Abstract
Dyssynchrony During Acute RV Apex Pacing. Introduction: Patients with heart block have conventionally received a pacemaker that stimulates the right ventricular apex (RVA) to restore heart rate control. While RVA pacing has been shown to create systolic dyssynchrony acutely, dyssynchrony can also occur in diastole. The effects of acute RVA pacing on diastolic synchrony have not been investigated. RVA pacing acutely impairs diastolic function by increasing the time constant of relaxation, decreasing the peak lengthening rate and decreasing peak negative dP/dt. We therefore hypothesized that acute RVA pacing would cause diastolic dyssynchrony in addition to creating systolic dyssynchrony.
Methods and Results
Fourteen patients (13 ± 4 years old) with non-preexcited supraventricular tachycardia underwent ablation therapy with subsequent testing to confirm elimination of the tachycardia substrate. Normal cardiac structure and function were then documented on two-dimensional echocardiography and 12-lead electrocardiography prior to enrollment. Tissue Doppler images were collected during normal sinus rhythm (NSR), right atrial appendage pacing (AAI), and VVI-RVA pacing during the postablation waiting interval. Systolic and diastolic dyssynchrony were quantified using cross-correlation analysis of tissue Doppler velocity curves. Systolic dyssynchrony increased 81% during RVA pacing relative to AAI and NSR (P < 0.01). Diastolic synchrony was not affected by the different pacing modes (P = 0.375).
Conclusion
Acute dyssynchronous activation of the LV created by RVA pacing resulted in systolic dyssynchrony with preserved diastolic synchrony in pediatric patients following catheter ablation for treatment of supraventricular tachycardia. Our results suggest that systolic and diastolic dyssynchrony are not tightly coupled and may develop through separate mechanisms.
Keywords: ventricular dyssynchrony, pacing, diastolic function, pediatrics, echocardiography
Introduction
Both adult and pediatric patients who require heart rate control with a pacemaker (due to AV block) are conventionally paced from the right ventricular apex (RVA). However, RVA pacing acutely reduces left ventricular (LV) function,1,2 and chronic RVA pacing causes histopathological changes in the myocardium3 as well as adverse LV remodeling.4 These acute and long-term effects may be linked to the mechanical dyssynchrony in the LV created by RVA pacing.5
Several studies have shown that acute RVA pacing creates systolic dyssynchrony in the LV.2,6,7 However, dyssynchrony can be present in both systole and diastole. The effect of acute RVA pacing on diastolic synchrony in normal human hearts has not been evaluated. Pacing-induced dyssynchronous activation of the LV acutely impairs diastolic function by increasing the time constant of relaxation,8–10 decreasing the peak lengthening rate,10 and decreasing peak negative dP/dt.10,11 We therefore hypothesized that acute RVA pacing would cause diastolic dyssynchrony in addition to creating systolic dyssynchrony. We tested this hypothesis by quantifying both systolic and diastolic dyssynchrony before and after acute dyssynchronous activation created by RVA pacing in structurally normal human hearts.
Methods
Subjects
Inclusion criteria were referral for catheter ablation of non-preexcited reentrant supraventricular tachycardia (i.e., atrioventricular nodal reentrant tachycardia or atrioventricular reentrant tachycardia) and normal cardiac structure and function documented on two-dimensional echocardiography and 12-lead electrocardiography following ablation of the arrhythmia substrate. Patients were excluded if they exhibited manifest preexcitation on baseline sinus 12-lead electrocardiogram. Fourteen patients (age 12.9 ± 3.6 years, 9 male) fit the criteria and were included. The study was approved by the Children’s Healthcare of Atlanta institutional review board and all subjects gave informed consent prior to enrollment.
Electrophysiology
Studies and ablations were performed with the patient under general anesthesia with continuous propofol infusion, which is standard at our institution. All cardiac medications were discontinued 5 days before the procedure. Quadrapolar catheters were placed in the RVA, right atrial appendage and adjacent to the His bundle, and a decapolar catheter was advanced into the coronary sinus. Tachycardia mechanism was confirmed with standard electrophysiologic testing.
The decision to ablate the arrhythmia substrate was based upon clinical indications. Catheter ablation was performed either with radiofrequency energy or cryotherapy at the discretion of the attending electrophysiologist. Subsequent electrophysiologic testing confirmed elimination of tachycardia substrate in all patients.
