Abstract
Thalassemia (thal) is an autosomal recessive, hereditary, chronic hemolytic anemia due to a partial or complete deficiency in the synthesis of α-globin chains (α-thal) or β-globin chains (β-thal) that compose the major adult hemoglobin (α 2β 2). It is caused by one or more mutations in the corresponding genes. The unpaired globin chains are unstable; they precipitate intracellularly, resulting in hemolysis, premature destruction of red blood cell [RBC] precursors in the bone marrow, and a short life-span of mature RBCs in the circulation. The state of anemia is treated by frequent RBC transfusions. This therapy results in the accumulation of iron (iron overload), a condition that is exacerbated by the breakdown products of hemoglobin (heme and iron) and the increased iron uptake for the chronic accelerated, but ineffective, RBC production. Iron catalyzes the generation of reactive oxygen species, which in excess are toxic, causing damage to vital organs such as the heart and liver and the endocrine system.
Herein, we review recent findings regarding the pathophysiology underlying the major symptoms of β-thal and potential therapeutic modalities for the amelioration of its complications, as well as new modalities that may provide a cure for the disease.
Keywords: Thalessemia, hemolytic anemia, β-globin
Introduction
Thalassemia (thal) is an autosomal recessive hereditary chronic hemolytic anemia due to a partial or complete deficiency in the synthesis of α-globin chains (α-thal) or β-globin chains (β-thal) which compose the major adult hemoglobin (HbA), a tetramer of α 2β 2. It is caused by one or more of several hundred mutations in the corresponding genes. The unpaired globin chains are unstable; they precipitate intracellularly, resulting in hemolysis, premature destruction (by apoptosis) of red blood cell (RBC) precursors in the bone marrow, and a short life-span of mature RBCs in the circulation. The breakdown products of Hb, heme and iron, catalyze chemical reactions that generate free radicals, including reactive oxygen species (ROS), which in excess are toxic, causing damage to vital organs such as the heart and liver and the endocrine system 1.
Thalassemia is the most common monogenic inherited disease worldwide. Historically, it originated and spread around the Mediterranean, the Middle East, and Southeast Asia, coincidental with the occurrence of malaria (carriers of the thal genes are considered to be resistant to the malaria parasite) 2. Today, because of vast migration, thal patients are present around the globe 3 and their incidence increases steadily.
Beta-thal is classified into three main subgroups based on their clinical expression: major, intermedia, and minor. β-thal major presents itself within the first 2 years of life with severe anemia, poor growth, and skeletal abnormalities and requires regular, lifelong blood transfusions. β-thal intermedia requires only periodic blood transfusions, while β-thal minor does not require a specific treatment. Alpha-thal presents with moderate anemia when there is a significant lack of synthesis of α-globin chains (HbH disease).
There are several combinations of β-thal with other diseases associated with abnormal β-globin, such as HbE and HbS (sickle cell disease) that can be expressed clinically with severe anemia. A combination of β-thal with α-thal results in a milder disease, most likely owing to the less severe α:β imbalance 4.
Thanks to the significant improvement in therapy, patients with β-thal may reach an advanced age. This is associated with clinical symptoms that are the consequence of the disease itself and the treatment modalities.
Herein, we review recent findings regarding the pathophysiology underlying the major symptoms of β-thal and potential therapeutic modalities for the amelioration of its complications, as well as new modalities that may provide a cure for the disease.
Chronic anemia – RBC transfusions
The basic clinical symptom of β-thal is chronic anemia—reduced number of RBCs and their Hb content, resulting from deficiency in Hb production and hemolysis. Chronic anemia is treated by RBC transfusion—in severe cases, every 2 weeks—which affects the patient’s quality of life, may cause recurrent infections and immune reactions, and—above all—iron overload (IO). Iron deposition in vital organs, through the generation of ROS, is a major cause of morbidity and mortality, especially among elderly patients 5.
Potential developments in the field of transfusion (beyond the scope of this review) include, among others, conditions of storage 6, ex vivo production of stem cell-derived RBCs 7, and transfusion of cell-free Hb 8.
Iron overload
The iron status reflects the balance among dietary iron uptake, its storage and mobilization, and its utilization. Iron overload is a common and serious problem in thal 9, as well as in other hereditary and acquired hemolytic anemias 10. The main causes of IO in thal are Hb instability, RBC transfusions, and increased iron absorption from the gastrointestinal tract.
Normally, 1–2 mg of iron is absorbed from the diet per day, with an equivalent amount lost by the turnover of gastrointestinal tract epithelial cells. The body has no mechanism for disposing of excess iron 11; therefore, in thal and other transfusion-dependent anemias, IO may accumulate in a relatively short time (transfusional IO). An RBC transfusion requirement of two units (200–250 mg of iron per unit) per month will result in over 20 g of excess body iron in 4 years 12.
Most of the iron in the body is bound to other molecules. In the plasma, it is bound to transferrin 13. When the transferrin iron-binding capacity is saturated (>80%), non-transferrin-bound iron (NTBI) forms appear 14. Most of the iron gets into cells through their surface transferrin receptor (TfR1) 15, but a small fraction is taken up by non-transferrin pathways 16. In erythroid cells, the incoming iron is mainly incorporated into heme to form the Hb molecule or is stored in ferritin 17. A small fraction remains free or loosely bound to other compounds as the labile iron pool (LIP) 18. The LIP has been suggested as a low-molecular-weight intermediate or transitory pool between extracellular iron and intracellular firmly bound iron 19. The intracellular LIP is redox active, catalyzing the Fenton and Haber-Weiss reactions that generate ROS 20. Excess ROS are cytotoxic through their interaction with cellular components, such as DNA, proteins, and lipids, causing damage to vital organs 21.
Removal of excess iron
Repeated bleeding (phlebotomy) is used to remove excess iron in patients with normal Hb levels, such as in patients with hereditary hemochromatosis, where IO is caused by mutations in the iron homeostasis system 22. Patients after hematopoietic stem cell (HSC) transplantation (HSCT) who had IO prior to transplantation due to multiple transfusions may also benefit from this treatment 23.
Most other IO patients are anemic (Hb <10 g/dL) and, therefore, particularly those who are transfusion dependent, will require iron chelation therapy in order to normalize their iron level (a transferrin saturation of <50% and serum ferritin <500 ng/mL). Iron chelators remove excess iron from the plasma and the cells by binding the labile, chelatable iron, thus facilitating its excretion through the urine and feces.
Three iron chelators are currently in clinical use. Deferoxamine, the first to be used clinically, is given by a slow, continuous, subcutaneous, overnight infusion through a portable pump. While its side effects are minimal, its mode of administration results in low compliance 24. Deferasirox, the first effective oral chelator, is given at 20–30 mg/kg once/day. Adverse effects (occurring in approximately 10% of patients) include nausea, abdominal pain, diarrhea, and rash as well as liver and kidney dysfunction. A new formulation of film-coated tablets provides better patient compliance, since it can be swallowed with a light meal without the need to disperse the tablet into a suspension prior to consumption 25. Deferiprone is an oral iron chelator effective in removing excess iron from the organs and mainly from the heart. The main potential complication is neutropenia that may rarely be followed by agranulocytosis. A liquid formulation has been recently introduced 26.
