Abstract
In the mosquito–human life cycle, the six species of malaria parasites infecting humans (Plasmodium falciparum, Plasmodium vivax, Plasmodium ovale wallickeri, Plasmodium ovale curtisi, Plasmodium malariae, and Plasmodium knowlesi) undergo 10 or more morphological states, replicate from single to 10,000+ cells, and vary in total population from one to many more than 106 organisms. In the human host, only a small number of these morphological stages lead to clinical disease and the vast majority of all malaria-infected patients in the world produce few (if any) symptoms in the human. Human clinical disease (e.g., fever, anemia, coma) is the result of the parasite preprogrammed biology in concert with the human pathophysiological response. Caveats and corollaries that add variation to this host–parasite interaction include parasite genetic diversity of key proteins, coinfections, comorbidities, delays in treatment, human polymorphisms, and environmental determinants.
Pathogenesis, the manner of development of a disease, for a human malaria clinical illness is a complex story that has many players, settings, and potential outcomes. As with any truly successful parasite, the observed outcome of evolution in malaria is the undisturbed transition from mosquito to human to mosquito with little impact on the vector and host. Although impact of malaria can be seen at the individual, community, country, and global level, from the parasite's perspective, a healthy host serving as two blood meals with a bit of fever in between is the norm. In fact, human clinical disease is quite rare relative to the global interaction network of mosquitoes and humans.
The biology of Plasmodium falciparum malaria parasites, as measured in vitro, is finite, predictable, and easily experimentally perturbed during the 48-hour life cycle (Bozdech et al. 2003a,b; Llinas and DeRisi 2004). In the mosquito–human life cycle, however, this parasite, along with the other five species infecting humans (Plasmodium vivax, Plasmodium ovale wallickeri, Plasmodium ovale curtisi, Plasmodium malariae, and Plasmodium knowlesi), undergoes 10 or more morphological states, replicate from a single to 10,000+ cells, and vary in total population from one to many more than 106 organisms (Liu et al. 2011; Cator et al. 2012; Dixon et al. 2012; Mohandas and An 2012; Antinori et al. 2013; Wright and Rayner 2014; Cui et al. 2015; Josling and Llinas 2015; Stone et al. 2015). In addition, all of these parasites (with the exception of P. knowlesi in humans) have been exposed for thousands of millennia to the physical, immunological, and more recently chemotherapeutic barriers in mosquitoes and humans, which places tremendous selection pressure across the species (Sabeti et al. 2002; Volkman et al. 2007; Bañuls et al. 2013; Perry 2014). It is clearly a finely tuned, well-rehearsed, and deftly executed program.
A similar selection pressure has been placed on humans and resulted in such fascinating evolutionary outcomes as sickle cell disease, hemoglobinopathies, cytokine mutations, and enzyme deficiency, which confer, as a conceptual group, the ability to survive to maturity and reproduction (Bañuls et al. 2013; Perry 2014). Death from malaria at an age less than 6 years (the current most common demographic) cannot be a goal of the parasite (speaking teleologically) and, thus, its occurrence should be cause for concern and investigation. However, the rarity of this event (438,000 out of 214,000,000 clinical cases or ∼0.2%) leaves the unfortunate mortality as an aberrant footnote in the overall biology of the species as a whole (WHO 2015). We should not, however, accept even one death from a preventable and treatable disease.
When we turn to the parasite inside the human host, only a small number of these morphological stages lead to clinical disease and the vast majority of all malaria-infected patients in the world produce few (if any) symptoms in the human (WHO 2015). This is a crucial point of the biology that is often missed or ignored by experimentalists and “single-mechanism” focused scientists. Every person who is infected with malaria (regardless of whether or not they show symptoms) has the parasite go through the exact same life-cycle morphological changes and human–parasite interactions. Disease, thus, must be the result of exaggeration of this baseline interaction, which, as mentioned, is beneficial to neither the parasite nor the human. Further evidence for this lies, obviously, within the overall rarity of such events. Moreover, there are relatively few physiological states the parasite can achieve inside the human host—all of this biology is accomplished with a meager 6000 genes, most of which have no known function (Bozdech et al. 2003a,b; Daily et al. 2007; Milner et al. 2012).
Human clinical disease is, thus, the result of the interaction of the parasite preprogrammed biology in concert with the human pathophysiological response. Caveats and corollaries that add variation to this host–parasite interaction include parasite genetic diversity of key proteins, coinfections, comorbidities, delays in treatment, human polymorphisms, and environmental determinants (Goncalves et al. 2014). The final clinical disease result includes a spectrum of fever, anemia, and coma, among many others (Hafalla et al. 2011; Oakley et al. 2011; Grau and Craig 2012).
When one questions, “how do we get rid of cerebral malaria?” (one of the more common causes of death), it is surprising to no one to hear the answer, “reduce the overall burden of malaria disease.” This may seem simple but, in fact, is a complex answer. Interventions with rapid drug treatment for anyone with a fever will drastically reduce the burden of mortality (sometimes to zero) in a given location (Clark et al. 2010). The treatment probably not only staves off a prolonged acute disease state (which may be a component of cerebral malaria [CM]) but also provides an antigen source to the immune system to create antibody and other responses that may quiet future infections. This effect, however, only lasts as long as the diversity of the parasite is stable (a result of endemicity) and the drug access continues (a result of infrastructure stability). In a world where eradication is a goal for malaria, the incidence of CM with multiple interventions may decrease or even vanish in the current at-risk population (children less than 6 years of age in sub-Saharan Africa). However, the risk of CM may simply shift to these same children at a later stage (or their children) as a region moves from high endemicity to low endemicity. During this entire process, however, the biology of the parasite will remain relatively stable and, thus, the risk for any of the currently observed diseases states will still exist. How, where, and why these disease states emerge (or vanish) is a product of many factors beyond the parasite, the vast majority of which are within our control.
