Chiu and colleagues review the primary evidence for divergent activation mechanisms and unitary properties of Pannexin 1 channels.
Abstract
Pannexin 1 (Panx1) forms plasma membrane ion channels that are widely expressed throughout the body. Panx1 activation results in the release of nucleotides such as adenosine triphosphate and uridine triphosphate. Thus, these channels have been implicated in diverse physiological and pathological functions associated with purinergic signaling, such as apoptotic cell clearance, blood pressure regulation, neuropathic pain, and excitotoxicity. In light of this, substantial attention has been directed to understanding the mechanisms that regulate Panx1 channel expression and activation. Here we review accumulated evidence for the various activation mechanisms described for Panx1 channels and, where possible, the unitary channel properties associated with those forms of activation. We also emphasize current limitations in studying Panx1 channel function and propose potential directions to clarify the exciting and expanding roles of Panx1 channels.
Introduction
Pannexins are a relatively recently identified class of membrane channels that are garnering interest across diverse fields of biology. The gene family was discovered by Yuri Panchin and colleagues, who used bioinformatics and cloning approaches to search for homologues of invertebrate innexins in Chordata; they proposed to name them pannexins, in recognition of their widespread presence across many phyla (i.e., in all eumetazoans, except echinoderms) and sequence similarity with innexins (Panchin et al., 2000; Abascal and Zardoya, 2012). At this time, family members from chordates are specified as pannexins, and homologues from nonchordate animals are referred as innexins. Members of the innexin/pannexin family contain four transmembrane (TM) domains with intracellular N and C termini and several conserved cysteines in the first extracellular loop. Despite this shared sequence homology and membrane topology, pannexins constitute plasma membrane ion channels under physiological conditions, as opposed to the gap junction channels formed by innexins (reviewed in Sosinsky et al., 2011). It is noteworthy that neither innexins nor pannexins share significant sequence homology with connexins, the vertebrate gap junction proteins that share the same general topological features (Panchin et al., 2000; Bruzzone et al., 2003; Baranova et al., 2004).
Additional members of this extended 4-TM channel family include the leucine-rich repeat-containing eight proteins (LRRC8A-LRRC8E, also known as SWELL), which are volume-regulating anion channels that allow permeation of anions and electrolytes in response to change of environmental osmolarity; and calcium homeostasis modulator 1 (CALHM1) channels, which generate mixed ionic currents with weak cation selectivity and allow ATP release when extracellular Ca2+ concentrations are reduced (Abascal and Zardoya, 2012; Siebert et al., 2013). N-glycosylation has been reported in Pannexin channels (Panx1–Panx3; Boassa et al., 2007; Penuela et al., 2007, 2009), as well as in LRRC8A (Voss et al., 2014) and CALHM1 (Dreses-Werringloer et al., 2008). Although the effect of N-glycosylation on channel properties remains unknown, it has been suggested to regulate cell-surface expression of Panx1 and may preclude the formation of gap junctions between Panx1 channels from neighboring cells (Boassa et al., 2007, 2008; Penuela et al., 2007). The diverse and physiologically important functions of the members of this family of channels is drawing tremendous attention, with much yet to be learned about their respective channel properties and regulatory mechanisms, both shared and distinct.
In this review, we seek to provide a comprehensive analysis of experimental evidence for functions and regulation of pannexins, particularly Panx1. In so doing, we call attention to potential pitfalls in earlier studies and challenge long-held views regarding activation mechanisms and channel properties of Panx1. We propose alternative explanations for disparate results and suggest interpretive cautions and best practices for future research. Although we focus primarily on pannexin channels, it is important to remain mindful of the related channels and how their shared properties could confound interpretations with respect to pannexin channels, as well as how their distinct characteristics might be used to help resolve confounding results.
Overview of Pannexin biology
In vertebrates, three pannexin paralogues (Panx1, Panx2, and Panx3) have been identified. Among these, Panx1 is the most widely represented in diverse tissues and cell types, including lymphocytes, adipocytes, muscle, endothelium, and epithelial cells (Baranova et al., 2004; Penuela et al., 2007; Chekeni et al., 2010; Billaud et al., 2011; Seminario-Vidal et al., 2011; Lohman et al., 2012a; Adamson et al., 2015). In contrast, expression of the other pannexins is limited to select tissues, with prominent expression of Panx2 primarily in the central nervous system and Panx3 in skin and skeletal tissues (Bruzzone et al., 2003; Baranova et al., 2004; Penuela et al., 2007; Iwamoto et al., 2010). These pannexin paralogues share ∼50% similarity in amino acid sequence, with higher similarity found in their N termini and TM regions (Penuela et al., 2009). The C-terminal tails harbor greater variability in both length and amino acid identity; of note, Panx2 possesses the longest C-tail, with proline-rich and hydrophilic regions (Yen and Saier, 2007). Thus, C-tails may present unique regulation sites that could underlie functional divergence among the different pannexin paralogues.
Panx1 forms oligomeric channels in the plasma membrane, potentially hexamers, as suggested by biochemical approaches (e.g., protein cross-linking, size exclusion chromatography) and single-molecule techniques (e.g., fluorescence photobleaching, negative stain electron microscopy; Boassa et al., 2007; Wang et al., 2014; Chiu et al., 2017). On the other hand, Panx2 reportedly forms octameric channels (Ambrosi et al., 2010), and a subunit stoichiometry for Panx3 has not been reported. Unlike Panx1, which is thought to form channels only on the plasma membrane, Panx3 may function as both a plasma membrane channel and an intracellular ion channel, localized at ER (Penuela et al., 2008; Ishikawa et al., 2011); to date, however, Panx3-dependent ionic currents have not been reported (Bruzzone et al., 2003; Poon et al., 2014).
The subcellular localization of Panx2 channels awaits determination. In favor of a role for Panx2 as an intracellular ion channel, a fluorescent protein-conjugated Panx2 was localized intracellularly after heterologous expression, and Panx2 was not detected at the cell surface of mouse neurons by immunostaining with an anti-Panx2 antibody (Lai et al., 2009; Le Vasseur et al., 2014; Boassa et al., 2015). However, functional expression of Panx2 channels at the plasma membrane was clearly demonstrated using cell-surface biotinylation and electrophysiological assays in other heterologous expression experiments (Penuela et al., 2009; Poon et al., 2014), indicating that Panx2 is capable of forming plasma membrane channels. The discrepancies may arise from different techniques or biological contexts (e.g., types of cells and tissues) used to investigate the expression of Panx2. For example, the subcellular localization of Panx2 may be influenced by expression levels or dependent on differentiation state of cells (Swayne et al., 2010).
In general, there is much less known about the regulation and function of Panx2 and Panx3, in comparison with Panx1. The (patho)physiological functions identified for Panx2 or Panx3 channels are currently limited to neuronal development and ischemia-reperfusion injury (Panx2) or skin/skeleton development (Panx3; Celetti et al., 2010; Swayne et al., 2010; Bargiotas et al., 2011; Ishikawa et al., 2011; Caskenette et al., 2016). Moreover, although different pannexins are often considered to be complementary (Bargiotas et al., 2011; Lohman and Isakson, 2014; Penuela et al., 2014), it is not at all certain that they share similar properties or could compensate for each other. For example, Panx1 and Panx2 have different basal activity, activation mechanisms, pharmacological sensitivity, and subcellular distribution (Penuela et al., 2008; Poon et al., 2014; Boassa et al., 2015); in addition, although suggestive evidence exists (Penuela et al., 2009; Celetti et al., 2010; Bargiotas et al., 2011), permeability to nucleotides or fluorescent dyes has not been definitively established for either Panx2 or Panx3.
In keeping with their broad distribution, Panx1 channels have now been implicated in a wide variety of physiological and pathological contexts, such as blood pressure regulation, glucose uptake, apoptotic cell clearance, tumor metastasis, neuropathic pain induction, ischemia-reperfusion injury, and morphine withdrawal responses (Chekeni et al., 2010; Bargiotas et al., 2011; Adamson et al., 2015; Billaud et al., 2015; Furlow et al., 2015; Weilinger et al., 2016; Burma et al., 2017; Weaver et al., 2017). These (patho)physiological roles of Panx1 channels are largely attributed to their ability to release ATP or other nucleotides, which support purinergic signaling in a paracrine or autocrine manner. In keeping with these diverse roles, multiple mechanisms have been implicated in Panx1 channel activation, including increased concentration of intracellular calcium or extracellular potassium, receptor-mediated signaling pathways, and proteolytic cleavage at the C termini of Panx1 proteins (Table 1; see Panx1 channels can be activated by distinct mechanism for discussion).
Table 1. Evidence for different activation mechanisms of Panx1 channels.
| Activation mechanism and stimulation | Model system | Electrophysiology | ATP release | Dye permeation | Reference |
|---|---|---|---|---|---|
| Pressure or stretch | |||||
| Negative pressure (∼40 mbar) | Recombinant PANX1 in Xenopus oocytes | Single-channel recording; inhibitor NA | NA | NA | Bao et al., 2004 |
| Pressure | Native PANX1 in human erythrocytes | Single-channel recording; inhibitor NA | NA | NA | Locovei et al., 2006a |
| Stretch (15 or 30 cmH2O) | Native Panx1 in rat bladders | NA | CBX (100 µM); BB FCF (100 µM) | NA | Beckel et al., 2015 |
| Hypotonicity | Native PANX1 in human erythrocytes | NA | CBX (100 µM) | Inhibitor NA | Locovei et al., 2006a |
| Hypotonicity | Native PANX1 in human bronchial epithelial cells | NA | CBX (10 µM); 10Panx1 (30 µM) | CBX (10 µM); 10Panx1 (30 µM) | Seminario-Vidal et al., 2011 |
| Hypotonicity | Native PANX1 in A549 cells | NA | PANX1 siRNA | PANX1 siRNA | Seminario-Vidal et al., 2011 |
| Hypotonicity | Native Panx1 in mouse tracheas | NA | Panx1−/− | Panx1−/− | Seminario-Vidal et al., 2011 |
| Hypotonicity | Native PANX1 in CN-LM1A and MDA-LM2 cellsa | NA | CBX (500 µM)b | NA | Furlow et al., 2015 |
| Hypotonicity and 75 mM extracellular K+ | Native Panx1 in mouse erythrocytes | NA | Probenecid (1 mM); Panx1−/−±probenecid | Panx1−/− | Qiu et al., 2011 |
| Extracellular K+ | |||||
| 20∼150 mM K+ | Recombinant Panx1 in Xenopus oocytes | Whole-cell current; CBX (100 µM); probenecid (1 mM) | CBX (100 µM); probenecid (100 or 500 µM) | NA | Silverman et al., 2009; Wang et al., 2014 |
| 150 mM K+ | Recombinant Panx1 in Xenopus oocytes | Single-channel recording; inhibitor NA | CBX (100 µM) | NA | Wang et al., 2014 |
| ? mM K+ | Recombinant Panx1 in Xenopus oocytes | NA | BB FCF (10 µM) | NA | Wang et al., 2013 |
| 50 mM K+ | Native PANX1 in 1321N1 cells | NA | NA | Panx1 shRNA | Silverman et al., 2009 |
| 10 mM K+ | Native Panx1 in mouse hippocampal slides | NA | Mefloquine (100 nM); Panx1−/− | Mefloquine (100 nM); Panx1−/− | Santiago et al., 2011 |
| 10 mM K+ | Native Panx1 in mouse astrocytes | Whole-cell current; Panx1−/− | Panx1−/− | Panx1−/− | Suadicani et al., 2012 |
| 75 mM extracellular K+ and hypotonicity | Native Panx1 in mouse erythrocytes | NA | Probenecid (1 mM); Panx1−/−±probenecid | Panx1−/− | Qiu et al., 2011 |
| Intracellular Ca2+ | |||||
| Ca2+ (0.1 µM∼10 mM) | Recombinant PANX1 in Xenopus oocytes | Inside-out macropatch; single-channel recording; inhibitor NA | NA | NA | Locovei et al., 2006b |
| A23187 (200 µM) | Recombinant Panx1 in Xenopus oocytes | Whole-cell current; inhibitor NA | NA | NA | Locovei et al., 2006b |
| Ionomycin (10 µM) | Recombinant PANX1 in N2A cells | NA | NA | Inhibitor NA | Kurtenbach et al., 2013 |
| Thrombin (30 nM) | Native PANX1 in A549 cells | NA | CBX (10 µM); BAPTA-AM (20 µM); thapsigargin (1 µM) | CBX (10 µM); BAPTA-AM (20 µM) | Seminario-Vidal et al., 2009 |
| Thrombin (1 or 1,000 U/ml) | Native PANX1 in HUVECs | NA | CBX (5 µM); PANX1 shRNA; BAPTA-AM (5 µM) | NA | Gödecke et al., 2012 |
| Caffeine (concentration NA) | Recombinant Panx1 in rat atrial myocytes | Single-channel recording; CBX (20 µM); probenecid (200 µM) | NA | NA | Kienitz et al., 2011 |
| SFK-mediated phosphorylation | |||||
| NMDA (100 µM) | Native Panx1 in rat hippocampal neurons | Whole-cell current; CBX (50 µM); 10Panx1 (100 µM); Panx1 shRNA | NA | CBX (50 µM); 10Panx1 (100 µM) | Thompson et al., 2008; Weilinger et al., 2016 |
| Anoxia | Native Panx1 in rat CA1 neurons | Whole-cell current; 10Panx1 (100 µM)±APV (50 µM); probenecid (500 µM); brain-specific Panx1−/−; TAT-Panx305-318 peptide (1 or 10 µM) | NA | NA | Weilinger et al., 2012 |
| TNFα (10 ng/ml) | Native PANX1 in HUVECs | NA | CBX (50 µM); 10Panx1 (200 µM); Panx1 siRNA; endothelial cell–specific Panx1−/− | 10Panx1 (200 µM) | Lohman et al., 2015 |
| TNFα (50 ng/ml) | Native Panx1 in mouse venous endothelia | NA | Endothelial cell–specific Panx1−/− | NA | Lohman et al., 2015 |
| BzATP (50 or 300 µM) | Native Panx1 in J774 cells | Whole-cell current; CBX (50 µM); mefloquine (100 nM); Panx1 siRNA | NA | CBX (50 µM); mefloquine (10 nM); Panx1 siRNA | Iglesias et al., 2008 |
| BzATP (300 µM) | Recombinant Panx1 in Xenopus oocytes | Whole-cell current; inhibitor NA | NA | Inhibitor NA | Iglesias et al., 2008 |
| BzATP (300 µM) | Native Panx1 in peritoneal macrophage from A/J mice | NA | NA | CBX (10 µM); 10Panx1 (300 µM) | Sorge et al., 2012 |
| BzATP (300 µM) | Native Panx1 in mouse astrocytes | Whole-cell current; CBX (50 µM); mefloquine (100 nM); Panx1−/− | Panx1−/− | Panx1−/− | Suadicani et al., 2012 |
| C-tail cleavage | |||||
| Anti-Fas (5 µg/ml) | Native PANX1 in Jurkat cells | Whole-cell current; CBX (10∼500 µM); probenecid (10 µM∼2 mM); CBX (100 µM)+PANX1 siRNA | CBX (500 µM)c; probenecid (2 mM); PANX1 siRNA | PANX1 siRNA; CBX (500 µM) | Chekeni et al., 2010 |
| Anti-Fas (5 µg/ml) | Native Panx1 in mouse thymocytes | NA | NA | CBX (500 µM) | Chekeni et al., 2010 |
| Anti-Fas (5 µg/ml) | Recombinant PANX1 in Jurkat cells | Whole-cell current; caspase site-deficient PANX1; CBX (100 µM) | Caspase site-deficient PANX1 | Caspase site-deficient PANX1 | Chekeni et al., 2010 |
| Anti-Fas (5 µg/ml) | Native Panx1 in mouse thymocytes | NA | NA | Panx1−/− | Qu et al., 2011 |
| Dexamethasone (0.5 µM) | Native Panx1 in mouse thymocytes | NA | Panx1−/− | NA | Qu et al., 2011 |
| C-tail truncationc | Recombinant PANX1 in Jurkat cells | Whole-cell current; CBX (100 µM) | NA | Inhibitor NA | Chekeni et al., 2010 |
| Caspase 3 | Native PANX1 in Jurkat cells; Recombinant PANX1 in HEK293T cells | Inside-out macropatch; Single-channel recording; CBX (50 µM) | NA | NA | Sandilos et al., 2012; Chiu et al., 2017 |
| TEV proteased | Recombinant PANX1 in HEK293T cells | Whole-cell current; Inside-out macropatch; CBX (50 µM) | NA | Inhibitor NA | Sandilos et al., 2012 |
| LPS (50 ng/ml) | Native Panx1 in bone marrow–derived macrophage | NA | CBX (50 µM); probenecid (100 µM); trovafloxacin (100 µM); Panx1−/− | NA | Yang et al., 2015 |
| LPS (50 ng/ml) | Recombinant Panx1 in bone marrow–derived macrophage | NA | Panx1−/− with Panx1-D378A | NA | Yang et al., 2015 |
NA, data not available or unknown.
