Abstract
Epithelia exist in the animal body since the onset of embryonic development; they generate tissue barriers and specify organs and glands. Through epithelial–mesenchymal transitions (EMTs), epithelia generate mesenchymal cells that form new tissues and promote healing or disease manifestation when epithelial homeostasis is challenged physiologically or pathologically. Transforming growth factor-βs (TGF-βs), activins, bone morphogenetic proteins (BMPs), and growth and differentiation factors (GDFs) have been implicated in the regulation of epithelial differentiation. These TGF-β family ligands are expressed and secreted at sites where the epithelium interacts with the mesenchyme and provide paracrine queues from the mesenchyme to the neighboring epithelium, helping the specification of differentiated epithelial cell types within an organ. TGF-β ligands signal via Smads and cooperating kinase pathways and control the expression or activities of key transcription factors that promote either epithelial differentiation or mesenchymal transitions. In this review, we discuss evidence that illustrates how TGF-β family ligands contribute to epithelial differentiation and induce mesenchymal transitions, by focusing on the embryonic ectoderm and tissues that form the external mammalian body lining.
TGF-β FAMILY MEMBERS IN EPITHELIAL DIFFERENTIATION
The transforming growth factor-β (TGF-β) family consists of secreted polypeptides that include TGF-β1, TGF-β2, and TGF-β3, activins, bone morphogenetic proteins (BMPs), and growth and differentiation factors (GDFs). These ligands signal after binding to type II and type I receptors that show protein kinase activity and activate intracellular effectors such as Smad proteins and various signaling branches of protein kinases, lipid kinases, and small GTPases (Hata and Chen 2016; Zhang 2017). The Smad branch of signaling mediators includes receptor-activated Smads (R-Smads), a common mediator Smad (co-Smad), and inhibitory Smads (I-Smads). Five different R-Smads (Smad1, Smad2, Smad3, Smad5, and Smad8) are directly phosphorylated by the type I receptors. Phosphorylated R-Smads form heteromeric complexes with Smad4, the only mammalian co-Smad. Smad6 and Smad7 are I-Smads, whose genes are transcriptionally induced by the TGF-β, activin, and BMP pathways and limit the activities of these pathways (Miyazawa and Miyazono 2016). The TGF-β family is implicated in multiple stages of early embryonic development; a prominent example is nodal, which signals the generation of proximodistal polarity in the early embryo (Schier 2003; Robertson 2014). As embryonic morphogenesis proceeds, nodal also specifies endodermal tissue differentiation, and controls the anteroposterior pattern of the embryo (Schier 2003; Robertson 2014). In addition, left–right body asymmetry is regulated by nodal and BMPs (Shiratori and Hamada 2014). On the other hand, dorsoventral embryonic polarity is controlled by BMP-specific extracellular antagonists, such as chordin and noggin, which limit the binding of BMPs to their signaling receptors (Bier and De Robertis 2015). Thus, BMPs secreted by dorsal embryonic tissue (i.e., the Spemann organizer) repress gene expression, and BMPs secreted by ventral tissue activate gene expression, generating a dorsoventral polar pattern of differentiation in the emerging embryonic tissue (Bier and De Robertis 2015). Nodal and BMPs and their extracellular antagonists, acting in concentration-dependent gradients across the early embryonic tissue, enable progenitor cells to generate the three embryonic lineages—ectoderm, endoderm and mesoderm. These three lineages subsequently generate directly or indirectly (i.e., through epithelial–mesenchymal interactions) differentiated epithelial cell types, which populate the various tissues discussed in this review.
EPITHELIAL–MESENCHYMAL AND MESENCHYMAL–EPITHELIAL TRANSITIONS IN EPITHELIAL ORGANOGENESIS
Epithelial morphogenesis proceeds through successive cycles of induction of epithelial proliferation by the adjacent mesenchymal layer, followed by differentiation cycles, which are also positively or negatively controlled by mesenchymal cells. In addition to the mesenchymal inputs, epithelial cells are also capable of transdifferentiating to other cell types, through processes that are collectively termed epithelial plasticity. When the transdifferentiation generates mesenchymal cells, the process is best known as epithelial–mesenchymal transition (EMT) (Hay 1995; Lim and Thiery 2012). EMT can be reversible and then leads to the transdifferentiation from mesenchymal to epithelial cells, known as mesenchymal–epithelial transition (MET) (Nieto 2013). However, cases of MET in which the starting cell source is a differentiated mesenchymal cell (e.g., a fibroblast) have been reported mainly in the field of induced pluripotent stem (iPS) cell technology (Sanchez-Alvarado and Yamanaka 2014). EMT and MET play critical roles in disease pathogenesis (Kalluri and Weinberg 2009), such as in cancer, in which TGF-β-induced EMT empowers prometastatic potential, and in tissue fibrosis, in which BMP-induced MET counteracts fibrosis. In this review, we focus on roles of EMT and MET during normal development of epithelial organs.
Hallmarks of EMT are the remodeling of tight, adherens, and desmosomal junctions at the plasma membrane and remodeling of the cytoskeleton, including actin microfilaments, microtubules, and keratin or vimentin intermediate filaments; these processes are driven by remodeling of polarity complexes in epithelial cells (Wheelock et al. 2008; Nelson 2009; Huang et al. 2012). The TGF-β family plays prominent roles in directing EMT and MET, and much is already understood in relation to the signaling pathways that mediate this epithelial response and the gene programs that control transdifferentiation (Lamouille et al. 2014).
Historically, evidence that TGF-β members induce EMT was gathered in studies of heart valve formation; TGF-β2 transforms endothelial cells to mesenchymal cells that generate the cushions that line the septa in the heart valves (Mercado-Pimentel and Runyan 2007). This example expands the concept of EMT beyond epithelial cells and informs us that plasticity in differentiation also takes place in the endothelium, thus named endothelial–mesenchymal transition (EndMT). Another critical observation of EMT induced by TGF-β1 involved mouse mammary epithelial NMuMG cells (Miettinen et al. 1994). Subsequent research defined molecular pathways and gene-based mechanisms that mediate the EMT program in NMuMG cells (Valcourt et al. 2005). In addition, TGF-β members drive EMT while arresting the cell cycle of NMuMG cells (Gal et al. 2008). It is worth noting that epithelial cell cycle arrest by TGF-βs, activins or BMPs is a hallmark response that characterizes this family of cytokines (Massagué 2012). It was also shown that BMPs, in a concentration-dependent manner, can overcome the positive effects of TGF-β in driving EMT, thus promoting the epithelial phenotype (Kowanetz et al. 2004), which is reminiscent of a positive effect of BMPs toward MET (Zeisberg et al. 2003). However, it should be kept in mind that BMP signaling can also promote EMT in cancer (Davis et al. 2016) or during neural crest delamination, as will be described later. Finally, studies in the cancer field revealed a role of EMT in the generation of epithelial stemness, and this concept has been extended to differentiation of normal mammary epithelium, and embryonic stem cell biology, including iPS cell technology (Watabe and Miyazono 2009; Samavarchi-Tehrani et al. 2010; Tam and Weinberg 2013).
EPIBLAST EPITHELIAL TO MESODERM DIFFERENTIATION
Gastrulation generates three layers of embryonic tissue—ectoderm, mesoderm, and endoderm. Each of these layers has distinct developmental fates as they generate the adult body tissues, with nerve and skin being derived from ectoderm, muscle from mesoderm, and gut from endoderm (Robertson 2014). During gastrulation, the epithelial cells of the epiblast generate, through EMT, motile mesenchymal cells that migrate into the blastocyst cavity to populate the space and give rise to the germ layers. In this manner, EMT gives rise to two embryonic tissue layers, mesoderm and endoderm, localized toward the center of the body; ectoderm lies toward the surface of the embryo surrounding the other two layers (Robertson 2014). However, gastrulation additionally encompasses substantial epithelial cell invagination and movement without EMT.
The epiblast EMT involves embryonic TGF-β family ligands such as mouse nodal and chicken Vg1, or the mammalian Vg1 orthologs GDF-1 and GDF-3 (Shah et al. 1997; Chea et al. 2005; Andersson et al. 2007). Classical studies established that loss-of-function Nodal mutations in mice lead to defective gastrulation (Robertson 2014). Moreover, transplantation experiments, aimed at rescuing the mutant phenotype, showed that only very low doses of nodal protein, expressed by few cells, are sufficient and required to mediate the epiblast EMT (Varlet et al. 1997). The binding of nodal to its receptors is controlled by the extracellular inhibitors lefty and cerberus-like, so that only cells positioned at the correct place during tissue ingression undergo EMT (Perea-Gomez et al. 2002; Bertocchini et al. 2004). Cerberus-like antagonizes not only nodal but also BMPs and Wnts, which is important as explained below. Similar to nodal, gain-of-function overexpression of Vg1 promotes epiblast EMT and causes partial duplication of the chicken primitive streak (Shah et al. 1997; Chea et al. 2005), whereas loss-of-function mutations in mouse Gdf1 and Gdf3 suppress epiblast EMT (Andersson et al. 2007). The embryonic epithelial cells need to be sensitized by Wnt signaling, which ensures that the cells express TGF-β family receptors (Skromne and Stern 2001). Thus, Wnt signaling acts upstream of nodal, Vg1, or GDF-1/3; therefore, altering Wnt levels in developing gastrulae mimics experimental perturbations of nodal, Vg1, or GDF-1/3 (Skromne and Stern 2001). In addition, the epiblast EMT must be regulated temporally, and this is partially achieved by activation of fibroblast growth factor (FGF) signaling in mesenchymal cells (Mathieu et al. 2004). FGF and nodal cooperate to sustain the expression of key transcription factors that drive the EMT during gastrulation, including Snail family proteins (Mathieu et al. 2004). Despite the defective gastrulation in embryos lacking the Snail1 gene, Snail transcriptional activity has not yet been firmly linked to induction of the mesodermal differentiation program (Acloque et al. 2009). Instead, the transcription factor eomesodermin (Eomes) may regulate the specification of mesoderm by EMT in the gastrulating embryo (Arnold et al. 2008). This has been shown by inactivation of Eomes, which does not perturb Snail expression in the embryonic cells, despite the loss of EMT and mesodermal differentiation in the mutant embryos (Arnold et al. 2008). More studies on the molecular cooperation between Snail and Eomes and the identification of distinct and common target genes during gastrulation are warranted. One such target gene is the polarity gene Crumbs3, whose expression is repressed by Snail, resulting in disassembly of tight junctions in embryonic epithelial cells (Whiteman et al. 2008). Furthermore, TGF-β family members induce Snail expression during the EMT and activate the p38 mitogen activated protein kinase (MAPK) through its binding protein, p38-interacting protein (p38IP) (Zohn et al. 2006). The function of p38IP relates to E-cadherin degradation during EMT (Zohn et al. 2006). However, similar to Snail, the exact roles of p38IP and the p38 MAPK downstream from nodal or GDF-1/3 remain to be clarified.
EMT during primitive streak formation shows mechanistic parallels with the changes observed in cell culture and in studies of EMT during tissue fibrosis or cancer progression. Deciphering molecular mechanisms of embryonic EMT is of direct relevance to the pathophysiology of adult diseases.
NEUROECTODERMAL DELAMINATION AND NEURAL CREST EMT
The neural crest represents an embryonic stem cell population, unique to vertebrates, that is located at the border of the neural plate in the early embryo and forms the central nervous system. After the neural tube closure, neural crest cells reside in the dorsal neural tube and undergo EMT to generate numerous derivatives, including peripheral neurons, glial and Schwann cells, pigment cells, and facial skeleton cells (Bronner-Fraser and Fraser 1988; Bronner 2012; Rogers et al. 2013).
Wnt, FGF, and BMP signaling pathways regulate gene expression during neural crest development (Sauka-Spengler and Bronner-Fraser 2008). Canonical and noncanonical Wnt signaling regulate the proliferation of neural crest precursors and the migration of delaminating cells (Garcia-Castro et al. 2002; Carmona-Fontaine et al. 2008). In avian embryos, BMP-4 is expressed in the dorsal neural tube that triggers the expression of the transcription factor c-Myb, and converts epithelial neural plate cells into mesenchymal neural crest cells (Sela-Donenfeld and Kalcheim 1999; Karafiat et al. 2005). The BMP inhibitor noggin, on the other hand, blocks the migration of neural crest cells until noggin levels are eventually down-regulated by signals from the somites (Sela-Donenfeld and Kalcheim 1999, 2000; Karafiat et al. 2005). The cross talk between BMP and Wnt signaling during delamination of the neural crest cells involves release of BMP signaling from noggin, leading to increases in Wnt1 expression, which eventually promotes cell cycle entry and delamination (Burstyn-Cohen et al. 2004).
At the transcription factor level, the Sox9, Foxd3, and Snail1 genes are necessary for the regulation of delamination during neural crest EMT (Dottori et al. 2001; Cheung et al. 2005). Sox9 is required for EMT and survival of the neural crest cells in the trunk of the crest (Cheung et al. 2005). Snail and Slug (encoded by the Snail2 gene), together with Sox9, induce neural crest delamination and EMT in neural epithelial cells, whereas FoxD3 regulates the neural crest cell migration by repressing the expression of cell–cell adhesion molecules and promoting the expression of migratory markers, including human natural killer antigen-1 (HNK-1) and cadherin-7 (Dottori et al. 2001; Cheung et al. 2005). In addition, BMP signaling generates complexes of Smad1 and/or Smad5 with the transcription factor Sox5, which activate the expression of several genes before the delamination of the neuroectodermal cells (Nordin and LaBonne 2014). The activity of these complexes is also important during other ectodermal tissue movements, in the blastula or during neural plate formation (Nordin and LaBonne 2014).
Similar to other EMTs, a positive forward regulatory loop has been revealed in the neural crest. Accordingly, BMP signaling induces the avian Snail2 gene using Sox9 as a transcriptional cofactor (Sakai et al. 2006). Sox9 and Slug also form complexes, and together with signaling input by protein kinase A, they amplify the expression of Snail2, resulting in sustained Slug protein activity throughout the neural crest delamination process (Sakai et al. 2006).
Another transcription factor that promotes EMT, zinc-finger E box–binding homeobox 2 (ZEB2, also known as SIP1), contributes to the neural crest mesenchymal transition (Rogers et al. 2013). Reduction of ZEB2 expression by morpholino oligonucleotides revealed that its activity is required for the proper expression of FoxD3 and subsequent transcriptional induction of Sox10 in the delaminating neural crest cells that engage into migration (Rogers et al. 2013). Overall, Snail and ZEB family transcription factors regulate the expression of cadherins and other junctional components (Cano et al. 2000; Ikenouchi et al. 2003; Peinado et al. 2007). These EMT transcription factors bind to conserved E-box sequences in regulatory promoter sequences of their target genes, such as the genes encoding E-cadherin, claudin, or occludin, and operate downstream from TGF-β signaling in cooperation with the activated Smads (Vincent et al. 2009).
ZEB2 mediates the switch from E-cadherin to N-cadherin expression (Rogers et al. 2013). In addition, several other cadherins mediate different functions in premigratory and migratory neural crest cells. Accordingly, cadherin-6B is important at the premigratory stage, and cadherin-7 and cadherin-11 facilitate cell migration during the mesenchymal transition (Nakagawa and Takeichi 1998; Vallin et al. 1998). Interestingly, cadherin-6B plays a dual regulatory role through cross talk with BMP signaling in the neural crest (Park and Gumbiner 2010, 2012). Cadherin-6B activates BMP signaling, which promotes the EMT, whereas BMP signaling induces cadherin-6B expression and represses N-cadherin expression in the premigratory neural crest (Park and Gumbiner 2010). In this embryonic tissue, N-cadherin antagonizes the EMT induced by BMP, whereas cadherin-6B contributes to the delamination process (Park and Gumbiner 2010). The mechanism of BMP receptor activation by cadherin-6B may not involve BMP ligands, but, rather, interaction of cadherin-6B with the BMP type II receptor (BMPRII), which recruits the LIM domain kinase LIMK1 to its carboxy-terminal tail, providing signals toward cofilin that mediate a reorganization of the actin cytoskeleton (Park and Gumbiner 2012). Smad signaling mediated by BMPRII and BMPRI is not required for this mechanism, and activation of actin reorganization is sufficient to cooperate with Sox9 for effective neural crest delamination (Park and Gumbiner 2012). Furthermore, loss of N-cadherin and gain of cadherin-6 by neural crest mesenchymal cells, results in accumulation of F-actin in specialized apical neural crest cell tails (Clay and Halloran 2014). This mechanism controls the distribution of active Rho GTPase at the apical membrane during cell detachment and thus promotes cell movement (Clay and Halloran 2014). Thus, neural crest EMT resembles the EMTs of epithelial tissues, but also shows distinct characteristics that require further understanding. In particular, the coordination of BMP signaling with other pathways remains to be fully characterized in the delaminating neural crest cells.