UNCOMPLICATED MALARIA
Within the geographic regions where the human population is at risk for malaria infection (2.5 billion), annually 215,000,000 clinical infections occur for which patients have symptoms and seek medical attention. Patient illness represents, however, a subset of all individuals who have been bitten by infected mosquitoes and a much larger portion of the “at-risk” population would show a positive malaria smear or other diagnostic test if they were screened (asymptomatic infection, true number variable and difficult to estimate) (malERA Consultative Group on Diagnoses and Diagnostics 2011; McMorrow et al. 2011; Laishram et al. 2012; Babiker et al. 2013; Lin et al. 2014; Stone et al. 2015). The exact malaria parasite biology within these two groups is probably very similar with the essential differences being due to the human immune response, number of prior infections, and exposure profile (Doolan and Martinez-Alier 2006; Dzikowski et al. 2006; Marsh and Kinyanjui 2006; De Leenheer and Pilyugin 2008; Punsawad 2013; de Souza 2014; Krzych et al. 2014). The symptoms of malaria infection can only begin in any ill patient with the first liver schizont rupture and release of merozoites into the peripheral circulation—this event is silent for the vast majority of patients who will become clinically ill. As the parasites continue through their asexual life cycle of merozoite reinvasion, trophozoite development, and schizont rupture over 24 to 48 hours, the level of parasitemia parallels the level of human response (i.e., fever, C-reactive protein [CRP], and tumor necrosis factor α [TNF-α]) until the patient crosses a threshold of awareness and “feels ill” (Oakley et al. 2011). Uncomplicated malaria is defined as symptoms present (fever) but no clinical or laboratory signs to indicate severity or vital organ dysfunction (WHO 2015).
Within the human host during an initial infection, macrophage ingestion of merozoites, ruptured schizonts, or antigen-presenting trophozoites in the circulation or spleen leads to release of TNF-α (Chakravorty et al. 2008; Randall and Engwerda 2010). The molecule, along with others in a cascade, is responsible for fever during infection. Other important molecules found during active infection include interleukin 10 (IL-10) and interferon γ (IFN-γ) among others (Clark et al. 2008; McCall and Sauerwein 2010; Freitas do Rosario and Langhorne 2012; Gun et al. 2014; Hunt et al. 2014). In subsequent infections, some degree of antibody production produced by the prior macrophage–T-cell–B-cell axis of the immune system confers additional macrophage activity leading to a more efficient clearance of parasites and production of new antibodies (Wykes and Good 2006; Freitas do Rosario and Langhorne 2012; Krzych et al. 2014; Hviid et al. 2015). As the human host immune system works its way through the continuously presented parasite protein repertoire, additional antibodies develop conferring additional protection.
Uncomplicated malaria is easily treated during each symptomatic episode with antimalarias specific to the parasite and the vast majority of patients easily clear the infection when treated with proper compliance.
P. falciparum
P. falciparum (Pf) modifies the surface of the infected red blood cell and creates an adhesive phenotype, which removes the parasite from circulation for nearly half of the asexual life cycle, a unique time frame among the malaria parasites (Grau and Craig 2012). The binding of the infected erythrocytes can occur with endothelium, platelets, or uninfected red blood cells (Fairhurst and Wellems 2006; Kraemer and Smith 2006; Smith et al. 2013). The parasite accomplishes this cytoadherant (“sticky cell”) state through the P. falciparum erythrocyte membrane protein 1 (PfEMP1), which is the product of var gene transcription (Smith et al. 2013). Within a given Pf parasite, there are ∼60 copies of the var gene, each highly variable and different from the others. These genes represent some of the most diverse within the parasite's genome and within the total parasite population. Their expression is driven by several mechanisms including immune selection pressure and epigenetics. This aspect of the parasites's biology (var gene expression) occurs in all infections including asymptomatic and uncomplicated malaria. The potential of this human–parasite interaction to cause disease in humans has a definite spectrum discussed below (Smith et al. 2013). Regardless of the disease variability, the sequestration (temporary removal of the parasite from circulation through red cell surface binding) of Pf occurs during every human infection for half of the asexual life cycle. Thus, in a low-level infection in which a single mosquito bite has introduced a single brood of synchronous parasites, patients may show negative peripheral blood smears. This may be especially true in the traveler or residents of low-endemicity regions. In highly endemic settings, however, patients are bitten repeatedly and can present with a continuous fever and an accompanying consistently positive blood smear during the first decades of life. As a local immunity to the Pf population evolves in a given host, smears may again drop to very low levels and even become undetectable despite ongoing transmission.
P. vivax
P. vivax (Pv) is the most common malaria parasite causing clinical disease outside of Africa (WHO 2015). Unlike Pf, but like all other human malaria parasites, Pv does not show a prolonged period of sequestration during infection (Costa et al. 2011). The parasite is, thus, probably more frequently exposed to clearance by the spleen and more commonly seen on a peripheral blood smear during an infection. One of the unique features of Pv is the red cell preference for reticulocytes and the use of predominantly the Duffy antigen for invasion although not absolutely (Moreno-Pérez et al. 2013; Zimmerman et al. 2013). This leads to a clinical infection with a lower level of parasitemia than is seen in Pf. Because reticulocytes are larger than mature red cells, the infected cells appear larger than the cells around them on peripheral blood smear. Characteristic Schuffner's dots, which are caveola–vesicle structures, are seen in both Pv and P. ovale (Udagama et al. 1988). The diagnostic form of Pv is the amoeboid form where the cytoplasm, unique to Pv, has finger-like projections without a typical round-to-oval structure.