The efficacy of chelation may be improved by the use of a combination of chelators. Thus, deferiprone may mobilize iron from tissues into the circulation, where deferoxamine binds and facilitates its excretion in the urine (the “shuttle mechanism”) 27.
An additional potential approach to reduce iron load, especially NTBI, is the administration of exogenous iron-free (apo)transferrin through the down regulation of TfR1 28.
In addition to free iron, some iron-containing compounds that are elevated in the plasma of thal patients due to hemolysis, such as free hemin and Hb, are of considerable toxicity. These compounds are neutralized by their scavengers, hemopexin and haptoglobin, respectively. In thal, these proteins are reduced, leaving free, un-neutralized hemin and Hb. The administration of hemopexin/haptoglobin may be suggested to reduce iron toxicity 29.
Modulation of iron absorption
Iron homeostasis is tightly regulated, mainly by hepcidin, a 25-amino-acid peptide which inhibits iron absorption and distribution. It binds to ferroportin, a surface iron exporter on absorptive enterocytes, macrophages, hepatocytes, and placenta cells. This binding induces ferroportin to be internalized and degraded, decreasing, consequently, the export of iron from these cells 30.
The level of hepcidin depends mainly on its rate of production in the liver, which is modulated mainly by the iron status and requirement. Iron loading increases hepcidin production, resulting in reduced intestinal iron absorption while iron deficiency has an opposite effect 30.
In β-thal, in spite of IO, hepcidin production is generally low, and consequently iron absorption is high 31. The reason for this anomaly is the inhibition of hepcidin gene expression caused by two factors. (A) High iron demand by the chronic stress erythropoiesis (see below) 32. Soluble factors such as the growth differentiation factor (GDF)-15 33, twisted gastrulation protein homolog 1 34, soluble transferrin receptor 35, and erythroferrone 36, which are overproduced by the proliferating erythroid precursors, inhibit hepcidin expression. (B) Oxidative stress (see below) due to the inactivation of transcription factors, including CCAAT/enhancer-binding protein a (C/EBPa) and signal transducer and activator of transcription 3 (STAT3) 37, histone deacetylase activation 38, and hypoxia-inducible factors 39. The decrease in hepcidin production is, however, balanced by other conditions prevalent in β-thal, including blood transfusions and inflammation that increase hepcidin production. In the latter case, pro-inflammatory cytokines, such as interleukins 6 and 1, turn on the hepcidin gene promoter by activating the STAT3 pathway 40.
Administration of hepcidin or stimulating its expression could improve IO by decreasing iron absorption. This was demonstrated in murine models of β-thal intermedia by (A) minihepcidins, small peptides that mimic hepcidin activity and act as agonists 41, (B) inhibition of negative regulators of matriptase-2 (TMPRSS6), a key regulator of hepcidin production, with silencing RNAs or antisense oligonucleotides 42, (C) exogenous transferrin through downregulation of TfR1 28, and (D) inhibition of erythroferrone 43.
Dyserythropoiesis
The chronic anemia and its associated hypoxia in thal stimulate increased production of RBCs (chronic stress erythropoiesis). This is mediated by overproduction of erythropoietin (EPO), the main erythropoietic stimulating hormone, and other factors, such as members of the transforming growth factor (TGF)-β and activin receptor-II (ActR-II) trap ligands 44. Binding of EPO to its surface receptor on erythroid precursors activates transduction pathways, including Jak2/Stat5, which inhibit apoptosis and induce proliferation and differentiation of the developing cells. However, this attempt is futile (“ineffective erythropoiesis”) due to oxidative stress-increased apoptosis and abortive differentiation.
Several therapeutic modalities aimed at reducing the dysery-thropoiesis in thal are currently under study and described below.
Activin receptor-II trap ligands
Luspatercept and Sotatercept, compounds that bind to trap ligands and GDF-11, developed in animal models, are currently in clinical trials 44. They prevent activins binding to ActR-II and the activation of the Smad 4-dependent signaling pathway, improving erythroid maturation and RBC production. A phase 3, multicenter, multinational study with luspatercept is ongoing in β-thal and HbE/β-thal subjects, with encouraging preliminary results 45.
JAK2 inhibitors
The EPO signaling of erythropoiesis involves Jak2 phosphorylation. Beta-thal mice have elevated EPO levels associated with increased Jak2 phosphorylation, resulting in ineffective erythropoiesis and extramedullary hematopoiesis 46. Jak2 inhibitors effectively reduce splenomegaly in such mice. Several Jak2 inhibitors have been developed with beneficial results in patients with myelofibrosis and Jak2-related polycythemia vera 47. Jak2 inhibitors could be also beneficial for non-transfusion-dependent thal patients with splenomegaly 48.
Induction of the Hsp70 chaperone machinery
The heat shock protein 70 (Hsp70) is a molecular chaperone needed for normal termination of erythropoiesis 49. It is predominant in the late erythroid precursors when it is translocated to the nucleus and protects the globin transcription factor-1 (GATA-1), the principal transcriptional factor for erythropoiesis, from caspase-3 cleavage 50. In β-thal major, HSP70 is sequestrated in the cytoplasm, leaving GATA-1 unprotected from cleavage, resulting in end-stage maturation arrest and apoptosis 51. Exportins, such as XPO1, are factors that control the nucleocytoplasmic trafficking of proteins and RNAs. The XPO1 inhibitors leptomycin B and KPT 251, recently tested in erythroid progenitors from β-thal major patients, demonstrated induction of HSP70 nuclear localization, GATA-1 expression, and improved terminal erythroid differentiation 52.
Reducing α-globin synthesis
The key pathophysiological mechanism leading to the ineffective erythropoiesis in β-thal is the continuous production and accumulation of free excess α-globin in the erythroid precursors. Indeed, reduction in α-globin chain output through co-inheritance of α-thal ameliorates the disease phenotype in patients with β-thal 53. The challenge here is selective silencing of the α-globin expression to an appropriate degree to be useful for patients with β-thal 54. Plausible approaches include post-transcriptional silencing through RNA interference (RNAi) using small interfering RNAs, short hairpin RNA, epigenetic drug targeting to alter the chromatin environment of the α-globin genes, and genome editing to disrupt the expression of the α-globin genes 55.
Oxidative stress
Although oxidative stress is not the primary etiology of thal, it plays a major role in its pathophysiology. The oxidative status of cells is regulated by the equilibrium between oxidants, such as the reactive oxygen species (ROS) that are produced mainly as byproducts of cellular respiration, and anti-oxidants, such as reduced glutathione. A balanced oxidative state is crucial for normal physiology. ROS serve as regulators in many processes, including proliferation and differentiation of the erythroid precursors. When this balance fails, such as in many pathological processes, oxidative stress ensues. The excess ROS bind to cell components such as the DNA, proteins, and membrane lipids, leading to cytotoxicity 21. In β-thal, oxidative stress is mainly the consequence of the unstable Hbs (hemichromes) and IO and it mediates many of its symptoms due to oxidative damage to RBCs (anemia), platelets, (hypercoagulable state) and leukocytes (recurrent infections) as well as cells in various vital organs (heart and liver) and the endocrine glands 21.
