Trailblazers and Inspiration
The most important focus of my research has been the epidemiologic and genetic factors that contribute to macular degeneration, the leading cause of visual impairment among the elderly in numerous countries around the world. For this work, I am extremely honored to receive the Mildred Weisenfeld Award. The Association for Research in Vision and Ophthalmology (ARVO) has been a large part of my career for approximately 3 decades, and I am very grateful for this recognition. I was Chair of the first ARVO committee on Women and Minorities when I was a trustee from 1992 to 1996, and then became vice president. Although there were only a few women in leadership positions at that time, ARVO is making strides in showcasing the research of all of its members, including the 40% of ARVO membership now composed of women. I share the distinct honor of being one of only two women who have received this award, joining Dr. Joan Miller among a group of 32 awardees since 1986.
Like so many of your stories, my story begins and ends with inspiration. I have been inspired to spend a large part of my career focused on the chronic disease, age-related macular degeneration (AMD), a progressive disease affecting the central part of the retina. I am both grateful and humbled by the inspiration I drew from great scientists in the past, from the many colleagues who have supported me, my family and friends, many of whom are here today, and my patients. I would like to tell their story as well as mine about how we began to focus on macular degeneration.
As one of only a few women in medicine at the beginning of my career, I was also inspired by historic trailblazers in the field. Dr. Isabel Barrows was the first female ophthalmologist in the United States and practiced in the late 1800s.1 Mildred Weisenfeld was also a pioneer and a trailblazer. She was diagnosed with retinitis pigmentosa in 1936. Her goal was to find more answers about her disease, and during her life, she led several efforts to support research. She founded the Fight for Sight organization in 1946 and in 1986, she established the Mildred Weisenfeld Award for Excellence in Ophthalmology.
ARVO also generated a lot of friendships and collaborations and many of those continue to this day. It was also an opportunity for some of our family to get together and I have drawn a lot of inspiration from my family. My parents, as first-generation immigrants, were role models for hard work and perseverance. They always encouraged me to pursue higher education and inspired me to go to medical school. During medical school, I became interested in why diseases occur at different rates within different populations, the causes of these diseases, and how they could be prevented, which then inspired me to get another degree in epidemiology.
Epidemiology and Application to Ophthalmology
Epidemiology is the study of the frequency, determinants, and patterns of disease in human populations. There have been many breakthroughs in epidemiology involving germ theory, disease transmission, vaccinations, study designs, and risk factors, and all of these are being used today. John Snow traced cholera to the Broad Street pump in the 1840s. Smallpox was the first disease eradicated by vaccination. The Framingham Heart Study led to the initial identification of heart disease risk factors that are very important to this day, and the Surgeon General Report in 1964 on smoking and lung cancer was preceded by 35 epidemiologic studies that provided evidence for this association.
I was the first ophthalmologist who obtained a degree in epidemiology at the Harvard School of Public Health. Before applying my knowledge to AMD research, as a resident I conducted a case-control study of ocular hypertension,2 collected the data, punched it onto the old computer cards, processed the data, and used logistic regression for the first time in ophthalmic research. Our study showed that family history of glaucoma, myopic refractive error, an absence of liquor intake, history of nonocular surgery, higher income, and a history of hypertension all increased risk for this diagnosis. Then during my ophthalmic pathology and retina fellowships, I applied my skills to the rare and potentially lethal malignancy, uveal melanoma.3–7 Using case-control methodology with sibling and population controls, as I proposed in my first National Institutes of Health (NIH) R01 grant, I evaluated the association between constitutional factors and environmental sources of UV radiation and risk of uveal melanoma.6 We found a higher risk of melanoma among patients of northern European ancestry, light skin color, and those who had at least 10 cutaneous nevi. In prospective studies, I also applied my ophthalmic pathology fellowship training and devised a novel quantitative system for counting and grading malignant epithelioid cells, measured height and diameters of the tumors, and used Kaplan-Meier survival curves and Cox proportional hazards analyses for the first time in this field, leading to new prognostic markers of this disease.3,7
Macular Degeneration
Following these early studies, I applied my epidemiology training to the much more common eye disease, macular degeneration. We did not know very much about the etiology of AMD back then, and questions from my patients were largely unanswered: “Why am I getting this disease?” “Is this disease going to progress?” “Are my family members at risk?” “How can I prevent it from getting worse?” We knew that the disease was related to aging, but there was a lot more to learn. My research goal then and still is, to provide some answers to why the disease occurs in some people and not others, why it progresses to advanced stages in a subgroup of those affected, how to prevent and delay its progression to reduce the burden of visual loss related to this potentially debilitating disease, and to use this knowledge to find new treatments.