Clinically, patients present almost identically to other malaria infections with fever plus a constellation of other possible symptoms. Unlike Pf and P. malariae (which have a single liver schizont rupture even shortly after sporozoite invasion), Pv, and Po may “reemerge” when hypnozoites (quiescent forms that last months to years in the liver from a single sporozoite exposure) release merozoites. Thus, the clinical timing of a disease (many months or years after exposure) could be a clue to one of these organisms.
P. ovale
P. ovale (Po) was shown to be two distinct species (P. ovale curtisi and P. ovale wallikeri), which only differ by a shorter latency period in P. ovale wallikeri and genetic sequence differences (Oguike et al. 2011). Thus, these two sympatric organisms are impossible to distinguish, present with the same clinical syndrome, and respond to the same therapy. Although their behavior is similar to Pv, Po does not require the Duffy blood group antigen for invasion of red blood cells. On peripheral blood smear, the diagnostic forms of Po are the comet form of the trophozoite as well as the oval appearance of infected red blood cells and the presence of fimbria or finger-like projects of the red cell membrane. The ring, schizont, and gametocyte stages of Po are very similar to Pv.
P. malariae
P. malariae (Pm) is the most benign form of malaria infection with several distinct clinical features (Collins and Jeffery 2007; Mueller et al. 2007; Das 2008). Patients have fever every 72 hours during an infection due to the longer parasite life cycle (Collins and Jeffery 2007). The number of merozoites produced with each schizont rupture is lower and, thus, the parasitemias are lower overall in these patient compared with others types of malaria (Collins and Jeffery 2007). This long life cycle and low level of infection leads to a more robust immune response. Thus, Pm is often considered to cause a chronic malaria they may last decades. One unique outcome of Pm is the deposition of immune complexes in the kidneys that can result in nephritis (Das 2008). On peripheral blood smear, the parasite shows the classic and diagnostic “band” form as well as a schizont with few merozoites and a central pigment globule (golden in color) refer to as a “daisy” form.
Clinically, patients who are ill with malaria symptoms and show forms suggestive of Pm should be evaluated for P. knowlesi as well as Pf because the detection of symptoms and/or the likelihood of co-infection is higher than a truly symptomatic Pm patient (Singh and Daneshvar 2013).
P. knowlesi
P. knowlesi (Pk) is found in a limited distribution in Malaysian/Indonesian Borneo with cases reported in other southeast Asian countries, including Vietnam, Singapore, Myanmar, Cambodia, Thailand, and the Philippines (Muller and Schlagenhauf 2014). Exposure to mosquitoes that feed on long-tailed and/or pig-tailed macaques (Singh and Daneshvar 2013) is required for transmission as no human-to-human (via mosquito) transmission has been reported. In vitro work has shown that the parasites prefer young red blood cells but can, over time, adapt to infect older human red blood cells, a phenomenon that currently limits rapid spread of the infection beyond the human:monkey milieu (Lim et al. 2013). The disease presents like other malarias with fever/chills and headache with uncommon features like nausea/vomiting, myalgia/arthralgia, upper respiratory symptoms, and jaundice (Muller and Schlagenhauf 2014). Although rare, fatal complications of Pk have occurred and do so with higher frequency than seen in Pv and Pf proportionally (Singh and Daneshvar 2013) owing to the new emergence in humans (zoonosis) and absence of sufficient time for human adaptability. Although Pk is not unique among the nonhuman vertebrate malarias that have been transmitted to humans, the current emergence of a large population distribution of a disease with high mortality has not been previously reported and warrants careful attention (Ta et al. 2014).
SEVERE MALARIA
P. vivax
During an infection with only Pv and no other comorbidities, death from the disease is exceedingly rare (if not unheard of). However, in the presence of comorbidities, severe disease and fatal outcomes are reported. Because of the relapsing phenotype of the liver, chronic disease can lead to severe anemia and malnutrition, which predispose to coinfections and a poor immune response (Dumas et al. 2009; Anstey et al. 2012; Costa et al. 2012). Like severe Pf and Pk (and any severe infection), the final common pathway can include respiratory distress, hepatorenal failure, and shock (Anstey et al. 2012). Coma has been reported rarely in Pv infection but the cause of this coma is not the same as is seen in Pf (in which parasite sequestration to a high level in the brain is seen in fatal cases).
P. knowlesi
The rate of severe disease in Pk is higher (∼8%), proportionally, than is seen in Pf or Pv and has higher mortality (3%) (Antinori et al. 2013). Similar to severe Pf malaria in adults, Pk-severe disease typically presents with the same initial constellation of fever, etc., and progresses in severe disease to include hypotension, respiratory distress, acute renal failure, hyperbilirubinemia, and shock (Rajahram et al. 2012; Antinori et al. 2013). Coma, as is required for a Pf cerebral malaria diagnosis, is not always seen in Pk fatal cases. The “common pathway” of any severe infection (i.e., may be seen in Pf, bacterial sepsis, etc.) is the result of an exaggerated human immune response in the presence of an untreated or delayed in treatment infection and probably not a result of specific mechanisms of the organisms. In fact, Pk as a cause of other morbid conditions (Gram-negative sepsis) has been reported. Pathologically, where Pf shows intense sequestration in the brain along with congestion and possibly brain swelling, Pk has a similar appearance in tissue with a curious lack of ICAM-1 in the brain (Cox-Singh et al. 2010; Menezes et al. 2012). The Pk family of genes homologous to Pf var genes is the SICAvars, which are larger in structure (12 vs. two exons) and quantity (>200 vs. 60) than the var genes of Pf (Lapp et al. 2009). The exact mechanism and interactions of Pk with human endothelium to produce sequestration are yet to be elucidated.