The cells endogenously express both wild-type and a truncated form of PANX1, which only expresses amino acid 1-89 of PANX1.
High concentration of CBX is required for inhibition of ATP release if albumin is present in the assay system (Chekeni et al., 2010).
A C-terminal truncated PANX1 (amino acid 1-371) was used in the study.
This study used a mutant human PANX1, with its caspase cleavage site replaced by a TEV protease cleavage site.
Despite increasing recognition of the importance of Panx1 activity in these multiple contexts, there have been remarkable discrepancies in studies describing single-channel properties of Panx1 that could serve as diagnostic features for identification of native channels. In addition, the pharmacological tools that are available for attributing effects to Panx1 in native environments are generally nonspecific, and their effects on the channels often remain poorly characterized. This lack of both consensus and reagents hinders advances in the field. Here, we examine critically the experimental evidence supporting specific Panx1 channel properties and activation mechanisms, and we discuss new advances and resources for identifying Panx1 channels and their specific roles. We hope this appraisal will be useful in raising concerns and limitations regarding current methodologies and guiding future investigations of Panx1 channels so that the field can collectively forge the consensus necessary to provide a consistent and comprehensive understanding of Panx1 channel biology.
Current limitations in studying Panx1 channels
Pharmacological tools have been used extensively, although not always definitively, to understand functions and regulation of Panx1 channels in native contexts. A variety of reagents, including chemicals and peptides, have been identified as inhibitors of Panx1 channels (see D’hondt et al., 2009 for an extended review). However, none of the most commonly used inhibitors are specific for Panx1. For example, although carbenoxolone (CBX) is a widely accepted Panx1 inhibitor, CBX also inhibits other closely related channels, such as connexins and LRRC8/SWELL1 (Ripps et al., 2004; Bruzzone et al., 2005; Ye et al., 2009; Voss et al., 2014). In addition, a peptide inhibitor, 10Panx1, has been widely used as a Panx1-specific inhibitor (Pelegrin and Surprenant, 2006; Lohman et al., 2015; Weilinger et al., 2016; Burma et al., 2017), even though its nonspecific inhibition of connexins has been reported (Wang et al., 2007). Several other compounds require high concentration for effective inhibition, such as probenecid (IC50 ∼350 µM; Ma et al., 2009), and issues associated with solubility as well as nonspecificity demand caution, particularly in applications involving systemic administration. Different reagents may preferentially inhibit Panx1 channels activated by different mechanisms or in certain locations. For example, CBX (100 µM) inhibits only ∼75% of Panx1 current induced by high K+ (75 mM; Jackson et al., 2014), and small peptides may not be able to pass blood–brain barrier.
To overcome these shortcomings in channel pharmacology, a panel of inhibitors with different chemical structure or modes of inhibition should be used to verify the results. Recent efforts have identified newer “specific” Panx1 inhibitors, including Brilliant Blue FCF (BB FCF) and trovafloxacin, which have little effect on various topologically or functionally related channels, such as connexins, Panx2, or P2X7 receptors (Wang et al., 2013; Poon et al., 2014); these can be included to implicate Panx1 activity. Moreover, in addition to common surrogate assays for pannexin activity, such as ATP release and dye uptake, direct electrophysiological recordings of channel activity should also be used. This is particularly important in light of reports that various channels, such as connexins or P2X7 receptors, are intrinsically capable of permeating fluorescent dye or ATP (Fiori et al., 2012; Karasawa et al., 2017). Thus, one can achieve greater confidence in attributing effects to Panx1, for example, by using trovafloxacin, a fluoroquinolone antibiotic that displays voltage-dependent inhibition of Panx1 currents (Poon et al., 2014), in parallel with other blockers (e.g., CBX, a steroid-like glycyrrhetinic acid that blocks across the voltage range). Finally, to further strengthen such conclusions, a combination of pharmacological and genetic approaches should also be considered (Burma et al., 2017; Weaver et al., 2017).
In addition to the paucity of selective inhibitors, the fidelity of currently available anti-Panx1 antibodies remains uncertain (Bargiotas et al., 2011; Cone et al., 2013); therefore, specificity of the antibody should be verified by using Panx1 knockout animals or other genetic approaches to prevent false-positive/negative results. Note that several independent Panx1 knockout mouse lines have been generated (Anselmi et al., 2008; Qu et al., 2011; Skarnes et al., 2011; Dvoriantchikova et al., 2012). Although studies using these mice agree on a common physiological function of Panx1 in releasing ATP (Qiu et al., 2011; Qu et al., 2011; Santiago et al., 2011; Seminario-Vidal et al., 2011), a hypomorphic phenotype has been reported in the KOMP knockout-first mouse line (Hanstein et al., 2013), which again emphasizes the importance of validating materials used for studying Panx1 channels. Given fast-developing implementations of CRISPR-Cas9 techniques (Yang et al., 2013), engineering an epitope-knock-in animal model would provide a useful resource to evaluate native expression patterns with well-characterized specific antibodies.
Meanwhile, ionic selectivity is generally determined by examining changes in reversal potential (Erev) upon altering ionic composition of recording solutions. Indeed, anionic permeability of Panx1 channels was claimed by using such a seemingly straightforward method (Ma et al., 2012; Romanov et al., 2012). However, an opposite interpretation was made in a separate paper, i.e., that Panx1 channels are permeable to sodium, even though both studies based their conclusions on the result that Erev was unchanged in solutions exchanging sodium for NMDG (N-methyl-d-glucamine; Pelegrin and Surprenant, 2006; Ma et al., 2012; Romanov et al., 2012). Arguing against either anion- or cation-selectivity, the activated Panx1 channel apparently allows permeation of large molecules with both positive and negative charge (i.e., TO-PRO-3 and ATP; Chekeni et al., 2010; Qu et al., 2011; Seminario-Vidal et al., 2011). Finally, ionic selectivity has not been rigorously examined, e.g., by determining changes in Erev of open channel currents (tail currents). Even so, interpretation of such experiments often relies on use of counter-ions that are impermeable, and that cannot be assumed in the case of Panx1 channels that reportedly pass large molecules with size >500 Da. Therefore, developing a reconstituted system, such as proteoliposomes, may provide a clean system to measure Panx1 permeability to molecules of different charge and size.
Panx1 channels can be activated by distinct mechanisms
Given the ubiquitous expression and multifaceted functions of Panx1 channels in diverse cell types and tissues, various mechanisms have been reported to activate Panx1 channels in either a reversible or irreversible manner. Here, we first describe experimental approaches and evidence that support the multimodal activation of Panx1 channels (Table 1). We then discuss distinct channel properties that have been attributed to the various activation mechanisms.
Pressure/stretch-induced activation of Panx1 channels
The first evidence suggesting that Panx1 might form a mechanosensitive channel came from a heterologous expression system. Using Xenopus laevis oocytes injected with cRNA of human PANX1, Bao et al. (2004) recorded increased channel activity in response to negative pressure (∼40 mbar), applied via the patch pipette. The increased open probability (PO) of these stretch-activated channels was associated with longer dwell time in larger subconductance states, with no obvious voltage-dependence of either PO or unitary conductance (Fig. 1 A). Subsequently, several papers reported increased ATP release and fluorescent dye uptake from airway epithelia or erythrocytes swollen by hypotonic solutions; this was attributed to mechanosensitive activation of Panx1, either by using cells derived from Panx1-deficient mice or a combination of pharmacological tools (Locovei et al., 2006a; Qiu et al., 2011; Seminario-Vidal et al., 2011). In addition, ATP release from metastatic breast cancer cells subjected to deformation as they transited the microvasculature or from distended rat bladders was also attributed to stretch-induced activation of Panx1, tested by using CBX or BB FCF (Beckel et al., 2015; Furlow et al., 2015). It is noteworthy, however, that electrophysiological recordings of the corresponding native ionic currents induced by pressure or hypotonicity have not yet been verified by knockout or pharmacological inhibition in any of these cells (i.e., erythrocytes or cancer cells). In fact, even in the original Xenopus oocyte experiments, the stretch-activated channels were not independently verified as Panx1 (e.g., by using channel blockers), so the possibility remains that this activity was from channels endogenous to the oocyte. Thus, the single-channel properties and cognate macroscopic activity of stretch-activated Panx1 channels awaits further investigation and validation.
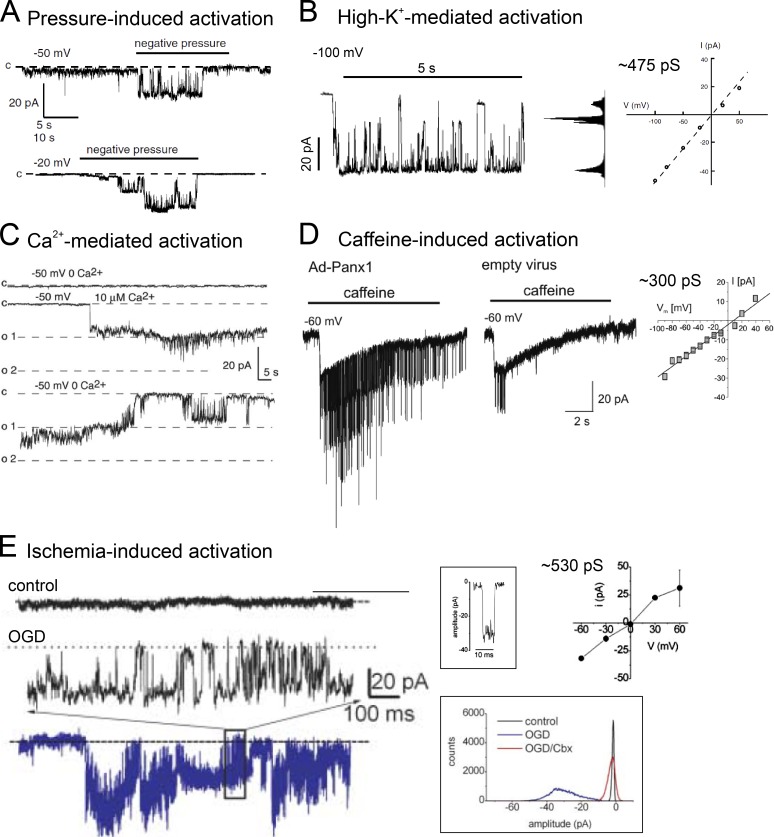
Figure 1.
Panx1 channels of large and linear unitary conductance. (A) Pressure-induced single-channel activity obtained from a Xenopus oocyte heterologously expressing human PANX1 (adapted from Bao et al., 2004). The unitary conductance of pressure/stretch-activated channels was reported elsewhere to be ∼475 pS (Locovei et al., 2006a). (B) High extracellular K+-activated single-channel activity obtained by using inside-out patch recording in Xenopus oocytes heterologously expressing human PANX1 (adapted from Bao et al., 2004). Membrane patch was exposed to symmetric 150 mM K+. The high-K+–activated channel visited multiple subconductance states and displayed a unitary conductance up to ∼475 pS. (C) Intracellular Ca2+-induced single-channel activity obtained by using inside-out patch recording in Xenopus oocytes heterologously expressing human PANX1 (adapted from Locovei et al., 2006b). The unitary conductance of Ca2+-activated channels was reported to be ∼550 pS (Locovei et al., 2006b). (D) Single-channel activity evoked by caffeine-induced Ca2+ release, obtained from rat atrial myocytes infected with adenovirus expressing mouse Panx1 or empty vector (adapted from Kienitz et al., 2011). The caffeine-activated channels showed a unitary conductance of ∼300 pS. (E) O2/glucose deprivation (OGD)-induced single-channel activity obtained by using cell-attached recording in rat hippocampal neurons (left). Boxed figures are exemplar single-channel opening (top right) and all-point histogram acquired from recordings under control, OGD, and OGD+CBX conditions (bottom right). The OGD-activated channels demonstrated a unitary conductance of ∼530 pS (adapted from Thompson et al., 2006). All figures are reproduced with permission.
