Abstract
Mucosal surfaces are lined by epithelial cells. In the intestine, the epithelium establishes a selectively permeable barrier that supports nutrient absorption and waste secretion while preventing intrusion by luminal materials. Intestinal epithelia therefore play a central role in regulating interactions between the mucosal immune system and luminal contents, which include dietary antigens, a diverse intestinal microbiome, and pathogens. The paracellular space is sealed by the tight junction, which is maintained by a complex network of protein interactions. Tight junction dysfunction has been linked to a variety of local and systemic diseases. Two molecularly and biophysically distinct pathways across the intestinal tight junction are selectively and differentially regulated by inflammatory stimuli. This review discusses the mechanisms underlying these events, their impact on disease, and the potential of using these as paradigms for development of tight junction-targeted therapeutic interventions.
Mucosal surfaces and the epithelial cells that line them are present at sites where tissues interface directly with the external environment or internal compartments that are contiguous with the external environment. Examples include the gastrointestinal tract, the pulmonary tree, and the genitourinary tract. In many cases the mucosa must balance the opposing goals of facilitating selective transport while also forming a barrier that restricts free exchange across the paracellular space. Crucial to both of these properties is the apical junctional complex. This structure, first described in 1963 (Farquhar and Palade 1963), is composed of three junctions that, from apical to basal, are known as the tight junction (zonula occludens), adherens junction (zonula adherens), and desmosome (macula adherens). The tight junction is a selectively permeable barrier that generally represents the rate-limiting step of paracellular transport. The adherens junction and desmosome provide essential adhesive and mechanical properties that contribute to barrier function but do not seal the paracellular space. The tight junction is the primary focus of this article.
This article will focus on the gastrointestinal tract. In part, this mirrors the state of knowledge regarding tight junction biology within organ systems. The gut has been studied in greatest detail due to the relative accessibility of the intestines by endoscopy, the highly ordered architecture, and the remarkable physical and biochemical stressors faced by the gastrointestinal mucosa. The latter include the most diverse microbiome of the body, peristalsis, and the continuous washing of luminal contents over the surface. Within this context, the intestinal epithelia must direct selective active and passive vectorial transport of ions, nutrients, water, and waste products (Ferraris and Diamond 1997; Kato and Romero 2011; Turner 2016). Finally, it should be recognized that the gastrointestinal tract is the primary site at which the immune system samples foreign materials that are essential to immune education (Chung et al. 2012; Hooper et al. 2012; Kim et al. 2016). This fact makes the regulatory systems that prevent aberrant immune activation while promoting appropriate immune responses particularly important within the gastrointestinal tract. It is therefore not surprising that almost any substantial defect in mucosal immune regulation results in enterocolitis in experimental animals and humans (Kuhn et al. 1993; Powrie et al. 1994; d'Hennezel et al. 2009; Glocker et al. 2009; Hayes et al. 2015; Kiesler et al. 2015; Mishima et al. 2015).
MUCOSAL ANATOMY
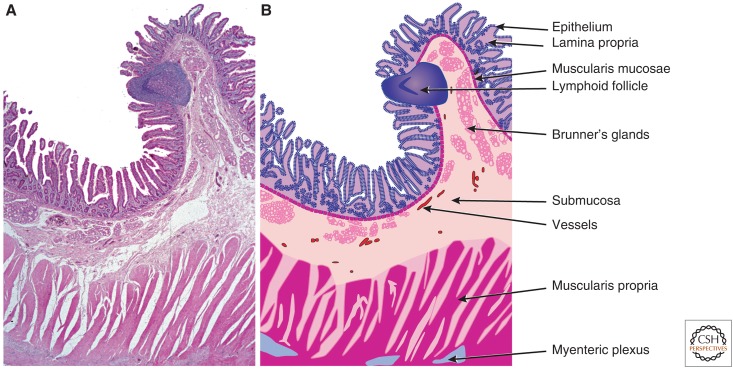
Mucosal surfaces share a common organization (Fig. 1). In general, from lumen to serosa, mucosae are composed of an epithelial layer that sits on an acellular basement membrane. Beneath this, a loose connective tissue layer, which may include blood vessels, lymphatics, immune cells, and other components, is termed the lamina propria. Both epithelium and lamina propria contribute to the villus and crypt architecture of the small intestine (Fig. 1). The underlying muscularis mucosae represents the deepest extent of the mucosa and separates the mucosa from the submucosa. The submucosa contains larger vessels, lymphatics, adipose tissue, and scattered immune cells. The submucosa is thought to also cushion the mucosa from forces exerted during peristalsis. The muscularis propria takes different forms depending on the tissue. For example, the gastric muscularis propria contains three distinct sets of muscle fibers with differing orientations, whereas the small intestine and colon possess only longitudinal and circumferential muscle fibers. In many, but not all sites, the muscularis propria is covered by a thin layer of epithelium, the serosa.
Figure 1.
Small intestinal mucosal architecture. (A) Low magnification image of hematoxylin and eosin-stained section of normal human duodenum. Many of the structural features that are common throughout the gastrointestinal tract and other mucosal surfaces can be appreciated. (B) Line diagram indicating specific structures that comprise the intestinal wall. The open spaces between fibers of the muscularis propria represent artefactual separation that occurred during tissue processing. (From Podolsky et al. 2015; reprinted, with permission from John Wiley & Sons ©2015.)
Epithelial organization varies widely across tissues. The airways (e.g., bronchioles), are lined by a pseudostratified columnar epithelium with abundant apical cilia, whereas air spaces (i.e., alveoli), are lined by a single layer of squamous type 1 and cuboidal, surfactant-producing type II epithelial cells. In contrast, the transitional epithelium of the bladder is stratified and contains specialized umbrella cells that allow the bladder to distend as needed. The oral cavity and esophagus are lined by stratified squamous epithelium, whereas the remainder of the gastrointestinal tract is lined by a simple (i.e., composed of a single layer), columnar epithelium. The organization of these epithelial cells and patterns of differentiation vary by site. For example, the stem and proliferative zone of the gastric mucosa is located within the glandular neck, with the specialized parietal and chief cells located in the deep glands. In contrast, stem cells and Paneth cells are concentrated within the base of intestinal glands (i.e., the crypts).
The small intestinal epithelium is composed of a single layer of columnar cells, many with a well-developed microvillus brush border. At least two populations of stem cells are present within the crypts, one that cycles actively and expresses the wnt pathway orphan receptor Lgr5, and a second, quiescent population that can replenish the Lgr5-positive pools after damage (e.g., radiation) (Barker et al. 2007; Powell et al. 2012; Wong et al. 2012). The crypts are also populated by Paneth cells, which release antimicrobial peptides and contribute to maintenance of the stem cell niche (Bevins and Salzman 2011), as well as enteroendocrine cells; neither of which are actively proliferative. Daughter cells produced by division of Lgr5-positive stem cells migrate out of the crypt and toward the villus. For the first part of this journey, proliferation continues as cells migrate through the transit amplifying zone. Although considered undifferentiated, epithelial cells within this region express abundant apical chloride channels as well as basolateral ion transport proteins necessary to support massive transcellular chloride secretion that generates an osmotic gradient to draw water into the lumen. Patients with cystic fibrosis, in which the most prominent chloride channel, cystic fibrosis transmembrane conductance regulator (CFTR), is defective, can develop intestinal obstruction as a result of insufficient luminal hydration (Collins 1992). Conversely, CFTR activation by cholera toxin results in voluminous, watery diarrhea (Gabriel et al. 1994). As epithelial cells exit the transit amplifying zone proliferation ceases and gene expression patterns change such that the same cells become specialized for nutrient absorption. Thus, in general terms, the villus is an absorptive compartment whereas the crypt is secretory.
INTESTINAL INTERCELLULAR JUNCTIONS
Migration along the crypt-villus axis is accompanied by expression of nutrient absorptive proteins, such as the apical sodium-glucose cotransporter SGLT1 (Hwang et al. 1991). Junctional proteins contribute to this coordinated migration and differentiation. For example, E-cadherin overexpression within the intestinal epithelium retards migration from crypt to villus, suppresses proliferation, induces apoptosis within the crypt, and delays absorptive cell differentiation (Hermiston et al. 1996). Conversely, disruption of E-cadherin function by transgenic expression of a dominant negative N-cadherin accelerates migration and is accompanied by aberrant differentiation, loss of polarity, and premature cell death (Hermiston and Gordon 1995). Complete E-cadherin knockout within intestinal epithelial cells results in a far more severe phenotype that includes villus blunting, a marker of premature epithelial death (i.e., before migration to the upper villus), and incomplete brush border development (Bondow et al. 2012; Kohlnhofer et al. 2016). Notably, E-cadherin-deficient intestinal epithelia also display loss of claudin-1 and increased claudin-4 expression. This indicates that, in addition to defects in transcellular transport protein expression, E-cadherin deficiency disrupts paracellular transport pathways. Consistent with this, electron dense probes are easily able to traverse the paracellular pathway within E-cadherin-deficient intestinal epithelia.
