Abstract
BACKGROUND
This study tested the effects of a multicomponent exercise training intervention called “Heart Failure Exercise And Training Camp” (HEART CAMP). The intervention was theoretically derived from Bandura’s social cognitive theory.
METHODS
An experimental repeated-measures design was used. Participants were randomized to the HEART CAMP intervention group (n = 22) or the attention control group (n = 20). Participants were compared on self-efficacy for exercise, symptoms, physical function, and quality of life over 12 weeks.
RESULTS
The intervention group had a 31% increase in cardiac exercise self-efficacy and significantly fewer symptoms compared with the attention control group. Quality of life increased significantly in both groups.
CONCLUSION
A theory-based intervention improved self-efficacy for exercise and symptoms in patients with heart failure.
Exercise intolerance, fatigue, and dyspnea are hallmark symptoms experienced by the approximately 5.8 million Americans with heart failure (HF).1 These symptoms frequently lead to decreased physical activity and physical deconditioning that impair functioning and erode self-confidence necessary to initiate and maintain regular exercise. Outpatient cardiac rehabilitation programs are not reimbursed for patients with HF, so initiation of individual exercise programs is difficult. Even those who participate in an exercise training program have problems with adherence.2–4 Creative and alternative models for initiating and maintaining regular exercise behavior in patients with HF are needed. The purpose of this pilot study was to test the effects of a multicomponent exercise training intervention called “Heart Failure Exercise And Training Camp” (HEART CAMP). The intervention was derived from Bandura’s social cognitive theory5 with the purpose of building self-efficacy for aerobic exercise and resistance training.
LITERATURE REVIEW
The American Heart Association’s Scientific Statement, Exercise and Heart Failure,6 recommends regular exercise for patients with stable chronic HF. Patients with HF have difficulty following this recommendation, and many do not engage in regular activity. Gary3 reported that 91% of women with HF are sedentary. Studies with both men and women reported that between 53% and 54% do not engage in regular exercise.7,8 As many as 80% of patients with HF report that exercise is an important health behavior,9 yet only 39% report actually engaging in exercise.8 A majority (63%) of patients with HF report a lack of skills for exercise.8 Strategies to assist patients to engage in regular exercise are needed.
The organizing framework for this study was derived from social cognitive theory,5 a model in which behavior, cognition, personal, and environmental influences are interactive determinants of self-efficacy. Self-efficacy (confidence in ability to perform a behavior) is concerned with self-regulation of thought processes, motivation, and affective and physiologic states. Self-efficacy beliefs (ie, beliefs in capabilities to perform a course of action to attain a desired outcome) are derived from 4 primary sources: enactive mastery experiences, vicarious experiences, verbal persuasion, and physiologic and affective states. Enactive mastery experiences are the most influential source of efficacy information. Successful performance builds self-efficacy. Feedback and self-monitoring of performance successes are also important to self-efficacy. Vicarious experiences are provided through modeling, observational learning, and social comparisons with persons of similar attributes and performance. Verbal persuasion involves provision of feedback on performance and personal capability. This feedback is especially important in the early stages of behavior adoption. Enhanced physical status and correct interpretations of bodily states are especially important in building self-efficacy.5
Self-efficacy for exercise (the degree of confidence that an individual has in his/her ability to perform exercise) has consistently been shown to relate to exercise behavior10,11 and is a strong predictor of physical activity in patients with HF.12 Gary3 reported that engaging in a walking program increased self-efficacy for exercise that resulted in improved functioning on the 6-Minute Walk Test and improved quality of life among patients with HF.13 Collins et al13 found a 17% improvement in self-efficacy after a 12-week exercise program. Few exercise studies are designed specifically to enhance self-efficacy as the primary outcome, despite the fact that patients report lack of skills as the primary barrier to exercise. Therefore, developing an intervention based on Bandura’s social cognitive theory is especially important for the patient with HF to increase self-efficacy and perceived success necessary to engage in regular exercise behavior.
Numerous studies have demonstrated the beneficial effects of exercise training in patients with HF.14,15 The positive effects on physical function, measured by the 6-Minute Walk Test, the Medical Outcomes Study 36-Item Short Form Health Survey Version 2 (MOS SF-36), and maximum oxygen consumption have consistently been demonstrated. Health-related quality of life, as measured by the Minnesota Living with Heart Failure Questionnaire and the Kansas City Cardiomyopathy Questionnaire (KCCQ), has also improved after exercise training.2,16–18
Studies evaluating HF symptoms as outcomes of exercise training in HF have been limited to date. None of the randomized controlled trials reviewed in the 2009 Cochrane review18 reported effects of exercise training on HF symptoms. The few studies that do report effects of exercise training on symptoms of fatigue and dyspnea have had inconsistent results. Some studies report improvements in symptoms of dyspnea and fatigue,19–21 whereas others show no change as a result of exercise training.22,23 Conflicting results from previous studies may be due to small sample sizes, variations in exercise protocols (eg, walking, cycling, and combination aerobic/resistance training), and use of various instruments to assess symptoms.
Only 1 large multicenter randomized controlled trial of exercise training in patients with HF has been completed to date. The Heart Failure: A Controlled Trial Investigating Outcomes of Exercise Training trial demonstrated that exercise training is safe for individuals with HF,24 but overall adherence was suboptimal.2 This finding suggests the need to improve patient’s self-confidence to increase adherence to exercise.
The aims of this study were to determine differences from baseline to 12 weeks in self-efficacy for exercise in the HEART CAMP intervention group compared with the attention control group and to determine differences from baseline to 12 weeks in symptoms (Dyspnea-Fatigue Index), physical function (SF-36 physical function subscale and KCCQ physical limitations subscale), and quality of life (KCCQ total score) in the HEART CAMP intervention group compared with the attention control group.
