Abstract
Objective
The objective of the study was to compare rotary chair and video head impulse test (vHIT) findings in patients with bilateral vestibular hypofunction (BVH) to determine whether vHIT can: 1) define severity of BVH and 2) accurately predict rotary chair findings in patients with BVH.
Study Design
Retrospective chart review
Setting
Research Hospital
Patients
Twenty subjects with bilateral vestibular hypofunction as assessed by rotary chair.
Intervention
Rotary chair and vHIT
Main Outcome Measures
The main outcome measures were rotary chair phase, gain, and symmetry and vHIT vestibulo-ocular reflex (VOR) gain. Rotary chair and vHIT results were assessed and subjects were stratified into groups according to the severity of their vestibular hypofunction. For rotary chair, subjects were classified as Mild, Moderate, or Severe BVH. For vHIT, subjects were classified as normal, unilateral or bilateral.
Results
Average lateral canal vHIT VOR gain: 1) significantly increased as severity of BVH decreased, and 2) demonstrated a significant and positive, linear relationship with rotary chair gains. vHIT was in disagreement with rotary chair in the classification of 5 subjects, which could be due to right-left asymmetry of BVH.
Conclusion
vHIT can serve as an initial tool for identifying patients with BVH. Lower vHIT gains are consistent with having Severe BVH. There was disagreement between vHIT and rotary chair, though not for any patients with Severe BVH. Compared to rotary chair, the clinical gold standard for identifying BVH, vHIT possesses 100% sensitivity for identifying Severe BVH when average vHIT gains are < 0.46.
Keywords: bilateral, vestibular, vHIT, rotary chair, gains, saccade
INTRODUCTION
Bilateral vestibular hypofunction (BVH) classically presents with symptoms of oscillopsia and darkness-evoked disequilibrium. Vestibular function tests are employed to confirm the clinical diagnosis.1 However, quantification of BVH severity remains an elusive target. Previous work has demonstrated that vestibular function in BVH may normalize at higher frequencies, if the condition is less severe.2,3 Therefore, rotary chair testing has been advocated as the most helpful diagnostic tool for quantifying BVH severity; however, it has limitations. Rotary chair assesses vestibular function at lower frequencies than those experienced physiologically and is limited to assessment of the lateral canal.4 Additionally, rotary chair testing is not widely available and remains practically inaccessible to providers outside of major metropolitan areas.
Head-impulse testing (HIT) has been incorporated in evaluating BVH.5 For many years, HIT has been limited to a bedside exam, which lacks the ability to detect “covert” saccades. As opposed to overt saccades that occur after the head impulse and are easily detect by an experienced clinician, covert saccades occur during the head impulse and are not visible to the naked eye. Recent introduction of the video head impulse test (vHIT) allows practitioners to assess covert saccades and provides an objective measure of vestibular function.6 vHIT has been validated against other vestibulo-ocular reflex (VOR) assessments in the diagnosis of both unilateral vestibular hypofunction (UVH) and BVH.6–8
The purpose of this study was to evaluate the relationship between rotary chair and vHIT findings in a population with clinical features consistent with BVH and to determine whether vHIT can: 1) define severity of BVH and 2) accurately predict rotary chair findings in patients with BVH. We hypothesize that vHIT can be used to identify BVH and that higher vHIT gains will correlate with higher rotary chair gains. If this relationship exists, vHIT is a more accessible tool to vestibular specialists to whom rotary chair is not readily available, and could serve as the initial tool for identifying and quantifying severity of BVH. Identifying BVH and further quantifying severity of BVH carries significant implications for patient counseling and rehabilitation.2,3
METHODS
Twenty subjects (9 males, mean age of 45.3 years, range 7 – 87 years) who presented to Boys Town National Research Hospital (BTNRH) from January 2013 through December 2015 were identified as having BVH based on retrospective review of charts and vestibular test findings. BVH etiologies were collected based on available documentation. This retrospective review was approved by the Institutional Review Board at BTNRH.
Rotary Chair
Rotary chair testing was completed in all subjects in a motorized rotational chair (Micromedical Technologies, Chatham, IL) in a light-proof booth. Eye movements were recorded by either an infrared, two-dimensional video system or with two-channel electrodes. Sinusoidal harmonic acceleration (SHA) testing was completed in response to a combination of the following frequencies (maximum velocities): 0.01 Hz (80°/sec), 0.02 Hz (70°/sec), 0.04 Hz (60°/sec), 0.08 Hz (50°/sec), 0.16 Hz (40°/sec), and 0.32 Hz (30°/sec). For each SHA test, gain (eye velocity/chair velocity), phase, and symmetry were recorded. The normative values for all test parameters were provided by the manufacturer.
