Abstract
The craniofacial complex is composed of fundamental components such as blood vessels and nerves, and also a variety of specialized tissues such as craniofacial bones, cartilages, muscles, ligaments, and the highly specialized and unique organs, the teeth. Together, these structures provide many functions including speech, mastication, and aesthetics of the craniofacial complex. Craniofacial defects not only influence the structure and function of the jaws and face, but may also result in deleterious psychosocial issues, emphasizing the need for rapid and effective, precise, and aesthetic reconstruction of craniofacial tissues. In a broad sense, craniofacial tissue reconstructions share many of the same issues as noncraniofacial tissue reconstructions. Therefore, many concepts and therapies for general tissue engineering can and have been used for craniofacial tissue regeneration. Still, repair of craniofacial defects presents unique challenges, mainly because of their complex and unique 3D geometry.
The most common causes of craniofacial defects are congenital birth defects (1/700 live births), trauma, inflammation, and cancer surgeries (Miura et al. 2006). Among these, the most prevalent is acute trauma, including falls, assaults, sports injuries, and vehicle crashes (Rocchi et al. 2007; Grayson et al. 2015; Hunter et al. 2015). Significant facial trauma can also result from battlefield injuries, particularly when combined with an increased survivability of wounded soldiers because of improved battlefield medical care and body armor. A recent report indicated that craniomaxillofacial injuries can represent up to 26% of all battlefield injuries, as occurred in Operation Iraqi Freedom/Operation Enduring Freedom (Afghanistan) (Lew et al. 2010). Congenital anomalies (CAs) are major causes of infant mortality and childhood morbidity, affecting 2%–3% of all babies (Mossey and Castilla 2003). Genetic birth defects, environmental exposure, and folic acid deficiency are the main causes of CA.
Craniofacial bone reconstruction plays a crucial role in craniofacial repairs, because they provide support for adjacent soft tissues and anchorage for dental structures, thereby guiding the structural stability and appearance of the face (Fig. 1) (Petrovic et al. 2012). Especially for extensive craniofacial injuries, successful regeneration of craniofacial bone is necessary to restore normal function of the craniofacial complex (Genden 2010; Ward et al. 2010). With sufficient bone structure, it is comparatively easier to restore the soft tissues of the craniofacial complex to form the facial features. An extreme example of this is the world’s first partial face transplant from a cadaver to a living human, which was performed on November 27, 2005, and which has been performed globally since then (Petrini 2015). In addition, in March 2010, a team of 30 Spanish doctors performed the first full-face transplant in the world (Garrett et al. 2015). These accomplishments stress the importance of craniofacial bone defect reconstruction as a fundamental first step for successful craniofacial regeneration.
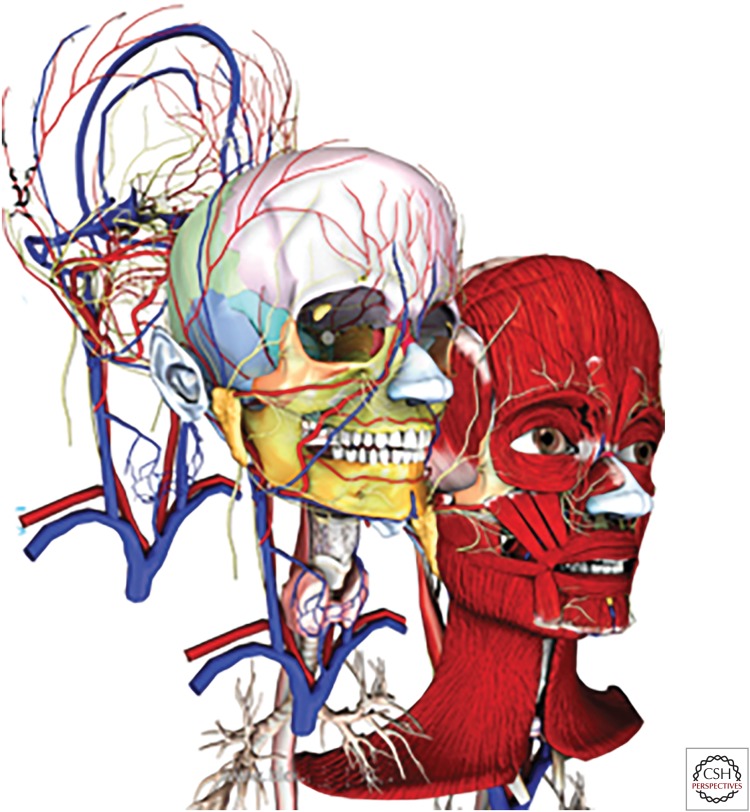
Figure 1.
Schematic of the layered structure of craniofacial tissues. The skull and craniofacial bones provide the structural support for the muscle, vascular network, and skin (from catalog.biodigital.com/storeImages/detail/cranio_dvd.jpg).
CRANIOFACIAL BONE ENGINEERING
As described above, bone repair is a crucial and fundamental step of craniofacial reconstruction. Living bone is in a continuous state of dynamic equilibrium consisting of bone resorption, regeneration, and remodeling. Bone has an innate ability for a limited amount of self-repair following traumatic injury. In 1982, Enlow hypothesized that some areas of the face remodel by bony deposition, whereas others remodel by bony resorption (Enlow 1982).
Bone Reconstruction
Bone reconstruction has a long history. The earliest attempt to repair bone defects was reported in Edgar Smith papyrus, ca. 2000 BC, using metals (Frommelt 1987). And, in 1668, the first bone graft was performed by Van Meekren on a patient with a cranial defect, via a xenograft (Van Meekren 1682). Since then, a variety of techniques using different combinations of autogenous, allogeneic, and prosthetic materials have been used to achieve bone reconstruction. By far, autogenous bone grafting has generally yielded the most favorable results (Hokugo et al. 2004; Vogelin et al. 2005; Gimbel et al. 2007). Still, a better understanding of the basic biology of autogenous bone graft procedures will allow for a more educated and predictable utilization of bone reconstruction procedures in clinical practice. What is currently known is that shortly after bone graft transplantation, hematoma formation occurs around the graft. Next, an inflammatory reaction occurs around the graft lasting for 5–7 days, at which time the hematoma and surrounding tissue reorganizes into a dense fibrovascular stroma around the graft. Vascular invasion by the host brings cells with osteogenic potential into the graft, after 10–14 days. Infiltrating host cells subsequently differentiate into osteoblasts that deposit new bone, and into osteoclasts that resorb necrotic bone, facilitating bone graft penetration by host vascular tissue. Cortical grafts in the onlay position show only superficial revascularization in the first 10 ∼ 21 days, and central revascularization by 8 ∼ 16 weeks (Chen et al. 1994; Ozaki and Buchman 1998). Since the earliest vessels do not enter the graft until later, incompletely revascularized regions of necrotic graft may persist indefinitely, sealed off from the viable regions of the graft. Entrapped cores of dead bone can only be resorbed with appropriate revascularizaion. Therefore, rapid and efficient revascularization plays a key role in bone graft survival, and can be influenced by a variety of conditions including prior irradiation, immune response, and conditions at the recipient site such as the presence of necrotic bone, scarring, and infection (Lukash et al. 1984). Existing healthy bone can also serve to direct healthy matrix bone deposition.
Histologically, two types of bone can easily be distinguished: cancellous bone and cortical bone. Cortical bone is the compact bone that covers the surface of most bones. Cancellous bone, which makes up much of the enlarged ends (epiphyses) of the long bones and flat bones of the skull, is less dense than cortical bone, because of its higher surface area to mass ratio. Revascularization of cancellous bone grafts occurs more rapidly and completely than cortical bone grafts, because the large pores between the trabeculae in cancellous bone grafts permit the unobstructed invasion of vascular tissue, and rapid diffusion of nutrients from the host bed, thereby promoting osteogenic cell survival and increased osteogenesis (Pinholt et al. 1994). Cancellous bone grafts can be completely revascularized and ultimately replaced with new bone in several weeks to months, whereas cortical bone graft revascularization can proceed slowly and incompletely (Teng et al. 2013; Martuscelli et al. 2014). The dense lamellar structure of cortical bone limits vascular invasion, in which vasculature invasion is constrained to preexisting pathways and proceeds from the graft periphery to the interior of the graft. Furthermore, osteoclastic enlargement of the Haversian and Volkmann’s canals must occur before blood vessels are able to penetrate the cortical bone graft, and vascular invasion is limited by the dense lamellar structure of cortical bone, where vasculature invasion is constrained to preexisting pores and proceeds from the graft periphery to the interior of the graft (Burchardt 1983). Therefore, cancellous bone grafts are well suited to treat bone gaps, because they revascularize quickly and stimulate significant new bone formation through osteoinduction. Conversely, cortical bone grafts are often used in cases of bone volume deficiency, where they can survive longer with limited resorption and retain a certain level of mechanical strength after transplantation (Neu 2000; Uhm et al. 2000).
The small population of cells present within autogenous bone grafts are what largely contribute to its superiority over other bone substitutes (Mulliken et al. 1984; Oklund et al. 1986). Osteogenic cells from the periosteum, endosteum, marrow, and intracortical elements of the graft contribute to graft viability and osteoid production (Burchardt 1987). And if the bone graft contains periosteum, the periosteum can provide blood supply and osteoprogenitor cells that further facilitate early vascularization and bone formation (Eyre-Brook 1984; Skawina and Gorczyca 1984). Unfortunately, adult periosteum loses this osteogenic ability after being detached from the ordinal bone surface (Melcher and Accursi 1971; Weng et al. 2000). Shortly after transplantation, most of the osteocytes present within the graft undergo necrosis, thereby rendering the graft relatively inert. One of the approaches used in clinic to increase the survival rate of large bone grafts is to first implant the grafts into highly vascularized muscle tissue, to facilitate vascular ingrowth and to develop a vascular pedicle suitable for microsurgical anastomosis (Warnke et al. 2004; Mesimäki et al. 2009). This practice is somewhat limited because of donor-site morbidity and the amount of bone that can be harvested (Burchardt 1987).
To overcome these issues, stem-cell-based bone-tissue engineering has been recognized as a promising alternative for bone reconstructions. As far back as 1968, Friedenstein’s team first reported that fibroblast-like cells isolated from bone marrow not only had the ability to differentiate to haematopoietic cells, but also had the potential for osteogenic differentiation (Friedenstein et al. 1968; Friedenstein 1976). At that time, the cells were named “mechanocytes.” Subsequent studies showed that these cells were chondrogenic and adipogenic (Owen and Friedenstein 1988). To date, these cells are commonly called mesenchymal stem cells (MSCs) (Beyer Nardi and da Silva Meirelles 2006). In addition to bone marrow, cells with MSC-like characteristics have been isolated from a variety of tissues including adult adipose, dental, and skeletal muscle tissues (Zuk et al. 2001; Qu-Petersen et al. 2002; Feisst et al. 2014). MSC-mediated bone regeneration has been widely tested in several clinical trials, demonstrating that local delivery of MSCs can enhance bone regeneration (Grayson et al. 2015).