In the normal intestine, E-cadherin expression is relatively constant over both longitudinal (i.e., proximal small intestine to distal colon), and vertical (i.e., crypt to villus), axes. In contrast, expression of tight junction proteins varies significantly over both axes as well as during development (Holmes et al. 2006). For example, claudin-8 expression is only detectable from the ileum to the distal colon, whereas claudin-18 is detectable in the stomach and duodenum but not in the ileum and colon (Holmes et al. 2006). Claudin-2 is highly expressed in the neonatal period by both crypt and villus, or surface in the colon, intestinal epithelial cells. At these times, claudin-15 expression is low and limited to the crypt. This pattern reverses over the first 30 to 90 days of life in mice (Holmes et al. 2006). As discussed below, definition of functions specific to each claudin protein is necessary to understand the physiological and pathophysiological significance of these temporal and spatial expression patterns. For example, whereas knockout mice lacking claudin-2 develop normally (Muto et al. 2010) and claudin-15 deficient mice display intestinal glucose malabsorption as a result of reduced luminal sodium (Tamura et al. 2011), knockout mice lacking both claudin-2 and claudin-15 die in the first month of life (Wada et al. 2013). These observations are easily understood with the knowledge that active nutrient uptake across the apical enterocyte membrane is energized by the gradient between luminal and intracellular sodium as well as recognition that the diet contains insufficient sodium to drive this absorption. Differential expression of claudins 2 and 15 is therefore essential to maintenance of the sodium gradient across the apical membrane. This is because the paracellular channels formed by these claudins are required for transcellularly absorbed sodium to recycle to the lumen and power further cycles of nutrient absorption. The unique functions of claudins 2 and 15 that might explain the need for their differential expression are incompletely understood.
TIGHT JUNCTION MOLECULAR ARCHITECTURE
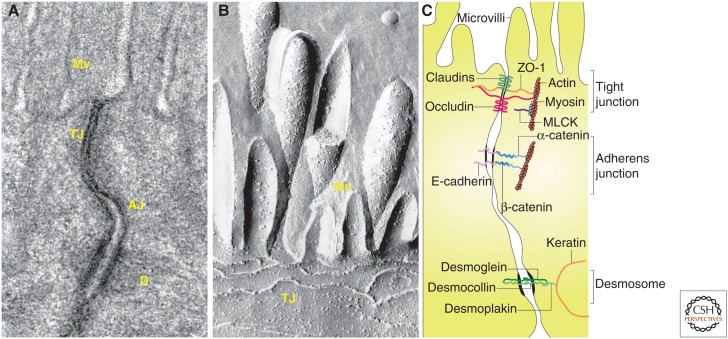
Tight junctions are not visible by light microscopy and are inconspicuous when viewed by transmission electron microscopy (Fig. 2A). In contrast, freeze fracture electron microscopy shows a complex three-dimensional structure composed of paired intramembranous strands within adjacent cells (Fig. 2B). The composition of the strands was a subject of debate, with protagonists of both lipids and proteins (Lingaraju et al. 2015). While not fully resolved, this debate was largely abandoned in 1986, when zonula occludens-1 (ZO-1) became the first identified tight junction protein (Stevenson et al. 1986, 1988). Shortly thereafter, a second peripheral membrane protein, cingulin, was reported (Citi et al. 1988). This cemented the field's focus on proteins (as shown in Fig. 2C), which has, for the most part, continued to the present.
Figure 2.
The apical junctional complex. (A) Transmission electron micrograph showing junctional complexes between two villous enterocytes. The tight junction (TJ) is just below the microvilli (Mv), followed by the adherens junction (AJ). The desmosomes (D) are located basolaterally. (B) Freeze-fracture electron micrograph showing apical microvilli (Mv) and tight junction strands (TJ) in a cultured intestinal epithelial cell. (C) Line drawing of the apical junctional complex of an intestinal epithelial cell. Tight junction proteins include claudins, zonula occludens-1 (ZO-1), and occludin, whereas E-cadherin, α-catenin, and β-catenin interact to form the adherens junction. Myosin light chain kinase (MLCK) is associated with the perijunctional actomyosin ring. Desmosomes are formed by interactions between desmoglein, desmocollin, desmoplakin and keratin filaments. (Parts A and C, from Turner 2009; reprinted, with permission; part B, from Shen et al. 2011; reprinted, with permission from Annual Reviews ©2011.)
When the first transmembrane protein of the tight junction, occludin, was discovered in 1993 (Furuse et al. 1993), it was heralded as the “Holy Grail” of tight junction biology (Gumbiner 1993). This was followed by a series of studies indicating critical roles for occludin and included reports that occludin overexpression could drive formation of intracellular multilamellar bodies with closely apposed lipid bilayers, similar to those in tight junctions (Furuse et al. 1996), and that occludin both enhanced steady-state barrier function in cultured monolayers and increased the number of strands seen by freeze-fracture electron microscopy (McCarthy et al. 1996). Occludin functions appear to require the cytoplasmic C terminus (Chen et al. 1997; Matter and Balda 1999; Odenwald et al. 2016), which is heavily phosphorylated in tight junction-associated occludin (Cordenonsi et al. 1997; Sakakibara et al. 1997; Wong 1997) and binds to ZO-1, ZO-2, and ZO-3 (Itoh et al. 1999). Further, introduction of a synthetic peptide corresponding to part of occludin's second extracellular loop (ECL2) disrupted epithelial paracellular barrier function (Wong and Gumbiner 1997). Finally, as discussed below, occludin is essential for some mechanisms of in vitro and in vivo tight junction regulation.
Despite the data above, many recognized that a single occludin isoform could not be the principal tight junction transmembrane protein, as tight junctions across different anatomical sites vary in size- and charge-selective permeability (Gumbiner 1993). Consistent with this, it was discovered that embryonic stem cells lacking occludin differentiated into polarized epithelia with functional tight junctions (Saitou et al. 1998). This was followed by a report that, despite multiple abnormalities, occludin knockout mice are viable and lack major defects in intestinal barrier function (Saitou et al. 2000). As a result, many dismissed occludin as an irrelevant protein. A more nuanced, inclusive interpretation is that occludin plays a regulatory rather than structural role. Consistent with this, two tight junction-associated, occludin-related proteins, tricellulin (marvelD2) and marvelD3, have been identified to complete the tight junction-associated MARVEL protein (TAMP) family (Ikenouchi et al. 2005; Raleigh et al. 2010). As implied by its name, tricellulin is concentrated at tricellular tight junctions. Tricellulin mutations have been linked to some forms of human hereditary deafness (Riazuddin et al. 2006), as have mutations in the tricellular junction protein angulin-2 (Higashi et al. 2013, 2015; Kim et al. 2015). Deafness also occurs in occludin knockout mice (Kitajiri et al. 2014).
Recognition that occludin was not absolutely required for barrier function and could not explain differential selectivities of tight junctions at different sites drove the continued search for transmembrane tight junction proteins. This led to the discovery of claudin-1 and claudin-2 (Furuse et al. 1998a). This family of 27 genes in mammals is characterized by four transmembrane domains, similar to the TAMP family, but is unrelated to the TAMPs or other tight junction proteins (Furuse et al. 1998a; Morita et al. 1999). The first extracellular loop (ECL1) of claudin proteins contains several conserved sequences, and most claudins also include a carboxy-terminal PDZ-binding sequence that interacts with the PDZ1 domain of ZO proteins (Itoh et al. 1999). It is well-documented that many claudin proteins form paracellular pores, and that the charge selectivity of these pores is defined by amino acids within ECL1 (Colegio et al. 2003; Li et al. 2014; Weber et al. 2015). The relationship of this to health and disease was initially shown by genetic linkage of paracellin-1, or claudin-16, to the autosomal recessive disease of renal hypomagnesemia with hypercalciuria and nephrocalcinosis (Simon et al. 1999). The divalent cation channel formed by claudin-16 (Gong et al. 2015) is essential for reabsorption of magnesium and calcium ions from the urinary space. Human claudin polymorphisms have also been linked to biliary disease (Hadj-Rabia et al. 2004) and deafness (Wilcox et al. 2001). Interestingly, a ZO-2 mutation that disrupts binding to claudins also causes biliary disease (Carlton et al. 2003).
While it is clear that claudin proteins form paracellular pores, the role of claudin proteins in forming the paracellular barrier is less clear-cut. The strongest evidence in favor of claudin-dependent barrier function comes from the severe epidermal barrier defects displayed by claudin-1 knockout mice (Furuse et al. 2002) and the striking ability of claudin proteins to form tight junction-like strands when expressed in fibroblasts, which do not normally form such strands (Furuse et al. 1998b). Notably, occludin is recruited into these claudin-dependent strands.
