Abstract
T helper (Th)17 cells are responsible for host defense against fungi and certain extracellular bacteria but have also been reported to play a role in a variety of autoimmune diseases. Th17 cells respond to environmental cues, are very plastic, and might also be involved in tissue homeostasis and regeneration. The imprinting of pathogenic properties in Th17 cells in autoimmunity seems highly dependent on interleukin (IL)-23. Since Th17 cells were first described in experimental autoimmune encephalomyelitis, they have been suggested to also promote tissue damage in multiple sclerosis (MS). Indeed, some studies linked Th17 cells to disease severity in MS, and the efficacy of anti-IL-17A therapy in MS supported this idea. In this review, we will summarize molecular features of Th17 cells and discuss the evidence for their function in experimental models of autoimmune diseases and MS.
The first report of interleukin (IL)-23 in 2000 (Oppmann et al. 2000) paved the way for the discovery of T helper (Th)17 cells (Aggarwal et al. 2003; Harrington et al. 2005; Park et al. 2005). Indeed, Il23p19−/− mice lack a population of IL-17-producing T cells at the site of chronic inflammatory lesions (Cua et al. 2003). Notably, IL-17 had already been cloned in 1993 (Rouvier et al. 1993) and its receptor, IL-17RA, in 1995 (Yao et al. 1995). However, only when IL-17 was identified as a product of Th cells that appeared to be induced by IL-23, interest in the IL-23/Th17 axis grew massively in the scientific community because it provided a potential pathogenetic framework for a variety of severely disabling organ-specific autoimmune diseases, including rheumatoid arthritis, diabetes mellitus, and multiples sclerosis (MS). The discovery of Th17 cells expanded the Th cell paradigm of Th1 and Th2 cells, and challenged the idea that chronic inflammatory diseases and organ-specific autoimmune diseases were caused by exaggerated Th1 responses. Th17 cells were first explored in experimental autoimmune encephalomyelitis (EAE), an animal model of MS, and were defined by their unique cytokine profile of IL-17A (IL-17), IL-17F, IL-21, IL-22, and their prototypical transcription factor retinoid-related orphan receptor-γt (ROR-γt) (Ivanov et al. 2006; Korn et al. 2009). However, Th17 cells are neither the only target of IL-23 (which is also sensed by γδ T cells, natural killer [NK] T cells, and innate lymphoid type 3 cells [ILC3s]), nor are they the only source of IL-17, which is also produced by γδ T cells, NK T cells, and ILC3s (reviewed in Cua and Tato 2010).
Early work suggested that IL-17 might be important in driving MS pathology because this cytokine was identified within lesions in the central nervous system (CNS) (Lock et al. 2002). However, the evidence for the correlation of Th17 frequencies in the peripheral blood or cerebrospinal fluid with disease activity in MS is limited. Even though genome-wide association studies (GWAS) have identified single nucleotide polymorphisms (SNPs) in the IL23 and IL23R genes in patients with inflammatory bowel disease (IBD) and psoriasis (Duerr et al. 2006; Cargill et al. 2007), associations of SNPs in genes involved in the IL-23R signaling pathway are only now beginning to be associated with MS with increasing numbers of patients and controls fed into GWAS analyses. Yet, an antibody that neutralizes both IL-12 and IL-23 was inefficient in MS patients as to the suppression of magnetic resonance imaging (MRI) activity (Segal et al. 2008). Nevertheless, neutralization of IL-17 suppressed disease activity in MS patients (Havrdová et al. 2012), and some reports propose that other products of Th17 cells (besides IL-17) play an important role in the inflammatory process in the CNS during MS.
In this review, we will highlight the factors that are responsible for the differentiation of pathogenic and nonpathogenic Th17 cells and compare the evidence for a role of IL-23 and Th17 cells in the pathogenesis of EAE and human MS.
THE BIOLOGY OF Th17 CELLS IN ANIMAL MODELS OF MS
Th17 cells were first discovered in EAE, and a substantial amount of knowledge about Th17 cell biology was gathered by using this model. It is the most common animal model for MS and is induced by immunization with a CNS-derived autoantigen emulsified in complete Freund's adjuvant (CFA). Because transfer of CNS antigen-specific Th1 cells induced EAE and interferon (IFN)-γ was found in CNS lesions of EAE mice, EAE was believed to be a Th1-driven autoimmune disease. Moreover, neutralizing polyclonal antibodies to IL-12 in rodents, and a monoclonal antibody to the p40 subunit of the human IL-12 heterodimer (p40/p35) in marmosets were able to suppress the induction of EAE (Leonard et al. 1995; Brok et al. 2002). Because IL-12 is crucial for Th1 differentiation, this further supported the idea that EAE was a Th1-mediated autoimmune disease. However, p35-deficient mice were still susceptible to EAE (Becher et al. 2002), and this was also true for a variety of other factors required for the differentiation of Th1 cells, including IFN-γ itself, fundamentally challenging the concept of EAE as a Th1 disease (Ferber et al. 1996; Zhang et al. 2003; Bettelli et al. 2004; Gutcher et al. 2006). IL-23 is a heterodimer that comprises the p40 subunit of IL-12 and a private p19 subunit. IL-23 promotes the expansion of Th17 cells (Aggarwal et al. 2003; Harrington et al. 2005; Park et al. 2005). Thus, it appeared an appealing idea to implicate Th17 cells (and not Th1 cells) as major inducers of autoimmune tissue inflammation because IL-23p19-deficient mice (in contrast to IL-12p35-deficient animals) were resistant to EAE (Cua et al. 2003).
Adoptive transfer studies showed that both in vitro–differentiated and restimulated myelin oligodendrocyte glycoprotein (MOG)-specific Th1 and Th17 cells were able to induce EAE in recipient mice. However, host animals that received antigen-specific Th17 cells had more lesions in the meninges and parenchyma and showed a mix of classical and atypical signs of EAE compared to recipients of Th1 cells (Jäger et al. 2009).
It has been difficult to address which are the main effector cytokines of Th17 cells in EAE pathogenesis. EAE onset was delayed in Il17a−/− mice, and disease severity was significantly reduced (Komiyama et al. 2006). Moreover, EAE severity was suppressed in wild-type (WT) SJL mice that were immunized with myelin proteolipid protein and received IL-17-neutralizing antibodies (Langrish et al. 2005). Because IL-17-deficient mice are only partly protected from EAE, the pathogenicity of Th17 cells can likely not be merely narrowed down to this one signature cytokine. IL-23 induces the expression of granulocyte macrophage colony-stimulating factor (GM-CSF) in Th17 cells (Codarri et al. 2011; El-Behi et al. 2011), although the expression of GM-CSF is not restricted to Th17 cells (Grifka-Walk et al. 2015). However, because GM-CSF-deficient mice are resistant to EAE (McQualter et al. 2001), GM-CSF was proposed to be a major effector molecule during Th17-mediated CNS inflammation. GM-CSF triggers a positive feedback loop, inducing the production of IL-23 by antigen-presenting cells (APCs) (El-Behi et al. 2011). However, there has been some debate on the major cellular targets of GM-CSF. Recently, a subset of monocyte-derived dendritic cells (DCs) was identified to be crucially activated by GM-CSF to promote inflammation in the CNS (Ko et al. 2014; Croxford et al. 2015).
Besides IL-23, IL-1β has also been involved in the expansion and late differentiation of pathogenic Th17 cells because the induction of Th17 cells in IL-1 receptor type 1 (IL-1R1)-deficient mice was abrogated and was not restored by IL-23 alone (Sutton et al. 2006). Expression of IL-1R1 in Th17 cells is induced by IL-6, and IL-1R1 signaling promotes expression of ROR-γt by maintaining high levels of IFN regulatory factor 4 (Irf4) (Chung et al. 2009). Co-overexpression of Irf4 and ROR-γt restored Th17-cell polarization in the absence of IL-1β-mediated signaling (Chung et al. 2009). In fact, Irf4 is essential for the production of IL-17 and IL-21, and Irf4-deficient mice are completely protected from EAE (Brüstle et al. 2007; Chen et al. 2008; Huber et al. 2008). Moreover, a recent study showed that IL-1β was able to skew T-cell development toward Th17 cells in the gut, where it was required to override retinoic acid–mediated Foxp3 expression in T cells and thereby tipped the Th17 cell/induced CD4+ regulatory T (iTreg) cell balance toward Th17 cells (Basu et al. 2015).
Interestingly, mice deficient for the Th1-associated transcription factor T-bet are protected from CNS autoimmunity (Bettelli et al. 2004). Fate-mapping studies showed that previous IL-17-producing T cells started expressing IFN-γ at sites of chronic inflammation (Lee et al. 2009; Kurschus et al. 2010; Hirota et al. 2011), raising the question whether the production of IFN-γ by late Th17 cells was dependent on T-bet (Duhen et al. 2013; Wang et al. 2014; Krausgruber et al. 2016). Because infections with Candida albicans supported sustained expression of IL-17 in antigen-specific T cells, the induction of effector cytokines previously unrelated to the Th17 signature portfolio, including IFN-γ, GM-CSF, and tumor necrosis factor (TNF), in “historic” IL-17 producers in autoimmunity was surprising and triggered a debate on the stability of the “Th17 lineage.” However, the presence of IL-17/IFN-γ double producing exTh17 cells in the inflamed CNS is a robust finding and is tightly dependent on IL-23 (Hirota et al. 2011).
In summary, the EAE model has been instrumental in the research of the IL-23/Th17 axis in CNS autoimmunity. The difficulties to translate some EAE findings into MS might, in part, be because of the strong bias toward Th17 responses induced by the adjuvants used for the induction of EAE. However, plasticity and effector mechanisms of Th17 cells were intensively studied in EAE and revealed a variety of molecular mechanisms that also applied in MS (Fig. 1).