Comparison of Craniofacial and Noncraniofacial Bone
Generally speaking, craniofacial bones are flat bones that share similar turnover and injury repair mechanisms of other skeletal bones, but with their own specific properties (Leucht et al. 2008). Whereas most of the knowledge gained from skeletal bone reconstruction can be applied to the craniofacial bone field, the unique features and properties of craniofacial bone regeneration require some additional considerations.
One of the major differences between craniofacial and noncraniofacial bones is their embryonic origin. The cranial neural crest gives rise to branchial arch–derived craniofacial bones and cartilages, whereas the axial skeleton is derived from the somites, and the lateral plate mesoderm forms the limb skeleton. Bone formation, or osteogenesis, is the transformation of preexisting mesenchymal tissue to calcified bone tissue. There are two major modes of bone formation: intramembranous and endochondral ossification. Intramembranous ossification is the direct conversion of mesenchymal tissue into bone, whereas endochondral ossification consists of mesenchymal cells that first differentiate to form a cartilage template that is later replaced by bone. Bones of the skull primarily form through intramembranous bone formation, with some contributions by endochondral ossification (Bilezikian et al. 2002). For example, Meckel’s cartilage forms at the proximal end of the mandible then largely disappears as the mandible ossifies, thereby playing an important role in mandibular morphogenesis (Ramaesh and Bard 2003; Tsutsui et al. 2008). During intramembranous ossification of the skull, neural crest–derived mesenchymal cells proliferate into compact condensations. Some of these cells develop into capillaries, whereas others change their shape to become osteoblasts, which secrete a collagen–proteoglycan matrix that is able to bind calcium salts. As calcification proceeds, bony spicules radiate out from regions where ossification began. Eventually, the entire region of calcified spicules becomes surrounded by compact mesenchymal cells that form the periosteum. The cells on the inner surface of the periosteum also become osteoblasts and deposit osteoid matrix parallel to that of the existing spicules. In this manner, many layers of bone are formed.
Craniofacial and noncraniofacial bones also show different homeostatic mechanisms. Several publications showed that bone grafts from the craniofacial skeleton had better survival and longer volumetric maintenance as compared to bone harvested from the iliac crest, rib, and tibia (Peer 1951; Sullivan and Szwajkun 1991). In addition, reports have indicated that membranous bone grafts retained their volume better than endochondral bone grafts (Zins and Whitaker 1979, 1983). In 1999, Buchman and Ozaki’s results suggested that bone volume maintenance may be the result of microarchitecture of the bone graft, based on the fact that intramembranous-derived craniofacial bone has a higher proportion of cortical bone as compared to endochondral bone (Buchman and Ozaki 1999). Evidence for this includes the fact that, under external stimuli such as ovariectomy and malnutrition, rat mandibular trabecular bone and bone mineral density loss occurs at a lower rate than in tibial primary spongiosa (Mavropoulos et al. 2007). Furthermore, the fact that certain bone diseases only affect the maxilla and mandible, such as bisphosphonate-related osteonecrosis of the jaw (BRONJ) (Price et al. 2004) and hyperparathyroid jaw tumor syndrome (Simonds et al. 2002), also suggest that different homeostatic mechanisms exist between craniofacial and noncraniofacial bones.
It remains ambiguous as to what causes these differences between craniofacial and skeletal bones, because osteoblast differentiation in both types of bone is regulated by the same key factors, including the transcription factors Runx2 and osterix (Ducy et al. 1997; Otto et al. 1997; Nakashima et al. 2002). Still, it has been shown that several growth factors, receptors, and associated signaling cascades play distinct roles in the craniofacial versus axial and appendicular skeleton (Abzhanov et al. 2007; De Coster et al. 2007; Kimmel et al. 2007).
CRANIOFACIAL BONE REGENERATION
Taken together, craniofacial and long bones are not only derived from different germ layers, but also show distinct characteristics. These results emphasize the need to take these differences into account when considering craniofacial bone–regeneration strategies.
Craniofacial Bone Graft
Similar to other bone reconstructions, autogenous bone grafts are considered to be the gold standard for reconstructing craniofacial bone defects (Gruss et al. 1985; Manson et al. 1985). The iliac crest and rib bones, all derived from the mesoderm, are among the more commonly used donor sites for bone grafting in craniofacial surgeries because they can be readily harvested while minimally impacting the host. Other than the common shortcomings of bone grafts, such as limited harvest amount and donor site morbidity, the major concern with using these bones is that they do not show the characteristics of natural jawbone.
As previously discussed, lack of vascularization can lead to graft resorption with resultant loss of the geometric structure of the bone graft (Peer 1951). Periosteum preservation can improve graft survival in the craniofacial region (Thompson and Casson 1970) by facilitating early revascularization (Knize 1974). Conditions at the implant site also influence graft revascularization (Zins et al. 1984). Although muscle coverage results in increased bone graft revascularization (Ermis and Poole 1992), facial muscles generally originate from the surface of the skull and craniofacial bones, and craniofacial injuries are commonly associated with muscle damage, which negatively influences the ingrowth of blood vessels. Previously reports have shown that for mandibular defects small bony defects (<4 to 6 cm) can successfully be treated with nonvascularized cortical-cancellous bone grafts, whereas larger continuity defects require vascularized grafts (Hidalgo 1989; Pogrel et al. 1997).
Another issue is that most craniofacial bones show extremely complex 3D shapes as compared to long bones. It is, therefore, exceedingly difficult to select and reshape vascularized autologous iliac crest, fibula, or ribs to precisely fit craniofacial bone defects.
Previous publications have shown that rigid fixation can improve bone graft volume retention, by facilitating primary bone healing and rapid revascularization, especially in bones required for motion such as the femur (Phillips and Rahn 1988; Lin et al. 1990). In contrast, most craniofacial bones show little motion, with the exception of the mandible. Even for the mandible, liquid food can be provided to restrain mandibular movement during the bone-healing process. While it may seem that rigid fixation would not significantly improve fixed craniofacial bone graft survival, a clinical study of 363 patients undergoing nasal reconstruction over a 14-year period showed that exceptional bone graft survival occurred when rigid interosseous stabilization was used (Jackson et al. 1998).
Craniofacial Bone Substitute
Stem Cells for Craniofacial Reconstruction
The main obstacles of using autologous bone for grafting is that it is only available in limited amounts (Neovius and Engstrand 2010). To overcome this critical shortcoming, and to create bone grafts of sufficient complex geometry, the field of bone-tissue engineering has been created as a practical approach to treat craniofacial skeletal defects by combining bioactive carriers, cells, and growth factors. Stem-cell-mediated bone repair has been used in clinical trials to regenerate large craniomaxillofacial defects to slow the process of bone degeneration in patients with osteonecrosis of the femoral head and for prophylactic treatment of distal tibial fractures (Grayson et al. 2015). To date, all of the MSC sources that have displayed promising osteogenic potential for bone regeneration also have been proposed as potential cell sources for craniofacial bone–tissue engineering (Cowan et al. 2004; Griffin et al. 2014).
The most well-characterized and used stem cells for bone regeneration are adult bone marrow stem cells (BMSCs). BMSCs are also the first type of cell tested for craniofacial bone regeneration. In 2001, augmented repair of cranial defects was observed by combining autologous sheep BMSCs with calcium alginate gel (Shang et al. 2001). Since then, MSCs from a variety of species, including mouse, rat, rabbit, canine, and porcine, have been confirmed as suitable cells for craniofacial bone repair (Abukawa et al. 2009; Zou et al. 2011; Lin et al. 2012). Successful craniofacial ossifications have been achieved using autologous BMSC-seeded bioscaffolds in several clinical studies (Kaigler et al. 2010; Behnia et al. 2012).
Adipose-derived mesenchymal cells (AMCs) are another type of commonly used cells for bone regeneration (Gimble et al. 2006; Sandor and Suuronen 2008). Recently, AMCs have emerged as a potential cell source for craniofacial tissue engineering (Zuk et al. 2001, 2002). Compared to BMCs, AMCs are more accessible and can be harvested in larger amounts. It has been shown that AMCs and BMCs isolated from the same donor showed similar growth kinetics and cell senescence (De Ugarte et al. 2003). In 2004, Longaker’s group first showed that the AMCs promoted bone regeneration of critical-size calvarial defects, when seeded onto hydroxyapatite-coated poly-lactic-co-glycolic acid scaffolds (Cowan et al. 2004). Many subsequent publications have shown that AMCs are a promising cell source for craniofacial bone regeneration when combined with a variety of scaffold materials (Gomes et al. 2012; Azevedo-Neto et al. 2013; Jin et al. 2014).
Certain types of perinatal stem cells, including umbilical cord–derived mesenchymal stem cells (Chen et al. 2013), amniotic epithelial cells (Barboni et al. 2013), and amniotic fluid mesenchymal cells (Berardinelli et al. 2013), have also shown osteogenic differentiation capacity and, therefore, the potential for craniofacial bone regeneration. One of the main advantages of these cells for promoting craniofacial bone regeneration is their ability to promote blood vessel formation (Maraldi et al. 2013).
As described above, most craniofacial tissues are derived from the neural crest. Paraxial mesoderm–derived iliac crest bone and osteoblasts show distinctly different properties than neural crest cell (NCC)-derived craniofacial bones and osteoblasts (Chai and Maxson 2006; Oppenheimer et al. 2008; Aghaloo et al. 2010), making them less desirable for craniofacial reparative bone applications. Therefore, a variety of cell populations harvested from the craniofacial complex have been examined for utility in craniofacial bone regeneration (Zhao and Chai 2015).
These studies showed that, similar to long bones, the periosteum of craniofacial bones contains progenitor cells that support craniofacial bone repair (Ochareon and Herring 2011). Craniofacial bone marrow cells have also been well characterized. A recent study using labeled NCC and mesoderm-derived bone marrow cells showed bone defect healing via the selective recruitment of cells from their respective tissues embryonic origin (Leucht et al. 2008), thereby reinforcing the presence of embryonic site-specific differences in craniofacial versus appendicular skeleton–derived BMSCs. A rat study showed that in vitro cultured mandible–derived BMSCs showed higher alkaline phosphatase activity, mineralization, and osteoblast gene expression, and formed 70% larger bone nodules containing threefold more mineralized bone, as compared to long-bone BMSCs (Aghaloo et al. 2010). It has also been shown that human stromal cells isolated from mandibular or maxillary marrow showed increased cell proliferation, stronger expression of osteoblastic markers, and delayed senescence as compared to iliac crest–derived marrow cells harvested from the same patient (Akintoye et al. 2006). But the relatively small size and anatomic complexity of the maxilla and mandible render the efficient isolation of bone marrow stem cells a difficult process (Yang et al. 2014).