Twenty-seven human claudin genes have been identified, but it is not clear that all of these are expressed as proteins (Liu et al. 2016). Notably, claudin-13 is expressed in rodents, but is absent in humans. The barrier and permeability properties of tight junctions are defined in significant part by the ensemble of claudin proteins expressed, and this varies between and within tissues (Holmes et al. 2006). The specific claudin proteins expressed within the gastrointestinal tract vary as a function of location, stage of pre- and postnatal development, and presence of disease (Heller et al. 2005; Holmes et al. 2006). In short, the molecular architecture of tight junctions is variable, with the specific composition reflecting the local environment and functional demands.
TUMOR NECROSIS FACTOR-MEDIATED REGULATION OF TIGHT JUNCTION PERMEABILITY
Organ-specific features of tight junction-dependent barrier function have been studied in the lungs (Schlingmann et al. 2016), kidneys (Pei et al. 2016), and gastrointestinal tract (Wada et al. 2013), but have been analyzed in greatest detail in the context of gastrointestinal disease (Clayburgh et al. 2005; Heller et al. 2005; Weber et al. 2008, 2010; Su et al. 2009; Marchiando et al. 2010a). In vitro, reductionist models of barrier loss in inflammatory bowel disease (IBD) include epithelial responses to exogenous tumor necrosis factor-α (TNF) and interleukin-13 (IL-13), which have each been implicated in Crohn's disease and ulcerative colitis, the two forms of IBD.
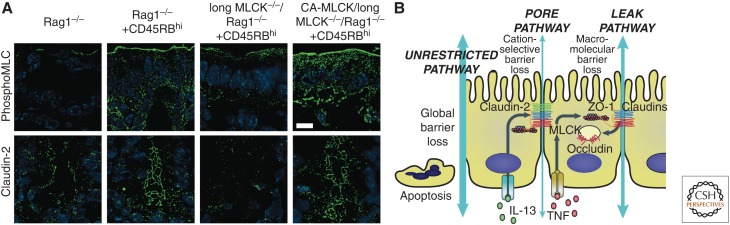
The mechanism of TNF-induced tight junction (i.e., non-apoptotic) barrier loss was initially reported in 2002 (Zolotarevsky et al. 2002). Previous work had determined that epithelial myosin light chain kinase (MLCK) was a critical physiological regulator of increased tight junction permeability induced by activation of sodium nutrient cotransport (Turner et al. 1997; Herrmann and Turner 2016). However, studies of MLCK were hampered by the lack of specific inhibitors, knockdown/knockout cell lines, and knockout mice, which resulted in the necessary reliance on nonspecific agents such as ML-7 (Bain et al. 2003). This changed when an exquisitely specific MLCK inhibitor was developed and tested in TNF-treated Caco-2 intestinal epithelial monolayers (Zolotarevsky et al. 2002). Remarkably, the inhibitory peptide, PIK, rapidly and completely reversed TNF-induced barrier loss of up to ∼30%. Greater degrees of barrier loss were not correctable by MLCK inhibition, likely because these were due to epithelial apoptosis. Subsequent work showed that TNF not only activated MLCK enzymatic activity acutely, but also enhanced MLCK expression in Caco-2 monolayers (Wang et al. 2005). It was subsequently shown that either enzymatic MLCK inhibition, using PIK, or genetic MLCK inhibition, using mice lacking the epithelial long MLCK isoform, was able to reverse or prevent, respectively, TNF-dependent, barrier loss and diarrhea in mice (Clayburgh et al. 2005). Further analysis showed that MLCK was upregulated within intestinal epithelia in mouse models (Wang et al. 2006) as well as human IBD, where magnitude of expression correlated with disease severity (Blair et al. 2006). Finally, long MLCK knockout mice were significantly protected from immune-mediated experimental IBD (Fig. 3A) (Su et al. 2013).
Figure 3.
Tight junction flux pathways in disease. Transgenic, intestinal epithelial-restricted expression of constitutively active-MLCK restores sensitivity of long MLCK−/− mice to CD4+CD45RBhi-adoptive transfer colitis, an immune-mediated experimental inflammatory bowel disease (IBD). (A) Long myosin light chain kinase (MLCK) is essential for myosin II regulatory light chain (MLC) phosphorylation and claudin-2 upregulation during immune-mediated experimental IBD. Long MLCK−/− mice are protected from disease-associated MLC phosphorylation and claudin-2 upregulation. Tissue specific, intestinal epithelial expression of a constitutively active MLCK catalytic domain (CA-MLCK) restores disease-associated MLC phosphorylation and claudin-2 upregulation. Serine-19-phosphorylated MLC (phosphoMLC) or claudin-2 (green) and nuclei (blue) are shown. Bar = 10 um. (B) Diagram of intestinal permeability pathways in disease. Pore and leak pathways are regulated by claudin-2 expression and MLCK-dependent occludin endocytosis, respectively. The unrestricted pathway is a tight junction-independent pathway at sites of epithelial damage (e.g., apoptosis) that is present in advanced disease. (From Su et al. 2013; adapted, with permission, from Elsevier ©2013.)
Three important aspects of this last study are worth noting. First, although the mice studied lacked long MLCK in all tissues, tissue-specific constitutively active MLCK expression within intestinal epithelial cells restored the disease phenotype of long MLCK knockout mice to one indistinguishable from wild-type mice (Su et al. 2013). This shows that the protection afforded by long MLCK knockout was caused by loss of intestinal epithelial long MLCK rather than long MLCK expressed in endothelium or other cell types.
Second, whereas they were protected from immune-mediated colitis, long MLCK knockout mice were not protected from dextran sulphate sodium (DSS) colitis. Notably, DSS induces colitis via direct epithelial injury, which is not the mechanism of human inflammatory bowel disease (IBD). This example can serve as an important warning when attempting to translate in vitro observations to an in vivo model. In the case of long MLCK knockout, the protection observed using an immune-mediated colitis model was due to preservation of the tight junction barrier. That model was appropriate for analysis of epithelial tight junctions because the initial disease-inducing signal came from the immune system. Use of a model where disease was initiated by direct epithelial damage (e.g., DSS colitis), would, in contrast, be inappropriate because the direct epithelial perturbation induced by DSS makes it impossible to study epithelial responses to biologically relevant perturbations (e.g., cytokines or cell-mediated attack). In addition, because DSS causes severe epithelial damage and mucosal ulceration, the barrier loss and disruption of other epithelial functions make it impossible to use this as a model of disease-driven epithelial dysfunction. Conversely, the immune-mediated colitis model used above, which relies on adoptive transfer of naïve effector T cells into an immunodeficient recipient, could be problematic for studies of immunoregulation because, for example, the immunodeficient recipients lack endogenous regulatory T cells. Nevertheless, exceptions to these generalizations must be considered. For example the adoptive transfer colitis model has been used in studies that transferred naïve effector T cells along with potential regulatory T-cell populations (Powrie et al. 1993). Those experiments allowed direct analysis of the effects of transferred cells without interference from endogenous immune regulatory elements. Similarly, the massive epithelial injury induced by DSS has been useful in analyses of mucosal repair mechanisms that mediate recovery from DSS colitis (Brown et al. 2007).
Third, the failure of long MLCK knockout to limit severity of DSS colitis is readily understood when one considers the critical role of MLCK in cell migration and wound healing (Russo et al. 2005; Chen et al. 2014). Thus, although some have expressed surprise at the failure of MLCK inhibition to prevent colitis induced by chemical injury or epithelial apoptosis and therefore questioned whether MLCK is a relevant therapeutic target, the protection of long MLCK knockout mice from immune-mediated experimental IBD, but not direct epithelial injury, is expected. Further, the inability of MLCK inhibition to prevent cell death-related barrier loss explains the ultimate development of immune-mediated colitis in long MLCK knockout mice as the result of immune attack that ultimately led to epithelial apoptosis-dependent, and tight junction-independent, barrier loss (Su et al. 2013).
Some investigators have argued that, because MLCK could, theoretically, phosphorylate substrates other than myosin II regulatory light chain, a more appropriate study would be to analyze mice lacking nonmuscle myosin IIA heavy chain specifically within the intestinal epithelium (Naydenov et al. 2016). However, these tissue-specific nonmuscle myosin IIA heavy chain-deficient mice display barrier defects and intestinal disease under basal (i.e., unstressed) conditions. This is likely because of chronic defects in epithelial cell migration and homeostatic wound repair, as the entire myosin motor apparatus is perturbed in these mice. These constitutive abnormalities limit the utility of these mice for study of tight junction physiology and pathophysiology. In contrast, targeting MLCK, an established regulator of tight junction permeability (Turner et al. 1997; Zolotarevsky et al. 2002; Clayburgh et al. 2005; Ma et al. 2005; Su et al. 2009, 2013), does not disrupt basal epithelial function in either long MLCK knockout mice or mice with intestinal epithelial specific constitutively active MLCK expression. Thus, despite some recent discussion, the critical role of MLCK in intestinal epithelial tight junction regulation remains, as does the value of targeting intestinal epithelial MLCK and defining the molecular mechanisms by which MLCK regulates tight junctions.