PLACENTAL MALARIA
The Pf parasite can uniquely cause a range of pathological changes in the setting of pregnancy due to the ability of the parasite to sequester paired with the large sink of novel placental molecules such as chondroitin sulfate (CSA). The parasite's PfEMP1 proteins that are products of the var2CSA genes bind to CSA as the parasites pass through the placenta, removing them from circulation, whereas non-CSA binding parasites continue circulating. Maternal antibody that has developed to malaria in previous infections appears to destroy the non-CSA binding parasites, whereas the placenta acts as a protected space for propagation. In addition to the direct effects of placental binding, mononuclear cell infiltrates may also be present and in very high numbers. Depending on when the malaria infection occurs during pregnancy, the placenta at examination may show pigment trapped in fibrin (older infection) or parasites and/or mononuclear cells (active infection). In addition to Pf, Pv has been reported to be associated with complications in pregnancy, including anemia, miscarriage, low birth weight, and congenital malaria (Anstey et al. 2012; Costa et al. 2012). The placental pathology in Pv, however, does not show the same degree of parasite or monocyte involvement and remains to be elucidated.
SEVERE MALARIA ANEMIA
The pediatric population in areas of high transmission is uniquely susceptible to severe malarial anemia (SMA) during the first 2 years of life. When the children present, blood transfusion can be life-saving along with antimalarial drugs. The exact mechanisms of the pathways that lead to SMA are not well understood. The disruption of the immune response of monocytes and lymphocytes in the presence of hemozoin may lead to an inappropriate regulation of erythropoietin (through IL-6, regulated on activation, normal T-cell expressed, and secreted [RANTES], and macrophage inflammatory protein 1s [MIP-1s]) (Perkins et al. 2011). The removal of red cell membrane and red cells completely by the spleen in an accelerated fashion due to the presence of malaria has also been suggested.
ACIDOSIS
Acidosis is a complex metabolic state with a range of etiologies (Planche and Krishna 2006; Taylor et al. 2012). Within malaria, acidosis is caused by a combination of several factors. The malaria parasite produces Plasmodium lactate dehydrogenase (pLDH), which creates lactic acid leading to decreased pH. Respiratory distress is a common feature of severe malaria and, through sequestration, somnolence, and/or brain swelling, direct central suppression of the respiratory centers leads to irregular breathing patterns in the setting of acidosis, which may contribute to the pH imbalance. Supportive therapy to protect the airway and more aggressively rebalance the pH may decrease mortality (Cheng and Yansouni 2013).
CEREBRAL MALARIA
The unique ability of P. falciparum to bind to endothelium produces the clinicopathological syndrome of CM in both children and adults. In highly endemic settings, children under 5 years are at highest risk for the disease with a mortality of 10% to 20%. In low endemic settings, all ages are at risk and mortality can be higher in adults. In the nonimmune population (e.g., travelers, military, etc.), a low level of infection (<1% parasitemia) can result in clinical signs of CM and be life-threatening. The clinical manifestations of CM may start with a typical malaria presentation and quickly (over minutes to hours) degenerate to a comatose state. After exclusion of other possibly causes of coma (e.g., postictal state, hypoglycemia, meningitis, bacterial sepsis, head trauma/cerebral bleed, etc.), a clinical diagnosis of CM can be made, which is best confirmed by examination of the retinal for signs of malaria retinopathy (Seydel et al. 2015). In any case, the diagnostic pathological feature of the disease—at autopsy—is the presence of P. falciparum parasites in greater than 20% of capillaries in the brain by either tissue smear or histological sections (Taylor et al. 2004). Other pathological features that are variably present include fibrin thrombi, ring hemorrhages, discoloration of the brain, axonal injury, and capillary leakage (Dorovini-Zis et al. 2011). Brain vessels will appear congested in all cases with brain swelling more prominent in acute deaths such as African pediatric patients (<48 hours). Multiorgan failure and acute respiratory distress syndrome with diffuse alveolar damage is more common in adult patients, particularly those that have a prolonged course of disease (Hanson et al. 2010, 2014; Medana et al. 2011; Ponsford et al. 2012; Prapansilp et al. 2013; Maude et al. 2014).
The pathobiology of CM is not completely understood but a large body of evidence from both clinical and pathological studies has implicated a series of events and pathways at work within the disease landscape. Endothelial activation to a more “sticky” state is the first step. During the early phases of any malaria infection, macrophage stimulation leads to TNF-α production, the increase of which stimulates display of adherence molecules in the brain endothelium like intracellular adhesion molecule 1 (ICAM-1). Other such stimulations lead to a variety of other up-regulation events as summarized in Figure 1. A large number of malaria parasites can bind, through PfEMP1, to ubiquitous molecules such as CD36 (platelets and endothelium outside of the brain), which logically explains both the thrombocytopenia of malaria infection as well as the very low incidence of CM compared with all malaria infections. In the African child under 5 years of age, the combination of factors leading to the increase in the “sticky” phenotype is most likely a delay in treatment of a malarial infection paired with a lack of well-developed protective specific antibodies in the setting of poor general health (e.g., malnutrition). For infected individuals outside of this setting, the total lack of immunity drives the disease. The capacity of the human immune system to clear a disease, which relies almost exclusively on macrophage phagocytosis is the macrophage compartment. Antibodies speed this process by increased uptake efficiency. In autopsy studies of children, the spleen, the primary site of clearance of the parasites in most CM patients shows a large burden of malaria pigment and macrophages but no parasites. This suggests that the clearance ability of the spleen is high and efficient. Where the process of clearance breaks down may be in the capacity of circulating monocytes/macrophages to keep up with the sequestration and parasite life cycle (every 48 hours).