THE TGF-β FAMILY IN THE DEVELOPMENT OF EXTERNAL EPITHELIAL TISSUES AND EMT
Skin and Hair Follicle Development
Defining Ectoderm
The epidermis and its appendages, such as hair follicles, sebaceous glands, and sweat glands, are derived from ectoderm. Ectoderm develops into epidermis and nervous system, and BMPs specify ectodermal identity (Fig. 1A). Based on a classical “default model” of neural induction, BMP, in particular BMP-4, is an epidermal inducer, and chordin, noggin, and follistatin are extracellular antagonists secreted from cells in the Spemann organizer, which block BMP signaling and induce neural development (reviewed in Stern 2005). Various studies have shown that activation followed by repression of BMP signaling is not sufficient to specify the fate of ectoderm, and that neural induction depends on FGF signaling in addition to the BMP signaling shutoff (Delaune et al. 2005; Stern 2005). Furthermore, Wnt-induced β-catenin signaling governs ectodermal patterning, by blocking ectodermal cells from responding to FGFs and preserving their response to BMPs (Stern 2005; Patthey and Gunhaga 2014). Appropriate formation of the border region between epidermis and nervous system, which develops into neural crest and peripheral nervous system, requires temporal activation of BMP signaling under the control of Wnt-induced β-catenin (reviewed in Patthey and Gunhaga 2014). Ectopic BMP narrows the border region, resulting in a wider epidermal region, and overexpressed BMP antagonists enlarge the neural plate arising from the border region (Stern 2005), which suggests that BMP controls ectodermal expression by defining the position of the border. At this developmental stage, the interaction of Sox5 with BMP-specific Smads plays important roles (Nordin and LaBonne 2014). In addition, Wnt-induced β-catenin activity in the dermis controls the generation of two types of dermal fibroblasts; the papillary fibroblast lineage responds to Hedgehog (Hh) signals and the reticular fibroblast lineage responds to TGF-β2 (Lichtenberger et al. 2016). The adjacent epidermal cells provide both Hh and TGF-β2, which promote proliferation and extracellular matrix synthesis in the two fibroblast types. Although the two fibroblast lineages develop independently from each other, they also maintain their responsiveness to the original Wnt signaling input (Lichtenberger et al. 2016). Later in skin development, basal progenitor cells divide and give rise to intermediate cells that migrate a short distance to enter the final differentiation zone of the dermal and epidermal layers (Zhu et al. 2014). This later phase is also driven by Wnt and β-catenin signaling, which induces expression of BMP-2, BMP-4, or BMP-7 in the dermal cells (Zhu et al. 2014). BMP–Smad signaling then induces expression of FGF7 and FGF10, which directly promote epidermal differentiation in the adjacent cell layer (Zhu et al. 2014). FGF signaling, in cooperation with the transcription factor p63, generates differentiated epidermal cells, which move away from the mitotic layer of the basal epidermal region, generating the stratified epidermal epithelium (Zhu et al. 2014).
Figure 1.
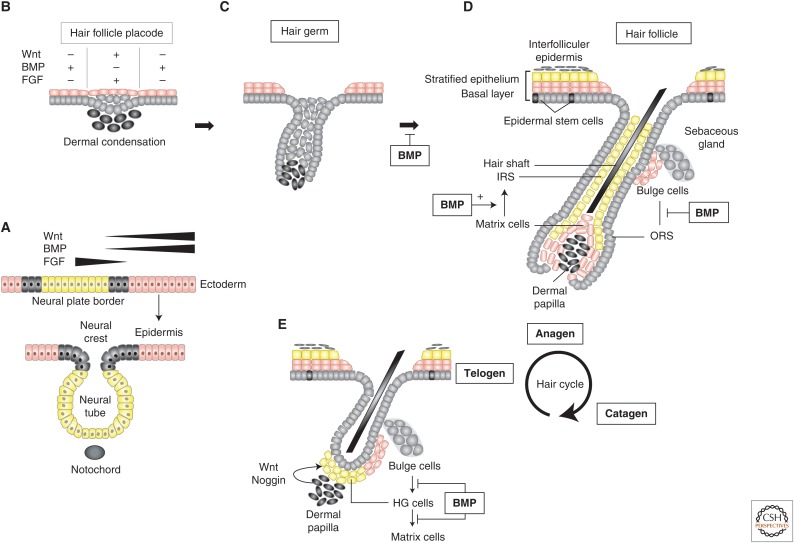
Roles of bone morphogenetic protein (BMP) signaling in epidermal development. (A) Ectoderm definition. Ectodermal cells (pink) are separated from the neural plate cells (yellow) by small patches of border cells (gray). These embryonic cell types are defined anatomically and based on the pathways that control their developmental fate. In ectodermal cells, Wnt-induced β-catenin signals are active, and BMP signaling is preserved and induces epidermis, whereas fibroblast growth factor (FGF) signaling promotes neural commitment in which Wnt–β-catenin signaling is silenced. Intermediate intensity of Wnt and BMP signals in the border region defines the limits of these embryonic territories. (B–E) Hair follicle development and hair cycling. (B) Wnt–β-catenin (+) and FGF (+) signaling induce placode formation accompanied by dermal condensation underneath. BMP (+) signaling on either side of the hair follicle placode inhibits its formation. (C) Hair follicle placode “downgrowth” away from the surface of the skin and engulfment of dermal condensations at the tip of the hair germ that grows downward. (D) Proliferation and differentiation of bulge cells toward outer root sheath (ORS) cells are blocked by BMP signals, whereas BMP signals are required for terminal differentiation of matrix cells to inner root sheath (IRS) cells that generate the hair shaft. Hair follicle stem cells (HFSCs) are accommodated in the bulge and hair germ. Bulge cells remain in a quiescent stage of their cell cycle and BMP signaling contributes to their lengthy life span. Hair germ cells are more prone to be activated by dermal signals and contribute postnatally to hair follicle regeneration. The three phases of the postnatal hair cycle are also shown. (E) At the end of the telogen phase, the dermal papilla secretes noggin, which inhibits BMP signaling; BMPs inhibit bulge cell differentiation to hair germ (HG) cells and matrix cells as explained in D. Noggin-mediated inhibition of BMP signaling leads to activation of HG cells and induction of their differentiation into matrix cells, whereas Wnt promotes HG cell proliferation.
Overview of Epidermal Development
Once epidermis is specified, hair follicle formation begins with local thickening of the epithelium (hair follicle placode), which is accompanied by condensation of the underlying mesenchyme (dermal condensation) that becomes the dermal papilla (Fig. 1B). The hair follicle placode grows downward, away from the skin surface, a process known as “follicular downgrowth,” and penetrates into the dermis, whereas the leading edge engulfs the underlying dermal condensation at the hair germ stage (Fig. 1C). The outermost cells give rise to the outer root sheath (ORS), which surrounds the hair follicles and is continuous with the basal layer of the interfollicular epidermis. The ORS is supposed to contain less differentiated cells than cells found in other compartments of the hair follicle. Rapidly proliferating transit-amplifying cells located in the matrix (root sheath) of the hair follicle emerge from the innermost cells, and differentiate into the supportive inner root sheath (IRS) and into the hair shaft forming seven concentric layers (Fig. 1D). The epidermis and its appendages consistently renew after birth as they derive from the multipotent epidermal stem cells (Moore and Lemischka 2006; Blanpain and Fuchs 2014). The interfollicular epidermis undergoes frequent turnover and is replenished by epidermal stem cells that are located in the innermost (basal) layer of stratified epidermis (Moore and Lemischka 2006; Blanpain and Fuchs 2014). In contrast, hair follicle stem cells (HFSCs) only periodically proliferate and regenerate through the hair cycle that consists of three phases—catagen (apoptosis-driven regression), telogen (quiescence), and anagen (growth and hair shaft formation) (Doma et al. 2013). Hair follicle development during embryogenesis and the postnatal anagen phase are thought to be regulated by similar mechanisms that generate the hair shaft–producing bulb (Fig. 1D,E; details about epidermal stem cells are presented below).
Mesenchymal and Epithelial Interaction
Epidermal development and postnatal hair cycling are orchestrated by reciprocal instructive interactions between epithelium (epidermis) and mesenchyme (dermis), as shown in coculture experiments of epidermal and dermal components from embryonic skin (Sengel 1990). Epidermal cells receive instructive signals from the underlying dermis, and the mesenchymal cells need epidermal signals to form dermal papillae (Davidson and Hardy 1952; Kollar 1970; Hardy 1992). Instructive signals consist of activators (ligands) and inhibitors (antagonists) for certain signaling pathways, and their balance determines the strength of signals and their effects. In epidermal development, BMP signaling is a pivotal instructive pathway that operates during mesenchymal and epithelial interactions.
The BMPs in Hair Follicle Development
Hair follicle formation is initiated by Wnt signaling through β-catenin (Fig. 1B) (Zhou et al. 1995; Gat et al. 1998; Andl et al. 2002). Wnt induces the expression of Wnt inhibitors, such as dickkopf (Dkk), whose concentration determines hair follicle position and patterning, perhaps through a reaction–diffusion model (Jiang et al. 2004; Sick et al. 2006; Zhang et al. 2008). Epidermal cells without Wnt or β-catenin signaling proliferate to form stratified interfollicular epithelium, and Wnt-induced β-catenin signaling correlates with the formation of the hair follicle placode. Epithelial signals including FGF enable the formation of a dermal condensation underneath the placode (Huh et al. 2013). Mice with an inactivated gene encoding the BMP antagonist noggin show, among other phenotypes, delayed hair follicle induction (Botchkarev et al. 1999; Mou et al. 2006). Conversely, addition of exogenous noggin on explanted embryonic skin cultures promotes hair follicle formation (Botchkarev et al. 1999; Mou et al. 2006). Accordingly, loss of the negative control of BMP-4 activity results in disturbed hair follicle formation and leads to progressive hair loss (Botchkarev et al. 1999; Mou et al. 2006). Furthermore, nuclear phospho-Smad1 cannot be detected in the follicular placode region where Wnt signaling through β-catenin is activated (Mou et al. 2006). These observations support an inhibitory role of BMP signaling in hair follicle formation. BMPs can attenuate the proliferation and migration and induce apoptosis of hair follicle epithelium (Blessing et al. 1993; Botchkarev et al. 1999).
BMP signaling activity also correlates with the loss of expression of essential factors that contribute to hair follicle development, such as Sonic Hedgehog (Shh), the tumor necrosis factor (TNF) family member ectodysplasin A (EDA), and its receptor EDAR (Kobielak et al. 2003; Andl et al. 2004). Shh is a key regulator of hair follicle morphogenesis during embryonic development and after birth during the anagen stage (Jung et al. 1998; St-Jacques et al. 1998; Chiang et al. 1999; Wang et al. 2000). BMP-4 signaling mediates repression of Shh expression, whereas noggin activates Shh expression (Botchkarev et al. 2001; Blanpain and Fuchs 2006; Gao et al. 2008). EDA and its receptor EDAR cooperate with Wnt–β-catenin signaling to give rise to the specialized mechanosensory guard hair follicles. EDA signaling through EDAR also increases the expression of connective tissue growth factor (CTGF) and moderately increases the expression of the BMP and activin antagonist follistatin (Pummila et al. 2007). Follistatin, acting as a BMP antagonist, can then block BMP signaling, and BMPs, in turn, repress epidermal EDAR expression (Mou et al. 2006; Pummila et al. 2007). CTGF has been shown to act as either a weak BMP antagonist or a factor that potentiates TGF-β signaling and promotes embryonic fibroblast condensation. Thus, CTGF can possibly act indirectly against the BMPs, through other BMP antagonists that are secreted by fibroblasts (Pummila et al. 2007), a mechanism of action that remains to be clarified.
By inactivating BMP signaling, noggin induces the expression of lymphoid enhancer-binding factor 1 (LEF1), a transcription factor downstream from the Wnt–β-catenin pathway (Botchkarev et al. 1999; Jamora et al. 2003). Lack of noggin attenuates the activity of Wnt-induced β-catenin signaling, and inactivation of the gene encoding the BMP receptor BMPRIA/ALK-3 results in sustained LEF1 expression (Kobielak et al. 2003; Andl et al. 2004), which confirms that blocking BMP signaling enhances and preserves the activity of the Wnt–β-catenin-LEF1 pathway (Fuchs 2007). Additionally, BMP signaling is essential for the terminal differentiation of the hair shaft and the IRS from the transit-amplifying matrix cells; BMP target genes, such as Msx2, Foxn1, and Hoxc13, are expressed in the differentiating IRS and hair shaft (Godwin and Capecchi 1998; Meier et al. 1999; Kulessa et al. 2000; Tkatchenko et al. 2001; Ma et al. 2003; Johns et al. 2005). GATA-binding protein 3 (GATA-3) expression is also induced by BMP-4 and maintains BMP-4 levels through a positive feedback loop, which is necessary for the differentiation of the IRS (Kobielak et al. 2003).
Expression of BMPs and Antagonists in Hair Follicles
BMP-2 expression is only observed in mouse whisker follicles (Lyons et al. 1990; Bitgood and McMahon 1995), and BMP-4 expression is more dominant in dermal condensations and papillae, although it is expressed in the IRS and precortical cells at later developmental stages (Fig. 1D) (Bitgood and McMahon 1995). BMP-6 is expressed in both the epithelial transit-amplifying matrix cells and the mesenchymal dermal papillae, which regulate each other through mesenchymal–epithelial interaction (Rendl et al. 2008). CTGF antagonizes the activity of BMP during the development of primary hair follicles (Mou et al. 2006), whereas other types of hair follicles are more dependent on noggin as BMP antagonist (van Genderen et al. 1994). Noggin is expressed in the dermal condensations and papillae (mesenchyme) throughout hair morphogenesis (Botchkarev et al. 1999; Jamora et al. 2005), and its expression is guided by epithelial signals, such as those induced by Shh, laminin-511, platelet-derived growth factor (PDGF), and dermal signals including those by integrin β1 and PDGF receptor α (PDGFRα) (Rishikaysh et al. 2014). The transcription factor Sox2 is expressed in dermal papillae and activates the expression of Sostdc1 (sclerostin domain-containing 1, also known as ectodin or USAG-1), a potent BMP inhibitor, leading to repression of BMP-6 expression in matrix cells (Clavel et al. 2012). Ablation of Sox2 in dermal papillae decreases the expression of Sostdc1, which leads to activation of BMP-6 expression in matrix cells, and thus inhibits their migration toward the hair shaft compartments, resulting in delay of hair growth.
Epidermal Development and Smads
R-Smads are highly expressed in the epidermis and hair follicles (He et al. 2001) and may have redundant roles. In contrast to inactivation of the expression of individual R-Smads, hair follicle development is severely abrogated when Smad4 expression is deleted in keratinocytes (Yang et al. 2005; Qiao et al. 2006). The phenotypes of hair follicles in Smad4−/− mice are very similar to those of Bmpr1a−/− mice, which suggests that hair follicle development is controlled by BMP–Smad4 signaling (Kobielak et al. 2003; Andl et al. 2004; Ming Kwan et al. 2004; Yuhki et al. 2004). Loss of Smad4 results in the depletion of HFSCs because of increased activity by β-catenin; increased β-catenin activity transcriptionally induces the expression of c-Myc, which causes rapid cell cycles and eventually exhausts the proliferative potential of the HFSCs, achieving their depletion (Yang et al. 2009). Smad7 expression is very low in normal keratinocytes (He et al. 2001) but is often increased under pathological conditions, such as in aged and photoaged human skin (He et al. 2002; Quan et al. 2002) and in skin cancer (He et al. 2001). Mice overexpressing Smad7 under the control of the keratin-5 (Krt5) promoter show multiple developmental defects in their stratified epithelia, including impairment of hair follicle development (He et al. 2002).
Epidermal Stem Cells and Hair Cycling
Accumulating evidence has revealed that stem cell niches are heterogeneous and compartmentalized. Stem cells residing in different epidermal niches show different levels of quiescence and fate (reviewed in Blanpain and Fuchs 2014). Among HFSCs, most quiescent cells reside in the upper half of the bulge region underneath the sebaceous glands, and the cells in the lower half of the bulge are more likely to proliferate (Morris et al. 2004; Tumbar et al. 2004; Levy et al. 2005; Zhang et al. 2009; Hsu et al. 2011; Rompolas et al. 2013). The hair germ (HG) just under the bulge and in close proximity to the dermal papilla represents another niche, where HFSCs are prone to be activated and proliferate (Fig. 1E) (Greco et al. 2009). The transition of HFSCs from the upper bulge toward the HG via the lower bulge is observed at the end of the telogen phase, and HG cells directly contribute to the regeneration of hair follicles during the anagen phase.
The transition of the HFSCs is regulated by dermal signals consisting of Wnts and noggin (Greco et al. 2009; Rompolas et al. 2013). Interestingly, the Wnt and BMP signaling pathways are cyclically activated out of phase during the hair regeneration cycle; this mechanism is maintained by the periodic regulation of expression of BMP-2 and BMP-4 by dermal cells and the out-of-phase, periodic Wnt-induced β-catenin signaling in the stem cells (Plikus et al. 2008). Activated Wnt–β-catenin signals induce HG cells to proliferate and differentiate into matrix cells leading to induction of anagen (Korinek et al. 1998; Pinto et al. 2003; Kuhnert et al. 2004; Blanpain and Fuchs 2006). Simultaneously, HG cells express Shh that promotes the differentiation of lower bulge cells into ORS and induces dermal papillae to express noggin (Ito et al. 2005; Jiang and Hui 2008; Rompolas et al. 2013; Hsu et al. 2014). In turn, noggin helps HG cells to express Shh (Botchkarev et al. 2001, 2002).
Inactivation of Bmpr1a, which encodes the only known BMP type I receptor expressed in hair follicles (Botchkarev et al. 1999), induces quiescent stem cells to enter the cell cycle leading to failure of hair follicle maintenance (Kobielak et al. 2003; Andl et al. 2004; Yuhki et al. 2004; Zhang et al. 2006). This result suggests that BMPs are essential for maintaining quiescence of HFSCs, which is in part explained by the transcriptional activation of the gene encoding the cell cycle inhibitor p27Kip1 by BMP signaling (Sharov et al. 2006). Supporting this notion, BMP-6 is expressed and Smad1 is activated in HFSCs in the bulge (Andl et al. 2002, 2004; Blanpain et al. 2004), and the expression of other Smads and their target genes is enriched in the bulge region (He et al. 2001; Morris 2004; Tumbar et al. 2004). Acting upstream of BMPs in the HFSCs, the transcription factor FoxC1 contributes to the self-renewal of the HFSCs by inducing the expression of the nuclear factor of activated T cells 1 (NFATc1) and BMP ligands (Wang et al. 2016). In this manner, FoxC1 promotes the quiescent state of the stem cells, and genetic loss of the Foxc1 gene in activated stem cells correlates with failure of the HFSCs to enter quiescence, whereas later ablation of Foxc1 in quiescent hair follicle cells residing in the stem cell niche does not impair the ability of younger HFSCs to enter the quiescent stage that relates to differentiation.