The pacing and echocardiographic acquisition were performed during the postablation waiting interval so as not to increase the duration of the patient’s procedure. Cardiac pacing was performed at identical rates with the following configurations: atrial pacing from the right atrial appendage (AAI) and ventricular VVI pacing from the right ventricular apex (RVA). The sequence of pacing modes was random for each patient and a washout time of 3 minutes was used between pacing modes. QRS morphology was used to exclude fusion and capture beats from analysis. The pacing rate was set to 25% over normal sinus rhythm to a minimum of 100 beats per minute to ensure intrinsic ventricular conduction did not occur.
Echocardiographic Acquisition
Apical 2-, 3-, and 4-chamber tissue Doppler color velocity images were collected during each pacing mode (AAI and RVA) and during normal sinus rhythm (NSR) with a 5 MHz GE (Horten, Norway) Vivid 7 system. Patients were paced for 1 minute prior to beginning image acquisition. Tissue Doppler was acquired at a minimum frame rate of 140 Hz, and the ventricular walls were aligned to within 20° from parallel with the Doppler beam. Valve timings during each pacing mode were determined from pulsed Doppler of the aortic outflow tract and color M-mode through the mitral valve. A minimum of three beats of velocity data was collected during a breath-hold.
Tissue Doppler Postprocessing
EchoPAC PC SW postprocessing software (version 4.0.3, GE Vingmed, Horten, Norway) was used to generate 3-beat averaged velocity curves of longitudinal velocity versus time from the six basal segments of the LV. The curves were processed with the default averaging filter (30 ms) within the EchoPAC software prior to export.
Quantification of Dyssynchrony
Dyssynchrony was quantified using cross-correlation methodology that has been previously described.12 In brief, a cross-correlation function was used to calculate the time delay that resulted in maximum correlation between velocity curves from opposing ventricular segments. One velocity curve was shifted relative to the other curve, and the cross-correlation value was computed for each time shift using a normalized scale. A value of 1 meant the two curves were perfectly synchronous in time, while a value of – 1 meant the two curves were dyssynchronous. A value of 1 is achieved when correlating a velocity curve with itself (“auto-correlation”), while a value of – 1 is achieved when correlating a velocity curve with its own negative. The time shift between the two curves that resulted in the maximum correlation value was defined as the cross-correlation delay (XCD) between the two curves.
Two dyssynchrony parameters were calculated with a cross-correlation function:
Systolic XCD—The maximum cross-correlation temporal delay between the systolic portion of the myocardial velocity curves from opposing basal sections in apical 2-, 3-, and 4-chamber views. Systole was defined to include the isovolumic contraction period from mitral valve closure to aortic valve closure.
Diastolic XCD—Diastolic XCD was calculated similarly to systolic XCD, except the diastolic portion of the velocity curve was used. Diastole was defined to include the isovolumic relaxation period from aortic valve closure to mitral valve closure.
Statistics
Statistical calculations were performed in SPSS version 15.0 (SPSS Inc., Chicago, IL, USA). A value of P < 0.05 was defined as statistically significant. Dyssynchrony was compared for the different pacing modes (NSR, AAI, RVA) using a repeated-measures ANOVA with a Huynh-Feldt correction followed by a Bonferroni multiple comparison post hoc test. Data are presented as mean ± one standard deviation.
Results
Figure 1 shows velocity curves from the septal and lateral wall of a representative patient. The systolic and diastolic cross-correlation plots for this patient are also shown to illustrate how the XCD parameters were calculated. This patient showed a large increase in systolic dyssynchrony due to acute RVA pacing with preserved diastolic synchrony.
Figure 1.