BVH was confirmed by rotary chair gain and phase at 0.08, 0.16, and 0.32 Hz, and at least one additional low frequency of rotation (0.01, 0.02, and/or 0.04 Hz). These frequencies were chosen because all participants had data at these 3 frequencies. Individual patient raw data were reviewed to ensure lines-of-best fit and accuracy of the automated data processing software for generating gain, phase, and symmetry values. In the low frequencies, all patients demonstrated reduced gain and either a phase lead (n = 1) or gain that was too low to generate a sufficient phase value (n = 19). In the high frequencies (0.08, 0.16, and 0.32 Hz), all patients had reduced gain at 0.08 Hz and either a phase lead (n = 4) or gain that was too low to generate a sufficient phase value (n = 16). For 0.16 Hz and 0.32 Hz, gain either normalized (n = 5) or remained reduced (n = 15) and phase either normalized (n = 4), demonstrated a lead (n = 12), or gain was too low to generate a sufficient phase value (n = 4). The differences in normalizing gain and phase in the high frequencies lead to a qualitative grouping of subjects, described below.
vHIT
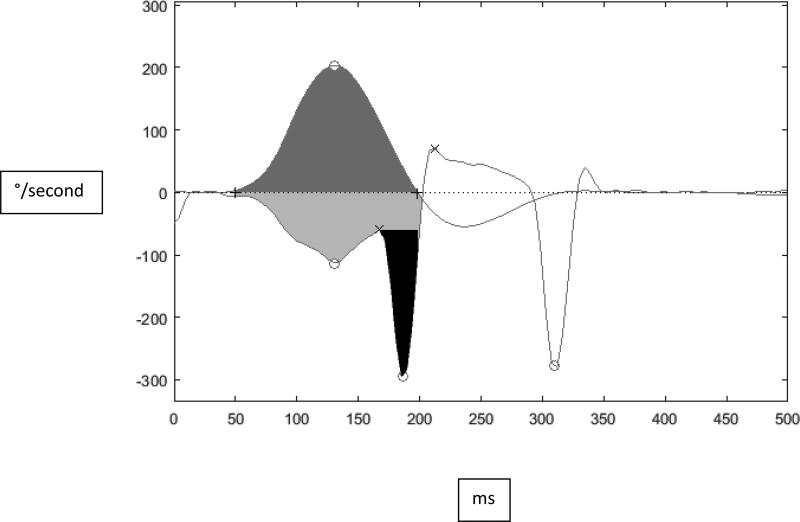
The vHIT was administered using either an ICS Impulse (Otometrics, Schaumberg, IL) or EyeSeeCam (Interacoustics, Denmark) unit. Subjects were seated 1 m from a visual target mounted at eye level on the wall. For each vHIT, the examiner stood behind the participant and delivered randomized (timing and direction) head impulses (100° to 250°/sec peak head velocity) in the plane of the lateral canals until approximately 20 acceptable head impulses were recorded for each direction (right and left). The outcome parameter was gain (eye velocity/head velocity). Gain was automatically calculated by the software. For the ICS Impulse, gain was calculated by dividing the area under the curve for eye velocity (with corrective saccades removed) by the area under the curve for head velocity. For the EyeSeeCam, the default method for calculating gain is to divide eye velocity by head velocity at 60 ms after the onset of the head impulse. There were 10 subjects evaluated with the EyeSeeCam and 10 subjects evaluated with the Otometrics device. Due to differences in the methods of gain calculations between the manufacturers, individual head impulses were analyzed in Matlab (2014a, Natick, MA), and gain was uniformly calculated for all head impulses by dividing the area under the curve for eye velocity (with corrective saccades removed) by the area under the curve for head velocity, see Figure 1. To validate this method, we compared the average vHIT gain generated in Matlab with average vHIT gain generated by the two devices. We found no statistically-significant difference between the gain calculations. Average right and left vHIT gain was calculated as a measure of overall lateral canal function.
Figure 1.
Example of area under the curve (AUC) gain calculation from the initiation of head movement until head velocity returns to 0 degrees/sec. The light gray (bottom panel) denotes AUC for eye velocity, dark gray (top panel) denotes AUC for head velocity, and the black (bottom panel) denotes the
Qualitative Assessment
Qualitative assessment of rotary chair results reflects common clinical practice and is supported by work of previous authors demonstrating retention of vestibular function in high frequencies in BVH patients9 with subsequent classifications of mild, moderate and severe.2,3,9 Therefore, 7 practitioners (3 authors and 4 non-authors, MD, PhD, or AuD) familiar with vestibular testing independently assessed and qualitatively classified each vHIT and rotary chair test, as follows:
1) Severe BVH; defined as abnormally low gain with no evidence of nystagmus in response to rotation across all frequencies, 2) Moderate BVH; defined as abnormally low gain across all frequencies with low amplitude nystagmus for some (usually higher) frequencies in response to rotation (Figure 2), and 3) Mild BVH; defined as abnormally low gain in the low frequencies that normalized in the high frequencies. Rotary chair phases were not used for this classification because in most of the subjects, rotary chair gains were too low for accurate quantification of phase.