BMPs also repress Wnt-induced β-catenin signaling in the hair follicles (Jamora et al. 2003; Kobielak et al. 2003; Blanpain et al. 2004). It has been suggested that paracrine BMP-4 and autocrine BMP-6 signaling maintain the quiescence in the niche (Blanpain et al. 2004). At the end of the anagen phase, regeneration of the hair shaft and IRS from matrix cells is completed, concomitantly with the terminal differentiation of the hair follicles. As mentioned, BMPs are required for terminal differentiation of the hair shaft. In the same way, the completion of regeneration requires active BMP signals. Targeted inactivation of Bmpr1a at this stage results in accumulation of highly proliferative and undifferentiated matrix cells and impaired differentiation of the IRS and hair shaft (Ming Kwan et al. 2004). Transgenic mice that express noggin in hair matrix cells and hair shaft precursor cells show accumulation of undifferentiated progenitor cells, leading to severely impaired hair shaft differentiation (Kulessa et al. 2000). Consistently, BMP-2 and BMP-4 are expressed during late anagen and early telogen, and are absent during late telogen and early anagen (Callahan and Oro 2001).
The expression of Smads and their target genes is diminished in HG, developing ORS, and proliferating matrix cells. The strongest BMP activity is observed in the differentiating IRS and hair shaft (Kobielak et al. 2003; Genander et al. 2014). Elimination of Smad1 and Smad5 expression during hair follicle morphogenesis by conditional double gene inactivation in mice results in lack of differentiated whiskers and associated death of the pups after birth (Kandyba et al. 2014). This experiment shows that BMPs maintain HFSC quiescence through Smad1 and Smad5 signaling, and that the presence of activated Smad8 in the HFSCs is not sufficient to mediate the biological effects of BMPs, causing a prolonged anagen phase in the follicles in the absence of Smad1 and Smad5. Additionally, BMP signaling is responsible for suppressing histone 3 methylation levels across the genome in HFSCs that enter quiescence and differentiate into hair follicle cells (Lee et al. 2016). BMPs induce the expression of specific histone demethylases and repress expression of some histone methyltransferases (Lee et al. 2016). An important question that remains is whether these epigenetic changes observed during the hair follicle cycle correlate with the expression of specific genes and/or may reflect a conformational state of the chromatin that adapts to each stage of differentiation.
In summary, BMPs maintain quiescent stem cells in the niche, and BMP signaling needs to be blocked in HG at the onset of anagen to allow HFSCs to proliferate and differentiate into matrix cells. Conversely, however, matrix cells and hair shaft progenitors require BMPs to complete the generation of new HFSCs. Similar to embryonic hair follicle development, spatiotemporal activation of BMP signals is important for postnatal hair cycling and maintenance of HFSCs.
Roles of TGF-βs and Activins in Epidermal Development
TGF-β plays an important role in skin regeneration (e.g., during wound healing) and is involved in normal epidermal development and maintenance of homeostasis, acting as a potent epithelial growth inhibitor. Transgenic mice that express Smad2 specifically in keratinocytes show severe thickening of their stratified epithelium and decreased number of hair follicles (Ito et al. 2001), suggesting that Smad2 activation in response to TGF-β or activin signaling promotes tissue proliferation. TGF-β signaling is also activated in HFSCs (Blanpain et al. 2004; Morris et al. 2004; Tumbar et al. 2004). At early stages of hair follicle development, neither the epithelium nor the mesenchyme expresses TGF-β at detectable levels (Lyons et al. 1990). As the follicles grow downward away from the skin surface, a process usually described as follicular “downgrowth,” TGF-β1 starts to be expressed in the placode epithelium and, at a later stage, the IRS expresses TGF-β1, and the ORS expresses TGF-β2 and TGF-β3 (Lyons et al. 1990). The expression of all three TGF-βs is gradually diminished after maturation (Lyons et al. 1990). TGF-β2 induces this “downgrowth” of hair follicles, which requires the Ras-extracellular signal-regulated kinase (Erk) MAPK pathway leading to induction of Snail expression (Foitzik et al. 1999; Jamora et al. 2005). In addition, follicular “downgrowth” or regression induced by TGF-β involves extensive apoptosis that appears as a gradient of cells with increasing death rates, so that a large number of follicular epithelial cells disappear (Mesa et al. 2015). The surviving basal epithelial cells phagocytose the dying layer of cells so that the follicle is ready for regeneration during the next cycle of growth and differentiation.
TGF-β1 is necessary for entry into the catagen phase (Foitzik et al. 2000), and its activity is controlled by the small leucine-rich repeat proteoglycan Tsukushi, which binds directly to TGF-β1 and mediates activation of its signaling pathway in the hair follicles (Niimori et al. 2012). Consequently, mice lacking Tsukushi expression caused by Tsku inactivation have a delayed hair cycle similar to the Tgfb1−/− mice (Foitzik et al. 2000; Niimori et al. 2012). TGF-β2 controls HFSC regeneration; it is secreted by the dermal papillae and activates the TGF-β receptors and Smad2 and Smad3 signaling in HFSCs, and induces the expression of the transmembrane protein TMEFF1 (transmembrane protein with EGF-like and two follistatin-like domains 1, also known as tomoregulin-1) (Oshimori and Fuchs 2012). TMEFF1 can associate with BMPs through its follistatin-homology domains, and inactivates BMP signaling, thus suppressing HFSC generation (Oshimori and Fuchs 2012), as mentioned earlier. Whether the activity of TGF-β2 is controlled extracellularly by Tsukushi, as for TGF-β1, remains to be examined (Niimori et al. 2012). Kindlin-1, which upon mutational inactivation of its gene causes the Kindler syndrome in humans, alters the homeostasis of stem cells by promoting integrin-mediated TGF-β activation and inhibiting the canonical Wnt pathway (Rognoni et al. 2014).
Inactivation of the Inhba gene, encoding inhibin βA (also known as activin βA), in mice leads to loss of activin A and inhibin A, causing lack of whiskers and their follicles (Matzuk 1995). Conversely, Fst−/− mice that lack activin inhibition by follistatin show a hyperkeratotic epidermis and abnormally shaped whiskers (Matzuk et al. 1995), which suggests that activin promotes normal hair follicle development. This conclusion is supported by analysis of the back skin of E18.5 mouse embryos carrying a Fst−/− germline mutation or expressing an Inhba transgene under the Krt14 promoter, the latter causing activin A overexpression in the prenatal mouse skin (Nakamura et al. 2003). Both mouse models show a delay in hair follicle morphogenesis (Nakamura et al. 2003). Ex vivo treatment of mouse hair follicle cultures with activin B (inhibin βB-βB) induces potent entry into the anagen stage (Jia et al. 2013). Keratinocytes and stromal cells in the developing skin respond to activins, as examined in transgenic mice expressing a dominant-negative mutant ActRIB/ALK-4 receptor in their keratinocytes (Bamberger et al. 2005). These mice show perturbed hair follicle morphogenesis; overexpressing inhibin βA in the hair follicles can at least partially rescue the effect of the dominant-negative ActRIB/ALK-4 (Nakamura et al. 2003; Bamberger et al. 2005), suggesting that activin signals in the epidermis through this receptor.
Transgenic mice expressing individual TGF-β family ligands have revealed roles in overall epidermal differentiation beyond effects on hair follicle morphogenesis. Analyses of mice overexpressing TGF-β1 support the growth inhibitory action of this cytokine in the epidermis, as it potently arrests the keratinocyte cell cycle, consistent with cell cycle studies of keratinocytes in culture (reviewed in Massagué 2012). Hyperkeratosis and a sparse distribution of hair follicles are seen in mice overexpressing activated TGF-β1 from the Krt1 promoter that drives keratin-1 expression, leading to a shiny and rigid skin that prohibits proper movement and breathing, and causes rapid postnatal death (Sellheyer et al. 1993). These mice show low numbers of dividing keratinocytes and few proliferating hair follicle cells in their skin (Sellheyer et al. 1993). In contrast, expression of active TGF-β1 from the Krt10 promoter results in mitotic activity of epidermal cells, indicative of hyperproliferation (Cui et al. 1995). However, when homeostasis is perturbed after chemical challenge that leads to skin hyperplasia, the transgenic TGF-β1 acts as a growth inhibitor requiring the function of the TGF-β type II receptor (TβRII) (Cui et al. 1995). Parallel approaches using inducible expression of active TGF-β1 in the epidermis confirm that TGF-β1 limits the proliferation of keratinocytes in vivo (Wang et al. 1999; Liu et al. 2001). Active TGF-β1 signaling in the epidermis can even resist to oncogenic stimuli that initiate neoplastic transformation (Wang et al. 1999). On the other hand, increased epidermal proliferation due to TGF-β1 overexpression is associated with a strong negative feedback response that includes Smad7 up-regulation (Liu et al. 2001). In a similar manner, expression of a dominant-negative TβRII mutant that blocks endogenous TGF-β signaling under the control of the Lor promoter (of the gene encoding loricrin) in the basal and suprabasal epidermal layers, leads to a strong hyperkeratotic phenotype (Wang et al. 1997). Expression of the same dominant-negative TβRII in the basal compartment of the skin and in hair follicle cells leads to relatively normal skin differentiation, but contributes synergisitcally to skin carcinogenesis when the mice are treated with radiation or chemical carcinogens (Amendt et al. 1998).
Overexpression of activin βA in mouse epidermis from the Krt14 promoter results in a dramatically enlarged epidermis because of increased proliferation of keratinocytes, and pronounced fibrosis, in part supported by an adipocyte to mesenchymal transdifferentiation (Munz et al. 1999). Mice expressing BMP-6 from the Krt10 promoter also show severe skin malformations leading to psoriasis, supporting the link between BMP-6 function and Wnt pathway activation in keratinocyte proliferation (Blessing et al. 1996), as discussed earlier. This study, similar to the TGF-β1 studies, revealed that the pattern of transgene expression within the tissue defines the response during epidermal differentiation. Thus, mice expressing the transgenic BMP-6 in small epidermal patches with low expression level show increased proliferation and hyperkeratosis, whereas mice expressing high and uniform levels of BMP-6 show potent growth inhibition during epidermal proliferation before or during birth (Blessing et al. 1996). Noggin expression from the Krt5 promoter in mouse epidermis blocks eyelid opening in the affected pups because of inefficient apoptosis in the eyelid skin and defective epidermal differentiation (Sharov et al. 2006). Thus, similar to the patterning of the skin and connective tissue around the digits of the arms and feet, BMPs control eyelid skin differentiation by coordinating epithelial apoptosis and differentiation.
An additional specification in skin differentiation is the generation of ciliated epithelial cells. Cilia have diverse physiological functions, by directing local fluid or air flow and serving as sensory appendages. TGF-β signaling through Smad2 and Smad3 controls the length of the cilium during embryogenesis, including in epidermal development (Tözser et al. 2015). In the epidermis, TGF-β signaling regulates the expression of the ciliary organizer, Meckel syndrome 1–related protein (MKSR), also known as B9 domain-containing 1 (B9D1) protein (Tözser et al. 2015). B9D1/MKSR is part of a multiprotein complex localized at the transition zone between the intracellular basal body and the membrane-protruding axoneme of the cilium, and regulates the assembly, growth, and length of the cilium. This mechanism of ciliogenesis is relevant in other developmental contexts ranging from gastrulation and neurulation to spermatid differentiation (Tözser et al. 2015). BMP signaling also controls differentiation of ciliary epithelia in embryos, through cooperation with Notch, specifying the localization of multiciliated and secretory cells in the outer layer of the embryonic epidermis (Cibois et al. 2015). Whereas BMPs drive differentiation, TGF-βs may control ciliary assembly and length.
Feather Development
Feathers, which enable birds to fly, are highly organized epidermal appendages with a hierarchical branched pattern, presenting some similarities to scales in fish and hairs in mammals (Fig. 2) (Yu et al. 2002). In situ RNA hybridization and immunohistochemistry revealed that TGF-β2 and TGF-β3 are expressed in the primordial buds of the feathers during late stages of chicken embryogenesis (Jakowlew et al. 1994). TGF-β2 is expressed in the junctional space where epithelium and mesenchyme interact (Fig. 2). When applied on chicken skin explants, TGF-β2 induces differentiation of feather buds, whereas TGF-β1 cannot show this response (Ting-Berreth and Chuong 1996).
Figure 2.
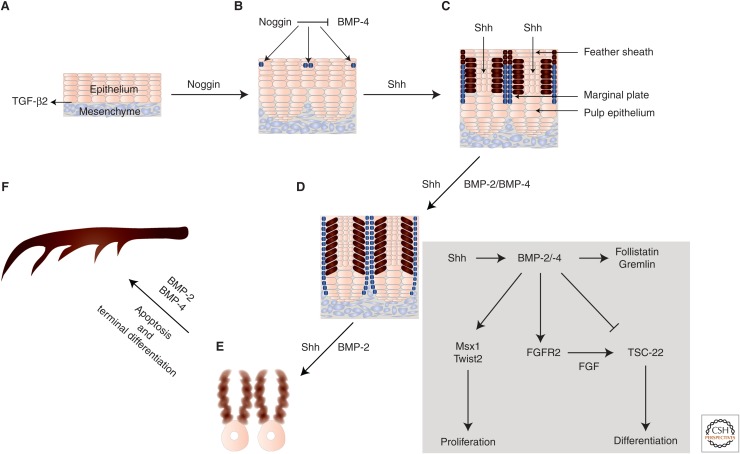
Branching and evolution of feathers. (A,B) TGF-β2 localizes in the border where epithelium and mesenchyme interact. The multilayered epidermis can be divided into epidermal ridges (dark blue cells) by the ridge-forming activator noggin and the ridge-forming inhibitor BMP-4. Noggin and BMP-4 are distributed in the mesenchyme (not shown) and their balanced action is crucial for normal specification of these primordial ridge cells. (B,C) High BMP-4 levels at the proliferative zone lead to the formation of a cylindrical structure by the epithelial cells. Within the epidermis, when the level of noggin is high, the epithelia gradually start to form multiple barb ridges (blue cells). The role and localization of the upstream Sonic Hedgehog (Shh) is also indicated. (D) Expression of BMP-2 and BMP-4 is regulated by Shh signaling. Bone morphogenetic proteins (BMPs) mediate a lateral inhibitory mechanism that leads to arrangement of feather buds in a periodic pattern. BMP signaling in the feather bud cells (brown) regulates the expression of FGFR2. Moreover, BMP induces the expression of transcriptional regulators Msx1 and Twist2 that drive feather bud proliferation. Follistatin and gremlin, secreted by feather interbud dermal cells moderate the autocrine action of BMP signaling via a feedback mechanism. TSC-22 expression is repressed by BMP signaling and induced by FGF signaling, and is required for feather cell differentiation. Marginal plate cells that express Shh and BMP-2 undergo apoptosis, and Shh-negative barb ridge cells continue proliferating. (E,F) Barb ridge cells express BMP-2 and BMP-4 and form the more differentiated structures known as barbules. Barbule plate cells form cilia and hooklets. Noggin activity toward the end of feather formation is reduced and leads to formation of the calamus without branches at the proximal end of the feather shaft. The site with higher BMP activity forms the bilateral symmetric feather. (The image has been inspired by the article of Yu et al. 2002.)
Opposing the activities of TGF-β2, BMPs induce responses that reflect antagonism to TGF-βs; BMP-2 and BMP-4 suppress feather primordium growth and differentiation in similar explant assays as those that were used to show induction of feather bud differentiation (Jung et al. 1998). Shh signaling induces the expression of BMP-2 and BMP-4, and BMP signaling suppresses Shh expression in a feedback mechanism (Fig. 2) (Harris et al. 2005). Additionally, microRNAs (miRNAs) control both Shh and BMP-2 expression in the developing feather bud (Zhang et al. 2013). Besides explant assays using TGF-β- or BMP-impregnated beads, retroviral delivery of BMP-2, BMP-4, or noggin to developing feather bud ectoderm confirmed the role of BMPs as suppressors of feather differentiation (Noramly and Morgan 1998). Feather buds are arranged in a periodic pattern along the central morphogenetic stalk that will develop into the rachis of the feather with evenly spaced barbs (Fig. 2) (Noramly and Morgan 1998; Yu et al. 2002). BMPs exert a lateral inhibitory mechanism that leads to segmental patterning of the feather tract, guaranteeing that feather buds do not grow in a dense and disorganized pattern, and are surrounded by space for future growth of differentiating tissue (Noramly and Morgan 1998; Yu et al. 2002).