The molecular mechanisms for mechanosensitivity of Panx1 remain to be established. A demonstrated interaction between F-actin and the intracellular C terminus of Panx1 (Bhalla-Gehi et al., 2010) provides a potential physical transduction mechanism for channel activation in response to membrane stretch. Consistent with such a cytoskeleton-tethering model, inhibition of RhoA or activation of myosin light chain kinase, which can disrupt the actin cytoskeleton, reduced ATP release from bronchial epithelial cells after hypotonic challenge (Seminario-Vidal et al., 2011). On the other hand, channel-intrinsic mechanosensitivity of Panx1 cannot be excluded because membrane deformation reportedly activated Panx1 channels in excised membrane patches where mechanical transduction via loosely anchored actin filaments is less likely (Bao et al., 2004). Further studies to examine the involvement of cytoskeleton proteins or to establish more reduced cell-free systems (e.g., Panx1-reconstituted proteoliposomes) could help clarify the physical basis for mechanosensitivity of Panx1 channels.
In sum, there is evidence for activation of Panx1 channels by membrane stretch, but the data are not definitive and the transduction mechanisms have not been elucidated. Importantly, electrophysiological experiments from verified recombinant channels, and in cells from wild-type and Panx1 knockout mice, would clarify the basic properties of stretch-activated channels and strengthen the conclusion that Panx1 indeed forms those native mechanosensitive channels. In this latter respect, it is critical to recognize that LRRC8/SWELL channels are also activated by hypotonicity and share multiple characteristics with Panx1, including carbenoxolone sensitivity and large molecule permeation (Qiu et al., 2014; Voss et al., 2014; Gaitán-Peñas et al., 2016; Syeda et al., 2016). Thus, the ATP release from human erythrocytes, which was attributed to Panx1 based only on CBX-sensitivity (Locovei et al., 2006a), could instead reflect LRRC8-mediated ATP release. These considerations demand extra caution in differentiating contributions from Panx1 or LRRC8/SWELL when examining stretch/pressure-activated channel currents, ATP release and dye uptake.
Panx1 activation by elevated extracellular potassium
High concentrations of extracellular K+ (7∼80 mM) have been observed in several pathological conditions, such as epileptiform convulsions or ischemic injury (Fisher et al., 1976; Hansen, 1978). Several studies have demonstrated that Panx1 channels can be activated by elevated levels of extracellular potassium, with a minimum concentration of 10 mM, by examining Panx1-mediated permeation of ions or large molecules (Silverman et al., 2009; Qiu et al., 2011; Santiago et al., 2011; Suadicani et al., 2012; Wang et al., 2014; Fig. 1 B). High-K+-induced Panx1 currents were first described by the Dahl laboratory using Xenopus oocytes heterologously expressing mouse Panx1 (Silverman et al., 2009), and later reported by the Scemes laboratory in mouse astrocytes (Suadicani et al., 2012). These K+-induced currents were reduced by CBX or probenecid and attenuated in astrocytes derived from Panx1 knockout mice. In addition to ionic currents, high-K+–induced dye uptake and ATP release were attenuated in a human astrocytoma cell line (1321N1) by shRNA-mediated PANX1 knockdown, and in hippocampal neurons or astrocytes derived from Panx1-knockout mice (Silverman et al., 2009; Santiago et al., 2011; Suadicani et al., 2012). This evidence provides support for elevated extracellular K+ as an activation mechanism for Panx1 channels and implicates potential roles of the channels in etiology of seizure or ischemic stroke (Bargiotas et al., 2011; Santiago et al., 2011).
The mechanism by which high extracellular K+ activates Panx1 channels remains to be determined. Increased Panx1 currents were observed under voltage clamp conditions, indicating that channel activation was not simply a result of membrane depolarization caused by elevated extracellular K+ (Silverman et al., 2009). It has been proposed that a direct association of K+ with the first extracellular loop of Panx1 may be required for high-K+–induced activation (Jackson et al., 2014). The rationale for this hypothesis follows from a series of studies: first, by using electrophysiological recordings in Xenopus oocytes heterologously expressing wild-type or mutant mouse Panx1, the Dahl laboratory found that Arg-75 in the first extracellular loop was critical for inhibition of Panx1 by high concentrations of ATP or ATP analogues, with IC50 ≥500 µM for ATP (Qiu and Dahl, 2009); second, in a later study, the same group also reported that high extracellular K+ attenuated the inhibition of Panx1 currents by high extracellular ATP (500 µM; Jackson et al., 2014). Based on these observations, these authors hypothesized an overlapping interaction site for K+ and ATP, and proposed that high K+ may activate Panx1 channels through direct binding. It will require further investigation to determine whether ATP and K+ compete for the same binding site. In this respect, it would be interesting to test whether high K+ is able to activate mutated Panx1 channels that are resistant to inhibition by ATP (e.g., R75A or W74A; Qiu and Dahl, 2009; Qiu et al., 2012). Intriguingly, high K+ also interferes with inhibition of Panx1 currents by other compounds with distinct chemical structures, such as CBX or probenecid (Jackson et al., 2014). It remains to be tested whether all these different chemical classes of Panx1 inhibitors modulate channel activity by competing for the proposed high-K+ binding site.
In collaboration with the Sosinsky laboratory, Dahl and colleagues also presented another line of evidence that supports a direct activation mechanism for high K+. In that work, by using electron microscopy, they found an enlarged “pore” diameter of purified, negatively stained mouse Panx1 channels in high-K+ solutions (Wang et al., 2014). The authors previously reported that maleimidobutyryl-biocytin (MBB), a thiol-modifying reagent, inhibited whole-cell currents in Xenopus oocytes heterologously expressing mouse Panx1 under normal K+ concentrations (Wang and Dahl, 2010), but not under high-K+ conditions (Wang et al., 2014). Because this was attributed to an interaction of MBB with Cys-426 at the distal end of C-tails (Wang and Dahl, 2010), the authors further suggested that the larger pore diameter might be explained by rearrangement of Panx1 C-tails in high-K+ (Wang et al., 2014). In general, a higher-resolution, 3-D structure of Panx1 in different K+ conditions would provide better understanding of high-K+–mediated activation of Panx1, and how high K+ might interact with ATP (and/or other blockers) on the channel.
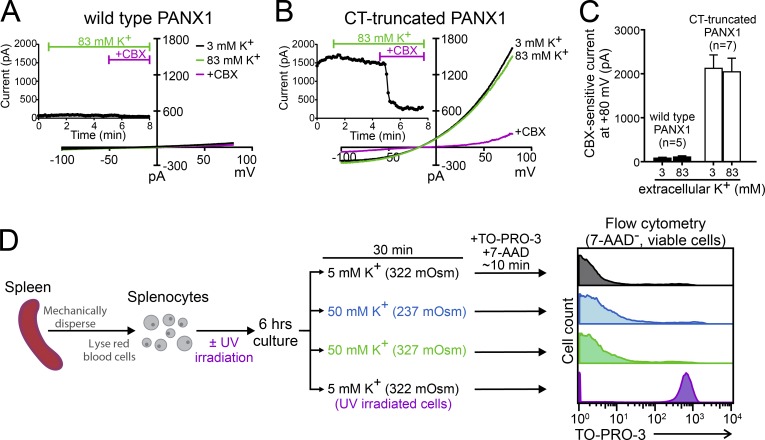
Despite the evidence cited above, it is important to acknowledge that we have been unable to verify high extracellular K+–induced Panx1 current or dye uptake (Chiu et al., 2017; Fig. 2). Thus, in HEK293T cells heterologously expressing either wild-type or C-terminally truncated human Panx1, current density was unaffected by large changes in K+ concentration in the bath solutions (3 vs. 83 mM; ∼300 mOsm). Moreover, by flow cytometry, negligible TO-PRO-3 uptake was found in mouse splenocytes (Fig. 2 D) and human Jurkat cells exposed to 50 mM K+. This was in sharp contrast to a pronounced TO-PRO-3 uptake by UV-irradiated, apoptotic splenocytes (Fig. 2 D). In addition, it appears that the ATP release observed from high-K+–treated erythrocytes (Locovei et al., 2006a; Qiu et al., 2011) is likely caused by hemolysis of erythrocytes upon exposure to the K-gluconate–based buffer (Keller et al., 2017). Together, these new results cast doubt on a long-held view that high-K+ is a universal activation mechanism for Panx1, and recommend additional examination of this mechanism by other independent research groups. In any case, one should exercise caution when attributing high-K+–induced activities to Panx1 channels.
Figure 2.
Raising extracellular K does not activate recombinant or native Panx1 channels. (A and B) Whole-cell currents were obtained from HEK293T cells expressing either wild-type PANX1 (A) or C-terminally truncated PANX1 (B) under control conditions (3 mM K+), high extracellular K+ (83 mM K+), and high extracellular K+ plus CBX (50 µM); insets show time series of current obtained at 80 mV under the indicated conditions. As previously reported (Chiu et al., 2017), whole-cell voltage-ramp I-Vs (−100 to 80 mV; 0.2 V/s at 0.14 Hz) were obtained at room temperature using borosilicate micropipettes (3∼5 MΩ) filled with internal solution containing (mM) 100 CsMeSO4, 30 TEACl, 4 NaCl, 1 MgCl2, 0.5 CaCl2, 10 HEPES, 10 EGTA, 3 ATP-Mg, and 0.3 GTP-Tris, pH 7.3. Control (3 mM K+) bath solution was composed of (mM) 140 NaCl, 3 KCl, 2 MgCl2, 2 CaCl2, 10 HEPES, and 10 glucose, pH 7.3. High-K+ solution included (mM) 60 NaCl, 83 KCl, 2 MgCl2, 2 CaCl2, and 10 HEPES at pH 7.3; glucose was added to maintain equal osmolarity with the control bath solution (∼300 mOsm). (C) Grouped data (mean ± SEM) shows that CBX-sensitive current from wild-type (n = 5) or CT-truncated PANX1 (n = 7) was unaffected by different extracellular K+ concentrations. These results were originally reported in the peer review file from Chiu et al. (2017). (D) Dye uptake measured by flow cytometry shows that viable (7-AAD negative) mouse splenocytes display negligible TO-PRO-3 uptake under control K+ conditions (5 mM K+; 322 mOsm), hypotonic high-K+ (50 mM K+, 237 mOsm; same ionic composition as Silverman et al., 2009), or osmolarity-adjusted high-K+ (50 mM K+ with 87 mM d-mannitol, 327 mOsm). In contrast, caspase-mediated Panx1 activation in UV-irradiated cells yields robust TO-PRO-3 uptake by viable cells. Splenocytes were freshly isolated from C57BL/6 mice, as previously described (Jin et al., 2008) and cultured in growth media (RPMI + 10% FBS); one group of cells was also exposed to UV irradiation (15 × 104 µJ). After 6 h culture at 37°C, cells were washed three times with RPMI, before a 30-min incubation in solutions containing different concentrations of K+. TO-PRO-3 (Panx1-permeable) and 7-AAD (Panx1-impermeable) were added ∼10 min before flow cytometry, as previously reported (Poon et al., 2014; Chiu et al., 2017). Note that necrotic cells (7-AAD+) were excluded from the analysis to avoid Panx1-independent TO-PRO-3 uptake.
Panx1 activation by increased intracellular calcium
Panx1 channels can be activated by multiple metabotropic receptors, specifically by those that couple via Gαq-containing heterotrimeric G proteins; in some cases (e.g., P2Y purinergic receptors; protease-activated receptors [PARs]), this receptor-mediated channel activation has been attributed to an associated increase in intracellular calcium.
For P2Y receptors, ATP-induced currents were obtained from Xenopus oocytes coexpressing P2Y1 or P2Y2 along with Panx1 channels, whereas ATP did not increase Panx1 currents in the absence of P2Y receptors (Locovei et al., 2006b). Increased whole-cell current was also observed by bath application of the calcium ionophore, A23187, in oocytes expressing Panx1, consistent with a role for elevated intracellular calcium in activating Panx1 channels. In the same study, Locovei et al. (2006b) found that increased activity of Panx1 channels can be observed in the inside-out patch configuration when the cytosolic side of membrane patches was exposed to elevated calcium in a dose-dependent manner (Fig. 1 C). This suggests that Panx1 might be activated directly by increased intracellular calcium expected from P2Y receptor activation, although this was not tested directly (e.g., by chelating intracellular Ca2+ in P2Y-stimulated cells).
In addition to P2Y receptor–induced currents, PAR1/3-mediated ATP release and fluorescent dye uptake are also considered to be Panx1-dependent events (Seminario-Vidal et al., 2009; Gödecke et al., 2012). PAR1-mediated ATP release from human umbilical vein endothelial cells (HUVECs) was reduced by shRNA-mediated knockdown of Panx1, but not by Connexin 43 (Cx43) shRNA (Gödecke et al., 2012); in a human lung epithelial cell line, A549, PAR3-mediated ATP release and propidium iodide uptake were attenuated by application of low concentration of CBX (10 µM; Seminario-Vidal et al., 2009). Bath application of A23187 increased ATP release from HUVECs, consistent with Ca2+-mediated activation of Panx1, although involvement of Panx1 in this A23187 effect was not examined concurrently using genetic or pharmacological tools (Gödecke et al., 2012). Also consistent with a role for Ca2+, the cell-permeable Ca2+ chelator, BAPTA-AM, reduced thrombin-induced ATP release from A549 cells (Seminario-Vidal et al., 2009). Furthermore, application of thapsigargin attenuated thrombin-induced ATP release from A549 cells (Seminario-Vidal et al., 2009), suggesting ER calcium stores might be a relevant calcium source for activating Panx1 channels. Although PAR-activated Panx1 channel currents have not yet been recorded directly, these results collectively support the idea that PAR receptors induce ATP release that is dependent on intracellular calcium and mediated via Panx1 channels.