Recently, a new type of BMSC, isolated from calvarial bone sutures, has shown utility for craniofacial bone regeneration (Zhao et al. 2015). In 2015, Chai’s group identified Gli1+ cells present within the calvarial suture mesenchyme of adult mice that can give rise to both an osteogenic front, periosteum, dura, and craniofacial bones. In humans, cranial sutures normally fuse between 20 and 30 years of age, whereas facial sutures fuse after ∼50 years of age (Senarath-Yapa et al. 2012; Badve et al. 2013), suggesting the possibility of isolating these cells for use in craniofacial bone regeneration. Still, the limited amount of suture mesenchyme prevents harvest of large numbers of cells.
All of the tissues found in teeth originate from the neural crest. Stem cells isolated from adult dental tissues, including dental pulp stem cells (DPSCs) (Gronthos et al. 2000; Papaccio et al. 2006; Zhang et al. 2008), stem cells from human exfoliated deciduous teeth (SHED) (Miura et al. 2003), periodontal ligament stem cells (PDLSCs) (Seo et al. 2004), and periapical stem cells (PSCs) (Han et al. 2010), all showed potential for osteo/odontogenic differentiation and the ability to form calcified dentin or osteodentin-like tissue. Therefore, these cell populations can be used not only to regenerate these same dental tissues, but also for craniofacial and noncraniofacial bone–tissue regeneration. In particular, DPSCs, which can be easily isolated from extracted wisdom teeth, showed a higher proliferation rate as compared to bone marrow–derived MSCs under the same culture conditions, potentially attributable to the strong expression of the cell-cycle activator, cyclin-dependent kinase 6 (Shi et al. 2001).
Widespread use of dental tissue–derived stem cells is somewhat limited by the size of the available tissues that can be used. Comparatively, it may be easier to harvest gingiva-derived mesenchymal stem cells (GMSCs), which also share the multilineage differentiation potential (Zhang et al. 2009). It has been shown that human GMSCs can support new bone formation in both mandibular and calvarial defects at 2 months in rat postsurgical reconstructions (Wang et al. 2011).
Cleft lip and palate (CL/P) are among the most common congenital malformations, occurring in 1/700 live births (Tolarova and Cervenka 1998). Standard surgical treatment of CL/P patients involves the removal of small pieces of orbicular oris muscle (Shkoukani et al. 2013). Bone reconstructive repair of a critical-size cranial defect was successfully achieved using muscle cells derived from these discarded tissues (Bueno et al. 2009).
Stem cells and/or scaffolds loaded with active growth factors, which stimulate osteoprogenitor cells to differentiate toward osteogenic support of the new bone formation, are also commonly used for bone-tissue engineering (Friedlaender et al. 2001; Terheyden et al. 2004). One of the most commonly used growth factors for craniofacial bone regeneration is bone morphogenic protein (BMP) (Terheyden et al. 2001a,b; Warnke and Coren 2003; Warnke et al. 2004).
Challenges for the Use of Stem Cells in Craniofacial Tissue–Engineering Applications
One of the main obstacles in translating experimental observations into clinical practice is the relatively poor mechanistic understanding of stem-cell-mediated therapies. In vivo stem-cell therapy requires large numbers of cells; insufficient cell numbers will not provide positive outcomes (Habisch et al. 2007). Despite the obvious benefits associated with cell delivery for bone regeneration, very few transplanted cells survive long term following transplantation (Dupont et al. 2010). The current lack of reliable methods to control MSC fate, especially in the in vivo condition, is one of the main problems that needs to be addressed. Better understanding of methods to optimize regenerative approaches is required before stem-cell-mediated therapies can be implemented as standard care in regenerative medicine.
MSC isolation and selection is another challenge. For most clinical applications, only a limited amount of viable tissue is available for stem-cell isolation (Sodek and McKee 2000). In addition, MSC selection and enrichment is hampered by lack of a single definitive stem-cell marker (Gronthos et al. 2003). Current isolation methods can achieve heterogeneous cell populations at best, and the proportions of MSC progenitors in these enriched MSC populations can vary even when the samples are obtained from the same donor at the same time (Digirolamo et al. 1999). Previous reports have indicated that only ∼30% of the initial adherent bone marrow–derived MSC clones showed trilineage (osteo/adipo/chondro) differentiation potential (Pittenger et al. 1999; Muraglia et al. 2000). Furthermore, only half of the single colony–derived clones transplanted with hydroxyapatite-tricalcium phosphate ceramic scaffolds showed bone formation in vivo (Kuznetsov et al. 1997). Although current clinical trials have shown that it is not necessary to generate purified cell populations to achieve positive results in the clinic (Prockop 2007), it is possible that more purified MSC populations will help to achieve more reliable clinical outcomes.
There also is a need for optimized methods to store and expand enriched MSC populations while maintaining the “stemness” of the MSCs, as required for advanced clinical application. This is because MSCs tend to spontaneously differentiate into various cell types over time in culture (Banfi et al. 2000; Izadpanah et al. 2008) and MSCs lose their multipotentiality after six or seven passages in in vitro cell culture (Colter et al. 2000; Sekiya et al. 2002). One of the most promising solutions is to create 3D culture conditions that resemble the in vivo stem-cell niche (Bara et al. 2015; Xiong et al. 2015). To date, no standardized methods have been achieved for this approach, despite many diverse attempts (Chow et al. 2001a,b). For example, a variety of tissue culture media have been tested, including those with low serum, various substitutes for fetal bovine serum (FBS) such as autologous serum, fresh frozen plasma, and human platelet lysates (Lange et al. 2007; Le Blanc et al. 2007), and a variety of serum-free media (Sekiya et al. 2002; Müller et al. 2006; Agata et al. 2009; Lindroos et al. 2009). A variety of 3D scaffold tissue culture methods have also been tested as in vitro 3D stem-cell niche systems, including the 3D hanging drop model (Schmal et al. 2013), bioreactor (Santos et al. 2016), and 3D printing (Alessandri et al. 2016).
CRANIOFACIAL RECONSTRUCTION
Craniofacial reconstruction involves the regeneration of multiple hard and soft tissues that are well vascularized and innervated. For better outcomes, it is ideal to regenerate the craniofacial tissues “all-in-one,” including both soft and hard tissues. A concept of the “biological boundary” was introduced in 1989 (Whitaker 1989), which emphasized that the soft tissue envelope has a genetically predetermined shape that is inclined to remain constant. However, soft connective tissues show faster growth rates as compared to hard tissues, because of the fact that soft tissue cellular components show higher rates of migration. Therefore, one of the main challenges for successful bone healing is preventing the ingrowth of connective tissue into the bone space, especially for large and complex craniofacial bone repair. To achieve this, the concept of guided tissue regeneration, which can be achieved by placing an inert membrane barrier over the defect to block the ingrowth of connective tissue, has been widely used for successful craniofacial reconstruction (Dahlin et al. 1988, 1990).
Computer-Aided Design (CAD)
One central challenge for successful craniofacial reconstruction is how to mimic the complex 3D structure and multicellular interactions that naturally occur in complex craniofacial structures (Thesleff et al. 2011). Resorption of bone grafts or regenerated bones often occurs after bone reconstruction. Minor bone resorption may not significantly influence the successful repair of long bones, but can seriously affect craniofacial reconstruction outcomes, again because of the complex geometry and small sizes of many craniofacial bones. Craniofacial reconstruction requires more accurate regenerative approaches to achieve desirable outcomes.
Currently, with the development of 3D computer tomography (CT) and CAD techniques, CT/CAD-based scaffold design can greatly benefit the accurate regeneration of craniofacial bones. One of the most commonly used strategies for accurate craniofacial reconstruction is the use of titanium scaffolds manufactured by the CT/CAD technique, based on the fact that nonabsorbable titanium scaffolds can achieve appropriately detailed complex craniofacial anatomical geometry. Terheyden’s group has used a titanium mesh cage filled with bone mineral blocks infiltrated with 7 mg recombinant hBMP7 and 20 ml of the patient’s bone marrow to successfully restore mandibular defects greater than 7 cm (Warnke et al. 2004). The titanium mesh cage was first implanted into the patient’s right latissimus dorsi muscle for 7 weeks before being transplanted as a free bone muscle flap. Eventually, the reconstructed mandible achieved the desired aesthetic and masticative functional levels. In another case, a titanium mesh was prefabricated and filled with β tricalcium phosphate (β-TCP) granules that were soaked in rhBMP-2 for 48 h before infiltrating with cultured adipose-derived stem cells (ASCs). In this instance, sufficient new bone developed to support dental implants in 10 months (Sandor et al. 2013). A combination of milled bone from the iliac crest, ASCs isolated from fat tissue harvested from the gluteal region, and autologous fibrin glue have also been used to treat multiple fractures (Lendeckel et al. 2004). Bone regeneration was observed within 3 months of surgery in these cases. Functional and anatomically correct repair of a maxillary defect was achieved using a microvascular custom-made ectopic bone flap developed from a titanium cage filled with β-TCP granules mixed autologous ASCs (Thesleff et al. 2011). To date, titanium cages filled with granules, cancellous bone chips, or bone blocks have been shown by far to be the best strategy for bone restoration of large craniofacial defects.
While conventional regenerative strategies have failed to regenerate or mimic the 3D complexity of the multicellular interactions occurring in native craniofacial tissues, 3D cell culture in bioreactors combined with gene therapy or growth factors have shown promise for increased survival of bone substitutes (Scherberich et al. 2007; Cordonnier et al. 2010; Sohier et al. 2010).