In the feather bud cells, BMPs signal through BMPRII, BMPRIA/ALK-3, and BMPRIB/ALK-6 (Ashique et al. 2002), and regulate the expression of the FGF receptor FGFR2, which is expressed in cells aligned along the area where the epithelium contacts the mesenchyme. Conversely, FGF signaling regulates the expression and secretion of BMPs along the patterned bud (Jung et al. 1998; Noramly and Morgan 1998). In addition to FGFR2 signaling, BMP signaling induces expression of transcription factors, such as Msx1 and Twist2 (also known as cDermo-1 in chicken), that drive proliferation of the whole feather bud (Scaal et al. 2002). Although BMP inhibits bud outgrowth in the neighboring space, the proliferation and differentiation of the primordial bud cells that secrete these BMPs is not affected at all. This lack of growth inhibition is maintained by bud cells, which locally secrete follistatin, thus protecting them from autocrine BMP actions (Patel et al. 1999). In fact, follistatin and another BMP inhibitor, gremlin, are secreted by interbud dermal cells in response to BMP through a feedback mechanism (Fig. 2) (Ohyama et al. 2001; Bardot et al. 2004). This patterning mechanism generates gradients of spatially organized BMP signaling that is interpreted by the transcription factor named transforming growth factor-β-stimulated clone-22 (TSC-22), whose expression is repressed by BMP signaling and activated by FGF signaling, and which is critical for feather cell differentiation (Dohrmann et al. 2002).
The studies of feather morphogenesis provide beautiful examples of feedback regulation and reciprocal cross talk between epithelial cells and adjacent mesenchyme that leads to the formation of highly patterned appendages.
Nail Morphogenesis
The development of vertebrate nails is intimately coordinated with the development of the extremities of limbs (fingers/toes-claws, etc.), a morphogenetic program that is regulated by BMPs. This has been shown by tissue-specific and developmental stage-specific overexpression of noggin from the Msx2 promoter, which targets expression to the apical ectodermal ridge that governs limb mesenchyme growth and patterning (Wang et al. 2004). As a result, endogenous BMPs acting during limb bud differentiation are inactivated and the embryo develops digital fusions or polydactyly, whereas the ventral footpads grow many nails (Wang et al. 2004). The apoptotic mechanisms that pattern the growing digits of these animals in response to BMP are inactivated because of the presence of noggin, and thus mesodermal differentiation is prolonged and unrestricted, causing these abnormalities (Wang et al. 2004).
Two key transcription factors drive mesenchymal stem cell differentiation into nail cells—Msx2 and FoxN1. Primordial epithelial cells secrete BMPs, which signal via their receptors on the adjacent mesenchymal stem cells and induce expression of Msx2 and FoxN1, promoting mesenchymal differentiation (Cai and Ma 2011). Although Msx2 promotes terminal nail cell differentiation, FoxN1 acts in a pathway that shares mechanistic aspects with hair follicle differentiation (Cai and Ma 2011). Accordingly, inactivation of Msx2 and Foxn1 in the germline causes extensive proliferation of primitive mesenchymal cells that cannot progress to terminal differentiation, and thus nail development is severely defective (Cai and Ma 2011). The common processes that shape differentiation into hairs and nails are also highlighted by studies of the transcription factor FoxI3, whose expression is induced by activin A in epithelial cells (Shirokova et al. 2013), and is required for hair, tooth, and nail development (Jussila et al. 2015; Shirokova et al. 2016).
Embryonic morphogenesis of digits and nails is also recapitulated, at least in part, in young children and in rodents following amputation of the distal digit (Han et al. 2008). Similar, but not identical, to embryonic morphogenesis, amputated digits regenerate based on the proliferative and differentiation activity of a blastema that secretes BMP-4 to pattern chondrogenesis and osteogenesis, so that the amputated digit will form again, albeit only to a partial size relative to the original size (Han et al. 2008). This regenerative mechanism takes place after birth and can last up to sexual maturity, which is the period of physiological growth and development of vertebrate digits (Han et al. 2008).
Palate Differentiation and Fusion
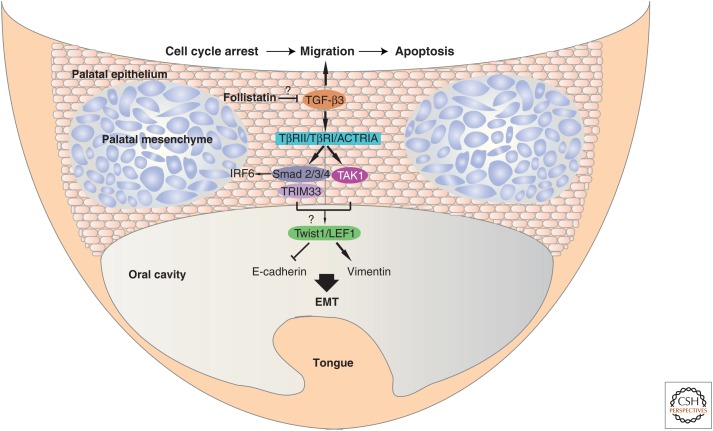
In situ RNA hybridization analysis during palate development and fusion revealed the expression of all three TGF-β isoforms in a cell type-specific manner (Pelton et al. 1990). These early studies suggested that TGF-β3 may mediate the transdifferentiation of palate epithelial cells to mesenchymal tissue, whereas TGF-β1 might regulate mesenchymal cell proliferation and extracellular matrix deposition (Pelton et al. 1990). The critical role of TGF-β3 is shown by the severe defects in palate development and closure observed in the Tgfb3−/− mice causing a cleft in the secondary palate (Fig. 3) (Kaartinen et al. 1995) that is not seen in mice lacking either of the other two TGF-β isoforms. Genetic replacement of TGF-β3 by TGF-β1 expression, by introducing a TGF-β1 cDNA into the deleted Tgfb3 exon, results in incomplete rescue of the cleft palate phenotype (Yang and Kaartinen 2007). TGF-β1 can initiate fusion of the two apposed epithelial sheets, but fails to complete the fusion process (Yang and Kaartinen 2007). Thus, TGF-β1 and TGF-β3 play roles in palatal differentiation, but TGF-β3 may preferentially induce the local apoptosis that is required for palate fusion at the seam, where the two sides of the tissue normally fuse (Murillo et al. 2009). Accordingly, treatment of primary epithelial cells from the palatal medial edge seam with TGF-β3 induces a program that starts with cell cycle arrest, followed by cell migration and local apoptosis, suggesting an in vitro reconstruction of the palatal fusion process (Ahmed et al. 2007). Such ex vivo effects of TGF-β3 would be expected to be recapitulated by treatment with exogenous TGF-β1 or TGF-β2, based on the redundant signaling pathways induced by these three isoforms, which remains to be studied. Furthermore, compound transgenic mice show that TGF-β3 signals via TβRI in the palate (Fig. 3), because an activated TβRI receptor can rescue the cleft palate of Tgfb3−/− mice, and targeted inactivation of TβRI expression in the palatal epithelium prevents completion of palatal differentiation and fusion (Dudas et al. 2004). Palatal fusion requires the coordinate signaling by Smads and TGF-β-activated kinase 1 (TAK1) downstream from TGF-β3 in vivo (Lane et al. 2015). Silencing Smad4 or Map3k7 (encoding TAK1) expression individually, leads to weak palatal phenotypes; combined inactivation of Smad4 and Map3k7 expression leads to the severe palatal phenotype seen in Tgfb3−/− mice (Lane et al. 2015). The chromatin regulator and ubiquitin ligase tripartite motif containing 33 (TRIM33, also known as TIF1γ or ectodermin) forms complexes with Smad2 and Smad3 and regulates expression of specific genes. Maximal cleft palate defects are scored when Trimm33 expression is silenced in combination with inactivation of Smad4 and Map3k7 expression (Lane et al. 2015), attesting to the physiological cooperation between three signaling branches, Smad2/3–TRIM33, Smad2/3–Smad4, and TAK1 downstream from TGF-β3 (Fig. 3).
Figure 3.
Molecular control of palatal development. TGF-β3 plays a crucial role in palatal development and closure. TGF-β3 signals through TβRII and TβRI receptors to stimulate the formation of Smad2–Smad3–Smad4 complexes. The ubiquitin ligase TRIM33 also participates in complexes with Smad2 and/or Smad3; cooperative actions between Smad, TRIM33, and TAK1 signaling may activate expression of the epithelial–mesenchymal transition (EMT) transcription factors Twist1 and LEF1 (note the “question mark,” as the latter mechanism is not firmly proven), which directly activate the expression of mesenchymal genes, such as the gene encoding vimentin, and suppress the expression of epithelial genes including the Cdh1 gene encoding E-cadherin during the palatal EMT. Smad complexes may also interact with IRF6 to regulate additional target genes during EMT. The possible involvement of follistatin, which may (note the “question mark”) indirectly limit TGF-β3 activity, and ActRI/ALK-2 as an alternative type I receptor, are also indicated to reflect the discussion in the main text. The sequence of events induced by TGF-β is epithelial cell cycle arrest, followed by cell migration and eventual apoptosis at the seam (top of the figure). (The image has been inspired by Bush and Jiang 2012.)
The type I receptor ActRI/ALK-2 has also been proposed to mediate TGF-β3 signals in the palate epithelium, possibly acting in a less potent manner (Fig. 3) (Noda et al. 2015). However, ActRI acts during palatal fusion as a BMP signaling receptor, which activates BMP-specific Smad proteins (Noda et al. 2015). An activated mutant of ActRI induces BMP Smad signaling and causes cleft palate because the epithelial layer in the medial edge fails to undergo apoptosis and has increased proliferation, and the mesenchymal and muscle layers fail to fuse (Noda et al. 2015). In a related but seemingly opposite scenario, mice with a gain-of-function mutation in the BMPRIA receptor show a variety of defects, mostly associated with craniofacial development, because of malformations in the neural crest (Hayano et al. 2015). A number of these mice also show defective closure of their secondary palate, suggesting that enhanced BMP–Smad signaling causes epithelial cell apoptosis (Hayano et al. 2015). Such palatal apoptosis depends on p53 activity, and those constitutively active Bmpr1a transgenic mice that show defective closure of their secondary palate can form relatively normal palatal fusion to the nasal septum when pharmacologically treated with pifithrin-α, which inhibits p53 activity. In contrast, only weak and partial rescue of the craniofacial defects of these constitutively active Bmpr1a transgenic mice is achieved following pifithrin-α treatment (Hayano et al. 2015). Thus, limited BMP signaling activity may be associated with normal completion of the palatal fusion to the overlaying nasal septum, whereas TGF-β promotes the overall process of secondary palate formation.
In addition, conditional inactivation of TβRII expression in the palatal epithelium also gives rise to cleft palate, with a concomitant reduction in muscle mass, and inactivation of Wnt signaling, through increased expression of the Wnt antagonists Dkk1 and Dkk4 that normally support palatal muscle growth (Iwata et al. 2014). A cleft palate phenotype has been observed in the mouse following inactivation of the Irf6 gene, which encodes the interferon regulatory factor 6 (IRF6) transcription factor (Iwata et al. 2013). This cleft palate phenotype can be rescued by concomitant deletion of the Smad4 gene, suggesting that Smads cooperate with IRF6 in the regulation of target genes (Iwata et al. 2013).
TGF-β3 induces expression of Twist1 in palatal epithelial cells (Fig. 3) during a time window before the fusion of the epithelial sheets (Yu et al. 2008). However, Twist1 expression alone is not sufficient to drive palatal fusion, as shown using siRNA-mediated silencing (Yu et al. 2008). In parallel, Smad-induced association of the transcription factor LEF1 to the promoter of the Cdh1 gene, which encodes E-cadherin, may enforce repression of E-cadherin expression and promote the onset of palatal EMT (Fig. 3) (Nawshad et al. 2007). The Smad–LEF1 complex may also directly regulate the expression of mesenchymal genes during palatal EMT, such as the gene encoding vimentin (Nawshad et al. 2007). Finally, uncontrolled EMT in the developing palate may be inhibited by the extracellular antagonist follistatin, which is coexpressed in the palatal epithelium with TGF-β3 (Nogai et al. 2008). However, the proposed inactivation of TGF-β3 by follistatin during palate development, based on a direct physical complex between TGF-β3 and follistatin, requires further investigation in vitro and in developing embryos (Nogai et al. 2008). Thus, EMT is an integral developmental program that mediates palatal fusion.
Tooth Development
Consistent with the inhibition of the activin and BMP functions by follistatin, Fst−/−mice show general growth retardation, and, among other affected organs, perturbed tooth development (Matzuk et al. 1995). Multiple TGF-β family members control tooth cell differentiation. Activin βA is required for normal tooth development, and several but not all teeth of Inhba−/− mice fail to progress into terminal development and remain arrested as undifferentiated primordial buds (Ferguson et al. 1998). The tooth phenotype of Inhba−/− mice is rescued by activin A released from beads that are implanted into the developing mandibles (Ferguson et al. 1998).
Conditional overexpression of the inhibitory Smad7 in stratified epithelia of mice also results in defective tooth development, in addition to other skin-related phenotypes (Klopcic et al. 2007). This study shows that TGF-β family signaling controls the terminal differentiation of ameloblasts and deposition of enamel layers in developing teeth (Klopcic et al. 2007). Additionally, conditional inactivation of the Tgfbr2 gene, encoding the TβRII receptor, in cells derived from the cranial neural crest prevents odontoblast differentiation and results in loss of dentin deposition that supports terminal tooth morphogenesis (Oka et al. 2007). Maturation of odontoblasts can be rescued by addition of TGF-β2 to tooth explants, confirming that TGF-β signaling controls terminal tooth development (Oka et al. 2007).
In this differentiation program, BMPs stored within the enamel matrix promote signaling in cells of the dental follicle (Kémoun et al. 2007). Dental follicle cells, cultured in the presence of exogenous BMP-2 and BMP-7, induce the expression of proteins that define cementoblast differentiation (Kémoun et al. 2007). Conversely, addition of noggin represses this induction, depletes the BMPs in the microenvironment, and blocks cementoblast protein expression (Kémoun et al. 2007). Additionally, transgenic mice expressing noggin in their dental epithelial cells show a block in early stage tooth development (Wang et al. 2012). BMP signaling activates the expression of the transcription factor Pitx2, which contributes to differentiation of dental epithelial cells in the growing dental bud (Wang et al. 2012). Pitx2 activates expression of the miR-200c microRNA, which, among other targets, represses noggin expression (Cao et al. 2013). Thus, silencing miR-200c expression results in defective terminal tooth differentiation that correlates with enhanced noggin levels in the developing dental epithelium (Cao et al. 2013). However, noggin interacts with and inhibits not only BMPs but also extracellular Wnts, thus blocking Wnt signaling in the dental epithelium (Yuan et al. 2015). Although interaction of noggin with several BMPs has been studied down to the level of crystallographic detail, the interaction between noggin and Wnts has only been analyzed using antibody-based techniques, such as proximity ligation assays and colocalization studies using immunohistochemistry (Yuan et al. 2015); thus, more rigorous biochemical analysis is awaited to further establish the noggin–Wnt interactions. Therefore, the phenotype resulting from transgenic noggin expression supports the interdependence of Wnt and BMP signaling, both controlling the expression of the Pitx2 gene, which is essential for odontogenesis (Yuan et al. 2015).
Further support on the role of BMP signaling in promoting tooth development stems from studies of conditional inactivation of the Bmpr1a gene (Yang et al. 2013). Doxycycline-inducible expression of the Cre recombinase from a hybrid Krt5-rtTA promoter and doxycycline treatment of embryos one day before the onset of dental epithelial differentiation, results in repression of BMP signaling from dental epithelial cells, including the ameloblasts, and causes a switch in differentiation (Yang et al. 2013). Thus, epithelial cells that normally generate the crown of the tooth give rise to cells of the root of the tooth, resulting in abnormal and excessive root growth (Yang et al. 2013). At this stage of tooth development, BMP signaling normally represses paracrine Wnt signaling, which is hyperactivated in Bmpr1a−/− odontoblasts (Yang et al. 2013). Furthermore, conditional Smad4 inactivation in odontoblasts blocks cell differentiation and extracellular matrix deposition, leading to rudimentary tooth development (Gao et al. 2009). Loss of Smad4 also results in reduced paracrine signaling by BMPs that are secreted by odontoblasts and act on neighboring epithelial cells of the developing tooth root. This perturbed signaling circuit results in keratocystic odontogenic tumors that may result from insufficient suppression of the proliferation of these epithelial cells caused by reduced BMP signaling (Gao et al. 2009). Following its conditional inactivation at distinct stages of odontogenesis, Smad4 loss does not affect early differentiation but disrupts the late stages of odontoblast differentiation (Kim et al. 2015). Thus, BMP signaling with Smad4 as coactivator may instruct odontoblasts to express collagens and deposit the secondary dentin layer that completes odontogenesis (Kim et al. 2015).
GDF-5, also known as cartilage-derived morphogenetic protein-1 or BMP-14, is postnatally expressed in cells of the periodontal ligament, especially after initiation of enamel synthesis (Liu et al. 2016). In this context, GDF-5 may act upstream of BMP-2, which then promotes ameloblast differentiation (Liu et al. 2016), although the exact role of GDF-5 as an upstream “weak” activator of BMP-2 activity remains to be clarified. The importance of BMP signaling in tooth development is also underscored by tooth phenotype resulting from genetic mutations in the human GREM2 gene, which encodes “protein related to DAN and cerberus” (PRDC), and the corresponding phenotype of Grem2−/− mice with characteristic dental defects, including small and malformed incisors (Kantaputra et al. 2015). Multiple human tooth defects have been associated with mutations in the GREM2 gene and can be discerned from contributions by other developmental regulators such as Wnt family members (Kantaputra et al. 2015). The autosomal-dominant character of these GREM2 mutations also underscores the importance of dose-dependence in the regulation of BMP signaling during dental development (Kantaputra et al. 2015).
