Abstract
Background
Hepatocellular carcinoma (HCC) is the most important cause of cancer-related deaths worldwide. Pirfenidone is an orally available small molecule with therapeutic potential for fibrotic diseases.
Material/Methods
In this study, we analyzed the effects of different pirfenidone concentrations on the proliferation of HepG2 HCC cells using Cell Counting Kit-8 (CCK-8) and colony formation assays. Flow cytometry was performed to measure the apoptotic effects of pirfenidone on HepG2 cells. Western blot analysis was performed to detect the expression of β-catenin and p-β-catenin.
Results
Pirfenidone inhibited proliferation and promoted HepG2 cell apoptosis. In addition, Western blot results indicated that pirfenidone suppressed β-catenin expression in HepG2 cells. To assess the mechanism, we treated HepG2 cells with pirfenidone, and pirfenidone plus the β-catenin activator, SB-216763. The results revealed that SB-216763 accelerated proliferation and inhibited apoptosis in HepG2 cells treated with pirfenidone. Western blot results showed that SB-216763 upregulated β-catenin expression in HepG2 cells treated with pirfenidone.
Conclusions
In conclusions, pirfenidone may be a potential drug for HCC treatment.
MeSH Keywords: Apoptosis; Carcinoma, Hepatocellular; Cell Proliferation
Background
Hepatocellular carcinoma (HCC) is a major health problem worldwide that develops mainly in cirrhotic livers. It is caused by genetic factors, alcoholic injury, and chronic infections such as with the hepatitis B and C viruses [1]. HCC is the fifth most frequently occurring cancer and the second leading cause of cancer-related deaths globally. Approximately 745 000 patients die from HCC yearly [2–4]. Because of the low detection rate of HCC at the curable stages, and a high rate of recurrence and metastasis, patient survival remains unsatisfactory despite recent developments in therapeutic strategies. Chemotherapy is one of the main treatment methods for terminal-stage HCC. However, most anticancer drugs have strong cytotoxic effects and lead to the emergence of drug resistance, which limits their widespread application and curative effect. Presently, adefovir dipivoxil, entecavir, and other antiviral drugs are used in clinical trials of HCC, but the incidence of HCC has not been reduced and even shows an increasing trend [5,6]. In comparison, pirfenidone shows unique advantages in the treatment of HCC.
Pirfenidone is an orally available small molecule that has been extensively evaluated in clinical trials for the treatment of several diseases. Numerous studies have shown that pirfenidone has important anti-inflammatory and antifibrotic effects in vivo and in vitro [2,3], such as on the lungs [4], renal system [5], liver [6], heart [7,8], muscle [9], and eyes [10]. Studies have indicated that pirfenidone can regulate transforming growth factor-β and tumor necrosis factor-α, and inhibit fibroblast proliferation and collagen synthesis [11,12].
Research has shown that β-catenin is overexpressed in HCC and can activate downstream cell signaling pathways, thereby regulating biological processes such as cell proliferation, apoptosis, and invasion. The effect of pirfenidone on the Wnt/β-catenin signaling pathway has not been reported. Therefore, in our study, we analyzed the effect of pirfenidone on cell proliferation and apoptosis, as well as the expression of Wnt/β-catenin signaling pathway proteins in HCC using HepG2 cells. Our results showed that pirfenidone inhibited the proliferation of HepG2 cells in a concentration-dependent manner, and promoted HepG2 cell apoptosis. Pirfenidone also suppressed β-catenin expression in HepG2 cells. In addition, the β-catenin activator, SB-216763, accelerated pirfenidone-mediated increases in proliferation and inhibited apoptosis in HepG2 cells. SB-216763 also upregulated β-catenin expression mediated by pirfenidone. Therefore, the results of our study indicate that pirfenidone might be a potential therapy for HCC.