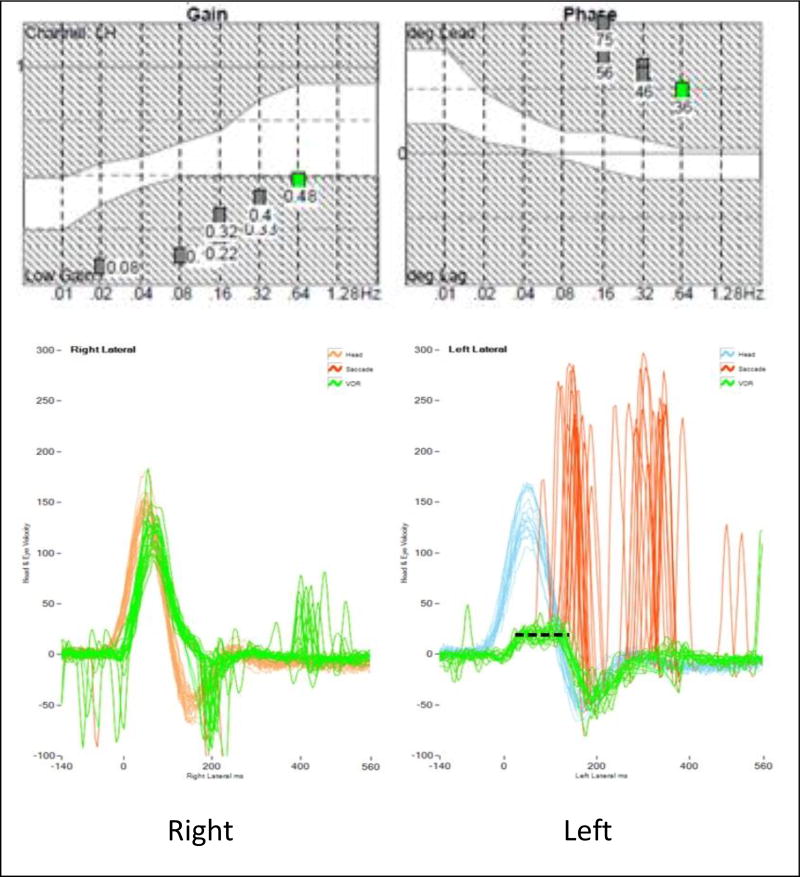
Figure 2.
Example of rotary chair and vHIT results depict: 1) clipping of the VOR on the left vHIT, highlighted by the dashed line, 2) an example of Moderate BVH on rotary chair, and 3) an example of discordant test findings between vHIT, consistent with UVH, and rotary chair, consistent with Moderate BVH.
vHIT eye velocity tracings were classified as: 1) Normal; defined as no reproducible corrective saccades10, 2) UVH; defined as presence of reproducible corrective saccades with clipping of the VOR eye velocity for head impulses in one direction only (Figure 2), and 3) BVH; defined as reproducible corrective saccades with clipping of the VOR eye velocity for head impulses in both directions. The clipping, or saturation of eye velocity shown in Figure 2, is due to the reduction or loss of excitatory neural responses for head impulses toward the side of lesion.7,11 In such cases, the inhibitory neural responses saturate quickly and the eye velocities cannot increase beyond a certain limit. For UVH, clipping occurs for impulses to one side, and for BVH, clipping occurs for both sides.
Statistical Analyses
A one-way ANOVA was completed to evaluate mean differences in rotary chair gain and vHIT gains between the rotary chair classification groups. Correlation analysis was completed to investigate the relationship between vHIT and rotary chair gain. Receiver Operating Characteristic (ROC) curves and Area under the ROC curve (AUC) were calculated to differentiate the BVH groups, using rotary chair as the clinical gold standard. All statistical analyses were completed using SPSS Version 22 (Chicago, IL).