IL-13-MEDIATED REGULATION OF TIGHT JUNCTION PERMEABILITY
Unlike TNF, which induces barrier loss within only a few hours, IL-13 requires at least 16 hours (Clayburgh et al. 2005; Heller et al. 2005; Wang et al. 2005; Weber et al. 2010). Further, whereas the MLCK inhibitor PIK can reverse TNF-induced barrier loss, it is ineffective against IL-13-induced barrier loss (Weber et al. 2010). The latter is explained by the observation that IL-13 augments paracellular permeability by increasing claudin-2 expression (Heller et al. 2005; Weber et al. 2010), which has been shown to create paracellular cation-selective pores (Furuse et al. 2001; Amasheh et al. 2002; Li et al. 2014; Weber et al. 2015). Intriguingly, claudin-2 is selectively upregulated by IL-13 (Weber et al. 2010), whereas other stimuli regulate expression of multiple tight junction-related proteins. For example, TNF enhances transcription of MLCK, multiple claudins (1, -2, -12, -15, and -16), all three tight junction-associated MARVEL proteins (TAMPs; occludin triceullin, and marvelD3), and ZO-1 (Raleigh et al. 2010; Weber et al. 2010).
It is worth noting that claudin-2 and MLCK do not operate in isolation. TNF, the prototypical regulator of MLCK in tight junction dysregulation, can enhance claudin-2 transcription (Mankertz et al. 2009; Weber et al. 2010). In addition, mice expressing constitutively active MLCK within the intestinal epithelium also display increased expression of claudin-2 (Weber et al. 2010). Finally, claudin-2 upregulation in the context of immune mediated colitis is markedly attenuated in long MLCK knockout mice (Fig. 3A) (Su et al. 2013).
PORE AND LEAK PATHWAYS
TNF and IL-13, which enhance paracellular permeability via distinct mechanisms that differentially regulate the biophysical properties of the tight junction barrier, have been remarkably useful tools in defining the cell biology of paracellular barrier function. This was initially apparent when, as noted above, it was found that MLCK inhibition could reverse TNF-, but not IL-13-, induced barrier loss (Weber et al. 2010). In contrast, claudin-2-targeted siRNA was able to attenuate IL-13-induced increases in claudin-2 expression and barrier loss (Weber et al. 2010). Further analyses showed that, whereas both cytokines induced similar reductions in transepithelial electrical resistance (TER), a measure of paracellular ion conductance, only TNF enhanced paracellular flux of the large paracellular probe 4 kD dextran (Weber et al. 2010). IL-13 specifically increased paracellular flux of monovalent cations (i.e., sodium), but not anions or 4 kD dextran. TNF, in contrast, increased paracellular flux of both cations and anions to similar degrees. These data indicate that two distinct pathways across the tight junction are preferentially activated by the model cytokines IL-13 and TNF. These pathways can be referred to as pore and leak (Anderson and Van Itallie 2009; Turner 2009; Shen et al. 2011). The pore pathway, which is selectively activated by IL-13, is a high-capacity, charge- and size-selective paracellular conductance route. Conversely, TNF increases flux across the low-capacity, charge-nonselective, and relatively size-nonselective leak pathway (Fig. 3B).
Beyond cytokines, permeability of pore and leak pathways can be regulated by modulating tight junction protein expression in cultured monolayers. Detailed analyses have shown that claudin-2 expression increases the density of cation-selective, paracellular pores with a diameter of 7-8 Å (Van Itallie et al. 2008; Li et al. 2014). Development of a novel paracellular patch clamp technique has recently allowed biophysical analyses demonstrating that these pores are actively gated and display a flickering behavior, with unstable open and closed conformations and a third, stable, closed conformation (Weber et al. 2015). This raises hope that it may be possible to develop pharmacologic agents that modulate these conformations and regulate claudin-dependent ion channels similar to drugs that regulate transmembrane ion channels (e.g., dihydropyridine derivatives). Notably, the existence of flickering paracellular pores was previously predicted on theoretical grounds (Cereijido et al. 2008), and mathematical modeling showed that independent opening and closing of these pores could explain the logarithmic relationship between number of tight junction strands and TER (Claude 1978; Weber and Turner 2017).
It is now evident that many of the claudins establish paracellular pores and that, whereas the charge-selectivities of these pores vary, they all display similar size-selectivities. However, the functions of other claudins remain somewhat controversial. Some investigators have concluded that claudin proteins should be classified as either pore-forming or sealing claudins (Amasheh et al. 2011; Krug et al. 2014). Others have argued that, whereas claudins can form strands, it may be the strands themselves that are responsible for the barrier (Lingaraju et al. 2015), or, alternatively, that the sealing claudins enhance the barrier by regulating pore-forming claudins (Van Itallie et al. 2001; Angelow et al. 2007; Turner et al. 2014). This remains an essential problem that must be solved if we are to fully understand the mechanisms by which tight junctions form selectively permeable barriers.
In contrast to the pore pathway, the molecular identity of the leak pathway, which conducts macromolecules, remains unclear. Flux across this pathway can, as indicated above, be enhanced by TNF. Similarly, knockdown of ZO-1 or occludin in cultured epithelial monolayers can increase leak pathway flux (Yu et al. 2005, 2010; Van Itallie et al. 2009; Al-Sadi et al. 2011; Buschmann et al. 2013). Given the occludin downregulation observed in TNF-treated cultured monolayers and human IBD, the known interactions between ZO-1 and the actin cytoskeleton (Fanning et al. 2002), and the essential contributions of MLCK to leak pathway regulation (Zolotarevsky et al. 2002; Clayburgh et al. 2005), one might conclude that critical interactions between these proteins are responsible for sealing of the leak pathway and that their loss enhances flux across this pathway in disease. While this may be correct, it does not exclude a separate compelling hypothesis that leak pathway flux occurs across tricellular junctions (Krug et al. 2009; Nayak et al. 2013; Higashi et al. 2015). These models might be linked by the observation that occludin deficiency results in redistribution of tricellulin from its normal localization at tricellular tight junctions to bicellular tight junctions (Ikenouchi et al. 2008; Buschmann et al. 2013). This is, however, not completely consistent with available data, as tricellulin overexpression leads to localization at bicellular and tricellular tight junctions and reduces leak pathway flux (Krug et al. 2009).
OCCLUDIN AS A MOLECULAR INTEGRATOR OF TIGHT JUNCTION REGULATION
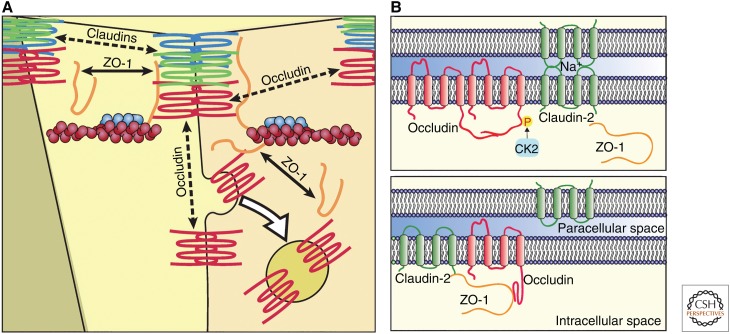
A series of in vitro and in vivo studies have showed that caveolin-dependent endocytosis is essential for barrier loss following actin depolymerization or TNF exposure (Fig. 4A) (Shen and Turner 2005; Schwarz et al. 2007; Marchiando et al. 2010b). Given the enigmatic nature of occludin function, one could debate whether occludin itself or some other molecule was the critical target of such endocytosis. However, in vivo occludin overexpression limited TNF-induced barrier loss and completely prevented TNF-induced diarrhea, thereby demonstrating the essential role of occludin (Marchiando et al. 2010b). Consistent with this, occludin knockdown in cultured epithelia increased leak pathway flux and prevented further cytokine-induced barrier loss (Van Itallie et al. 2010; Buschmann et al. 2013). While it invites more speculation than available data allow, it is interesting to note that the 125 Å diameter of the enhanced paracellular flux pathway in occludin-deficient intestinal epithelial cells is close to the 100 Å (i.e., 10 nm) diameter of a potential tricellular channel described in freeze-fracture studies over 40 years ago (Staehelin 1973).
Figure 4.
Molecular mechanisms of leak and pore pathway regulation. (A) Tight junction complex remodeling at steady-state and in response to tumor necrosis factor (TNF). Dashed black lines indicate energy-independent diffusion of claudins (green, blue) and occludin (red) within the membrane; the mobile fractions of occludin and claudin are both increased by TNF. Solid black lines indicate energy-dependent zonula occludens-1 (ZO-1) (orange) exchange between tight junction and cytosolic pools. Occludin endocytosis (white arrow) is driven by TNF-induced myosin light chain kinase (MLCK) activation and requires both caveolin-1 and ZO-1. (B) Phosphorylation regulates interactions between tight junction proteins and can be exploited to modify claudin-2 channel activity. Diagram shows interactions and dynamic behavior of proteins involved in tight junction regulation by casein kinase 2 (CK2). Upper panel. CK2-mediated phosphorylation of occludin S408 facilitates dimerization and diffusion within the membrane, thereby limiting occludin binding to ZO-1 and claudin-2 and allowing flux across claudin-2 pores. Lower panel. CK2 inhibition and occludin dephosphorylation promotes formation of occludin:ZO-1:claudin-2 complexes that reduce claudin-2 anchoring and pore function at the tight junction. (Part B, from Raleigh et al. 2011; reprinted, with permission from The Rockefeller University Press © 2011.)