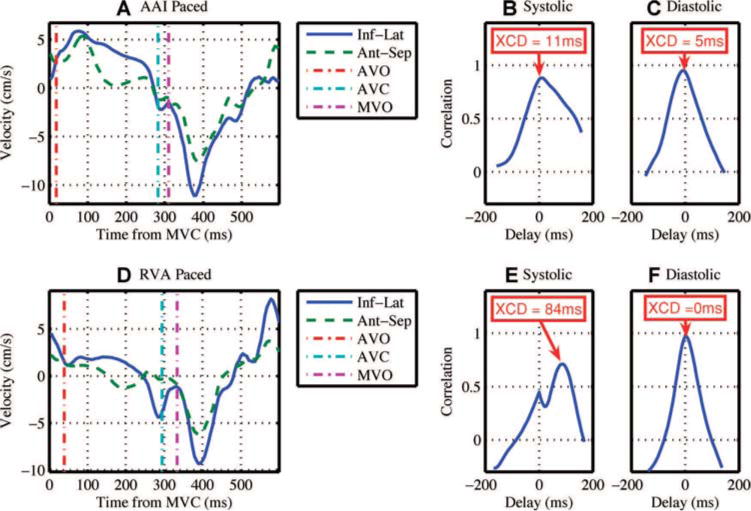
Acute pacing from the right ventricular apex creates systolic dyssynchrony with preserved diastolic synchrony. (A) Basal inferolateral (Inf-Lat) and anteroseptal (Ant-Sep) segmental velocity curves collected from a patient during right atrial appendage pacing (AAI). Valve timings are shown with mitral valve closure at time zero. (B) The cross-correlation plot of the systolic portion of the velocity curves in A. Systolic dyssynchrony is 11 ms. (C) The cross-correlation plot of the diastolic portion of the velocity curves in A. Diastolic dyssynchrony is 5 ms. (D) Basal inferolateral and anteroseptal segmental velocity curves collected from the same patient during right ventricular apex (RVA) pacing. Note that the segments are moving in opposite direction to each other for a significant portion of systole. (E) The cross-correlation plot of the systolic portion of the velocity curves in D. Systolic dyssynchrony has increased to 84 ms due to RVA pacing. (F) The cross-correlation plot of the diastolic portion of the velocity curves in D. Diastolic dyssynchrony is 0 ms. AVC = aortic valve closure; AVO = aortic valve opening; MVC = mitral valve closure; MVO = mitral valve opening; XCD = cross-correlation delay.
AAI Versus NSR
Systolic and diastolic dyssynchrony were not different between AAI and NSR (Fig. 2 and Table 1). Thus, acute atrial pacing did not change the level of synchrony from that present at baseline during NSR.
Figure 2.
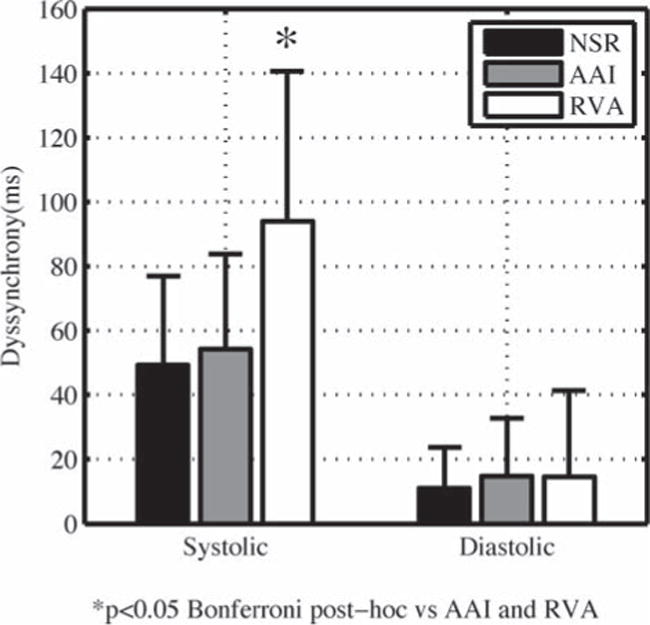
Acute right ventricular apex pacing creates systolic dyssynchrony with preserved diastolic synchrony. Bars are mean ± SD, n = 14. AAI = right atrial appendage pacing; NSR = normal sinus rhythm with no pacing; RVA = right ventricular apex pacing.
TABLE 1.