Downstream from BMP signaling, the Shh pathway contributes to the generation of dental epithelial stem cells that slowly self-renew and contribute to the continuous growth of the mouse incisors (Li et al. 2015b). Shh signaling via transcriptional elevation of Gli levels promotes the expression of Sox2, a transcription factor that defines stemness in many cell lineages (Li et al. 2015b). BMP-induced Smad signaling suppresses expression of Shh, thus limiting the self-renewing potential of the dental stem cells (Li et al. 2015b). Accordingly, cell type-specific ablation of Smad4 promotes Shh signaling through Gli proteins, and enhances the stem cell population, causing continuous growth of incisors, and aberrant growth of molar teeth, whose growth is normally suppressed by BMP signaling (Li et al. 2015b).
The contribution of Wnt signaling in controlling tooth development, which often results in effects that are opposite to those of BMPs, has been analyzed at the genome-wide level and through mathematical modeling (O’Connell et al. 2012). This study defines reciprocal Wnt and BMP pathway cross talk that regulates large numbers of genes in the two juxtaposed tissues, the dental epithelium and the adjacent mesenchyme, during progression of tooth development (O’Connell et al. 2012).
TGF-β1-induced Smad signaling has been analyzed in odontoblast culture differentiation experiments (Lee et al. 2011). TGF-β activates, and recruits, ubiquitin ligases to the transcriptional regulator, nuclear factor I-C (NFI-C), whose activity is important for differentiation of the dental root (Lee et al. 2011). Thus, TGF-β1 signaling represses the expression of NFI-C, which in turn represses TGF-β signaling by reducing the phosphorylated Smad2 and Smad3 levels, through an undefined mechanism (Lee et al. 2011). This may generate a double-negative regulatory network that is important for odontobast differentiation.
Teeth grow continuously during adult mouse life, a process promoted by adult dental stem cells and cytokines. TGF-β signaling through TβRI regulates the expression and signaling by FGF family members, which are required for self-renewal of the dental stem cells (Zhao et al. 2011). Conditional Tgfbr1 inactivation in the dental mesenchyme, which is derived from the embryonic neural crest, drastically reduces the expression of FGFs and the population of dental stem cells, whereas addition of FGF10 to explanted developing teeth rescues the defect caused by TβRI loss (Zhao et al. 2011).
GDF-11/BMP-11, a distant member of the TGF-β family, has been studied for its role in craniofacial development (Lee and Lee 2015). The binding of GDF-11 to its receptors is inhibited by the extracellular GDF-associated serum protein 1 (GASP1) (Lee and Lee 2013). Tooth buds start expressing GASP1 before embryonic age of E17.5, and mice with loss-of-function mutations in both the Gdf11 and Gasp1 genes show enhanced cleft palate and abnormal incisors in the lower mandible (Lee and Lee 2015). The incisor defect is due to lack of proliferation and differentiation in the tooth buds for these specific incisors only. Loss of Gdf11 only does not result in this phenotype, suggesting that individual tooth buds selectively control their development by the coordinate activity of GASP1 and GDF-11 (Lee and Lee 2015).
THE TGF-β FAMILY IN EPITHELIAL DIFFERENTIATION AND EMT IN THE EYE
TGF-β family members also regulate epithelial differentiation in eye development. This is illustrated by their activities in lens and retinal epithelial cell differentiation.
Lens Differentiation
Although BMPs control embryonic lens differentiation, the TGF-βs regulate late lens development and have been linked to pathogenetic processes in the lens (de Iongh et al. 2001; Saika et al. 2001). Genetic ablation of the individual TGF-β genes in the mouse corneal epithelium showed that Tgfb2 inactivation, but not inactivation of Tgfb1 or Tgfb3, results in abnormal differentiation and defective fusion of the cornea to the neighboring lens (Saika et al. 2001). TGF-β2 regulates extracellular matrix deposition in the developing stroma of the corneal epithelium, and excessive macrophage recruitment to the Tgfb2−/− corneal epithelium suggests that the mutant phenotype results from abnormal macrophage function (Saika et al. 2001). Furthermore, overexpression of dominant-negative mutants of TβRI or TβRII in the lens epithelium results in a strong block in lens epithelial differentiation, which causes extensive cell death and leads to cataract formation (de Iongh et al. 2001). During lens differentiation, the dominant-negative TGF-β receptors disrupt the expression and structural organization of critical filamentous proteins in the mature lens fibers, because of lack of architectural coordination between the actin cytoskeleton and the assembly of fibrous proteins (de Iongh et al. 2001). On the other hand, lens-specific Tgfbr2 ablation results in normal lens development (Beebe et al. 2004), suggesting that TGF-β family members other than the TGF-β isoforms (e.g., activins) might control the differentiation program that generates the lens fibers. Perhaps, dominant-negative TGF-β receptor expression might interfere with signaling components downstream from such alternative ligands, whose identity remains to be firmly established.
The BMPs play instructive roles in the differentiation of the lens epithelium. In developing chick eyes, BMP-induced Smad1 activation is seen during elongation and differentiation of the lens fiber cells (Belecky-Adams et al. 2002). Overexpression of noggin in the chick retina perturbs lens cell differentiation and induces apoptosis, causing tissue degradation (Belecky-Adams et al. 2002). The impaired differentiation of the lens epithelium can be rescued ex vivo by BMP-2, BMP-4 or BMP-7, which overcome the defect caused by noggin (Belecky-Adams et al. 2002). Supporting the role of BMPs during lens epithelial differentiation, mouse lens epithelium-specific Bmpr1a inactivation results in small lenses with thin cell layers (Beebe et al. 2004). A similar mouse lens-specific Actr1 inactivation also results in small lenses, because of enhanced apoptosis (Rajagopal et al. 2008). In this context, ActRI/ALK-2 activation promotes cell proliferation at early stages of lens development, and cell cycle arrest during late stages of lens development, which is required for lens fiber differentiation (Rajagopal et al. 2008).
The molecular programs regulated by BMP signaling in lens epithelial cells are not well characterized; however, in chick lens explant cultures, BMP signaling cooperates with FGF signaling to induce the expression of CCDC80/equarin, a cell-cycle and differentiation regulator (Jarrin et al. 2012). BMP signaling also regulates the expression of FGF receptors so that lens epithelial cells gain responsiveness to FGFs (Boswell and Musil 2015). Subsequently, FGF receptor signaling induces BMP4 expression, thus forming a positive feedback loop, which provides sustained BMP responses during lens formation (Boswell and Musil 2015).
Similar and conserved mechanisms may operate in mouse and chick lens development. Accordingly, inactivation of the expression of BMPRIA or ActRI in ectodermal tissue that generates the lens progenitor cells prevents lens placode formation and subsequent lens cell invagination, highlighting the requirement of these two BMP receptors (Huang et al. 2015). The embryonic ectodermal tissue attempts to compensate the loss of receptor signaling by secreting high amounts of BMPs, such as BMP-7, leading to perturbed BMP morphogenetic gradients, aberrant regulation of target genes, especially those of the apoptotic program, and abnormalities in the fission of the optic cavity that surrounds the lens (Huang et al. 2015). The physiological requirement of BMP signaling during lens development is also supported by studies of myopic eye development in guinea pigs, caused by lens injury (Li et al. 2015a). During progression of myopia, BMP-2 expression and phospho-Smad1/5 levels decrease (Li et al. 2015a). The requirement of BMP-2 signaling is thought to depend on the regulation of extracellular matrix biosynthesis (e.g., of collagens or glycosaminoglycans) and the induction of chondrogenic genes (Li et al. 2015a). Thus, reduced BMP signaling and low chondrogenic gene expression prevent terminal differentiation during lens injury progression and cause myopia.
Although low level transgenic expression of TGF-β1 in the mouse lens and cornea has no apparent phenotype, high level TGF-β1 expression results in the development of small eyes with enhanced mesenchymal cell numbers, excessive extracellular matrix deposition, and dramatic apoptosis of lens epithelial cells, suggesting a role of TGF-β1 in promoting fibrotic EMT (Flügel-Koch et al. 2002). Studies of lens epithelial cells in culture highlight a role of Smad3 as mediator of the TGF-β-induced EMT response in tissue injury and fibrosis (Saika et al. 2004). In addition to Smad3, the phosphatidylinositol 3′ kinase and Erk MAPK pathways contribute to the regulation of target genes, such as those encoding Snail, Slug or α-smooth muscle actin, by TGF-β during lens EMT (Cho et al. 2007; Choi et al. 2007; Chen et al. 2014). BMP-7 signaling antagonizes TGF-β-induced EMT in in lens epithelial cell cultures, and protects epithelial differentiation by inducing the expression of the transcription factors Id2 and Id3 (Kowanetz et al. 2004). Adenoviral BMP-7 delivery directly to the adult mouse lens induces Id2 and Id3 expression in the lens epithelium, whereas adenoviral Id2 or Id3 expression promotes endogenous BMP-7 expression (Saika et al. 2006). These three interventions protect the lens epithelium from injury-mediated EMT and resulting fibrosis.
Retinal Epithelium Formation
Early studies using whole rat embryo cultures at several developmental stages and treatment of the embryos with antibodies against BMP-7 gave evidence for an overt defect in eye development (Solursh et al. 1996). BMP-7 is expressed in the rat retinal epithelium, lens, and cornea, as shown by in situ hybridization and immunohistochemistry (Solursh et al. 1996).
Human embryonic stem cells have been differentiated into retinal epithelial cells using a mixture of growth factors, including TGF-β family proteins, showing their need in retinal epithelial differentiation (Idelson et al. 2009). TGF-β can induce EMT in retinal epithelial cells at least in part via transcriptional induction of Snail1 (Li et al. 2011). Smad signaling induces expression of the guanine exchange factor, GEF-H1 that activates the small GTPase RhoA and promotes actin cytoskeletal remodeling that couples retinal EMT to cell migration (Tsapara et al. 2010). Furthermore, retinal epithelial cells undergo cell cycle arrest in response to TGF-β; however, the concomitant transcriptional induction of survivin expression by TGF-β overcomes cytostasis and promotes cell survival during EMT (Lee et al. 2013).
The retinal epithelium receives important cues for its differentiation from the juxtaposed lens (Heermann et al. 2015). The optic cup develops around the differentiating lens, and this involves cell proliferation in three dimensions that gradually decreases (Heermann et al. 2015). In zebrafish eyes, epithelial cells migrate from the apices of the lens and form streams of tissue remodeling along the rims of the optic cup (Heermann et al. 2015). BMP signaling is essential for the movement of these epithelial cells, which comprise retinal stem cells, and inhibition of BMP signaling by antagonists, such as follistatin and the decoy receptor, BMP and activin membrane-bound inhibitor (BAMBI), disrupts their morphogenetic fate (Heermann et al. 2015). Completion of this differentiation program in the developing eye, which ensures physiological fission between the lens and the optic cup cells, therefore relies on controlled BMP activity (Heermann et al. 2015). Chick eye studies have clarified that, at early stages of eye development, lens-derived BMP activity is required for the specification of the retinal epithelial layer and the generation of the neural retina that contacts the optic nerve cells in the brain (Pandit et al. 2015). Lens-derived BMPs signal in cross talk with Wnts, and specify retinal epithelial cell differentiation (Pandit et al. 2015). In addition, this signaling cross talk limits the expansion of telencephalic neuronal cells, a step required for the development of the neural retina and its contacts with eye and brain cells (Pandit et al. 2015).
Thus, the control of epithelial cell differentiation in the eye by TGF-β family members is best documented in the case of the lens and cornea. Retinal epithelial cells also receive signals from these growth factors in vivo, and EMT in retinal tissue, which has been studied in culture, has been linked to pathological changes.
CONCLUSIONS AND PERSPECTIVES
This review illustrates roles of TGF-β family members in an array of, but not all, epithelial tissues. Unfortunately, it is no longer possible to comprehensively review the overwhelming literature on the topic. Mouse models with cell-specific gene inactivation or overexpression have revealed the sequential action of multiple TGF-β family members and their cross talk with other pathways, primarily Wnt, FGF, Hh, or MAPK signaling modules. Many details on signal transduction mechanisms are derived from cell culture studies. However, their translation into the in vivo situation increases steadily and presents a firm requirement for future research. In addition, processes characterized using small rodent models do not always represent human physiology, especially in relation to adult tissue homeostasis and pathogenesis. Thus, it is important to further define the TGF-β family biology in a human context, possibly with high affinity reagents that allow sensitive detection of proteins and their complexes in tissues and biological fluids.
We also touched on the process of EMT, without covering its importance in cancer progression or fibrosis. It is evident that EMT contributes to organ development, and it is even clearer that TGF-β family members are potent regulators of various transdifferentiations during organogenesis. Distinct from the EMTs, but equally important, are the abundant epithelial–mesenchymal interactions that specify the proliferation and differentiation of new cell types. This area of biology will continue to shed light on fundamental processes that depend on the plastic interaction between neighboring cell types. The time approaches when the developmental processes that generate new epithelia, and the mechanisms by which mesenchyme specifies epithelial differentiation, will be completed. Not all TGF-β family members have yet been studied for their roles in epithelial biology. Future studies are awaited to enlighten this exciting front in developmental and cell biology.
ACKNOWLEDGMENTS
We thank all past and present members of the TGF-β signaling and STEP groups for their contributions to the scientific work emanating from our laboratory. We thank Carl-Henrik Heldin for his support, guidance, and a thorough reading of the manuscript during preparation. This review summarizes work from a large number of published papers; however, because of space limitations we have been unable to include all relevant publications in our discussion and thus cite other review articles. We apologize to those authors whose relevant work has not been included in this review. We acknowledge funding by the Ludwig Institute for Cancer Research, the Swedish Cancer Society, the Swedish Research Council, and the Marie Curie Initial Training Network “IT-Liver” under the European Union FP7 program.
Footnotes
Editors: Rik Derynck and Kohei Miyazono
Additional Perspectives on The Biology of the TGF-β Family available at www.cshperspectives.org
REFERENCES
*Reference is also in this subject collection.