The data discussed above show that occludin internalization, and likely downstream degradation, is essential for TNF-induced barrier loss but do not explain the underlying mechanisms. One possible explanation comes from the observation that the many described interprotein interactions at tight junctions are continuously remodeled, even at steady-state (Shen et al. 2008). These highly dynamic interactions and subtle changes in their kinetics (Fig. 4A) may link the observations that occludin or ZO-1 knockdown induce similar increases in leak pathway permeability (Van Itallie et al. 2009; Yu et al. 2010). Such a model could also explain the role of specific residues that link occludin to ZO-1 in TNF-induced acceleration of occludin exchange at the tight junction (Tash et al. 2012; Buschmann et al. 2013). Notably, occludin has also been reported to interact directly with caveolin-1 (Van Itallie and Anderson 2012).
Occludin interactions with ZO-1 might also be regulated by casein kinase 2 (CK2) phosphorylation of residues near and within the occludin carboxy-terminal coiled-coil domain (Smales et al. 2003; Raleigh et al. 2011; Dorfel et al. 2013). Inhibition of occludinS408 phosphorylation reduced occludin exchange (i.e., enhanced anchoring) at the tight junction (Fig. 4B). Remarkably, this also reduced pore pathway conductance of small cations (Raleigh et al. 2011). A complex series of experiments showed that, when phosphorylated at residue S408, tight junction-associated occludin forms dimers that are freely diffusible within the plane of the membrane. In contrast, S408 dephosphorylation resulting from CK2 inhibition caused occludin to associate with ZO-1 and, in turn, ZO-1 to associate with claudin-2 (Fig. 4B). This complex diffused less freely than occludin dimers within the junction. However, the mobile fraction of claudin-2, which is normally very low, nearly doubled (Raleigh et al. 2011). This suggests that claudin-2 is only able to form paracellular cation pores when residing within the stable (i.e., immobile) pool at the tight junction. While complicated, this model shows how subtle changes in tight junction protein interactions can cause dramatic changes in barrier function. Consistent with the proposed inhibition of claudin-2 pore function, CK2 inhibition was able to completely reverse IL-13-induced increases in paracellular cation flux without blocking increased claudin-2 expression (Raleigh et al. 2011). Thus, as MLCK and occludin∶ZO-1 interactions are potential targets for restoration of leak pathway defects, correction of disease-associated increases in pore pathway permeability may be accomplished by regulation of tripartite interactions between occludin, ZO-1, and claudin-2, perhaps by modulating CK2 activity.
CONCLUDING REMARKS
It has been 30 years since the original identification of the first tight junction protein, ZO-1 (Stevenson et al. 1986). Our understanding of tight junctions and their remarkable functions has grown exponentially during that time. From the first anti-ZO-1 antibodies, which could not recognize the human protein, to contemporary studies of knockout mice, human specimens, and patients with genetically encoded diseases of the tight junction, the field has traveled a great distance. However, significant frontiers remain to be explored. These range from detailed understanding of tight junction protein structures and interactions to biological functions in health and disease to the development of agents that modify function. Several examples have been discussed, but many mechanisms remain to be discovered. The success of such endeavors is an essential prerequisite to unlocking the potential of tight junction modulation as a therapeutic intervention.
ACKNOWLEDGMENTS
This review summarizes experimental data in major advances by many investigators working on all aspects of tight junction biology. While we have tried to cite the contributions accurately, it is likely that some have been missed and we apologize for any oversight. We are grateful to many in the field who generously collaborated, discussed, and debated these ideas. These include scientists within our own group as well as innumerable individuals worldwide. Supported by National Institute of Health grants R01DK61931 and R01DK68271 and a Crohn's and Colitis Foundation of America (CCFA) Senior Investigator Award.
Footnotes
Editors: Carien M. Niessen and Alpha S. Yap
Additional Perspectives on Cell–Cell Junctions available at www.cshperspectives.org
REFERENCES
- Al-Sadi R, Khatib K, Guo S, Ye D, Youssef M, Ma T. 2011. Occludin regulates macromolecule flux across the intestinal epithelial tight junction barrier. Am J Physiol Gastrointest Liver Physiol 300: G1054–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amasheh S, Meiri N, Gitter AH, Schoneberg T, Mankertz J, Schulzke JD, Fromm M. 2002. Claudin-2 expression induces cation-selective channels in tight junctions of epithelial cells. J Cell Sci 115: 4969–4976. [DOI] [PubMed] [Google Scholar]
- Amasheh S, Fromm M, Gunzel D. 2011. Claudins of intestine and nephron—a correlation of molecular tight junction structure and barrier function. Acta Physiol (Oxf) 201: 133–140. [DOI] [PubMed] [Google Scholar]
- Anderson JM, Van Itallie CM. 2009. Physiology and function of the tight junction. Cold Spring Harb Perspect Biol 1: a002584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelow S, Schneeberger EE, Yu AS. 2007. Claudin-8 expression in renal epithelial cells augments the paracellular barrier by replacing endogenous claudin-2. J Membr Biol 215: 147–159. [DOI] [PubMed] [Google Scholar]
- Bain J, McLauchlan H, Elliott M, Cohen P. 2003. The specificities of protein kinase inhibitors: An update. Biochem J 371: 199–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, et al. 2007. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449: 1003–1007. [DOI] [PubMed] [Google Scholar]
- Bevins CL, Salzman NH. 2011. Paneth cells, antimicrobial peptides and maintenance of intestinal homeostasis. Nat Rev Microbiol 9: 356–368. [DOI] [PubMed] [Google Scholar]
- Blair SA, Kane SV, Clayburgh DR, Turner JR. 2006. Epithelial myosin light chain kinase expression and activity are upregulated in inflammatory bowel disease. Lab Invest 86: 191–201. [DOI] [PubMed] [Google Scholar]
- Bondow BJ, Faber ML, Wojta KJ, Walker EM, Battle MA. 2012. E-cadherin is required for intestinal morphogenesis in the mouse. Dev Biol 371: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SL, Riehl TE, Walker MR, Geske MJ, Doherty JM, Stenson WF, Stappenbeck TS. 2007. Myd88-dependent positioning of Ptgs2-expressing stromal cells maintains colonic epithelial proliferation during injury. J Clin Invest 117: 258–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschmann MM, Shen L, Rajapakse H, Raleigh DR, Wang Y, Wang Y, Lingaraju A, Zha J, Abbott E, McAuley EM, et al. 2013. Occludin OCEL-domain interactions are required for maintenance and regulation of the tight junction barrier to macromolecular flux. Mol Biol Cell 24: 3056–3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlton VE, Harris BZ, Puffenberger EG, Batta AK, Knisely AS, Robinson DL, Strauss KA, Shneider BL, Lim WA, Salen G, et al. 2003. Complex inheritance of familial hypercholanemia with associated mutations in TJP2 and BAAT. Nat Genet 34: 91–96. [DOI] [PubMed] [Google Scholar]
- Cereijido M, Contreras RG, Shoshani L, Flores-Benitez D, Larre I. 2008. Tight junction and polarity interaction in the transporting epithelial phenotype. Biochim Biophys Acta 1778: 770–793. [DOI] [PubMed] [Google Scholar]
- Chen Y, Merzdorf C, Paul DL, Goodenough DA. 1997. COOH terminus of occludin is required for tight junction barrier function in early Xenopus embryos. J Cell Biol 138: 891–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Tao T, Wen C, He WQ, Qiao YN, Gao YQ, Chen X, Wang P, Chen CP, Zhao W, et al. 2014. Myosin light chain kinase (MLCK) regulates cell migration in a myosin regulatory light chain phosphorylation-independent mechanism. J Biol Chem 289: 28478–28488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung H, Pamp SJ, Hill JA, Surana NK, Edelman SM, Troy EB, Reading NC, Villablanca EJ, Wang S, Mora JR, et al. 2012. Gut immune maturation depends on colonization with a host-specific microbiota. Cell 149: 1578–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citi S, Sabanay H, Jakes R, Geiger B, Kendrick-Jones J. 1988. Cingulin, a new peripheral component of tight junctions. Nature 333: 272–276. [DOI] [PubMed] [Google Scholar]