Dyssynchrony During Each Pacing Mode
| Parameter (Units) | NSR | AAI | RVA | *P (ANOVA) |
|---|---|---|---|---|
| Systolic dyssynchrony (ms) | 49 ± 28 | 54 ± 29 | 94 ± 47†‡ | 0.004 |
| Diastolic dyssynchrony (ms) | 11 ± 13 | 15 ± 18 | 15 ± 27 | 0.375 |
| QRS duration (ms) | 80 ± 15 | 78 ± 16 | 140 ± 15†‡ | <0.001 |
| QT(c) interval (ms) | 423 ± 26 | 429 ± 24 | 479 ± 26†‡ | <0.001 |
| Heart rate (bpm) | 86 ± 19 | 109 ± 15‡ | 109 ± 15‡ | <0.001 |
Data are mean ± SD, n = 14 for all pacing modes.
Repeated-measures ANOVA for comparing the values between pacing modes,
P < 0.05 versus AAI by Bonferroni post-hoc multiple comparison.
P < 0.05 versus NSR by Bonferroni post hoc multiple comparison. AAI = right atrial appendage pacing; NSR = normal sinus rhythm with no pacing; QT(c) = QT interval corrected for heart rate; RVA = right ventricular apex pacing.
Systolic Dyssynchrony
Acute RVA pacing created systolic dyssynchrony (uncorrected post hoc P = 0.016 vs AAI and P = 0.007 vs NSR) (Fig. 2). Systolic dyssynchrony increased 73% during RVA pacing relative to AAI and 90% relative to NSR.
Diastolic Dyssynchrony
Acute RVA pacing did not create diastolic dyssynchrony. Diastolic dyssynchrony did not change between the three pacing modes (Fig. 2 and Table 1).
Discussion
We sought to explore the acute effects of dyssynchronous activation on diastolic synchrony in the left ventricle. We quantified systolic and diastolic dyssynchrony in 14 patients with structurally normal hearts during normal sinus rhythm, right atrial appendage pacing, and right ventricular apex pacing. Our data demonstrate that acute right ventricular apex pacing creates systolic dyssynchrony with preserved diastolic synchrony.
Diastolic Function During Ventricular Pacing
Ventricular pacing acutely impairs diastolic function by increasing the time constant of relaxation,8–10 decreasing the peak lengthening rate,10 and decreasing peak negative dP/dt.10,11 Thus, we hypothesized that acute RVA pacing would cause diastolic dyssynchrony. However, our results lead us to reject this hypothesis and conclude that acute RVA pacing does not affect diastolic synchrony.
Our results can be explained by a discussion of the predominant factors that control relaxation in the heart. Diastolic relaxation is controlled both by the sensitivity of the contractile system to the prevailing load (load dependence) and the decaying activation (inactivation dependence).13 Several energy-dependent processes (such as the detachment of actin from myosin and sequestering of calcium into the sarcoplasmic reticulum) determine the inactivation dependence of the ventricle.14 This inactivation-dependent control mechanism modulates the load-dependent control, which is dominant during early diastolic relaxation.15 This early-diastolic load dependence enables the fibers to elongate instantaneously in unison during early diastole despite potentially inhomogeneous, or dyssynchronous, activation.15 Thus, dyssynchronous activation of healthy myocardium where the load dependence of relaxation is maintained leads to systolic dyssynchrony with preserved diastolic synchrony.
Diastolic Dyssynchrony
While most studies on dyssynchrony have focused on systole, diastolic dyssynchrony is more frequent than systolic dyssynchrony in dilated cardiomyopathy.16,17 In addition, diastolic dyssynchrony has been reported in other cardiovascular diseases such as hypertrophic cardiomyopathy.18 Thus, evaluation of diastolic synchrony may be important, and few studies have investigated this phenomenon.
This is the first study to demonstrate the effect of acute RVA pacing on diastolic synchrony in structurally normal human hearts. We demonstrated that acute RVA pacing does not affect diastolic synchrony despite the large increase in systolic dyssynchrony. Our results suggest that systolic and diastolic dyssynchrony develop through separate mechanisms. This finding is corroborated by a recent study on 373 patients with congestive heart failure in which isolated diastolic dyssynchrony was present in 18% of patients and isolated systolic dyssynchrony was present in 30% of patients.19 Other studies have also shown a lack of correlation between systolic and diastolic dyssynchrony.20 Thus, systolic and diastolic dyssynchrony do not appear to be tightly coupled and potentially develop through separate mechanisms.