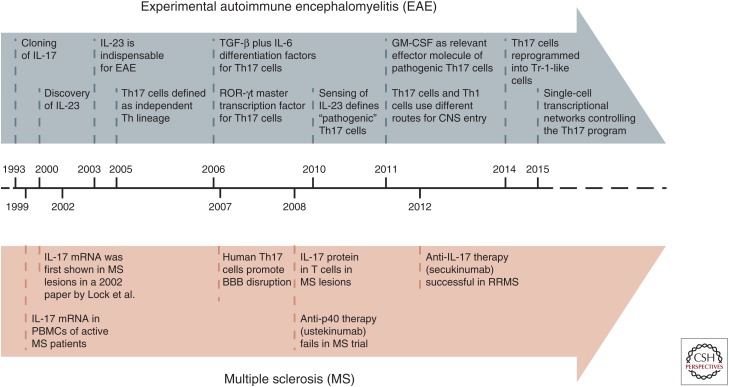
Figure 1.
Timeline. Scientific discoveries that led to the concept of the “interleukin (IL)-23/T helper (Th)17 axis” in experimental autoimmune encephalomyelitis (EAE) and multiple sclerosis (MS). TGF, Transforming growth factor; GM-CSF, granulocyte macrophage colony-stimulating factor; CNS, central nervous system; PBMCs, peripheral blood mononuclear cells; BBB, blood–brain barrier; RRMS, relapsing remitting multiple sclerosis.
CYTOKINE MILIEUS FOR Th17-CELL DIFFERENTIATION AND PLASTICITY
Despite the fact that IL-23 is necessary for the induction of EAE (Cua et al. 2003; Bettelli et al. 2006), it is no bona fide differentiation factor for Th17 cells because naïve T cells lack IL-23R and do not respond to IL-23 (Mangan et al. 2006). Instead, a cocktail of transforming growth factor-β (TGF-β) and IL-6 is sufficient to differentiate murine naïve T cells into Th17 cells (Bettelli et al. 2006; Mangan et al. 2006; Veldhoen et al. 2006). In addition, IL-21 cooperates with TGF-β to induce Th17 cells in a milieu devoid of IL-6, and thus presents an IL-6-independent mode of Th17-cell generation (Korn et al. 2007; Nurieva et al. 2007).
The role of TGF-β for Th17-cell differentiation has been controversial. Human T cells were reported to require IL-1β, IL-6, and IL-23 to differentiate into Th17 cells, and TGF-β even inhibited Th17 cell polarization (Acosta-Rodriguez et al. 2007; Wilson et al. 2007). However, TGF-β was clearly required for the differentiation of naïve human T cells into Th17 cells and in particular for the induction of RORC (the human homolog of ROR-γt) in serum-free medium (Manel et al. 2008; Yang et al. 2008). IL-1β and IL-6 alone only induced IL-17 secretion in precommitted central memory CD4+ T cells. Because T cells produce TGF-β themselves, an autocrine or paracrine TGF-β-dependent loop must be considered during Th17 differentiation (Li et al. 2007; Gutcher et al. 2011), and more recent reports have shown that Th17 cell differentiation can occur in the absence of exogenous TGF-β in murine T cells. A combination of IL-1β, IL-6, and IL-23 may induce Th17 cell differentiation (Ghoreschi et al. 2010), and these Th17 cells coexpressed T-bet and ROR-γt and were highly pathogenic in inducing EAE upon adoptive transfer.
These experiments supported the idea that distinct “stable” subsets of Th17 cells might exist (Lee et al. 2012). It had earlier been proposed that naïve T cells that were stimulated with TGF-β1, and IL-6 produced IL-17 and IL-10 but did not induce autoimmunity upon adoptive transfer into host animals unless previously exposed to IL-23 (McGeachy et al. 2007). In fact, IL-23R signaling was shown to be essential for the pathogenicity of Th17 cells (McGeachy et al. 2009). It is currently under investigation whether pathogenic versus nonpathogenic Th17 cells represent terminally differentiated Th cell subsets or are different stages of the Th17 developmental program. Also, the conditions under which different subsets of Th17 cells are generated in vivo remain to be determined.
One concept, which has been elaborated to some extent, is the idea that different subsets of Th17 cells likely reflect Th17 differentiation at different priming sites in vivo. For instance, Th17 differentiation in draining lymph nodes and mucosal tissues, like the intestinal lamina propria, is IL-6-dependent, whereas Th17 priming in the spleen seems to be independent of IL-6 (Hu et al. 2011). The need for IL-6 in Th17 differentiation was associated with the presence of CD103+ DCs, which produce retinoic acid and are proficient in inducing iTregs. CD103+ DCs are relatively abundant in the gut but only comprise a small fraction of splenic DCs. Thus, the local milieu in the spleen is less permissive for iTreg programming of naïve conventional T cells obviating the need for IL-6 to start the Th17 developmental program. However, it remains to be determined how naïve T cells sense IL-6 and whether IL-6, which is produced by many cellular sources, must be provided by the same APC that also presents antigen in the context of major histocompatibility complex (MHC) class II molecules to result in productive Th17 differentiation. IL-1β appears to be essential for the differentiation of Th17 cells in all tissue compartments, which is in line with previous studies and the finding that pertussis toxin promotes Th1/Th17 cell differentiation by inducing the production of IL-1β (Ronchi et al. 2016). However, in contrast to IL-6, IL-1β might have more “mitogenic” properties rather than representing an instructive cue for the specific differentiation of Th17 cells (see below and Kim et al. 2006).
In contrast to Th1 cells that only express IL-12R (heterodimeric IL-12Rβ1/IL-12Rβ2) but lack IL-23R (heterodimeric IL-12Rβ1/IL-23R), Th17 cells express both receptors and respond to both IL-12 and IL-23. In addition, Th17 cells also respond to IL-27, another IL-12 family cytokine (Stumhofer et al. 2006, 2007; Diveu et al. 2009). This exquisite responsiveness to IL-12 family cytokines might in part explain the plasticity of Th17 cells. For example, IL-12 and IL-27 have been shown to “reprogram” precommitted Th17 cells to a Tr1-like phenotype by inducing the transcriptional modulator Blimp1 (Heinemann et al. 2014). IL-23 failed to induce Blimp1, but promoted the expression of ROR-γt and induced the generation of pathogenic Th17 cells, characterized by the production of IL-17, IFN-γ, and GM-CSF (Heinemann et al. 2014). Another pathway to skew Th17 cells into Tr-1 like cells is dependent on the aryl hydrocarbon receptor (Ahr) (Gagliani et al. 2015). Tr-1 cells are defined by the expression of IFN-γ and IL-10, but lack of IL-17 has been shown to critically contribute to the containment of immunopathology in the context of chronic inflammatory diseases (Groux et al. 1997; Battaglia et al. 2004, 2006; Mayo et al. 2016).
Other “nonimmune” (environmental) cues, including salt concentration and even fatty acids, were also reported to modulate the differentiation and plasticity of Th17 cells (Kleinewietfeld et al. 2013; Wu et al. 2013; Berod et al. 2014). Although this has not been rigorously tested, it appears that Th17 cells are more responsive to a variety of external factors than Th1 cells or Th2 cells, suggesting that interventional strategies targeting these instructive cues (many of which are modulated by lifestyle) might exert their effects via modulation of Th17 immunity.
TRANSCRIPTION FACTOR NETWORKS IN Th17 CELLS
The idea that Th17 cells are a Th cell lineage of their own received a lot of attention when it was shown that IL-17 production in CD4+ T cells could be induced in the absence of the master transcription factors for Th1 and Th2 cells, T-bet and Gata-3, respectively (Harrington et al. 2005; Park et al. 2005).
Later, ROR-γt was discovered to be the “key” transcription factor for Th17 cell differentiation (Ivanov et al. 2006). Besides genetic loss-of-function studies, small-molecule ROR-γt antagonists were shown to impair Th17 cell differentiation, emphasizing the role of ROR-γt as a crucial transcription factor for Th17 cells (Xiao et al. 2014). Network analyses of Th17 cells that were differentiated with TGF-β and IL-6 showed the involvement of Irf4 and basic leucine zipper transcription factor ATF-like (Batf), enabling transcriptional activity of Stat3 and ROR-γt (Ciofani et al. 2012). Irf4-deficient mice were protected from EAE and showed crippled Th17 development, as a result of reduced ROR-γt expression (Brüstle et al. 2007). Batf is a member of the activator protein 1 (AP-1) transcription factor family and is believed to inhibit AP-1 activity. Batf-deficient mice are also resistant to EAE and Batf−/− T cells fail to induce ROR-γt and IL-21 (Schraml et al. 2009). Overexpression of ROR-γt or IL-21 could not fully restore IL-17 production in Batf−/− T cells, suggesting that ROR-γt and Batf may have to cooperate in Th17 development. There is remarkable overlap between Batf and Irf4 promoter regions and cooperative binding of Batf and Irf4 promotes chromatin accessibility for Stat3 and ROR-γt (Ciofani et al. 2012; Li et al. 2012). Moreover, ROR-γt only exclusively regulates a few Th17 genes, namely, Il17, Il17f, and Il23r, but modulates and fine tunes expression at key loci in T cells with a preestablished Th17 lineage program, suppressing alternative fate decisions toward other lineages (Ciofani et al. 2012). Importantly, this suggests that a transcription factor complex regulates lineage fate decisions in Th17 cell development instead of only one master transcription factor.
IL-1β signaling in Th17 cells contributes to reinforcing Th17 responses. Mechanistically, IL-1β promotes the phosphorylation of mammalian target of rapamycin (mTOR), a central regulator of cellular metabolism, and therefore enhances the metabolic fitness of Th17 cells during inflammation (Gulen et al. 2010). In detail, mTOR induces the expression of myelocytomatosis oncogene (Myc), which switches the metabolic pathway from fatty acid β-oxidation to the glycolytic, pentose phosphate, and glutaminolytic pathways to provide enough energy for proliferating cells during T-cell priming. Moreover, signaling through mTOR induces the expression of hypoxia-inducible factor 1α (Hif1α), a transcription factor that promotes Th17 cell differentiation by direct transcriptional activation of ROR-γt and forms a complex with ROR-γt and p300 to induce IL-17 expression (Dang et al. 2011). Additionally, the Th17/Treg balance (Bettelli et al. 2006) is skewed by Hif1α toward Th17 cells by binding Foxp3 and targeting it for proteasomal degradation (Dang et al. 2011).