In settings that do not also involve concurrent receptor stimulation, the effects of Ca2+ on Panx1 channels are less clear. For example, ethidium bromide uptake by N2A cells heterologously expressing mouse or zebrafish Panx1 was increased by ionomycin, another cell-permeable calcium ionophore (Kurtenbach et al., 2013). However, ionomycin was unable to induce ATP release from A549 cells, even though calcium-mediated activation of Panx1 was suggested based on inhibition of thrombin-induced ATP release by BAPTA-AM and thapsigargin in those same cells (Seminario-Vidal et al., 2009). This discrepancy may reflect the specificity of calcium-mediated Panx1 activation by receptor-associated mechanisms. For example, concurrent receptor-mediated activation of the Rho GTPase pathway may also be required to increase ATP release from human A549 cells, in addition to elevated intracellular calcium (Seminario-Vidal et al., 2009). Also not linked to a receptor-mediated process, it was reported that Panx1 constitutes a caffeine-activated, large conductance cation channel in cardiomyocytes (Kienitz et al., 2011; Fig. 1 D), for which channel activation is dependent on elevated intracellular calcium (Loirand et al., 1991). However, those caffeine-activated channels display unitary properties distinct from P2Y receptor–activated channels (Locovei et al., 2006b), which is surprising if both depend on increased intracellular Ca2+. Thus, it remains uncertain whether Ca2+ can activate Panx1 directly, or modulates the channel differently in the context of concurrent receptor signaling.
A mechanism for Panx1 activation by calcium has not yet been provided. Because Panx1 channels do not have a conventional calcium-binding domain (i.e., EF hand or Ca2+ bowl), some undefined calcium binding motif or a potential calcium/calmodulin interaction may instead explain Ca2+-mediated activation of Panx1. Understanding the mechanisms underlying activation of Panx1 channels by intracellular Ca2+ will require further studies to interrogate the effect of raised intracellular calcium itself on Panx1 channel properties and determine precisely how Ca2+ interacts with Panx1.
Panx1 activation by Src family kinase–mediated phosphorylation
In addition to their activation by G protein–coupled metabotropic receptors, there is evidence that Panx1 channels mediate effects downstream of ionotropic receptors and chemokine receptors, such as NMDA, TNFα, and P2X7 receptors (Iglesias et al., 2008; Thompson et al., 2008; Lohman et al., 2015); in these cases, channel activation is attributed to signaling pathways linked to posttranslational modification of Panx1, particularly phosphorylation.
Thompson and colleagues reported that anoxia activates Panx1 channels in hippocampal pyramidal neurons via NMDA receptors (NMDARs; Thompson et al., 2008; Weilinger et al., 2012). In hippocampal CA1 neurons, NMDA- or anoxia-induced secondary inward currents were attenuated by bath application of CBX or 10Panx1 to rat brain slices, or by conditional deletion of Panx1 in mouse brain, supporting the presence of Panx1-dependent currents (Thompson et al., 2008; Weilinger et al., 2012). In addition, NMDA- or anoxia-induced inward currents were reduced by NMDAR antagonists, D-APV or R-CPP, as well as by a Src family kinase (SFK) inhibitor, PP2. Interestingly, however, an NMDAR pore blocker, MK-801, did not affect Panx1-dependent inward currents, suggesting that ion permeation via NMDA receptor channels was not required. These results implicate a metabotropic role for NMDARs, separate from their ion channel function, in activating Panx1 channels via SFKs (Weilinger et al., 2012, 2016).
A likely phosphorylation site on Panx1 was identified by using an antibody specifically recognizing phosphorylation at tyrosine-308 (pY308). Activation of NMDARs increased pY308-Panx1 immunoreactivity in N2A cells heterologously expressing wild-type Panx1 and Src, and this NMDAR-dependent increase in pY308 was not observed in cells treated with PP2 or expressing a Panx1 mutant resistant to phosphorylation at Tyr-308 (Panx1Y308F; Weilinger et al., 2016). Additionally, application of a cell-permeable peptide containing 14 amino acids of Panx1 surrounding Tyr-308 (305–318) interfered with NMDAR-dependent increases in pY308 on Panx1. This peptide did not affect pY416-Src autophosphorylation after NMDAR stimulation in N2A cells (Weilinger et al., 2016), indicating that its presumed competitor function was downstream of Src activation. Collectively, these results support a model whereby NMDARs activate Panx1 channels metabotropically, via SFKs (Weilinger et al., 2012, 2016). Of note, phosphorylation of Panx1 at Tyr-308 and a constitutive interaction between NMDAR, Src, and Panx1 were all present before NMDA stimulation (Weilinger et al., 2016). This supports the possibility of a preexisting signalosome for NMDAR-mediated activation of Panx1 and further implies that some critical number of phosphorylation events at Y308 of Panx1 subunits in the oligomeric channel may be required for NMDA- or anoxia-induced activation of Panx1 channels.
A role for Src family kinases was also proposed for TNFα-induced activation of Panx1 channels, which promotes emigration of leukocytes across the venous microcirculation during inflammation. In this case, SFK-dependent phosphorylation of Panx1 occurs at Tyr-198, a site distinct from that targeted downstream of NMDARs (Lohman et al., 2015). TNFα-induced ATP release was reduced in human umbilical vein endothelial cells (HUVECs) after Panx1 siRNA-mediated knockdown or in mesenteric venules obtained from endothelium-specific Panx1 knockout mice. After application of TNFα, phosphorylation at Tyr-198 of Panx1 was increased, as assessed with an anti-pY198 antibody; this pY198 immunoreactivity was diminished by SFK inhibitors (Lohman et al., 2015), suggesting that Tyr-198 is phosphorylated downstream of SFKs in response to TNFα stimulation. It remains to be determined whether SFKs phosphorylate the channel directly. Further studies to analyze the structural and single-channel properties of these phosphorylated Panx1 channels, at either Tyr-308 or Tyr-198, will provide important fundamental information on activation mechanisms by receptor-mediated tyrosine phosphorylation.
In a further example, SFKs are also implicated in Panx1 channel activation downstream of P2X7 receptors (P2X7Rs; Iglesias et al., 2008). First, it is important to note that this P2X7–Panx1 functional interaction is controversial. For example, ATP-induced YO-PRO-1 uptake is retained in bone marrow-derived macrophages (BMDMs) from Panx1−/− mice, even though it is diminished in BMDMs from P2X7−/− mice (Qu et al., 2011). In addition, it was recently demonstrated that purified P2X7 receptors are themselves sufficient to permeate fluorescent dye in reconstituted proteoliposomes (YO-PRO-1; Karasawa et al., 2017). These findings suggest that Panx1 is dispensable for P2X7R-induced large pore formation and dye uptake. Nevertheless, in J774 mouse macrophage cells, whole-cell inward currents and YO-PRO-1 uptake was induced by BzATP, a P2X7R agonist, and these effects were reduced by Panx1 inhibition (i.e., by using CBX and mefloquine, and also by siRNA-mediated knockdown). Implicating SFKs in P2X7R-mediated Panx1 activation, BzATP-induced inward currents and YO-PRO-1 uptake were both attenuated by PP2 (Iglesias et al., 2008). The phosphorylation site of Panx1 relevant for this proposed P2X7R-SFK signaling mechanism has not been identified; potentially this could involve either (or both) of the Tyr-198 or Tyr-308 sites implicated in TNFα or NMDAR pathways.
A further relevant observation is that functional coupling of P2X7R and Panx1 are seemingly dependent on a single nucleotide polymorphism (SNP) of P2X7Rs: only P2X7Rs that contain Pro-451, but not Leu-451, can mediate Panx1 channel activation (Iglesias et al., 2008; Sorge et al., 2012). The P2X7R(Leu-451) variants retain calcium permeability and phospholipase D (PLD)-coupling ability, so calcium-mediated or PLD-induced activation of Panx1 channels is unlikely (Le Stunff et al., 2004; Sorge et al., 2012). It remains to be determined if direct binding of P2X7Rs and Panx1 channels is required for channel activation, and whether functional coupling between SFK and Panx1 is also dependent on the P2X7R SNP. Note that, although a preexisting interaction between P2X7R and Panx1 was suggested by coimmunoprecipitation (Pelegrin and Surprenant, 2006; Iglesias et al., 2008), it is not clear that this presumed physical interaction is required for P2X7R-mediated Panx1 activation.
In consideration of the possibility that SFKs act as direct activators for Panx1 in multiple pathways that evidently lead to phosphorylation at distinct channel residues, a stimulus-dependent coupling between SFKs and Panx1 channels may exist in order to render specificity among the different signaling pathways. Thus, it will be critical to identify how these various SFK-dependent pathways and phosphorylation sites differentially modulate Panx1 channel activity and properties.
Regulation of Panx1 activity by other kinases
In addition to SFKs, other kinases, such as c-Jun NH2-terminal kinase (JNK; Xiao et al., 2012) and protein kinase G (PKG; Poornima et al., 2015) have been implicated in regulating the activity of Panx1 channels. Xiao et al. (2012) reported that palmitic acid–induced ATP release and YO-PRO-1 uptake were reduced by a JNK inhibitor, SP600125, or by knocking down Panx1 in HTC rat hepatoma cells using shRNA. Even though JNK has been implicated in palmitic acid–induced apoptosis in hepatocytes (Malhi et al., 2006; Wei et al., 2006), it is unlikely that palmitic acid–induced ATP release from HTC cells is mediated by caspase cleavage-activated Panx1 because the ATP release was not decreased by a pan-caspase inhibitor, zVAD-fmk (Xiao et al., 2012). It remains to be determined whether JNK can activate Panx1 channels through a direct phosphorylation or via other signaling messengers.
In contrast to the Panx1 channel activation reported for most signaling pathways, Poornima et al. (2015) showed that nitric oxide (NO) reduced constitutive whole-cell current in HEK293 cells heterologously expressing rat Panx1; this effect was abrogated by inhibiting soluble guanylyl cyclase or PKG, whereas a PKA inhibitory peptide had no effect. Mutational analysis of potential phosphorylation sites indicated that rat Panx1(S206A) was resistant to NO-induced inhibition (Poornima et al., 2015). These data suggest that NO may cause inhibition of Panx1 current by PKG-mediated phosphorylation at Ser-206 and further imply a potential role for phosphatases in maintaining basal activity of the channels. Note, also, that a previous study found that Panx1 channels were inhibited by NO-induced S-nitrosylation on cysteine residues (Lohman et al., 2012b). Thus, multiple different types of channel modification may contribute to inhibition of Panx1 by NO.
A unique activation mechanism by irreversible C-tail cleavage
In addition to the aforementioned reversible activation mechanisms, Panx1 channels can be activated by caspase-mediated cleavage, an irreversible process. During apoptosis of T lymphocytes, activated caspase 3 or 7 cleaves Panx1 channels at a C terminal site (376DVVD379 of human Panx1); the cleaved channel then mediates release of nucleotides to serve as “find-me” signals that attract phagocytes for clearance of the dying cells (Chekeni et al., 2010). Subsequently, this effect of caspase attributed to Panx1 channels was independently verified in lymphocytes derived from Panx1 knockout mice (Qu et al., 2011). Panx1 channels recorded in inside-out patch configuration are activated by bath application of activated caspase 3, and dissociation of the cleaved C-terminal tail from the channel is required for channel activation, supporting an intrinsic channel mechanism whereby the C-tail functions as an autoinhibitory region (Sandilos et al., 2012).
Caspase 11 can also cleave and activate Panx1 channel, as demonstrated in bone marrow–derived macrophages (BMDMs) undergoing lipopolysaccharide (LPS)-induced pyroptosis (Yang et al., 2015). In this study, BMDMs obtained from various lines of knockout mice, including P2X7−/−, caspase 11−/−, and Panx1−/− mice, were deficient in ATP release and YO-PRO-1 uptake after LPS stimulation. Reexpression of wild-type caspase 11, but not a catalytically inactive (C254S) mutant, rescued LPS-induced cleavage of Panx1 in caspase 11−/− BMDMs. Likewise, reconstitution of wild-type Panx1, but not a caspase cleavage-resistant mutant (D378A), rescued LPS-stimulated ATP release and cytotoxicity in Panx1−/− BMDMs. Thus, caspase 11 activates Panx1 channels during pyroptosis by C-terminal cleavage, attacking the same site accessed by caspases 3/7 during apoptosis.
Other unresolved activation mechanisms for Panx1 channels
Beyond those described above, molecular mechanisms for activation of Panx1 channels by other receptors, including α1 adrenergic receptors, insulin receptors, and CXCR4 chemokine receptors, await further elucidation (Billaud et al., 2011, 2015; Adamson et al., 2015; Velasquez et al., 2016). The ATP release or fluorescent dye uptake induced by these receptors was diminished by Panx1 inhibitors (probenecid or 10Panx1; Adamson et al., 2015; Velasquez et al., 2016) or in Panx1-deleted cells (Billaud et al., 2015; Velasquez et al., 2016). For α1D receptor or insulin receptor, complementary results from whole-cell recordings in a heterologous system also support activation of Panx1 channels by receptor-activated pathways (Adamson et al., 2015; Billaud et al., 2015). Aforementioned molecular mechanisms, such as phosphorylation of Panx1 or elevated intracellular Ca2+, may provide potential directions for future investigations.
Other recent work suggests that Panx1 channels may be regulated by pH (Kurtenbach et al., 2013). In N2A cells expressing zebrafish Panx1 (drPanx1a or drPanx1b), ethidium bromide uptake was increased by extracellular alkalization and reduced by extracellular acidification. It would be intriguing to test whether a similar regulation by pH can be observed in other Panx1 homologues and by using other measurements of Panx1 function (i.e., ATP release or ionic current); in addition, it would be important to determine whether pH regulation represents an intrinsic mechanism that involves direct channel titration by protons, or whether it reflects effects mediated indirectly by other channel modulators that are sensitive to the prevailing pH.
Activation mode-dependent unitary conductance and ATP permeation
Unique single-channel properties, such as unitary conductance or activation/inactivation kinetics, are often considered a signature for specific ion channels and provide fundamental information for identifying and characterizing ion channels in their native environment. Unlike other channels, however, Panx1 has been associated with strikingly divergent unitary properties. This includes major differences reported for channel conductance, open–closed kinetics, voltage dependence, and permeant selectivity in different modes of activation. Here we discuss different lines of evidence that attribute divergent unitary properties to Panx1 and compare those earlier findings with our recent discovery demonstrating a graded increase in conductance and gating of Panx1 upon sequential removal of C-tails.