I explain to my patients that the retina lines the back of the eye like a film in a camera, and the macula is the center of the retina, which is needed for clear, straight-ahead central vision. Macular degeneration associated with aging is called age-related macular degeneration, referred to as AMD. This disease makes our vision blurry, dark, and distorted, rendering it difficult to read, write, drive, use a computer, and watch TV, essentially interfering with our activities of daily living.
AMD is a leading cause of visual impairment worldwide.8–11 Our population is getting older as people are living longer, and the number of older people in the United States is expected to double in the next 30 years. So, many more people get this disease. There are now approximately 2 million individuals with visual loss due to AMD in the United States, and this will rise to approximately 5 million in a few decades.8 The global prevalence of AMD is estimated at 196 million affected individuals, and this disease burden is projected to rise by 40%, to 288 million, by 2040.11 Thus, AMD has been called an epidemic.
Many of my patients are affected by this disease and they have provided another major source of inspiration to me throughout my career. One man with AMD had tears in his eyes when he looked at me and said, “The most disturbing impact is that I can no longer see the faces of the people I love.”
For our studies, we developed a five-stage classification system for evaluating the clinical aspects of AMD. The Clinical Age-Related Maculopathy Staging (CARMS) system12 was implemented which is used today by my research team as well as others.13,14 CARMS grades were defined as follows: grade 1 (no AMD, no drusen or only a few small drusen <63 μm), grade 2 (early AMD, intermediate-size drusen 63–124 μm), grade 3 (intermediate AMD, large drusen ≥125 μm), grade 4 (advanced dry AMD, or geographic atrophy [GA], including both central and noncentral forms), and grade 5 (advanced exudative AMD, or neovascular disease [NV], with choroidal neovascularization).
In the 1980s, I initiated and submitted grant proposals for case-control and longitudinal cohort studies on nutrition, dietary intake, and other modifiable risk factors to identify markers of progression to advanced stages of AMD, and also launched twin and familial studies to dissect the genetic and environmental components of this disease. Grant reviews said, “the disease is not genetic.” Then, I resubmitted and emphasized the nongenetic factors and they said, “the disease is genetic.” For the prospective longitudinal proposal to study risk factors for progression, reviewers raised concerns that outcomes would still occur after the 5-year grant period, the maximum time frame for a grant. All of you who write and depend on grants understand what I mean, but we persevered and marched on, and eventually we were successful in obtaining funding to look at the genetic and nongenetic factors underlying the disease.
I learned in epidemiology courses that a twin study is the classic way to study and dissect genes and environment. I developed protocols to study the elderly twins in the World War II (WWII) Veteran Twin Registry, surveyed more than 12,000 twins, and collected clinical data and ocular photographs with the assistance of ophthalmologists nationwide (Seddon JM, et al. IOVS 1997;37:ARVO Abstract 676). This was the first population-based twin study of eye disease and provided an opportunity to evaluate the presence of AMD in a large cohort of both monozygotic and dizygotic twin pairs. The underlying assumption was that in a twin study, if a given disease is 100% genetic, the same AMD phenotypes would be observed among all monozygotic twins sharing the same genetic profile, and only approximately 50% of the dizygotic twins would have the same phenotype. However, this was not the case. Results demonstrated a substantial genetic component to macular degeneration, but there was also an important environmental influence. Our analyses showed that a high proportion of AMD was attributable to genetics, with heritability ranging from 46% to 71%, depending on the stage of the disease. More advanced disease, as well as larger drusen and greater drusen area measuring 175 μm or larger were highly heritable, with estimates of 71%. The environmental influence on this disease is also notable (19% to 37%).15 Therefore, both nature and nurture were important in the development of AMD. This underlying premise that both lifestyle and genetic factors contributed to AMD provided the “guiding light” for my research objectives over the past 30 years: to evaluate the impact of both lifestyle and genetic factors on AMD outcomes, and to determine how these two aspects might work together in their contribution to individual risk.