Oxidative stress may be ameliorated by endogenous and exogenous antioxidants. Their effects include scavenging and inactivating ROS and correcting their damage to cellular components. Many antioxidants are supplied by nutrition. A "Mediterranean diet" and moderate wine consumption are thought to have a protective effect ("the French Paradox") due to their high content of antioxidants 56, 57. Antioxidants can also be taken as food additives, either as pure compounds, such as vitamins C and E and Q10, or as crude extracts, such as the fermented papaya preparation and curcumin 9. Using such additives succeeded in ameliorating various parameters of oxidative stress in thal, but a clear clinical benefit, such as reducing transfusion dependence, was less successful. Effective outcome may require a combination of drugs, especially those affecting both the oxidative stress and the IO.
A newly discovered therapeutic target is the interaction between the oxidative state and the processes of erythroid cell proliferation and differentiation, which, as mentioned above, is defective (dyserythropoiesis) in β-thal. Several agents have been tested recently, especially in murine models of β-thal intermedia (β-thal mice). Although promising, the findings of these studies need careful interpretation, given the difference in globin gene composition and erythroid differentiation between human and mouse 58.
The transcription factor forkhead box O3 (Foxo3) is a key player in the development of erythroid precursors. At early stages, it is phosphorylated by proteins of the EPOR-PI3K/AKT/mTOR pathway, translocated out of the nucleus, and remains inactivated. At late stages, it is relocated into the nucleus, gets activated, and induces the production of antioxidants that neutralize ROS to allow efficient erythropoiesis 59, 60.
In β-thal mice, Foxo3 is downregulated in late erythroid precursors owing to hyper-activation of the EPOR-PI3K/AKT/mTOR pathway, which leads to oxidative damage and ineffective erythropoiesis 61.
Rapamycin, an mTOR inhibitor, has been shown in β-thal mice to improve erythroid cell maturation, β-globin production, and anemia through Foxo3 activation 61. In another study, rapamycin increased γ-globin expression and HbF production in cultured erythroid precursors from β-thal intermedia patients 62. Another Foxo3-activating agent is resveratrol (3,5,4’-trihydroxy-trans-stilbene), a non-flavonoid polyphenol that upregulates antioxidants. The use of resveratrol in β-thal mice has been shown to increase RBC survival and Hb levels and reduce reticulocyte count 63. In contrast, a study in double mutant Foxo3 –/–/Th3/1 mice showed that a loss of Foxo3 leads to improved early erythropoiesis 64.
Consequently, further laboratory and clinical investigations are required in this field.
The eukaryotic initiation factor 2 (eIF2) is a factor required for the initiation of translation through the binding of tRNA to the ribosomes. In the erythroid precursors, its activity is regulated by a mechanism involving phosphorylation at its α-subunit by heme-regulated eIF2a kinase (HRI). Stress (such as heme deficiency and oxidative stress) 65 during the late stage of erythroid differentiation activates HRI that coordinates the syntheses of heme and globin. Furthermore, the phosphorylated α-subunit of eIF2 has been demonstrated to turn on the activating transcription factor 4 (ATF4) to diminish oxidative stress in erythroid precursors 66, 67.
This kinase has been found to be decreased in β-thal mice, leading to embryonic lethality 68. Salubrinal, a selective inhibitor of eIF2aP dephosphorylation, has been found to augment the HRI signaling pathway and to reduce the production of hemichromes in β-thal erythroid precursors 67. It has also been shown to increase HbF production with a concomitant decrease of HbA in differentiating human CD34 cells by a post-transcriptional mechanism 69. These findings provide the basis for manipulating the HRI-eIF2aP signaling pathway for the treatment of β-thal.
Peroxiredoxin-2 (Prx2) is an essential antioxidant protein that scavenges and inactivates ROS throughout erythropoiesis. It has been found to be upregulated during both murine and human β-thal 70. Knockout of Prx2 in β-thal mice worsened their phenotype, while administration of fused recombinant PEP1Prx2 ameliorated their symptoms, with activation of the Erk signaling pathway towards Tfr2 and the Sma and Mad (SMAD) system 71.
Heme oxygenase-1 (HO-1) is an enzyme that catalyzes the degradation of heme in response to stress, such as oxidative stress or hypoxia, both of which occur in β-thal 72. Its expression has been found to be augmented in EPO-dependent fetal liver erythropoietic cells from β-thal mice. Administration of tin protoporphyrin IX, an HO-1 inhibitor, improved ineffective erythropoiesis and Hb levels, and decreased spleen size and liver iron 73.
Stimulation of HbF production
During prenatal life in humans, the major Hb is fetal Hb (HbF), a tetramer of α- and γ-globin (α 2γ 2) which is replaced during the first year of life by HbA (α 2β 2) (Hb switching). Thus, the clinical features of β-hemoglobinopathies, including β-thal, are not apparent at birth; only as HbF levels wane are the symptoms manifested 74. Patients with β-thal produce high but variable levels of HbF compared to normal individuals. High levels of HbF ameliorate the severity of the disease, mainly by reducing the surplus of α-globin chains.
These findings have motivated the research of the mechanisms of Hb switching as well as for pharmacological and gene modification modalities to reactivate the expression of the γ-globin genes and production of HbF 75.
Pharmacological approach
Various compounds have been tested in vitro and in animal models for their capacity to reactivate the γ-globin genes 76. Currently, the only compound in clinical use is hydroxyurea, an S-phase cell cycle inhibitor. However, its mechanism of action on HbF remains elusive, a subset of patients are resistant, its effect in β-thal is inferior compared to that of sickle cell disease, and being myelosuppressive necessitates careful monitoring of patients 77.
New agents include those that affect chromatin regulators (such as decitabine on DNA methylation and histone deacetylase inhibitors) and others that affect DNA-binding transcription factors.
Gene modification approach
The patient's HSCs are subjected to gene editing ex vivo and then returned to the patient for reconstitution 78, 79.
Increased production of γ-globin has been accomplished using lentiviral vectors that express a zinc finger protein which interacts with the promoter of the γ-globin gene 80 or by carrying microRNAs that silence its repressors 81, 82.
Two potent transcriptional repressors of γ-globin, BCL11A and ZBTB7A, have been identified. They act with additional trans-acting epigenetic repressive complexes, lineage-defining factors, and developmental programs to silence the γ-globin genes by working on cis-acting sequences at the globin gene loci. Inhibition of these repressors could reactivate γ-globin production in adult patients.
Most of the studies targeted BCL11A. Its inhibiting antisense oligonucleotides were administered in an erythroleukemia series expressing BCL11A and Krüppel-like factor 1 (KLF1) 83, 84. KLF1 activates the expression of the β-globin gene and it plays a role in the transcriptional silencing of the γ-globin gene, possibly through BCL11A 85, 86.
Genome editing of the promoter of BCL11A can be accomplished by several nucleases, such as engineered zinc finger nucleases (ZFNs), transcription activator-like effector nucleases (TALENs), and clustered regularly interspaced short palindromic repeats linked to Cas9 nucleases (CRISPR-Cas9) 87. It has been shown recently that ZFN-driven BCL11A enhancer ablation leads to increased production of HbF in erythroid precursors derived from β-thal HSCs, which could be used for autologous transplantation 88. A similar effect has been achieved with CRISPR-Cas9-mediated BCL11A enhancer inactivation in a human adult-stage erythroid cell line 89.