3D Printing
3D bioprinting of tissue provides a promising approach to customize various combinations of biomaterials and cell sources to achieve complex 3D architectures. 3D printing is a method that fabricates objects by building consecutive layers of scaffold material plus cells layer by layer, until the desired 3D volumetric structures are achieved (Derby 2012). In this way, 3D printing techniques can provide precise spatial control of the functional components. For the purpose of tissue regeneration, it is possible to print cells directly, to print cell-laden biomaterials, or to print scaffold-free cell aggregates. Currently, there are three types of commercially available 3D printers for tissue engineering: inkjet printers (Klebe 1988), laser-based printers (Guillemot et al. 2010), and microextrusion printers (Fig. 2) (Cohen et al. 2006). Inkjet printers, the most commonly used type of printer for both nonbiological and biological applications, print structures using a drop-on-demand process (Murphy and Atala 2014). Inkjet bioprinters have successfully been used to fabricate bone. This technique can greatly benefit craniofacial bone constructs because it provides fairly rapid and “customer-fit” reconstruction of complex surface bone (Saijo et al. 2009; Azuma et al. 2014). Inkjet bioprinting techniques have also been tested for functional in situ skin regeneration (Skardal et al. 2012). Microextrusion printers use pneumatic or mechanical (piston or screw) dispensing systems to extrude continuous beads of material and/or cells (Chang et al. 2008; Visser et al. 2013). Microextrusion bioprinters have been used to fabricate multiple tissue types, including branched vascular trees (Norotte et al. 2009), islet tissue (Xu et al. 2010), and aortic valves (Duan et al. 2013). Laser-based bioprinter design is based on a laser-induced forward transfer technique using a laser beam (Chrisey 2000). To date, laser-based bioprinters have been successfully used to print cellularized skin (Colina et al. 2005).
Figure 2.
Components of inkjet, microextrusion, and laser-assisted bioprinters. (A) Thermal inkjet printers electrically heat the printhead to produce air-pressure pulses that force droplets from the nozzle, whereas acoustic printers use pulses formed by piezoelectric or ultrasound pressure. (B) Microextrusion printers use pneumatic or mechanical (piston or screw) dispensing systems to extrude continuous beads of material and/or cells. (C) Laser-assisted printers use lasers focused on an absorbing substrate to generate pressures that propel cell-containing materials onto a collector substrate. (From Murphy and Atala 2014; adapted, with permission, from Nature Publishing Group © 2014.)
Current 3D techniques for craniofacial regeneration are largely limited to bone and cartilage tissues (Schek et al. 2005; Reichert et al. 2012). Hierarchical composite scaffolds, consisting of both soft and hard tissue components, have successfully been used for periodontal complex regeneration (Vaquette et al. 2012; Costa et al. 2014; Ivanovski et al. 2014). Laser-based bioprinters have been used to deposit nanohydroxyapatite in a mouse calvarial 3D defect model (Keriquel et al. 2010). Similarly, 3D-printed biphasic scaffolds containing poly-(epsilon)-caprolactone and hydroxyapatite in the size and shape of teeth have been tested, as well as 3D-printed dental epithelial (DE) and dental mesenchymal (DM) cell aggregates, to use as in vitro models for DE–DM cell interactions observed in natural tooth development (Kim et al. 2010).
The 3D printing techniques designed to achieve functional organ regeneration is still in its infancy (Ho et al. 2015). 3D-printed scaffolds, tissue analogs, and organs have been proposed as exciting alternatives to address some of these key challenges now facing the fields of regenerative medicine and dentistry (Derby 2012; Murphy and Atala 2014). This technique has the advantages of enabling the precise positioning of cells and biomaterials in 3D with finely tuned internal and external architectures, while being customizable to patient-specific needs (Obregon et al. 2015).
The craniofacial complex contains multiple types of highly integrated hard and soft tissues. It would be beneficial to regenerate composite hard and soft tissues at the same time, to achieve rapid functional recovery of regenerated craniofacial tissues. Teeth are somewhat unique components of the craniofacial complex. Since teeth are anchored in maxillary and mandibular jawbones, methods to generate jawbone together with dental tissues, in addition to 3D printing, are currently being investigated, with some success (Fig. 3) (Duailibi et al. 2008; Abukawa et al. 2009). A major obstacle in this approach is the design of methods and approaches that maintain the integrity of both the bone and tooth structures, while allowing them to be functionally integrated. Prior reports showed that bioengineered tooth constructs were surrounded by newly formed bone, precluding tooth eruption and proper function (Cowan et al. 2004). The clinical use of composite tissue regeneration, such as cartilage and bone and vascularized muscle, is still in its infancy, and further studies are needed to perfect these highly promising approaches.
Figure 3.
Composite bone–tooth constructs. (A) Tooth scaffolds composed of a dental mesenchymal (DM) cell seeded polyglycolide/poly-l-lactide (PGA/PLLA) scaffold sphere to mimic the dental papilla, and a dental epithelial (DE) cell seeded gelfoam strip to mimic the enamel organ. (B) Lattice bone scaffolds made by poly-dl-lactic-co-glycolic acid (PLGA), seeded with iliac crest–derived mesenchymal stem cells (MSCs) and grown in the rotational oxygen-permeable bioreactor system (ROBS) for 6 weeks. (C) Fabricated tooth–bone construct seeded with cells before implantation. (D) Surgical implant site before wound closure. (E) Bioengineered dental tissues that closely resembled those of naturally formed pig tooth tissues surrounded by alveolar bone. Scale bar = 100 µm. b, bone; bm, bone marrow; d, dentin; e, enamel; p, pulp; pdl, periodontal ligament.
One of the main obstacles of current 3D printing techniques is the size limit of the printout structure, mainly because hydrogel, the most commonly used injecting material, provides inadequate structural support (Chang et al. 2011). Recently, a new integrated tissue-organ printer (ITOP) has been developed to target the generation of stable human-scale tissue constructs (Kang et al. 2016). The key to this method is to print a sacrificial scaffold adjacent to the cell-laden hydrogel. The cell-laden hydrogel protects cell viability and supports cell expansion and differentiation. The sacrificial scaffolding material provides the initial structural and architectural integrity and, on removal, also provides diffusable microchannels to facilitate nutrient perfusion. This technique has been used to fabricate human-scale mandibular bone, ear-shaped cartilage, and organized skeletal muscle.
Another major challenge of the 3D printing technique is how to precisely integrate biological components and gradients for composite tissue engineering. Compared to other tissue-regeneration methods, 3D printing can provide fairly accurate control of different tissues. Still, 3D printing has its own limitations, mainly because the formation of 3D structures is based on the accumulation of 2D structures. For example, accurately mimicking the branching patterns of the vascular tree has remained a big challenge of 3D printing (Kolesky et al. 2016).
CONCLUSIONS
Successful reconstruction of the craniofacial complex requires full restoration of both functional and aesthetic aspects of the face. Stem-cell-mediated tissue-regeneration approaches provide the potential for highly successful craniofacial tissue-regeneration. Despite increased knowledge and characterization of MSCs, and the mounting enthusiasm for the use of MSCs in regenerative therapies in humans, the detailed mechanisms of MSCs proliferation and differentiation are still not fully understood.
This lack of understanding has not permitted the full use of stromal cells to facilitate or enhance tissue repair in clinical practice because of fear of potential unwarranted deleterious behaviors of the transplanted cells (Grayson et al. 2015). Despite these concerns, stem-cell-mediated regenerative strategies, combined with precise CAD approaches, are anticipated to eventually provide many new and promising methods for successful craniofacial reparative therapies.
Footnotes
Editor: Joseph P. Vacanti
Additional Perspectives on Tissue Engineering and Regenerative Medicine available at www.perspectivesinmedicine.org
REFERENCES
- Abukawa H, Zhang W, Young CS, Asrican R, Vacanti JP, Kaban LB, Troulis MJ, Yelick PC. 2009. Reconstructing mandibular defects using autologous tissue-engineered tooth and bone constructs. J Oral Maxillofac Surg 67: 335–347. [DOI] [PubMed] [Google Scholar]
- Abzhanov A, Rodda SJ, McMahon AP, Tabin CJ. 2007. Regulation of skeletogenic differentiation in cranial dermal bone. Development 134: 3133–3144. [DOI] [PubMed] [Google Scholar]
- Agata H, Watanabe N, Ishii Y, Kubo N, Ohshima S, Yamazaki M, Tojo A, Kagami H. 2009. Feasibility and efficacy of bone tissue engineering using human bone marrow stromal cells cultivated in serum-free conditions. Biochem Biophys Res Commun 382: 353–358. [DOI] [PubMed] [Google Scholar]
- Aghaloo TL, Chaichanasakul T, Bezouglaia O, Kang B, Franco R, Dry SM, Atti E, Tetradis S. 2010. Osteogenic potential of mandibular vs. long-bone marrow stromal cells. J Dent Res 89: 1293–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akintoye SO, Lam T, Shi S, Brahim J, Collins MT, Robey PG. 2006. Skeletal site-specific characterization of orofacial and iliac crest human bone marrow stromal cells in same individuals. Bone 38: 758–768. [DOI] [PubMed] [Google Scholar]