In contrast to systolic dyssynchrony, diastolic dyssynchrony in heart failure may develop over a longer duration. A recent study by Kang et al. compared patients with acquired LBBB with patients with RV pacemakers and normal function.20 The RV-paced patients had a low amount of diastolic dyssynchrony that was comparable with that of the patients with acquired LBBB and normal EF. However, patients with LBBB and low EF (<35%) had nearly three times the level of diastolic dyssynchrony, compared with patients with LBBB and normal EF. The authors concluded that conduction system disease alone cannot induce diastolic dyssynchrony, and pathology of the myocardium may be the cause of both diastolic dyssynchrony and LV systolic dysfunction.20 Our results further support the hypothesis that conduction system disease alone cannot induce diastolic dyssynchrony by showing that diastolic dyssynchrony does not develop during acute dyssynchronous activation of the LV (which is a model for LBBB conduction system disease).
Dyssynchronous electrical activation of the LV leads to pathological changes in the myocardium,3 which may be the cause of diastolic dyssynchrony. Dyssynchronous activation induces hypertrophy of the LV wall in late-activated regions both in dogs21 and in humans with congenital complete heart block and pacing-induced dyssynchrony.4 LV mass index correlates with diastolic dyssynchrony in patients with heart failure and normal ejection fraction.17 Therefore, the hypertrophy due to long-term dyssynchronous activation in the LV may play an important role in causing diastolic dyssynchrony in heart failure.
The process of calcium binding and uptake is also disturbed in the failing myocardium.22,23 This may alter the load sensitivity following the isovolumic relaxation period, since efficient calcium sequestering in the sarcoplasmic reticulum is required to generate the early diastolic load dependence.15 Thus, regional inhomogeneities in the failing heart may lead to diastolic dyssynchrony due to the changes in early diastolic load dependence.
There are two studies that conflict with our results. First, Betocchi et al. found that acute ventricular pacing increases diastolic dyssynchrony relative to atrial pacing in patients with coronary artery disease.24 However, in patients with coronary disease, early onset of lengthening may occur in hypoxic muscle supplied by partially occluded coronary arteries.25,26 In addition, hypoxia suppresses the load dependence of relaxation,15 suggesting that patients with coronary artery disease may have a different response to acute pacing relative to the healthy patients in our study.
Aoyagi et al. reported that diastolic dyssynchrony increased from 12 ± 4 during right atrial pacing to 27 ± 6 during RVA sequential pacing in seven open-chest dogs.8 However, the authors only examined dyssynchrony between three segments on the anterior wall, as opposed to our study in which we examined dyssynchrony from all six walls of the LV. Also, we quantified dyssynchrony using cross-correlation methodology that is potentially more accurate in identifying dyssynchrony than time to peak analysis12 such as that employed by Aoyagi et al.
Systolic Dyssynchrony
Systolic dyssynchrony appears to be an acute effect of dyssynchronous activation. Our study and previous studies2,6,7 have shown that systolic dyssynchrony is acutely created by RVA pacing. Conduction system disease in patients with congestive heart failure can also lead to systolic dyssynchrony and worsened LV function that can be treated with cardiac resynchronization therapy (CRT) utilizing biventricular pacemakers.27 CRT acutely reduces systolic dyssynchrony,28 and the reduction can be reversed immediately when the pacemaker is turned off.29 Thus, LV systolic dyssynchrony is an acutely induced, reversible consequence of dyssynchronous activation.
Limitations
The patients in this study underwent an invasive cardiac procedure involving catheter ablation, which may have an unknown effect on structure and/or function. However, we documented normal cardiac structure and function with two-dimensional echocardiography and 12-lead electrocardiography following ablation of the arrhythmia substrate. The patients also served as their own controls, which should minimize any effect the procedure had on the data.
Threshold values to diagnose dyssynchrony in children have not yet been developed. Therefore, we could not report the individual number of patients who developed dyssynchrony due to RVA pacing and we only reported mean values.
We paced patients for 1 minute before acquiring tissue Doppler images to assess dyssynchrony. We did not pace patients for a longer duration in order to minimize the length of the catheterization procedure. Diastolic dyssynchrony may develop after a longer duration of pacing. However, multiple studies have documented the acute decline in systolic and diastolic function due to RVA pacing after only 15–30 seconds.8,10 Thus, we felt it was important to also document the effect of ventricular pacing on diastolic dyssynchrony after a similar duration of 1 minute prior to exploring the longer-term effects.