BCL11A has important roles in physiology; therefore, reducing its expression in vivo requires novel vectors that can restrict its effect to the erythroid lineage 82. Dissection of the erythroid-specific enhancer down to a small region in the gene offers such possibility 90. The same applies to other factors, like KLF1 and MYB, which are involved in HbF production.
One could also consider de-repressing γ-globin expression by forcing interaction of the β-locus control region with the γ-gene using a synthetic DNA-binding protein 91, 92.
Gene therapy
Gene therapy involves in vivo genetic manipulation of the autologous HSCs, which are then transplanted to the patient for reconstitution 78, 79. This approach has focused on two areas. (A) Increasing the production of γ-globin by the addition of its gene, overexpression of its endogenous activating transcription factors, and silencing of its repressors, as discussed above. (B) Increasing the production of β-globin by the addition of a normal gene or correction of the mutated gene. Studies of gene therapy have utilized mainly lentivirus vectors in experimental systems, including cultured CD34 HSCs from β-thal patients and β-thal mouse models. Yet the safety profile of such technologies is still uncertain.
From the current gene-modifying approaches, only β-globin addition has been tried in β-thal patients. Transfusion-dependent βE/β 0 patients have been transplanted with autologous CD34 progenitor cells transduced ex vivo with lentiviral β-globin vectors. To date, there are a total of seven patients who have been treated with encouraging results in terms of engraftment and transfusion independence; long-term follow up will clarify the possible insertional mutagenesis issues 93, 94.
Phase 1 clinical trials have been initiated in order to assess these issues. Early phase, open label clinical trials of LentiGlobin BB305 will assess its efficacy and safety in patients with β-thal major or sickle cell disease. Safety and tolerability of autologous CD34 HSCs transduced with TNS9.3.55 or GLOBE lentiviral vectors are also being assessed in ongoing trials. Preliminary data from the latter trial have been recently presented regarding three patients with β-thal major. All patients showed a satisfactory engraftment, with mild and reversible adverse events 95.
Genomic editing has been demonstrated to modify the β-globin gene. Thus, TALEN-mediated gene correction has been used in induced HSCs from β-thal patients 96.
Allogeneic hematopoietic stem cell transplantation
Allogeneic HSCT (allo-HSCT) is currently the only definitive cure for transfusion-dependent young patients before the development of IO-related tissue damage 97. β-thal major patients with good risk features have a >90% chance of a successful outcome 98, 99, but allo-HSCT in high-risk patients is challenging because of graft rejection and transplant-related mortality 14. Novel modified or reduced-intensity conditioning regimens are being evaluated in an attempt to improve the outcome in such patients with favorable results 100– 102.
Traditionally, fully matched human leukocyte antigen (HLA)-identical siblings have been used as donors, but matched unrelated donors have also been tried in low-risk patients. Bone marrow is the preferable source of HSCs, but HSCs from the peripheral blood and cord blood are also being tried in low-risk cases.
Prevention
In spite of the advent of therapeutic modalities that alleviate the symptoms and may even cure the disease, the incidence of affected newborns is expected to increase. Most of the new cases are in underdeveloped countries where the standard of medical care is low and in communities where consanguinity is high. Therefore, the prevention of the homozygous state presents an important endeavor. Comprehensively preventive programs involve carrier detections, molecular diagnostics, genetic counseling, and prenatal diagnosis. Currently, prenatal diagnosis is performed, for couples at risk, by obtaining fetal material by chorionic villus sampling in the first trimester and by amniocentesis or cordocentesis in the second trimester. An additional procedure for obtaining fetal material is aspiration of celomic fluid (celocentesis) followed by selection of embryo-fetal erythroid precursors by an anti-CD71 MicroBeads method or by direct micromanipulator pickup of the cells selected on the basis of their morphology 103. Molecular analysis, aimed at the detection of mutations in the globin genes, is then performed 104.
Recently, the possibility of safer and cheaper prenatal diagnosis procedures emerged. Fetal-derived genetic material (cells or cell-free DNA) is obtained from the maternal blood and tested. These non-invasive procedures that present no risk to the fetus and reduce cost (no special procedures and staff are required for sampling) may allow future screening for thal as well as other genetic diseases 105.
Indeed, non-invasive prenatal testing using maternal plasma cell-free DNA has already been applied for screening for common chromosomal aneuploidies. Progress has also been made in screening for monogenic diseases, using thal as a model disease. One approach focuses on the detection or exclusion of paternally inherited fetal mutations that are absent from the mother's genome. Testing maternally inherited mutations requires highly sensitive DNA quantification. The relative mutation/haplotype dosage approach might detect fetal mutations even when the parents share the same mutation.
Another approach is pre-implantation genetic diagnosis of cells (usually single cells) that had been biopsied from oocytes/zygotes or embryos generated by in vitro fertilization. With respect to thal, this technique aims at giving birth to an unaffected newborn, and, when relevant, for cord blood cells compatible with an existing affected sibling to support HSCT. Pre-implantation diagnosis precludes the need for abortion 106.
Conclusion
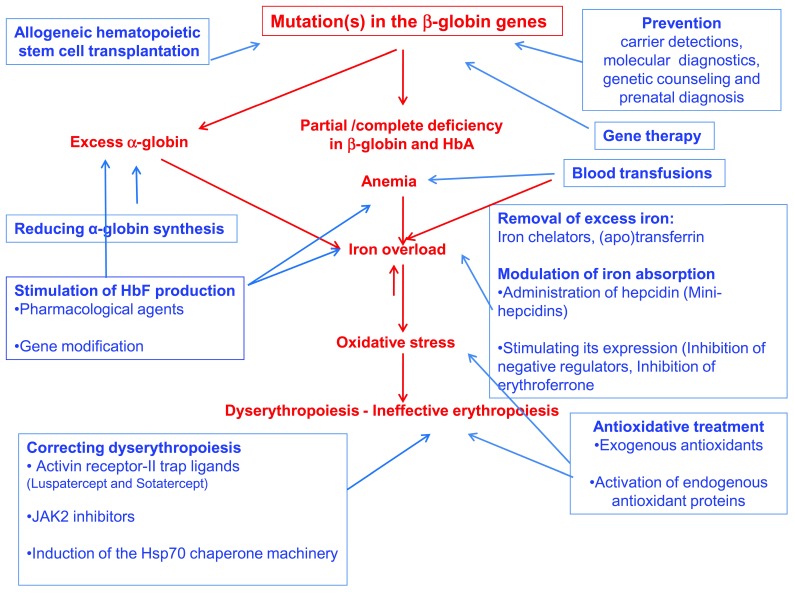
Beta-thalassemia is caused by mutations in the β-globin gene, resulting in partial or complete deficiency of its product. This deficiency and the accompanying excess of the unmatched α-globin chains result in oxidative stress, dyserythropoiesis, and chronic anemia. The main therapeutic modality is blood transfusion that improves the anemia but exacerbates IO. To date, the only curative measure is allo-HSCT. New modalities, aimed at various targets, are being developed. These include the means to stimulate the synthesis of γ-globin and reduce the synthesis of α-globin, as well as the iron excess, oxidative stress, and dyserythropoiesis. Attempts to increase β-globin synthesis focus on gene manipulation. However, the most likely approach to reduce the patients' load is efficient prevention: carrier detection, prenatal diagnosis, and genetic counseling ( Figure 1).