- Alessandri K, Feyeux M, Gurchenkov B, Delgado C, Trushko A, Krause KH, Vignjević D, Nassoy P, Roux A. 2016. A 3D printed microfluidic device for production of functionalized hydrogel microcapsules for culture and differentiation of human neuronal stem cells (hNSC). Lab Chip 16: 1593–1604. [DOI] [PubMed] [Google Scholar]
- Azevedo-Neto RD, Gonzaga CC, Deliberador TM, Klug LG, da Costa Oliveira L, Zielak JC, de Andrade Urban C, de Araujo MR, Giovanini AF. 2013. Fragmented adipose tissue transplanted to craniofacial deformities induces bone repair associated with immunoexpression of adiponectin and parathyroid hormone 1-receptor. Cleft Palate Craniofac J 50: 639–647. [DOI] [PubMed] [Google Scholar]
- Azuma M, Yanagawa T, Ishibashi-Kanno N, Uchida F, Ito T, Yamagata K, Hasegawa S, Sasaki K, Adachi K, Tabuchi K, et al. 2014. Mandibular reconstruction using plates prebent to fit rapid prototyping 3-dimensional printing models ameliorates contour deformity. Head Face Med 10: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badve CA, Mallikarjunappa, MK, Iyer RS, Ishak GE, Khanna PC. 2013. Craniosynostosis: Imaging review and primer on computed tomography. Pediatr Radiol 43: 728–742; quiz 725–727. [DOI] [PubMed] [Google Scholar]
- Banfi A, Muraglia A, Dozin B, Mastrogiacomo M, Cancedda R, Quarto R. 2000. Proliferation kinetics and differentiation potential of ex vivo expanded human bone marrow stromal cells: Implications for their use in cell therapy. Exp Hematol 28: 707–715. [DOI] [PubMed] [Google Scholar]
- Bara JJ, Herrmann M, Menzel U, Benneker L, Alini M, Stoddart MJ. 2015. Three-dimensional culture and characterization of mononuclear cells from human bone marrow. Cytotherapy 17: 458–472. [DOI] [PubMed] [Google Scholar]
- Barboni B, Mangano C, Valbonetti L, Marruchella G, Berardinelli P, Martelli A, Muttini A, Mauro A, Bedini R, Turriani M, et al. 2013. Synthetic bone substitute engineered with amniotic epithelial cells enhances bone regeneration after maxillary sinus augmentation. PLoS ONE 8: e63256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behnia H, Khojasteh A, Soleimani M, Tehranchi A, Atashi A. 2012. Repair of alveolar cleft defect with mesenchymal stem cells and platelet derived growth factors: A preliminary report. J Craniomaxillofac Surg 40: 2–7. [DOI] [PubMed] [Google Scholar]
- Berardinelli P, Valbonetti L, Muttini A, Martelli A, Peli R, Zizzari V, Nardinocchi D, Vulpiani MP, Tetè S, Barboni B, et al. 2013. Role of amniotic fluid mesenchymal cells engineered on MgHA/collagen-based scaffold allotransplanted on an experimental animal study of sinus augmentation. Clin Oral Investig 17: 1661–1675. [DOI] [PubMed] [Google Scholar]
- Beyer Nardi N, da Silva Meirelles L. 2006. Mesenchymal stem cells: Isolation, in vitro expansion and characterization. Handb Exp Pharmacol 2006: 249–282. [PubMed] [Google Scholar]
- Bilezikian JP, Raisz LG, Rodan GA (ed). 2002. Principles of bone biology, 2nd ed Academic, San Diego. [Google Scholar]
- Buchman SR, Ozaki W. 1999. The ultrastructure and resorptive pattern of cancellous onlay bone grafts in the craniofacial skeleton. Ann Plast Surg 43: 49–56. [DOI] [PubMed] [Google Scholar]
- Bueno DF, Kerkis I, Costa AM, Martins MT, Kobayashi GS, Zucconi E, Fanganiello RD, Salles FT, Almeida AB, do Amaral CE, et al. 2009. New source of muscle-derived stem cells with potential for alveolar bone reconstruction in cleft lip and/or palate patients. Tissue Eng Part A 15: 427–435. [DOI] [PubMed] [Google Scholar]
- Burchardt H. 1983. The biology of bone graft repair. Clin Orthop Relat Res 1983: 28–42. [PubMed] [Google Scholar]
- Burchardt H. 1987. Biology of bone transplantation. Orthop Clin North Am 18: 187–196. [PubMed] [Google Scholar]
- Chai Y, Maxson RE Jr. 2006. Recent advances in craniofacial morphogenesis. Dev Dyn 235: 2353–2375. [DOI] [PubMed] [Google Scholar]
- Chang R, Nam J, Sun W. 2008. Effects of dispensing pressure and nozzle diameter on cell survival from solid freeform fabrication-based direct cell writing. Tissue Eng Part A 14: 41–48. [DOI] [PubMed] [Google Scholar]
- Chang CC, Boland ED, Williams SK, Hoying JB. 2011. Direct-write bioprinting three-dimensional biohybrid systems for future regenerative therapies. J Biomed Mater Res B Appl Biomater 98: 160–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen NT, Glowacki J, Bucky LP, Hong HZ, Kim WK, Yaremchuk MJ. 1994. The roles of revascularization and resorption on endurance of craniofacial onlay bone grafts in the rabbit. Plast Reconstr Surg 93: 714–722; discussion 23–24. [PubMed] [Google Scholar]
- Chen W, Liu J, Manuchehrabadi N, Weir MD, Zhu Z, Xu HH. 2013. Umbilical cord and bone marrow mesenchymal stem cell seeding on macroporous calcium phosphate for bone regeneration in rat cranial defects. Biomaterials 34: 9917–9925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow DC, Wenning LA, Miller WM, Papoutsakis ET. 2001a. Modeling pO2 distributions in the bone marrow hematopoietic compartment. I: Krogh’s model. Biophys J 81: 675–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow DC, Wenning LA, Miller WM, Papoutsakis ET. 2001b. Modeling pO2 distributions in the bone marrow hematopoietic compartment. II: Modified Kroghian models. Biophys J 81: 685–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrisey DB. 2000. Materials processing: The power of direct writing. Science 289: 879–881. [DOI] [PubMed] [Google Scholar]
- Cohen DL, Malone E, Lipson H, Bonassar LJ. 2006. Direct freeform fabrication of seeded hydrogels in arbitrary geometries. Tissue Eng 12: 1325–1335. [DOI] [PubMed] [Google Scholar]
- Colina M, Serra P, Fernandez-Pradas JM, Sevilla L, Morenza JL. 2005. DNA deposition through laser induced forward transfer. Biosens Bioelectron 20: 1638–1642. [DOI] [PubMed] [Google Scholar]
- Colter DC, Class R, DiGirolamo CM, Prockop DJ. 2000. Rapid expansion of recycling stem cells in cultures of plastic-adherent cells from human bone marrow. Proc Natl Acad Sci 97: 3213–3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordonnier T, Layrolle P, Gaillard J, Langonné A, Sensebé L, Rosset P, Sohier J. 2010. 3D environment on human mesenchymal stem cells differentiation for bone tissue engineering. J Mater Sci Mater Med 21: 981–987. [DOI] [PubMed] [Google Scholar]
- Costa PF, Vaquette C, Zhang Q, Reis RL, Ivanovski S, Hutmacher DW. 2014. Advanced tissue engineering scaffold design for regeneration of the complex hierarchical periodontal structure. J Clin Periodontol 41: 283–294. [DOI] [PubMed] [Google Scholar]
- Cowan CM, Shi YY, Aalami OO, Chou YF, Mari C, Thomas R, Quarto N, Contag CH, Wu B, Longaker MT. 2004. Adipose-derived adult stromal cells heal critical-size mouse calvarial defects. Nat Biotechnol 22: 560–567. [DOI] [PubMed] [Google Scholar]
- Dahlin C, Linde A, Gottlow J, Nyman S. 1988. Healing of bone defects by guided tissue regeneration. Plast Reconstr Surg 81: 672–676. [DOI] [PubMed] [Google Scholar]
- Dahlin C, Gottlow J, Linde A, Nyman S. 1990. Healing of maxillary and mandibular bone defects using a membrane technique. An experimental study in monkeys. Scand J Plast Reconstr Surg Hand Surg 24: 13–19. [DOI] [PubMed] [Google Scholar]
- De Coster PJ, Mortier G, Marks LA, Martens LC. 2007. Cranial suture biology and dental development: Genetic and clinical perspectives. J Oral Pathol Med 36: 447–455. [DOI] [PubMed] [Google Scholar]
- Derby B. 2012. Printing and prototyping of tissues and scaffolds. Science 338: 921–926. [DOI] [PubMed] [Google Scholar]
- De Ugarte DA, Morizono K, Elbarbary A, Alfonso Z, Zuk PA, Zhu M, Draggo JL, Ashjian P, Thomas B, Benhaim P, et al. 2003. Comparison of multi-lineage cells from human adipose tissue and bone marrow. Cells Tissues Organs 174: 101–109. [DOI] [PubMed] [Google Scholar]
- Digirolamo CM, Stokes D, Colter D, Phinney DG, Class R, Prockop DJ. 1999. Propagation and senescence of human marrow stromal cells in culture: A simple colony-forming assay identifies samples with the greatest potential to propagate and differentiate. Br J Haematol 107: 275–281. [DOI] [PubMed] [Google Scholar]
- Duailibi SE, Duailibi MT, Zhang W, Asrican R, Vacanti JP, Yelick PC. 2008. Bioengineered dental tissues grown in the rat jaw. J Dent Res 87: 745–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan B, Hockaday LA, Kang KH, Butcher JT. 2013. 3D bioprinting of heterogeneous aortic valve conduits with alginate/gelatin hydrogels. J Biomed Mater Res A 101: 1255–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G. 1997. Osf2/Cbfa1: A transcriptional activator of osteoblast differentiation. Cell 89: 747–754. [DOI] [PubMed] [Google Scholar]
- Dupont KM, Sharma K, Stevens HY, Boerckel JD, Garcia AJ, Guldberg RE. 2010. Human stem cell delivery for treatment of large segmental bone defects. Proc Natl Acad Sci 107: 3305–3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enlow DH. 1982. The handbook of facial growth. WB Saunders, Philadelphia. [Google Scholar]
- Ermis I, Poole M. 1992. The effects of soft tissue coverage on bone graft resorption in the craniofacial region. Br J Plast Surg 45: 26–29. [DOI] [PubMed] [Google Scholar]
- Eyre-Brook AL. 1984. The periosteum: Its function reassessed. Clin Orthop Relat Res 1984: 300–307. [PubMed] [Google Scholar]
- Feisst V, Brooks AE, Chen CJ, Dunbar PR. 2014. Characterization of mesenchymal progenitor cell populations directly derived from human dermis. Stem Cells Dev 23: 631–642. [DOI] [PubMed] [Google Scholar]
- Friedenstein AJ. 1976. Precursor cells of mechanocytes. Int Rev Cytol 47: 327–359. [DOI] [PubMed] [Google Scholar]