This study is limited by a small sample size (14 patients). However, the sample size was large enough to show statistical differences in systolic dyssynchrony between pacing modes.
RVA pacing was performed in VVI mode, which desynchronizes the atria and ventricles. We chose VVI over AV synchronous pacing to ensure that conduction from the atria through the AV node did not occur during RVA pacing. This atrioventricular desynchronization may affect LV diastolic synchrony. However, we found no difference in LV diastolic synchrony between AAI and VVI-RVA pacing modes. Thus, if atrioventricular desynchronization does affect diastolic synchrony, the effect is very small.
Conclusion
Acute pacing from the right ventricular apex creates systolic dyssynchrony but does not affect diastolic synchrony in pediatric patients following catheter ablation for treatment of supraventricular tachycardia. This supports the hypothesis that systolic and diastolic dyssynchrony are not tightly coupled and have separate mechanisms underlying their development.
Acknowledgments
This work was supported by grants from the Wallace H. Coulter Foundation (Miami, Florida), the American Heart Association (Dallas, TX, Pre-doctoral Fellowship for BKF, Award 0615089B), and NIH MSTP grant T32 GM08169.
Footnotes
Emory University has applied for a patent on using cross-correlation to quantify dyssynchrony. B.K. Fornwalt and Drs. Fyfe and Oshinski are coauthors on the patent.
References
- 1.Karpawich PP, Mital S. Comparative left ventricular function following atrial, septal, and apical single chamber heart pacing in the young. Pacing Clin Electrophysiol. 1997;20:1983–1988. doi: 10.1111/j.1540-8159.1997.tb03605.x. [DOI] [PubMed] [Google Scholar]
- 2.Cojoc A, Reeves JG, Schmarkey L, Strieper MJ, Joyner RW, Wagner MB, Campbell RM, Vinten-Johansen J, Frias PA. Effects of single-site versus biventricular epicardial pacing on myocardial performance in an immature animal model of atrioventricular block. J Cardiovasc Electrophysiol. 2006;17:884–889. doi: 10.1111/j.1540-8167.2006.00504.x. [DOI] [PubMed] [Google Scholar]
- 3.Karpawich PP, Rabah R, Haas JE. Altered cardiac histology following apical right ventricular pacing in patients with congenital atrioventricular block. Pacing Clin Electrophysiol. 1999;22:1372–1377. doi: 10.1111/j.1540-8159.1999.tb00631.x. [DOI] [PubMed] [Google Scholar]
- 4.Thambo JB, Bordachar P, Garrigue S, Lafitte S, Sanders P, Reuter S, Girardot R, Crepin D, Reant P, Roudaut R, Jais P, Haissaguerre M, Clementy J, Jimenez M, Thambo J-B, Bordachar P, Garrigue S, Lafitte S, Sanders P, Reuter S, Girardot R, Crepin D, Reant P, Roudaut R, Jais P, Haissaguerre M, Clementy J, Jimenez M. Detrimental ventricular remodeling in patients with congenital complete heart block and chronic right ventricular apical pacing [see comment] Circulation. 2004;110:3766–3772. doi: 10.1161/01.CIR.0000150336.86033.8D. [DOI] [PubMed] [Google Scholar]
- 5.Vanagt WY, Verbeek XA, Delhaas T, Mertens L, Daenen WJ, Prinzen FW. The left ventricular apex is the optimal site for pediatric pacing: Correlation with animal experience. Pacing Clin Electrophysiol. 2004;27:837–843. doi: 10.1111/j.1540-8159.2004.00544.x. [DOI] [PubMed] [Google Scholar]
- 6.Catanzariti D, Maines M, Cemin C, Broso G, Marotta T, Vergara G. Permanent direct his bundle pacing does not induce ventricular dyssynchrony unlike conventional right ventricular apical pacing. An intrapatient acute comparison study. J Interv Card Electrophysiol. 2006;16:81–92. doi: 10.1007/s10840-006-9033-5. [DOI] [PubMed] [Google Scholar]