Figure 1. Beta-thalassemia: causes, symptoms, and therapeutic modalities.
Causes and symptoms are marked in red; therapeutic modalities are marked in blue.
Acknowledgements
The article is in memory of Prof. Eliezer A. Rachmilewitz who recently passed away.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
Aurelio Maggio, Division of Haematology II, Villa Sofia-Cervello Hospital, Palermo, Italy
Ali Taher, American University of Beirut Medical Center, Beirut, Lebanon
Funding Statement
The author(s) declared that no grants were involved in supporting this work.
[version 1; referees: 2 approved]
References
- 1. Rund D, Rachmilewitz E: Beta-thalassemia. N Engl J Med. 2005;353(11):1135–46. 10.1056/NEJMra050436 [DOI] [PubMed] [Google Scholar]
- 2. Clegg JB, Weatherall DJ: Thalassemia and malaria: new insights into an old problem. Proc Assoc Am Physicians. 1999;111(4):278–82. 10.1046/j.1525-1381.1999.99235.x [DOI] [PubMed] [Google Scholar]
- 3. Modell B, Darlison M: Global epidemiology of haemoglobin disorders and derived service indicators. Bull World Health Organ. 2008;86(6):480–7. 10.2471/BLT.06.036673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kan YW, Nathan DG: Mild thalassemia: the result of interactions of alpha and beta thalassemia genes. J Clin Invest. 1970;49(4):635–42. 10.1172/JCI106274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Porter JB, Garbowski M: The pathophysiology of transfusional iron overload. Hematol Oncol Clin North Am. 2014;28(4):683–701, vi. 10.1016/j.hoc.2014.04.003 [DOI] [PubMed] [Google Scholar]
- 6. van de Watering L: Red cell storage and prognosis. Vox Sang. 2011;100(1):36–45. 10.1111/j.1423-0410.2010.01441.x [DOI] [PubMed] [Google Scholar]
- 7. Kim HO: In-vitro stem cell derived red blood cells for transfusion: are we there yet? Yonsei Med J. 2014;55(2):304–9. 10.3349/ymj.2014.55.2.304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Winslow RM: Red cell substitutes. Semin Hematol. 2007;44(1):51–9. 10.1053/j.seminhematol.2006.09.013 [DOI] [PubMed] [Google Scholar]
- 9. Fibach E, Rachmilewitz EA: The role of antioxidants and iron chelators in the treatment of oxidative stress in thalassemia. Ann N Y Acad Sci. 2010;1202:10–6. 10.1111/j.1749-6632.2010.05577.x [DOI] [PubMed] [Google Scholar]
- 10. Fibach E, Rachmilewitz EA: Iron overload in hematological disorders. Presse Med.In Press,2017;46(12 Pt 2):e296–e305. 10.1016/j.lpm.2017.10.007 [DOI] [PubMed] [Google Scholar]
- 11. Hentze MW, Muckenthaler MU, Andrews NC: Balancing acts: molecular control of mammalian iron metabolism. Cell. 2004;117(3):285–97. 10.1016/S0092-8674(04)00343-5 [DOI] [PubMed] [Google Scholar]
- 12. Hellström-Lindberg E: Management of anemia associated with myelodysplastic syndrome. Semin Hematol. 2005;42(2 Suppl 1):S10–3. 10.1053/j.seminhematol.2005.01.002 [DOI] [PubMed] [Google Scholar]
- 13. Wang J, Pantopoulos K: Regulation of cellular iron metabolism. Biochem J. 2011;434(3):365–81. 10.1042/BJ20101825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Breuer W, Hershko C, Cabantchik ZI: The importance of non-transferrin bound iron in disorders of iron metabolism. Transfus Sci. 2000;23(3):185–92. 10.1016/S0955-3886(00)00087-4 [DOI] [PubMed] [Google Scholar]
- 15. Richardson DR, Ponka P: The molecular mechanisms of the metabolism and transport of iron in normal and neoplastic cells. Biochim Biophys Acta. 1997;1331(1):1–40. 10.1016/S0304-4157(96)00014-7 [DOI] [PubMed] [Google Scholar]
- 16. Prus E, Fibach E: Uptake of non-transferrin iron by erythroid cells. Anemia. 2011;2011: 945289. 10.1155/2011/945289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Konijn AM: Iron metabolism in inflammation. Baillieres Clin Haematol. 1994;7(4):829–49. 10.1016/S0950-3536(05)80127-1 [DOI] [PubMed] [Google Scholar]
- 18. Prus E, Fibach E: The labile iron pool in human erythroid cells. Br J Haematol. 2008;142(2):301–7. 10.1111/j.1365-2141.2008.07192.x [DOI] [PubMed] [Google Scholar]
- 19. Jacobs A: Low molecular weight intracellular iron transport compounds. Blood. 1977;50(3):433–9. [PubMed] [Google Scholar]
- 20. Jacobs A: An intracellular transit iron pool. Ciba Found Symp. 1976; (51):91–106. [DOI] [PubMed] [Google Scholar]
- 21. Fibach E, Rachmilewitz E: The role of oxidative stress in hemolytic anemia. Curr Mol Med. 2008;8(7):609–19. 10.2174/156652408786241384 [DOI] [PubMed] [Google Scholar]
- 22. Rombout-Sestrienkova E, van Kraaij MG, Koek GH: How we manage patients with hereditary haemochromatosis. Br J Haematol. 2016;175(5):759–70. 10.1111/bjh.14376 [DOI] [PubMed] [Google Scholar]
- 23. Leitch HA, Fibach E, Rachmilewitz E: Toxicity of iron overload and iron overload reduction in the setting of hematopoietic stem cell transplantation for hematologic malignancies. Crit Rev Oncol Hematol. 2017;113:156–70. 10.1016/j.critrevonc.2017.03.002 [DOI] [PubMed] [Google Scholar]
- 24. Haghpanah S, Zarei T, Zahedi Z, et al. : Compliance and satisfaction with deferasirox (Exjade®) compared with deferoxamine in patients with transfusion-dependent beta-thalassemia. Hematology. 2014;19(4):187–91. 10.1179/1607845413Y.0000000121 [DOI] [PubMed] [Google Scholar]
- 25. Taher AT, Origa R, Perrotta S, et al. : New film-coated tablet formulation of deferasirox is well tolerated in patients with thalassemia or lower-risk MDS: Results of the randomized, phase II ECLIPSE study. Am J Hematol. 2017;92(5):420–8. 10.1002/ajh.24668 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 26. Chuansumrit A, Songdej D, Sirachainan N, et al. : Safety profile of a liquid formulation of deferiprone in young children with transfusion-induced iron overload: a 1-year experience. Paediatr Int Child Health. 2016;36(3):209–13. 10.1179/2046905515Y.0000000040 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 27. Vlachodimitropoulou Koumoutsea E, Garbowski M, Porter J: Synergistic intracellular iron chelation combinations: mechanisms and conditions for optimizing iron mobilization. Br J Haematol. 2015;170(6):874–83. 10.1111/bjh.13512 [DOI] [PubMed] [Google Scholar]