Panx1 as large-conductance, ATP-releasing channels
For many years, Panx1 channels have been routinely described as large-conductance (i.e., 300–500 pS), nonselective, voltage-activated ion channels (Bao et al., 2004; Iglesias et al., 2008; Santiago et al., 2011; Kurtenbach et al., 2014). Large-conductance, nonrectifying channels attributed to recombinant Panx1 were initially described in inside-out recordings from Xenopus oocytes injected with human Panx1 cRNA (isoform 2, 422 amino acids) during symmetrical exposure to 150 mM potassium gluconate (Bao et al., 2004; Fig. 1 B). This channel activity was reportedly not observed in uninjected oocytes. In addition, potassium gluconate–induced ATP release is significantly higher in Panx1 cRNA-injected oocytes than in Cx43 cRNA–injected or uninjected oocytes (Bao et al., 2004). In the same study, Bao et al. (2004) also suggested that ATP can permeate through Panx1 channels, because they observed a reversal potential of the channels in asymmetrical concentration of K2ATP that could not be explained by an exclusive permeation of K+. Similar large-conductance channels were observed in (a) rat hippocampal neurons during oxygen-glucose deprivation (∼530 pS; Thompson et al., 2006; Fig. 1 E), conditions that also activated a probenecid- and 10Panx1-sensitive whole-cell current (Weilinger et al., 2012); (b) human erythrocytes subjected to high-K+ or hypertonic challenge (∼450 pS; Locovei et al., 2006a); and (c) rat atrial cardiac myocytes upon caffeine stimulation, only under conditions when Panx1 was exogenously expressed (∼300 pS; Kienitz et al., 2011; Fig. 1 D). This evidence supported a very large unitary conductance as a common characteristic for Panx1 channels.
However, even though a large, nonrectifying conductance was a common feature shared by these recorded channels, it is noteworthy that they displayed other single-channel properties that were quite dissimilar. First, the high-K+–activated Panx1 channels visited multiple subconductance states (Bao et al., 2004; Fig. 1 B) whereas the ischemia- or caffeine-induced channels appeared to have only a single conductance state (Thompson et al., 2006; Kienitz et al., 2011; Fig. 1, D and E). Second, the caffeine-induced, Ca2+-activated high-conductance channels display short open times that contrast with the prolonged openings observed for either high-K+-activated or ischemia-activated channels (Fig. 1 D vs. Fig. 1, B and E). To date, the reasons for the divergence of unitary properties among the high-conductance Panx1 channels remain unknown and await further exploration.
Panx1 as small-conductance channels that do not release ATP
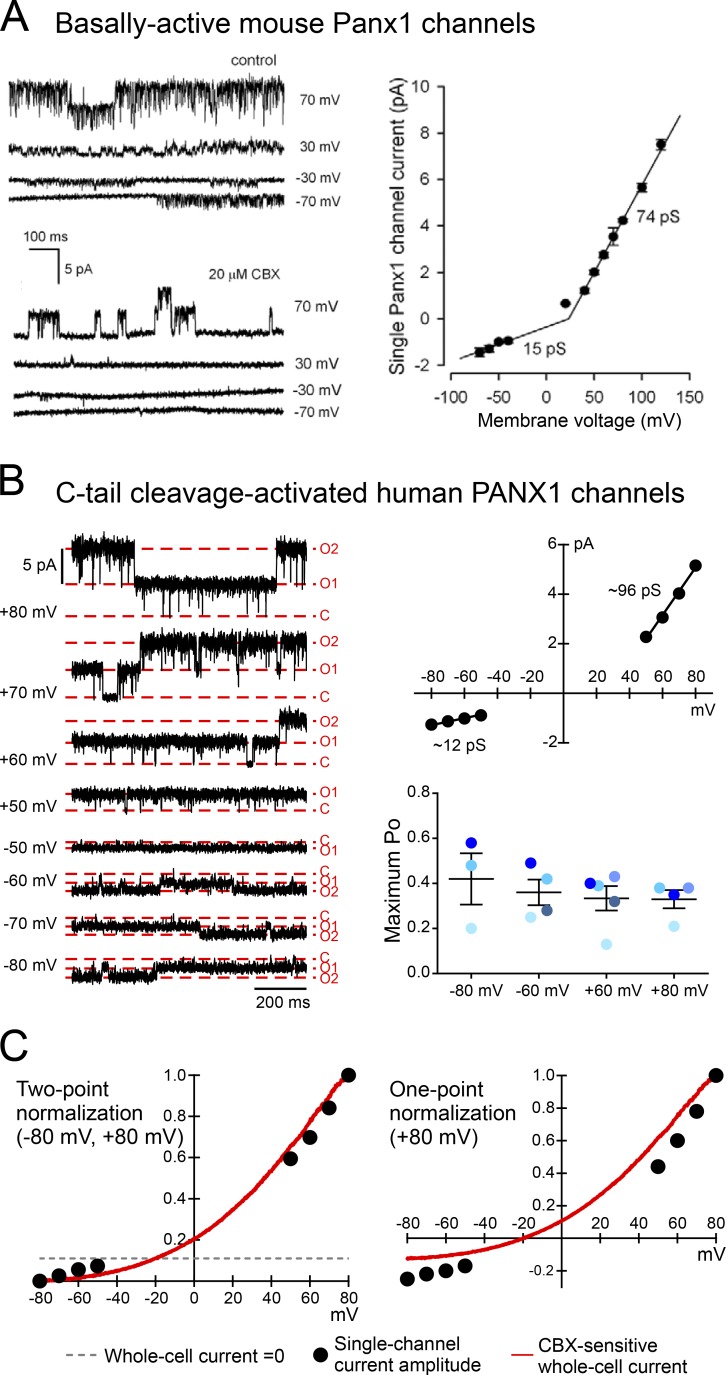
Several recent studies described constitutively active Panx1 channels with a much smaller and outwardly rectifying unitary conductance (i.e., ∼15 pS at hyperpolarized potentials and <100 pS at depolarized potentials) after heterologous expression in mammalian cells, challenging the common characterization of Panx1 as a large-conductance channel. For example, multiple laboratories have reported inside-out or cell-attached recording of wild-type mouse Panx1 channels expressed heterologously in HEK293 cells with a unitary conductance of ∼70 pS at depolarized potentials and ∼15 pS at hyperpolarized potentials (Ma et al., 2012; Romanov et al., 2012; Fig. 3 A). These relatively small-conductance Panx1 channels were recorded in the absence of additional stimulation; they were reportedly anion-selective (Ma et al., 2012; Romanov et al., 2012) and unable to release ATP at detectable levels (Romanov et al., 2012).
Figure 3.
Panx1 channels of ≤100 pS and outwardly rectifying unitary conductance. (A) Single-channel activity and outwardly rectifying unitary conductance of basally active mouse Panx1 heterologously expressed in HEK293 cells (adapted from Romanov et al., 2012). (B) Single-channel activity of human PANX1 heterologously expressed in HEK293T cells, activated by caspase 3–mediated C-tail cleavage in an inside-out configuration (left). The C-tail cleavage-activated channels demonstrated an outwardly rectifying unitary conductance (top right), whereas the open probability (PO) remained unchanged across a wide range of membrane voltage (bottom right). Figures were adapted from Chiu et al. (2017). (C) Unitary current amplitudes closely overlay CBX-sensitive whole-cell currents using two-point normalization (left), suggesting that the outwardly rectifying whole-cell current is mainly attributed to the outwardly rectifying unitary conductance. Note that the same data points are not well aligned when normalized to the peak current amplitude at 80 mV (right). All figures are reproduced with permission.
The discrepancies observed in unitary conductance (300∼500 pS vs. maximum of ∼70 pS) may stem from their relative states of activation. Indeed, it was suggested based on single-particle electron microscopy that mouse Panx1 channels exist basally as a small-pore, small-conductance channel that cannot release ATP and transition to a large-pore, large-conductance channel when activated by high extracellular K+ to release ATP (Wang et al., 2014). The cation/anion selectivity was not directly examined under different K+ concentrations. Nevertheless, a general conclusion was advanced that only large-conductance Panx1 channels are capable of permeating large molecules, such as ATP or fluorescent dyes (Wang et al., 2014). As discussed in the next section, this does not appear to be the case.
Large conductance is not a prerequisite for ATP permeation
The suggestion that large molecule permeation (ATP, dye) is only a property of large-conductance Panx1 channels is not supported by recent work. Specifically, by examining native and engineered human Panx1 channels, our group demonstrated that smaller-conductance Panx1 channels are compatible with release of ATP or permeation of large dyes (Chiu et al., 2017).
We found that cleavage-activated recombinant human PANX1 channels recorded under inside-out conditions displayed an outwardly rectifying unitary conductance, with a maximum of ∼96 pS at depolarized potentials (between 50 and 80 mV; Fig. 3 B) and ∼15 pS at hyperpolarized potentials (between −50 and −80 mV; Fig. 3 B); native hPANX1 channels activated by caspase cleavage in a lymphocyte cell line (Jurkat cells) revealed generally similar properties, but with a slightly lower peak conductance under cell-attached recording conditions (∼80 pS; Chiu et al., 2017). In addition, we found that caspase-mediated cleavage activates PANX1 channels in a stepwise manner; this was clearly demonstrated by sequentially removing C-tails from individual subunits in the oligomeric channel, which led to graded increases in both unitary conductance and open probability. A corresponding graded increase in permeation of both ATP and fluorescent dyes (i.e., TO-PRO-3) was observed from PANX1 channels as more C-tails were removed, even from channels that displayed maximum conductance ranging from ∼50 to ∼96 pS. Of note, because of the pronounced outward rectification in cleavage-activated channels, the unitary conductance is even smaller at the negative potentials expected under conditions in which ATP release and dye uptake were measured. Thus, a large unitary conductance is not required for ATP release or dye uptake via activated PANX1 channels. Finally, because both ATP (negatively charged) and TO-PRO-3 (positively charged) permeate through cleavage-activated Panx1 channels, it is unlikely that these lower-conductance channels could be strictly anion selective.
Release of ATP by smaller-conductance Panx1 channels is not unique to C-tail cleavage-mediated activation. In human PANX1 channels activated by α1D adrenergic receptor signaling, a similar conductance (∼80 pS) also supports ATP release (Billaud et al., 2015). Intriguingly, in the absence of additional activation, mouse Panx1 channels show constitutive activity with a unitary conductance comparable to ATP-releasing human PANX1 channels partially activated by C-tail cleavage (Ma et al., 2012; Romanov et al., 2012; Wang et al., 2014; Chiu et al., 2017). However, these basally active mouse Panx1 channels appear to be ATP-impermeable (Romanov et al., 2012; Wang et al., 2014; Billaud et al., 2015). Together, these data indicate that ATP release by Panx1 does not require formation of large-conductance channels but is dependent on the activation status of the channels. Thus, by itself, single-channel conductance is not a reliable indicator of large molecule permeation; the specific changes in channel properties that allow for ATP release after different activation mechanisms remain enigmatic.
In summary, Panx1 channels appear to display different properties under basal, unstimulated conditions and when activated by different mechanisms. Although this may well be the case, caveats abound. In many instances, especially in native systems, but even also in some heterologous contexts, the recorded channels have not been identified definitively as Panx1. In native systems, this is largely a function of imperfect pharmacology and/or failure to apply existing molecular tools to verify that recorded channels are indeed Panx1. Future work attempting to reconcile these discrepancies should use a combination of currently available tools, especially when studying native channels. For example, even though multiple pharmacological and molecular approaches were used to implicate Panx1 as the ischemia- or caffeine-activated channels in cardiomyocytes (e.g., Panx1 inhibitors, siRNA knockdown, viral-mediated expression of exogenous Panx1; Kienitz et al., 2011), a more compelling identification could be obtained by using the now widely available Panx1 knockout mice.
Panx1 channel gating is voltage independent
Another discrepancy regarding Panx1 channel properties within the extant scientific literature is the question of whether the channels are gated by changes in membrane potential, i.e., voltage-gated. Whereas it is widely recognized that Panx1 channels generate a voltage-dependent current, whereby outward currents at positive membrane potentials are relatively larger than inward currents at negative potentials, such voltage dependence can reflect effects of membrane potential on single-channel conductance, channel open probability, or both. However, only the latter effect on PO constitutes voltage gating. This is not a semantic issue because it has clear mechanistic implications: voltage gating implies the existence of a “voltage sensor” that can react to changes in membrane potential to initiate conformational changes that favor either open or closed states of the channel. Given that Panx1 channels typically mediate voltage-dependent, outwardly rectifying whole-cell currents (Bruzzone et al., 2003; Romanov et al., 2012; Sandilos et al., 2012; Jackson et al., 2014), and in light of early observations suggesting that channel unitary conductance of Panx1 was linear and unaffected by membrane potential (Bao et al., 2004; Thompson et al., 2006; Fig. 1, B and E), it was reasonable to suggest that voltage-dependent currents reflected an underlying voltage gating of the channel. This view garnered indirect support when normalized current–voltage (I-V) relationships of single-channel amplitudes from basally active mouse Panx1 (i.e., unitary conductance) were out of register with superimposed I-V relationships of Panx1 whole-cell currents. It was suggested that this misalignment of the single-channel and whole-cell I-V curves could be explained if channel PO is reduced at negative membrane potential, i.e., if Panx1 channels are voltage-gated (Romanov et al., 2012). A recent study also suggested that the PO of basally active mouse Panx1 increased with membrane depolarization and invoked an unconventional mechanism in which voltage-dependent anion flux modulates gating (Nomura et al., 2017).
A different conclusion was reached, however, when voltage gating was assessed by direct measurement of channel PO in human PANX1 channels activated by C-terminal cleavage. Specifically, those cleavage-activated channels display essentially identical PO over a wide range of membrane potentials (i.e., from −80 to 80 mV; Fig. 3 B), indicating that channel gating is independent of voltage (Chiu et al., 2017). Although one could argue that caspase-mediated cleavage of the C-tail might eliminate the voltage-gating mechanism, this is not supported by the observation that whole-cell I-V relationships were unaltered in Panx1 channels that retain varying numbers of C-tails. In addition, the unitary conductance and whole cell I-V relationships of human PANX1 channels show essentially identical outward rectification when activated by caspase cleavage (C-tail removed) or α1-adrenoceptor signaling (C-tail intact; Chiu et al., 2017). These observations suggest that it is unlikely that the C-tail of PANX1 is responsible for voltage-dependent gating. In sum, direct measures of PO provide little evidence for voltage-dependent gating of human PANX1 channels.