MATERIALS AND METHODS
Design and sample
The study used an experimental repeated-measures design. Participants were randomized to either the HEART CAMP intervention or the attention control group. Primary outcome measures were obtained at baseline and at 12 and 24 weeks for both groups. The study was conceptualized in 2 phases of 12 weeks each. Phase 1 involved structured aerobic exercise 3 times weekly in a hospital-based rehabilitation setting and resistance training twice weekly at home. Group meetings were held weekly for the first 3 weeks. Exercise in weeks 4 to 12 was self-scheduled in a maintenance rehabilitation setting (3 times weekly), and group sessions were decreased to biweekly. During phase 1, the group sessions were designed to teach subjects specific strategies to build self-efficacy for exercise behavior. In phase 2 of the study (weeks 13–24), the subjects continued exercise behaviors in a self-directed manner according to the learned behaviors in phase 1. This article reports findings from phase 1 of the study (baseline to week 12).
Subjects were recruited from a regional cardiology center in the Midwestern United States. The sample consisted of patients with chronic HF randomly assigned to the HEART CAMP intervention group or the attention control group. Participants were recruited for the study and randomized in 3 blocks of 16 subjects (8 subjects per group) to facilitate small group educational meetings.
Inclusion criteria were (a) age 21 years or greater; (b) oriented to person, place, and time; (c) able to speak and read English; (d) resting left ventricular ejection fraction < 40%; and (e) optimum medical therapy with no changes in medications within the past 30 days. Exclusion criteria included (a) clinical evidence of decompensated HF; (b) unstable angina pectoris; (c) myocardial infarction, coronary artery bypass surgery, or biventricular pacemaker less than 3 months previously; (d) orthopedic or neuromuscular disorders preventing participation in exercise and strength/resistance training; and (e) participation in aerobic exercise more than 3 times per week during the past 12 months. Participants were also excluded if they were currently participating in another study.
Figure 1 depicts the study enrollment and participant flow. Of the 139 patients with HF assessed for eligibility, there were 38 who did not meet inclusion criteria. The primary reason was a resting left ventricular ejection fraction more than 40%. Reasons for refusal included lack of time (n = 19) and unwillingness to commit to scheduled 3 days per week exercise if randomized to intervention group (n = 12) and other (n = 8). All participants who were enrolled completed the 12 weeks of phase 1.
Fig 1.
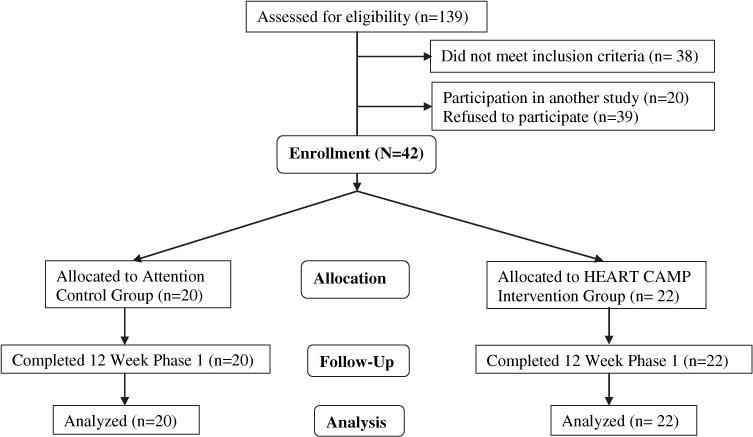
Consort flow chart.
Intervention
Attention control group
The attention control group was designed to minimize the potential confound of human interaction.25 The attention control and intervention groups both participated in small group educational sessions with topics pertinent to HF. Materials used in these sessions were adapted from the educational modules provided by the Heart Failure Society of America.26 Group sessions were held in the same frequency and format (ie, weekly for the first 3 weeks and biweekly for weeks 4–12), with an equivalent amount of time spent with the intervention nurse for both groups (Table I).
Table I.
Topical listing for group sessions and intervention timeline
| Weekly group sessions both groups | HEART CAMP intervention group exercise training intervention | |
|---|---|---|
| Week 1 | Topic 1 Introduction | Hospital-based cardiac rehabilitation sessions (60 min each) 3 times weekly |
| Week 2 | Topic 2 What is HF | 60-min exercise sessions (15 min warm-up, 30-min aerobic at 40%–70% heart rate reserve, and 15-min cool-down) |
| Week 3 | Topic 3 Symptoms of HF | Resistance training sessions once weekly at exercise facility and home-based once weekly |
| Week 4 | Topic 4 Diet | |
| Week 6 | Topic 5 Medications | Maintenance rehabilitation 3 times weekly for exercise 60-min exercise sessions (15-min warm up, 30-min aerobic at 40%–70% heart rate reserve, and 15-min cool-down) |
| Week 8 | Topic 6 Stress management | Home-based resistance training twice weekly |
| Week 10 | Topic 7 Health maintenance | Self-efficacy strategies (Table II) |
| Week 12 | Conclusion of phase 1 Review and summary |
HF, heart failure.
Multicomponent HEART CAMP intervention group
The HEART CAMP intervention was a multicomponent intervention designed to build self-efficacy for aerobic and resistance exercise. Participants met weekly during weeks 1 to 3 and biweekly during weeks 4 to 12 in small group sessions with a physical therapist and a nurse to guide the training.
Intervention strategies to build self-efficacy were derived from Bandura’s cognitive behavioral theory and centered on 4 main sources: enactive mastery experiences, vicarious experiences, verbal persuasions, and physiologic and affective states. Specific strategies for each of these sources of self-efficacy were provided in the HEART CAMP intervention and are summarized in Table II. Cohort groups of 8 participants were used for the small group sessions. Small group sessions were used to incorporate the processes of social learning, social exchange, and social comparison that are recommended as methods to increase self-efficacy.5 Adherence to health habits such as exercise requires self-regulation that is achieved through the sub-skills of goal setting, feedback, and relapse management.5 Participants, with the guidance of the physical therapist, established goals for exercise each week. Exercise performance data were compared with goals, and the physical therapist provided feedback on goals and exercise performance. Bar graphs were created to depict weekly adherence to goals (number and duration of sessions and intensity of exercise). Problem-solving strategies for any perceived difficulties or barriers to exercise were addressed in the group sessions. The adherence management strategies and adherence results from the intervention are more fully described in another report.27
Table II.