Material and Methods
Cell culture and treatments
Human hepatoma HepG2 cells were purchased from the American Type Culture Collection (Manassas, VA, USA) and cultured in Dulbecco’s modified Eagle’s medium (Invitrogen, Carlsbad, CA, USA) containing 10% fetal bovine serum (Gibco, Grand Island, NY, USA), 100 U/mL penicillin, and 1 μg/mL streptomycin (Invitrogen) in an incubator with an atmosphere of 5% CO2 at 37°C. HepG2 cells were seeded in 6-well plates (1×105 cells/well) and treated with 0, 100, 200, 400, 600, and 800 μmol/L pirfenidone for 0, 6, 12, 24, 36, and 48 h.
Cell proliferation
Cell proliferation was determined using Cell Counting Kit-8 (CCK-8, Shanghai Beyotime Biotechnology, Shanghai, China). HepG2 cells (1×104 cells/well) were cultured in 96-well plates with 100 μL Dulbecco’s modified Eagle’s medium containing 10% fetal bovine serum and treated with 0, 100, 200, 400, 600, and 800 μmol/L pirfenidone for 0, 12, 24, 36, and 48 h. CCK-8 solution (10 μL) was added to each well at a specific time, followed by a 3-h incubation at 37°C. Absorbance was detected at 450 nm using a microplate reader (Molecular Devices, Sunnyvale, CA, USA). All experiments were repeated at least 3 times.
Colony formation assay
HepG2 cells were seeded on a fresh 6-well plate at a density of 1000 cells/well and cultured in complete medium at 37°C under 5% CO2. After 14 days, cells were fixed in methanol and stained with 0.1% crystal violet. The number of colonies was counted manually.
Flow cytometry
Cells were collected and seeded into 6-well plates. Cells were digested by EDTA-free trypsin (Shanghai Beyotime Biotechnology), stained with Annexin V-FITC and propidium iodide (Shanghai BestBio Science, Shanghai, China), and incubated in the dark for 15 min at room temperature. The cell cycle and apoptosis of each group were detected by an EPICS XL-MCL flow cytometer (Beckman Coulter, Brea, CA, USA) with an excitation wavelength of 488 nm and an emission wavelength of 530 nm.
Western blot analysis
HepG2 cells (4×105/well) were seeded in a 6-well plate and treated for 24 h with 0, 100, 200, 400, 600, and 800 μmol/L pirfenidone at 37°C in a 5% CO2 incubator. Total proteins were acquired using a lysis buffer containing a protease inhibitor cocktail (Roche Applied Science, Indianapolis, IN, USA). Total protein concentrations were detected using a bicinchoninic acid assay kit (Thermo Fisher Scientific, Waltham, MA, USA). Equivalent amounts of protein were loaded onto the lanes of the sodium dodecyl sulfate-polyacrylamide gel using a micropipette. After electrophoresis, the separated proteins were transferred to polyvinylidene difluoride membranes (Millipore, Billerica, MA, USA) blocked with 5% skim milk (BD Biosciences, Franklin Lakes, NJ, USA) and incubated with primary antibodies overnight at 4°C. The next day, the membranes were incubated with anti-rabbit horseradish peroxidase-conjugated secondary antibodies (cat. no. ab6721). The signals were visualized using an enhanced chemiluminescence substrate kit (GE Healthcare, Pittsburgh, PA, USA) and Western blotting system (ComWin Biotech, Beijing, China). The following monoclonal primary antibodies and dilutions were used: anti-β-catenin (1: 1000, cat. no. ab32572), anti-phosphorylated (p)-β-catenin (1: 300, cat. no: ab27798), and anti-human GAPDH (1: 3000, cat. no. ab181602) as the internal control. All antibodies were from Abcam (Cambridge, MA, USA).
Statistical analysis
All data were expressed as the means ± standard deviations (SD) of 3 independent experiments. Statistical significance was determined using a one-way analysis of variance and Student’s t-test using SPSS version 19.0 software (IBM, Chicago, IL, USA). All data are presented as means ± standard deviation. P<0.05 was considered statistically significant.
Results
Pirfenidone inhibits HepG2 cell proliferation
We investigated the effects of various concentrations of pirfenidone (0, 100, 200, 400, 600, and 800 μmol/L) for 48 h on the growth of HepG2 cells. The CCK-8 assay revealed a significant time- and concentration-dependent inhibition of HepG2 cell proliferation (Figure 1).