Nongenetic Factors
Now we know that the most important nongenetic factors related to AMD are essentially an individual's behaviors and lifestyles, including history of cigarette smoking, dietary patterns, and anthropometric measurements. These factors can be modified in a direction that is either detrimental or protective, with corresponding impact on AMD risk. Attention to these modifiable factors is, therefore, of utmost importance in reducing the onset of AMD as well as its progression. Studies I initiated in the 1980s included various epidemiologic designs, case-control and longitudinal cohort studies, to determine risk and preventive factors that precede development of advanced AMD, and also family and twin studies to assess genes and environment.
Cigarette Smoking
The leading modifiable risk factor is cigarette smoking. In the first large prospective study of AMD,16 we determined that women who currently smoked at least 25 cigarettes per day had a significantly higher risk of AMD causing visual loss of 20/30 or worse, compared with women who never smoked (risk ratio [RR] 2.4; 95% confidence interval [CI] 1.4–4.0; P trend = 0.004). Past smokers of the same amount had two times the risk of AMD compared with never smokers (RR 2.0; 95% CI 1.2–3.4; P trend = 0.002). Even more striking was the observation that approximately 29% of AMD cases in this study were attributable to this modifiable factor. So, cigarette smoking was an independent risk factor for AMD. Other studies have shown that cigarette smoking increases oxidative stress and lipid peroxidation, reduces plasma antioxidant levels and levels of high-density lipoprotein (HDL) cholesterol, and increases inflammation and risk of vascular disease, which are all mechanisms associated with AMD. So, do not smoke, and tell your family, friends, and your patients that smoking also affects their eyes.
Nutrition
Back in the same time period, there was an interest in the potential impact of vitamins on cancer and heart disease and other diseases of aging. We are exposed to daily insults, such as high-energy visible light and normal metabolic processes, that lead to free radicals and harmful oxidation. The retina is highly susceptible to oxidative processes, because the photoreceptor outer segment membranes are rich in polyunsaturated fatty acids. So, we hypothesized that nutritional factors and vitamins and minerals with antioxidant properties could reduce risk of AMD. I was the principal investigator at one of the clinical centers in the National Eye Institute (NEI)-sponsored Eye Disease Case-Control Study in the mid-1980s. This study did not have any nutritional component. I initiated and developed the Nutritional Ancillary Study, as well as the nutritional biomarker component of the study, and designed a food frequency questionnaire (FFQ) for use in eye research, which we demonstrated to be reliable and reproducible.17 All centers collected diet data using this FFQ, as well as blood samples for nutritional assays. Our center analyzed the data and focused initially on the antioxidants, dietary carotenoids, lutein and zeaxanthin, and vitamins A, C, and E.18
Our results showed that dietary carotenoids reduced risk of AMD. The highest quintile of dietary carotenoid intake was significantly associated with a 43% lower risk of AMD (odds ratio [OR] 0.57; 95% CI 0.35–0.92; P trend = 0.02) compared with the lowest intake, adjusting for age, sex, clinic location, education, systolic blood pressure, self-reported physical activity level, alcohol intake, body mass index (BMI), and smoking status. Among the specific carotenoids, lutein and zeaxanthin, which are primarily obtained from dark green, leafy vegetables, were most strongly associated with a reduced risk of AMD, and the higher the quintile of intake, the lower the risk, a significant dose-response relationship (P trend < 0.001). On the other hand, dietary intake of beta-carotene had no effect on risk of AMD, and the highest intake group did not differ from the lowest intake group.18
Several food items rich in carotenoids were inversely associated with AMD. In particular, a higher frequency of intake of spinach or collard greens was associated with a substantially lower risk for AMD. Results suggested an 88% lower risk with higher intake, defined as eating a one-half cup serving at least five times per week. Other foods that are high in lutein and zeaxanthin include dark green leafy vegetables, kale, turnip greens, and collard greens. These results were biologically plausible because the carotenoid, lutein, is located in the macula and it has anti-inflammatory and antioxidant properties, mechanisms that can contribute to a reduction in AMD. Diets containing 6 mg per day of lutein and zeaxanthin had the lowest risk in our study, which has become the dose used in many nutraceuticals on the market.