- Claude P. 1978. Morphological factors influencing transepithelial permeability: A model for the resistance of the zonula occludens. J Membr Biol 39: 219–232. [DOI] [PubMed] [Google Scholar]
- Clayburgh DR, Barrett TA, Tang Y, Meddings JB, Van Eldik LJ, Watterson DM, Clarke LL, Mrsny RJ, Turner JR. 2005. Epithelial myosin light chain kinase-dependent barrier dysfunction mediates T cell activation-induced diarrhea in vivo. J Clin Invest 115: 2702–2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colegio OR, Van Itallie C, Rahner C, Anderson JM. 2003. Claudin extracellular domains determine paracellular charge selectivity and resistance but not tight junction fibril architecture. Am J Physiol Cell Physiol 284: C1346–1354. [DOI] [PubMed] [Google Scholar]
- Collins FS. 1992. Cystic fibrosis: Molecular biology and therapeutic implications. Science 256: 774–779. [DOI] [PubMed] [Google Scholar]
- Cordenonsi M, Mazzon E, De Rigo L, Baraldo S, Meggio F, Citi S. 1997. Occludin dephosphorylation in early development of Xenopus laevis. J Cell Sci 110: 3131–3139. [DOI] [PubMed] [Google Scholar]
- d'Hennezel E, Ben-Shoshan M, Ochs HD, Torgerson TR, Russell LJ, Lejtenyi C, Noya FJ, Jabado N, Mazer B, Piccirillo CA. 2009. FOXP3 forkhead domain mutation and regulatory T cells in the IPEX syndrome. N Engl J Med 361: 1710–1713. [DOI] [PubMed] [Google Scholar]
- Dorfel MJ, Westphal JK, Bellmann C, Krug SM, Cording J, Mittag S, Tauber R, Fromm M, Blasig IE, Huber O. 2013. CK2-dependent phosphorylation of occludin regulates the interaction with ZO-proteins and tight junction integrity. Cell Commun Signal 11: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanning AS, Ma TY, Anderson JM. 2002. Isolation and functional characterization of the actin binding region in the tight junction protein ZO-1. FASEB J 16: 1835–1837. [DOI] [PubMed] [Google Scholar]
- Farquhar M, Palade G. 1963. Junctional complexes in various epithelia. J Cell Biol 17: 375–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraris RP, Diamond J. 1997. Regulation of intestinal sugar transport. Physiol Rev 77: 257–302. [DOI] [PubMed] [Google Scholar]
- Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, Tsukita S, Tsukita S. 1993. Occludin: a novel integral membrane protein localizing at tight junctions. J Cell Biol 123: 1777–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuse M, Fujimoto K, Sato N, Hirase T, Tsukita S. 1996. Overexpression of occludin, a tight junction-associated integral membrane protein, induces the formation of intracellular multilamellar bodies bearing tight junction-like structures. J Cell Sci 109: 429–435. [DOI] [PubMed] [Google Scholar]
- Furuse M, Fujita K, Hiiragi T, Fujimoto K, Tsukita S. 1998a. Claudin-1 and -2: novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J Cell Biol 141: 1539–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuse M, Sasaki H, Fujimoto K, Tsukita S. 1998b. A single gene product, claudin-1 or -2, reconstitutes tight junction strands and recruits occludin in fibroblasts. J Cell Biol 143: 391–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuse M, Furuse K, Sasaki H, Tsukita S. 2001. Conversion of zonulae occludentes from tight to leaky strand type by introducing claudin-2 into Madin-Darby canine kidney I cells. J Cell Biol 153: 263–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuse M, Hata M, Furuse K, Yoshida Y, Haratake A, Sugitani Y, Noda T, Kubo A, Tsukita S. 2002. Claudin-based tight junctions are crucial for the mammalian epidermal barrier: A lesson from claudin-1-deficient mice. J Cell Biol 156: 1099–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel SE, Brigman KN, Koller BH, Boucher RC, Stutts MJ. 1994. Cystic fibrosis heterozygote resistance to cholera toxin in the cystic fibrosis mouse model. Science 266: 107–109. [DOI] [PubMed] [Google Scholar]
- Glocker EO, Kotlarz D, Boztug K, Gertz EM, Schaffer AA, Noyan F, Perro M, Diestelhorst J, Allroth A, Murugan D, et al. 2009. Inflammatory bowel disease and mutations affecting the interleukin-10 receptor. N Engl J Med 361: 2033–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Y, Renigunta V, Zhou Y, Sunq A, Wang J, Yang J, Renigunta A, Baker LA, Hou J. 2015. Biochemical and biophysical analyses of tight junction permeability made of claudin-16 and claudin-19 dimerization. Mol Biol Cell 26: 4333–4346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumbiner BM. 1993. Breaking through the tight junction barrier. J Cell Biol 123: 1631–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadj-Rabia S, Baala L, Vabres P, Hamel-Teillac D, Jacquemin E, Fabre M, Lyonnet S, De Prost Y, Munnich A, Hadchouel M, et al. 2004. Claudin-1 gene mutations in neonatal sclerosing cholangitis associated with ichthyosis: A tight junction disease. Gastroenterol 127: 1386–1390. [DOI] [PubMed] [Google Scholar]
- Hayes P, Dhillon S, O'Neill K, Thoeni C, Hui KY, Elkadri A, Guo CH, Kovacic L, Aviello G, Alvarez LA, et al. 2015. Defects in NADPH oxidase genes NOX1 and DUOX2 in very early onset inflammatory bowel disease. Cell Mol Gastroenterol Hepatol 1: 489–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller F, Florian P, Bojarski C, Richter J, Christ M, Hillenbrand B, Mankertz J, Gitter AH, Burgel N, Fromm M, et al. 2005. Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterol 129: 550–564. [DOI] [PubMed] [Google Scholar]
- Hermiston ML, Gordon JI. 1995. In vivo analysis of cadherin function in the mouse intestinal epithelium: Essential roles in adhesion, maintenance of differentiation, and regulation of programmed cell death. J Cell Biol 129: 489–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermiston ML, Wong MH, Gordon JI. 1996. Forced expression of E-cadherin in the mouse intestinal epithelium slows cell migration and provides evidence for nonautonomous regulation of cell fate in a self-renewing system. Genes Dev 10: 985–996. [DOI] [PubMed] [Google Scholar]
- Herrmann JR, Turner JR. 2016. Beyond Ussing's chambers: Contemporary thoughts on integration of transepithelial transport. Am J Physiol Cell Physiol 310: C423–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashi T, Katsuno T, Kitajiri S, Furuse M. 2015. Deficiency of angulin-2/ILDR1, a tricellular tight junction-associated membrane protein, causes deafness with cochlear hair cell degeneration in mice. PLoS ONE 10: e0120674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashi T, Tokuda S, Kitajiri S, Masuda S, Nakamura H, Oda Y, Furuse M. 2013. Analysis of the 'angulin' proteins LSR, ILDR1 and ILDR2--tricellulin recruitment, epithelial barrier function and implication in deafness pathogenesis. J Cell Sci 126: 966–977. [DOI] [PubMed] [Google Scholar]
- Holmes JL, Van Itallie CM, Rasmussen JE, Anderson JM. 2006. Claudin profiling in the mouse during postnatal intestinal development and along the gastrointestinal tract reveals complex expression patterns. Gene Expr Patterns 6: 581–588. [DOI] [PubMed] [Google Scholar]
- Hooper LV, Littman DR, Macpherson AJ. 2012. Interactions between the microbiota and the immune system. Science 336: 1268–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang ES, Hirayama BA, Wright EM. 1991. Distribution of the SGLT1 Na+/glucose cotransporter and mRNA along the crypt-villus axis of rabbit small intestine. Biochem Biophys Res Commun 181: 1208–1217. [DOI] [PubMed] [Google Scholar]
- Ikenouchi J, Furuse M, Furuse K, Sasaki H, Tsukita S, Tsukita S. 2005. Tricellulin constitutes a novel barrier at tricellular contacts of epithelial cells. J Cell Biol 171: 939–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikenouchi J, Sasaki H, Tsukita S, Furuse M, Tsukita S. 2008. Loss of occludin affects tricellular localization of tricellulin. Mol Biol Cell 19: 4687–4693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh M, Furuse M, Morita K, Kubota K, Saitou M, Tsukita S. 1999. Direct binding of three tight junction-associated MAGUKs, ZO-1, ZO-2, and ZO-3, with the COOH termini of claudins. J Cell Biol 147: 1351–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato A, Romero MF. 2011. Regulation of electroneutral NaCl absorption by the small intestine. Annu Rev Physiol 73: 261–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiesler P, Fuss IJ, Strober W. 2015. Experimental Models of inflammatory bowel diseases. Cell Mol Gastroenterol Hepatol 1: 154–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KS, Hong SW, Han D, Yi J, Jung J, Yang BG, Lee JY, Lee M, Surh CD. 2016. Dietary antigens limit mucosal immunity by inducing regulatory T cells in the small intestine. Science 351: 858–863. [DOI] [PubMed] [Google Scholar]
