subunit of N-type Ca2+ channels. Neuroreport. 12:2423–2427. 10.1097/00001756-200108080-00027 [DOI] [PubMed] [Google Scholar]
- Hille B. 1994. Modulation of ion-channel function by G-protein-coupled receptors. Trends Neurosci. 17:531–536. 10.1016/0166-2236(94)90157-0 [DOI] [PubMed] [Google Scholar]
- Hirning L.D., Fox A.P., McCleskey E.W., Olivera B.M., Thayer S.A., Miller R.J., and Tsien R.W.. 1988. Dominant role of N-type Ca2+ channels in evoked release of norepinephrine from sympathetic neurons. Science. 239:57–61. 10.1126/science.2447647 [DOI] [PubMed] [Google Scholar]
- Holz G.G. IV, Dunlap K., and Kream R.M.. 1988. Characterization of the electrically evoked release of substance P from dorsal root ganglion neurons: methods and dihydropyridine sensitivity. J. Neurosci. 8:463–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inchauspe C.G., Martini F.J., Forsythe I.D., and Uchitel O.D.. 2004. Functional compensation of P/Q by N-type channels blocks short-term plasticity at the calyx of Held presynaptic terminal. J. Neurosci. 24:10379–10383. 10.1523/JNEUROSCI.2104-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun K., Piedras-Rentería E.S., Smith S.M., Wheeler D.B., Lee S.B., Lee T.G., Chin H., Adams M.E., Scheller R.H., Tsien R.W., and Shin H.S.. 1999. Ablation of P/Q-type Ca2+ channel currents, altered synaptic transmission, and progressive ataxia in mice lacking the α1A-subunit. Proc. Natl. Acad. Sci. USA. 96:15245–15250. 10.1073/pnas.96.26.15245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki H., and Kretsinger R.H.. 1995. Calcium-binding proteins 1: EF-hands. Protein Profile. 2:297–490. [PubMed] [Google Scholar]
- Kim E.Y., Rumpf C.H., Fujiwara Y., Cooley E.S., Van Petegem F., and Minor D.L. Jr. 2008. Structures of CaV2 Ca2+/CaM-IQ domain complexes reveal binding modes that underlie calcium-dependent inactivation and facilitation. Structure. 16:1455–1467. 10.1016/j.str.2008.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E.Y., Rumpf C.H., Van Petegem F., Arant R.J., Findeisen F., Cooley E.S., Isacoff E.Y., and Minor D.L. Jr. 2010. Multiple C-terminal tail Ca2+/CaMs regulate CaV1.2 function but do not mediate channel dimerization. EMBO J. 29:3924–3938. 10.1038/emboj.2010.260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Ghosh S., Nunziato D.A., and Pitt G.S.. 2004. Identification of the components controlling inactivation of voltage-gated Ca2+ channels. Neuron. 41:745–754. 10.1016/S0896-6273(04)00081-9 [DOI] [PubMed] [Google Scholar]
- Lee A., Wong S.T., Gallagher D., Li B., Storm D.R., Scheuer T., and Catterall W.A.. 1999. Ca2+/calmodulin binds to and modulates P/Q-type calcium channels. Nature. 399:155–159. 10.1038/20194 [DOI] [PubMed] [Google Scholar]
- Lee A., Scheuer T., and Catterall W.A.. 2000. Ca2+/calmodulin-dependent facilitation and inactivation of P/Q-type Ca2+ channels. J. Neurosci. 20:6830–6838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A., Zhou H., Scheuer T., and Catterall W.A.. 2003. Molecular determinants of Ca2+/calmodulin-dependent regulation of Cav2.1 channels. Proc. Natl. Acad. Sci. USA. 100:16059–16064. 10.1073/pnas.2237000100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B., Zhong H., Scheuer T., and Catterall W.A.. 2004. Functional role of a C-terminal Gbetagamma-binding domain of Ca(v)2.2 channels. Mol. Pharmacol. 66:761–769. [DOI] [PubMed] [Google Scholar]
- Liang H., DeMaria C.D., Erickson M.G., Mori M.X., Alseikhan B.A., and Yue D.T.. 2003. Unified mechanisms of Ca2+ regulation across the Ca2+ channel family. Neuron. 39:951–960. 10.1016/S0896-6273(03)00560-9 [DOI] [PubMed] [Google Scholar]
- Lipscombe D., Allen S.E., and Toro C.P.. 2013. Control of neuronal voltage-gated calcium ion channels from RNA to protein. Trends Neurosci. 36:598–609. 10.1016/j.tins.2013.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi C.A., Tramontana M., Cecconi R., and Santicioli P.. 1990. Neurochemical evidence for the involvement of N-type calcium channels in transmitter secretion from peripheral endings of sensory nerves in guinea pigs. Neurosci. Lett. 114:203–206. 10.1016/0304-3940(90)90072-H [DOI] [PubMed] [Google Scholar]