- 7.Wyman BT, Hunter WC, Prinzen FW, McVeigh ER. Mapping propagation of mechanical activation in the paced heart with MRI tagging. Am J Physiol. 1999;276:H881–H891. doi: 10.1152/ajpheart.1999.276.3.H881. [DOI] [PubMed] [Google Scholar]
- 8.Aoyagi T, Iizuka M, Takahashi T, Ohya T, Serizawa T, Momomura S, Sato H, Mochizuki T, Matsui H, Ikenouchi H, et al. Wall motion asynchrony prolongs time constant of left ventricular relaxation. Am J Physiol. 1989;257:H883–H890. doi: 10.1152/ajpheart.1989.257.3.H883. [DOI] [PubMed] [Google Scholar]
- 9.Blaustein AS, Gaasch WH. Myocardial relaxation. VI. Effects of beta-adrenergic tone and asynchrony on LV relaxation rate. Am J Physiol. 1983;244:H417–H422. doi: 10.1152/ajpheart.1983.244.3.H417. [DOI] [PubMed] [Google Scholar]
- 10.Zile MR, Blaustein AS, Shimizu G, Gaasch WH. Right ventricular pacing reduces the rate of left ventricular relaxation and filling. J Am Coll Cardiol. 1987;10:702–709. doi: 10.1016/s0735-1097(87)80215-2. [DOI] [PubMed] [Google Scholar]
- 11.Bedotto JB, Grayburn PA, Black WH, Raya TE, McBride W, Hsia HH, Eichhorn EJ. Alterations in left ventricular relaxation during atrioventricular pacing in humans. J Am Coll Cardiol. 1990;15:658–664. doi: 10.1016/0735-1097(90)90642-3. [DOI] [PubMed] [Google Scholar]
- 12.Fornwalt BK, Arita T, Bhasin M, Voulgaris G, Merlino JD, León AR, Fyfe DA, Oshinski JN. Cross-correlation quantification of dyssynchrony: A new method for quantifying the synchrony of contraction and relaxation in the heart. J Am Soc Echocardiogr. 2007;20:1330–1337. e1331. doi: 10.1016/j.echo.2007.04.030. [DOI] [PubMed] [Google Scholar]
- 13.Brutsaert DL, Sys SU. Relaxation and diastole of the heart. Physiol Rev. 1989;69:1228–1315. doi: 10.1152/physrev.1989.69.4.1228. [DOI] [PubMed] [Google Scholar]
- 14.Brutsaert DL, de Clerck NM, Goethals MA, Housmans PR. Relaxation of ventricular cardiac muscle. J Physiol. 1978;283:469–480. doi: 10.1113/jphysiol.1978.sp012513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brutsaert DL, Housmans PR, Goethals MA. Dual control of relaxation. Its role in the ventricular function in the mammalian heart. Circ Res. 1980;47:637–652. doi: 10.1161/01.res.47.5.637. [DOI] [PubMed] [Google Scholar]
- 16.Schuster I, Habib G, Jego C, Thuny F, Avierinos J-F, Derumeaux G, Beck L, Medail C, Franceschi F, Renard S, Ferracci A, Lefevre J, Luccioni R, Deharo J-C, Djiane P. Diastolic asynchrony is more frequent than systolic asynchrony in dilated cardiomyopathy and is less improved by cardiac resynchronization therapy. J Am Coll Cardiol. 2005;46:2250–2257. doi: 10.1016/j.jacc.2005.02.096. [DOI] [PubMed] [Google Scholar]
- 17.Wang J, Kurrelmeyer KM, Torre-Amione G, Nagueh SF. Systolic and diastolic dyssynchrony in patients with diastolic heart failure and the effect of medical therapy. J Am Coll Cardiol. 2007;49:88–96. doi: 10.1016/j.jacc.2006.10.023. [DOI] [PubMed] [Google Scholar]
- 18.Pacileo G, De Cristofaro M, Russo MG, Sarubbi B, Pisacane C, Calabro R. Hypertrophic cardiomyopathy in pediatric patients: Effect of verapamil on regional and global left ventricular diastolic function. Can J Cardiol. 2000;16:146–152. [PubMed] [Google Scholar]