Figure 1.
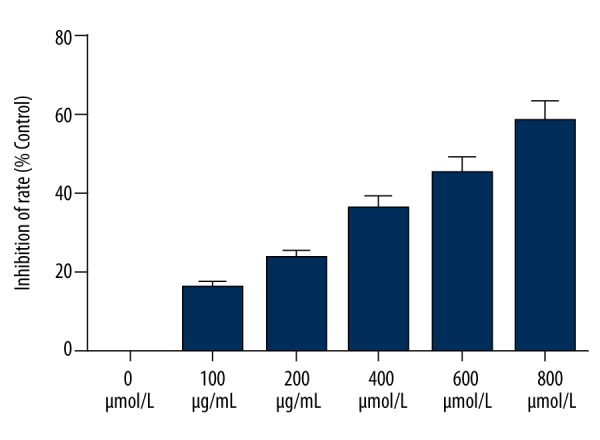
Pirfenidone inhibits proliferation of HepG2 cells. The Cell Counting Kit-8 assay was performed to determine the viability of HepG2 cells treated with various concentrations of pirfenidone (0, 100, 200, 400, 600, and 800 μmol/L) for 48 h.
Pirfenidone promotes apoptosis of HepG2 cells
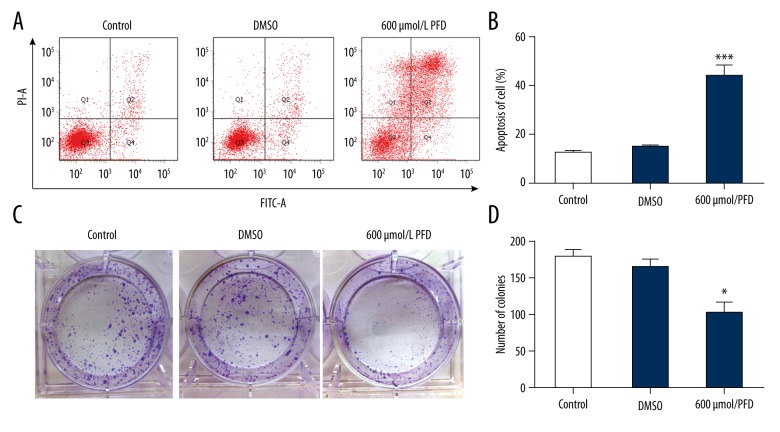
Based on the inhibitory effect of pirfenidone on HepG2 cell proliferation, we then determined whether pirfenidone induced apoptosis. The results indicated that, compared with phosphate-buffered saline (PBS)- and DMSO-treated controls, pirfenidone significantly promoted HepG2 cell apoptosis (*** P<0.001) (Figure 2A, 2B). The viability of HepG2 cells was also determined by the colony formation assay. The results revealed that the number of colonies was significantly decreased in pirfenidone-treated cells compared with PBS- and DMSO-treated control cells (* P<0.05) (Figure 2C, D).
Figure 2.
Pirfenidone (PFD) promotes apoptosis of HepG2 cells. HepG2 cells were treated with phosphate-buffered saline (control), an equivalent amount of DMSO, or 600 μmol/L PFD in DMSO for 48 h. (A) Apoptosis was detected by flow cytometry in treated HepG2 cells. (B) The extent of HepG2 cell apoptosis was calculated in the different treatment groups. (C) Colony formation assay in treated HepG2 cells. (D) The number of colonies was counted. (* P<0.05 vs. control and DMSO).