HEART CAMP intervention strategies
| Intervention strategy (Bandura 1997) | HEART CAMP intervention | Time frame |
|---|---|---|
| Enactive mastery Experiences (performance accomplishment) | Return demonstration of use of exercise equipment Return demonstration of self-monitoring techniques (heart rate, rating of perceived exertion, symptoms) Mutual goal setting for weekly exercise and review of graphs of goal accomplishment Reward and celebration of accomplishments |
Weeks 1–3 Weeks 1–3 Weeks 1–12 Weeks 3 and 12 |
| Vicarious experience | Initial demonstration of exercise techniques by physical therapist Present weekly progress of the cohort group related to goals (progressive mastery) Education and debriefing sessions with cohort group (sharing of strategies to overcome difficulties, problem solving for relapse management) Role modeling by physical therapist of cognitive processes to overcome difficulties with exercise |
Week 1 Weeks 1–12 Weeks 1–12 Weeks 3, 6, and 10 |
| Verbal persuasion | Presentation of exercise benefits by cardiologist Educational materials on benefits of exercise Web-based educational material related to exercise (HFSA module on exercise) Coaching by physical therapist (feedback related to individual progress) Cohort group picture in t-shirts with HEART CAMP logo at celebration ceremony |
Week 1 Week 1 Week 1 Weeks 3, 6, and 10 Weeks 3 and 12 |
| Physiologic and affective states | Symptom assessment education Recognition of warning symptoms to moderate or stop exercise Symptom management strategies for exercise Education and debriefing sessions by physical therapist and nurse related to symptom assessment, recognition of warning signs, and problem solving Relapse management for physiologic and affective interference with exercise |
Week 1–3 Week 1–3 Week 1–3 Weeks 1–12 Weeks 1–12 |
HEART CAMP, Heart Failure Exercise And Training Camp; HFSA, Heart Failure Society of America.
The exercise training component of HEART CAMP consisted of aerobic exercise 3 times per week and resistance training twice per week. Each exercise session lasted 60 minutes (ie, 15 minutes warm up, 30 minutes exercise, 15 minutes cool down). Aerobic exercise intensity was set using 40% to 70% heart rate reserve as determined with a baseline cardiopulmonary exercise test. Resistance training was based on the acclimation method with 1 to 3 sets of 8 repetitions each for 8 to 10 exercises involving upper and lower body.28 Resistance training was initiated with 2- to 3-lb hand weights or resistance bands and progressed according to individual acclimation. The first 3 weeks of aerobic training (9 sessions) took place in a hospital-based rehabilitation setting and participants had electrocardiographic monitoring, consistent with the recommendation of the American Association for Cardiovascular and Pulmonary Rehabilitation.29 Three weeks of continuous electrocardiographic monitoring and supervision allow for detection of any adverse effects that may occur during initiation of exercise.29 During the remaining 9 weeks, participants exercised 3 times weekly according to their own schedules in a maintenance rehabilitation facility. Participants completed resistance exercises at home after an initial training session at the rehabilitation facility. Hand weights and resistance bands for provided for use at home. Participants completed an exercise diary to document completion of exercise sessions.
Procedure
Approval was obtained from the institutional review board of the University of Nebraska Medical Center and the study site. Subjects were recruited from a mid-western cardiology HF clinic that had a patient population of more than 800 patients. Patients with HF were recruited and screened for inclusion by the principal investigator, who is employed in a faculty practice as a nurse practitioner. Three separate groups of 12 to 16 subjects were recruited. After providing written informed consent, each group was randomized to either the intervention or the attention control arms of the study.
Measures to address intervention fidelity
Intervention fidelity strategies were based on the recommendations of Bellg et al.30 The theoretic basis for specific intervention strategies was an important first step in assuring fidelity. Every participant in the treatment group received the same number of group sessions that were equal in frequency and length. The principal investigator led the group sessions for the treatment and control groups using a protocol developed from the Heart Failure Society of America’s educational modules.26 The exercise training protocol was guided by a physical therapist in a cardiac rehabilitation setting during the first 3 weeks of the study. The principal investigator taught resistance training during the first 3 weeks of the study using detailed pictures and guidelines. Receipt of the treatment was assessed during each group session through questioning and verification of participant understanding. The principal investigator or physical therapist observed enactment of skills on a regular basis during the first 3 weeks of the study and every 4 weeks during the remaining 9 weeks.
Instruments
Self-efficacy for exercise
The 16-item Cardiac Exercise Self-Efficacy Instrument (CESEI)31 measures perceived self-efficacy for exercise in patients with cardiac disease. Items from the original tool related to pre-hospital level of activity and enduring strenuous exercise were revised. Participants in this study had not been recently hospitalized, so assessment of pre-hospital level of activity was not appropriate. The intensity of exercise was set at 40% to 70% heart rate reserve, and participants were not being taught to endure strenuous exercise. The CESEI uses a 5-point Likert response scale of 1 “very little confidence” to 5 “quite a lot of confidence” from the original instrument.31 The CESEI had internal consistency reliability (r = .90) and a test–retest reliability estimate of .87 in a sample of cardiac rehabilitation participants.31 Known group validity was documented in a sample of marathon runners, and this sample reported significantly (P < .01) higher self-efficacy scores than the participants receiving cardiac rehabilitation. Physical activity was a predictor of self-efficacy, as measured by the CESEI, in cardiac rehabilitation.32 Cronbach’s alphas for the revised 18-item CESEI instrument were .97 at baseline and .98 at 12 weeks in data obtained in this study.
Dyspnea/Fatigue Index
The Dyspnea/Fatigue Index measures a patient’s subjective perception of the impact of the symptoms of dyspnea and fatigue on function during daily activities or at rest. The index is composed of 3 items, each of which has 5 ordered categories from which subjects choose the category that best describes their perceptions of dyspnea and fatigue. Item 1 describes the magnitude of the task at which the patient becomes short of breath or fatigued. Item 2 categorizes the pace at which the patient can carry out physical tasks of varying magnitude. In Item 3, categories are defined by degree of functional impairment and reflect whether the patient has modified or given up usual activities as a result of their symptoms. Ratings are summed across the 3 items with a possible range of 0 (severely impaired) to 12 (no impairment). Reliability and validity have been established with patients with HF.33 Cronbach’s alpha reliability for this study was .85 at baseline and .82 at 12 weeks.