RESULTS
Etiologies for the 20 BVH subjects included BVH associated with progressive hearing loss (n = 4), BVH associated with congenital hearing loss (n = 2), meningitis (n = 4), Ototoxic exposure (n=4) Vestibular atelectasis (n = 1), Usher syndrome (n = 1), Inner ear malformation (n = 1), and Unknown/Idiopathic (n = 3). Subject classification groups for rotary chair and vHIT are presented in Table 1. vHIT was in agreement with rotary chair classification of BVH for 15 (75%) subjects. The remaining 5 subjects (shown by an asterisk) were classified as UVH. An example of such disagreement is also shown in Figure 2, where rotary chair is consistent with Moderate BVH; however, vHIT demonstrates UVH.
Table 1.
Rotary Chair and vHIT Classifications
| vHIT Group | Total | |||
|---|---|---|---|---|
| Unilateral | Bilateral | |||
| Rotary Chair Group | Mild | 1 | 3 | 4 |
| Moderate | 4 | 4 | 8 | |
| Severe | 0 | 8 | 8 | |
| Total | 5* | 15 | 20 | |
Mean rotary chair and vHIT gains are presented in Table 2. There was a significant mean difference in the 3-frequency rotary chair gain average between rotary chair classification groups (F (2, 19) = 11.935, p = 0.001). Post hoc analysis using Tukey’s procedure suggests the mean 3-frequency rotary chair gain average was significantly different between Severe BVH and both Mild and Moderate BVH, with gain decreasing as the severity of BVH increases.
Table 2.
Mean (and range) of horizontal vHIT gain and rotary chair 3-frequency averages for Bilateral Vestibular Hypofunction Groups
| BVH Group |
n | Mean horizontal vHIT gain |
Mean rotary chair 3- frequency averages |
||
|---|---|---|---|---|---|
| Mean | Range | Mean | Range | ||
| Mild | 4 | 0.68 | 0.52–0.86 | 0.42 | 0.28 – 0.50 |
| Moderate | 8 | 0.51 | 0.26–0.69 | 0.28 | 0.11 – 0.40 |
| Severe | 8 | 0.29 | 0.17–0.45 | 0.15 | 0.03 – 0.29 |
There was also a significant mean difference in average vHIT gain between rotary chair classification groups (Figure 3A, F (2, 19) = 13.376, p < 0.001). Post hoc analysis using Tukey’s procedure suggests that average vHIT gain was significantly different between Severe BVH and both Mild and Moderate BVH, with average vHIT gain decreasing as the severity of BVH increases. Average vHIT gain also demonstrated a significant and positive correlation with rotary chair gain at each frequency of rotation (0.08 Hz: r = 0.722, p < 0.001, 0.16 Hz: r = 0.752, p < 0.001, 0.32 Hz r = 0.777, p < 0.001). The correlation increased as the frequency of rotation increased, suggesting that higher mean vHIT gains are associated with less severe BVH. This also emphasizes the role of vHIT in assessing high-frequency VOR.
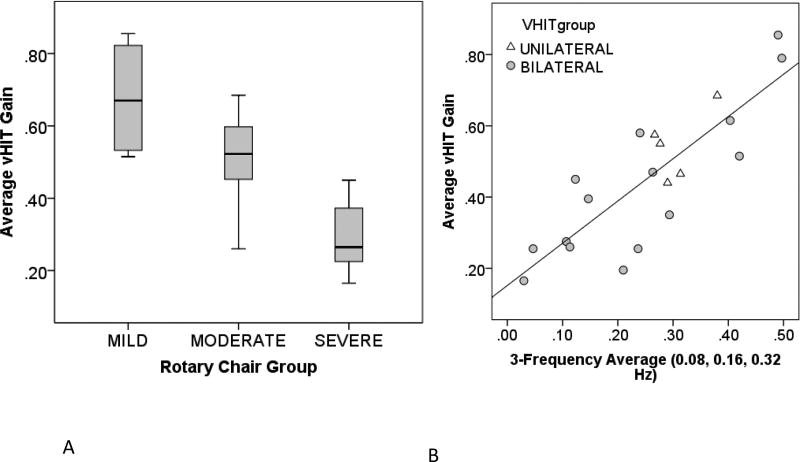
Figure 3.
Panel A) Average vHIT gain for each of the rotary chair classification groups. The horizontal line indicates the median, and the gray boxes indicate the middle interquartile range (50% of the data). Panel B) Average vHIT gain as a function of the rotary chair 3-frequency (0.08, 0.16, 0.32 Hz) average for different vHIT classifications.