We also observed differential effects on AMD risk for specific dietary fats, not only in the case-control study (Seddon JM, et al. IOVS 1994;34:ARVO Abstract 2003), but also in a prospective longitudinal study of early and intermediate disease to identify why some people develop the late stages over time and others do not.19–21 High total fat intake was associated with almost a three-fold higher risk of progression and saturated and trans-unsaturated fats conferred over a 2-fold higher rate of progression from nonadvanced to advanced stages of AMD.20 Higher intake of omega-3 fats, which are found in high levels in fish and some nuts, reduced risk of progression to advanced AMD by 25% to 40%, particularly among participants with lower linoleic acid intake. These associations between AMD and dietary fats, as well as the association with smoking, were also found in our analyses of the twin cohort.21 The attributable risk was 32% for smoking and 22% for dietary omega-3.
Following initiation of our studies on nutrition and macular degeneration, the Age-Related Eye Disease Study (AREDS) was launched by NEI in 1991.22 This was a multicenter clinical trial of supplements containing vitamin C, vitamin E, zinc, and beta-carotene led by Dr. Rick Ferris at NEI. Our Clinical Center in Boston also played a role. The combination of supplements reduced progression from intermediate to advanced AMD by 25% over 5 years.22 Our 1994 results of a beneficial intake of 6 mg per day of lutein and lack of any benefit for intake of beta-carotene18 were supported in the analyses of the AREDS dietary data in 2007.23 A subsequent clinical trial, AREDS2, led by Dr. Emily Chew at NIH, included supplements with lutein, zeaxanthin, and omega-3 fatty acids. Supplements containing vitamin C, E, zinc, as well as lutein and zeaxanthin are now recommended for individuals with intermediate-level AMD.24
Anthropometric Factors and Exercise
We also evaluated modifiable anthropometric factors, including BMI, waist circumference, and waist-to-hip ratio, in our prospective cohort.25 A BMI defined as obese (≥30) was significantly associated with a higher risk of progression to advanced stages of AMD (RR 2.35; 95% CI 1.27–1.34), as was the overweight classification (RR 2.32; 95% CI 1.32–4.07). A significant trend was observed for higher risk with higher BMI (P trend = 0.007). The highest tertile of waist circumference significantly increased risk of progression (RR 2.04; 95% CI 1.12–3.72; P trend = 0.02) compared with the lowest tertile. A higher waist-to-hip ratio also increased risk of progression (RR [tertile 3 vs. tertile 1] 1.84; 95% CI 1.07–3.15; P trend = 0.02). In contrast, higher levels of physical activity tended to reduce risk of progression.
Parallels to Cardiovascular Disease
All of these associations (diet, obesity, smoking) pointed to a similarity between dietary and lifestyle factors associated with AMD and cardiovascular disease.26 We therefore hypothesized that biomarkers related to cardiovascular disease, such as C-reactive protein (CRP), a marker of inflammation, and homocysteine, also would be related to AMD, and found that higher levels of both of these biomarkers and other inflammatory cardiovascular markers were associated with AMD risk.27–29 Unhealthy lifestyle factors, including smoking and higher BMI, were associated with higher levels of these inflammatory cytokines. Vitamin C, lutein/zeaxanthin, and fish consumption were associated with lower serum CRP levels, and vitamin E, alpha-carotene, antioxidants, and vitamin B6 were associated with lower levels of homocysteine.28 Because AMD and cardiovascular disease appeared to share common antecedents,26 targeting these risk factors has the potential to reduce the burden of both major chronic diseases.
Our epidemiologic data on nutrition and eye disease were incorporated into a science-based cookbook, Eat Right for Your Sight,30 with the help of the American Macular Degeneration Foundation. Many of the recipes contain the healthy nutrients that reduce inflammation, act as antioxidants, and play a mechanistic role in the etiology and prevention of AMD. Eating right for your sight is very much like eating right for your life.