- 28. Li H, Rybicki AC, Suzuka SM, et al. : Transferrin therapy ameliorates disease in beta-thalassemic mice. Nat Med. 2010;16(2):177–82. 10.1038/nm.2073 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 29. Vinchi F, Vercellotti GM, Belcher JD, et al. : Elevated systemic heme and iron levels as risk factor for vascular dysfunction and atherosclerosis: Evidence from a beta-thalassemia cohort study. Atherosclerosis. 2017;263:e107–e108. 10.1016/j.atherosclerosis.2017.06.344 [DOI] [Google Scholar]
- 30. Ganz T, Nemeth E: Hepcidin and iron homeostasis. Biochim Biophys Acta. 2012;1823(9):1434–43. 10.1016/j.bbamcr.2012.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gardenghi S, Marongiu MF, Ramos P, et al. : Ineffective erythropoiesis in beta-thalassemia is characterized by increased iron absorption mediated by down-regulation of hepcidin and up-regulation of ferroportin. Blood. 2007;109(11):5027–35. 10.1182/blood-2006-09-048868 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 32. De Franceschi L, Daraio F, Filippini A, et al. : Liver expression of hepcidin and other iron genes in two mouse models of beta-thalassemia. Haematologica. 2006;91(10):1336–42. [PubMed] [Google Scholar]
- 33. Tanno T, Bhanu NV, Oneal PA, et al. : High levels of GDF15 in thalassemia suppress expression of the iron regulatory protein hepcidin. Nat Med. 2007;13(9):1096–101. 10.1038/nm1629 [DOI] [PubMed] [Google Scholar]
- 34. Gu S, Song X, Zhao Y, et al. : The evaluation of iron overload through hepcidin level and its related factors in myelodysplastic syndromes. Hematology. 2013;18(5):286–94. 10.1179/1607845412Y.0000000064 [DOI] [PubMed] [Google Scholar]
- 35. Fertrin KY, Lanaro C, Franco-Penteado CF, et al. : Erythropoiesis-driven regulation of hepcidin in human red cell disorders is better reflected through concentrations of soluble transferrin receptor rather than growth differentiation factor 15. Am J Hematol. 2014;89(4):385–90. 10.1002/ajh.23649 [DOI] [PubMed] [Google Scholar]
- 36. Kautz L, Jung G, Valore EV, et al. : Identification of erythroferrone as an erythroid regulator of iron metabolism. Nat Genet. 2014;46(7):678–84. 10.1038/ng.2996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Choi SO, Cho YS, Kim HL, et al. : ROS mediate the hypoxic repression of the hepcidin gene by inhibiting C/EBPalpha and STAT-3. Biochem Biophys Res Commun. 2007;356(7):312–7. 10.1016/j.bbrc.2007.02.137 [DOI] [PubMed] [Google Scholar]
- 38. Miura K, Taura K, Kodama Y, et al. : Hepatitis C virus-induced oxidative stress suppresses hepcidin expression through increased histone deacetylase activity. Hepatology. 2008;48(5):1420–9. 10.1002/hep.22486 [DOI] [PubMed] [Google Scholar]
- 39. Peyssonnaux C, Zinkernagel AS, Schuepbach RA, et al. : Regulation of iron homeostasis by the hypoxia-inducible transcription factors (HIFs). J Clin Invest. 2007;117(7):1926–32. 10.1172/JCI31370 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 40. Fraenkel PG: Anemia of Inflammation: A Review. Med Clin North Am. 2017;101(2):285–96. 10.1016/j.mcna.2016.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Preza GC, Ruchala P, Pinon R, et al. : Minihepcidins are rationally designed small peptides that mimic hepcidin activity in mice and may be useful for the treatment of iron overload. J Clin Invest. 2011;121(12):4880–8. 10.1172/JCI57693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nai A, Pagani A, Mandelli G, et al. : Deletion of TMPRSS6 attenuates the phenotype in a mouse model of β-thalassemia. Blood. 2012;119(21):5021–9. 10.1182/blood-2012-01-401885 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 43. Kautz L, Jung G, Du X, et al. : Erythroferrone contributes to hepcidin suppression and iron overload in a mouse model of β-thalassemia. Blood. 2015;126(17):2031–7. 10.1182/blood-2015-07-658419 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 44. Dussiot M, Maciel TT, Fricot A, et al. : An activin receptor IIA ligand trap corrects ineffective erythropoiesis in β-thalassemia. Nat Med. 2014;20(4):398–407. 10.1038/nm.3468 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 45. Motta I, Scaramellini N, Cappellini MD: Investigational drugs in phase I and phase II clinical trials for thalassemia. Expert Opin Investig Drugs. 2017;26(7):793–802. 10.1080/13543784.2017.1335709 [DOI] [PubMed] [Google Scholar]
- 46. Libani IV, Guy EC, Melchiori L, et al. : Decreased differentiation of erythroid cells exacerbates ineffective erythropoiesis in beta-thalassemia. Blood. 2008;112(3):875–85. 10.1182/blood-2007-12-126938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Savona MR: Are we altering the natural history of primary myelofibrosis? Leuk Res. 2014;38(9):1004–12. 10.1016/j.leukres.2014.04.012 [DOI] [PubMed] [Google Scholar]
- 48. Casu C, Oikonomidou PR, Lo Presti V, et al. : POTENTIAL THERAPEUTIC APPLICATIONS OF JAK2 INHIBITORS AND HIF2a-ASO FOR THE TREATMENT OF beta-THALASSEMIA INTERMEDIA AND MAJOR. Am J Hematol. 2017;92:E221–E221. [Google Scholar]
- 49. Walters RW, Parker R: Coupling of Ribostasis and Proteostasis: Hsp70 Proteins in mRNA Metabolism. Trends Biochem Sci. 2015;40(10):552–9. 10.1016/j.tibs.2015.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ribeil J, Zermati Y, Vandekerckhove J, et al. : Hsp70 regulates erythropoiesis by preventing caspase-3-mediated cleavage of GATA-1. Nature. 2007;445(7123):102–5. 10.1038/nature05378 [DOI] [PubMed] [Google Scholar]
- 51. Arlet JB, Ribeil JA, Guillem F, et al. : HSP70 sequestration by free α-globin promotes ineffective erythropoiesis in β-thalassaemia. Nature. 2014;514(7521):242–6. 10.1038/nature13614 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 52. Guillem F, Dussiot M, Causse S, et al. : XPO1 (Exportin-1) Is a Major Regulator of Human Erythroid Differentiation. Potential Clinical Applications to Decrease Ineffective Erythropoiesis of Beta-Thalassemia. Blood. 2015;126(23):2368 Reference Source [Google Scholar]
- 53. Quek L, Thein SL: Molecular therapies in beta-thalassaemia. Br J Haematol. 2007;136(3):353–65. 10.1111/j.1365-2141.2006.06408.x [DOI] [PubMed] [Google Scholar]
- 54. Mettananda S, Gibbons RJ, Higgs DR: Understanding α-globin gene regulation and implications for the treatment of β-thalassemia. Ann N Y Acad Sci. 2016;1368(1):16–24. 10.1111/nyas.12988 [DOI] [PubMed] [Google Scholar]