- Acloque H, Adams MS, Fishwick K, Bronner-Fraser M, Nieto MA. 2009. Epithelial–mesenchymal transitions: The importance of changing cell state in development and disease. J Clin Invest 119: 1438–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed S, Liu CC, Nawshad A. 2007. Mechanisms of palatal epithelial seam disintegration by transforming growth factor (TGF) β3. Dev Biol 309: 193–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amendt C, Schirmacher P, Weber H, Blessing M. 1998. Expression of a dominant negative type II TGF-β receptor in mouse skin results in an increase in carcinoma incidence and an acceleration of carcinoma development. Oncogene 17: 25–34. [DOI] [PubMed] [Google Scholar]
- Andersson O, Bertolino P, Ibanez CF. 2007. Distinct and cooperative roles of mammalian Vg1 homologs GDF1 and GDF3 during early embryonic development. Dev Biol 311: 500–511. [DOI] [PubMed] [Google Scholar]
- Andl T, Reddy ST, Gaddapara T, Millar SE. 2002. WNT signals are required for the initiation of hair follicle development. Dev Cell 2: 643–653. [DOI] [PubMed] [Google Scholar]
- Andl T, Ahn K, Kairo A, Chu EY, Wine-Lee L, Reddy ST, Croft NJ, Cebra-Thomas JA, Metzger D, Chambon P, et al. 2004. Epithelial Bmpr1a regulates differentiation and proliferation in postnatal hair follicles and is essential for tooth development. Development 131: 2257–2268. [DOI] [PubMed] [Google Scholar]
- Arnold SJ, Hofmann UK, Bikoff EK, Robertson EJ. 2008. Pivotal roles for eomesodermin during axis formation, epithelium-to-mesenchyme transition and endoderm specification in the mouse. Development 135: 501–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashique AM, Fu K, Richman JM. 2002. Signalling via type IA and type IB bone morphogenetic protein receptors (BMPR) regulates intramembranous bone formation, chondrogenesis and feather formation in the chicken embryo. Int J Dev Biol 46: 243–253. [PubMed] [Google Scholar]
- Bamberger C, Scharer A, Antsiferova M, Tychsen B, Pankow S, Muller M, Rulicke T, Paus R, Werner S. 2005. Activin controls skin morphogenesis and wound repair predominantly via stromal cells and in a concentration-dependent manner via keratinocytes. Am J Pathol 167: 733–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardot B, Lecoin L, Fliniaux I, Huillard E, Marx M, Viallet JP. 2004. Drm/Gremlin, a BMP antagonist, defines the interbud region during feather development. Int J Dev Biol 48: 149–156. [PubMed] [Google Scholar]
- Beebe D, Garcia C, Wang X, Rajagopal R, Feldmeier M, Kim JY, Chytil A, Moses H, Ashery-Padan R, Rauchman M. 2004. Contributions by members of the TGFβ superfamily to lens development. Int J Dev Biol 48: 845–856. [DOI] [PubMed] [Google Scholar]
- Belecky-Adams TL, Adler R, Beebe DC. 2002. Bone morphogenetic protein signaling and the initiation of lens fiber cell differentiation. Development 129: 3795–3802. [DOI] [PubMed] [Google Scholar]
- Bertocchini F, Skromne I, Wolpert L, Stern CD. 2004. Determination of embryonic polarity in a regulative system: Evidence for endogenous inhibitors acting sequentially during primitive streak formation in the chick embryo. Development 131: 3381–3390. [DOI] [PubMed] [Google Scholar]
- Bier E, De Robertis EM. 2015. BMP gradients: A paradigm for morphogen-mediated developmental patterning. Science 348: aaa5838. [DOI] [PubMed] [Google Scholar]
- Bitgood MJ, McMahon AP. 1995. Hedgehog and Bmp genes are coexpressed at many diverse sites of cell–cell interaction in the mouse embryo. Dev Biol 172: 126–138. [DOI] [PubMed] [Google Scholar]
- Blanpain C, Fuchs E. 2006. Epidermal stem cells of the skin. Annu Rev Cell Dev Biol 22: 339–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanpain C , Fuchs E. 2014. Plasticity of epithelial stem cells in tissue regeneration. Science 344: 1242281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanpain C, Lowry WE, Geoghegan A, Polak L, Fuchs E. 2004. Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell 118: 635–648. [DOI] [PubMed] [Google Scholar]
- Blessing M, Nanney LB, King LE, Jones CM, Hogan BL. 1993. Transgenic mice as a model to study the role of TGF-β-related molecules in hair follicles. Genes Dev 7: 204–215. [DOI] [PubMed] [Google Scholar]
- Blessing M, Schirmacher P, Kaiser S. 1996. Overexpression of bone morphogenetic protein-6 (BMP-6) in the epidermis of transgenic mice: Inhibition or stimulation of proliferation depending on the pattern of transgene expression and formation of psoriatic lesions. J Cell Biol 135: 227–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boswell BA, Musil LS. 2015. Synergistic interaction between the fibroblast growth factor and bone morphogenetic protein signaling pathways in lens cells. Mol Biol Cell 26: 2561–2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botchkarev VA, Botchkareva NV, Roth W, Nakamura M, Chen LH, Herzog W, Lindner G, McMahon JA, Peters C, Lauster R, et al. 1999. Noggin is a mesenchymally derived stimulator of hair-follicle induction. Nat Cell Biol 1: 158–164. [DOI] [PubMed] [Google Scholar]
- Botchkarev VA, Botchkareva NV, Nakamura M, Huber O, Funa K, Lauster R, Paus R, Gilchrest BA. 2001. Noggin is required for induction of the hair follicle growth phase in postnatal skin. FASEB J 15: 2205–2214. [DOI] [PubMed] [Google Scholar]
- Botchkarev VA, Botchkareva NV, Sharov AA, Funa K, Huber O, Gilchrest BA. 2002. Modulation of BMP signaling by noggin is required for induction of the secondary (nontylotrich) hair follicles. J Invest Dermatol 118: 3–10. [DOI] [PubMed] [Google Scholar]
- Bronner ME. 2012. Formation and migration of neural crest cells in the vertebrate embryo. Histochem Cell Biol 138: 179–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronner-Fraser M, Fraser SE. 1988. Cell lineage analysis reveals multipotency of some avian neural crest cells. Nature 335: 161–164. [DOI] [PubMed] [Google Scholar]
- Burstyn-Cohen T, Stanleigh J, Sela-Donenfeld D, Kalcheim C. 2004. Canonical Wnt activity regulates trunk neural crest delamination linking BMP/noggin signaling with G1/S transition. Development 131: 5327–5339. [DOI] [PubMed] [Google Scholar]
- Bush JO, Jiang R. 2012. Palatogenesis: Morphogenetic and molecular mechanisms of secondary palate development. Development 139: 231–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai J, Ma L. 2011. Msx2 and Foxn1 regulate nail homeostasis. Genesis 49: 449–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan CA, Oro AE. 2001. Monstrous attempts at adnexogenesis: Regulating hair follicle progenitors through Sonic Hedgehog signaling. Curr Opin Genet Dev 11: 541–546. [DOI] [PubMed] [Google Scholar]
- Cano A, Perez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, Portillo F, Nieto MA. 2000. The transcription factor snail controls epithelial–mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol 2: 76–83. [DOI] [PubMed] [Google Scholar]
- Cao H, Jheon A, Li X, Sun Z, Wang J, Florez S, Zhang Z, McManus MT, Klein OD, Amendt BA. 2013. The Pitx2:miR-200c/141:noggin pathway regulates Bmp signaling and ameloblast differentiation. Development 140: 3348–3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmona-Fontaine C, Matthews HK, Kuriyama S, Moreno M, Dunn GA, Parsons M, Stern CD, Mayor R. 2008. Contact inhibition of locomotion in vivo controls neural crest directional migration. Nature 456: 957–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chea HK, Wright CV, Swalla BJ. 2005. Nodal signaling and the evolution of deuterostome gastrulation. Dev Dyn 234: 269–278. [DOI] [PubMed] [Google Scholar]
- Chen X, Ye S, Xiao W, Wang W, Luo L, Liu Y. 2014. ERK1/2 pathway mediates epithelial–mesenchymal transition by cross-interacting with TGFβ/Smad and Jagged/Notch signaling pathways in lens epithelial cells. Int J Mol Med 33: 1664–1670. [DOI] [PubMed] [Google Scholar]
- Cheung M, Chaboissier MC, Mynett A, Hirst E, Schedl A, Briscoe J. 2005. The transcriptional control of trunk neural crest induction, survival, and delamination. Dev Cell 8: 179–192. [DOI] [PubMed] [Google Scholar]
- Chiang C, Swan RZ, Grachtchouk M, Bolinger M, Litingtung Y, Robertson EK, Cooper MK, Gaffield W, Westphal H, Beachy PA, et al. 1999. Essential role for Sonic Hedgehog during hair follicle morphogenesis. Dev Biol 205: 1–9. [DOI] [PubMed] [Google Scholar]
- Cho HJ, Baek KE, Saika S, Jeong MJ, Yoo J. 2007. Snail is required for transforming growth factor-β-induced epithelial–mesenchymal transition by activating PI3 kinase/Akt signal pathway. Biochem Biophys Res Commun 353: 337–343. [DOI] [PubMed] [Google Scholar]
- Choi J, Park SY, Joo CK. 2007. Transforming growth factor-β1 represses E-cadherin production via slug expression in lens epithelial cells. Invest Ophthalmol Vis Sci 48: 2708–2718. [DOI] [PubMed] [Google Scholar]
- Cibois M, Luxardi G, Chevalier B, Thome V, Mercey O, Zaragosi LE, Barbry P, Pasini A, Marcet B, Kodjabachian L. 2015. BMP signalling controls the construction of vertebrate mucociliary epithelia. Development 142: 2352–2363. [DOI] [PubMed] [Google Scholar]
- Clavel C, Grisanti L, Zemla R, Rezza A, Barros R, Sennett R, Mazloom AR, Chung CY, Cai X, Cai CL, et al. 2012. Sox2 in the dermal papilla niche controls hair growth by fine-tuning BMP signaling in differentiating hair shaft progenitors. Dev Cell 23: 981–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clay MR, Halloran MC. 2014. Cadherin 6 promotes neural crest cell detachment via F-actin regulation and influences active Rho distribution during epithelial-to-mesenchymal transition. Development 141: 2506–2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui W, Fowlis DJ, Cousins FM, Duffie E, Bryson S, Balmain A, Akhurst RJ. 1995. Concerted action of TGF-β1 and its type II receptor in control of epidermal homeostasis in transgenic mice. Genes Dev 9: 945–955. [DOI] [PubMed] [Google Scholar]
- Davidson P, Hardy MH. 1952. The development of mouse vibrissae in vivo and in vitro. J Anat 86: 342–356. [PMC free article] [PubMed] [Google Scholar]
- Davis H, Raja E, Miyazono K, Tsubakihara Y, Moustakas A. 2016. Mechanisms of action of bone morphogenetic proteins in cancer. Cytokine Growth Factor Rev 27: 81–92. [DOI] [PubMed] [Google Scholar]
- de Iongh RU, Lovicu FJ, Overbeek PA, Schneider MD, Joya J, Hardeman ED, McAvoy JW. 2001. Requirement for TGFβ receptor signaling during terminal lens fiber differentiation. Development 128: 3995–4010. [DOI] [PubMed] [Google Scholar]
- Delaune E, Lemaire P, Kodjabachian L. 2005. Neural induction in Xenopus requires early FGF signalling in addition to BMP inhibition. Development 132: 299–310. [DOI] [PubMed] [Google Scholar]
- Dohrmann CE, Noramly S, Raftery LA, Morgan BA. 2002. Opposing effects on TSC-22 expression by BMP and receptor tyrosine kinase signals in the developing feather tract. Dev Dyn 223: 85–95. [DOI] [PubMed] [Google Scholar]
- Doma E, Rupp C, Baccarini M. 2013. EGFR-Ras-Raf signaling in epidermal stem cells: Roles in hair follicle development, regeneration, tissue remodeling and epidermal cancers. Int J Mol Sci 14: 19361–19384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dottori M, Gross MK, Labosky P, Goulding M. 2001. The winged-helix transcription factor Foxd3 suppresses interneuron differentiation and promotes neural crest cell fate. Development 128: 4127–4138. [DOI] [PubMed] [Google Scholar]
- Dudas M, Nagy A, Laping NJ, Moustakas A, Kaartinen V. 2004. Tgf-β3-induced palatal fusion is mediated by Alk-5/Smad pathway. Dev Biol 266: 96–108. [DOI] [PubMed] [Google Scholar]
- Ferguson CA, Tucker AS, Christensen L, Lau AL, Matzuk MM, Sharpe PT. 1998. Activin is an essential early mesenchymal signal in tooth development that is required for patterning of the murine dentition. Genes Dev 12: 2636–2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flügel-Koch C, Ohlmann A, Piatigorsky J, Tamm ER. 2002. Disruption of anterior segment development by TGF-β1 overexpression in the eyes of transgenic mice. Dev Dyn 225: 111–125. [DOI] [PubMed] [Google Scholar]
- Foitzik K, Paus R, Doetschman T, Dotto GP. 1999. The TGF-β2 isoform is both a required and sufficient inducer of murine hair follicle morphogenesis. Dev Biol 212: 278–289. [DOI] [PubMed] [Google Scholar]
- Foitzik K, Mueller-Roever S, Maurer M, Botchkareva N, Botchkarev V, Handjiski B, Metz M, Hibino T, Soma T, Dotto GP, et al. 2000. Control of murine hair follicle regression (catagen) by TGF-β1 in vivo. FASEB J 14: 752–760. [DOI] [PubMed] [Google Scholar]
- Fuchs E. 2007. Scratching the surface of skin development. Nature 445: 834–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gal A, Sjöblom T, Fedorova L, Imreh S, Beug H, Moustakas A. 2008. Sustained TGFβ exposure suppresses Smad and non-Smad signalling in mammary epithelial cells, leading to EMT and inhibition of growth arrest and apoptosis. Oncogene 27: 1218–1230. [DOI] [PubMed] [Google Scholar]
- Gao J, DeRouen MC, Chen CH, Nguyen M, Nguyen NT, Ido H, Harada K, Sekiguchi K, Morgan BA, Miner JH, et al. 2008. Laminin-511 is an epithelial message promoting dermal papilla development and function during early hair morphogenesis. Genes Dev 22: 2111–2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Yang G, Weng T, Du J, Wang X, Zhou J, Wang S, Yang X. 2009. Disruption of Smad4 in odontoblasts causes multiple keratocystic odontogenic tumors and tooth malformation in mice. Mol Cell Biol 29: 5941–5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Castro MI, Marcelle C, Bronner-Fraser M. 2002. Ectodermal Wnt function as a neural crest inducer. Science 297: 848–851. [DOI] [PubMed] [Google Scholar]
- Gat U, DasGupta R, Degenstein L, Fuchs E. 1998. De novo hair follicle morphogenesis and hair tumors in mice expressing a truncated β-catenin in skin. Cell 95: 605–614. [DOI] [PubMed] [Google Scholar]
- Genander M, Cook PJ, Ramsköld D, Keyes BE, Mertz AF, Sandberg R, Fuchs E. 2014. BMP signaling and its pSMAD1/5 target genes differentially regulate hair follicle stem cell lineages. Cell Stem Cell 15: 619–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godwin AR, Capecchi MR. 1998. Hoxc13 mutant mice lack external hair. Genes Dev 12: 11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco V, Chen T, Rendl M, Schober M, Pasolli HA, Stokes N, Dela Cruz-Racelis J, Fuchs E. 2009. A two-step mechanism for stem cell activation during hair regeneration. Cell Stem Cell 4: 155–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han M, Yang X, Lee J, Allan CH, Muneoka K. 2008. Development and regeneration of the neonatal digit tip in mice. Dev Biol 315: 125–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy MH. 1992. The secret life of the hair follicle. Trends Genet 8: 55–61. [DOI] [PubMed] [Google Scholar]
- Harris MP, Williamson S, Fallon JF, Meinhardt H, Prum RO. 2005. Molecular evidence for an activator–inhibitor mechanism in development of embryonic feather branching. Proc Natl Acad Sci 102: 11734–11739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Hata A, Chen YG. 2016. TGF-β signaling from receptors to Smads. Cold Spring Harb Perspect Biol 8: a022061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay ED. 1995. An overview of epithelio–mesenchymal transformation. Acta Anat 154: 8–20. [DOI] [PubMed] [Google Scholar]
- Hayano S, Komatsu Y, Pan H, Mishina Y. 2015. Augmented BMP signaling in the neural crest inhibits nasal cartilage morphogenesis by inducing p53-mediated apoptosis. Development 142: 1357–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He W, Cao T, Smith DA, Myers TE, Wang XJ. 2001. Smads mediate signaling of the TGFβ superfamily in normal keratinocytes but are lost during skin chemical carcinogenesis. Oncogene 20: 471–483. [DOI] [PubMed] [Google Scholar]
- He W, Wang D, Han S, Zheng B, ten Dijke P. 2002. Overexpression of Smad7 results in severe pathological alterations in multiple epithelial tissues. EMBO J 21: 2580–2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heermann S, Schutz L, Lemke S, Krieglstein K, Wittbrodt J. 2015. Eye morphogenesis driven by epithelial flow into the optic cup facilitated by modulation of bone morphogenetic protein. eLife 4: e05216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu YC, Pasolli HA, Fuchs E. 2011. Dynamics between stem cells, niche, and progeny in the hair follicle. Cell 144: 92–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu YC, Li L, Fuchs E. 2014. Transit-amplifying cells orchestrate stem cell activity and tissue regeneration. Cell 157: 935–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang RY, Guilford P, Thiery JP. 2012. Early events in cell adhesion and polarity during epithelial–mesenchymal transition. J Cell Sci 125: 4417–4422. [DOI] [PubMed] [Google Scholar]
- Huang J, Liu Y, Filas B, Gunhaga L, Beebe DC. 2015. Negative and positive auto-regulation of BMP expression in early eye development. Dev Biol 407: 256–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh SH, Närhi K, Lindfors PH, Häärä O, Yang L, Ornitz DM, Mikkola ML. 2013. Fgf20 governs formation of primary and secondary dermal condensations in developing hair follicles. Genes Dev 27: 450–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idelson M, Alper R, Obolensky A, Ben-Shushan E, Hemo I, Yachimovich-Cohen N, Khaner H, Smith Y, Wiser O, Gropp M, et al. 2009. Directed differentiation of human embryonic stem cells into functional retinal pigment epithelium cells. Cell Stem Cell 5: 396–408. [DOI] [PubMed] [Google Scholar]
- Ikenouchi J, Matsuda M, Furuse M, Tsukita S. 2003. Regulation of tight junctions during the epithelium–mesenchyme transition: Direct repression of the gene expression of claudins/occludin by Snail. J Cell Sci 116: 1959–1967. [DOI] [PubMed] [Google Scholar]