In light of this, we reexamined the data and assumptions inherent in the previous indirect analysis that inferred voltage-gating of Panx1 based on comparisons between normalized single-channel and whole cell I-V relationships and identified a critical factor that likely yielded misleading information (Romanov et al., 2012). That analysis involved a normalization procedure that was based on a single data point (i.e., peak current at 80 mV); this single-point normalization does not recognize the outwardly rectifying nature of the whole-cell and single-channel currents, and disproportionally skews the normalized single channel I-V relationship, especially at hyperpolarized potentials. By using a more appropriate two-point normalization procedure and making comparisons with the CBX-sensitive whole cell current component, we found that unitary conductance and whole cell currents of C-terminally cleaved human PANX1 are closely aligned across a wide range of voltages (Fig. 3 C). This indicates that the single channel rectification can account essentially entirely for the whole-cell rectification and obviates any need to invoke voltage-gating of the channel. Moreover, given that Panx1 channels allow both cation and anion permeation (Pelegrin and Surprenant, 2006; Santiago et al., 2011; Seminario-Vidal et al., 2011), the unconventional voltage-gating mechanism recently proposed for the channels, which depends on anionic selectivity, should receive additional scrutiny (Nomura et al., 2017).
A structure–function model for Panx1 activation by displacement of C-tails
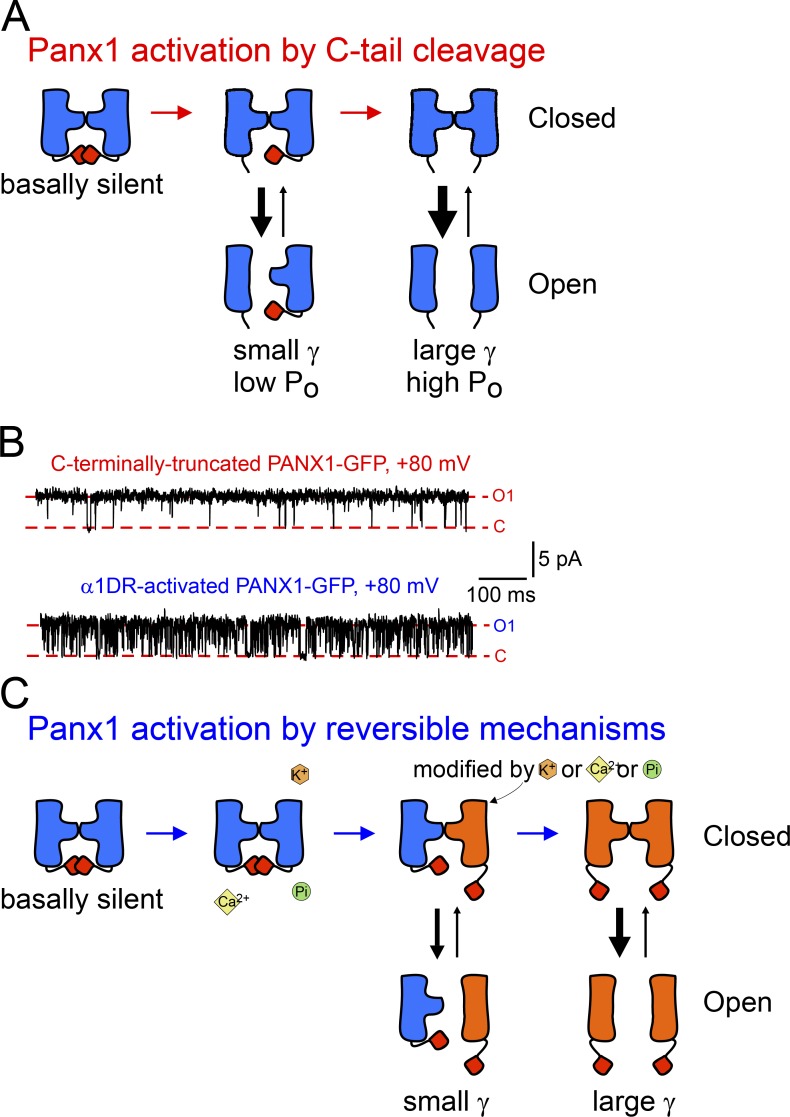
Using the substituted cysteine accessibility method (SCAM) to analyze inhibitory effects of thiol-modifying reagents on mouse Panx1 current in Xenopus oocytes, Wang and Dahl (2010) suggested that a region near the first TM domain and the first extracellular loop forms the outer pore lining, and the C-tail is localized within the pore itself. In agreement with this work, our group later demonstrated that currents from C-terminally truncated PANX1 channels were inhibited by overexpression of C-tails, suggesting that they have an autoinhibitory role (Sandilos et al., 2012), and that physical dissociation of the autoinhibitory C-tail is necessary for cleavage-mediated PANX1 activation (Sandilos et al., 2012). By analyzing unitary properties of Panx1 channels, we further demonstrated that both unitary conductance and PO, along with ATP/dye permeation, are gradually increased when C-tails are sequentially removed from the channel complex (Chiu et al., 2017). These observations led us to propose a structure–function model that envisions the process of Panx1 activation by either irreversible or reversible displacement of C-tails. In such a model (Fig. 4 A), increased conductance may reflect reduced steric hindrance associated with C-tail removal; such a steric effect is implied by the stepwise increase in single-channel currents as additional tails were removed, and the observation that passage of small ions (i.e., current) occurred when only a single C-tail was removed but permeation to larger molecules required removal of at least two C-tails (Chiu et al., 2017). Cleavage of the C-tails may also decrease resistance by shortening the length or increasing the width of the permeation pathway. Note, however, that C-tails appear to have a negligible effect on ion selectivity, as indicated by a similar reversal potential among channels containing varying numbers of intact C-tails (Chiu et al., 2017).
Figure 4.
A general model for Panx1 activation by progressive displacement of autoinhibitory C-tails. (A) Diagram depicts an irreversible activation of Panx1 channels by progressive removal of C-tails. (B) C-tail cleavage-activated human PANX1 channels showed longer open time (top) in contrast to α1D receptor-activated PANX1 channels that demonstrated flickering openings (bottom). (C) A proposed model for activation of Panx1 channels by reversible mechanisms, involving sequential posttranslational modifications or binding of extracellular K+ or intracellular Ca2+ on Panx1 subunits. All figures are adapted from Chiu et al. (2017) and reproduced with permission.
In addition to the quantized increases in unitary conductance, removal of C-tails also increased channel open probability in a stepwise fashion. Even so, it is clear that the C-tails cannot form the actual channel gate because fully cleaved channels transition between open and closed states, with a maximum PO of ∼0.4. Interestingly, human PANX1 channels activated by α1 adrenoceptors achieve a unitary conductance similar to that of fully cleaved channels, despite their intact C termini. The major difference is that α1 receptor–activated channels show substantially shorter open time than C-tail–cleaved channels (Chiu et al., 2017; Fig. 4 B). The more flickering behavior of α1 adrenoceptor–activated channels may reflect repeated association and dissociation of C-tails from the channel pore (e.g., in response to rapid addition or removal of the posttranslational modifications) or a reduced binding affinity caused by stable posttranslational modifications. On the other hand, the flickering opening may be caused by allosteric effects on Panx1 channel gating by receptor-induced posttranslational modifications (Chiu et al., 2017; Fig. 4 C). Interestingly, ATP permeation has been reported in α1D adrenoceptor-activated mouse Panx1 despite similar unitary conductance of the unstimulated and receptor-activated channels (Billaud et al., 2015). High-resolution, 3-D structures of Panx1 channels activated by different mechanisms would help reveal the specific conformations that account for these different channel properties.
Remaining questions for activation of Panx1 channels
Several questions remain unanswered regarding the various gating mechanisms of Panx1 channels, especially for reversible modes of channel activation such as high extracellular K+ or NMDAR-Src signaling. For example, does the model of C-tail displacement apply in these circumstances, and/or does high K+ or tyrosine phosphorylation activate Panx1 channels in a progressive manner that reflects sequential subunit modification? For NMDAR-Src signaling, where there appears to be basal phosphorylation of the unstimulated channel, is there a specific number of phospho-tyrosines that support channel activation? For these and similar questions, a comprehensive analyses of the unitary properties of Panx1 channels activated by these mechanisms is required.
Another set of intriguing questions relate to differences between mouse and human Panx1 channels. Unlike human homologues, mouse Panx1 is basally active without additional stimulation (Ma et al., 2012; Romanov et al., 2012; Sandilos et al., 2012; Jackson et al., 2014); this may be partially explained by a nonconserved region following the C-terminal caspase cleavage site (Sandilos et al., 2012). Most puzzling, however, is the observation that unstimulated mouse Panx1 channels have conductance properties that appear to be quite similar to those of human PANX1 after cleavage or α1 adrenoceptor activation: the basally active channels are also outwardly rectifying, with a peak conductance ∼70 pS. The flickering behavior and the mean open time of unstimulated mouse Panx1 channels resemble the gating kinetics of full-length human Panx1 channels activated by α1 adrenoceptors (Romanov et al., 2012; Chiu et al., 2017; Nomura et al., 2017). Nevertheless, despite unitary properties that are generally similar to receptor-activated human PANX1, the unstimulated mouse Panx1 channels do not appear to support ATP release or dye uptake (Romanov et al., 2012; Wang et al., 2014; Billaud et al., 2015). The reasons for this are currently not known. It is possible that the lower PO or shorter open time of unstimulated mouse Panx1 is incompatible with permeation of ATP or fluorescent dyes.
Nevertheless, given that similar gating mechanisms are likely shared by mouse and human Panx1, further exploration of the structural and biophysical differences between the mouse and human homologues may provide better understanding of Panx1 gating. In addition, it is known that mouse Panx1 can be further activated by caspase-mediated cleavage or by α1 adrenoceptor signaling (Qu et al., 2011; Billaud et al., 2015). Whether mouse Panx1 channels undergo a graded increase in both unitary conductance and PO in response to various activators requires further analysis. Aside from a relative hypotension during the active period, genetic deletion of Panx1 in mice is generally benign in the absence of an insult or physiological challenge (e.g., with ischemia-reperfusion or nerve injury; Bargiotas et al., 2011; Billaud et al., 2015; Weaver et al., 2017). Are native mouse Panx1 channels basally silent in vivo because of unidentified inhibitory mechanisms? Do native Panx1 channels contribute to cellular homeostasis and membrane potential? Also note that Panx1 orthologues from different species (e.g., rat, mouse, or human) have very different splice variants (Bruzzone et al., 2003; Ma et al., 2009; Li et al., 2011), and caution should be used when extrapolating between these different channel orthologues.
Outlook
Studies of Panx1 are drawing tremendous attention in various fields because of the diverse (patho)physiological functions of the channel. Although many channel activation mechanisms have been described, other regulatory mechanisms remain relatively unexplored. For example, little is known regarding transcriptional or translational regulation (Dufresne and Cyr, 2014; Zhang et al., 2015), channel protein trafficking and degradation (Boassa et al., 2007, 2008; Gehi et al., 2011), or context-specific interactomes. In addition, current views on Panx1 have largely focused on its role in purinergic signaling; however, its role as an ion channel, affecting cell membrane potential dynamics, or its potential ability to release other large molecules, such as glutamate, osmolytes, or other electro-neutral permeants, remain to be examined. To date, Panx1 activity has been investigated primarily by loss-of-function approaches, either pharmacological or genetic, and there may be great value in taking the converse approach if Panx1 channel activators could be discovered. Moreover, efforts to elucidate the biophysical mechanisms for various inhibitors and to search for specific activators would provide new information regarding channel function, and may present a potential avenue for designing better agents to treat various Panx1-related defects in a context-dependent manner.
Acknowledgments
This work was supported by grants from the National Institutes of Health: R01 GM107848 (to D.A. Bayliss) and P01 HL120840 (to D.A. Bayliss and B.N. Desai).
The authors declare no competing financial interests.
Lesley C. Anson served as editor.