Genetic Factors
As the modifiable risk factors in AMD pathology evolved, collectively with others in the field, we began to put the pieces of this very complex puzzle of AMD together. In addition to the identification of lifestyle factors, we hypothesized early on that genetic factors also played a role. Our research team began to collect DNA specimens along with epidemiologic data about modifiable factors in the 1980s in twin and longitudinal cohort studies. To determine if there was familial aggregation and to support our grant proposals, we evaluated relatives of patients and found that relatives of individuals with advanced disease had a 3-fold higher risk of AMD, compared with relatives of controls who did not have AMD.31 We also began the twin study of elderly twins in the WWII registry, as described above. Now over the past 30 years, we have accrued approximately 10,000 individuals, including 600 families throughout the country, in our study cohort. The biorepository and database include blood samples, ocular imaging, phenotypes classified using our CARMS grading system,12 and many have longitudinal follow-up for studies of progression over time.
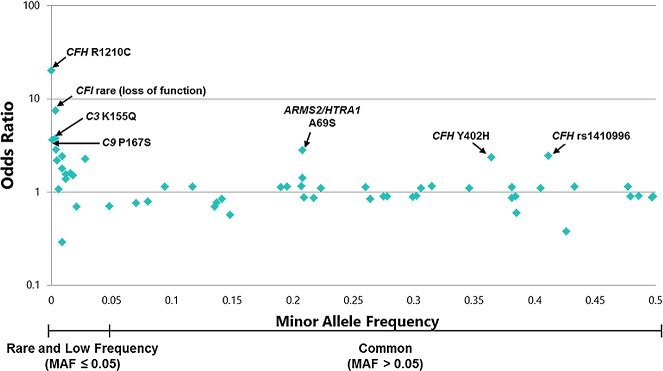
Genetic analyses of AMD evolved over time,32 and included the following types of study designs: linkage studies pointing to numerous chromosomes,33–36 case-control association studies,37–49 genome-wide association studies (GWAS),50–56 and, later, whole-exome sequencing studies.57,58 Candidate gene studies also were done early on, but did not demonstrate a common link between monogenic retinal diseases and AMD. We now know that AMD is a common, polygenic disease wherein multiple common variants, defined as variants with a minor allele frequency >5%, contribute varying amounts to personal risk. Rare and low-frequency variants are found in fewer than 5% of the population, and are more likely to be deleterious. As depicted in Figure 1, there are numerous common and rare variants associated with AMD to date. The effect sizes are much larger for the rare variants, conferring up to a 20-fold increased risk, compared with up to a 3-fold risk for common variants.
Figure 1.
Display of genetic variants associated with AMD risk according to minor allele frequency and estimated OR. All ORs are calculated based on risk per effective allele and are on a logarithmic scale.
Why look for genes? They shed light on disease pathogenesis, aid in diagnosis, and can point to new avenues of treatment. Mechanisms and pathways by which many of these genetic variants confer risk include dysregulation of the complement pathway, disruption of the extracellular collagen matrix, dysfunction of genes regulating lipids and cholesterol, increased growth of new blood vessels (angiogenesis), and DNA repair mechanisms.
Common Variants
GWAS and case-control studies have been instrumental for the identification of the common variants, including complement factor H (CFH) Y402H variant identified by four independent groups,37–40 as well as others in the complement pathway.44–48 Our laboratory identified a previously unrecognized, intronic genetic variant in CFH (rs1410996) that increased the influence of this locus on AMD,44 which has been replicated in numerous studies. We also discovered a new common variant in C3 that showed a significant association with AMD,46 as well as a common variant near complement factor I.48 These common variants, as well as additional loci identified in complement factor B (CFB) and complement component 2 (C2) are well established in their roles to confer AMD risk.44,45 The discovery of several complement-related loci lend further support to the theory that inflammation and immune mechanisms play a critical role in the pathogenesis of AMD.