- Ito Y, Sarkar P, Mi Q, Wu N, Bringas P, Liu Y, Reddy S, Maxson R, Deng C, Chai Y. 2001. Overexpression of Smad2 reveals its concerted action with Smad4 in regulating TGF-β-mediated epidermal homeostasis. Dev Biol 236: 181–194. [DOI] [PubMed] [Google Scholar]
- Ito M, Liu Y, Yang Z, Nguyen J, Liang F, Morris RJ, Cotsarelis G. 2005. Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nat Med 11: 1351–1354. [DOI] [PubMed] [Google Scholar]
- Iwata J, Suzuki A, Pelikan RC, Ho TV, Sanchez-Lara PA, Urata M, Dixon MJ, Chai Y. 2013. Smad4-Irf6 genetic interaction and TGFβ-mediated IRF6 signaling cascade are crucial for palatal fusion in mice. Development 140: 1220–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata J, Suzuki A, Yokota T, Ho TV, Pelikan R, Urata M, Sanchez-Lara PA, Chai Y. 2014. TGFβ regulates epithelial–mesenchymal interactions through WNT signaling activity to control muscle development in the soft palate. Development 141: 909–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakowlew SB, Ciment G, Tuan RS, Sporn MB, Roberts AB. 1994. Expression of transforming growth factor-β2 and β3 mRNAs and proteins in the developing chicken embryo. Differentiation 55: 105–118. [DOI] [PubMed] [Google Scholar]
- Jamora C, DasGupta R, Kocieniewski P, Fuchs E. 2003. Links between signal transduction, transcription and adhesion in epithelial bud development. Nature 422: 317–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamora C, Lee P, Kocieniewski P, Azhar M, Hosokawa R, Chai Y, Fuchs E. 2005. A signaling pathway involving TGF-β2 and snail in hair follicle morphogenesis. PLoS Biol 3: e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrin M, Pandit T, Gunhaga L. 2012. A balance of FGF and BMP signals regulates cell cycle exit and equarin expression in lens cells. Mol Biol Cell 23: 3266–3274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Q, Zhang M, Kong Y, Chen S, Chen Y, Wang X, Zhang L, Lang W, Zhang L, Zhang L. 2013. Activin B promotes initiation and development of hair follicles in mice. Cells Tissues Organs 198: 318–326. [DOI] [PubMed] [Google Scholar]
- Jiang J, Hui CC. 2008. Hedgehog signaling in development and cancer. Dev Cell 15: 801–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang TX, Widelitz RB, Shen WM, Will P, Wu DY, Lin CM, Jung HS, Chuong CM. 2004. Integument pattern formation involves genetic and epigenetic controls: Feather arrays simulated by digital hormone models. Int J Dev Biol 48: 117–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns SA, Soullier S, Rashbass P, Cunliffe VT. 2005. Foxn1 is required for tissue assembly and desmosomal cadherin expression in the hair shaft. Dev Dyn 232: 1062–1068. [DOI] [PubMed] [Google Scholar]
- Jung HS, Francis-West PH, Widelitz RB, Jiang TX, Ting-Berreth S, Tickle C, Wolpert L, Chuong CM. 1998. Local inhibitory action of BMPs and their relationships with activators in feather formation: Implications for periodic patterning. Dev Biol 196: 11–23. [DOI] [PubMed] [Google Scholar]
- Jussila M, Aalto AJ, Sanz Navarro M, Shirokova V, Balic A, Kallonen A, Ohyama T, Groves AK, Mikkola ML, Thesleff I. 2015. Suppression of epithelial differentiation by Foxi3 is essential for molar crown patterning. Development 142: 3954–3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaartinen V, Voncken JW, Shuler C, Warburton D, Bu D, Heisterkamp N, Groffen J. 1995. Abnormal lung development and cleft palate in mice lacking TGF-β3 indicates defects of epithelial–mesenchymal interaction. Nat Genet 11: 415–421. [DOI] [PubMed] [Google Scholar]
- Kalluri R, Weinberg RA. 2009. The basics of epithelial–mesenchymal transition. J Clin Invest 119: 1420–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandyba E, Hazen VM, Kobielak A, Butler SJ, Kobielak K. 2014. Smad1 and 5 but not Smad8 establish stem cell quiescence which is critical to transform the premature hair follicle during morphogenesis toward the postnatal state. Stem Cells 32: 534–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantaputra PN, Kaewgahya M, Hatsadaloi A, Vogel P, Kawasaki K, Ohazama A, Ketudat Cairns JR. 2015. GREMLIN 2 mutations and dental anomalies. J Dent Res 94: 1646–1652. [DOI] [PubMed] [Google Scholar]
- Karafiat V, Dvorakova M, Krejci E, Kralova J, Pajer P, Snajdr P, Mandikova S, Bartunek P, Grim M, Dvorak M. 2005. Transcription factor c-Myb is involved in the regulation of the epithelial–mesenchymal transition in the avian neural crest. Cell Mol Life Sci 62: 2516–2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kémoun P, Laurencin-Dalicieux S, Rue J, Farges JC, Gennero I, Conte-Auriol F, Briand-Mesange F, Gadelorge M, Arzate H, Narayanan AS, et al. 2007. Human dental follicle cells acquire cementoblast features under stimulation by BMP-2/-7 and enamel matrix derivatives (EMD) in vitro. Cell Tissue Res 329: 283–294. [DOI] [PubMed] [Google Scholar]
- Kim TH, Bae CH, Lee JY, Lee JC, Ko SO, Chai Y, Cho ES. 2015. Temporo-spatial requirement of Smad4 in dentin formation. Biochem Biophys Res Commun 459: 706–712. [DOI] [PubMed] [Google Scholar]
- Klopcic B, Maass T, Meyer E, Lehr HA, Metzger D, Chambon P, Mann A, Blessing M. 2007. TGF-β superfamily signaling is essential for tooth and hair morphogenesis and differentiation. Eur J Cell Biol 86: 781–799. [DOI] [PubMed] [Google Scholar]
- Kobielak K, Pasolli HA, Alonso L, Polak L, Fuchs E. 2003. Defining BMP functions in the hair follicle by conditional ablation of BMP receptor IA. J Cell Biol 163: 609–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollar EJ. 1970. The induction of hair follicles by embryonic dermal papillae. J Invest Dermatol 55: 374–378. [DOI] [PubMed] [Google Scholar]
- Korinek V, Barker N, Moerer P, van Donselaar E, Huls G, Peters PJ, Clevers H. 1998. Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nat Genet 19: 379–383. [DOI] [PubMed] [Google Scholar]
- Kowanetz M, Valcourt U, Bergström R, Heldin CH, Moustakas A. 2004. Id2 and Id3 define the potency of cell proliferation and differentiation responses to transforming growth factor β and bone morphogenetic protein. Mol Cell Biol 24: 4241–4254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhnert F, Davis CR, Wang HT, Chu P, Lee M, Yuan J, Nusse R, Kuo CJ. 2004. Essential requirement for Wnt signaling in proliferation of adult small intestine and colon revealed by adenoviral expression of Dickkopf-1. Proc Natl Acad Sci 101: 266–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulessa H, Turk G, Hogan BL. 2000. Inhibition of Bmp signaling affects growth and differentiation in the anagen hair follicle. EMBO J 19: 6664–6674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamouille S, Xu J, Derynck R. 2014. Molecular mechanisms of epithelial–mesenchymal transition. Nat Rev Mol Cell Biol 15: 178–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane J, Yumoto K, Azhar M, Ninomiya-Tsuji J, Inagaki M, Hu Y, Deng CX, Kim J, Mishina Y, Kaartinen V. 2015. Tak1, Smad4 and Trim33 redundantly mediate TGF-β3 signaling during palate development. Dev Biol 398: 231–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YS, Lee SJ. 2013. Regulation of GDF-11 and myostatin activity by GASP-1 and GASP-2. Proc Natl Acad Sci 110: E3713–3722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YS, Lee SJ. 2015. Roles of GASP-1 and GDF-11 in dental and craniofacial development. J Oral Med Pain 40: 110–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DS, Yoon WJ, Cho ES, Kim HJ, Gronostajski RM, Cho MI, Park JC. 2011. Crosstalk between nuclear factor I-C and transforming growth factor-β1 signaling regulates odontoblast differentiation and homeostasis. PLoS ONE 6: e29160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Choi JH, Joo CK. 2013. TGF-β1 regulates cell fate during epithelial–mesenchymal transition by upregulating survivin. Cell Death Dis 4: e714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Kang S, Lilja KC, Colletier KJ, Scheitz CJ, Zhang YV, Tumbar T. 2016. Signalling couples hair follicle stem cell quiescence with reduced histone H3 K4/K9/K27me3 for proper tissue homeostasis. Nat Commun 7: 11278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy V, Lindon C, Harfe BD, Morgan BA. 2005. Distinct stem cell populations regenerate the follicle and interfollicular epidermis. Dev Cell 9: 855–861. [DOI] [PubMed] [Google Scholar]
- Li H, Wang H, Wang F, Gu Q, Xu X. 2011. Snail involves in the transforming growth factor β1-mediated epithelial–mesenchymal transition of retinal pigment epithelial cells. PLoS ONE 6: e23322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Cui D, Zhao F, Huo L, Hu J, Zeng J. 2015a. BMP-2 is involved in scleral remodeling in myopia development. PLoS ONE 10: e0125219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Feng J, Liu Y, Ho TV, Grimes W, Ho HA, Park S, Wang S, Chai Y. 2015b. BMP-SHH signaling network controls epithelial stem cell fate via regulation of its niche in the developing tooth. Dev Cell 33: 125–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenberger BM, Mastrogiannaki M, Watt FM. 2016. Epidermal β-catenin activation remodels the dermis via paracrine signalling to distinct fibroblast lineages. Nat Commun 7: 10537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim J, Thiery JP. 2012. Epithelial–mesenchymal transitions: Insights from development. Development 139: 3471–3486. [DOI] [PubMed] [Google Scholar]
- Liu X, Alexander V, Vijayachandra K, Bhogte E, Diamond I, Glick A. 2001. Conditional epidermal expression of TGFβ 1 blocks neonatal lethality but causes a reversible hyperplasia and alopecia. Proc Natl Acad Sci 98: 9139–9144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Saito K, Maruya Y, Nakamura T, Yamada A, Fukumoto E, Ishikawa M, Iwamoto T, Miyazaki K, Yoshizaki K, et al. 2016. Mutant GDF5 enhances ameloblast differentiation via accelerated BMP2-induced Smad1/5/8 phosphorylation. Sci Rep 6: 23670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons KM, Pelton RW, Hogan BL. 1990. Organogenesis and pattern formation in the mouse: RNA distribution patterns suggest a role for bone morphogenetic protein-2A (BMP-2A). Development 109: 833–844. [DOI] [PubMed] [Google Scholar]
- Ma L, Liu J, Wu T, Plikus M, Jiang TX, Bi Q, Liu YH, Müller-Röver S, Peters H, Sundberg JP, et al. 2003. “Cyclic alopecia” in Msx2 mutants: Defects in hair cycling and hair shaft differentiation. Development 130: 379–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massagué J. 2012. TGFβ signalling in context. Nat Rev Mol Cell Biol 13: 616–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathieu J, Griffin K, Herbomel P, Dickmeis T, Strahle U, Kimelman D, Rosa FM, Peyrieras N. 2004. Nodal and Fgf pathways interact through a positive regulatory loop and synergize to maintain mesodermal cell populations. Development 131: 629–641. [DOI] [PubMed] [Google Scholar]
- Matzuk MM. 1995. Functional analysis of mammalian members of the transforming growth factor-β superfamily. Trends Endocrinol Metab 6: 120–127. [DOI] [PubMed] [Google Scholar]
- Matzuk MM, Lu N, Vogel H, Sellheyer K, Roop DR, Bradley A. 1995. Multiple defects and perinatal death in mice deficient in follistatin. Nature 374: 360–363. [DOI] [PubMed] [Google Scholar]
- Meier N, Dear TN, Boehm T. 1999. Whn and mHa3 are components of the genetic hierarchy controlling hair follicle differentiation. Mech Dev 89: 215–221. [DOI] [PubMed] [Google Scholar]
- Mercado-Pimentel ME, Runyan RB. 2007. Multiple transforming growth factor-β isoforms and receptors function during epithelial–mesenchymal cell transformation in the embryonic heart. Cells Tissues Organs 185: 146–156. [DOI] [PubMed] [Google Scholar]
- Mesa KR, Rompolas P, Zito G, Myung P, Sun TY, Brown S, Gonzalez DG, Blagoev KB, Haberman AM, Greco V. 2015. Niche-induced cell death and epithelial phagocytosis regulate hair follicle stem cell pool. Nature 522: 94–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miettinen PJ, Ebner R, Lopez AR, Derynck R. 1994. TGF-β induced transdifferentiation of mammary epithelial cells to mesenchymal cells: Involvement of type I receptors. J Cell Biol 127: 2021–2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming Kwan K, Wurst W, Behringer RR. 2004. Essential roles of BMPR-IA signaling in differentiation and growth of hair follicles and in skin tumorigenesis. Genesis 39: 10–25. [DOI] [PubMed] [Google Scholar]
- *.Miyazawa K, Miyazono K. 2016. Regulation of TGF-β family signaling by inhibitory Smads. Cold Spring Harb Perspect Biol 10.1101/a022095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore KA, Lemischka IR. 2006. Stem cells and their niches. Science 311: 1880–1885. [DOI] [PubMed] [Google Scholar]
- Morris RJ. 2004. A perspective on keratinocyte stem cells as targets for skin carcinogenesis. Differentiation 72: 381–386. [DOI] [PubMed] [Google Scholar]
- Morris RJ, Liu Y, Marles L, Yang Z, Trempus C, Li S, Lin JS, Sawicki JA, Cotsarelis G. 2004. Capturing and profiling adult hair follicle stem cells. Nat Biotechnol 22: 411–417. [DOI] [PubMed] [Google Scholar]
- Mou C, Jackson B, Schneider P, Overbeek PA, Headon DJ. 2006. Generation of the primary hair follicle pattern. Proc Natl Acad Sci 103: 9075–9080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munz B, Smola H, Engelhardt F, Bleuel K, Brauchle M, Lein I, Evans LW, Huylebroeck D, Balling R, Werner S. 1999. Overexpression of activin A in the skin of transgenic mice reveals new activities of activin in epidermal morphogenesis, dermal fibrosis and wound repair. EMBO J 18: 5205–5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murillo J, Maldonado E, Barrio MC, Del Rio A, Lopez Y, Martinez-Sanz E, Gonzalez I, Martin C, Casado I, Martinez-Alvarez C. 2009. Interactions between TGF-β1 and TGF-β3 and their role in medial edge epithelium cell death and palatal fusion in vitro. Differentiation 77: 209–220. [DOI] [PubMed] [Google Scholar]
- Nakagawa S, Takeichi M. 1998. Neural crest emigration from the neural tube depends on regulated cadherin expression. Development 125: 2963–2971. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Matzuk MM, Gerstmayer B, Bosio A, Lauster R, Miyachi Y, Werner S, Paus R. 2003. Control of pelage hair follicle development and cycling by complex interactions between follistatin and activin. FASEB J 17: 497–499. [DOI] [PubMed] [Google Scholar]
- Nawshad A, Medici D, Liu CC, Hay ED. 2007. TGFβ3 inhibits E-cadherin gene expression in palate medial-edge epithelial cells through a Smad2-Smad4-LEF1 transcription complex. J Cell Sci 120: 1646–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson WJ. 2009. Remodeling epithelial cell organization: Transitions between front-rear and apical-basal polarity. Cold Spring Harb Perspect Biol 1: a000513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto MA. 2013. Epithelial plasticity: A common theme in embryonic and cancer cells. Science 342: 1234850. [DOI] [PubMed] [Google Scholar]
- Niimori D, Kawano R, Felemban A, Niimori-Kita K, Tanaka H, Ihn H, Ohta K. 2012. Tsukushi controls the hair cycle by regulating TGF-β1 signaling. Dev Biol 372: 81–87. [DOI] [PubMed] [Google Scholar]
- Noda K, Mishina Y, Komatsu Y. 2015. Constitutively active mutation of ACVR1 in oral epithelium causes submucous cleft palate in mice. Dev Biol 415: 306–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogai H, Rosowski M, Grun J, Rietz A, Debus N, Schmidt G, Lauster C, Janitz M, Vortkamp A, Lauster R. 2008. Follistatin antagonizes transforming growth factor-β3-induced epithelial–mesenchymal transition in vitro: Implications for murine palatal development supported by microarray analysis. Differentiation 76: 404–416. [DOI] [PubMed] [Google Scholar]
- Noramly S, Morgan BA. 1998. BMPs mediate lateral inhibition at successive stages in feather tract development. Development 125: 3775–3787. [DOI] [PubMed] [Google Scholar]
- Nordin K, LaBonne C. 2014. Sox5 is a DNA-binding cofactor for BMP R-Smads that directs target specificity during patterning of the early ectoderm. Dev Cell 31: 374–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell DJ, Ho JW, Mammoto T, Turbe-Doan A, O’Connell JT, Haseley PS, Koo S, Kamiya N, Ingber DE, Park PJ, et al. 2012. A Wnt-Bmp feedback circuit controls intertissue signaling dynamics in tooth organogenesis. Sci Signal 5: ra4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohyama A, Saito F, Ohuchi H, Noji S. 2001. Differential expression of two BMP antagonists, gremlin and follistatin, during development of the chick feather bud. Mech Dev 100: 331–333. [DOI] [PubMed] [Google Scholar]
- Oka S, Oka K, Xu X, Sasaki T, Bringas P Jr, Chai Y. 2007. Cell autonomous requirement for TGF-β signaling during odontoblast differentiation and dentin matrix formation. Mech Dev 124: 409–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshimori N, Fuchs E. 2012. Paracrine TGF-β signaling counterbalances BMP-mediated repression in hair follicle stem cell activation. Cell Stem Cell 10: 63–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandit T, Jidigam VK, Patthey C, Gunhaga L. 2015. Neural retina identity is specified by lens-derived BMP signals. Development 142: 1850–1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park KS, Gumbiner BM. 2010. Cadherin 6B induces BMP signaling and de-epithelialization during the epithelial mesenchymal transition of the neural crest. Development 137: 2691–2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park KS, Gumbiner BM. 2012. Cadherin-6B stimulates an epithelial mesenchymal transition and the delamination of cells from the neural ectoderm via LIMK/cofilin mediated non-canonical BMP receptor signaling. Dev Biol 366: 232–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel K, Makarenkova H, Jung HS. 1999. The role of long range, local and direct signalling molecules during chick feather bud development involving the BMPs, follistatin and the Eph receptor tyrosine kinase Eph-A4. Mech Dev 86: 51–62. [DOI] [PubMed] [Google Scholar]