MOS SF-36 physical function subscale
The MOS SF-36 is a multidimensional scale designed to measure subjective functioning from a generic perspective. The physical function subscale measures subject limitations in performance of physical activities.34,35 Standardized response choices are used for each item. Each subscale is scored on a scale of 0 to 100, with a higher score indicating better health. Internal consistency reliability estimates range from .78 to .95.34,36 Patients with HF or myocardial infarction have the poorest physical function and role physical function in patients with selected chronic illnesses.37
KCCQ
The KCCQ was developed as a disease-specific health status measure of quality of life for patients with HF.38 It is self-administered and consists of 23 items. There are 7 subscales that measure the domains of physical limitations, symptoms, quality of life, social limitation, self-efficacy, functional status, and clinical status (clinical summary). The physical limitations and quality of life subscales have Cronbach’s alphas of .90 and .78, respectively.38 Three-month follow-up data indicated no significant change for patients with stable HF who remained stable.38 The validity of subscales of KCCQ has been supported by comparison of scores with other quality of life instruments (SF-36, New York Heart Association, Minnesota Living with Heart Failure), and the sensitivity of the KCCQ was higher than that of the Minnesota Living with Heart Failure questionnaire.38
Sample size
The target sample size of 21 patients per intervention group was determined by the need to have sufficient subjects to evaluate feasibility and obtain preliminary estimates of effect sizes in this pilot study.
Data analysis
Because instruction and discussion occurred in small groups of patients who progressed through the study together, it is likely that the scores of individuals within the same group were not independent. To account for this dependency in the data, the analysis included cohort as a random effect nested within intervention group and crossed with time, with both treated as fixed effects. In the primary analysis, initial level was included as a time point rather than as a covariate to evaluate change from baseline. In this population, an untreated group would be expected to show declines in physical function and quality of life during a 3-month period.
Within each intervention group (collapsed across cohorts), distributions were evaluated graphically and with tests of significance of skewness and kurtosis for departures from univariate normality. Data were analyzed using a traditional repeated-measures analysis of variance (ANOVA), with a focus on the intervention × time interaction. Homogeneity of the variance/covariance matrix was evaluated using Box’s M test. To account for the nesting in this analysis, the test of this interaction involves forming a quasi-F statistic using the mean square for the cohort (intervention) × time effect as the error term instead of the usual within-subject error.39,40 When the interaction of the nested effect and time is not significant, pooling of error term sums of squares and degrees of freedom is sometimes suggested as a strategy to improve power.41 However, because not all statisticians support this method and that approach made no difference in the results for any of the outcomes in this study, only the results of the more conservative analysis are presented. Significant interaction effects were followed by tests of simple main effects of time within group. Because this was a pilot study and overlooking important effects (type II error) was as great a concern as type I error, a liberal α level of .20 was used to identify outcomes for further investigation.
Effect sizes were estimated using η2, the ratio of the sums-of-squares for the effects of interest to the total within-subject sums-of-squares. When the P value for an interaction effect was less than .20, effect sizes for the simple main effect of time within each group were also calculated, using the separate within-subject sum of squares for each group.
Supplemental analyses were conducted with change from baseline to 12 weeks as the dependent variable, adjusting for gender, age, and baseline score on the outcome. Initially, we fit a main-effects only model that included the nested effect; conclusions were the same as in the primary analysis, and results from this analysis will not be reported. With this model, a rigorous check of the homogeneity of regression assumption for all covariates and all cells in the design was impractical given the small sample size. A compromise model without the nested factor (which had not been a significant effect in the original analyses) and including only main effects and 2-way interaction terms was fit to explore possible differential effects of the intervention by age, gender, or initial status on the outcome.
RESULTS
The sample included 21 patients with ischemic HF and 21 patients with nonischemic HF with a total of 24 men and 18 women. Subjects had New York Heart Association class II (n = 23) and III (n = 19) HF. Participants were evenly distributed by gender in the 2 groups. Participants in the intervention group completed an average of 85.6% ± 17.5% of the 5 day per week exercise sessions. Group sessions were attended 88.8% ± 12.5% of the time by the intervention group and 88.6% ± 15.7% of the time by the attention control group. There were no gender-related differences in exercise participation or group session participation.
Descriptive statistics for the demographic and clinical variables are presented by group in Table III. There were no significant differences in demographic or clinical variables between groups. None of the distributions exhibited significant skewness or kurtosis, and no outliers were identified using the criterion of z-scores ± 3.0.40 The observed probability for the Box’s M test of homogeneity of the variance/covariance matrix was ≥ .20 for all outcomes, except the SF-36 physical function subscale (P = .06). Because the cells with the fewest participants were among those having the largest variance, the F-tests reported for this outcome are likely to be liberal.
Table III.
Demographic and clinical variables
| Variables | HEART CAMP intervention group (n = 22) |
Attention control group (n = 20) |
Total group (n = 42) |
|---|---|---|---|
| Mean ± SD n (%) |
Mean ± SD n (%) |
Mean ± SD | |
| Age (y) | 57.0 ± 12.3 | 63.0 ± 15.1 | 59.9 ± 13.8 |
| LVEF (%) | 33.2 ± 6.6 | 32.3 ± 5.5 | 32.7 ± 6.1 |
| Duration of Diagnosis (mo) | 39.1 ± 27.1 | 27.1 ± 29.8 | 32.2 ± 28.1 |
| Charlson Comorbidity Index | 2.0 ± 1.1 | 2.2 ± 1.1 | 2.1 ± 1.1 |
| Male | 12 (54.5) | 12 (54.5) | |
| NYHA class | |||
| Class II | 14 (63.6) | 9 (45.0) | |
| Class III | 8 (36.4) | 11 (55.0) | |
| Cause of HF | |||
| Ischemic | 8 (36.4) | 13 (65.0) | |
| Nonischemic | 14 (63.6) | 7 (35.0) | |
| Ethnicity | |||
| Caucasian | 21 (95.5) | 20 (100.0) | |
| African-American | 1 (4.5) | 0 (.0) |
HEART CAMP, Heart Failure Exercise And Training Camp; SD, standard deviation; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; HF, heart failure.