Loci implicating other pathways also have been associated with AMD with genome-wide significance.41,50–53,55,59,60 We discovered the association of LIPC with AMD, a variant in the hepatic lipase gene that functions in the HDL pathway.50,51 In collaborative efforts, our teams discovered additional relationships with variants in CETP and ABCA1, which also have the potential to modify HDL cholesterol.50,51,61 Collectively, in a parallel study, we found a relationship with TIMP3,51 a gene that was previously associated with Sorsby's fundus macular dystrophy, although we did not detect linkage or association with this gene in a smaller size study several years before.62 In a large meta-analysis, we discovered additional new loci in the genes FRK/COL10A1 and COL8A1 in the collagen matrix pathway and VEGFA, the first genome-wide significant AMD gene in the angiogenesis or new blood vessel growth pathway.52 More recently, using an exome array design that included 4332 cases and 25,268 controls, we discovered three protective variants.59 In this study, two protective, low-frequency variants were significantly associated with a lower risk of AMD: A307V in PELI3 and N1050Y in CFH. We additionally identified a common variant near CTRB1 (rs8056814) that reduced risk of AMD. With international consortium efforts also using an exome array design, 52 genetic variants in 34 loci were associated with AMD, including those previously reported.60
The emerging genetic results to date indicate that AMD has contributions from numerous pathways and mechanisms: complement and immune response; lipids, including HDL cholesterol; angiogenesis; the collagen, connective tissue, and extracellular matrices; and DNA repair and protein binding. Discovery of genes conferring higher or lower risk of disease has important implications for understanding disease pathogenesis. Targeting these pathways can lead to novel and improved treatments, and knowledge of genetic susceptibilities could lead to more tailored drug therapies.
AMD Phenotypes Associated With Common Genetic Variants
The studies above focused mainly on advanced AMD, so we explored genetic associations with other sub-phenotypes. In our large national cohort of twins and families, we found that peripheral retinal drusen beyond the macula and posterior retinal vascular arcades and peripheral retinal reticular pigment changes are associated with the diagnosis of AMD. These phenotypes were also associated with AMD genotypes. Two variants in the gene CFH, CFHY402H, and rs1410996, conferred a 2-fold higher risk of having these peripheral retinal phenotypes, even after adjusting for the presence or absence of AMD.63 These associations were not seen for the genes CFB, C2, or C3.
Another important question was whether genetic susceptibility differed between the two advanced subtypes of AMD: GA and NV. Analyses of our cohort showed that the ARMS2 locus conferred increased risk for both advanced AMD subtypes, but was the only 1 among the 115 single nucleotide polymorphisms (SNPs) evaluated that imparted greater risk for NV compared with GA.64 We confirmed this association in a GWAS meta-analysis: the genetic variant in ARMS2 conferred increased risk for both types of AMD, with somewhat greater risk for the neovascular form.54 This finding was also confirmed in larger meta-analyses.
Rare Variants
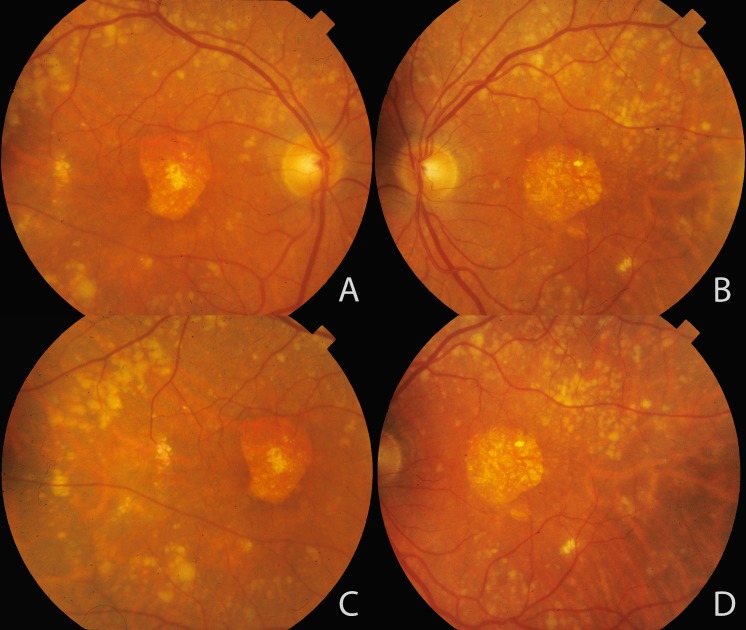
The common variants explained approximately 65% of heritability of AMD, yet a notable proportion of the heritability of AMD remained unexplained,44,55 so we hypothesized that the remaining amount may be explained by rare variation (minor allele frequency <5%). As these rare variants are found in a smaller proportion of the population, they can have much larger effects on AMD risk, and the impact of a rare variant is more likely to be causal.10,65 We therefore searched for the impact of rare variants on risk of AMD and used genotype data and high-throughput sequencing to discover the first confirmed association between a rare, highly penetrant variant and AMD. This high-risk CFH haplotype with a c.3628C>T mutation resulted in an R1210C substitution. This mutation is like a spelling error in the genome or an amino acid substitution at a specific location: arginine is converted to a cysteine at position 1210. This rare variant is highly penetrant and confers the strongest genetic risk for AMD to date, with an OR greater than 20 (Fig. 1).65 We compared the phenotypes of carriers versus noncarriers and found that this mutation is associated with an earlier age of diagnosis of advanced AMD compared with noncarriers,65,66 as well as extensive drusen accumulation in the macula and throughout the fundus, high risk for advanced disease, and symmetrical disease in the two eyes, as depicted in Figure 2.66
Figure 2.