Pirfenidone suppresses β-catenin expression HepG2 cells
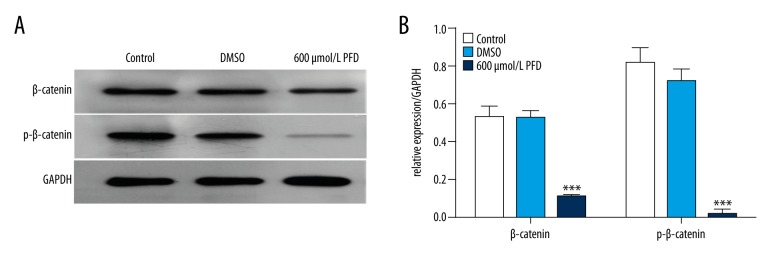
Researchers have shown that Wnt/β-catenin signaling plays an important role in the progression of cancer [7–9]. In our study, to explore the molecular mechanism of action of pirfenidone in HepG2 cells, we assessed the expression levels of β-catenin and p-β-catenin. HepG2 cells were treated with PBS (control), an equivalent amount of DMSO, or 600 μmol/L pirfenidone in DMSO for 48 h. Expression levels of β-catenin and p-β-catenin proteins were measured using Western blot analysis. Pirfenidone dramatically downregulated the expression levels of β-catenin and p-β-catenin proteins (*** P<0.001) (Figure 3A, 3B).
Figure 3.
Pirfenidone (PFD) suppresses β-catenin expression in HepG2 cells. HepG2 cells were treated with phosphate-buffered saline (control), an equivalent amount of DMSO, or 600 μmol/L PFD in DMSO for 48 h. (A) Treated HepG2 cells were harvested for Western blot analysis using β-catenin and p-β-catenin antibodies. (B) The Western blot densities were normalized to the density of GAPDH in each lane. (*** P<0.001 vs. control and DMSO).
The β-catenin activator, SB-216763, inhibits apoptosis of HepG2 cells mediated by pirfenidone
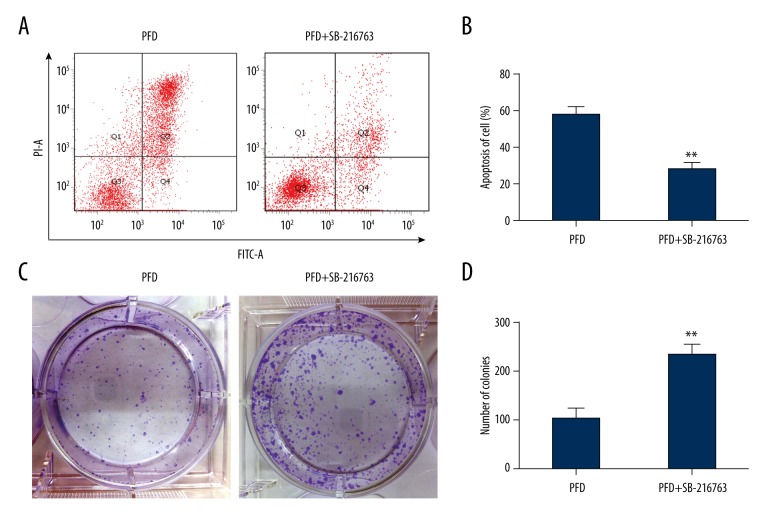
To further explore the molecular mechanism by which pirfenidone affected apoptosis of HepG2 cells, flow cytometry was used. The results indicated that SB-216763, a β-catenin activator, significantly inhibited pirfenidone-induced HepG2 cell apoptosis (** P<0.01) (Figure 4A, 4B). We then analyzed the effect of SB-216763 on HepG2 cell proliferation using the colony formation assay. The results revealed that SB-216763 increased HepG2 cell proliferation in the presence of pirfenidone (** P<0.01) (Figure 4C, 4D).
Figure 4.
The β-catenin activator, SB-216763, inhibits pirfenidone (PFD) effects in HepG2 cells. (A) Apoptosis was detected by flow cytometry in HepG2 cells treated with PFD (600 μmol/L) with and without SB-216763. (B) The extent of apoptosis was quantified in HepG2 cells treated with PFD (600 μmol/L) with and without SB-216763. (C) Colony formation assays were performed in HepG2 cells treated with PFD (600 μmol/L) with and without SB-216763. (D) The number of colonies was determined. (** P<0.01).