References
- Abascal F., and Zardoya R.. 2012. LRRC8 proteins share a common ancestor with pannexins, and may form hexameric channels involved in cell-cell communication. BioEssays. 34:551–560. 10.1002/bies.201100173 [DOI] [PubMed] [Google Scholar]
- Adamson S.E., Meher A.K., Chiu Y.H., Sandilos J.K., Oberholtzer N.P., Walker N.N., Hargett S.R., Seaman S.A., Peirce-Cottler S.M., Isakson B.E., et al. 2015. Pannexin 1 is required for full activation of insulin-stimulated glucose uptake in adipocytes. Mol. Metab. 4:610–618. 10.1016/j.molmet.2015.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrosi C., Gassmann O., Pranskevich J.N., Boassa D., Smock A., Wang J., Dahl G., Steinem C., and Sosinsky G.E.. 2010. Pannexin1 and Pannexin2 channels show quaternary similarities to connexons and different oligomerization numbers from each other. J. Biol. Chem. 285:24420–24431. 10.1074/jbc.M110.115444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anselmi F., Hernandez V.H., Crispino G., Seydel A., Ortolano S., Roper S.D., Kessaris N., Richardson W., Rickheit G., Filippov M.A., et al. 2008. ATP release through connexin hemichannels and gap junction transfer of second messengers propagate Ca2+ signals across the inner ear. Proc. Natl. Acad. Sci. USA. 105:18770–18775. 10.1073/pnas.0800793105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao L., Locovei S., and Dahl G.. 2004. Pannexin membrane channels are mechanosensitive conduits for ATP. FEBS Lett. 572:65–68. 10.1016/j.febslet.2004.07.009 [DOI] [PubMed] [Google Scholar]
- Baranova A., Ivanov D., Petrash N., Pestova A., Skoblov M., Kelmanson I., Shagin D., Nazarenko S., Geraymovych E., Litvin O., et al. 2004. The mammalian pannexin family is homologous to the invertebrate innexin gap junction proteins. Genomics. 83:706–716. 10.1016/j.ygeno.2003.09.025 [DOI] [PubMed] [Google Scholar]
- Bargiotas P., Krenz A., Hormuzdi S.G., Ridder D.A., Herb A., Barakat W., Penuela S., von Engelhardt J., Monyer H., and Schwaninger M.. 2011. Pannexins in ischemia-induced neurodegeneration. Proc. Natl. Acad. Sci. USA. 108:20772–20777. 10.1073/pnas.1018262108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckel J.M., Daugherty S.L., Tyagi P., Wolf-Johnston A.S., Birder L.A., Mitchell C.H., and de Groat W.C.. 2015. Pannexin 1 channels mediate the release of ATP into the lumen of the rat urinary bladder. J. Physiol. 593:1857–1871. 10.1113/jphysiol.2014.283119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhalla-Gehi R., Penuela S., Churko J.M., Shao Q., and Laird D.W.. 2010. Pannexin1 and pannexin3 delivery, cell surface dynamics, and cytoskeletal interactions. J. Biol. Chem. 285:9147–9160. 10.1074/jbc.M109.082008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billaud M., Lohman A.W., Straub A.C., Looft-Wilson R., Johnstone S.R., Araj C.A., Best A.K., Chekeni F.B., Ravichandran K.S., Penuela S., et al. 2011. Pannexin1 regulates α1-adrenergic receptor-mediated vasoconstriction. Circ. Res. 109:80–85. 10.1161/CIRCRESAHA.110.237594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billaud M., Chiu Y.H., Lohman A.W., Parpaite T., Butcher J.T., Mutchler S.M., DeLalio L.J., Artamonov M.V., Sandilos J.K., Best A.K., et al. 2015. A molecular signature in the pannexin1 intracellular loop confers channel activation by the α1 adrenoreceptor in smooth muscle cells. Sci. Signal. 8:ra17 10.1126/scisignal.2005824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boassa D., Ambrosi C., Qiu F., Dahl G., Gaietta G., and Sosinsky G.. 2007. Pannexin1 channels contain a glycosylation site that targets the hexamer to the plasma membrane. J. Biol. Chem. 282:31733–31743. 10.1074/jbc.M702422200 [DOI] [PubMed] [Google Scholar]
- Boassa D., Qiu F., Dahl G., and Sosinsky G.. 2008. Trafficking dynamics of glycosylated pannexin 1 proteins. Cell Commun. Adhes. 15:119–132. 10.1080/15419060802013885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boassa D., Nguyen P., Hu J., Ellisman M.H., and Sosinsky G.E.. 2015. Pannexin2 oligomers localize in the membranes of endosomal vesicles in mammalian cells while Pannexin1 channels traffic to the plasma membrane. Front. Cell. Neurosci. 8:468 10.3389/fncel.2014.00468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruzzone R., Hormuzdi S.G., Barbe M.T., Herb A., and Monyer H.. 2003. Pannexins, a family of gap junction proteins expressed in brain. Proc. Natl. Acad. Sci. USA. 100:13644–13649. 10.1073/pnas.2233464100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruzzone R., Barbe M.T., Jakob N.J., and Monyer H.. 2005. Pharmacological properties of homomeric and heteromeric pannexin hemichannels expressed in Xenopus oocytes. J. Neurochem. 92:1033–1043. 10.1111/j.1471-4159.2004.02947.x [DOI] [PubMed] [Google Scholar]
- Burma N.E., Bonin R.P., Leduc-Pessah H., Baimel C., Cairncross Z.F., Mousseau M., Shankara J.V., Stemkowski P.L., Baimoukhametova D., Bains J.S., et al. 2017. Blocking microglial pannexin-1 channels alleviates morphine withdrawal in rodents. Nat. Med. 23:355–360. 10.1038/nm.4281 [DOI] [PubMed] [Google Scholar]
- Caskenette D., Penuela S., Lee V., Barr K., Beier F., Laird D.W., and Willmore K.E.. 2016. Global deletion of Panx3 produces multiple phenotypic effects in mouse humeri and femora. J. Anat. 228:746–756. 10.1111/joa.12437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celetti S.J., Cowan K.N., Penuela S., Shao Q., Churko J., and Laird D.W.. 2010. Implications of pannexin 1 and pannexin 3 for keratinocyte differentiation. J. Cell Sci. 123:1363–1372. 10.1242/jcs.056093 [DOI] [PubMed] [Google Scholar]
- Chekeni F.B., Elliott M.R., Sandilos J.K., Walk S.F., Kinchen J.M., Lazarowski E.R., Armstrong A.J., Penuela S., Laird D.W., Salvesen G.S., et al. 2010. Pannexin 1 channels mediate ‘find-me’ signal release and membrane permeability during apoptosis. Nature. 467:863–867. 10.1038/nature09413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu Y.H., Jin X., Medina C.B., Leonhardt S.A., Kiessling V., Bennett B.C., Shu S., Tamm L.K., Yeager M., Ravichandran K.S., and Bayliss D.A.. 2017. A quantized mechanism for activation of pannexin channels. Nat. Commun. 8:14324 10.1038/ncomms14324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cone A.C., Ambrosi C., Scemes E., Martone M.E., and Sosinsky G.E.. 2013. A comparative antibody analysis of pannexin1 expression in four rat brain regions reveals varying subcellular localizations. Front. Pharmacol. 4:6 10.3389/fphar.2013.00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’hondt C., Ponsaerts R., De Smedt H., Bultynck G., and Himpens B.. 2009. Pannexins, distant relatives of the connexin family with specific cellular functions? BioEssays. 31:953–974. 10.1002/bies.200800236 [DOI] [PubMed] [Google Scholar]
- Dreses-Werringloer U., Lambert J.C., Vingtdeux V., Zhao H., Vais H., Siebert A., Jain A., Koppel J., Rovelet-Lecrux A., Hannequin D., et al. 2008. A polymorphism in CALHM1 influences Ca2+ homeostasis, Abeta levels, and Alzheimer’s disease risk. Cell. 133:1149–1161. 10.1016/j.cell.2008.05.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufresne J., and Cyr D.G.. 2014. Regulation of the pannexin-1 promoter in the rat epididymis. Biol. Reprod. 91:143 10.1095/biolreprod.114.122168 [DOI] [PubMed] [Google Scholar]
- Dvoriantchikova G., Ivanov D., Barakat D., Grinberg A., Wen R., Slepak V.Z., and Shestopalov V.I.. 2012. Genetic ablation of Pannexin1 protects retinal neurons from ischemic injury. PLoS One. 7:e31991 10.1371/journal.pone.0031991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiori M.C., Figueroa V., Zoghbi M.E., Saéz J.C., Reuss L., and Altenberg G.A.. 2012. Permeation of calcium through purified connexin 26 hemichannels. J. Biol. Chem. 287:40826–40834. 10.1074/jbc.M112.383281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher R.S., Pedley T.A., Moody W.J. Jr., and Prince D.A.. 1976. The role of extracellular potassium in hippocampal epilepsy. Arch. Neurol. 33:76–83. 10.1001/archneur.1976.00500020004002 [DOI] [PubMed] [Google Scholar]
- Furlow P.W., Zhang S., Soong T.D., Halberg N., Goodarzi H., Mangrum C., Wu Y.G., Elemento O., and Tavazoie S.F.. 2015. Mechanosensitive pannexin-1 channels mediate microvascular metastatic cell survival. Nat. Cell Biol. 17:943–952. 10.1038/ncb3194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaitán-Peñas H., Gradogna A., Laparra-Cuervo L., Solsona C., Fernández-Dueñas V., Barrallo-Gimeno A., Ciruela F., Lakadamyali M., Pusch M., and Estévez R.. 2016. Investigation of LRRC8-mediated volume-regulated anion currents in Xenopus oocytes. Biophys. J. 111:1429–1443. 10.1016/j.bpj.2016.08.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehi R., Shao Q., and Laird D.W.. 2011. Pathways regulating the trafficking and turnover of pannexin1 protein and the role of the C-terminal domain. J. Biol. Chem. 286:27639–27653. 10.1074/jbc.M111.260711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gödecke S., Roderigo C., Rose C.R., Rauch B.H., Gödecke A., and Schrader J.. 2012. Thrombin-induced ATP release from human umbilical vein endothelial cells. Am. J. Physiol. Cell Physiol. 302:C915–C923. 10.1152/ajpcell.00283.2010 [DOI] [PubMed] [Google Scholar]
- Hansen A.J. 1978. The extracellular potassium concentration in brain cortex following ischemia in hypo- and hyperglycemic rats. Acta Physiol. Scand. 102:324–329. 10.1111/j.1748-1716.1978.tb06079.x [DOI] [PubMed] [Google Scholar]
- Hanstein R., Negoro H., Patel N.K., Charollais A., Meda P., Spray D.C., Suadicani S.O., and Scemes E.. 2013. Promises and pitfalls of a Pannexin1 transgenic mouse line. Front. Pharmacol. 4:61 10.3389/fphar.2013.00061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglesias R., Locovei S., Roque A., Alberto A.P., Dahl G., Spray D.C., and Scemes E.. 2008. P2X7 receptor-Pannexin1 complex: pharmacology and signaling. Am. J. Physiol. Cell Physiol. 295:C752–C760. 10.1152/ajpcell.00228.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa M., Iwamoto T., Nakamura T., Doyle A., Fukumoto S., and Yamada Y.. 2011. Pannexin 3 functions as an ER Ca(2+) channel, hemichannel, and gap junction to promote osteoblast differentiation. J. Cell Biol. 193:1257–1274. 10.1083/jcb.201101050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto T., Nakamura T., Doyle A., Ishikawa M., de Vega S., Fukumoto S., and Yamada Y.. 2010. Pannexin 3 regulates intracellular ATP/cAMP levels and promotes chondrocyte differentiation. J. Biol. Chem. 285:18948–18958. 10.1074/jbc.M110.127027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson D.G., Wang J., Keane R.W., Scemes E., and Dahl G.. 2014. ATP and potassium ions: A deadly combination for astrocytes. Sci. Rep. 4:4576 10.1038/srep04576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J., Desai B.N., Navarro B., Donovan A., Andrews N.C., and Clapham D.E.. 2008. Deletion of Trpm7 disrupts embryonic development and thymopoiesis without altering Mg2+ homeostasis. Science. 322:756–760. 10.1126/science.1163493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasawa A., Michalski K., Mikhelzon P., and Kawate T.. 2017. The P2X7 receptor forms a dye-permeable pore independent of its intracellular domain but dependent on membrane lipid composition. eLife. 6:e31186 10.7554/eLife.31186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller A.S., Diederich L., Panknin C., DeLalio L.J., Drake J.C., Sherman R., Jackson E.K., Yan Z., Kelm M., Cortese-Krott M.M., and Isakson B.E.. 2017. Possible roles for ATP release from RBCs exclude the cAMP-mediated Panx1 pathway. Am. J. Physiol. Cell Physiol. 313:C593–C603. 10.1152/ajpcell.00178.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kienitz M.C., Bender K., Dermietzel R., Pott L., and Zoidl G.. 2011. Pannexin 1 constitutes the large conductance cation channel of cardiac myocytes. J. Biol. Chem. 286:290–298. 10.1074/jbc.M110.163477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtenbach S., Prochnow N., Kurtenbach S., Klooster J., Zoidl C., Dermietzel R., Kamermans M., and Zoidl G.. 2013. Pannexin1 channel proteins in the zebrafish retina have shared and unique properties. PLoS One. 8:e77722 10.1371/journal.pone.0077722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtenbach S., Kurtenbach S., and Zoidl G.. 2014. Emerging functions of pannexin 1 in the eye. Front. Cell. Neurosci. 8:263 10.3389/fncel.2014.00263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C.P., Bechberger J.F., and Naus C.C.. 2009. Pannexin2 as a novel growth regulator in C6 glioma cells. Oncogene. 28:4402–4408. 10.1038/onc.2009.283 [DOI] [PubMed] [Google Scholar]
- Le Stunff H., Auger R., Kanellopoulos J., and Raymond M.N.. 2004. The Pro-451 to Leu polymorphism within the C-terminal tail of P2X7 receptor impairs cell death but not phospholipase D activation in murine thymocytes. J. Biol. Chem. 279:16918–16926. 10.1074/jbc.M313064200 [DOI] [PubMed] [Google Scholar]
- Le Vasseur M., Lelowski J., Bechberger J.F., Sin W.C., and Naus C.C.. 2014. Pannexin 2 protein expression is not restricted to the CNS. Front. Cell. Neurosci. 8:392 10.3389/fncel.2014.00392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Tomić M., and Stojilkovic S.S.. 2011. Characterization of novel Pannexin 1 isoforms from rat pituitary cells and their association with ATP-gated P2X channels. Gen. Comp. Endocrinol. 174:202–210. 10.1016/j.ygcen.2011.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locovei S., Bao L., and Dahl G.. 2006a Pannexin 1 in erythrocytes: function without a gap. Proc. Natl. Acad. Sci. USA. 103:7655–7659. 10.1073/pnas.0601037103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locovei S., Wang J., and Dahl G.. 2006b Activation of pannexin 1 channels by ATP through P2Y receptors and by cytoplasmic calcium. FEBS Lett. 580:239–244. 10.1016/j.febslet.2005.12.004 [DOI] [PubMed] [Google Scholar]