- 55. Mettananda S, Fisher CA, Sloane-Stanley JA, et al. : Selective silencing of α-globin by the histone demethylase inhibitor IOX1: a potentially new pathway for treatment of β-thalassemia. Haematologica. 2017;102(3):e80–e84. 10.3324/haematol.2016.155655 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 56. Khemayanto H, Shi B: Role of Mediterranean diet in prevention and management of type 2 diabetes. Chin Med J (Engl). 2014;127(20):3651–6. [PubMed] [Google Scholar]
- 57. Hu X, Wang H, Lv X, et al. : Cardioprotective Effects of Tannic Acid on Isoproterenol-Induced Myocardial Injury in Rats: Further Insight into 'French Paradox'. Phytother Res. 2015;29(9):1295–1303. 10.1002/ptr.5376 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 58. Bank A: Regulation of human fetal hemoglobin: new players, new complexities. Blood. 2006;107(2):435–43. 10.1182/blood-2005-05-2113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Marinkovic D, Zhang X, Yalcin S, et al. : Foxo3 is required for the regulation of oxidative stress in erythropoiesis. J Clin Invest. 2007;117(8):2133–44. 10.1172/JCI31807 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 60. Wang H, Li Y, Wang S, et al. : Knockdown of transcription factor forkhead box O3 (FOXO3) suppresses erythroid differentiation in human cells and zebrafish. Biochem Biophys Res Commun. 2015;460(4):923–30. 10.1016/j.bbrc.2015.03.128 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 61. Zhang X, Campreciós G, Rimmelé P, et al. : FOXO3-mTOR metabolic cooperation in the regulation of erythroid cell maturation and homeostasis. Am J Hematol. 2014;89(10):954–63. 10.1002/ajh.23786 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 62. Pecoraro A, Troia A, Calzolari R, et al. : Efficacy of Rapamycin as Inducer of Hb F in Primary Erythroid Cultures from Sickle Cell Disease and β-Thalassemia Patients. Hemoglobin. 2015;39(4):225–9. 10.3109/03630269.2015.1036882 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 63. Franco SS, De Falco L, Ghaffari S, et al. : Resveratrol accelerates erythroid maturation by activation of FoxO3 and ameliorates anemia in beta-thalassemic mice. Haematologica. 2014;99(2):267–75. 10.3324/haematol.2013.090076 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 64. Liang R, Campreciós G, Bigarella C, et al. : Loss of Foxo3 reduces erythroblast apoptosis and enhances RBC production in beta-thalassemic mice. Blood. 2015;126(23):756 Reference Source [Google Scholar]
- 65. Donnelly N, Gorman AM, Gupta S, et al. : The eIF2α kinases: their structures and functions. Cell Mol Life Sci. 2013;70(19):3493–511. 10.1007/s00018-012-1252-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Chen JJ: Translational control by heme-regulated eIF2α kinase during erythropoiesis. Curr Opin Hematol. 2014;21(3):172–8. 10.1097/MOH.0000000000000030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Suragani RN, Zachariah RS, Velazquez JG, et al. : Heme-regulated eIF2α kinase activated Atf4 signaling pathway in oxidative stress and erythropoiesis. Blood. 2012;119(22):5276–84. 10.1182/blood-2011-10-388132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Han AP, Fleming MD, Chen JJ: Heme-regulated eIF2alpha kinase modifies the phenotypic severity of murine models of erythropoietic protoporphyria and beta-thalassemia. J Clin Invest. 2005;115(6):1562–70. 10.1172/JCI24141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Hahn CK, Lowrey CH: Induction of fetal hemoglobin through enhanced translation efficiency of γ-globin mRNA. Blood. 2014;124(17):2730–4. 10.1182/blood-2014-03-564302 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 70. De Franceschi L, Bertoldi M, De Falco L, et al. : Oxidative stress modulates heme synthesis and induces peroxiredoxin-2 as a novel cytoprotective response in β-thalassemic erythropoiesis. Haematologica. 2011;96(11):1595–604. 10.3324/haematol.2011.043612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Matte A, De Falco L, Iolascon A, et al. : The Interplay Between Peroxiredoxin-2 and Nuclear Factor-Erythroid 2 Is Important in Limiting Oxidative Mediated Dysfunction in β-Thalassemic Erythropoiesis. Antioxid Redox Signal. 2015;23(16):1284–97. 10.1089/ars.2014.6237 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 72. Pittalà V, Salerno L, Romeo G, et al. : A focus on heme oxygenase-1 (HO-1) inhibitors. Curr Med Chem. 2013;20(30):3711–32. 10.2174/0929867311320300003 [DOI] [PubMed] [Google Scholar]
- 73. Santos DG, Mikhael M, Rivella S, et al. : Heme Oxygenase 1 Plays a Role In The Pathophysiology Of beta-Thalassemia. Blood. 2015;122.26531164 [Google Scholar]
- 74. Weatherall DJ: Mechanisms for the heterogeneity of the thalassemias. IJPHO. 1997;4:3–10. Reference Source [Google Scholar]
- 75. Sripichai O, Fucharoen S: Fetal hemoglobin regulation in β-thalassemia: heterogeneity, modifiers and therapeutic approaches. Expert Rev Hematol. 2016;9(12):1129–37. 10.1080/17474086.2016.1255142 [DOI] [PubMed] [Google Scholar]
- 76. Gambari R, Fibach E: Medicinal chemistry of fetal hemoglobin inducers for treatment of beta-thalassemia. Curr Med Chem. 2007;14(2):199–212. 10.2174/092986707779313318 [DOI] [PubMed] [Google Scholar]
- 77. Fucharoen S, Inati A, Siritanaratku N, et al. : A randomized phase I/II trial of HQK-1001, an oral fetal globin gene inducer, in β-thalassaemia intermedia and HbE/β-thalassaemia. Br J Haematol. 2013;161(4):587–93. 10.1111/bjh.12304 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 78. Smith EC, Orkin SH: Hemoglobin genetics: recent contributions of GWAS and gene editing. Hum Mol Genet. 2016;25(R2):R99–R105. 10.1093/hmg/ddw170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Wilber A, Hargrove PW, Kim YS, et al. : Therapeutic levels of fetal hemoglobin in erythroid progeny of β-thalassemic CD34 + cells after lentiviral vector-mediated gene transfer. Blood. 2011;117(10):2817–26. 10.1182/blood-2010-08-300723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Costa FC, Fedosyuk H, Chazelle AM, et al. : Mi2β is required for γ-globin gene silencing: temporal assembly of a GATA-1-FOG-1-Mi2 repressor complex in β-YAC transgenic mice. PLoS Genet. 2012;8(12):e1003155. 10.1371/journal.pgen.1003155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Lulli V, Romania P, Morsilli O, et al. : MicroRNA-486-3p regulates γ-globin expression in human erythroid cells by directly modulating BCL11A. PLoS One. 2013;8(4):e60436. 10.1371/journal.pone.0060436 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 82. Guda S, Brendel C, Renella R, et al. : miRNA-embedded shRNAs for Lineage-specific BCL11A Knockdown and Hemoglobin F Induction. Mol Ther. 2015;23(9):1465–74. 10.1038/mt.2015.113 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 83. Guo SL, Aghajan M, Casu C, et al. : Targeting TMPRSS6 Using Antisense Technology for the Treatment of Beta-Thalassemia. Blood. 2015;126(23):753 Reference Source [Google Scholar]