- Kim NK, Higashi T, Lee KY, Kim AR, Kitajiri S, Kim MY, Chang MY, Kim V, Oh SH, Kim D, et al. 2015. Downsloping high-frequency hearing loss due to inner ear tricellular tight junction disruption by a novel ILDR1 mutation in the Ig-like domain. PLoS ONE 10: e0116931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitajiri S, Katsuno T, Sasaki H, Ito J, Furuse M, Tsukita S. 2014. Deafness in occludin-deficient mice with dislocation of tricellulin and progressive apoptosis of the hair cells. Biol Open 3: 759–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlnhofer BM, Thompson CA, Walker EM, Battle MA. 2016. GATA4 regulates epithelial cell proliferation to control intestinal growth and development in mice. Cell Mol Gastroenterol Hepatol 2: 189–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krug SM, Amasheh S, Richter JF, Milatz S, Gunzel D, Westphal JK, Huber O, Schulzke JD, Fromm M. 2009. Tricellulin forms a barrier to macromolecules in tricellular tight junctions without affecting ion permeability. Mol Biol Cell 20: 3713–3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krug SM, Schulzke JD, Fromm M. 2014. Tight junction, selective permeability, and related diseases. Semin Cell Dev Biol 36: 166–176. [DOI] [PubMed] [Google Scholar]
- Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W. 1993. Interleukin-10-deficient mice develop chronic enterocolitis. Cell 75: 263–274. [DOI] [PubMed] [Google Scholar]
- Li J, Zhuo M, Pei L, Rajagopal M, Yu AS. 2014. Comprehensive cysteine-scanning mutagenesis reveals Claudin-2 pore-lining residues with different intrapore locations. J Biol Chem 289: 6475–6484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingaraju A, Long TM, Wang Y, Austin JR II, Turner JR. 2015. Conceptual barriers to understanding physical barriers. Semin Cell Dev Biol 42: 13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Koval M, Ranganathan S, Fanayan S, Hancock WS, Lundberg EK, Beavis RC, Lane L, Duek P, McQuade L, et al. 2016. Systems proteomics view of the endogenous human claudin protein family. J Proteome Res 15: 339–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma TY, Boivin MA, Ye D, Pedram A, Said HM. 2005. Mechanism of TNF-α modulation of Caco-2 intestinal epithelial tight junction barrier: Role of myosin light-chain kinase protein expression. Am J Physiol - Gastrointest Liver Physiol 288: G422–430. [DOI] [PubMed] [Google Scholar]
- Mankertz J, Amasheh M, Krug SM, Fromm A, Amasheh S, Hillenbrand B, Tavalali S, Fromm M, Schulzke JD. 2009. TNFα up-regulates claudin-2 expression in epithelial HT-29/B6 cells via phosphatidylinositol-3-kinase signaling. Cell Tissue Res 336: 67–77. [DOI] [PubMed] [Google Scholar]
- Marchiando AM, Graham WV, Turner JR. 2010a. Epithelial barriers in homeostasis and disease. Annu Rev Pathol 5: 119–144. [DOI] [PubMed] [Google Scholar]
- Marchiando AM, Shen L, Graham WV, Weber CR, Schwarz BT, Austin JR II, Raleigh DR, Guan Y, Watson AJ, Montrose MH, et al. 2010b. Caveolin-1-dependent occludin endocytosis is required for TNF-induced tight junction regulation in vivo. J Cell Biol 189: 111–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matter K, Balda MS. 1999. Occludin and the functions of tight junctions. Int Rev Cytol 186: 117–146. [DOI] [PubMed] [Google Scholar]
- McCarthy KM, Skare IB, Stankewich MC, Furuse M, Tsukita S, Rogers RA, Lynch RD, Schneeberger EE. 1996. Occludin is a functional component of the tight junction. J Cell Sci 109: 2287–2298. [DOI] [PubMed] [Google Scholar]
- Mishima Y, Liu B, Hansen JJ, Sartor RB. 2015. Resident bacteria-stimulated IL-10-secreting B cells ameliorate T cell-mediated colitis by inducing Tr-1 cells that require IL-27-signaling. Cell Mol Gastroenterol Hepatol 1: 295–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita K, Furuse M, Fujimoto K, Tsukita S. 1999. Claudin multigene family encoding four-transmembrane domain protein components of tight junction strands. Proc Natl Acad Sci 96: 511–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muto S, Hata M, Taniguchi J, Tsuruoka S, Moriwaki K, Saitou M, Furuse K, Sasaki H, Fujimura A, Imai M, et al. 2010. Claudin-2-deficient mice are defective in the leaky and cation-selective paracellular permeability properties of renal proximal tubules. Proc Natl Acad Sci 107: 8011–8016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak G, Lee SI, Yousaf R, Edelmann SE, Trincot C, Van Itallie CM, Sinha GP, Rafeeq M, Jones SM, Belyantseva IA, et al. 2013. Tricellulin deficiency affects tight junction architecture and cochlear hair cells. J Clin Invest 123: 4036–4049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naydenov NG, Feygin A, Wang D, Kuemmerle JF, Harris G, Conti MA, Adelstein RS, Ivanov AI. 2016. Nonmuscle myosin IIA regulates intestinal epithelial barrier in vivo and plays a protective role during experimental colitis. Sci Rep 6: 24161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odenwald MA, Choi W, Buckley A, Shashikanth N, Joseph NE, Wang Y, Warren MH, Buschmann MM, Pavlyuk R, Hildebrand J, et al. 2016. ZO-1 interactions with F-actin and occludin direct epithelial polarization and single lumen specification in 3D culture. J Cell Sci 130: 243–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei L, Solis G, Nguyen MT, Kamat N, Magenheimer L, Zhuo M, Li J, Curry J, McDonough AA, Fields TA, et al. 2016. Paracellular epithelial sodium transport maximizes energy efficiency in the kidney. J Clin Invest 126: 2509–2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell AE, Wang Y, Li Y, Poulin EJ, Means AL, Washington MK, Higginbotham JN, Juchheim A, Prasad N, Levy SE, et al. 2012. The pan-ErbB negative regulator Lrig1 is an intestinal stem cell marker that functions as a tumor suppressor. Cell 149: 146–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powrie F, Leach MW, Mauze S, Caddle LB, Coffman RL. 1993. Phenotypically distinct subsets of CD4+ T cells induce or protect from chronic intestinal inflammation in C. B-17 scid mice. Int Immunol 5: 1461–1471. [DOI] [PubMed] [Google Scholar]
- Powrie F, Leach MW, Mauze S, Menon S, Caddle LB, Coffman RL. 1994. Inhibition of Th1 responses prevents inflammatory bowel disease in scid mice reconstituted with CD45RBhi CD4+ T cells. Immunity 1: 553–562. [DOI] [PubMed] [Google Scholar]
- Raleigh DR, Marchiando AM, Zhang Y, Shen L, Sasaki H, Wang Y, Long M, Turner JR. 2010. Tight junction-associated MARVEL proteins marveld3, tricellulin, and occludin have distinct but overlapping functions. Mol Biol Cell 21: 1200–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raleigh DR, Boe DM, Yu D, Weber CR, Marchiando AM, Bradford EM, Wang Y, Wu L, Schneeberger EE, Shen L, et al. 2011. Occludin S408 phosphorylation regulates tight junction protein interactions and barrier function. J Cell Biol 193: 565–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riazuddin S, Ahmed ZM, Fanning AS, Lagziel A, Kitajiri S, Ramzan K, Khan SN, Chattaraj P, Friedman PL, Anderson JM, et al. 2006. Tricellulin is a tight-junction protein necessary for hearing. Am J Hum Genet 79: 1040–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo JM, Florian P, Shen L, Graham WV, Tretiakova MS, Gitter AH, Mrsny RJ, Turner JR. 2005. Distinct temporal-spatial roles for ρ kinase and myosin light chain kinase in epithelial purse-string wound closure. Gastroenterol 128: 987–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitou M, Fujimoto K, Doi Y, Itoh M, Fujimoto T, Furuse M, Takano H, Noda T, Tsukita S. 1998. Occludin-deficient embryonic stem cells can differentiate into polarized epithelial cells bearing tight junctions. J Cell Biol 141: 397–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitou M, Furuse M, Sasaki H, Schulzke JD, Fromm M, Takano H, Noda T, Tsukita S. 2000. Complex phenotype of mice lacking occludin, a component of tight junction strands. Mol Biol Cell 11: 4131–4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakakibara A, Furuse M, Saitou M, Ando-Akatsuka Y, Tsukita S. 1997. Possible involvement of phosphorylation of occludin in tight junction formation. J Cell Biol 137: 1393–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlingmann B, Overgaard CE, Molina SA, Lynn KS, Mitchell LA, Dorsainvil White S, Mattheyses AL, Guidot DM, Capaldo CT, Koval M. 2016. Regulation of claudin/zonula occludens-1 complexes by hetero-claudin interactions. Nat Commun 7: 12276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz BT, Wang F, Shen L, Clayburgh DR, Su L, Wang Y, Fu YX, Turner JR. 2007. LIGHT signals directly to intestinal epithelia to cause barrier dysfunction via cytoskeletal and endocytic mechanisms. Gastroenterol 132: 2383–2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L, Turner JR. 2005. Actin depolymerization disrupts tight junctions via caveolae-mediated endocytosis. Mol Biol Cell 16: 3919–3936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L, Weber CR, Turner JR. 2008. The tight junction protein complex undergoes rapid and continuous molecular remodeling at steady state. J Cell Biol 181: 683–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L, Weber CR, Raleigh DR, Yu D, Turner JR. 2011. Tight junction pore and leak pathways: A dynamic duo. Annu Rev Physiol 73: 283–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon DB, Lu Y, Choate KA, Velazquez H, Al-Sabban E, Praga M, Casari G, Bettinelli A, Colussi G, Rodriguez-Soriano J, et al. 1999. Paracellin-1, a renal tight junction protein required for paracellular Mg2+ resorption. Science 285: 103–106. [DOI] [PubMed] [Google Scholar]