Descriptive statistics for baseline and 12-week outcomes and results of the ANOVA are shown in Table IV for the primary outcome of cardiac exercise self-efficacy. By using the criterion of α = .20, there was a significant group × time interaction for cardiac exercise self-efficacy (F[1,4] = 3.72, P = .13). Simple effects tests showed that the intervention group had a significant increase in cardiac exercise self-efficacy (.9 increase on the 5-point scale) (F[1,2] = 31.25, P = .03, η2 = .47), compared with a nonsignificant change (.2 increase on the 5-point scale) for the attention-control group (F[1,2] = 2.33, P = .27, η2 = .15).
Table IV.
Repeated-measures analysis of variance summary table for cardiac exercise self-efficacy
| Cardiac exercise self-efficacy | n | Baseline Mean ± SD |
12 wks Mean ± SD |
Fa | P | η2 |
|---|---|---|---|---|---|---|
| Intervention (HEART CAMP) | ||||||
| Cohort 1 | 7 | 3.0 ± .8 | 4.0 ± .8 | |||
| Cohort 2 | 7 | 2.8 ± 1.0 | 3.8 ± 1.3 | |||
| Cohort 3 | 6 | 3.0 ± .4 | 3.6 ± 1.0 | |||
| Total HEART CAMP intervention group | 20 | 2.9 ± .8 | 3.8 ± 1.0 | |||
| Attention control | ||||||
| Cohort 1 | 7 | 2.5 ± .5 | 2.6 ± .6 | |||
| Cohort 2 | 4 | 2.0 ± .6 | 2.9 ± 1.4 | |||
| Cohort 3 | 7 | 2.2 ± .6 | 2.3 ± 1.0 | |||
| Total attention control group | 18 | 2.3 ± .6 | 2.5 ± .9 | |||
| Time | 20.33 | .01 | .31 | |||
| Group × time | 3.72 | .13 | .06 | |||
| Cohort (group) × time | .50 | .06 | .85 |
SD, standard deviation; HEART CAMP, Heart Failure Exercise And Training Camp.
Time and group × time effects are each tested using the mean square of the cohort (group) × time effect as the denominator (df = 1.4). Cohort (group) × time effect was tested using the residual mean square error (dfnumerator = 4, dfdenominator = 32)
The group × time interaction was significant for the Dyspnea-Fatigue Index (F[1,4] = 30.49, P < .01). Simple effects tests showed that the intervention group reported less fatigue or dyspnea during activity at 12 weeks compared with baseline (F[1,2] = 25.15, P = .04, η2 = .34), whereas the control group reported slightly more fatigue or dyspnea (F[1,2] = 6.07, P = .13, η2 = .02). Descriptive statistics and results of the ANOVA are summarized by group in Table V.
Table V.
Repeated-measures analysis of variance summary table for Dyspnea-Fatigue Index
| Dyspnea fatigue index | n | Baseline Mean ± SD |
12 wks Mean ± SD |
Fa | P | η2 |
|---|---|---|---|---|---|---|
| Intervention (HEART CAMP) | ||||||
| Cohort 1 | 5 | 6.4 ± 1.1 | 7.2 ± 1.6 | |||
| Cohort 2 | 5 | 7.6 ± 1.5 | 9.2 ± 1.6 | |||
| Cohort3 | 5 | 6.2 ± 3.8 | 7.8 ± 3.0 | |||
| Total HEART CAMP intervention group | 15 | 6.7 ± 2.4 | 8.1 ± 2.2 | |||
| Attention control | ||||||
| Cohort 1 | 7 | 7.6 ± 2.0 | 7.4 ± 2.0 | |||
| Cohort 2 | 3 | 5.3 ± 1.2 | 4.7 ± 1.2 | |||
| Cohort 3 | 6 | 6.5 ± 3.9 | 6.3 ± 2.8 | |||
| Total attention control group | 16 | 6.8 ± 2.8 | 6.5 ± 2.3 | |||
| Time | 10.32 | .03 | .04 | |||
| Group × time | 30.49 | <.01 | .12 | |||
| Cohort (group) × time | .12 | .97 | .02 |
SD, standard deviation; HEART CAMP, Heart Failure Exercise And Training Camp.
Time and group × time effects are each tested using the mean square of the cohort (group) × time effect as the denominator (df = 1.4). Cohort (group) × time effect was tested using the residual mean square error (dfnumerator = 4, dfdenominator = 25).
The group × time interaction was not significant for the physical function subscale of the SF-36 (F[1,4] = .96, P = .39) or the physical limitations sub-scale of the KCCQ (F[1,4] = .54, P = .50).
Although, the group × time interaction was not significant (F[1,4] = .01, P = .94) for quality of life, the effect for time was significant (F[1,4] = 12.27, P = .02). Both the intervention and the attention control groups showed significant increases in quality of life over the 12-week study period. Descriptive statistics and results of the statistical tests are summarized by group in Table VI.
Table VI.
Repeated-measures analysis of variance summary table for quality of life (Kansas City Cardiomyopathy Questionnaire quality of life subscale score)
| KCCQ quality of life | n | Baseline Mean ± SD |
12 wks Mean ± SD |
Fa | P | η2 |
|---|---|---|---|---|---|---|
| Intervention (HEART CAMP) | ||||||
| Cohort 1 | 7 | 52.4 ± 17.8 | 63.1 ± 17.9 | |||
| Cohort 2 | 8 | 70.8 ± 20.4 | 87.5 ± 14.1 | |||
| Cohort 3 | 6 | 56.9 ± 23.2 | 63.9 ± 27.2 | |||
| Total HEART CAMP intervention group | 21 | 60.7 ± 21.1 | 72.6 ± 22.2 | |||
| Attention control | ||||||
| Cohort 1 | 7 | 61.9 ± 12.6 | 78.6 ± 12.6 | |||
| Cohort 2 | 5 | 60.0 ± 30.3 | 79.2 ± 16.1 | |||
| Cohort 3 | 7 | 66.7 ± 13.6 | 66.7 ± 16.7 | |||
| Total attention control group | 19 | 63.2 ± 18.1 | 74.3 ± 15.5 | |||
| Time | 12.27 | .02 | .31 | |||
| Group × time | .01 | .94 | <.01 | |||
| Cohort (group) × time | 1.48 | .23 | .10 |
KCCQ, Kansas City Cardiomyopathy Questionnaire; SD, standard deviation; HEART CAMP, Heart Failure Exercise And Training Camp.