- 84. Peralta R, Low A, Kim A, et al. : Targeting BCL11A and KLF1 For The Treatment Of Sickle Cell Disease and beta-Thalassemia In Vitro using Antisense Oligonucleotides. Blood. 2013;122(21):1022 Reference Source [Google Scholar]
- 85. Tallack MR, Perkins AC: KLF1 directly coordinates almost all aspects of terminal erythroid differentiation. IUBMB Life. 2010;62(12):886–90. 10.1002/iub.404 [DOI] [PubMed] [Google Scholar]
- 86. Esteghamat F, Gillemans N, Bilic I, et al. : Erythropoiesis and globin switching in compound Klf1::Bcl11a mutant mice. Blood. 2013;121(13):2553–62. 10.1182/blood-2012-06-434530 [DOI] [PubMed] [Google Scholar]
- 87. McNutt M: Breakthrough to genome editing. Science. 2015;350(6267):1445. 10.1126/science.aae0479 [DOI] [PubMed] [Google Scholar]
- 88. Reik A, Chang K, Vierstra J, et al. : 53. From GWAS To the Clinic: Genome-Editing the Human BCL11A Erythroid Enhancer for Fetal Globin Elevation in the Hemoglobinopathies. Molecular Therapy. 2015;23(Supplement 1):S23–S24. 10.1016/S1525-0016(16)33658-9 [DOI] [Google Scholar]; F1000 Recommendation
- 89. Bauer DE, Canver MC, Smith EC, et al. : Crispr-Cas9 Saturating Mutagenesis Reveals an Achilles Heel in the BCL11A Erythroid Enhancer for Fetal Hemoglobin Induction (by Genome Editing). Blood. 2015;126(23):638 Reference Source [Google Scholar]
- 90. Vierstra J, Reik A, Chang K, et al. : Functional footprinting of regulatory DNA. Nat Methods. 2015;12(10):927–30. 10.1038/nmeth.3554 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 91. Breda L, Motta I, Lourenco S, et al. : Forced chromatin looping raises fetal hemoglobin in adult sickle cells to higher levels than pharmacologic inducers. Blood. 2016;128(8):1139–43. 10.1182/blood-2016-01-691089 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 92. Deng W, Rupon JW, Krivega I, et al. : Reactivation of developmentally silenced globin genes by forced chromatin looping. Cell. 2014;158(4):849–60. 10.1016/j.cell.2014.05.050 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 93. Cavazzana M, Ribeil JA, Payen E, et al. : Outcomes of Gene Therapy for Severe Sickle Disease and Beta-Thalassemia Major Via Transplantation of Autologous Hematopoietic Stem Cells Transduced Ex Vivo with a Lentiviral Beta AT87Q-Globin Vector. Blood. 2015;126(23):202 Reference Source [Google Scholar]
- 94. Walters MC, Rasko J, Hongeng S, et al. : Update of Results from the Northstar Study (HGB-204): A Phase 1/2 Study of Gene Therapy for Beta-Thalassemia Major Via Transplantation of Autologous Hematopoietic Stem Cells Transduced Ex-Vivo with a Lentiviral Beta AT87Q-Globin Vector (LentiGlobin BB305 Drug Product). Blood. 2015;126(23):201 Reference Source [Google Scholar]
- 95. Marktel S, Giglio F, Cicalese MP, et al. : A Phase I/Ii Study of Autologous Hematopoietic Stem Cells Genetically Modified with Globe Lentiviral Vector for the Treatment of Transfusion Dependent Beta-Thalassemia. Haematologica. 2016;101:168–168. [Google Scholar]
- 96. Ma N, Liao B, Zhang H, et al. : Transcription activator-like effector nuclease (TALEN)-mediated gene correction in integration-free β-thalassemia induced pluripotent stem cells. J Biol Chem. 2013;288(48):34671–9. 10.1074/jbc.M113.496174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Lucarelli G, Isgrò A, Sodani P, et al. : Hematopoietic stem cell transplantation in thalassemia and sickle cell anemia. Cold Spring Harb Perspect Med. 2012;2(5):a011825. 10.1101/cshperspect.a011825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Angelucci E, Matthes-Martin S, Baronciani D, et al. : Hematopoietic stem cell transplantation in thalassemia major and sickle cell disease: indications and management recommendations from an international expert panel. Haematologica. 2014;99(5):811–20. 10.3324/haematol.2013.099747 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 99. Goussetis E, Peristeri I, Kitra V, et al. : HLA-matched sibling stem cell transplantation in children with β-thalassemia with anti-thymocyte globulin as part of the preparative regimen: the Greek experience. Bone Marrow Transplant. 2012;47(8):1061–6. 10.1038/bmt.2011.219 [DOI] [PubMed] [Google Scholar]
- 100. Gaziev J, De Angelis G, Isgro A, et al. : Transplant Outcomes in High-Risk (Class 3) Patients with Thalassemia Treated with a Modified Protocol Are Equivalent to Low/Intermediate-Risk (Class 1/Class 2) Patients. Blood. 2015;126:620 Reference Source 26012568 [Google Scholar]
- 101. King AA, Kamani N, Bunin N, et al. : Successful matched sibling donor marrow transplantation following reduced intensity conditioning in children with hemoglobinopathies. Am J Hematol. 2015;90(12):1093–8. 10.1002/ajh.24183 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 102. Mohanan EP, Panetta JC, Royan SSB, et al. : Population Pharmacokinetics of Fludarabine and Treosulfan in Patients with Thalassemia Undergoing Hematopoietic Stem Cell Transplantation. Blood. 2015;126(23):3120 Reference Source [Google Scholar]
- 103. Giambona A, Leto F, Damiani G, et al. : Identification of embryo-fetal cells in celomic fluid using morphological and short-tandem repeats analysis. Prenat Diagn. 2016;36(10):973–978. 10.1002/pd.4922 [DOI] [PubMed] [Google Scholar]
- 104. Li DZ, Yang YD: Invasive prenatal diagnosis of fetal thalassemia. Best Pract Res Clin Obstet Gynaecol. 2017;39:41–52. 10.1016/j.bpobgyn.2016.10.011 [DOI] [PubMed] [Google Scholar]
- 105. Hudecova I, Chiu RW: Non-invasive prenatal diagnosis of thalassemias using maternal plasma cell free DNA. Best Pract Res Clin Obstet Gynaecol. 2017;39:63–73. 10.1016/j.bpobgyn.2016.10.016 [DOI] [PubMed] [Google Scholar]
- 106. Traeger-Synodinos J: Pre-implantation genetic diagnosis. Best Pract Res Clin Obstet Gynaecol. 2017;39:74–88. 10.1016/j.bpobgyn.2016.10.010 [DOI] [PubMed] [Google Scholar]