Recent years have shed some light on the underlying molecular processes that are executed during Th17 cell differentiation. The Th17 transcriptional program is more complex than originally envisioned and is not only dependent on Stat3 and ROR-γt to generate fully committed Th17 cells. Despite all these insights, the role of IL-23 in producing pathogenic Th17 cells is still not fully understood and cannot merely be reduced to its induction of Stat3.
THE PIVOTAL ROLE OF IL-23 IN Th17 CELL PROGRAMMING
IL-23 has been described in the context of the differentiation of pathogenic Th17 cells in many studies and it seems clear that the IL-23/Th17 axis and autoimmunity are strongly intertwined in EAE. The spectrum of actions of IL-23 in the context of Th17 cell development includes expansion and stabilization of pathogenic Th17 cells, maintenance of IL-17 production, induction of GM-CSF expression, and generation of IL-17/IFN-γ double-positive T cells.
Whereas Il23r−/− mice are resistant to EAE (Awasthi et al. 2009; McGeachy et al. 2009), mixed WT plus Il23r−/− bone marrow chimeric mice develop EAE with regular disease severity, which allows for the analysis of IL-23R functions on T cells in an inflammatory milieu. Here, Il23r−/− T cells started expressing IL-17, but were arrested at an early activation stage (marked by impaired down-regulation of IL-2 and CD27 expression) (McGeachy et al. 2009). Eventually, Il23r−/− Th17 cells failed to be recruited to the inflamed CNS. In contrast to CD4+ T cells, the IL-23R-deficient myeloid compartment was fully functional as compared to its WT counterpart. Thus, while Il23r−/− T cells exhibit impaired effector functions, the mechanistic underpinning for this phenomenon remains to be identified.
In a model of colitis, IL-23R signaling in T cells in the gut increased the accumulation of Th17 cells, as well as the percentage of IL-17/IFN-γ double-positive T cells, and inhibited the differentiation of Foxp3-positive T cells (Ahern et al. 2010). However, while in the CNS, IL-23 signaling in Th17 cells is believed to promote IL-17/IFN-γ double producers in a T-bet-dependent manner (Hirota et al. 2011; Wang et al. 2014); T-bet was dispensable for the IL-23-dependent induction of pathogenic IL17/IFN-γ-positive T cells in the colon (Krausgruber et al. 2016). Notably, T cells from T-bet-deficient donors injected into Rag1−/− mice showed even higher frequencies of IL-17/IFN-γ double-positive T cells in the colon. Interestingly, this is in contrast to earlier studies wherein transfer of T-bet-deficient T cells into host mice failed to induce colitis (Neurath et al. 2002). Whereas different genetic backgrounds and microbial differences, such as colonization with segmented filamentous bacteria, might in part be responsible for these conflicting results, further investigation is required to identify the “nonredundant” pathogenic function of IL-23 in the context of T-cell-mediated autoimmunity.
The serum glucocorticoid kinase 1 (Sgk1) was identified in a transcriptional profiling study to be an essential node downstream of IL-23 signaling (Wu et al. 2013). Sgk1 is a serine/threonine protein kinase (Waldegger et al. 1997) and its expression is increased by elevated salt concentrations (Wu et al. 2013). The increase in salt concentrations was associated with higher levels of Th17 cells in mice and humans, and resulted in a more severe form of EAE in mice fed with a high-salt diet (Kleinewietfeld et al. 2013). Mechanistically, Sgk1 inhibits a direct repressor of IL-23R expression (i.e., Foxo1), and thereby stabilizes the Th17 phenotype through enhanced induction of IL-23R expression (Wu et al. 2013). However, Sgk1 is also a stress response gene (Miyata et al. 2011; Yuen et al. 2011) and might constitute a more general link between environmental stressors and inflammatory responses.
Furthermore, recombination of signal-binding protein for the immunoglobulin κJ region (RBPJ), which is a downstream regulator of Notch signaling, has been linked to the development of Th17 cells (Yosef et al. 2013). In fact, inhibition of Notch signaling results in a reduction of Th17-associated cytokines in murine and human Th17 cells and ameliorates EAE (Keerthivasan et al. 2011). The role of RBPJ-mediated Notch signaling in the development of Th17 cells has recently been addressed in more detail. RBPJ was found to promote the differentiation of pathogenic Th17 cells by directly up-regulating IL-23R expression through binding and transactivating the IL23r promoter together with ROR-γt. Conversely, RBPJ repressed Il10 in Th17 cells (Meyer Zu Horste et al. 2016).
Taken together, multiple upstream signals that are associated with enhanced pathogenicity of Th17 cells converge (either directly or indirectly) in the transactivation of the Il23r gene. Yet, despite the clear evidence of IL-23 being associated with the development of pathogenic Th17 cells and the recent advances in how the expression of IL-23R is induced in Th17 cells, its functions and downstream transcriptional targets in T cells in different settings of inflammation still remain to be fully determined.
THE IL-23/Th17 AXIS IN HUMAN MULTIPLE SCLEROSIS
While GWAS in MS patients indicated that MS is a T-cell-mediated disorder, a predominant role of Th17 cells in MS has not been directly evident (International Multiple Sclerosis Genetics Consortium (IMSGC) et al. 2011, 2013). Interestingly, an SNP within the STAT3 gene was even protective and was linked to a decreased odds ratio for the development of MS while the same SNP (rs744166) constitutes a risk allele for Crohn's disease (Jakkula et al. 2010). However, replication in independent MS cohorts has been difficult (Cenit et al. 2010; Lill et al. 2012), and two other SNPs (rs9891119 and rs4796791) within the STAT3 gene were associated with marginally increased risks for MS (International Multiple Sclerosis Genetics Consortium (IMSGC) et al. 2011, 2013). For none of these haplotypes, immunologic analyses as to the modulation of Th17 responses are available in MS patients. In contrast to MS, both GWAS data and functional analyses of the IL-23/Th17 pathway provide clear evidence of its importance in the pathogenic process of IBD and psoriasis (Duerr et al. 2006; Cargill et al. 2007).
In fact, in early work, no SNP in the IL23 gene or IL23R gene was found to be associated with MS susceptibility (Roos et al. 2008). However, larger GWAS now provide evidence for the association of several molecules involved in the IL-23R signaling pathway (including STAT3 and TYK2) with MS. Despite the efficacy of IL-12/IL-23p40-neutralizing antibodies in EAE, treatment in human MS with ustekinumab, a human monoclonal antibody against the p40 subunit (shared between IL-23 and IL-12), did not lower the cumulative number of new gadolinium-enhancing T1-weighted MRI lesions in a phase II clinical trial with relapsing-remitting multiple sclerosis (RRMS) patients (Segal et al. 2008). This lack of efficacy might at least in part be a result of the inclusion of patients with advanced disease where inflammation may no longer be driven by T-cell activation (reviewed in Longbrake and Racke 2009; Mahad et al. 2015). In addition, IL-23 is likely involved in the programming of pathogenic Th17 cells at the site of inflammation and it is unknown to what extent ustekinumab is able to cross the blood–brain and blood–cerebrospinal fluid barriers to access relevant anatomical compartments and block IL-23 at these sites (see comment by Martin 2008).
A number of correlative studies linked Th17 cells with human MS. IL-17 messenger RNA (mRNA) and protein were detected in perivascular lymphocytes and in CD4+ and CD8+ T cells in active brain lesions of MS patients (Lock et al. 2002; Tzartos et al. 2008). Early studies with small numbers of MS patients indicated that mononuclear cells expressing Th17-associated molecules, including IL-17 mRNA, were more frequent in the peripheral blood and cerebrospinal fluid of RRMS patients as compared with controls and further increased during clinical exacerbations (Matusevicius et al. 1999; Hedegaard et al. 2008; Brucklacher-Waldert et al. 2009). Molecules identified to specifically shape the Th17-expression profile, such as, for example, microRNA-326, showed a higher expression in peripheral blood lymphocytes of MS patients as compared with healthy controls (Du et al. 2009). Furthermore, the ex vivo frequency of Th17 (but not Th1) cells in the cerebrospinal fluid (but not in the peripheral blood) correlated with disease activity in early MS and RRMS patients (Brucklacher-Waldert et al. 2009). Also, in comparison to Th1 clones, Th17 clones generated from the peripheral blood and cerebrospinal fluid of RRMS patients showed higher levels of activation markers, costimulatory molecules, as well as molecules involved in homing of lymphocytes to the CNS (Brucklacher-Waldert et al. 2009). Even more convincing correlations of Th17-associated molecules with disease severity were detected when myelin-specific T-cell populations instead of polyclonal repertoires were interrogated for the expression of Th17-associated factors. Chemokine receptors were used as surface markers to identify Th cell subsets in humans, and Th17 cells express CCR6 while Th1 cells express CXCR3 (Sallusto et al. 1998; Annunziato et al. 2007). Comparing myelin-reactive CCR6+ CD4+ T cells in the peripheral blood of MS patients versus healthy controls on the single-cell level revealed that MS-derived CCR6+ T cells expressed more IL-17 and GM-CSF while control-derived CCR6+ T cells were higher in IL-10 expression (Cao et al. 2015).
Whereas many studies focused on “classic” effector cytokines of Th17 cells to account for immunopathology in MS, it is increasingly becoming clear that Th17 cells also have additional functions during chronic inflammatory processes. Interestingly, Th17 cells have been suggested to be particularly well equipped to give help to B cells (Mitsdoerffer et al. 2010) and, in pediatric patients with demyelinating events (acute disseminated encephalomyelitis and transverse myelitis), the amount of Th17-associated cytokines (including IL-17) were significantly higher in the cerebrospinal fluid of those patients with positive-serum anti-MOG antibodies as compared with seronegative patients (Kothur et al. 2016). It remains to be determined whether antigen-specific Th17 cells contribute to the formation of tertiary lymphoid follicles that have been observed in the meningeal compartment of chronic MS patients (Magliozzi et al. 2007, 2010; Howell et al. 2011). Some results from mechanistic studies in experimental models indicated that Th17 cells might indeed be involved in the formation of these structures and, by analogy, human Th17 also expressed some of the molecules, including IL-17 itself and lymphotoxin β receptor ligands that are responsible for the induction of tertiary lymphoid follicles (Peters et al. 2011; Lee et al. 2015; Pikor et al. 2015).