Time and group × time effects are each tested using the mean square of the cohort (group) × time effect as the denominator (df = 1.4). Cohort (group) × time effect was tested using the residual mean square error (dfnumerator = 4, dfdenominator = 34).
Evaluation of normality of the difference scores by group indicated no extreme outliers or large skewness or kurtosis values. Possible heterogeneity of variance (Levene’s test P < .20) was found only for change in cardiac exercise self-efficacy. The smallest cell size was associated with the smallest variance, which should result in the F-test being conservative.
When change from baseline to 12 weeks was adjusted for age, gender, and baseline level of the outcome (ignoring nesting), groups differed significantly on cardiac exercise self-efficacy (F[1, 30] = 2.06, P = .16, η2 = .05), as they had in the primary analysis. The intervention group increased an average of .9 ± .9 points compared with an increase of .2 ± .8 in the attention control group.
The effect of the intervention on change in dyspnea-fatigue scores depended on gender (intervention × gender F[1, 23] = 8.31, P = .01, η2 = .15). For men, the intervention groups differed significantly (F[1, 11] = 2.36, P = .15, η2 = .08], with improvement in the exercise group (2.5 ± 1.4) but not in the attention control group (−.4 ± 2.2). Change in dyspnea-fatigue did not differ for women in either group.
A disordinal intervention × gender effect was significant for the SF-36 physical function subscale (F[1,31] = 2.67, P = .11, η2 = .07). The mean of physical function for men increased more in the intervention (7.8 ± 7.2) than in the attention control group (1.0 ± 13.9). Women improved on physical function in the attention control group (4.2 ± 12.7), but there was a decline in the intervention group (−1.1 ± 8.0). However, neither of these simple main effects of group within gender was significant.
Results of the analyses of the KCCQ physical limitation and quality of life subscales suggested that the intervention’s effect may depend on the patient’s level on the outcome on entry into the study (F[1,30] = 1.77, P = .19, η2 = .04 for physical limitations; F[1, 32] = 3.18, P = .08, η2 = .07 for quality of life). These interactions were explored with regression plots of the outcome on the covariate in each group, but the sparseness of data in critical areas of the plots precluded further interpretation. In addition, 2 influential cases were identified in each analysis. With these cases removed, all main effects and interactions are nonsignificant, suggesting that the findings may not be robust.
DISCUSSION
Findings from this pilot study show that the HEART CAMP intervention improved patient self-efficacy for exercise over 12 weeks. The 31% improvement in self-efficacy in the intervention group was larger than the 17% improvement reported by Collins et al,13 who conducted a study of a 12-week program of cardiopulmonary training with an exercise physiologist or nurse supervising the 3 days per week exercise training sessions. The HEART CAMP intervention differed in terms of the additional group sessions with specific strategies focused on enactive mastery experiences, vicarious experiences, verbal persuasions, and physiologic and affective states that were derived from Bandura’s social cognitive theory. The large effect of the intervention on exercise self-efficacy suggests that the strategies to build confidence in the ability to exercise were effective. Given that patients with HF report lack of skills as the primary reason for not exercising,8 it seems important to build confidence in the ability to exercise (self-efficacy). Although previous research demonstrates a relationship between self-efficacy and exercise behavior in patients with HF,10,11 and -self-efficacy is the strongest predictor of physical activity,12 little is known about the effects of self-efficacy on long-term exercise behavior. The relationship between self-efficacy and long-term exercise adherence should be evaluated in future studies.
The HEART CAMP intervention resulted in 21% improvement in dyspnea-fatigue in the HEART CAMP group compared with the attention control group, whose scores worsened by 4%. Previous studies of exercise in HF had small samples, few women, varied exercise protocols (eg, walking, cycling, and combination aerobic/resistance training), and instruments to assess symptoms that make it difficult to compare findings.
The supplemental analyses suggest that change in symptoms and physical function may depend on gender. Past studies included only men22 or had less than 30% female participants.19,21,23 Future exercise studies should include measures of symptoms and adequate proportions of women to further explore potential gender effects.
Unlike the majority of past studies of exercise in HF,18 quality of life (KCCQ quality of life subscale) significantly improved over time in both groups in this study. A key difference between this study and previous research is the study design. In contrast with past studies that included a usual care comparison group, 18 this study used an attention control group with HF education delivered in small group sessions. Because participants had been on optimum medical therapy with no changes in medications over the 30 days before the study and had no clinical evidence of decompensated HF, it is unlikely that changes in quality of life were a result of improved stability of HF. The finding of improved quality of life in both groups suggests that HF educational content and the HEART CAMP intervention may improve health-related quality of life, whereas only the HEART CAMP intervention improves symptoms and self-efficacy for exercise.
The strengths of this study are the experimental repeated-measures design with use of an attention-control group and the rigorous measures to address intervention fidelity. The intervention dose was well controlled, as delivered by the study investigators and cardiac rehabilitation staff. Subject adherence of greater than 85% to the exercise sessions (5 days per week) and to small group educational sessions ensure treatment fidelity in this study. Finally, all subjects were retained throughout the 12 weeks of the intervention.
LIMITATIONS
The results of the study have limited generalizability because of the small, racially homogenous sample. Although there was approximately equal representation of men and women, there was a predominance of white participants and all subjects had documented systolic HF (ie, left ventricular ejection fraction < 40%).