SB-216763 upregulates β-catenin expression mediated by pirfenidone in HepG2 cells
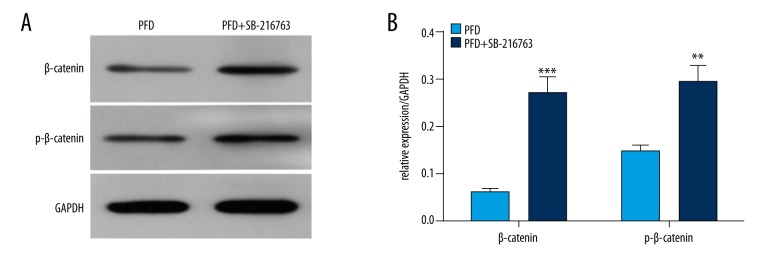
We next determined the influence of SB-216763 on the expression of β-catenin and p-β-catenin in HepG2 cells. The results showed that SB-216763 dramatically increased the protein expression levels of β-catenin (*** P<0.001) and p-β-catenin compared with the pirfenidone alone group (** P<0.01) (Figure 5A, 5B).
Figure 5.
The β-catenin activator, SB-216763, upregulates β-catenin expression mediated by pirfenidone (PFD) in HepG2 cells. HepG2 cells were treated with PFD (600 μmol/L) with or without SB-216763. (A) Total protein was isolated. The expression levels of β-catenin and p-β-catenin proteins were measured using Western blotting. GAPDH was used as the loading control. (B) The Western blot densities were normalized to GAPDH. (** P<0.01; *** P<0.001).
Discussion
Hepatitis is an important pathogenic factor in the development of HCC. Statistically, 90% of patients with HCC are infected with the hepatitis B virus and always have evidence of liver fibrosis. Presently, because HCC is often not detected at curable stages, and there is a high rate of recurrence and metastasis, patient survival remains unsatisfactory. Pirfenidone as a small molecule with therapeutic potential for use in the treatment of fibrotic diseases and can reduce transforming growth factor-β expression, antagonize tumor necrosis factor-α signaling [10], and eliminate reactive oxygen species [11]. Research suggests that pirfenidone affects the expression of extracellular matrix components, with the gradual destruction of the fibrotic process in normal tissues, which is a factor influencing the progression of chronic disease, including HCC.
Cellular proliferation is vital for the initiation and development of fibrosis and cancer. In vitro studies have demonstrated the inhibitory role of pirfenidone in the proliferation of a variety of normal cell types, including rat cardiac fibroblasts, human lens epithelial cell line, and human myometrial cells [12–14]. Furthermore, pirfenidone has been shown to inhibit the proliferation of some tumor cell types, such as human leiomyoma cells, pancreatic cancer cells, and glioma cell [15–17]. In the present study, by using cell viability and colonies formation assays, we revealed that pirfenidone inhibited HepG2 proliferation in a dose-dependent manner and reached about 50% inhibition of cell viability at 600 μM. Furthermore, at the tested doses inducing about 50% cell viability, inhibition was used for further research. HCC has high tumor heterogeneity, and a number of pathways have been implicated in the maintenance of HCC stems cells, including RAF/MEK/ERK, PI3K/AKT/mTOR, and Wnt/β-catenin signaling [9]. In this study, it was observed that the Wnt/β-catenin pathway was highly activated only when exposed to vehicle. This was consistent with the previous studies that found aberrant accumulation of β-catenin for constitutive activation of the Wnt/β-catenin pathway was present in HepG2 cells [18,19]. These results indicated that HepG2 was suitable for this study. With the dose of 50% cell viability inhibition, it was shown that marked and significant reductions of β-catenin expression and its phosphorylation were induced. β-Catenin is an essential component of canonical Wnt signaling, in which it serves a role in activating gene transcription [20]. It has been demonstrated that in the presence of Wnt-ligands, β-catenin protein can act as a co-transcription factor for the transcription of its own promoter [21]. Therefore, the decrease in β-catenin protein observed in the cells was potentially caused by a positive feedback regulation. In addition, various growth factors, including TGF-β, can activate β-catenin signaling via autocrine Wnt ligand production [22] It is well known that pirfenidone exerts its anti-fibrotic effect mainly by inhibiting TGF-β signaling. Therefore, TGF-β inhibition might critically contribute to the decreased Wnt/β-catenin pathway, thus inducing decreased expression and phosphorylation of β-catenin.