As noted, lateral vHIT was in disagreement with rotary chair in the classification of BVH for 5 subjects. In Figure 3B, average vHIT gain is plotted as a function of the rotary chair 3-frequency (0.08, 0.16, 0.32 Hz) average for different vHIT classifications. Average vHIT gain demonstrated a significant and positive correlation (r = 0.836, p < 0.001) with the rotary chair 3-frequency average. As shown, regardless of whether lateral vHIT qualitatively appears bilateral (n = 15, gray circles) or unilateral (n = 5, triangles), average vHIT gain continues to be a reliable indicator of severity of BVH.
The disagreement between rotary chair and vHIT findings could be due to 1) the lack of objective criterion for quantifying BVH severity (Table 1), or 2) existence of bilateral but asymmetric vestibular hypofunction. Because rotary chair is considered the gold standard for identifying BVH and determining BVH severity, ROC curves were analyzed for average vHIT gain to differentiate between the Severe BVH (n = 8) and Moderate and Mild BVH groups combined (n = 12). The area under the ROC curve was 0.948. An average vHIT gain value of 0.46 shows sensitivity of 100% and specificity of 83.3% and gains below 0.26 shows specificity of 100% and sensitivity of 50%. These findings suggest average vHIT gain is an excellent tool for identifying Severe BVH.
DISCUSSION
BVH defies complete characterization due to its low incidence, multiple etiologies, vague symptomatology, and poorly defined diagnostic criteria.3,12,13 In addition, the most common method of evaluating vestibular function, the caloric test, is not suitable for BVH because 1) it generates low-frequency stimulation of the vestibular system, and 2) it does not accurately characterize the absolute function of each canal due to individual differences in heat transfer from the external auditory canal to the labyrinth.14 Whereas rotary chair provides multi-frequency assessment of vestibular function, only its higher frequencies overlap with the natural operational range of the VOR. vHIT on the other hand, provides assessment of high-frequency VOR function.15 Rotary chair is considered by most as the gold standard for identifying BVH and determining its severity, with caloric, dynamic visual acuity, and HIT as adjuvant tests.16–18 Some have hypothesized that “rotary chair testing is probably the best quantitative method for evaluating the severity of bilateral vestibular function” and have advocated for its role in confirming results of other high-frequency vestibular testing.2,19,20 However, rotary chair is not commonly available outside of large academic practices and is thus limited in application. This has led investigators to consider alternatives for quantification of BVH severity.
The first purpose of this study was to determine whether vHIT can define BVH severity as classified by rotary chair. In subjects classified as BVH on rotary chair, average vHIT gains were positively correlated with rotary chair gains, suggesting that in patients with BVH, average vHIT gain is predictive of BVH severity. Similarly, Weber et al6 demonstrated good agreement between vHIT gain and caloric responses in patients with BVH due to gentamicin vestibulotoxicity, where patients with average vHIT gains less than 0.3 were unresponsive to caloric testing. Moon et al21 also noted increasing average vHIT gain in patient groups as their caloric responses improved from bilateral to unilateral to normal.
Quantifying BVH severity carries significance for patients when discussing prognosis and outcome expectations, especially when prescribing vestibular rehabilitation. Patients with BVH are at higher risk for falls compared to those with UVH.22 Approximately 35–50% of BVH patients have a history of falls.2,23 BVH patients may be refractory to the benefits of vestibular rehabilitation.2,3,24–27 Quantification of severity may help stratify these patients as candidates for future therapies related to vestibular implants or gene therapy.28,29 Based on our results, vHIT could be a useful tool for this stratification.
The second purpose of this study was to determine if vHIT could accurately identify BVH based on rotary chair classifications. In fifteen (75%) subjects vHIT and rotary chair findings were in agreement, including all eight subjects with Severe BVH. The remaining five subjects (25%) had vHIT results that were in disagreement with rotary chair findings. We offer two reasons for this disagreement. First, caloric testing, rotary chair, and vHIT assess the vestibular system at increasingly higher frequencies, respectively. Hain et al19 propose that BVH is initiated at lower frequencies and progresses to high-frequency loss, which may render tests of high frequency function (i.e., vHIT, dynamic visual acuity, etc.) less likely to produce abnormal results in early stages. Therefore, we speculate the discrepancy between roatry chair and vHIT results is attributable to the severity BVH, which is frequency dependent (i.e., abnormal function in response to low frequency stimulation and normalizing function in the high frequencies). Consistent with this hypothesis, the five subjects with discordant rotary and vHIT demonstrated normal rotary chair gains in the higher frequencies. Likewise, compared to caloric testing, vHIT gains tend to decrease with increasing caloric asymmetry.30 However, there are inconsistencies with this frequency-dependent model, especially in Ménière's Disease (MD), often characterized by normal vHIT gains and abnormal caloric testing.31 While varying models have been purposed, this is an area of active research.30–32
The second possibility for the incongruent results between vHIT and rotary chair is difficulty in distinguishing mild asymmetric BVH versus UVH using rotary chair alone.5,33–35 In five of our subjects, rotary chair was consistent with BVH while vHIT was consistent with UVH. In these instances, vHIT could reflect the asymmetric nature of BVH. Understanding this relationship requires further research between vHIT, rotary chair, and caloric testing in BVH, whereas most vHIT investigations have focused on UVH patients.