The small sample size also limited data analytic options for the nested design. Rigorous evaluation of distributional assumptions was not possible with a sample of this size, nor can robustness to violations of assumptions be assumed. A larger sample would be required to follow up interaction effects and to explore the relationships between self-efficacy and exercise behavior.
Research assistants who were blinded to group assignment assisted in some of the data collection. However, because of budget constraints, the investigators who were not blinded to group assignment were also involved in data collection. This threat to validity would need to be eliminated in a larger study in which sufficient research staff could be hired so that all data collection was completed by blinded personnel.
Cardiac rehabilitation staff directed the aerobic exercise training during the first 3 weeks of the intervention, which increased the difficulty of ensuring fidelity of the intervention. Cardiac rehabilitation staff were trained in the protocol, but subjects were integrated into regular sessions with other cardiac rehabilitation patients and a researcher was not present for all sessions to ensure adherence to the protocol.
Although the intervention and attention control groups participated in an equal number of the group sessions, the intervention group received more time with study personnel. This may have had an impact on the study outcomes.
CONCLUSIONS
This study demonstrates that an exercise intervention based on Bandura’s theory can successfully improve self-efficacy for exercise in patients with HF. The small sample size precluded an evaluation of the relationship between self-efficacy and exercise, but this should be examined in future research. Cost-effectiveness should also be evaluated.
The potential for improvement in the symptoms of dyspnea and fatigue in response to exercise is important to communicate to patients. The idea of expending energy through exercise to improve symptoms is counterintuitive to patients with HF. Therefore, explaining that exercise may improve symptoms and quality of life is important to patients living with HF because they consider maintaining quality of life as important as survival.42
Interventions such as HEART CAMP are needed to build the confidence or self-efficacy beliefs to engage in regular exercise behavior. Helping patients to gain confidence in the ability to exercise and to realize improvements in symptoms and quality of life may be important factors in achieving long-term behavior change related to exercise.
Acknowledgments
Grant Support: National Institutes of Health/National Institutes of Nursing Research R15 NR009215.
References
- 1.Heart disease and stroke statistics—2010 update. Available at: http://circ.ahajournals.org/cgi/reprint/CIRCULATIONAHA.108.191261. Accessed February 26, 2010.
- 2.Flynn KE, Pina IL, Whellan DJ, et al. Effects of exercise training on health status in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. 2009;301:1451–9. doi: 10.1001/jama.2009.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gary R. Exercise self-efficacy in older women with diastolic heart failure: results of a walking program and education intervention. J Gerontol Nurs. 2006;32:31–9. doi: 10.3928/00989134-20060701-05. [DOI] [PubMed] [Google Scholar]
- 4.McKelvie RS, Teo KK, Roberts R, et al. Effects of exercise training in patients with heart failure: The Exercise Rehabilitation Trial (EXERT) Am Heart J. 2002;144:23–30. doi: 10.1067/mhj.2002.123310. [DOI] [PubMed] [Google Scholar]
- 5.Bandura A. Self-efficacy: the exercise of control. New York: WH Freeman and Company; 1997. [Google Scholar]
- 6.Pina IL, Apstein CS, Balady GJ, et al. Exercise and heart failure: a statement from the American Heart Association Committee on Exercise, Rehabilitation, and Prevention. Circulation. 2003;107:1210–25. doi: 10.1161/01.cir.0000055013.92097.40. [DOI] [PubMed] [Google Scholar]
- 7.Evangelista LS, Kagawa-Singer M, Dracup K. Gender differences in health perceptions and meaning in persons living with heart failure. Heart Lung. 2001;30:167–76. doi: 10.1067/mhl.2001.114893. [DOI] [PubMed] [Google Scholar]
- 8.Jaarsma T, Halfens R, Tan F, Abu-Saad HH, Dracup K, Diederiks J. Self-care and quality of life in patients with advanced heart failure: the effect of a supportive educational intervention. Heart Lung. 2000;29:319–30. doi: 10.1067/mhl.2000.108323. [DOI] [PubMed] [Google Scholar]
- 9.van der Wal MH, Jaarsma T. Adherence in heart failure in the elderly: problems and possible solutions. Int J Cardiol. 2008;125:203–8. doi: 10.1016/j.ijcard.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 10.Conn VS, Valentine JC, Cooper HM. Interventions to increase physical activity among aging adults: a meta-analysis. Ann Behav Med. 2002;24:190–200. doi: 10.1207/S15324796ABM2403_04. [DOI] [PubMed] [Google Scholar]
- 11.Oka RK, DeMarco T, Haskell WL. Perceptions of physical fitness in patients with heart failure. Prog Cardiovasc Nurs. 1999;14:97–102. [PubMed] [Google Scholar]
- 12.Oka RK, Gortner SR, Stotts NA, Haskell WL. Predictors of physical activity in patients with chronic heart failure secondary to either ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol. 1996;77:159–63. doi: 10.1016/s0002-9149(96)90588-3. [DOI] [PubMed] [Google Scholar]
- 13.Collins E, Langbein WE, Dilan-Koetje J, et al. Effects of exercise training on aerobic capacity and quality of life in individuals with heart failure. Heart Lung. 2004;33:154–61. doi: 10.1016/j.hrtlng.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 14.Smart N, Marwick TH. Exercise training for patients with heart failure: a systematic review of factors that improve mortality and morbidity. Am J Med. 2004;116:693–706. doi: 10.1016/j.amjmed.2003.11.033. [DOI] [PubMed] [Google Scholar]