- Friedenstein AJ, Petrakova KV, Kurolesova AI, Frolova GP. 1968. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation 6: 230–247. [PubMed] [Google Scholar]
- Friedlaender GE, Perry CR, Cole JD, Cook SD, Cierny G, Muschler GF, Zych GA, Calhoun JH, LaForte AJ, Yin S. 2001. Osteogenic protein-1 (bone morphogenetic protein-7) in the treatment of tibial nonunions. J Bone Joint Surg Am 83-A: S151–S158. [PMC free article] [PubMed] [Google Scholar]
- Frommelt H. 1987. Polymers for medical applications. Makromol Chem Macromol Symp 12: 281–301. [Google Scholar]
- Garrett GL, Beegun I, D’Souza A. 2015. Facial transplantation: Historical developments and future directions. J Laryngol Otol 129: 206–211. [DOI] [PubMed] [Google Scholar]
- Genden EM. 2010. Reconstruction of the mandible and the maxilla: The evolution of surgical technique. Arch Facial Plast Surg 12: 87–90. [DOI] [PubMed] [Google Scholar]
- Gimbel M, Ashley RK, Sisodia M, Gabbay JS, Wasson KL, Heller J, Wilson L, Kawamoto HK, Bradley JP. 2007. Repair of alveolar cleft defects: Reduced morbidity with bone marrow stem cells in a resorbable matrix. J Craniofac Surg 18: 895–901. [DOI] [PubMed] [Google Scholar]
- Gimble JM, Zvonic S, Floyd ZE, Kassem M, Nuttall ME. 2006. Playing with bone and fat. J Cell Biochem 98: 251–266. [DOI] [PubMed] [Google Scholar]
- Gomes SP, Deliberador TM, Gonzaga CC, Klug LG, da Costa Oliveira L, de Andrade Urban C, Zielak JC, Giovanini AF. 2012. Bone healing in critical-size defects treated with immediate transplant of fragmented autogenous white adipose tissue. J Craniofac Surg 23: 1239–1244. [DOI] [PubMed] [Google Scholar]
- Grayson WL, Bunnell BA, Martin E, Frazier T, Hung BP, Gimble JM. 2015. Stromal cells and stem cells in clinical bone regeneration. Nat Rev Endocrinol 11: 140–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin M, Kalaskar DM, Butler PE, Seifalian AM. 2014. The use of adipose stem cells in cranial facial surgery. Stem Cell Rev 10: 671–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. 2000. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci 97: 13625–13630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronthos S, Zannettino AC, Hay SJ, Shi S, Graves SE, Kortesidis A, Simmons PJ. 2003. Molecular and cellular characterisation of highly purified stromal stem cells derived from human bone marrow. J Cell Sci 116: 1827–1835. [DOI] [PubMed] [Google Scholar]
- Gruss JS, Mackinnon SE, Kassel EE, Cooper PW. 1985. The role of primary bone grafting in complex craniomaxillofacial trauma. Plast Reconstr Surg 75: 17–24. [DOI] [PubMed] [Google Scholar]
- Guillemot F, Souquet A, Catros S, Guillotin B, Lopez J, Faucon M, Pippenger B, Bareille R, Rémy M, Bellance S, et al. 2010. High-throughput laser printing of cells and biomaterials for tissue engineering. Acta Biomater 6: 2494–2500. [DOI] [PubMed] [Google Scholar]
- Habisch HJ, Janowski M, Binder D, Kuzma-Kozakiewicz M, Widmann A, Habich A, Schwalenstöcker B, Hermann A, Brenner R, Lukomska B, et al. 2007. Intrathecal application of neuroectodermally converted stem cells into a mouse model of ALS: Limited intraparenchymal migration and survival narrows therapeutic effects. J Neural Transm 114: 1395–1406. [DOI] [PubMed] [Google Scholar]
- Han C, Yang Z, Zhou W, Jin F, Song Y, Wang Y, Huo N, Chen L, Qian H, Hou R, et al. 2010. Periapical follicle stem cell: A promising candidate for cementum/periodontal ligament regeneration and bio-root engineering. Stem Cells Dev 19: 1405–1415. [DOI] [PubMed] [Google Scholar]
- Hidalgo DA. 1989. Fibula free flap: A new method of mandible reconstruction. Plast Reconstr Surg 84: 71–79. [PubMed] [Google Scholar]
- Ho TV, Iwata J, Ho HA, Grimes WC, Park S, Sanchez-Lara PA, Chai Y. 2015. Integration of comprehensive 3D microCT and signaling analysis reveals differential regulatory mechanisms of craniofacial bone development. Dev Biol 400: 180–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hokugo A, Kubo Y, Takahashi Y, Fukuda A, Horiuchi K, Mushimoto K, Morita S, Tabata Y. 2004. Prefabrication of vascularized bone graft using guided bone regeneration. Tissue Eng 10: 978–986. [DOI] [PubMed] [Google Scholar]
- Hunter C, Januszyk M, Wan DC, Momeni A. 2015. Systematic reviews in craniofacial trauma-strengths and weaknesses. Ann Plast Surg 77: 363–368. [DOI] [PubMed] [Google Scholar]
- Ivanovski S, Vaquette C, Gronthos S, Hutmacher DW, Bartold PM. 2014. Multiphasic scaffolds for periodontal tissue engineering. J Dent Res 93: 1212–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izadpanah R, Kaushal D, Kriedt C, Tsien F, Patel B, Dufour J, Bunnell BA. 2008. Long-term in vitro expansion alters the biology of adult mesenchymal stem cells. Cancer Res 68: 4229–4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson IT, Choi HY, Clay R, Bevilacqua R, TerKonda S, Celik M, Smith AW. 1998. Long-term follow-up of cranial bone graft in dorsal nasal augmentation. Plast Reconstr Surg 102: 1869–1873. [DOI] [PubMed] [Google Scholar]
- Jin Y, Zhang W, Liu Y, Zhang M, Xu L, Wu Q, Zhang X, Zhu Z, Huang Q, Jiang X. 2014. rhPDGF-BB via ERK pathway osteogenesis and adipogenesis balancing in ADSCs for critical-sized calvarial defect repair. Tissue Eng Part A 20: 3303–3313. [DOI] [PubMed] [Google Scholar]
- Kaigler D, Pagni G, Park CH, Tarle SA, Bartel RL, Giannobile WV. 2010. Angiogenic and osteogenic potential of bone repair cells for craniofacial regeneration. Tissue Eng Part A 16: 2809–2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang HW, Lee SJ, Ko IK, Kengla C, Yoo JJ, Atala A. 2016. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat Biotechnol 34: 312–319. [DOI] [PubMed] [Google Scholar]
- Keriquel V, Guillemot F, Arnault I, Guillotin B, Miraux S, Amédée J, Fricain JC, Catros S. 2010. In vivo bioprinting for computer- and robotic-assisted medical intervention: Preliminary study in mice. Biofabrication 2: 014101. [DOI] [PubMed] [Google Scholar]
- Kim K, Lee CH, Kim BK, Mao JJ. 2010. Anatomically shaped tooth and periodontal regeneration by cell homing. J Dent Res 89: 842–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel CB, Walker MB, Miller CT. 2007. Morphing the hyomandibular skeleton in development and evolution. J Exp Zool B Mol Dev Evol 308: 609–624. [DOI] [PubMed] [Google Scholar]
- Klebe RJ. 1988. Cytoscribing: A method for micropositioning cells and the construction of two- and three-dimensional synthetic tissues. Exp Cell Res 179: 362–373. [DOI] [PubMed] [Google Scholar]
- Knize DM. 1974. The influence of periosteum and calcitonin on onlay bone graft survival. A roentgenographic study. Plast Reconstr Surg 53: 190–199. [DOI] [PubMed] [Google Scholar]
- Kolesky DB, Homan KA, Skylar-Scott MA, Lewis JA. 2016. Three-dimensional bioprinting of thick vascularized tissues. Proc Natl Acad Sci 113: 3179–3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznetsov SA, Krebsbach PH, Satomura K, Kerr J, Riminucci M, Benayahu D, Robey PG. 1997. Single-colony derived strains of human marrow stromal fibroblasts form bone after transplantation in vivo. J Bone Miner Res 12: 1335–1347. [DOI] [PubMed] [Google Scholar]
- Lange C, Cakiroglu F, Spiess AN, Cappallo-Obermann H, Dierlamm J, Zander AR. 2007. Accelerated and safe expansion of human mesenchymal stromal cells in animal serum-free medium for transplantation and regenerative medicine. J Cell Physiol 213: 18–26. [DOI] [PubMed] [Google Scholar]
- Le Blanc K, Samuelsson H, Lonnies L, Sundin M, Ringden O. 2007. Generation of immunosuppressive mesenchymal stem cells in allogeneic human serum. Transplantation 84: 1055–1059. [DOI] [PubMed] [Google Scholar]
- Lendeckel S, Jödicke A, Christophis P, Heidinger K, Wolff J, Fraser JK, Hedrick MH, Berthold L, Howaldt HP. 2004. Autologous stem cells (adipose) and fibrin glue used to treat widespread traumatic calvarial defects: Case report. J Craniomaxillofac Surg 32: 370–373. [DOI] [PubMed] [Google Scholar]
- Leucht P, Kim JB, Amasha R, James AW, Girod S, Helms JA. 2008. Embryonic origin and Hox status determine progenitor cell fate during adult bone regeneration. Development 135: 2845–2854. [DOI] [PubMed] [Google Scholar]
- Lew TA, Walker JA, Wenke JC, Blackbourne LH, Hale RG. 2010. Characterization of craniomaxillofacial battle injuries sustained by United States service members in the current conflicts of Iraq and Afghanistan. J Oral Maxillofac Surg 68: 3–7. [DOI] [PubMed] [Google Scholar]
- Lin KY, Bartlett SP, Yaremchuk MJ, Fallon M, Grossman RF, Whitaker LA. 1990. The effect of rigid fixation on the survival of onlay bone grafts: An experimental study. Plast Reconstr Surg 86: 449–456. [DOI] [PubMed] [Google Scholar]
- Lin CY, Chang YH, Kao CY, Lu CH, Sung LY, Yen TC, Lin KJ, Hu YC. 2012. Augmented healing of critical-size calvarial defects by baculovirus-engineered MSCs that persistently express growth factors. Biomaterials 33: 3682–3692. [DOI] [PubMed] [Google Scholar]
- Lindroos B, Boucher S, Chase L, Kuokkanen H, Huhtala H, Haataja R, Vemuri M, Suuronen R, Miettinen S. 2009. Serum-free, xeno-free culture media maintain the proliferation rate and multipotentiality of adipose stem cells in vitro. Cytotherapy 11: 958–972. [DOI] [PubMed] [Google Scholar]
- Lukash FN, Zingaro EA, Salig J. 1984. The survival of free nonvascularized bone grafts in irradiated areas by wrapping in muscle flaps. Plast Reconstr Surg 74: 783–788. [DOI] [PubMed] [Google Scholar]
- Manson PN, Crawley WA, Yaremchuk MJ, Rochman GM, Hoopes JE, French JH Jr. 1985. Midface fractures: Advantages of immediate extended open reduction and bone grafting. Plast Reconstr Surg 76: 1–12. [PubMed] [Google Scholar]