When using rotary chair as the clinical gold standard for identifying BVH, comparison of average vHIT gains between Severe BVH and the remaining BVH subjects resulted in ROC curves that provide 100% sensitivity at 0.46, indicating that patients with vHIT gains above this range are unlikely to have Severe BVH. Furthermore, the positive correlation between average vHIT gain and average rotary chair gain suggests that average vHIT gain in can be used to determine level of severity. This contrasts Moon et al21 who identified 65 patients with bilateral vHIT abnormalities by either gain reduction or observable corrective saccade. Using ROC curves, they found no consistency between caloric testing, vHIT, and rotary chair for diagnosing BVH. They concluded that vHIT alone was not an adequate tool for diagnosis of BVH. The main difference between their study and the current study is that they did not separate their patients based on the severity of BVH. In addition, they did not utilize VOR gain and relied solely on the corrective saccades for vHIT.
It has typically been recommended to perform rotary chair on patients with abnormal bedside HIT and abnormal bedside dynamic visual acuity for validation of BVH29; however, not all clinics have a rotary chair. vHIT provides objective data regarding VOR function. Therefore, while we acknowledge that disagreement does occur, we propose that if vHIT is used as a first tier assessment, average vHIT gain can be used to identify severe BVH and additional testing may not be warranted. Our results suggest that in patients with average vHIT gain below 0.46 Severe BVH is likely. Further testing would be warranted in patients with unilateral vHIT to help differentiate between UVH, or asymmetrical BVH.
A limitation of our study is that it was retrospective. The subjects did not have additional uniform vestibular testing to solidify a diagnosis of BVH (i.e., consistent frequencies on rotary chair, calorics, VEMP, dynamic visual acuity etc.). The use of three frequencies with one variable low frequency was able to establish a reliable trend in this study. Nonetheless, recent literature has demonstrated the clinical and research benefit of uniform multi-test assessments in these patients as it provides a characterization of the spectrum of vestibular frequencies and how they relate to each other.21,36
Conclusion
vHIT can serve as an initial tool for identifying patients with BVH. Lower average vHIT gains are consistent with more severe BVH. When vHIT demonstrates a UVH, rotary chair testing may still be needed in patients suspected of having BVH. There was disagreement between vHIT and rotary chair, though not for any patients with Severe BVH. Compared to rotary chair, the clinical gold standard for identifying BVH, vHIT possesses 100% sensitivity for identifying Severe BVH when average vHIT gains are < 0.46.
Acknowledgments
Research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under award number P20GM109023 and by the National Institute on Deafness and Other Communication Disorders under award number R03DC015318.
We thank the vestibular audiologists at BTNRH for their qualitative review of the data.
KLJ does consulting regarding vestibular testing through Audiology Systems.
KB is a consultant to Otometrics and Bertec Corp.
Footnotes
Conflicts of Interest:
PDJ has no disclosures.
This work was presented at the following meeting:
Podium presentation at American Balance Society meeting in Scottsdale, AZ, USA on March 2th, 2016
Contributor Information
Paul D. Judge, University of Nebraska Medical Center, 989250 Nebraska Medical Center, Omaha, NE 68198, Phone: 402.559.7005; Fax: 402.559.8288.
Kristen L. Janky, Boys Town National Research Hospital, 555 N 30th St, Omaha, NE 68131.
Kamran Barin, The Ohio State University, Eye and Ear Institute, 915 Olentangy River Road, Columbus, Ohio 43212.