As in animal models of autoimmunity and chronic inflammation, Th17 cells are not the only cellular source of IL-17 in humans. In mice, γδ T cells have been reported in numerous studies to produce IL-17 in response to IL-23 (Shibata et al. 2007; Nakamura et al. 2008; Reinhardt et al. 2016) and, just recently, the gut microbiota was linked to affect migration of IL-17-producing γδ T cells from the small intestine into the brain (Benakis et al. 2016) in a model of poststroke inflammation. γδ T cells were also shown to restrain Tregs in an IL-23-dependent manner and thereby increased EAE severity (Petermann et al. 2010). Interestingly, IL-17-producing γδ T cells accumulate in the cerebrospinal fluid of MS patients (Schirmer et al. 2013) and γδ T cells have long been found within MS lesions (Wucherpfennig et al. 1992). Yet, it remains to be determined whether γδ T cells are relevant sources of IL-17 in the CNS during human disease.
Interestingly, efficient therapeutic interventions in MS patients were associated with the reduction of Th17 cells in the peripheral blood. While this phenomenon has been observed in the treatment of acute relapses with steroids (Liu et al. 2009) and also in treatment strategies with disease-modifying drugs, including type I IFNs (Durelli et al. 2009) and fingolimod (Mehling et al. 2010), none of these trials allow for the conclusion of a causal relationship of Th17 cells with immunopathology in MS because no specific effector molecules of Th17 cells were direct targets of these therapies. Conversely, the most striking evidence for a pathogenic role of IL-17 in MS comes from the clinical and radiologic benefit noticed in RRMS patients treated with a neutralizing antibody to IL-17. Secukinumab showed efficacy in a phase II clinical trial with RRMS patients (Havrdová et al. 2012), and more advanced antibodies to IL-17 with improved pharmacodynamic properties are being considered as disease-modifying therapies in MS patients (Wiendl et al. 2015).
In summary, it is highly likely that Th17 cells play a pathogenic role in MS. Th17 cells are elevated at sites of inflammation-mediated tissue damage in the CNS of MS patients and are capable of inducing a broad tissue response with widespread immunopathology by recruitment of other lymphoid and myeloid cells (Carlson et al. 2008). Of course, the mechanistic underpinning of Th17-mediated tissue damage in humans is not worked out to the same extent as in mouse models. However, many hints point to similar functions of effector molecules of Th17 cells in mice and men.
Th17 CELLS IN NEUROMYELITIS OPTICA
Th17 cells have also been linked to the pathogenesis of neuromyelitis optica (NMO) and NMO spectrum disorder (NMOSD), which are characterized by serum antibodies to aquaporin-4 (AQP4) (Wingerchuk et al. 2015; Hinson et al. 2016). A study with patients with RRMS, relapsing NMO, and healthy control participants showed that Th17- and IL-17-producing CD8+ T cells were elevated in the blood of both RRMS and relapsing NMO patients compared to healthy control participants, but this elevation was more profound in NMO patients (Wang et al. 2011). In a recent study in which a wide range of cytokines, chemokines, and growth factors were measured in the cerebrospinal fluid of patients with NMO/NMOSD, RRMS, primary progressive MS (PPMS), and noninflammatory neurological diseases (ONDs), increased expression of Th17- and Th1-mediated proinflammatory cytokines was detected in the cerebrospinal fluid of NMO/NMOSD patients compared to OND patients, whereas proinflammatory cytokines were only mildly elevated in the cerebrospinal fluid of RRMS patients during relapses (Matsushita et al. 2013).
It is widely accepted that the immunopathology in NMO is mediated by binding of anti-AQP4 antibodies to target epitopes within the CNS, mainly astrocytic foot processes at the glia limitans, followed by complement-dependent lysis of astrocytes (reviewed in Papadopoulos and Verkman 2012). Therefore, the role of Th17 cells in NMO is likely restricted to Th functions in the formation of anti-AQP4 antibodies in the peripheral immune compartment and to the inflammation of the glial vascular unit to facilitate the access of anti-AQP4 antibodies to their target structures in the CNS. Interestingly, cortical pathology and tertiary lymphoid follicles in the meninges have never been observed in NMO patients (Lucchinetti et al. 2002; Popescu et al. 2010; Misu et al. 2013). Again, this points to a differential role of pathogenic T cells in MS and NMO. Consistent with these observations, a series of therapeutic strategies that are beneficial in MS have failed in NMO patients, including type I IFNs and natalizumab (Tanaka et al. 2009; Palace et al. 2010; Barnett et al. 2012). Indeed, some NMOSD patients even reacted with adverse effects when treated with natalizumab (Barnett et al. 2012; Kleiter et al. 2012; Kitley et al. 2014). Natalizumab blocks α4-integrins and has been shown to disrupt VLA-4 (α4β1)-mediated Th1 migration into the spinal cord in EAE and MS, while being less efficient in preventing the access of Th17 cells to the CNS compartment (Fig. 2) (Rothhammer et al. 2011). Thus, Th17 cells might be particularly important for the induction of an inflammatory milieu at the blood–brain barrier in NMOSD, an idea that is also supported by the infiltration of NMO lesions by neutrophils, which are bona fide effector cells that are typically recruited into Th17-type responses (Liang et al. 2007; Pelletier et al. 2010; Griffin et al. 2012).
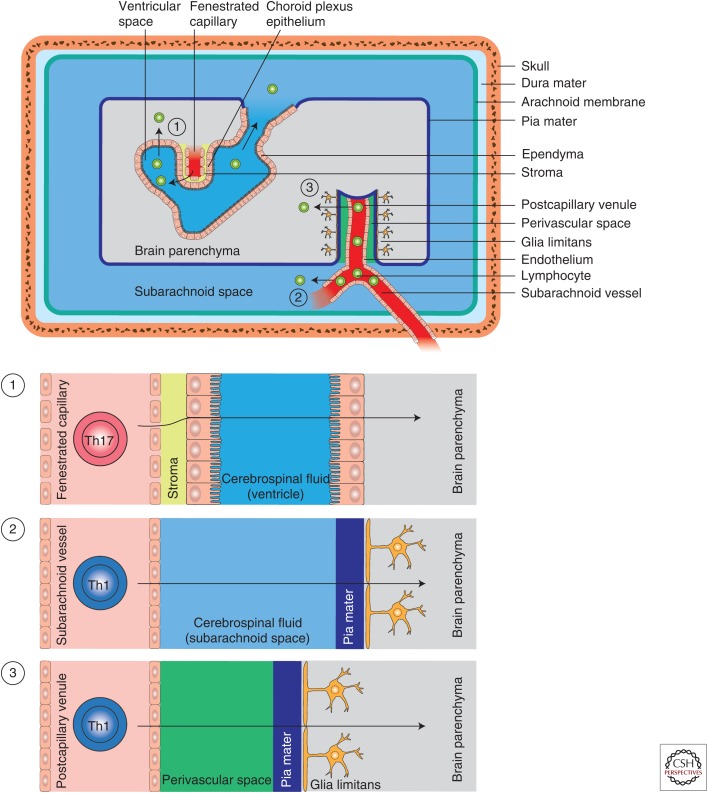
Figure 2.
Potential entry routes of lymphocytes into the central nervous system (CNS) in multiple sclerosis. The CNS compartment is separated from the systemic immune compartment by the blood cerebrospinal fluid and blood–brain barriers. In homeostasis and during inflammation, three distinct routes of entry of immune cells into the CNS have been described: (1) T cells may enter the brain parenchyma through the choroid plexus by crossing the choroid plexus epithelium; this route is potentially VLA-4 (α4β1-integrin)-independent and might favor T helper (Th) 17 cell entry. (2) T cells are also able to access the cerebrospinal fluid space through subarachnoid vessels. Once in the cerebrospinal fluid, certain lymphocytes are able to cross the glia limitans (pia mater) to directly infiltrate into the cerebrospinal fluid parenchyma. This route of entry is likely VLA-4-dependent. (3) Finally, entry of T cells into the brain parenchyma also occurs through postcapillary venules of the cerebrospinal fluid parenchyma (via the perivascular space [Virchow–Robin space]). This route has previously been considered as the most important port of entry for lymphocytes in CNS inflammation.
CONCLUDING REMARKS
Over a decade of research on Th17 cells has yielded exciting new therapy options by neutralizing either IL-17 or IL-17RA in psoriasis (Leonardi et al. 2012; Papp et al. 2012). These therapeutic strategies might also become available for rheumatoid arthritis and ankylosing spondylitis. While IL-17 is a primordial pathogenicity factor in the skin and in the joints, the CNS is less prone to support IL-17-driven inflammation. Still, it is likely that immune cells that produce IL-17 and perhaps other cytokines like GM-CSF (as Th17 cells do as well) are important mediators of disease in MS. However, targeting individual cytokines might be insufficient in MS and the recent success of B-cell-depleting therapies (Hauser et al. 2017; Montalban et al. 2017) supports the idea that elimination of B cells as APCs might be an efficient means to inhibit pathogenic T cells altogether. In fact, Th17 cells closely interact with B cells (Mitsdoerffer et al. 2010), and it remains to be determined whether depletion of B cells is particularly inhibitory to the reactivation of Th17 cells. For example, anti-CD20 therapy might affect Th17 responses by depleting a subset of APCs (i.e., B cells) that produce IL-6 and thus particularly promote Th17 responses.
Many studies were performed to elucidate the connection of Th17 cells with human MS. To date, the understanding of mechanistic processes of Th17 cells and its hallmark cytokine IL-17 in the context of MS is still elusive and needs further effort to depict the role of Th17 cells in the early phases of the disease, during relapses, and in remission. Whether Th17 cells drive the disease, whether they sustain neuroinflammation, whether they just act as bystanders, or even have a role in tissue regeneration is not yet definitively answered. Solving these questions will provide the means for designing more advanced treatment options.