- Maraldi T, Riccio M, Pisciotta A, Zavatti M, Carnevale G, Beretti F, La Sala GB, Motta A, De Pol A. 2013. Human amniotic fluid-derived and dental pulp-derived stem cells seeded into collagen scaffold repair critical-size bone defects promoting vascularization. Stem Cell Res Ther 4: 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martuscelli R, Toti P, Sbordone L, Guidetti F, Ramaglia L, Sbordone C. 2014. Five-year outcome of bone remodelling around implants in the maxillary sinus: Assessment of differences between implants placed in autogenous inlay bone blocks and in ungrafted maxilla. Int J Oral Maxillofac Surg 43: 1117–1126. [DOI] [PubMed] [Google Scholar]
- Mavropoulos A, Rizzoli R, Ammann P. 2007. Different responsiveness of alveolar and tibial bone to bone loss stimuli. J Bone Miner Res 22: 403–410. [DOI] [PubMed] [Google Scholar]
- Melcher AH, Accursi GE. 1971. Osteogenic capacity of periosteal and osteoperiosteal flaps elevated from the parietal bone of the rat. Arch Oral Biol 16: 573–580. [DOI] [PubMed] [Google Scholar]
- Mesimäki K, Lindroos B, Törnwall J, Mauno J, Lindqvist C, Kontio R, Miettinen S, Suuronen R. 2009. Novel maxillary reconstruction with ectopic bone formation by GMP adipose stem cells. Int J Oral Maxillofac Surg 38: 201–209. [DOI] [PubMed] [Google Scholar]
- Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG, Robey PG, Shi S. 2003. SHED: Stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci 100: 5807–5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura M, Miura Y, Sonoyama W, Yamaza T, Gronthos S, Shi S. 2006. Bone marrow-derived mesenchymal stem cells for regenerative medicine in craniofacial region. Oral Dis 12: 514–522. [DOI] [PubMed] [Google Scholar]
- Mossey P, Castilla E (ed.). 2003. Global registry and database on craniofacial anomalies. In Report of a WHO registry meeting on craniofacial anomalies. World Health Organization, Geneva, Switzerland. [Google Scholar]
- Müller I, Kordowich S, Holzwarth C, Spano C, Isensee G, Staiber A, Viebahn S, Gieseke F, Langer H, Gawaz MP, et al. 2006. Animal serum-free culture conditions for isolation and expansion of multipotent mesenchymal stromal cells from human BM. Cytotherapy 8: 437–444. [DOI] [PubMed] [Google Scholar]
- Mulliken JB, Kaban LB, Glowacki J. 1984. Induced osteogenesis—The biological principle and clinical applications. J Surg Res 37: 487–496. [DOI] [PubMed] [Google Scholar]
- Muraglia A, Cancedda R, Quarto R. 2000. Clonal mesenchymal progenitors from human bone marrow differentiate in vitro according to a hierarchical model. J Cell Sci 113: 1161–1166. [DOI] [PubMed] [Google Scholar]
- Murphy SV, Atala A. 2014. 3D bioprinting of tissues and organs. Nat Biotechnol 32: 773–785. [DOI] [PubMed] [Google Scholar]
- Nakashima M, Mizunuma K, Murakami T, Akamine A. 2002. Induction of dental pulp stem cell differentiation into odontoblasts by electroporation-mediated gene delivery of growth/differentiation factor 11 (Gdf11). Gene Ther 9: 814–818. [DOI] [PubMed] [Google Scholar]
- Neovius E, Engstrand T. 2010. Craniofacial reconstruction with bone and biomaterials: Review over the last 11 years. J Plast Reconstr Aesthet Surg 63: 1615–1623. [DOI] [PubMed] [Google Scholar]
- Neu BR. 2000. Segmental bone and cartilage reconstruction of major nasal dorsal defects. Plast Reconstr Surg 106: 160–170. [DOI] [PubMed] [Google Scholar]
- Norotte C, Marga FS, Niklason LE, Forgacs G. 2009. Scaffold-free vascular tissue engineering using bioprinting. Biomaterials 30: 5910–5917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obregon F, Vaquette C, Ivanovski S, Hutmacher DW, Bertassoni LE. 2015. Three-dimensional bioprinting for regenerative dentistry and craniofacial tissue engineering. J Dent Res 94: 143S–152S. [DOI] [PubMed] [Google Scholar]
- Ochareon P, Herring SW. 2011. Cell replication in craniofacial periosteum: Appositional vs. resorptive sites. J Anat 218: 285–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oklund SA, Prolo DJ, Gutierrez RV, King SE. 1986. Quantitative comparisons of healing in cranial fresh autografts, frozen autografts and processed autografts, and allografts in canine skull defects. Clin Orthop Relat Res 1986: 269–291. [PubMed] [Google Scholar]
- Oppenheimer AJ, Tong L, Buchman SR. 2008. Craniofacial bone grafting: Wolff’s Law revisited. Craniomaxillofac Trauma Reconstr 1: 49–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto F, Thornell AP, Crompton T, Denzel A, Gilmour KC, Rosewell IR, Stamp GW, Beddington RS, Mundlos S, Olsen BR, et al. 1997. Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell 89: 765–771. [DOI] [PubMed] [Google Scholar]
- Owen M, Friedenstein AJ. 1988. Stromal stem cells: Marrow-derived osteogenic precursors. Ciba Found Symp 136: 42–60. [DOI] [PubMed] [Google Scholar]
- Ozaki W, Buchman SR. 1998. Volume maintenance of onlay bone grafts in the craniofacial skeleton: Micro-architecture versus embryologic origin. Plast Reconstr Surg 102: 291–299. [DOI] [PubMed] [Google Scholar]
- Papaccio G, Graziano A, d’Aquino R, Graziano MF, Pirozzi G, Menditti D, De Rosa A, Carinci F, Laino G. 2006. Long-term cryopreservation of dental pulp stem cells (SBP-DPSCs) and their differentiated osteoblasts: A cell source for tissue repair. J Cell Physiol 208: 319–325. [DOI] [PubMed] [Google Scholar]
- Peer LA. 1951. Fate of autogenous human bone grafts. Br J Plast Surg 3: 233–243. [DOI] [PubMed] [Google Scholar]
- Petrini C. 2015. Facial transplants: Current situation and ethical issues. Clin Ter 166: 215–217. [DOI] [PubMed] [Google Scholar]
- Petrovic V, Zivkovic P, Petrovic D, Stefanovic V. 2012. Craniofacial bone tissue engineering. Oral Surg Oral Med Oral Pathol Oral Radiol 114: e1–e9. [DOI] [PubMed] [Google Scholar]
- Phillips JH, Rahn BA. 1988. Fixation effects on membranous and endochondral onlay bone-graft resorption. Plast Reconstr Surg 82: 872–877. [DOI] [PubMed] [Google Scholar]
- Pinholt EM, Solheim E, Talsnes O, Larsen TB, Bang G, Kirkeby OJ. 1994. Revascularization of calvarial, mandibular, tibial, and iliac bone grafts in rats. Ann Plast Surg 33: 193–197. [DOI] [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. 1999. Multilineage potential of adult human mesenchymal stem cells. Science 284: 143–147. [DOI] [PubMed] [Google Scholar]
- Pogrel MA, Podlesh S, Anthony JP, Alexander J. 1997. A comparison of vascularized and nonvascularized bone grafts for reconstruction of mandibular continuity defects. J Oral Maxillofac Surg 55: 1200–1206. [DOI] [PubMed] [Google Scholar]
- Price N, Lipton A, Jain VK, Ruggiero S. 2004. Prevention and management of osteonecrosis of the jaw associated with bisphosphonate therapy. Support Cancer Ther 2: 14–17. [DOI] [PubMed] [Google Scholar]
- Prockop DJ. 2007. “Stemness” does not explain the repair of many tissues by mesenchymal stem/multipotent stromal cells (MSCs). Clin Pharmacol Ther 82: 241–243. [DOI] [PubMed] [Google Scholar]
- Qu-Petersen Z, Deasy B, Jankowski R, Ikezawa M, Cummins J, Pruchnic R, Mytinger J, Cao B, Gates C, Wernig A, et al. 2002. Identification of a novel population of muscle stem cells in mice: Potential for muscle regeneration. J Cell Biol 157: 851–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaesh T, Bard JB. 2003. The growth and morphogenesis of the early mouse mandible: A quantitative analysis. J Anat 203: 213–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichert JC, Cipitria A, Epari DR, Saifzadeh S, Krishnakanth P, Berner A, Woodruff MA, Schell H, Mehta M, Schuetz MA, et al. 2012. A tissue engineering solution for segmental defect regeneration in load-bearing long bones. Sci Transl Med 4: 141ra93. [DOI] [PubMed] [Google Scholar]
- Rocchi G, Fadda MT, Marianetti TM, Reale G, Iannetti G. 2007. Craniofacial trauma in adolescents: Incidence, etiology, and prevention. J Trauma 62: 404–409. [DOI] [PubMed] [Google Scholar]
- Saijo H, Igawa K, Kanno Y, Mori Y, Kondo K, Shimizu K, Suzuki S, Chikazu D, Lino M, Anzai M, et al. 2009. Maxillofacial reconstruction using custom-made artificial bones fabricated by inkjet printing technology. J Artif Organs 12: 200–205. [DOI] [PubMed] [Google Scholar]
- Sandor GK, Suuronen R. 2008. Combining adipose-derived stem cells, resorbable scaffolds and growth factors: An overview of tissue engineering. J Can Dent Assoc 74: 167–170. [PubMed] [Google Scholar]
- Sandor GK, Tuovinen VJ, Wolff J, Patrikoski M, Jokinen J, Nieminen E, Mannerström B, Lappalainen OP, Seppänen R, Miettinen S. 2013. Adipose stem cell tissue-engineered construct used to treat large anterior mandibular defect: A case report and review of the clinical application of good manufacturing practice-level adipose stem cells for bone regeneration. J Oral Maxillofac Surg 71: 938–950. [DOI] [PubMed] [Google Scholar]
- Santos CA, Andrade LR, Costa MH, Souza HS, Granjeiro JM, Takiya CM, Borojevic R, Nasciutti LE. 2016. Gastrospheres of human gastric mucosa cells: An in vitro model of stromal and epithelial stem cell niche reconstruction. Histol Histopathol 31: 879–895. [DOI] [PubMed] [Google Scholar]
- Schek RM, Taboas JM, Hollister SJ, Krebsbach PH. 2005. Tissue engineering osteochondral implants for temporomandibular joint repair. Orthod Craniofac Res 8: 313–319. [DOI] [PubMed] [Google Scholar]
- Scherberich A, Galli R, Jaquiery C, Farhadi J, Martin I. 2007. Three-dimensional perfusion culture of human adipose tissue-derived endothelial and osteoblastic progenitors generates osteogenic constructs with intrinsic vascularization capacity. Stem Cells 25: 1823–1829. [DOI] [PubMed] [Google Scholar]
- Schmal O, Seifert J, Schaffer TE, Walter CB, Aicher WK, Klein G. 2013. Hematopoietic stem and progenitor cell expansion in contact with mesenchymal stromal cells in a hanging drop model uncovers disadvantages of 3D culture. Stem Cells Int 2016: 4148093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiya I, Larson BL, Smith JR, Pochampally R, Cui JG, Prockop DJ. 2002. Expansion of human adult stem cells from bone marrow stroma: Conditions that maximize the yields of early progenitors and evaluate their quality. Stem Cells 20: 530–541. [DOI] [PubMed] [Google Scholar]