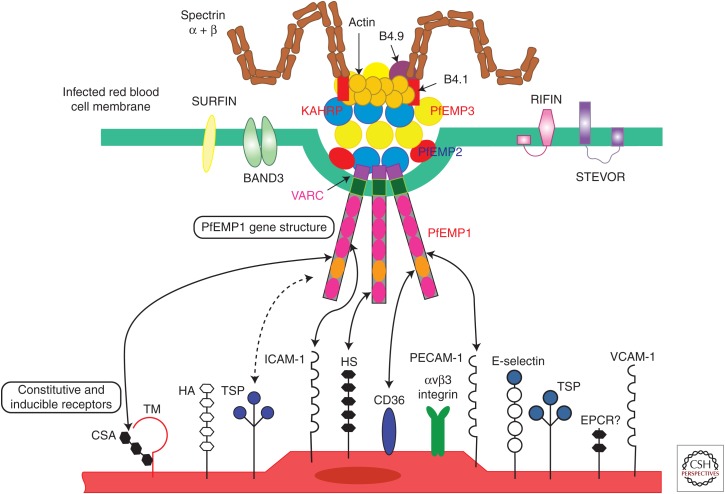
Figure 1.
A model of the interactions between Plasmodium parasites (predominantly from Plasmodium falciparum, top) and the human endothelium (bottom) is shown. The prominent feature is the P. falciparum erythrocyte membrane protein 1 (PfEMP1) molecules that protrude from the raised knob structures, which in themselves are made of a combination of human and parasite proteins in a tight complex. Surfins, rifins, and stevors from the parasite are also located in the red cell membrane. On the human side, a range of molecules, depending on tissue, are involved infected parasite interactions, including those that are always present on endothelium (e.g., CD36, outside of the brain and on platelets), are present during activation (e.g., intracellular adhesion molecule 1 [ICAM-1]), and are activated during interaction with other molecules (e.g., endothelial protein C receptor [EPCR] and thrombospondin [TSP]). Special tissue situations include chondroitin sulfate (CSA) in the placenta and CR-1 on uninfected red blood cells (which mediates rosette formation).
OVERLAP IN SEVERE DISEASE
Severe malaria in African children is often a monosyndromic presentation with little, if any, overt complications beyond coma, anemia, and/or acidosis. Studies suggest that in adult patients, the disease has multiple modalities at play in fatal cases including respiratory, hepatic, renal, and vascular complications. Equivalent studies in pediatric cases suggest that evidence of early changes in these same pathways may be at work; however, the rapid pace from presentation to either death or recovery does not allow these additional processes to manifest. In pediatric patients in Africa, an overlap of SMA, acidosis, and/or CM can occur, which may lead to higher mortality in overlap groups. CM, as there is not current ancillary treatment beyond antimalarias and supportive therapy, remains a key factor in mortality outcomes.
CONCLUDING REMARKS
Malaria pathogenesis has a broad and narrow context depending on the frame of reference. For fatal disease, the sequestration of Pf in tissues along with up-regulation of cytokines, toxic substances, and a lack of adequate, timely therapy, are key features of the process. For the remainder of malaria infections, the negative aspects of the disease are results of imbalance in the parasite–human interaction for a given species with the exception of Pk, a true zoonosis. As eradication moves from a goal to a rational plan of action, a firm understanding of the pathogenesis of malaria in all patient groups is required to not only predict where disease (especially severe disease) will occur but be able to prevent it.
ACKNOWLEDGMENTS
We thank the citizens of Malawi for their enormous contribution to understanding the pathogenesis of malaria in both pediatric and placental disease as well as the ongoing contributions from the citizens and our colleagues in Southeast Asia for their paramount work. Every illness with malaria affects the person, their family, and their society and our hope through this review is to bring us many steps closer to an end to malaria for everyone.