ACKNOWLEDGMENTS
T.K. is supported by the German Research Foundation (DFG) (Heisenberg, TR128, SFB1054, and SyNergy) and the European Research Council (ERC) (CoG 647215, Exodus).
Footnotes
Editors: Howard L. Weiner and Vijay K. Kuchroo
Additional Perspectives on Multiple Sclerosis available at www.perspectivesinmedicine.org
REFERENCES
- Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. 2007. Interleukins 1β and 6 but not transforming growth factor-β are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol 8: 942–949. [DOI] [PubMed] [Google Scholar]
- Aggarwal S, Ghilardi N, Xie MH, de Sauvage FJ, Gurney AL. 2003. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J Biol Chem 278: 1910–1914. [DOI] [PubMed] [Google Scholar]
- Ahern PP, Schiering C, Buonocore S, McGeachy MJ, Cua DJ, Maloy KJ, Powrie F. 2010. Interleukin-23 drives intestinal inflammation through direct activity on T cells. Immunity 33: 279–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annunziato F, Cosmi L, Santarlasci V, Maggi L, Liotta F, Mazzinghi B, Parente E, Fili L, Ferri S, Frosali F, et al. 2007. Phenotypic and functional features of human Th17 cells. J Exp Med 204: 1849–1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awasthi A, Riol-Blanco L, Jager A, Korn T, Pot C, Galileos G, Bettelli E, Kuchroo VK, Oukka M. 2009. Cutting edge: IL-23 receptor gfp reporter mice reveal distinct populations of IL-17-producing cells. J Immunol 182: 5904–5908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett MH, Prineas JW, Buckland ME, Parratt JD, Pollard JD. 2012. Massive astrocyte destruction in neuromyelitis optica despite natalizumab therapy. Mult Scler 18: 108–112. [DOI] [PubMed] [Google Scholar]
- Basu R, Whitley SK, Bhaumik S, Zindl CL, Schoeb TR, Benveniste EN, Pear WS, Hatton RD, Weaver CT. 2015. IL-1 signaling modulates activation of STAT transcription factors to antagonize retinoic acid signaling and control the TH17 cell–iTreg cell balance. Nat Immunol 16: 286–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battaglia M, Gianfrani C, Gregori S, Roncarolo MG. 2004. IL-10-producing T regulatory type 1 cells and oral tolerance. Ann NY Acad Sci 1029: 142–153. [DOI] [PubMed] [Google Scholar]
- Battaglia M, Stabilini A, Draghici E, Migliavacca B, Gregori S, Bonifacio E, Roncarolo MG. 2006. Induction of tolerance in type 1 diabetes via both CD4+CD25+ T regulatory cells and T regulatory type 1 cells. Diabetes 55: 1571–1580. [DOI] [PubMed] [Google Scholar]
- Becher B, Durell BG, Noelle RJ. 2002. Experimental autoimmune encephalitis and inflammation in the absence of interleukin-12. J Clin Invest 110: 493–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benakis C, Brea D, Caballero S, Faraco G, Moore J, Murphy M, Sita G, Racchumi G, Ling L, Pamer EG, et al. 2016. Commensal microbiota affects ischemic stroke outcome by regulating intestinal γδ T cells. Nat Med 22: 516–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berod L, Friedrich C, Nandan A, Freitag J, Hagemann S, Harmrolfs K, Sandouk A, Hesse C, Castro CN, Bahre H, et al. 2014. De novo fatty acid synthesis controls the fate between regulatory T and T helper 17 cells. Nat Med 20: 1327–1333. [DOI] [PubMed] [Google Scholar]
- Bettelli E, Sullivan B, Szabo SJ, Sobel RA, Glimcher LH, Kuchroo VK. 2004. Loss of T-bet, but not STAT1, prevents the development of experimental autoimmune encephalomyelitis. J Exp Med 200: 79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. 2006. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441: 235–238. [DOI] [PubMed] [Google Scholar]
- Brok HP, van Meurs M, Blezer E, Schantz A, Peritt D, Treacy G, Laman JD, Bauer J, Hart BA. 2002. Prevention of experimental autoimmune encephalomyelitis in common marmosets using an anti-IL-12p40 monoclonal antibody. J Immunol 169: 6554–6563. [DOI] [PubMed] [Google Scholar]
- Brucklacher-Waldert V, Stuerner K, Kolster M, Wolthausen J, Tolosa E. 2009. Phenotypical and functional characterization of T helper 17 cells in multiple sclerosis. Brain 132: 3329–3341. [DOI] [PubMed] [Google Scholar]
- Brüstle A, Heink S, Huber M, Rosenplanter C, Stadelmann C, Yu P, Arpaia E, Mak TW, Kamradt T, Lohoff M. 2007. The development of inflammatory TH-17 cells requires interferon-regulatory factor 4. Nat Immunol 8: 958–966. [DOI] [PubMed] [Google Scholar]
- Cao Y, Goods BA, Raddassi K, Nepom GT, Kwok WW, Love JC, Hafler DA. 2015. Functional inflammatory profiles distinguish myelin-reactive T cells from patients with multiple sclerosis. Sci Transl Med 7: 287ra274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cargill M, Schrodi SJ, Chang M, Garcia VE, Brandon R, Callis KP, Matsunami N, Ardlie KG, Civello D, Catanese JJ, et al. 2007. A large-scale genetic association study confirms IL12B and leads to the identification of IL23R as psoriasis-risk genes. Am J Hum Genet 80: 273–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson T, Kroenke M, Rao P, Lane TE, Segal B. 2008. The Th17-ELR+ CXC chemokine pathway is essential for the development of central nervous system autoimmune disease. J Exp Med 205: 811–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenit MC, Alcina A, Marquez A, Mendoza JL, Diaz-Rubio M, de las Heras V, Izquierdo G, Arroyo R, Fernandez O, de la Concha EG, et al. 2010. STAT3 locus in inflammatory bowel disease and multiple sclerosis susceptibility. Genes Immun 11: 264–268. [DOI] [PubMed] [Google Scholar]
- Chen Q, Yang W, Gupta S, Biswas P, Smith P, Bhagat G, Pernis AB. 2008. IRF-4-binding protein inhibits interleukin-17 and interleukin-21 production by controlling the activity of IRF-4 transcription factor. Immunity 29: 899–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung Y, Chang SH, Martinez GJ, Yang XO, Nurieva R, Kang HS, Ma L, Watowich SS, Jetten AM, Tian Q, et al. 2009. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity 30: 576–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciofani M, Madar A, Galan C, Sellars M, Mace K, Pauli F, Agarwal A, Huang W, Parkurst CN, Muratet M, et al. 2012. A validated regulatory network for Th17 cell specification. Cell 151: 289–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codarri L, Gyulveszi G, Tosevski V, Hesske L, Fontana A, Magnenat L, Suter T, Becher B. 2011. RORγt drives production of the cytokine GM-CSF in helper T cells, which is essential for the effector phase of autoimmune neuroinflammation. Nat Immunol 12: 560–567. [DOI] [PubMed] [Google Scholar]
- Croxford AL, Lanzinger M, Hartmann FJ, Schreiner B, Mair F, Pelczar P, Clausen BE, Jung S, Greter M, Becher B. 2015. The cytokine GM-CSF drives the inflammatory signature of CCR2+ monocytes and licenses autoimmunity. Immunity 43: 502–514. [DOI] [PubMed] [Google Scholar]
- Cua DJ, Tato CM. 2010. Innate IL-17-producing cells: The sentinels of the immune system. Nat Rev Immunol 10: 479–489. [DOI] [PubMed] [Google Scholar]
- Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, Seymour B, Lucian L, To W, Kwan S, Churakova T, et al. 2003. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature 421: 744–748. [DOI] [PubMed] [Google Scholar]
- Dang EV, Barbi J, Yang HY, Jinasena D, Yu H, Zheng Y, Bordman Z, Fu J, Kim Y, Yen HR, et al. 2011. Control of TH17/Treg balance by hypoxia-inducible factor 1. Cell 146: 772–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diveu C, McGeachy MJ, Boniface K, Stumhofer JS, Sathe M, Joyce-Shaikh B, Chen Y, Tato CM, McClanahan TK, de Waal Malefyt R, et al. 2009. IL-27 blocks RORc expression to inhibit lineage commitment of Th17 cells. J Immunol 182: 5748–5756. [DOI] [PubMed] [Google Scholar]
- Du C, Liu C, Kang J, Zhao G, Ye Z, Huang S, Li Z, Wu Z, Pei G. 2009. MicroRNA miR-326 regulates TH-17 differentiation and is associated with the pathogenesis of multiple sclerosis. Nat Immunol 10: 1252–1259. [DOI] [PubMed] [Google Scholar]
- Duerr RH, Taylor KD, Brant SR, Rioux JD, Silverberg MS, Daly MJ, Steinhart AH, Abraham C, Regueiro M, Griffiths A, et al. 2006. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science 314: 1461–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duhen R, Glatigny S, Arbelaez CA, Blair TC, Oukka M, Bettelli E. 2013. Cutting edge: The pathogenicity of IFN-γ-producing Th17 cells is independent of T-bet. J Immunol 190: 4478–4482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durelli L, Conti L, Clerico M, Boselli D, Contessa G, Ripellino P, Ferrero B, Eid P, Novelli F. 2009. T-helper 17 cells expand in multiple sclerosis and are inhibited by interferon-β. Ann Neurol 65: 499–509. [DOI] [PubMed] [Google Scholar]
- El-Behi M, Ciric B, Dai H, Yan Y, Cullimore M, Safavi F, Zhang GX, Dittel BN, Rostami A. 2011. The encephalitogenicity of TH17 cells is dependent on IL-1- and IL-23-induced production of the cytokine GM-CSF. Nat Immunol 12: 568–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferber IA, Brocke S, Taylor-Edwards C, Ridgway W, Dinisco C, Steinman L, Dalton D, Fathman CG. 1996. Mice with a disrupted IFN-γ gene are susceptible to the induction of experimental autoimmune encephalomyelitis (EAE). J Immunol 156: 5–7. [PubMed] [Google Scholar]