- Patthey C, Gunhaga L. 2014. Signaling pathways regulating ectodermal cell fate choices. Exp Cell Res 321: 11–16. [DOI] [PubMed] [Google Scholar]
- Peinado H, Olmeda D, Cano A. 2007. Snail, Zeb and bHLH factors in tumour progression: An alliance against the epithelial phenotype? Nat Rev Cancer 7: 415–428. [DOI] [PubMed] [Google Scholar]
- Pelton RW, Hogan BL, Miller DA, Moses HL. 1990. Differential expression of genes encoding TGFs β1, β2, and β3 during murine palate formation. Dev Biol 141: 456–460. [DOI] [PubMed] [Google Scholar]
- Perea-Gomez A, Vella FD, Shawlot W, Oulad-Abdelghani M, Chazaud C, Meno C, Pfister V, Chen L, Robertson E, Hamada H, et al. 2002. Nodal antagonists in the anterior visceral endoderm prevent the formation of multiple primitive streaks. Dev Cell 3: 745–756. [DOI] [PubMed] [Google Scholar]
- Pinto D, Gregorieff A, Begthel H, Clevers H. 2003. Canonical Wnt signals are essential for homeostasis of the intestinal epithelium. Genes Dev 17: 1709–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plikus MV, Mayer JA, de la Cruz D, Baker RE, Maini PK, Maxson R, Chuong CM. 2008. Cyclic dermal BMP signalling regulates stem cell activation during hair regeneration. Nature 451: 340–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pummila M, Fliniaux I, Jaatinen R, James MJ, Laurikkala J, Schneider P, Thesleff I, Mikkola ML. 2007. Ectodysplasin has a dual role in ectodermal organogenesis: Inhibition of Bmp activity and induction of Shh expression. Development 134: 117–125. [DOI] [PubMed] [Google Scholar]
- Qiao W, Li AG, Owens P, Xu X, Wang XJ, Deng CX. 2006. Hair follicle defects and squamous cell carcinoma formation in Smad4 conditional knockout mouse skin. Oncogene 25: 207–217. [DOI] [PubMed] [Google Scholar]
- Quan T, He T, Kang S, Voorhees JJ, Fisher GJ. 2002. Ultraviolet irradiation alters transforming growth factor β/Smad pathway in human skin in vivo. J Invest Dermatol 119: 499–506. [DOI] [PubMed] [Google Scholar]
- Rajagopal R, Dattilo LK, Kaartinen V, Deng CX, Umans L, Zwijsen A, Roberts AB, Böttinger EP, Beebe DC. 2008. Functions of the type 1 BMP receptor Acvr1 (Alk2) in lens development: Cell proliferation, terminal differentiation, and survival. Invest Ophthalmol Vis Sci 49: 4953–4960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendl M, Polak L, Fuchs E. 2008. BMP signaling in dermal papilla cells is required for their hair follicle-inductive properties. Genes Dev 22: 543–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rishikaysh P, Dev K, Diaz D, Qureshi WMS, Filip S, Mokry J. 2014. Signaling involved in hair follicle morphogenesis and development. Int J Mol Sci 15: 1647–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson EJ. 2014. Dose-dependent Nodal/Smad signals pattern the early mouse embryo. Semin Cell Dev Biol 32: 73–79. [DOI] [PubMed] [Google Scholar]
- Rogers CD, Saxena A, Bronner ME. 2013. Sip1 mediates an E-cadherin-to-N-cadherin switch during cranial neural crest EMT. J Cell Biol 203: 835–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rognoni E, Widmaier M, Jakobson M, Ruppert R, Ussar S, Katsougkri D, Böttcher RT, Lai-Cheong JE, Rifkin DB, McGrath JA, et al. 2014. Kindlin-1 controls Wnt and TGF-β availability to regulate cutaneous stem cell proliferation. Nat Med 20: 350–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rompolas P, Mesa KR, Greco V. 2013. Spatial organization within a niche as a determinant of stem-cell fate. Nature 502: 513–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saika S, Saika S, Liu CY, Azhar M, Sanford LP, Doetschman T, Gendron RL, Kao CW, Kao WW. 2001. TGFβ2 in corneal morphogenesis during mouse embryonic development. Dev Biol 240: 419–432. [DOI] [PubMed] [Google Scholar]
- Saika S, Kono-Saika S, Ohnishi Y, Sato M, Muragaki Y, Ooshima A, Flanders KC, Yoo J, Anzano M, Liu CY, et al. 2004. Smad3 signaling is required for epithelial–mesenchymal transition of lens epithelium after injury. Am J Pathol 164: 651–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saika S, Ikeda K, Yamanaka O, Flanders KC, Ohnishi Y, Nakajima Y, Muragaki Y, Ooshima A. 2006. Adenoviral gene transfer of BMP-7, Id2, or Id3 suppresses injury-induced epithelial-to-mesenchymal transition of lens epithelium in mice. Am J Physiol Cell Physiol 290: C282–289. [DOI] [PubMed] [Google Scholar]
- Sakai D, Suzuki T, Osumi N, Wakamatsu Y. 2006. Cooperative action of Sox9, Snail2 and PKA signaling in early neural crest development. Development 133: 1323–1333. [DOI] [PubMed] [Google Scholar]
- Samavarchi-Tehrani P, Golipour A, David L, Sung HK, Beyer TA, Datti A, Woltjen K, Nagy A, Wrana JL. 2010. Functional genomics reveals a BMP-driven mesenchymal-to-epithelial transition in the initiation of somatic cell reprogramming. Cell Stem Cell 7: 64–77. [DOI] [PubMed] [Google Scholar]
- Sanchez-Alvarado A, Yamanaka S. 2014. Rethinking differentiation: Stem cells, regeneration, and plasticity. Cell 157: 110–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauka-Spengler T, Bronner-Fraser M. 2008. A gene regulatory network orchestrates neural crest formation. Nat Rev Mol Cell Biol 9: 557–568. [DOI] [PubMed] [Google Scholar]
- Scaal M, Prols F, Fuchtbauer EM, Patel K, Hornik C, Kohler T, Christ B, Brand-Saberi B. 2002. BMPs induce dermal markers and ectopic feather tracts. Mech Dev 110: 51–60. [DOI] [PubMed] [Google Scholar]
- Schier AF. 2003. Nodal signaling in vertebrate development. Annu Rev Cell Dev Biol 19: 589–621. [DOI] [PubMed] [Google Scholar]
- Sela-Donenfeld D and Kalcheim C. 1999. Regulation of the onset of neural crest migration by coordinated activity of BMP4 and noggin in the dorsal neural tube. Development 126: 4749–4762. [DOI] [PubMed] [Google Scholar]
- Sela-Donenfeld D, Kalcheim C. 2000. Inhibition of noggin expression in the dorsal neural tube by somitogenesis: A mechanism for coordinating the timing of neural crest emigration. Development 127: 4845–4854. [DOI] [PubMed] [Google Scholar]
- Sellheyer K, Bickenbach JR, Rothnagel JA, Bundman D, Longley MA, Krieg T, Roche NS, Roberts AB, Roop DR. 1993. Inhibition of skin development by overexpression of transforming growth factor β1 in the epidermis of transgenic mice. Proc Natl Acad Sci 90: 5237–5241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengel P. 1990. Pattern formation in skin development. Int J Dev Biol 34: 33–50. [PubMed] [Google Scholar]
- Shah SB, Skromne I, Hume CR, Kessler DS, Lee KJ, Stern CD, Dodd J. 1997. Misexpression of chick Vg1 in the marginal zone induces primitive streak formation. Development 124: 5127–5138. [DOI] [PubMed] [Google Scholar]
- Sharov AA, Sharova TY, Mardaryev AN, Tommasi di Vignano A, Atoyan R, Weiner L, Yang S, Brissette JL, Dotto GP, Botchkarev VA. 2006. Bone morphogenetic protein signaling regulates the size of hair follicles and modulates the expression of cell cycle-associated genes. Proc Natl Acad Sci 103: 18166–18171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiratori H, Hamada H. 2014. TGFβ signaling in establishing left–right asymmetry. Semin Cell Dev Biol 32: 80–84. [DOI] [PubMed] [Google Scholar]
- Shirokova V, Jussila M, Hytonen MK, Perala N, Drogemuller C, Leeb T, Lohi H, Sainio K, Thesleff I, Mikkola ML. 2013. Expression of Foxi3 is regulated by ectodysplasin in skin appendage placodes. Dev Dyn 242: 593–603. [DOI] [PubMed] [Google Scholar]
- Shirokova V, Biggs LC, Jussila M, Ohyama T, Groves AK, Mikkola ML. 2016. Foxi3 deficiency compromises hair follicle stem cell specification and activation. Stem Cells 34: 1896–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sick S, Reinker S, Timmer J, Schlake T. 2006. WNT and DKK determine hair follicle spacing through a reaction-diffusion mechanism. Science 314: 1447–1450. [DOI] [PubMed] [Google Scholar]
- Skromne I, Stern CD. 2001. Interactions between Wnt and Vg1 signalling pathways initiate primitive streak formation in the chick embryo. Development 128: 2915–2927. [DOI] [PubMed] [Google Scholar]
- Solursh M, Langille RM, Wood J, Sampath TK. 1996. Osteogenic protein-1 is required for mammalian eye development. Biochem Biophys Res Commun 218: 438–443. [DOI] [PubMed] [Google Scholar]
- Stern CD. 2005. Neural induction: Old problem, new findings, yet more questions. Development 132: 2007–2021. [DOI] [PubMed] [Google Scholar]
- St-Jacques B, Dassule HR, Karavanova I, Botchkarev VA, Li J, Danielian PS, McMahon JA, Lewis PM, Paus R, McMahon AP. 1998. Sonic Hedgehog signaling is essential for hair development. Curr Biol 8: 1058–1068. [DOI] [PubMed] [Google Scholar]
- Tam WL, Weinberg RA. 2013. The epigenetics of epithelial–mesenchymal plasticity in cancer. Nat Med 19: 1438–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting-Berreth SA, Chuong CM. 1996. Local delivery of TGF β2 can substitute for placode epithelium to induce mesenchymal condensation during skin appendage morphogenesis. Dev Biol 179: 347–359. [DOI] [PubMed] [Google Scholar]
- Tkatchenko AV, Visconti RP, Shang L, Papenbrock T, Pruett ND, Ito T, Ogawa M, Awgulewitsch A. 2001. Overexpression of Hoxc13 in differentiating keratinocytes results in downregulation of a novel hair keratin gene cluster and alopecia. Development 128: 1547–1558. [DOI] [PubMed] [Google Scholar]
- Tözser J, Earwood R, Kato A, Brown J, Tanaka K, Didier R, Megraw TL, Blum M, Kato Y. 2015. TGF-β signaling regulates the differentiation of motile cilia. Cell Rep 11: 1000–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsapara A, Luthert P, Greenwood J, Hill CS, Matter K, Balda MS. 2010. The RhoA activator GEF-H1/Lfc is a transforming growth factor-β target gene and effector that regulates α-smooth muscle actin expression and cell migration. Mol Biol Cell 21: 860–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumbar T, Guasch G, Greco V, Blanpain C, Lowry WE, Rendl M, Fuchs E. 2004. Defining the epithelial stem cell niche in skin. Science 303: 359–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valcourt U, Kowanetz M, Niimi H, Heldin CH, Moustakas A. 2005. TGF-β and the Smad signaling pathway support transcriptomic reprogramming during epithelial–mesenchymal cell transition. Mol Biol Cell 16: 1987–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallin J, Girault JM, Thiery JP, Broders F. 1998. Xenopus cadherin-11 is expressed in different populations of migrating neural crest cells. Mech Dev 75: 171–174. [DOI] [PubMed] [Google Scholar]
- van Genderen C, Okamura RM, Fariñas I, Quo RG, Parslow TG, Bruhn L, Grosschedl R. 1994. Development of several organs that require inductive epithelial–mesenchymal interactions is impaired in LEF-1-deficient mice. Genes Dev 8: 2691–2703. [DOI] [PubMed] [Google Scholar]
- Varlet I, Collignon J, Robertson EJ. 1997. Nodal expression in the primitive endoderm is required for specification of the anterior axis during mouse gastrulation. Development 124: 1033–1044. [DOI] [PubMed] [Google Scholar]
- Vincent T, Neve EPA, Johnson JR, Kukalev A, Rojo F, Albanell J, Pietras K, Virtanen I, Philipson L, Leopold PL, et al. 2009. A SNAIL1-SMAD3/4 transcriptional repressor complex promotes TGF-β mediated epithelial–mesenchymal transition. Nat Cell Biol 11: 943–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CK, Omi M, Ferrari D, Cheng HC, Lizarraga G, Chin HJ, Upholt WB, Dealy CN, Kosher RA. 2004. Function of BMPs in the apical ectoderm of the developing mouse limb. Dev Biol 269: 109–122. [DOI] [PubMed] [Google Scholar]
- Wang XJ, Greenhalgh DA, Bickenbach JR, Jiang A, Bundman DS, Krieg T, Derynck R, Roop DR. 1997. Expression of a dominant-negative type II transforming growth factor β (TGF-β) receptor in the epidermis of transgenic mice blocks TGF-β-mediated growth inhibition. Proc Natl Acad Sci 94: 2386–2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XJ, Liefer KM, Tsai S, O’Malley BW, Roop DR. 1999. Development of gene-switch transgenic mice that inducibly express transforming growth factor β1 in the epidermis. Proc Natl Acad Sci 96: 8483–8488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LC, Liu Zy, Gambardella L, Delacour A, Shapiro R, Yang J, Sizing I, Rayhorn P, Garber EA, Benjamin CD, et al. 2000. Conditional disruption of hedgehog signaling pathway defines its critical role in hair development and regeneration. J Invest Dermatol 114: 901–908. [DOI] [PubMed] [Google Scholar]
- Wang Y, Li L, Zheng Y, Yuan G, Yang G, He F, Chen Y. 2012. BMP activity is required for tooth development from the lamina to bud stage. J Dent Res 91: 690–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Siegenthaler JA, Dowell RD, Yi R. 2016. Foxc1 reinforces quiescence in self-renewing hair follicle stem cells. Science 351: 613–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watabe T, Miyazono K. 2009. Roles of TGF-β family signaling in stem cell renewal and differentiation. Cell Res 19: 103–115. [DOI] [PubMed] [Google Scholar]
- Wheelock MJ, Shintani Y, Maeda M, Fukumoto Y, Johnson KR. 2008. Cadherin switching. J Cell Sci 121: 727–735. [DOI] [PubMed] [Google Scholar]
- Whiteman EL, Liu CJ, Fearon ER, Margolis B. 2008. The transcription factor snail represses Crumbs3 expression and disrupts apico-basal polarity complexes. Oncogene 27: 3875–3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang LT, Kaartinen V. 2007. Tgfb1 expressed in the Tgfb3 locus partially rescues the cleft palate phenotype of Tgfb3 null mutants. Dev Biol 312: 384–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Mao C, Teng Y, Li W, Zhang J, Cheng X, Li X, Han X, Xia Z, Deng H, et al. 2005. Targeted disruption of Smad4 in mouse epidermis results in failure of hair follicle cycling and formation of skin tumors. Cancer Res 65: 8671–8678. [DOI] [PubMed] [Google Scholar]
- Yang L, Wang L, Yang X. 2009. Disruption of Smad4 in mouse epidermis leads to depletion of follicle stem cells. Mol Biol Cell 20: 882–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Hai B, Qin L, Ti X, Shangguan L, Zhao Y, Wiggins L, Liu Y, Feng JQ, Chang JY, et al. 2013. Cessation of epithelial Bmp signaling switches the differentiation of crown epithelia to the root lineage in a β-catenin-dependent manner. Mol Cell Biol 33: 4732–4744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M, Wu P, Widelitz RB, Chuong CM. 2002. The morphogenesis of feathers. Nature 420: 308–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W, Kamara H, Svoboda KK. 2008. The role of twist during palate development. Dev Dyn 237: 2716–2725. [DOI] [PubMed] [Google Scholar]
- Yuan G, Yang G, Zheng Y, Zhu X, Chen Z, Zhang Z, Chen Y. 2015. The non-canonical BMP and Wnt/β-catenin signaling pathways orchestrate early tooth development. Development 142: 128–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuhki M, Yamada M, Kawano M, Iwasato T, Itohara S, Yoshida H, Ogawa M, Mishina Y. 2004. BMPR1A signaling is necessary for hair follicle cycling and hair shaft differentiation in mice. Development 131: 1825–1833. [DOI] [PubMed] [Google Scholar]
- Zeisberg M, Hanai J, Sugimoto H, Mammoto T, Charytan D, Strutz F, Kalluri R. 2003. BMP-7 counteracts TGF-β1-induced epithelial-to-mesenchymal transition and reverses chronic renal injury. Nat Med 9: 964–968. [DOI] [PubMed] [Google Scholar]
- *.Zhang YE. 2017. Non-Smad signaling pathways of the TGF-β family. Cold Spring Harb Perspect Biol 9: a022129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, He XC, Tong WG, Johnson T, Wiedemann LM, Mishina Y, Feng JQ, Li L. 2006. Bone morphogenetic protein signaling inhibits hair follicle anagen induction by restricting epithelial stem/progenitor cell activation and expansion. Stem Cells 24: 2826–2839. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Andl T, Yang SH, Teta M, Liu F, Seykora JT, Tobias JW, Piccolo S, Schmidt-Ullrich R, Nagy A, et al. 2008. Activation of β-catenin signaling programs embryonic epidermis to hair follicle fate. Development 135: 2161–2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YV, Cheong J, Ciapurin N, McDermitt DJ, Tumbar T. 2009. Distinct self-renewal and differentiation phases in the niche of infrequently dividing hair follicle stem cells. Cell Stem Cell 5: 267–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Nie Q, Su Y, Xie X, Luo W, Jia X, Zhang X. 2013. MicroRNA profile analysis on duck feather follicle and skin with high-throughput sequencing technology. Gene 519: 77–81. [DOI] [PubMed] [Google Scholar]
- Zhao H, Li S, Han D, Kaartinen V, Chai Y. 2011. Alk5-mediated transforming growth factor β signaling acts upstream of fibroblast growth factor 10 to regulate the proliferation and maintenance of dental epithelial stem cells. Mol Cell Biol 31: 2079–2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P, Byrne C, Jacobs J, Fuchs E. 1995. Lymphoid enhancer factor 1 directs hair follicle patterning and epithelial cell fate. Genes Dev 9: 700–713. [DOI] [PubMed] [Google Scholar]
- Zhu XJ, Liu Y, Dai ZM, Zhang X, Yang X, Li Y, Qiu M, Fu J, Hsu W, Chen Y, et al. 2014. BMP-FGF signaling axis mediates Wnt-induced epidermal stratification in developing mammalian skin. PLoS Genet 10: e1004687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zohn IE, Li Y, Skolnik EY, Anderson KV, Han J, Niswander L. 2006. p38 and a p38-interacting protein are critical for downregulation of E-cadherin during mouse gastrulation. Cell 125: 957–969. [DOI] [PubMed] [Google Scholar]