- Senarath-Yapa K, Chung MT, McArdle A, Wong VW, Quarto N, Longaker MT, Wan DC. 2012. Craniosynostosis: Molecular pathways and future pharmacologic therapy. Organogenesis 8: 103–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, Young M, Robey PG, Wang CY, Shi S. 2004. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 364: 149–155. [DOI] [PubMed] [Google Scholar]
- Shang Q, Wang Z, Liu W, Shi Y, Cui L, Cao Y. 2001. Tissue-engineered bone repair of sheep cranial defects with autologous bone marrow stromal cells. J Craniofac Surg 12: 586–593; discussion 94–95. [DOI] [PubMed] [Google Scholar]
- Shi S, Robey PG, Gronthos S. 2001. Comparison of human dental pulp and bone marrow stromal stem cells by cDNA microarray analysis. Bone 29: 532–539. [DOI] [PubMed] [Google Scholar]
- Shkoukani MA, Chen M, Vong A. 2013. Cleft lip—A comprehensive review. Front Pediatr 1: 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonds WF, James-Newton LA, Agarwal SK, Yang B, Skarulis MC, Hendy GN, Marx SJ. 2002. Familial isolated hyperparathyroidism: Clinical and genetic characteristics of 36 kindreds. Medicine (Baltimore) 81: 1–26. [DOI] [PubMed] [Google Scholar]
- Skawina A, Gorczyca W. 1984. The role of nutrient and periosteal blood vessels in the vascularization of the cortex of shafts of the long bones in human fetuses. Folia Morphol (Warsz) 43: 159–164. [PubMed] [Google Scholar]
- Skardal A, Mack D, Kapetanovic E, Atala A, Jackson JD, Yoo J, Soker S. 2012. Bioprinted amniotic fluid-derived stem cells accelerate healing of large skin wounds. Stem Cells Transl Med 1: 792–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodek J, McKee MD. 2000. Molecular and cellular biology of alveolar bone. Periodontol 2000 24: 99–126. [DOI] [PubMed] [Google Scholar]
- Sohier J, Corre P, Weiss P, Layrolle P. 2010. Hydrogel/calcium phosphate composites require specific properties for three-dimensional culture of human bone mesenchymal cells. Acta Biomater 6: 2932–2939. [DOI] [PubMed] [Google Scholar]
- Sullivan WG, Szwajkun PR. 1991. Revascularization of cranial versus iliac crest bone grafts in the rat. Plast Reconstr Surg 87: 1105–1109. [DOI] [PubMed] [Google Scholar]
- Teng M, Liang X, Yuan Q, Nie J, Ye J, Cheng Q, Zhai J, Liao J, Sun X, Wen C, et al. 2013. The inlay osteotome sinus augmentation technique for placing short implants simultaneously with reduced crestal bone height. A short-term follow-up. Clin Implant Dent Relat Res 15: 918–926. [DOI] [PubMed] [Google Scholar]
- Terheyden H, Knak C, Jepsen S, Palmie S, Rueger DR. 2001a. Mandibular reconstruction with a prefabricated vascularized bone graft using recombinant human osteogenic protein.1: An experimental study in miniature pigs. Part I: Prefabrication. Int J Oral Maxillofac Surg 30: 373–379. [DOI] [PubMed] [Google Scholar]
- Terheyden H, Warnke P, Dunsche A, Jepsen S, Brenner W, Palmie S, Toth C, Rueger DR. 2001b. Mandibular reconstruction with prefabricated vascularized bone grafts using recombinant human osteogenic protein.1: An experimental study in miniature pigs. Part II: Transplantation. Int J Oral Maxillofac Surg 30: 469–478. [DOI] [PubMed] [Google Scholar]
- Terheyden H, Menzel C, Wang H, Springer IN, Rueger DR, Acil Y. 2004. Prefabrication of vascularized bone grafts using recombinant human osteogenic protein.1: Part 3: Dosage of rhOP-1, the use of external and internal scaffolds. Int J Oral Maxillofac Surg 33: 164–172. [DOI] [PubMed] [Google Scholar]
- Thesleff T, Lehtimaki K, Niskakangas T, Mannerström B, Miettinen S, Suuronen R, Öhman J. 2011. Cranioplasty with adipose-derived stem cells and biomaterial: A novel method for cranial reconstruction. Neurosurgery 68: 1535–1540. [DOI] [PubMed] [Google Scholar]
- Thompson N, Casson JA. 1970. Experimental onlay bone grafts to the jaws. A preliminary study in dogs. Plast Reconstr Surg 46: 341–349. [DOI] [PubMed] [Google Scholar]
- Tolarova MM, Cervenka J. 1998. Classification and birth prevalence of orofacial clefts. Am J Med Genet 75: 126–137. [PubMed] [Google Scholar]
- Tsutsui TW, Riminucci M, Holmbeck K, Bianco P, Robey PG. 2008. Development of craniofacial structures in transgenic mice with constitutively active PTH/PTHrP receptor. Bone 42: 321–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhm KI, Hwang SH, Choi BG. 2000. Cleft lip nose correction with onlay calvarial bone graft and suture suspension in Oriental patients. Plast Reconstr Surg 105: 499–503. [DOI] [PubMed] [Google Scholar]
- Van Meekran MJ. 1682. Observationes medicochirurgicae [Observations of medicine and surgery]. Henrici and Bloom, Amsterdam. [Google Scholar]
- Vaquette C, Fan W, Xiao Y, Hamlet S, Hutmacher DW, Ivanovski S. 2012. A biphasic scaffold design combined with cell sheet technology for simultaneous regeneration of alveolar bone/periodontal ligament complex. Biomaterials 33: 5560–5573. [DOI] [PubMed] [Google Scholar]
- Visser J, Peters B, Burger TJ, Boomstra J, Dhert WJ, Melchels FP, Malda J. 2013. Biofabrication of multi-material anatomically shaped tissue constructs. Biofabrication 5: 035007. [DOI] [PubMed] [Google Scholar]
- Vogelin E, Jones NF, Huang JI, Brekke JH, Lieberman JR. 2005. Healing of a critical-sized defect in the rat femur with use of a vascularized periosteal flap, a biodegradable matrix, and bone morphogenetic protein. J Bone Joint Surg Am 87: 1323–1331. [DOI] [PubMed] [Google Scholar]
- Wang F, Yu M, Yan X, Wen Y, Zeng Q, Yue W, Yang P, Pei X. 2011. Gingiva-derived mesenchymal stem cell-mediated therapeutic approach for bone tissue regeneration. Stem Cells Dev 20: 2093–2102. [DOI] [PubMed] [Google Scholar]
- Ward BB, Brown SE, Krebsbach PH. 2010. Bioengineering strategies for regeneration of craniofacial bone: A review of emerging technologies. Oral Dis 16: 709–716. [DOI] [PubMed] [Google Scholar]
- Warnke PH, Coren AJ. 2003. First experiences with recombinant human bone morphogenetic protein 7 (osteogenic protein 1) in a human case in maxillofacial surgery. Plast Reconstr Surg 111: 2471–2472. [DOI] [PubMed] [Google Scholar]
- Warnke PH, Springer IN, Wiltfang J, Acil Y, Eufinger H, Wehmöller M, Russo PA, Bolte H, Sherry E, Behrens E, et al. 2004. Growth and transplantation of a custom vascularised bone graft in a man. Lancet 364: 766–770. [DOI] [PubMed] [Google Scholar]
- Weng D, Hurzeler MB, Quinones CR, Ohlms A, Caffesse RG. 2000. Contribution of the periosteum to bone formation in guided bone regeneration. A study in monkeys. Clin Oral Implants Res 11: 546–554. [DOI] [PubMed] [Google Scholar]
- Whitaker LA. 1989. Biological boundaries: A concept in facial skeletal restructuring. Clin Plast Surg 16: 1–10. [PubMed] [Google Scholar]
- Xiong Y, He J, Zhang W, Zhou G, Cao Y, Liu W. 2015. Retention of the stemness of mouse adipose-derived stem cells by their expansion on human bone marrow stromal cell-derived extracellular matrix. Tissue Eng Part A 21: 1886–1894. [DOI] [PubMed] [Google Scholar]
- Xu M, Wang X, Yan Y, Yao R, Ge Y. 2010. A cell-assembly derived physiological 3D model of the metabolic syndrome, based on adipose-derived stromal cells and a gelatin/alginate/fibrinogen matrix. Biomaterials 31: 3868–3877. [DOI] [PubMed] [Google Scholar]
- Yang M, Zhang H, Gangolli R. 2014. Advances of mesenchymal stem cells derived from bone marrow and dental tissue in craniofacial tissue engineering. Curr Stem Cell Res Ther 9: 150–161. [DOI] [PubMed] [Google Scholar]
- Zhang W, Walboomers XF, van Osch GJ, van den Dolder J, Jansen JA. 2008. Hard tissue formation in a porous HA/TCP ceramic scaffold loaded with stromal cells derived from dental pulp and bone marrow. Tissue Eng Part A 14: 285–294. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Shi S, Liu Y, Uyanne J, Shi Y, Shi S, Le AD. 2009. Mesenchymal stem cells derived from human gingiva are capable of immunomodulatory functions and ameliorate inflammation-related tissue destruction in experimental colitis. J Immunol 183: 7787–7798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Chai Y. 2015. Stem cells in teeth and craniofacial bones. J Dent Res 94: 1495–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Feng J, Ho TV, Grimes W, Urata M, Chai Y. 2015. The suture provides a niche for mesenchymal stem cells of craniofacial bones. Nat Cell Biol 17: 386–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zins JE, Whitaker LA. 1979. Membranous vs endochondral bone autografts: Implications for craniofacial reconstruction. Surg Forum 30: 521–523. [PubMed] [Google Scholar]
- Zins JE, Whitaker LA. 1983. Membranous versus endochondral bone: Implications for craniofacial reconstruction. Plast Reconstr Surg 72: 778–785. [DOI] [PubMed] [Google Scholar]
- Zins JE, Kusiak JF, Whitaker LA, Enlow DH. 1984. The influence of the recipient site on bone grafts to the face. Plast Reconstr Surg 73: 371–381. [DOI] [PubMed] [Google Scholar]
- Zou D, Zhang Z, Ye D, Tang A, Deng L, Han W, Zhao J, Wang S, Zhang W, Zhu C, et al. 2011. Repair of critical-sized rat calvarial defects using genetically engineered bone marrow-derived mesenchymal stem cells overexpressing hypoxia-inducible factor-1α. Stem Cells 29: 1380–1390. [DOI] [PubMed] [Google Scholar]
- Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. 2001. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng 7: 211–228. [DOI] [PubMed] [Google Scholar]
- Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hendrick MH. 2002. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell 13: 4279–4295. [DOI] [PMC free article] [PubMed] [Google Scholar]