- 15.Lloyd-Williams F, Mair FS, Leitner M. Exercise training and heart failure: a systematic review of current evidence. Br J Gen Pract. 2002;52:47–55. [PMC free article] [PubMed] [Google Scholar]
- 16.Pozehl B, Duncan K, Krueger S, VerMaas P. Adjunctive effects of exercise training in heart failure patients receiving maximum pharmacologic therapy. Prog Cardiovasc Nurs. 2003;18:177–83. doi: 10.1111/j.0889-7204.2003.02414.x. [DOI] [PubMed] [Google Scholar]
- 17.Belardinelli R, Georgiou D, Cianci G, Purcaro A. Randomized, controlled trial of long-term moderate exercise training in chronic heart failure: effects on functional capacity, quality of life, and clinical outcome. Circulation. 1999;99:1173–82. doi: 10.1161/01.cir.99.9.1173. [DOI] [PubMed] [Google Scholar]
- 18.Rees K, Taylor RRS, Singh S, Coats AJS, Ebrahim S. Exercise-based rehabilitation for heart failure. Cochrane Database Syst Rev. 2004;(3):1–39. doi: 10.1002/14651858.CD003331.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pozehl B, Duncan K, Hertzog M. The effects of exercise training on fatigue and dyspnea in heart failure. Eur J Cardiovasc Nurs. 2008;7:127–32. doi: 10.1016/j.ejcnurse.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 20.Beniaminovitz A, Lang CC, LaManca J, Mancini DM. Selective low-level leg muscle training alleviates dyspnea in patients with heart failure. J Am Coll Cardiol. 2002;40:1602–8. doi: 10.1016/s0735-1097(02)02342-2. [DOI] [PubMed] [Google Scholar]
- 21.Oka RK, De Marco T, Haskell WL, et al. Impact of a home-based walking and resistance training program on quality of life in patients with heart failure. Am J Cardiol. 2000;85:365–9. doi: 10.1016/s0002-9149(99)00748-1. [DOI] [PubMed] [Google Scholar]
- 22.Corvera-Tindel T, Doering LV, Woo MA, Khan S, Dracup K. Effects of a home walking exercise program on functional status and symptoms in heart failure. Am Heart J. 2004;147:339–46. doi: 10.1016/j.ahj.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 23.Willenheimer R, Rydberg E, Cline C, et al. Effects on quality of life, symptoms and daily activity 6 months after termination of an exercise training programme in heart failure patients. Int J Cardiol. 2001;77:25–31. doi: 10.1016/s0167-5273(00)00383-1. [DOI] [PubMed] [Google Scholar]
- 24.O’Connor CM, Whellan DJ, Lee KL, et al. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. 2009;301:11439–50. doi: 10.1001/jama.2009.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thompson B, Geller NL, Hunsberger S, et al. Behavioral and pharmacologic interventions: the Raynaud’s treatment study. Control Clin Trials. 1999;20:52–63. doi: 10.1016/s0197-2456(98)00046-4. [DOI] [PubMed] [Google Scholar]
- 26.Educational modules on heart failure. Available at: http://www.hfsa.org/heart_failure_education_modules.asp. Accessed February 26, 2010.
- 27.Duncan K, Pozehl B, Norman J, Hertzog M. A self-directed adherence management program for patients’ with heart failure completing combined aerobic and resistance exercise training. Appl Nurs Res. 2009 doi: 10.1016/j.apnr.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.American College of Sports Medicine. ACSM’s guidelines for exercise testing and prescription. 7th. Philadelphia: Lippincott Williams & Wilkins; 2005. [Google Scholar]
- 29.American College of Sports Medicine position stand. Exercise for patients with coronary artery disease. Med Sci Sports Exerc. 1994;26:i–v. [PubMed] [Google Scholar]
- 30.Bellg AJ, Resnick B, Hecht J, et al. Enhancing treatment fidelity in health behavior change studies: best practices and recommendations from the NIH behavior change consortium. Health Psychol. 2004;23:443–51. doi: 10.1037/0278-6133.23.5.443. [DOI] [PubMed] [Google Scholar]
- 31.Hickey ML, Owen SV, Froman RD. Instrument development: cardiac diet and exercise self-efficacy. Nurs Res. 1992;41:347–51. [PubMed] [Google Scholar]
- 32.Burns KJ, Camaione DN, Froman RD, Clark BA., 3rd Predictors of referral to cardiac rehabilitation and cardiac exercise self-efficacy. Clin Nurs Res. 1998;7:147–63. doi: 10.1177/105477389800700205. [DOI] [PubMed] [Google Scholar]
- 33.Feinstein AR, Fisher MB, Pigeon JG. Changes in dyspnea-fatigue ratings as indicators of quality of life in the treatment of congestive heart failure. Am J Cardiol. 1989;64:50–5. doi: 10.1016/0002-9149(89)90652-8. [DOI] [PubMed] [Google Scholar]
- 34.McHorney CA, Ware JE, Jr, Lu JF, Sherbourne CD. The MOS 36-item short-form health survey (SF-36): III. tests of data quality, scaling assumptions, and reliability across diverse patient groups. Med Care. 1994;32:40–66. doi: 10.1097/00005650-199401000-00004. [DOI] [PubMed] [Google Scholar]
- 35.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36) I conceptual framework and item selection. Med Care. 1992;30:473–83. [PubMed] [Google Scholar]
- 36.Ware J, Kosinski M, Dewey J. How to score Version Two of the SF-36 Health Survey. Lincoln, RI: Quality Metric Incorporated; 2000. [Google Scholar]
- 37.Stewart AL, Greenfield S, Hays RD, et al. Functional status and well-being of patients with chronic conditions results from the medical outcomes study. JAMA. 1989;262:907–13. [PubMed] [Google Scholar]
- 38.Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol. 2000;35:1245–55. doi: 10.1016/s0735-1097(00)00531-3. [DOI] [PubMed] [Google Scholar]
- 39.Keppel G. Design and analysis: a researcher’s handbook. 3rd. Upper Saddle River, NJ: Prentice-Hall, Inc; 1991. [Google Scholar]
- 40.Tabachnick BG, Fidel LS. Experimental designs using ANOVA. Belmont, CA: Duxbury Press; 2007. [Google Scholar]
- 41.Brown DR, Michels KM, Winer BJ. Statistical principles in experimental design. 3rd. New York: McGraw-Hill; 1991. [Google Scholar]
- 42.Lewis EF, Johnson PA, Johnson W, Collins C, Griffin L, Stevenson LW. Preferences for quality of life or survival expressed by patients with heart failure. J Heart Lung Transplant. 2001;20:1016–24. doi: 10.1016/s1053-2498(01)00298-4. [DOI] [PubMed] [Google Scholar]