Wnt signaling has an important role in the development of a variety of tumors. Upon binding to the receptor, Wnt induces a cascade of intracellular signaling events containing a major component, Glycogen Synthase Kinase-3 (GSK-3), which mediates β-catenin phosphorylation, leading to its degradation via ubiquitin/proteasome pathway [23]. SB-216763 is a selective GSK-3 inhibitor, which has been demonstrated to inhibit the Wnt/β-catenin signaling pathway [24]. In our study, it was observed that the inhibitory effects of pirfenidone on cell growth and on the expression and phosphorylation of β-catenin were abolished by SB-216763 exposure. These findings indicated that pirfenidone inhibited HCC cell growth via the Wnt/β-catenin signaling pathway.
Conclusions
Our results revealed that pirfenidone inhibited the growth HCC cells in a concentration-dependent manner. Furthermore, it was found to suppress the expression of Wnt/β-catenin signaling proteins. Previous findings show that pirfenidone can reduce lung cancer in patients with pulmonary fibrosis [25]. We conclude that pirfenidone might be a potentially therapeutic agent for treatment of HCC.
Footnotes
Source of support: This study was supported by funds from the Program for Scientific Cooperation in Guizhou Province (LH for Scientific Cooperation in Guizhou) (no: 20147147), Talents in the Platform of Scientific Cooperation in Guizhou Province (no: 20165603), the Program for Scientific Cooperation in Guizhou Province (LH for Scientific Cooperation in Guizhou) (no: 20167405), the National Natural Science Foundation (no: 81560297), the National Natural Science Foundation (no: 81460276), the Program for Scientific Cooperation in Guizhou Province (LH for Scientific Cooperation in Guizhou) (no: 20157384), and the National Natural Science Foundation (no: 81460365; no: 81760325)
Conflict of interest
None.
References
- 1.Baffy G. Hepatocellular carcinoma in non-alcoholic fatty liver disease: Epidemiology, pathogenesis, and prevention. J Clin Transl Hepatol. 2013;1:131–37. doi: 10.14218/JCTH.2013.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li Z, Zhang C, Lou C, et al. Comparison of percutaneous cryosurgery and surgical resection for the treatment of small hepatocellular carcinoma. Oncol Lett. 2013;6:239–45. doi: 10.3892/ol.2013.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 4.Asia-Pacific Working Party on Prevention of Hepatocellular Carcinoma. Prevention of hepatocellular carcinoma in the Asia-Pacific region: consensus statements. J Gastroenterol Hepatol. 2010;25:657–63. doi: 10.1111/j.1440-1746.2009.06167.x. [DOI] [PubMed] [Google Scholar]
- 5.Hosaka T, Suzuki F, Kobayashi M, et al. Development of HCC in patients receiving adefovir dipivoxil for lamivudine-resistant hepatitis B virus mutants. Hepatol Res. 2010;40:145–52. doi: 10.1111/j.1872-034X.2009.00582.x. [DOI] [PubMed] [Google Scholar]
- 6.Papatheodoridis GV, Yurdaydin C, Dalekos GN, et al. The risk of hepatocellular carcinoma (HCC) is decreasing after the first 5 years of entecavir (ETV) or tenofovir (TDF) therapy in Caucasian chronic hepatitis B (CHB) patients. Hepatology. 2016;63:923a. [Google Scholar]
- 7.Fu Y, Zheng S, An N, et al. beta-catenin as a potential key target for tumor suppression. Int J Cancer. 2011;129:1541–51. doi: 10.1002/ijc.26102. [DOI] [PubMed] [Google Scholar]
- 8.Valenta T, Hausmann G, Basler K. The many faces and functions of beta-catenin. The EMBO journal. 2012;31:2714–36. doi: 10.1038/emboj.2012.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anson M, Crain-Denoyelle AM, Baud V, et al. Oncogenic beta-catenin triggers an inflammatory response that determines the aggressiveness of hepatocellular carcinoma in mice. J Clin Invest. 2012;122:586–99. doi: 10.1172/JCI43937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cain WC, Stuart RW, Lefkowitz DL, et al. Inhibition of tumor necrosis factor and subsequent endotoxin shock by pirfenidone. Int J Immunopharmacol. 1998;20:685–95. doi: 10.1016/s0192-0561(98)00042-3. [DOI] [PubMed] [Google Scholar]