- Gagliani N, Amezcua Vesely MC, Iseppon A, Brockmann L, Xu H, Palm NW, de Zoete MR, Licona-Limon P, Paiva RS, Ching T, et al. 2015. Th17 cells transdifferentiate into regulatory T cells during resolution of inflammation. Nature 523: 221–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghoreschi K, Laurence A, Yang XP, Tato CM, McGeachy MJ, Konkel JE, Ramos HL, Wei L, Davidson TS, Bouladoux N, et al. 2010. Generation of pathogenic TH17 cells in the absence of TGF-β signalling. Nature 467: 967–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin GK, Newton G, Tarrio ML, Bu DX, Maganto-Garcia E, Azcutia V, Alcaide P, Grabie N, Luscinskas FW, Croce KJ, et al. 2012. IL-17 and TNF-α sustain neutrophil recruitment during inflammation through synergistic effects on endothelial activation. J Immunol 188: 6287–6299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grifka-Walk HM, Giles DA, Segal BM. 2015. IL-12-polarized Th1 cells produce GM-CSF and induce EAE independent of IL-23. Eur J Immunol 45: 2780–2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groux H, O’Garra A, Bigler M, Rouleau M, Antonenko S, de Vries JE, Roncarolo MG. 1997. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature 389: 737–742. [DOI] [PubMed] [Google Scholar]
- Gulen MF, Kang Z, Bulek K, Youzhong W, Kim TW, Chen Y, Altuntas CZ, Sass Bak-Jensen K, McGeachy MJ, Do JS, et al. 2010. The receptor SIGIRR suppresses Th17 cell proliferation via inhibition of the interleukin-1 receptor pathway and mTOR kinase activation. Immunity 32: 54–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutcher I, Urich E, Wolter K, Prinz M, Becher B. 2006. Interleukin 18-independent engagement of interleukin 18 receptor-α is required for autoimmune inflammation. Nat Immunol 7: 946–953. [DOI] [PubMed] [Google Scholar]
- Gutcher I, Donkor MK, Ma Q, Rudensky AY, Flavell RA, Li MO. 2011. Autocrine transforming growth factor-β1 promotes in vivo Th17 cell differentiation. Immunity 34: 396–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. 2005. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol 6: 1123–1132. [DOI] [PubMed] [Google Scholar]
- Hauser SL, Bar-Or A, Comi G, Giovannoni G, Hartung H-P, Hemmer B, Lublin F, Montalban X, Rammohan KW, Selmaj K, et al. 2017. Ocrelizumab versus interferon β-1a in relapsing multiple sclerosis. N Engl J Med 376: 221–234. [DOI] [PubMed] [Google Scholar]
- Havrdová E, Belova A, Goloborodko A, Tisserant A, Jones I, Garren H, Johns D. 2012. Positive proof of concept of AIN457, an antibody against interleukin-17A, in relapsing-remitting multiple sclerosis. ECTRIMS 2012, Abstract 168. Lyon, France. [Google Scholar]
- Hedegaard CJ, Krakauer M, Bendtzen K, Lund H, Sellebjerg F, Nielsen CH. 2008. T helper cell type 1 (Th1), Th2 and Th17 responses to myelin basic protein and disease activity in multiple sclerosis. Immunology 125: 161–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinemann C, Heink S, Petermann F, Vasanthakumar A, Rothhammer V, Doorduijn E, Mitsdoerffer M, Sie C, Prazeres da Costa O, Buch T, et al. 2014. IL-27 and IL-12 oppose pro-inflammatory IL-23 in CD4+ T cells by inducing Blimp1. Nat Commun 5: 3770. [DOI] [PubMed] [Google Scholar]
- Hinson SR, Lennon VA, Pittock SJ. 2016. Autoimmune AQP4 channelopathies and neuromyelitis optica spectrum disorders. Handb Clin Neurol 133: 377–403. [DOI] [PubMed] [Google Scholar]
- Hirota K, Duarte JH, Veldhoen M, Hornsby E, Li Y, Cua DJ, Ahlfors H, Wilhelm C, Tolaini M, Menzel U, et al. 2011. Fate mapping of IL-17-producing T cells in inflammatory responses. Nat Immunol 12: 255–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell OW, Reeves CA, Nicholas R, Carassiti D, Radotra B, Gentleman SM, Serafini B, Aloisi F, Roncaroli F, Magliozzi R, et al. 2011. Meningeal inflammation is widespread and linked to cortical pathology in multiple sclerosis. Brain 134: 2755–2771. [DOI] [PubMed] [Google Scholar]
- Hu W, Troutman TD, Edukulla R, Pasare C. 2011. Priming microenvironments dictate cytokine requirements for T helper 17 cell lineage commitment. Immunity 35: 1010–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber M, Brustle A, Reinhard K, Guralnik A, Walter G, Mahiny A, von Low E, Lohoff M. 2008. IRF4 is essential for IL-21-mediated induction, amplification, and stabilization of the Th17 phenotype. Proc Natl Acad Sci 105: 20846–20851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Multiple Sclerosis Genetics Consortium (IMSGC); Wellcome Trust Case Control Consortium 2; Sawcer S, Hellenthal G, Pirinen M, Spencer CC, Patsopoulos NA, Moutsianas L, Dilthey A, Su Z, et al. 2011. Genetic risk and a primary role for cell-mediated immune mechanisms in multiple sclerosis. Nature 476: 214–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Multiple Sclerosis Genetics Consortium (IMSGC); Beecham AH, Patsopoulos NA, Xifara DK, Davis MF, Kemppinen A, Cotsapas C, Shah TS, Spencer C, Booth D, et al. 2013. Analysis of immune-related loci identifies 48 new susceptibility variants for multiple sclerosis. Nat Genet 45: 1353–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. 2006. The orphan nuclear receptor RORγt directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 126: 1121–1133. [DOI] [PubMed] [Google Scholar]
- Jäger A, Dardalhon V, Sobel RA, Bettelli E, Kuchroo VK. 2009. Th1, Th17, and Th9 effector cells induce experimental autoimmune encephalomyelitis with different pathological phenotypes. J Immunol 183: 7169–7177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakkula E, Leppa V, Sulonen AM, Varilo T, Kallio S, Kemppinen A, Purcell S, Koivisto K, Tienari P, Sumelahti ML, et al. 2010. Genome-wide association study in a high-risk isolate for multiple sclerosis reveals associated variants in STAT3 gene. Am J Hum Genet 86: 285–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keerthivasan S, Suleiman R, Lawlor R, Roderick J, Bates T, Minter L, Anguita J, Juncadella I, Nickoloff BJ, Le Poole IC, et al. 2011. Notch signaling regulates mouse and human Th17 differentiation. J Immunol 187: 692–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YJ, Hwang SY, Oh ES, Oh S, Han IO. 2006. IL-1β, an immediate early protein secreted by activated microglia, induces iNOS/NO in C6 astrocytoma cells through p38 MAPK and NF-κB pathways. J Neurosci Res 84: 1037–1046. [DOI] [PubMed] [Google Scholar]
- Kitley J, Evangelou N, Kuker W, Jacob A, Leite MI, Palace J. 2014. Catastrophic brain relapse in seronegative NMO after a single dose of natalizumab. J Neurol Sci 339: 223–225. [DOI] [PubMed] [Google Scholar]
- Kleinewietfeld M, Manzel A, Titze J, Kvakan H, Yosef N, Linker RA, Muller DN, Hafler DA. 2013. Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells. Nature 496: 518–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiter I, Hellwig K, Berthele A, Kumpfel T, Linker RA, Hartung HP, Paul F, Aktas O, Neuromyelitis Optica Study G. 2012. Failure of natalizumab to prevent relapses in neuromyelitis optica. Arch Neurol 69: 239–245. [DOI] [PubMed] [Google Scholar]
- Ko HJ, Brady JL, Ryg-Cornejo V, Hansen DS, Vremec D, Shortman K, Zhan Y, Lew AM. 2014. GM-CSF-responsive monocyte-derived dendritic cells are pivotal in Th17 pathogenesis. J Immunol 192: 2202–2209. [DOI] [PubMed] [Google Scholar]
- Komiyama Y, Nakae S, Matsuki T, Nambu A, Ishigame H, Kakuta S, Sudo K, Iwakura Y. 2006. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J Immunol 177: 566–573. [DOI] [PubMed] [Google Scholar]
- Korn T, Bettelli E, Gao W, Awasthi A, Jager A, Strom TB, Oukka M, Kuchroo VK. 2007. IL-21 initiates an alternative pathway to induce proinflammatory TH17 cells. Nature 448: 484–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn T, Bettelli E, Oukka M, Kuchroo VK. 2009. IL-17 and Th17 Cells. Annu Rev Immunol 27: 485–517. [DOI] [PubMed] [Google Scholar]
- Kothur K, Wienholt L, Tantsis EM, Earl J, Bandodkar S, Prelog K, Tea F, Ramanathan S, Brilot F, Dale RC. 2016. B cell, Th17, and neutrophil related cerebrospinal fluid cytokine/chemokines are elevated in MOG antibody associated demyelination. PLoS ONE 11: e0149411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krausgruber T, Schiering C, Adelmann K, Harrison OJ, Chomka A, Pearson C, Ahern PP, Shale M, Oukka M, Powrie F. 2016. T-bet is a key modulator of IL-23-driven pathogenic CD4+ T cell responses in the intestine. Nat Commun 7: 11627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurschus FC, Croxford AL, Heinen AP, Wortge S, Ielo D, Waisman A. 2010. Genetic proof for the transient nature of the Th17 phenotype. Eur J Immunol 40: 3336–3346. [DOI] [PubMed] [Google Scholar]
- Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, Cua DJ. 2005. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med 201: 233–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YK, Turner H, Maynard CL, Oliver JR, Chen D, Elson CO, Weaver CT. 2009. Late developmental plasticity in the T helper 17 lineage. Immunity 30: 92–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Awasthi A, Yosef N, Quintana FJ, Xiao S, Peters A, Wu C, Kleinewietfeld M, Kunder S, Hafler DA, et al. 2012. Induction and molecular signature of pathogenic TH17 cells. Nat Immunol 13: 991–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Mitsdoerffer M, Xiao S, Gu G, Sobel RA, Kuchroo VK. 2015. IL-21R signaling is critical for induction of spontaneous experimental autoimmune encephalomyelitis. J Clin Invest 125: 4011–4020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard JP, Waldburger KE, Goldman SJ. 1995. Prevention of experimental autoimmune encephalomyelitis by antibodies against interleukin 12. J Exp Med 181: 381–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonardi C, Matheson R, Zachariae C, Cameron G, Li L, Edson-Heredia E, Braun D, Banerjee S. 2012. Anti-interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasis. N Engl J Med 366: 1190–1199. [DOI] [PubMed] [Google Scholar]
- Li MO, Wan YY, Flavell RA. 2007. T cell-produced transforming growth factor-β1 controls T cell tolerance and regulates Th1- and Th17-cell differentiation. Immunity 26: 579–591. [DOI] [PubMed] [Google Scholar]
- Li P, Spolski R, Liao W, Wang L, Murphy TL, Murphy KM, Leonard WJ. 2012. BATF-JUN is critical for IRF4-mediated transcription in T cells. Nature 490: 543–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang SC, Long AJ, Bennett F, Whitters MJ, Karim R, Collins M, Goldman SJ, Dunussi-Joannopoulos K, Williams CM, Wright JF, et al. 2007. An IL-17F/A heterodimer protein is produced by mouse Th17 cells and induces airway neutrophil recruitment. J Immunol 179: 7791–7799. [DOI] [PubMed] [Google Scholar]
- Lill CM, Schjeide BM, Akkad DA, Blaschke P, Winkelmann A, Gerdes LA, Hoffjan S, Luessi F, Dorner T, Li SC, et al. 2012. Independent replication of STAT3 association with multiple sclerosis risk in a large German case-control sample. Neurogenetics 13: 83–86. [DOI] [PubMed] [Google Scholar]
- Liu M, Hu X, Wang Y, Peng F, Yang Y, Chen X, Lu Z, Zheng X. 2009. Effect of high-dose methylprednisolone treatment on Th17 cells in patients with multiple sclerosis in relapse. Acta Neurol Scand 120: 235–241. [DOI] [PubMed] [Google Scholar]
- Lock C, Hermans G, Pedotti R, Brendolan A, Schadt E, Garren H, Langer-Gould A, Strober S, Cannella B, Allard J, et al. 2002. Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nat Med 8: 500–508. [DOI] [PubMed] [Google Scholar]
- Longbrake EE, Racke MK. 2009. Why did IL-12/IL-23 antibody therapy fail in multiple sclerosis? Expert Rev Neurother 9: 319–321. [DOI] [PubMed] [Google Scholar]
- Lucchinetti CF, Mandler RN, McGavern D, Bruck W, Gleich G, Ransohoff RM, Trebst C, Weinshenker B, Wingerchuk D, Parisi JE, et al. 2002. A role for humoral mechanisms in the pathogenesis of Devic's neuromyelitis optica. Brain 125: 1450–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magliozzi R, Howell O, Vora A, Serafini B, Nicholas R, Puopolo M, Reynolds R, Aloisi F. 2007. Meningeal B-cell follicles in secondary progressive multiple sclerosis associate with early onset of disease and severe cortical pathology. Brain 130: 1089–1104. [DOI] [PubMed] [Google Scholar]
- Magliozzi R, Howell OW, Reeves C, Roncaroli F, Nicholas R, Serafini B, Aloisi F, Reynolds R. 2010. A Gradient of neuronal loss and meningeal inflammation in multiple sclerosis. Ann Neurol 68: 477–493. [DOI] [PubMed] [Google Scholar]
- Mahad DH, Trapp BD, Lassmann H. 2015. Pathological mechanisms in progressive multiple sclerosis. Lancet Neurol 14: 183–193. [DOI] [PubMed] [Google Scholar]
- Manel N, Unutmaz D, Littman DR. 2008. The differentiation of human TH-17 cells requires transforming growth factor-β and induction of the nuclear receptor RORγt. Nat Immunol 9: 641–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangan PR, Harrington LE, O’Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. 2006. Transforming growth factor-β induces development of the TH17 lineage. Nature 441: 231–234. [DOI] [PubMed] [Google Scholar]
- Martin R. 2008. Recommendation of Segal BM, et al. Lancet Neurol. 2008 Sep; 7(9):796–804. F1000Prime, 14 Nov 2008. [DOI] [PubMed] [Google Scholar]
- Matsushita T, Tateishi T, Isobe N, Yonekawa T, Yamasaki R, Matsuse D, Murai H, Kira J. 2013. Characteristic cerebrospinal fluid cytokine/chemokine profiles in neuromyelitis optica, relapsing remitting or primary progressive multiple sclerosis. PLoS ONE 8: e61835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matusevicius D, Kivisakk P, He B, Kostulas N, Ozenci V, Fredrikson S, Link H. 1999. Interleukin-17 mRNA expression in blood and CSF mononuclear cells is augmented in multiple sclerosis. Mult Scler 5: 101–104. [DOI] [PubMed] [Google Scholar]
- Mayo L, Cunha AP, Madi A, Beynon V, Yang Z, Alvarez JI, Prat A, Sobel RA, Kobzik L, Lassmann H, et al. 2016. IL-10-dependent Tr1 cells attenuate astrocyte activation and ameliorate chronic central nervous system inflammation. Brain 139: 1939–1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeachy MJ, Bak-Jensen KS, Chen Y, Tato CM, Blumenschein W, McClanahan T, Cua DJ. 2007. TGF-β and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain TH-17 cell-mediated pathology. Nat Immunol 8: 1390–1397. [DOI] [PubMed] [Google Scholar]
- McGeachy MJ, Chen Y, Tato CM, Laurence A, Joyce-Shaikh B, Blumenschein WM, McClanahan TK, O’Shea JJ, Cua DJ. 2009. The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nat Immunol 10: 314–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQualter JL, Darwiche R, Ewing C, Onuki M, Kay TW, Hamilton JA, Reid HH, Bernard CC. 2001. Granulocyte macrophage colony-stimulating factor: A new putative therapeutic target in multiple sclerosis. J Exp Med 194: 873–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehling M, Lindberg R, Raulf F, Kuhle J, Hess C, Kappos L, Brinkmann V. 2010. Th17 central memory T cells are reduced by FTY720 in patients with multiple sclerosis. Neurology 75: 403–410. [DOI] [PubMed] [Google Scholar]
- Meyer Zu, Horste G, Wu C, Wang C, Cong L, Pawlak M, Lee Y, Elyaman W, Xiao S, Regev A, Kuchroo VK. 2016. RBPJ controls development of pathogenic Th17 cells by regulating IL-23 receptor expression. Cell Rep 16: 392–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misu T, Hoftberger R, Fujihara K, Wimmer I, Takai Y, Nishiyama S, Nakashima I, Konno H, Bradl M, Garzuly F, et al. 2013. Presence of six different lesion types suggests diverse mechanisms of tissue injury in neuromyelitis optica. Acta Neuropathol 125: 815–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsdoerffer M, Lee Y, Jager A, Kim HJ, Korn T, Kolls JK, Cantor H, Bettelli E, Kuchroo VK. 2010. Proinflammatory T helper type 17 cells are effective B-cell helpers. Proc Natl Acad Sci 107: 14292–14297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata S, Koyama Y, Takemoto K, Yoshikawa K, Ishikawa T, Taniguchi M, Inoue K, Aoki M, Hori O, Katayama T, et al. 2011. Plasma corticosterone activates SGK1 and induces morphological changes in oligodendrocytes in corpus callosum. PLoS ONE 6: e19859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montalban X, Hauser SL, Kappos L, Arnold DL, Bar-Or A, Comi G, de Seze J, Giovannoni G, Hartung H-P, Hemmer B, et al. 2017. Ocrelizumab versus placebo in primary progressive multiple sclerosis. N Engl J Med 376: 209–220. [DOI] [PubMed] [Google Scholar]
- Nakamura R, Shibata K, Yamada H, Shimoda K, Nakayama K, Yoshikai Y. 2008. Tyk2-signaling plays an important role in host defense against Escherichia coli through IL-23-induced IL-17 production by γδ T cells. J Immunol 181: 2071–2075. [DOI] [PubMed] [Google Scholar]
- Neurath MF, Weigmann B, Finotto S, Glickman J, Nieuwenhuis E, Iijima H, Mizoguchi A, Mizoguchi E, Mudter J, Galle PR, et al. 2002. The transcription factor T-bet regulates mucosal T cell activation in experimental colitis and Crohn's disease. J Exp Med 195: 1129–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurieva R, Yang XO, Martinez G, Zhang Y, Panopoulos AD, Ma L, Schluns K, Tian Q, Watowich SS, Jetten AM, et al. 2007. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature 448: 480–483. [DOI] [PubMed] [Google Scholar]
- Oppmann B, Lesley R, Blom B, Timans JC, Xu Y, Hunte B, Vega F, Yu N, Wang J, Singh K, et al. 2000. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity 13: 715–725. [DOI] [PubMed] [Google Scholar]
- Palace J, Leite MI, Nairne A, Vincent A. 2010. Interferon β treatment in neuromyelitis optica: Increase in relapses and aquaporin 4 antibody titers. Arch Neurol 67: 1016–1017. [DOI] [PubMed] [Google Scholar]
- Papadopoulos MC, Verkman AS. 2012. Aquaporin 4 and neuromyelitis optica. Lancet Neurol 11: 535–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papp KA, Leonardi C, Menter A, Ortonne JP, Krueger JG, Kricorian G, Aras G, Li J, Russell CB, Thompson EH, et al. 2012. Brodalumab, an anti-interleukin-17-receptor antibody for psoriasis. N Engl J Med 366: 1181–1189. [DOI] [PubMed] [Google Scholar]
- Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, et al. 2005. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol 6: 1133–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier M, Maggi L, Micheletti A, Lazzeri E, Tamassia N, Costantini C, Cosmi L, Lunardi C, Annunziato F, Romagnani S, et al. 2010. Evidence for a cross-talk between human neutrophils and Th17 cells. Blood 115: 335–343. [DOI] [PubMed] [Google Scholar]
- Petermann F, Rothhammer V, Claussen MC, Haas JD, Blanco LR, Heink S, Prinz I, Hemmer B, Kuchroo VK, Oukka M, et al. 2010. γδ T cells enhance autoimmunity by restraining regulatory T cell responses via an interleukin-23-dependent mechanism. Immunity 33: 351–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A, Pitcher LA, Sullivan JM, Mitsdoerffer M, Acton SE, Franz B, Wucherpfennig K, Turley S, Carroll MC, Sobel RA, et al. 2011. Th17 cells induce ectopic lymphoid follicles in central nervous system tissue inflammation. Immunity 35: 986–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pikor NB, Prat A, Bar-Or A, Gommerman JL. 2015. Meningeal tertiary lymphoid tissues and multiple sclerosis: A gathering place for diverse types of immune cells during CNS autoimmunity. Front Immunol 6: 657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popescu BF, Parisi JE, Cabrera-Gomez JA, Newell K, Mandler RN, Pittock SJ, Lennon VA, Weinshenker BG, Lucchinetti CF. 2010. Absence of cortical demyelination in neuromyelitis optica. Neurology 75: 2103–2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt A, Yevsa T, Worbs T, Lienenklaus S, Sandrock I, Oberdorfer L, Korn T, Weiss S, Forster R, Prinz I. 2016. IL-23-dependent γδ T cells produce IL-17 and accumulate in enthesis, aortic valve, and ciliary body. Arthritis Rheumatol 68: 2476–2486. [DOI] [PubMed] [Google Scholar]
- Ronchi F, Basso C, Preite S, Reboldi A, Baumjohann D, Perlini L, Lanzavecchia A, Sallusto F. 2016. Experimental priming of encephalitogenic Th1/Th17 cells requires pertussis toxin-driven IL-1β production by myeloid cells. Nat Commun 7: 11541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos IM, Kockum I, Hillert J. 2008. The interleukin 23 receptor gene in multiple sclerosis: A case-control study. J Neuroimmunol 194: 173–180. [DOI] [PubMed] [Google Scholar]
- Rothhammer V, Heink S, Petermann F, Srivastava R, Claussen MC, Hemmer B, Korn T. 2011. Th17 lymphocytes traffic to the central nervous system independently of α4 integrin expression during EAE. J Exp Med 208: 2465–2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouvier E, Luciani MF, Mattei MG, Denizot F, Golstein P. 1993. CTLA-8, cloned from an activated T cell, bearing AU-rich messenger RNA instability sequences, and homologous to a herpesvirus saimiri gene. J Immunol 150: 5445–5456. [PubMed] [Google Scholar]
- Sallusto F, Lenig D, Mackay CR, Lanzavecchia A. 1998. Flexible programs of chemokine receptor expression on human polarized T helper 1 and 2 lymphocytes. J Exp Med 187: 875–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirmer L, Rothhammer V, Hemmer B, Korn T. 2013. Enriched CD161high CCR6+ γδ T cells in the cerebrospinal fluid of patients with multiple sclerosis. JAMA Neurol 70: 345–351. [DOI] [PubMed] [Google Scholar]
- Schraml BU, Hildner K, Ise W, Lee WL, Smith WA, Solomon B, Sahota G, Sim J, Mukasa R, Cemerski S, et al. 2009. The AP-1 transcription factor Batf controls TH17 differentiation. Nature 460: 405–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal BM, Constantinescu CS, Raychaudhuri A, Kim L, Fidelus-Gort R, Kasper LH, Ustekinumab MSI. 2008. Repeated subcutaneous injections of IL12/23 p40 neutralising antibody, ustekinumab, in patients with relapsing-remitting multiple sclerosis: A phase II, double-blind, placebo-controlled, randomised, dose-ranging study. Lancet Neurol 7: 796–804. [DOI] [PubMed] [Google Scholar]
- Shibata K, Yamada H, Hara H, Kishihara K, Yoshikai Y. 2007. Resident Vδ1+ γδ T cells control early infiltration of neutrophils after Escherichia coli infection via IL-17 production. J Immunol 178: 4466–4472. [DOI] [PubMed] [Google Scholar]
- Stumhofer JS, Laurence A, Wilson EH, Huang E, Tato CM, Johnson LM, Villarino AV, Huang Q, Yoshimura A, Sehy D, et al. 2006. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat Immunol 7: 937–945. [DOI] [PubMed] [Google Scholar]
- Stumhofer JS, Silver JS, Laurence A, Porrett PM, Harris TH, Turka LA, Ernst M, Saris CJ, O’Shea JJ, Hunter CA. 2007. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nat Immunol 8: 1363–1371. [DOI] [PubMed] [Google Scholar]
- Sutton C, Brereton C, Keogh B, Mills KH, Lavelle EC. 2006. A crucial role for interleukin (IL)-1 in the induction of IL-17-producing T cells that mediate autoimmune encephalomyelitis. J Exp Med 203: 1685–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Tanaka K, Komori M. 2009. Interferon-β(1b) treatment in neuromyelitis optica. Eur Neurol 62: 167–170. [DOI] [PubMed] [Google Scholar]
- Tzartos JS, Friese MA, Craner MJ, Palace J, Newcombe J, Esiri MM, Fugger L. 2008. Interleukin-17 production in central nervous system-infiltrating T cells and glial cells is associated with active disease in multiple sclerosis. Am J Pathol 172: 146–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. 2006. TGFβ in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity 24: 179–189. [DOI] [PubMed] [Google Scholar]
- Waldegger S, Barth P, Raber G, Lang F. 1997. Cloning and characterization of a putative human serine/threonine protein kinase transcriptionally modified during anisotonic and isotonic alterations of cell volume. Proc Natl Acad Sci 94: 4440–4445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HH, Dai YQ, Qiu W, Lu ZQ, Peng FH, Wang YG, Bao J, Li Y, Hu XQ. 2011. Interleukin-17-secreting T cells in neuromyelitis optica and multiple sclerosis during relapse. J Clin Neurosci 18: 1313–1317. [DOI] [PubMed] [Google Scholar]
- Wang Y, Godec J, Ben-Aissa K, Cui K, Zhao K, Pucsek AB, Lee YK, Weaver CT, Yagi R, Lazarevic V. 2014. The transcription factors T-bet and Runx are required for the ontogeny of pathogenic interferon-γ-producing T helper 17 cells. Immunity 40: 355–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiendl A, Dahlke F, Bennett D, Rosenkranz G, Wolf C, Bar-Or A. 2015. IL-17 neutralization by subcutaneous CJM112, a fully human anti IL-17A monoclonal antibody for the treatment of relapsing-remitting multiple sclerosis: Study design of a phase 2 trial. ECTRIMS Online Library, Abstract P655, Oct. 8, 2015. [Google Scholar]
- Wilson NJ, Boniface K, Chan JR, McKenzie BS, Blumenschein WM, Mattson JD, Basham B, Smith K, Chen T, Morel F, et al. 2007. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol 8: 950–957. [DOI] [PubMed] [Google Scholar]
- Wingerchuk DM, Banwell B, Bennett JL, Cabre P, Carroll W, Chitnis T, de Seze J, Fujihara K, Greenberg B, Jacob A, et al. 2015. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology 85: 177–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C, Yosef N, Thalhamer T, Zhu C, Xiao S, Kishi Y, Regev A, Kuchroo VK. 2013. Induction of pathogenic TH17 cells by inducible salt-sensing kinase SGK1. Nature 496: 513–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wucherpfennig KW, Newcombe J, Li H, Keddy C, Cuzner ML, Hafler DA. 1992. γδ T-cell receptor repertoire in acute multiple sclerosis lesions. Proc Natl Acad Sci 89: 4588–4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao S, Yosef N, Yang J, Wang Y, Zhou L, Zhu C, Wu C, Baloglu E, Schmidt D, Ramesh R, et al. 2014. Small-molecule RORγt antagonists inhibit T helper 17 cell transcriptional network by divergent mechanisms. Immunity 40: 477–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Anderson DE, Baecher-Allan C, Hastings WD, Bettelli E, Oukka M, Kuchroo VK, Hafler DA. 2008. IL-21 and TGF-β are required for differentiation of human TH17 cells. Nature 454: 350–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Z, Fanslow WC, Seldin MF, Rousseau AM, Painter SL, Comeau MR, Cohen JI, Spriggs MK. 1995. Herpesvirus Saimiri encodes a new cytokine, IL-17, which binds to a novel cytokine receptor. Immunity 3: 811–821. [DOI] [PubMed] [Google Scholar]
- Yosef N, Shalek AK, Gaublomme JT, Jin H, Lee Y, Awasthi A, Wu C, Karwacz K, Xiao S, Jorgolli M, et al. 2013. Dynamic regulatory network controlling TH17 cell differentiation. Nature 496: 461–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen EY, Liu W, Karatsoreos IN, Ren Y, Feng J, McEwen BS, Yan Z. 2011. Mechanisms for acute stress-induced enhancement of glutamatergic transmission and working memory. Mol Psychiatry 16: 156–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang GX, Gran B, Yu S, Li J, Siglienti I, Chen X, Kamoun M, Rostami A. 2003. Induction of experimental autoimmune encephalomyelitis in IL-12 receptor-β 2-deficient mice: IL-12 responsiveness is not required in the pathogenesis of inflammatory demyelination in the central nervous system. J Immunol 170: 2153–2160. [DOI] [PubMed] [Google Scholar]