- Smales C, Ellis M, Baumber R, Hussain N, Desmond H, Staddon JM. 2003. Occludin phosphorylation: identification of an occludin kinase in brain and cell extracts as CK2. FEBS Lett 545: 161–166. [DOI] [PubMed] [Google Scholar]
- Staehelin LA. 1973. Further observations on the fine structure of freeze-cleaved tight junctions. J Cell Sci 13: 763–786. [DOI] [PubMed] [Google Scholar]
- Stevenson BR, Siliciano JD, Mooseker MS, Goodenough DA. 1986. Identification of ZO-1: A high molecular weight polypeptide associated with the tight junction (zonula occludens) in a variety of epithelia. J Cell Biol 103: 755–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson BR, Anderson JM, Goodenough DA, Mooseker MS. 1988. Tight junction structure and ZO-1 content are identical in two strains of Madin-Darby canine kidney cells which differ in transepithelial resistance. J Cell Biol 107: 2401–2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su L, Shen L, Clayburgh DR, Nalle SC, Sullivan EA, Meddings JB, Abraham C, Turner JR. 2009. Targeted epithelial tight junction dysfunction causes immune activation and contributes to development of experimental colitis. Gastroenterol 136: 551–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su L, Nalle SC, Shen L, Turner ES, Singh G, Breskin LA, Khramtsova EA, Khramtsova G, Tsai PY, Fu YX, et al. 2013. TNFR2 activates MLCK-dependent tight junction dysregulation to cause apoptosis-mediated barrier loss and experimental colitis. Gastroenterol 145: 407–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura A, Hayashi H, Imasato M, Yamazaki Y, Hagiwara A, Wada M, Noda T, Watanabe M, Suzuki Y, Tsukita S. 2011. Loss of claudin-15, but not claudin-2, causes Na+ deficiency and glucose malabsorption in mouse small intestine. Gastroenterol 140: 913–923. [DOI] [PubMed] [Google Scholar]
- Tash BR, Bewley MC, Russo M, Keil JM, Griffin KA, Sundstrom JM, Antonetti DA, Tian F, Flanagan JM. 2012. The occludin and ZO-1 complex, defined by small angle X-ray scattering and NMR, has implications for modulating tight junction permeability. Proc Natl Acad Sci 109: 10855–10860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner JR. 2009. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol 9: 799–809. [DOI] [PubMed] [Google Scholar]
- Turner JR. 2016. Epithelia and gastrointestinal function. In Yamada's Textbook of Gastroenterology (eds. Podolsky DK, et al. ), pp. 317–329. John Wiley & Sons, NJ. [Google Scholar]
- Turner JR, Rill BK, Carlson SL, Carnes D, Kerner R, Mrsny RJ, Madara JL. 1997. Physiological regulation of epithelial tight junctions is associated with myosin light-chain phosphorylation. Am J Physiol 273: C1378–1385. [DOI] [PubMed] [Google Scholar]
- Turner JR, Buschmann MM, Romero-Calvo I, Sailer A, Shen L. 2014. The role of molecular remodeling in differential regulation of tight junction permeability. Semin Cell Dev Biol 36: 204–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Itallie CM, Anderson JM. 2012. Caveolin binds independently to claudin-2 and occludin. Ann N Y Acad Sci 1257: 103–107. [DOI] [PubMed] [Google Scholar]
- Van Itallie C, Rahner C, Anderson JM. 2001. Regulated expression of claudin-4 decreases paracellular conductance through a selective decrease in sodium permeability. J Clin Invest 107: 1319–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Itallie CM, Holmes J, Bridges A, Gookin JL, Coccaro MR, Proctor W, Colegio OR, Anderson JM. 2008. The density of small tight junction pores varies among cell types and is increased by expression of claudin-2. J Cell Sci 121: 298–305. [DOI] [PubMed] [Google Scholar]
- Van Itallie CM, Fanning AS, Bridges A, Anderson JM. 2009. ZO-1 stabilizes the tight junction solute barrier through coupling to the perijunctional cytoskeleton. Mol Biol Cell 20: 3930–3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Itallie CM, Fanning AS, Holmes J, Anderson JM. 2010. Occludin is required for cytokine-induced regulation of tight junction barriers. J Cell Sci 123: 2844–2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada M, Tamura A, Takahashi N, Tsukita S. 2013. Loss of claudins 2 and 15 from mice causes defects in paracellular Na+ flow and nutrient transport in gut and leads to death from malnutrition. Gastroenterol 144: 369–380. [DOI] [PubMed] [Google Scholar]
- Wang F, Graham WV, Wang Y, Witkowski ED, Schwarz BT, Turner JR. 2005. Interferon-γ and tumor necrosis factor-α synergize to induce intestinal epithelial barrier dysfunction by up-regulating myosin light chain kinase expression. Am J Pathol 166: 409–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Schwarz BT, Graham WV, Wang Y, Su L, Clayburgh DR, Abraham C, Turner JR. 2006. IFN-γ-induced TNFR2 expression is required for TNF-dependent intestinal epithelial barrier dysfunction. Gastroenterol 131: 1153–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber CR, Nalle SC, Tretiakova M, Rubin DT, Turner JR. 2008. Claudin-1 and claudin-2 expression is elevated in inflammatory bowel disease and may contribute to early neoplastic transformation. Lab Invest 88: 1110–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber CR, Raleigh DR, Su L, Shen L, Sullivan EA, Wang Y, Turner JR. 2010. Epithelial myosin light chain kinase activation induces mucosal interleukin-13 expression to alter tight junction ion selectivity. J Biol Chem 285: 12037–12046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber CR, Liang GH, Wang Y, Das S, Shen L, Yu AS, Nelson DJ, Turner JR. 2015. Claudin-2-dependent paracellular channels are dynamically gated. eLife 4: e09906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber CR, Turner JR. 2017. Dynamic modeling of the tight junction pore pathway. Ann N Y Acad Sci (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox ER, Burton QL, Naz S, Riazuddin S, Smith TN, Ploplis B, Belyantseva I, Ben-Yosef T, Liburd NA, Morell RJ, et al. 2001. Mutations in the gene encoding tight junction claudin-14 cause autosomal recessive deafness DFNB29. Cell 104: 165–172. [DOI] [PubMed] [Google Scholar]
- Wong V. 1997. Phosphorylation of occludin correlates with occludin localization and function at the tight junction. Am J Physiol 273: C1859–1867. [DOI] [PubMed] [Google Scholar]
- Wong V, Gumbiner BM. 1997. A synthetic peptide corresponding to the extracellular domain of occludin perturbs the tight junction permeability barrier. J Cell Biol 136: 399–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong VW, Stange DE, Page ME, Buczacki S, Wabik A, Itami S, van de Wetering M, Poulsom R, Wright NA, Trotter MW, et al. 2012. Lrig1 controls intestinal stem-cell homeostasis by negative regulation of ErbB signalling. Nat Cell Biol 14: 401–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu AS, McCarthy KM, Francis SA, McCormack JM, Lai J, Rogers RA, Lynch RD, Schneeberger EE. 2005. Knockdown of occludin expression leads to diverse phenotypic alterations in epithelial cells. Am J Physiol Cell Physiol 288: C1231–1241. [DOI] [PubMed] [Google Scholar]
- Yu D, Marchiando AM, Weber CR, Raleigh DR, Wang Y, Shen L, Turner JR. 2010. MLCK-dependent exchange and actin binding region-dependent anchoring of ZO-1 regulate tight junction barrier function. Proc Natl Acad Sci 107: 8237–8241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolotarevsky Y, Hecht G, Koutsouris A, Gonzalez DE, Quan C, Tom J, Mrsny RJ, Turner JR. 2002. A membrane-permeant peptide that inhibits MLC kinase restores barrier function in in vitro models of intestinal disease. Gastroenterol 123: 163–172. [DOI] [PubMed] [Google Scholar]