Footnotes
Editors: Dyann F. Wirth and Pedro L. Alonso
Additional Perspectives on Malaria: Biology in the Era of Eradication available at www.perspectivesinmedicine.org
REFERENCES
- Anstey NM, Douglas NM, Poespoprodjo JR, Price RN. 2012. Plasmodium vivax: Clinical spectrum, risk factors and pathogenesis. Adv Parasitol 80: 151–201. [DOI] [PubMed] [Google Scholar]
- Antinori S, Galimberti L, Milazzo L, Corbellino M. 2013. Plasmodium knowlesi: The emerging zoonotic malaria parasite. Acta Tropica 125: 191–201. [DOI] [PubMed] [Google Scholar]
- Babiker HA, Gadalla AA, Ranford-Cartwright LC. 2013. The role of asymptomatic P. falciparum parasitaemia in the evolution of antimalarial drug resistance in areas of seasonal transmission. Drug Resist Updat 16: 1–9. [DOI] [PubMed] [Google Scholar]
- Bañuls AL, Thomas F, Renaud F. 2013. Of parasites and men. Infect Genet Evol 20: 61–70. [DOI] [PubMed] [Google Scholar]
- Bozdech Z, Llinas M, Pulliam BL, Wong ED, Zhu J, DeRisi JL. 2003a. The transcriptome of the intraerythrocytic developmental cycle of Plasmodium falciparum. PLoS Biol 1: E5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozdech Z, Zhu J, Joachimiak MP, Cohen FE, Pulliam B, DeRisi JL. 2003b. Expression profiling of the schizont and trophozoite stages of Plasmodium falciparum with a long-oligonucleotide microarray. Genome Biol 4: R9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cator LJ, Lynch PA, Read AF, Thomas MB. 2012. Do malaria parasites manipulate mosquitoes? Trends Parasitol 28: 466–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravorty SJ, Hughes KR, Craig AG. 2008. Host response to cytoadherence in Plasmodium falciparum. Biochem Soc Trans 36: 221–228. [DOI] [PubMed] [Google Scholar]
- Cheng MP, Yansouni CP. 2013. Management of severe malaria in the intensive care unit. Crit Care Clin 29: 865–885. [DOI] [PubMed] [Google Scholar]
- Clark IA, Alleva LM, Budd AC, Cowden WB. 2008. Understanding the role of inflammatory cytokines in malaria and related diseases. Travel Med Infect Dis 6: 67–81. [DOI] [PubMed] [Google Scholar]
- Clark TD, Njama-Meya D, Nzarubara B, Maiteki-Sebuguzi C, Greenhouse B, Staedke SG, Kamya MR, Dorsey G, Rosenthal PJ. 2010. Incidence of malaria and efficacy of combination antimalarial therapies over 4 years in an urban cohort of Ugandan children. PLoS ONE 5: e11759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins WE, Jeffery GM. 2007. Plasmodium malariae: Parasite and disease. Clin Microbiol Rev 20: 579–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa FT, Lopes SC, Ferrer M, Leite JA, Martin-Jaular L, Bernabeu M, Nogueira PA, Mourao MP, Fernandez-Becerra C, Lacerda MV, et al. 2011. On cytoadhesion of Plasmodium vivax: Raison d'etre? Mem Inst Oswaldo Cruz 106: 79–84. [DOI] [PubMed] [Google Scholar]
- Costa FT, Lopes SC, Albrecht L, Ataide R, Siqueira AM, Souza RM, Russell B, Renia L, Marinho CR, Lacerda MV. 2012. On the pathogenesis of Plasmodium vivax malaria: Perspectives from the Brazilian field. Int J Parasitol 42: 1099–1105. [DOI] [PubMed] [Google Scholar]
- Cox-Singh J, Hiu J, Lucas SB, Divis PC, Zulkarnaen M, Chandran P, Wong KT, Adem P, Zaki SR, Singh B, et al. 2010. Severe malaria—A case of fatal Plasmodium knowlesi infection with post-mortem findings: A case report. Malaria J 9: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui L, Lindner S, Miao J. 2015. Translational regulation during stage transitions in malaria parasites. Ann NY Acad Sci 1342: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daily JP, Scanfeld D, Pochet N, Le Roch K, Plouffe D, Kamal M, Sarr O, Mboup S, Ndir O, Wypij D, et al. 2007. Distinct physiological states of Plasmodium falciparum in malaria-infected patients. Nature 450: 1091–1095. [DOI] [PubMed] [Google Scholar]
- Das BS. 2008. Renal failure in malaria. J Vector Borne Dis 45: 83–97. [PubMed] [Google Scholar]
- De Leenheer P, Pilyugin SS. 2008. Immune response to a malaria infection: Properties of a mathematical model. J Biol Dyn 2: 102–120. [DOI] [PubMed] [Google Scholar]
- de Souza JB. 2014. Protective immunity against malaria after vaccination. Parasite Immunol 36: 131–139. [DOI] [PubMed] [Google Scholar]
- Dixon MW, Dearnley MK, Hanssen E, Gilberger T, Tilley L. 2012. Shape-shifting gametocytes: How and why does P. falciparum go banana-shaped? Trends Parasitol 28: 471–478. [DOI] [PubMed] [Google Scholar]
- Doolan DL, Martinez-Alier N. 2006. Immune response to pre-erythrocytic stages of malaria parasites. Curr Mol Med 6: 169–185. [DOI] [PubMed] [Google Scholar]
- Dorovini-Zis K, Schmidt K, Huynh H, Fu W, Whitten RO, Milner D, Kamiza S, Molyneux M, Taylor TE. 2011. The neuropathology of fatal cerebral malaria in Malawian children. Am J Pathol 178: 2146–2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumas JF, Simard G, Flamment M, Ducluzeau PH, Ritz P. 2009. Is skeletal muscle mitochondrial dysfunction a cause or an indirect consequence of insulin resistance in humans? Diabetes Metab 35: 159–167. [DOI] [PubMed] [Google Scholar]
- Dzikowski R, Templeton TJ, Deitsch K. 2006. Variant antigen gene expression in malaria. Cell Microbiol 8: 1371–1381. [DOI] [PubMed] [Google Scholar]
- Fairhurst RM, Wellems TE. 2006. Modulation of malaria virulence by determinants of Plasmodium falciparum erythrocyte membrane protein-1 display. Curr Opin Hematol 13: 124–130. [DOI] [PubMed] [Google Scholar]