- 19.Yu CM, Zhang Q, Yip GW, Lee PW, Kum LC, Lam YY, Fung JW. Diastolic and systolic asynchrony in patients with diastolic heart failure: A common but ignored condition. J Am Coll Cardiol. 2007;49:97–105. doi: 10.1016/j.jacc.2006.10.022. [DOI] [PubMed] [Google Scholar]
- 20.Kang SJ, Song JK, Yang HS, Song JM, Kang DH, Rhee KS, Nam GB, Choi KJ, Kim JJ, Kim YH. Systolic and diastolic regional myocardial motion of pacing-induced versus idiopathic left bundle branch block with and without left ventricular dysfunction. Am J Cardiol. 2004;93:1243–1246. doi: 10.1016/j.amjcard.2004.01.068. [DOI] [PubMed] [Google Scholar]
- 21.van Oosterhout MF, Prinzen FW, Arts T, Schreuder JJ, Vanagt WY, Cleutjens JP, Reneman RS. Asynchronous electrical activation induces asymmetrical hypertrophy of the left ventricular wall. Circulation. 1998;98:588–595. doi: 10.1161/01.cir.98.6.588. [DOI] [PubMed] [Google Scholar]
- 22.Harigaya S, Schwartz A. Rate of calcium binding and uptake in normal animal and failing human cardiac muscle. Membrane vesicles (relaxing system) and mitochondria. Circ Res. 1969;25:781–794. doi: 10.1161/01.res.25.6.781. [DOI] [PubMed] [Google Scholar]
- 23.Lindenmayer GE, Sordahl LA, Harigaya S, Allen JC, Besch HR, Jr, Schwartz A. Some biochemical studies on subcellular systems isolated from fresh recipient human cardiac tissue obtained during transplantation. Am J Cardiol. 1971;27:277–283. doi: 10.1016/0002-9149(71)90303-1. [DOI] [PubMed] [Google Scholar]
- 24.Betocchi S, Piscione F, Villari B, Pace L, Ciarmiello A, Perrone-Filardi P, Salvatore C, Salvatore M, Chiariello M. Effects of induced asynchrony on left ventricular diastolic function in patients with coronary artery disease. J Am Coll Cardiol. 1993;21:1124–1131. doi: 10.1016/0735-1097(93)90234-r. [DOI] [PubMed] [Google Scholar]
- 25.Theroux P, Ross J, Jr, Franklin D, Kemper WS, Sasyama S. Regional myocardial function in the conscious dog during acute coronary occlusion and responses to morphine, propranolol, nitroglycerin, and lidocaine. Circulation. 1976;53:302–314. doi: 10.1161/01.cir.53.2.302. [DOI] [PubMed] [Google Scholar]
- 26.Wiegner AW, Allen GJ, Bing OH. Weak and strong myocardium in series: Implications for segmental dysfunction. Am J Physiol. 1978;235:H776–H783. doi: 10.1152/ajpheart.1978.235.6.H776. [DOI] [PubMed] [Google Scholar]
- 27.Bax JJ, Abraham T, Barold SS, Breithardt OA, Fung JW, Garrigue S, Gorcsan J, 3rd, Hayes DL, Kass DA, Knuuti J, Leclercq C, Linde C, Mark DB, Monaghan MJ, Nihoyannopoulos P, Schalij MJ, Stellbrink C, Yu CM. Cardiac resynchronization therapy: Part 1— Issues before device implantation. J Am Coll Cardiol. 2005;46:2153–2167. doi: 10.1016/j.jacc.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 28.Yu CM, Lin H, Fung WH, Zhang Q, Kong SL, Sanderson JE. Comparison of acute changes in left ventricular volume, systolic and diastolic functions, and intraventricular synchronicity after biventricular and right ventricular pacing for heart failure. Am Heart J. 2003;145:E18. doi: 10.1016/S0002-8703(03)00071-1. [DOI] [PubMed] [Google Scholar]
- 29.Bax JJ, Molhoek SG, van Erven L, Voogd PJ, Somer S, Boersma E, Steendijk P, Schalij MJ, Van der Wall EE. Usefulness of myocardial tissue Doppler echocardiography to evaluate left ventricular dyssynchrony before and after biventricular pacing in patients with idiopathic dilated cardiomyopathy. Am J Cardiol. 2003;91:94–97. doi: 10.1016/s0002-9149(02)03009-6. [DOI] [PubMed] [Google Scholar]