- Maximov A., and Bezprozvanny I.. 2002. Synaptic targeting of N-type calcium channels in hippocampal neurons. J. Neurosci. 22:6939–6952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintz I.M., Sabatini B.L., and Regehr W.G.. 1995. Calcium control of transmitter release at a cerebellar synapse. Neuron. 15:675–688. 10.1016/0896-6273(95)90155-8 [DOI] [PubMed] [Google Scholar]
- Mori M.X., Vander Kooi C.W., Leahy D.J., and Yue D.T.. 2008. Crystal structure of the CaV2 IQ domain in complex with Ca2+/calmodulin: high-resolution mechanistic implications for channel regulation by Ca2+. Structure. 16:607–620. 10.1016/j.str.2008.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanou E., Yan J., Whitehead N.P., Kim M.J., Froehner S.C., Scheuer T., and Catterall W.A.. 2016. Altered short-term synaptic plasticity and reduced muscle strength in mice with impaired regulation of presynaptic CaV2.1 Ca2+ channels. Proc. Natl. Acad. Sci. USA. 113:1068–1073. 10.1073/pnas.1524650113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil P.G., Brody D.L., and Yue D.T.. 1998. Preferential closed-state inactivation of neuronal calcium channels. Neuron. 20:1027–1038. 10.1016/S0896-6273(00)80483-3 [DOI] [PubMed] [Google Scholar]
- Pedigo S., and Shea M.A.. 1995. Quantitative endoproteinase GluC footprinting of cooperative Ca2+ binding to calmodulin: Proteolytic susceptibility of E31 and E87 indicates interdomain interactions. Biochemistry. 34:1179–1196. 10.1021/bi00004a011 [DOI] [PubMed] [Google Scholar]
- Peterson B.Z., DeMaria C.D., Adelman J.P., and Yue D.T.. 1999. Calmodulin is the Ca2+ sensor for Ca2+-dependent inactivation of L-type calcium channels. Neuron. 22:549–558. 10.1016/S0896-6273(00)80709-6 [DOI] [PubMed] [Google Scholar]
- Peterson B.Z., Lee J.S., Mulle J.G., Wang Y., de Leon M., and Yue D.T.. 2000. Critical determinants of Ca2+-dependent inactivation within an EF-hand motif of L-type Ca2+ channels. Biophys. J. 78:1906–1920. 10.1016/S0006-3495(00)76739-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitt G.S., Zühlke R.D., Hudmon A., Schulman H., Reuter H., and Tsien R.W.. 2001. Molecular basis of calmodulin tethering and Ca2+-dependent inactivation of L-type Ca2+ channels. J. Biol. Chem. 276:30794–30802. 10.1074/jbc.M104959200 [DOI] [PubMed] [Google Scholar]
- Sakaba T., and Neher E.. 2001. Quantitative relationship between transmitter release and calcium current at the calyx of held synapse. J. Neurosci. 21:462–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sang L., Dick I.E., and Yue D.T.. 2016. Protein kinase A modulation of CaV1.4 calcium channels. Nat. Commun. 7:12239 10.1038/ncomms12239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simms B.A., and Zamponi G.W.. 2014. Neuronal voltage-gated calcium channels: structure, function, and dysfunction. Neuron. 82:24–45. 10.1016/j.neuron.2014.03.016 [DOI] [PubMed] [Google Scholar]
- Tang Q., Bangaru M.L., Kostic S., Pan B., Wu H.E., Koopmeiners A.S., Yu H., Fischer G.J., McCallum J.B., Kwok W.M., et al. . 2012. Ca2+-dependent regulation of Ca2+ currents in rat primary afferent neurons: Role of CaMKII and the effect of injury. J. Neurosci. 32:11737–11749. 10.1523/JNEUROSCI.0983-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theoharis N.T., Sorensen B.R., Theisen-Toupal J., and Shea M.A.. 2008. The neuronal voltage-dependent sodium channel type II IQ motif lowers the calcium affinity of the C-domain of calmodulin. Biochemistry. 47:112–123. 10.1021/bi7013129 [DOI] [PubMed] [Google Scholar]
- Thomas J.R., and Lee A.. 2016. Measuring Ca2+-dependent modulation of voltage-gated Ca2+ channels in HEK-293T cells. Cold Spring Harb. Protoc. 2016:pdb.prot087213 10.1101/pdb.prot087213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujimoto T., Jeromin A., Saitoh N., Roder J.C., and Takahashi T.. 2002. Neuronal calcium sensor 1 and activity-dependent facilitation of P/Q-type calcium currents at presynaptic nerve terminals. Science. 295:2276–2279. 10.1126/science.1068278 [DOI] [PubMed] [Google Scholar]
- Wheeler D.B., Randall A., and Tsien R.W.. 1994. Roles of N-type and Q-type Ca2+ channels in supporting hippocampal synaptic transmission. Science. 264:107–111. 10.1126/science.7832825 [DOI] [PubMed] [Google Scholar]
- Wu J., Yan Z., Li Z., Qian X., Lu S., Dong M., Zhou Q., and Yan N.. 2016. Structure of the voltage-gated calcium channel Cav1.1 at 3.6 Å resolution. Nature. 537:191–196. 10.1038/nature19321 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.