- 11.Giri SN, Leonard S, Shi X, et al. Effects of pirfenidone on the generation of reactive oxygen species in vitro. J Environ Pathol Toxicol Oncol. 1999;18:169–77. [PubMed] [Google Scholar]
- 12.Shi Q, Liu X, Bai Y, et al. In vitro effects of pirfenidone on cardiac fibroblasts: proliferation, myofibroblast differentiation, migration and cytokine secretion. PLoS One. 2011;6:e28134. doi: 10.1371/journal.pone.0028134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang Y, Ye Y, Lin X, et al. Inhibition of pirfenidone on TGF-beta2 induced proliferation, migration and epithlial-mesenchymal transition of human lens epithelial cells line SRA01/04. PLoS One. 2013;8:e56837. doi: 10.1371/journal.pone.0056837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Conte E, Gili E, Fagone E, et al. Effect of pirfenidone on proliferation, TGF-beta-induced myofibroblast differentiation and fibrogenic activity of primary human lung fibroblasts. Eur J Pharm Sci. 2014;58:13–19. doi: 10.1016/j.ejps.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 15.Lee BS, Margolin SB, Nowak RA. Pirfenidone: A novel pharmacological agent that inhibits leiomyoma cell proliferation and collagen production. J Clin Edocrinol Metab. 1998;83:219–23. doi: 10.1210/jcem.83.1.4503. [DOI] [PubMed] [Google Scholar]
- 16.Kozono S, Ohuchida K, Eguchi D, et al. Pirfenidone inhibits pancreatic cancer desmoplasia by regulating stellate cells. Cancer Res. 2013;73:2345–56. doi: 10.1158/0008-5472.CAN-12-3180. [DOI] [PubMed] [Google Scholar]
- 17.Burghardt I, Tritschler F, Opitz CA, et al. Pirfenidone inhibits TGF-beta expression in malignant glioma cells. Biochem Biophys Res Commun. 2007;354:542–47. doi: 10.1016/j.bbrc.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 18.Gao C, Xiao G, Hu J. Regulation of Wnt/beta-catenin signaling by posttranslational modifications. Cell Biosci. 2014;4:13. doi: 10.1186/2045-3701-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de La Coste A, Romagnolo B, Billuart P, et al. Somatic mutations of the beta-catenin gene are frequent in mouse and human hepatocellular carcinomas. Proc Natl Acad Sci USA. 1998;95:8847–51. doi: 10.1073/pnas.95.15.8847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–80. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 21.Bandapalli OR, Dihlmann S, Helwa R, et al. Transcriptional activation of the beta-catenin gene at the invasion front of colorectal liver metastases. J Pathol. 2009;218:370–79. doi: 10.1002/path.2539. [DOI] [PubMed] [Google Scholar]
- 22.Guo X, Wang XF. Signaling cross-talk between TGF-beta/BMP and other pathways. Cell Res. 2009;19:71–88. doi: 10.1038/cr.2008.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amit S, Hatzubai A, Birman Y, et al. Axin-mediated CKI phosphorylation of beta-catenin at Ser 45: A molecular switch for the Wnt pathway. Genes Dev. 2002;16:1066–76. doi: 10.1101/gad.230302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zeng J, Liu X, Li X, et al. Daucosterol inhibits the proliferation, migration, and invasion of hepatocellular carcinoma cells via Wnt/beta-catenin signaling. Molecules. 2017;22(6) doi: 10.3390/molecules22060862. pii: E862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hu M, Wang Y, Zhang Y, et al. Reduced lung cancer incidence attributable to decreased tobacco use in urban Shanghai. Cancer Causes Control. 2013;24:2021–25. doi: 10.1007/s10552-013-0269-y. [DOI] [PubMed] [Google Scholar]