- Freitas do Rosario AP, Langhorne J. 2012. T cell-derived IL-10 and its impact on the regulation of host responses during malaria. Int J Parasitol 42: 549–555. [DOI] [PubMed] [Google Scholar]
- Goncalves RM, Lima NF, Ferreira MU. 2014. Parasite virulence, co-infections and cytokine balance in malaria. Pathog Glob Health 108: 173–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grau GE, Craig AG. 2012. Cerebral malaria pathogenesis: Revisiting parasite and host contributions. Future Microbiol 7: 291–302. [DOI] [PubMed] [Google Scholar]
- Gun SY, Claser C, Tan KS, Renia L. 2014. Interferons and interferon regulatory factors in malaria. Mediators Inflamm 2014: 243713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafalla JC, Silvie O, Matuschewski K. 2011. Cell biology and immunology of malaria. Immunol Rev 240: 297–316. [DOI] [PubMed] [Google Scholar]
- Hanson J, Lee SJ, Mohanty S, Faiz MA, Anstey NM, Charunwatthana P, Yunus EB, Mishra SK, Tjitra E, Price RN, et al. 2010. A simple score to predict the outcome of severe malaria in adults. Clin Infect Dis 50: 679–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson J, Anstey NM, Bihari D, White NJ, Day NP, Dondorp AM. 2014. The fluid management of adults with severe malaria. Crit Care 18: 642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt NH, Ball HJ, Hansen AM, Khaw LT, Guo J, Bakmiwewa S, Mitchell AJ, Combes V, Grau GE. 2014. Cerebral malaria: γ-Interferon redux. Front Cell Infect Microbiol 4: 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hviid L, Barfod L, Fowkes FJ. 2015. Trying to remember: Immunological B cell memory to malaria. Trends Parasitol 31: 89–94. [DOI] [PubMed] [Google Scholar]
- Josling GA, Llinas M. 2015. Sexual development in Plasmodium parasites: Knowing when it's time to commit. Nat Rev Microbiol 13: 573–587. [DOI] [PubMed] [Google Scholar]
- Kraemer SM, Smith JD. 2006. A family affair: var genes, PfEMP1 binding, and malaria disease. Curr Opin Microbiol 9: 374–380. [DOI] [PubMed] [Google Scholar]
- Krzych U, Zarling S, Pichugin A. 2014. Memory T cells maintain protracted protection against malaria. Immunol Lett 161: 189–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laishram DD, Sutton PL, Nanda N, Sharma VL, Sobti RC, Carlton JM, Joshi H. 2012. The complexities of malaria disease manifestations with a focus on asymptomatic malaria. Malaria J 11: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapp SA, Korir CC, Galinski MR. 2009. Redefining the expressed prototype SICAvar gene involved in Plasmodium knowlesi antigenic variation. Malaria J 8: 181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim C, Hansen E, DeSimone TM, Moreno Y, Junker K, Bei A, Brugnara C, Buckee CO, Duraisingh MT. 2013. Expansion of host cellular niche can drive adaptation of a zoonotic malaria parasite to humans. Nat Commun 4: 1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JT, Saunders DL, Meshnick SR. 2014. The role of submicroscopic parasitemia in malaria transmission: What is the evidence? Trends Parasitol 30: 183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Miao J, Cui L. 2011. Gametocytogenesis in malaria parasite: Commitment, development and regulation. Future Microbiol 6: 1351–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinas M, DeRisi JL. 2004. Pernicious plans revealed: Plasmodium falciparum genome wide expression analysis. Curr Opin Microbiol 7: 382–387. [DOI] [PubMed] [Google Scholar]
- malERA Consultative Group on Diagnoses and Diagnostics. 2011. A research agenda for malaria eradication: Diagnoses and diagnostics. PLoS Med 8: e1000396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh K, Kinyanjui S. 2006. Immune effector mechanisms in malaria. Parasite Immunol 28: 51–60. [DOI] [PubMed] [Google Scholar]
- Maude RJ, Barkhof F, Hassan MU, Ghose A, Hossain A, Abul Faiz M, Choudhury E, Rashid R, Abu Sayeed A, Charunwatthana P, et al. 2014. Magnetic resonance imaging of the brain in adults with severe falciparum malaria. Malaria J 13: 177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall MB, Sauerwein RW. 2010. Interferon-γ—Central mediator of protective immune responses against the pre-erythrocytic and blood stage of malaria. J Leukocyte Biol 88: 1131–1143. [DOI] [PubMed] [Google Scholar]
- McMorrow ML, Aidoo M, Kachur SP. 2011. Malaria rapid diagnostic tests in elimination settings—Can they find the last parasite? Clin Microbiol Infect 17: 1624–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medana IM, Day NP, Sachanonta N, Mai NT, Dondorp AM, Pongponratn E, Hien TT, White NJ, Turner GD. 2011. Coma in fatal adult human malaria is not caused by cerebral oedema. Malaria J 10: 267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menezes RG, Pant S, Kharoshah MA, Senthilkumaran S, Arun M, Nagesh KR, Bhat NB, Mahadeshwara Prasad DR, Karki RK, Subba SH, et al. 2012. Autopsy discoveries of death from malaria. Legal Med 14: 111–115. [DOI] [PubMed] [Google Scholar]
- Milner DA Jr, Pochet N, Krupka M, Williams C, Seydel K, Taylor TE, Van de Peer Y, Regev A, Wirth D, Daily JP, et al. 2012. Transcriptional profiling of Plasmodium falciparum parasites from patients with severe malaria identifies distinct low vs. high parasitemic clusters. PLoS ONE 7: e40739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohandas N, An X. 2012. Malaria and human red blood cells. Med Microbiol Immunol 201: 593–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Pérez DA, Ruíz JA, Patarroyo MA. 2013. Reticulocytes: Plasmodium vivax target cells. Biol Cell 105: 251–260. [DOI] [PubMed] [Google Scholar]