References
- 1.Jorns-Häderli M, Straumann D, Palla A. Accuracy of the bedside head impulse test in detecting vestibular hypofunction. J Neurol Neurosurg Psychiatry. 2007;78(10):1113–1118. doi: 10.1136/jnnp.2006.109512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gillespie MB, Minor LB. Prognosis in bilateral vestibular hypofunction. Laryngoscope. 1999;109(1):35–41. doi: 10.1097/00005537-199901000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Telian SA, Shepard NT, Smith-Wheelock M, Hoberg M. Bilateral vestibular paresis: Diagnosis and treatment. Otolaryngol -- Head Neck Surg. 1991;104(1):67–71. doi: 10.1177/019459989110400113. [DOI] [PubMed] [Google Scholar]
- 4.Ahmed MF, Goebel JA, Sinks BC. Caloric Test Versus Rotational Sinusoidal Harmonic Acceleration and Step-Velocity Tests in Patients With and Without Suspected Peripheral Vestibulopathy. Otol Neurotol. 2009;30(6):800–805. doi: 10.1097/MAO.0b013e3181b0d02d. [DOI] [PubMed] [Google Scholar]
- 5.Petersen JA, Straumann D, Weber KP. Clinical diagnosis of bilateral vestibular loss: three simple bedside tests. Ther Adv Neurol Disord. 2013;6(1):41–45. doi: 10.1177/1756285612465920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weber KP, Aw ST, Todd MJ, McGarvie LA, Curthoys IS, Halmagyi GM. Horizontal head impulse test detects gentamicin vestibulotoxicity. Neurology. 2009;72(16):1417–1424. doi: 10.1212/WNL.0b013e3181a18652. [DOI] [PubMed] [Google Scholar]
- 7.Weber KP, Aw ST, Todd MJ, McGarvie LA, Curthoys IS, Halmagyi GM. Head impulse test in unilateral vestibular loss: vestibulo-ocular reflex and catch-up saccades. Neurology. 2008;70(6):454–463. doi: 10.1212/01.wnl.0000299117.48935.2e. [DOI] [PubMed] [Google Scholar]
- 8.MacDougall HG, Weber KP, McGarvie LA, Halmagyi GM, Curthoys IS. The video head impulse test: diagnostic accuracy in peripheral vestibulopathy. Neurology. 2009;73(14):1134–1141. doi: 10.1212/WNL.0b013e3181bacf85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Black FO, Peterka RJ, Elardo SM. Vestibular reflex changes following aminoglycoside induced ototoxicity. Laryngoscope. 1987;97(5):582–586. doi: 10.1288/00005537-198705000-00008. [DOI] [PubMed] [Google Scholar]
- 10.MacDougall HG, Curthoys IS. Plasticity during Vestibular Compensation: The Role of Saccades. Front Neurol. 2012;3:21. doi: 10.3389/fneur.2012.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldberg JM. The Vestibular System : A Sixth Sense. Oxford University Press; 2012. [Google Scholar]
- 12.Rinne T, Bronstein AM, Rudge P, Gresty MA, Luxon LM. Bilateral loss of vestibular function: clinical findings in 53 patients. J Neurol. 245(6–7):314–321. doi: 10.1007/s004150050225. [DOI] [PubMed] [Google Scholar]
- 13.Jen JC. Bilateral vestibulopathy: clinical, diagnostic, and genetic considerations. Semin Neurol. 2009;29(5):528–533. doi: 10.1055/s-0029-1241035. [DOI] [PubMed] [Google Scholar]
- 14.van de Berg R, van Tilburg M, Kingma H. Bilateral Vestibular Hypofunction: Challenges in Establishing the Diagnosis in Adults. ORL J Otorhinolaryngol Relat Spec. 2015;77(4):197–218. doi: 10.1159/000433549. [DOI] [PubMed] [Google Scholar]
- 15.Alhabib SF, Saliba I. Video head impulse test: a review of the literature. Eur Arch Oto-Rhino-Laryngology. 2016 Jun; doi: 10.1007/s00405-016-4157-4. [DOI] [PubMed] [Google Scholar]
- 16.Zingler VC, Cnyrim C, Jahn K, et al. Causative factors and epidemiology of bilateral vestibulopathy in 255 patients. Ann Neurol. 2007;61(6):524–532. doi: 10.1002/ana.21105. [DOI] [PubMed] [Google Scholar]
- 17.Fife TD, Tusa RJ, Furman JM, et al. Assessment: vestibular testing techniques in adults and children: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2000;55(10):1431–1441. doi: 10.1212/wnl.55.10.1431. [DOI] [PubMed] [Google Scholar]
- 18.Kim S, Oh Y-M, Koo J-W, Kim JS. Bilateral vestibulopathy: clinical characteristics and diagnostic criteria. Otol Neurotol. 2011;32(5):812–817. doi: 10.1097/MAO.0b013e31821a3b7d. [DOI] [PubMed] [Google Scholar]
- 19.Hain TC, Cherchi M, Yacovino DA. Bilateral vestibular loss. Semin Neurol. 2013;33(3):195–203. doi: 10.1055/s-0033-1354597. [DOI] [PubMed] [Google Scholar]
- 20.Timothy C Hain. [Accessed November 14, 2016];Bilateral Vestibulopathy | American Hearing Research Foundation. http://american-hearing.org/disorders/bilateral-vestibulopathy/. Published 2012.