- Lohman A.W., and Isakson B.E.. 2014. Differentiating connexin hemichannels and pannexin channels in cellular ATP release. FEBS Lett. 588:1379–1388. 10.1016/j.febslet.2014.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohman A.W., Billaud M., Straub A.C., Johnstone S.R., Best A.K., Lee M., Barr K., Penuela S., Laird D.W., and Isakson B.E.. 2012a Expression of pannexin isoforms in the systemic murine arterial network. J. Vasc. Res. 49:405–416. 10.1159/000338758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohman A.W., Weaver J.L., Billaud M., Sandilos J.K., Griffiths R., Straub A.C., Penuela S., Leitinger N., Laird D.W., Bayliss D.A., and Isakson B.E.. 2012b S-nitrosylation inhibits pannexin 1 channel function. J. Biol. Chem. 287:39602–39612. 10.1074/jbc.M112.397976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohman A.W., Leskov I.L., Butcher J.T., Johnstone S.R., Stokes T.A., Begandt D., DeLalio L.J., Best A.K., Penuela S., Leitinger N., et al. 2015. Pannexin 1 channels regulate leukocyte emigration through the venous endothelium during acute inflammation. Nat. Commun. 6:7965 10.1038/ncomms8965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loirand G., Pacaud P., Baron A., Mironneau C., and Mironneau J.. 1991. Large conductance calcium-activated non-selective cation channel in smooth muscle cells isolated from rat portal vein. J. Physiol. 437:461–475. 10.1113/jphysiol.1991.sp018606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma W., Hui H., Pelegrin P., and Surprenant A.. 2009. Pharmacological characterization of pannexin-1 currents expressed in mammalian cells. J. Pharmacol. Exp. Ther. 328:409–418. 10.1124/jpet.108.146365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma W., Compan V., Zheng W., Martin E., North R.A., Verkhratsky A., and Surprenant A.. 2012. Pannexin 1 forms an anion-selective channel. Pflugers Arch. 463:585–592. 10.1007/s00424-012-1077-z [DOI] [PubMed] [Google Scholar]
- Malhi H., Bronk S.F., Werneburg N.W., and Gores G.J.. 2006. Free fatty acids induce JNK-dependent hepatocyte lipoapoptosis. J. Biol. Chem. 281:12093–12101. 10.1074/jbc.M510660200 [DOI] [PubMed] [Google Scholar]
- Nomura T., Taruno A., Shiraishi M., Nakahari T., Inui T., Sokabe M., Eaton D.C., and Marunaka Y.. 2017. Current-direction/amplitude-dependent single channel gating kinetics of mouse pannexin 1 channel: A new concept for gating kinetics. Sci. Rep. 7:10512 10.1038/s41598-017-10921-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panchin Y., Kelmanson I., Matz M., Lukyanov K., Usman N., and Lukyanov S.. 2000. A ubiquitous family of putative gap junction molecules. Curr. Biol. 10:R473–R474. 10.1016/S0960-9822(00)00576-5 [DOI] [PubMed] [Google Scholar]
- Pelegrin P., and Surprenant A.. 2006. Pannexin-1 mediates large pore formation and interleukin-1beta release by the ATP-gated P2X7 receptor. EMBO J. 25:5071–5082. 10.1038/sj.emboj.7601378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penuela S., Bhalla R., Gong X.Q., Cowan K.N., Celetti S.J., Cowan B.J., Bai D., Shao Q., and Laird D.W.. 2007. Pannexin 1 and pannexin 3 are glycoproteins that exhibit many distinct characteristics from the connexin family of gap junction proteins. J. Cell Sci. 120:3772–3783. 10.1242/jcs.009514 [DOI] [PubMed] [Google Scholar]
- Penuela S., Celetti S.J., Bhalla R., Shao Q., and Laird D.W.. 2008. Diverse subcellular distribution profiles of pannexin 1 and pannexin 3. Cell Commun. Adhes. 15:133–142. 10.1080/15419060802014115 [DOI] [PubMed] [Google Scholar]
- Penuela S., Bhalla R., Nag K., and Laird D.W.. 2009. Glycosylation regulates pannexin intermixing and cellular localization. Mol. Biol. Cell. 20:4313–4323. 10.1091/mbc.E09-01-0067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penuela S., Kelly J.J., Churko J.M., Barr K.J., Berger A.C., and Laird D.W.. 2014. Panx1 regulates cellular properties of keratinocytes and dermal fibroblasts in skin development and wound healing. J. Invest. Dermatol. 134:2026–2035. 10.1038/jid.2014.86 [DOI] [PubMed] [Google Scholar]
- Poon I.K., Chiu Y.H., Armstrong A.J., Kinchen J.M., Juncadella I.J., Bayliss D.A., and Ravichandran K.S.. 2014. Unexpected link between an antibiotic, pannexin channels and apoptosis. Nature. 507:329–334. 10.1038/nature13147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poornima V., Vallabhaneni S., Mukhopadhyay M., and Bera A.K.. 2015. Nitric oxide inhibits the pannexin 1 channel through a cGMP-PKG dependent pathway. Nitric Oxide. 47:77–84. 10.1016/j.niox.2015.04.005 [DOI] [PubMed] [Google Scholar]
- Qiu F., and Dahl G.. 2009. A permeant regulating its permeation pore: Inhibition of pannexin 1 channels by ATP. Am. J. Physiol. Cell Physiol. 296:C250–C255. 10.1152/ajpcell.00433.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu F., Wang J., Spray D.C., Scemes E., and Dahl G.. 2011. Two non-vesicular ATP release pathways in the mouse erythrocyte membrane. FEBS Lett. 585:3430–3435. 10.1016/j.febslet.2011.09.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu F., Wang J., and Dahl G.. 2012. Alanine substitution scanning of pannexin1 reveals amino acid residues mediating ATP sensitivity. Purinergic Signal. 8:81–90. 10.1007/s11302-011-9263-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Z., Dubin A.E., Mathur J., Tu B., Reddy K., Miraglia L.J., Reinhardt J., Orth A.P., and Patapoutian A.. 2014. SWELL1, a plasma membrane protein, is an essential component of volume-regulated anion channel. Cell. 157:447–458. 10.1016/j.cell.2014.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu Y., Misaghi S., Newton K., Gilmour L.L., Louie S., Cupp J.E., Dubyak G.R., Hackos D., and Dixit V.M.. 2011. Pannexin-1 is required for ATP release during apoptosis but not for inflammasome activation. J. Immunol. 186:6553–6561. 10.4049/jimmunol.1100478 [DOI] [PubMed] [Google Scholar]
- Ripps H., Qian H., and Zakevicius J.. 2004. Properties of connexin26 hemichannels expressed in Xenopus oocytes. Cell. Mol. Neurobiol. 24:647–665. 10.1023/B:CEMN.0000036403.43484.3d [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanov R.A., Bystrova M.F., Rogachevskaya O.A., Sadovnikov V.B., Shestopalov V.I., and Kolesnikov S.S.. 2012. The ATP permeability of pannexin 1 channels in a heterologous system and in mammalian taste cells is dispensable. J. Cell Sci. 125:5514–5523. 10.1242/jcs.111062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandilos J.K., Chiu Y.H., Chekeni F.B., Armstrong A.J., Walk S.F., Ravichandran K.S., and Bayliss D.A.. 2012. Pannexin 1, an ATP release channel, is activated by caspase cleavage of its pore-associated C-terminal autoinhibitory region. J. Biol. Chem. 287:11303–11311. 10.1074/jbc.M111.323378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago M.F., Veliskova J., Patel N.K., Lutz S.E., Caille D., Charollais A., Meda P., and Scemes E.. 2011. Targeting pannexin1 improves seizure outcome. PLoS One. 6:e25178 10.1371/journal.pone.0025178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seminario-Vidal L., Kreda S., Jones L., O’Neal W., Trejo J., Boucher R.C., and Lazarowski E.R.. 2009. Thrombin promotes release of ATP from lung epithelial cells through coordinated activation of rho- and Ca2+-dependent signaling pathways. J. Biol. Chem. 284:20638–20648. 10.1074/jbc.M109.004762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seminario-Vidal L., Okada S.F., Sesma J.I., Kreda S.M., van Heusden C.A., Zhu Y., Jones L.C., O’Neal W.K., Penuela S., Laird D.W., et al. 2011. Rho signaling regulates pannexin 1-mediated ATP release from airway epithelia. J. Biol. Chem. 286:26277–26286. 10.1074/jbc.M111.260562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebert A.P., Ma Z., Grevet J.D., Demuro A., Parker I., and Foskett J.K.. 2013. Structural and functional similarities of calcium homeostasis modulator 1 (CALHM1) ion channel with connexins, pannexins, and innexins. J. Biol. Chem. 288:6140–6153. 10.1074/jbc.M112.409789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman W.R., de Rivero Vaccari J.P., Locovei S., Qiu F., Carlsson S.K., Scemes E., Keane R.W., and Dahl G.. 2009. The pannexin 1 channel activates the inflammasome in neurons and astrocytes. J. Biol. Chem. 284:18143–18151. 10.1074/jbc.M109.004804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skarnes W.C., Rosen B., West A.P., Koutsourakis M., Bushell W., Iyer V., Mujica A.O., Thomas M., Harrow J., Cox T., et al. 2011. A conditional knockout resource for the genome-wide study of mouse gene function. Nature. 474:337–342. 10.1038/nature10163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorge R.E., Trang T., Dorfman R., Smith S.B., Beggs S., Ritchie J., Austin J.S., Zaykin D.V., Vander Meulen H., Costigan M., et al. 2012. Genetically determined P2X7 receptor pore formation regulates variability in chronic pain sensitivity. Nat. Med. 18:595–599. 10.1038/nm.2710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosinsky G.E., Boassa D., Dermietzel R., Duffy H.S., Laird D.W., MacVicar B., Naus C.C., Penuela S., Scemes E., Spray D.C., et al. 2011. Pannexin channels are not gap junction hemichannels. Channels (Austin). 5:193–197. 10.4161/chan.5.3.15765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suadicani S.O., Iglesias R., Wang J., Dahl G., Spray D.C., and Scemes E.. 2012. ATP signaling is deficient in cultured Pannexin1-null mouse astrocytes. Glia. 60:1106–1116. 10.1002/glia.22338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swayne L.A., Sorbara C.D., and Bennett S.A.. 2010. Pannexin 2 is expressed by postnatal hippocampal neural progenitors and modulates neuronal commitment. J. Biol. Chem. 285:24977–24986. 10.1074/jbc.M110.130054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syeda R., Qiu Z., Dubin A.E., Murthy S.E., Florendo M.N., Mason D.E., Mathur J., Cahalan S.M., Peters E.C., Montal M., and Patapoutian A.. 2016. LRRC8 proteins form volume-regulated anion channels that sense ionic strength. Cell. 164:499–511. 10.1016/j.cell.2015.12.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson R.J., Zhou N., and MacVicar B.A.. 2006. Ischemia opens neuronal gap junction hemichannels. Science. 312:924–927. 10.1126/science.1126241 [DOI] [PubMed] [Google Scholar]
- Thompson R.J., Jackson M.F., Olah M.E., Rungta R.L., Hines D.J., Beazely M.A., MacDonald J.F., and MacVicar B.A.. 2008. Activation of pannexin-1 hemichannels augments aberrant bursting in the hippocampus. Science. 322:1555–1559. 10.1126/science.1165209 [DOI] [PubMed] [Google Scholar]
- Velasquez S., Malik S., Lutz S.E., Scemes E., and Eugenin E.A.. 2016. Pannexin1 channels are required for chemokine-mediated migration of CD4+ T lymphocytes: Role in inflammation and experimental autoimmune encephalomyelitis. J. Immunol. 196:4338–4347. 10.4049/jimmunol.1502440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss F.K., Ullrich F., Münch J., Lazarow K., Lutter D., Mah N., Andrade-Navarro M.A., von Kries J.P., Stauber T., and Jentsch T.J.. 2014. Identification of LRRC8 heteromers as an essential component of the volume-regulated anion channel VRAC. Science. 344:634–638. 10.1126/science.1252826 [DOI] [PubMed] [Google Scholar]
- Wang J., and Dahl G.. 2010. SCAM analysis of Panx1 suggests a peculiar pore structure. J. Gen. Physiol. 136:515–527. 10.1085/jgp.201010440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Ma M., Locovei S., Keane R.W., and Dahl G.. 2007. Modulation of membrane channel currents by gap junction protein mimetic peptides: size matters. Am. J. Physiol. Cell Physiol. 293:C1112–C1119. 10.1152/ajpcell.00097.2007 [DOI] [PubMed] [Google Scholar]
- Wang J., Jackson D.G., and Dahl G.. 2013. The food dye FD&C Blue No. 1 is a selective inhibitor of the ATP release channel Panx1. J. Gen. Physiol. 141:649–656. 10.1085/jgp.201310966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Ambrosi C., Qiu F., Jackson D.G., Sosinsky G., and Dahl G.. 2014. The membrane protein Pannexin1 forms two open-channel conformations depending on the mode of activation. Sci. Signal. 7:ra69 10.1126/scisignal.2005431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver J.L., Arandjelovic S., Brown G., K Mendu S., S Schappe M., Buckley M.W., Chiu Y.H., Shu S., Kim J.K., Chung J., et al. 2017. Hematopoietic pannexin 1 function is critical for neuropathic pain. Sci. Rep. 7:42550 10.1038/srep42550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y., Wang D., Topczewski F., and Pagliassotti M.J.. 2006. Saturated fatty acids induce endoplasmic reticulum stress and apoptosis independently of ceramide in liver cells. Am. J. Physiol. Endocrinol. Metab. 291:E275–E281. 10.1152/ajpendo.00644.2005 [DOI] [PubMed] [Google Scholar]
- Weilinger N.L., Tang P.L., and Thompson R.J.. 2012. Anoxia-induced NMDA receptor activation opens pannexin channels via Src family kinases. J. Neurosci. 32:12579–12588. 10.1523/JNEUROSCI.1267-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weilinger N.L., Lohman A.W., Rakai B.D., Ma E.M., Bialecki J., Maslieieva V., Rilea T., Bandet M.V., Ikuta N.T., Scott L., et al. 2016. Metabotropic NMDA receptor signaling couples Src family kinases to pannexin-1 during excitotoxicity. Nat. Neurosci. 19:432–442. 10.1038/nn.4236 [DOI] [PubMed] [Google Scholar]
- Xiao F., Waldrop S.L., Khimji A.K., and Kilic G.. 2012. Pannexin1 contributes to pathophysiological ATP release in lipoapoptosis induced by saturated free fatty acids in liver cells. Am. J. Physiol. Cell Physiol. 303:C1034–C1044. 10.1152/ajpcell.00175.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D., He Y., Muñoz-Planillo R., Liu Q., and Núñez G.. 2015. Caspase-11 requires the pannexin-1 channel and the purinergic P2X7 pore to mediate pyroptosis and endotoxic shock. Immunity. 43:923–932. 10.1016/j.immuni.2015.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H., Wang H., Shivalila C.S., Cheng A.W., Shi L., and Jaenisch R.. 2013. One-step generation of mice carrying reporter and conditional alleles by CRISPR/Cas-mediated genome engineering. Cell. 154:1370–1379. 10.1016/j.cell.2013.08.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Z.C., Oberheim N., Kettenmann H., and Ransom B.R.. 2009. Pharmacological “cross-inhibition” of connexin hemichannels and swelling activated anion channels. Glia. 57:258–269. 10.1002/glia.20754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen M.R., and Saier M.H. Jr. 2007. Gap junctional proteins of animals: The innexin/pannexin superfamily. Prog. Biophys. Mol. Biol. 94:5–14. 10.1016/j.pbiomolbio.2007.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Laumet G., Chen S.R., Hittelman W.N., and Pan H.L.. 2015. Pannexin-1 up-regulation in the dorsal root ganglion contributes to neuropathic pain development. J. Biol. Chem. 290:14647–14655. 10.1074/jbc.M115.650218 [DOI] [PMC free article] [PubMed] [Google Scholar]