- Mueller I, Zimmerman PA, Reeder JC. 2007. Plasmodium malariae and Plasmodium ovale—The “bashful” malaria parasites. Trends Parasitol 23: 278–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller M, Schlagenhauf P. 2014. Plasmodium knowlesi in travellers, update 2014. Int J Infect Dis 22: 55–64. [DOI] [PubMed] [Google Scholar]
- Oakley MS, Gerald N, McCutchan TF, Aravind L, Kumar S. 2011. Clinical and molecular aspects of malaria fever. Trends Parasitol 27: 442–449. [DOI] [PubMed] [Google Scholar]
- Oguike MC, Betson M, Burke M, Nolder D, Stothard JR, Kleinschmidt I, Proietti C, Bousema T, Ndounga M, Tanabe K, et al. 2011. Plasmodium ovale curtisi and Plasmodium ovale wallikeri circulate simultaneously in African communities. Int J Parasitol 41: 677–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins DJ, Were T, Davenport GC, Kempaiah P, Hittner JB, Ong'echa JM. 2011. Severe malarial anemia: Innate immunity and pathogenesis. Int J Biol Sci 7: 1427–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry GH. 2014. Parasites and human evolution. Evol Anthropol 23: 218–228. [DOI] [PubMed] [Google Scholar]
- Planche T, Krishna S. 2006. Severe malaria: Metabolic complications. Curr Mol Med 6: 141–153. [DOI] [PubMed] [Google Scholar]
- Ponsford MJ, Medana IM, Prapansilp P, Hien TT, Lee SJ, Dondorp AM, Esiri MM, Day NP, White NJ, Turner GD. 2012. Sequestration and microvascular congestion are associated with coma in human cerebral malaria. J Infect Dis 205: 663–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prapansilp P, Medana I, Mai NT, Day NP, Phu NH, Yeo TW, Hien TT, White NJ, Anstey NM, Turner GD. 2013. A clinicopathological correlation of the expression of the angiopoietin-Tie-2 receptor pathway in the brain of adults with Plasmodium falciparum malaria. Malaria J 12: 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punsawad C. 2013. Effect of malaria components on blood mononuclear cells involved in immune response. Asian Pac J Trop Biomed 3: 751–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajahram GS, Barber BE, William T, Menon J, Anstey NM, Yeo TW. 2012. Deaths due to Plasmodium knowlesi malaria in Sabah, Malaysia: Association with reporting as Plasmodium malariae and delayed parenteral artesunate. Malaria J 11: 284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall LM, Engwerda CR. 2010. TNF family members and malaria: Old observations, new insights and future directions. Exp Parasitol 126: 326–331. [DOI] [PubMed] [Google Scholar]
- Sabeti PC, Reich DE, Higgins JM, Levine HZ, Richter DJ, Schaffner SF, Gabriel SB, Platko JV, Patterson NJ, McDonald GJ, et al. 2002. Detecting recent positive selection in the human genome from haplotype structure. Nature 419: 832–837. [DOI] [PubMed] [Google Scholar]
- Seydel KB, Kampondeni SD, Valim C, Potchen MJ, Milner DA, Muwalo FW, Birbeck GL, Bradley WG, Fox LL, Glover SJ, et al. 2015. Brain swelling and death in children with cerebral malaria. N Engl J Med 372: 1126–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh B, Daneshvar C. 2013. Human infections and detection of Plasmodium knowlesi. Clin Microbiol Rev 26: 165–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JD, Rowe JA, Higgins MK, Lavstsen T. 2013. Malaria's deadly grip: Cytoadhesion of Plasmodium falciparum–infected erythrocytes. Cell Microbiol 15: 1976–1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone W, Goncalves BP, Bousema T, Drakeley C. 2015. Assessing the infectious reservoir of falciparum malaria: Past and future. Trends Parasitol 31: 287–296. [DOI] [PubMed] [Google Scholar]
- Ta TH, Hisam S, Lanza M, Jiram AI, Ismail N, Rubio JM. 2014. First case of a naturally acquired human infection with Plasmodium cynomolgi. Malaria J 13: 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor TE, Fu WJ, Carr RA, Whitten RO, Mueller JS, Fosiko NG, Lewallen S, Liomba NG, Molyneux ME. 2004. Differentiating the pathologies of cerebral malaria by postmortem parasite counts. Nat Med 10: 143–145. [DOI] [PubMed] [Google Scholar]
- Taylor WR, Hanson J, Turner GD, White NJ, Dondorp AM. 2012. Respiratory manifestations of malaria. Chest 142: 492–505. [DOI] [PubMed] [Google Scholar]
- Udagama PV, Atkinson CT, Peiris JS, David PH, Mendis KN, Aikawa M. 1988. Immunoelectron microscopy of Schuffner's dots in Plasmodium vivax–infected human erythrocytes. Am J Pathol 131: 48–52. [PMC free article] [PubMed] [Google Scholar]
- Volkman SK, Sabeti PC, DeCaprio D, Neafsey DE, Schaffner SF, Milner DA Jr, Daily JP, Sarr O, Ndiaye D, Ndir O, et al. 2007. A genome-wide map of diversity in Plasmodium falciparum. Nat Genet 39: 113–119. [DOI] [PubMed] [Google Scholar]
- WHO. 2015. World malaria report 2015. World Health Organization, Geneva. [Google Scholar]
- Wright GJ, Rayner JC. 2014. Plasmodium falciparum erythrocyte invasion: Combining function with immune evasion. PLoS Pathog 10: e1003943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wykes M, Good MF. 2006. Memory B cell responses and malaria. Parasite Immunol 28: 31–34. [DOI] [PubMed] [Google Scholar]
- Zimmerman PA, Ferreira MU, Howes RE, Mercereau-Puijalon O. 2013. Red blood cell polymorphism and susceptibility to Plasmodium vivax. Adv Parasitol 81: 27–76. [DOI] [PMC free article] [PubMed] [Google Scholar]