- 21.Moon M, Chang SO, Kim M-B. Diverse clinical and laboratory manifestations of bilateral vestibulopathy. Laryngoscope. 2016 Mar; doi: 10.1002/lary.25946. [DOI] [PubMed] [Google Scholar]
- 22.Herdman SJ, Hall CD, Maloney B, Knight S, Ebert M, Lowe J. Variables associated with outcome in patients with bilateral vestibular hypofunction: Preliminary study. J Vestib Res. 2015;25(3–4):185–194. doi: 10.3233/VES-150556. [DOI] [PubMed] [Google Scholar]
- 23.Herdman SJ, Blatt P, Schubert MC, Tusa RJ. Falls in patients with vestibular deficits. Am J Otol. 2000;21(6):847–851. [PubMed] [Google Scholar]
- 24.Shepard NT, Telian SA, Smith-Wheelock M, Raj A. Vestibular and balance rehabilitation therapy. Ann Otol Rhinol Laryngol. 1993;102(3 Pt 1):198–205. doi: 10.1177/000348949310200306. [DOI] [PubMed] [Google Scholar]
- 25.Brown KE, Whitney SL, Wrisley DM, Furman JM. Physical therapy outcomes for persons with bilateral vestibular loss. Laryngoscope. 2001;111(10):1812–1817. doi: 10.1097/00005537-200110000-00027. [DOI] [PubMed] [Google Scholar]
- 26.Whitney SL, Alghadir AH, Anwer S. Recent Evidence About the Effectiveness of Vestibular Rehabilitation. Curr Treat Options Neurol. 2016;18(3):13. doi: 10.1007/s11940-016-0395-4. [DOI] [PubMed] [Google Scholar]
- 27.Krebs DE, Gill-Body KM, Riley PO, Parker SW. Double-blind, placebo-controlled trial of rehabilitation for bilateral vestibular hypofunction: preliminary report. Otolaryngol Head Neck Surg. 1993;109(4):735–741. doi: 10.1177/019459989310900417. [DOI] [PubMed] [Google Scholar]
- 28.Staecker H, Praetorius M, Brough DE. Development of gene therapy for inner ear disease: Using bilateral vestibular hypofunction as a vehicle for translational research. Hear Res. 2011;276(1–2):44–51. doi: 10.1016/j.heares.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Phillips JO, Ling L, Nie K, et al. Vestibular implantation and longitudinal electrical stimulation of the semicircular canal afferents in human subjects. J Neurophysiol. 2015;113(10):3866–3892. doi: 10.1152/jn.00171.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCaslin DL, Jacobson GP, Bennett ML, Gruenwald JM, Green AP. Predictive properties of the video head impulse test: measures of caloric symmetry and self-report dizziness handicap. Ear Hear. 35(5):e185–91. doi: 10.1097/AUD.0000000000000047. [DOI] [PubMed] [Google Scholar]
- 31.Blödow A, Blödow J, Bloching MB, Helbig R, Walther LE. Horizontal VOR function shows frequency dynamics in vestibular schwannoma. Eur Arch Otorhinolaryngol. 2015;272(9):2143–2148. doi: 10.1007/s00405-014-3042-2. [DOI] [PubMed] [Google Scholar]
- 32.McGarvie LA, Curthoys IS, MacDougall HG, Halmagyi GM. What does the head impulse test versus caloric dissociation reveal about vestibular dysfunction in Ménière’s disease? Ann N Y Acad Sci. 2015;1343:58–62. doi: 10.1111/nyas.12687. [DOI] [PubMed] [Google Scholar]
- 33.Herdman SJ, Tusa RJ, Blatt P, Suzuki A, Venuto PJ, Roberts D. Computerized dynamic visual acuity test in the assessment of vestibular deficits. Am J Otol. 1998;19(6):790–796. [PubMed] [Google Scholar]
- 34.Barin K. Rotational Tests of Vestibular Function. Semin Hear. 2009;20(4):253–266. [Google Scholar]
- 35.Barin K. Clinical Neurophysiology of Vestibular Compensation. In: Jacobson G, Shepard NT, editors. Balance Function Assessment and Management. Plural Publishing; 2015. pp. 77–98. [Google Scholar]
- 36.Ahmed MF, Goebel JA, Sinks BC. Caloric test versus rotational sinusoidal harmonic acceleration and step-velocity tests in patients with and without suspected peripheral vestibulopathy. Otol Neurotol. 2009;30(6):800–805. doi: 10.1097/MAO.0b013e3181b0d02d. [DOI] [PubMed] [Google Scholar]