Extensive drusen accumulation throughout retinal vascular arcades associated with the complement factor H (CFH) R1210C rare variant. Color fundus photographs of a patient with the CFH R1210C rare variant and geographic atrophy in the right (A, C) and left (B, D) eyes are shown. Large confluent soft drusen are seen in the macular area and extending throughout the topography of retinal vascular arcades and beyond. Reprinted with permission from Ferrara D, Seddon JM. Phenotypic characterization of complement factor H R1210C rare genetic variant in age-related macular degeneration. JAMA Ophthalmol. 2015;133:785–791. Copyright 2015 American Medical Association.
Given this success, we continued on the path toward searching for other rare variants. We applied a novel analysis strategy described in our 2010 report in which we evaluated the degree to which known variants explain the clustering of AMD in a group of 322 densely affected families.67 For a subset of the families, the mean genotypic load was significantly lower than the expected load determined from simulation. We hypothesized that these families may harbor rare, more penetrant variants for AMD and suggested that these families undergo linkage analysis and resequencing to find the implicated genes. We used this approach and focused on families with high disease burden and low AMD genetic risk,67 and applied whole-exome sequencing. We found additional novel rare variants in the gene CFH: R53C and D90G, which explained disease in families and cases carrying those variants.57 Furthermore, we determined that four rare loss-of-function variants in CFH were associated with disease in four other families.58 These rare variants and the common Y402H variant in CFH are depicted in Figure 3.68 We then conducted a large targeted sequencing study evaluating the exons in 681 genes using 2493 samples in our cohort from extreme phenotypes of advanced cases and unaffected controls, and replication genotyping in 5115 independent samples.69 This effort led to novel discoveries of rare variants in C3 (K155Q) and complement component 9 (C9, P167S). The C3 rare variant was also reported simultaneously in independent cohorts.70,71 In our study, we also found a burden of 59 rare variants in CFI, with a 7.5-fold elevated risk of advanced AMD for rare CFI variants predicted to either damage and reduce the protein or cause loss of function and abolish the protein.69
Figure 3.

Factor H is a plasma protein (300 μg/mL) consisting entirely of 20 homologous complement control proteins (CCPs). Each CCP module contains approximately 60 amino acids, linked together (like beads on a string) by 3 to 8 amino acids in an extended head-to-tail fashion. Selected SNPs are shown, including the common Y402H polymorphism and several rare variants with high penetrance identified in familial AMD and independent cases. The three major functional sites of the rare variants are noted, including the complement regulatory site (CCPs 1–4) mediating cofactor activity and two regions that mediate transfer of this plasma protein to a surface, such as a drusen or a damaged RPE cell. Adapted from Triebwasser MP, Roberson EDO, Yu Y, et al. Rare variants in the functional domains of complement factor H are associated with age-related macular degeneration. Invest Ophthalmol Vis Sci. 2015;56:6873–6878. Copyright 2015 Association for Research in Vision and Ophthalmology.
Functional Impact of Rare Variants
Unlike common variants, these rare variants have clear functional significance, provide mechanistic insights, and can point to therapeutic targets. We initially explored functional impact of these variants through analyses of plasma complement components and activation fragments leveraging our sample biorepository. Increased levels of activation fragments were independently associated with AMD.72 Functional experiments in our targeted sequencing study with our collaborator, John Atkinson at Washington University in St. Louis, showed that the novel rare mutation we found in C3 resulted in excessive alternative complement activation.69 Some rare variants in CFI led to lower serum factor I (FI) levels (Fig. 4) and low FI levels were associated with higher risk of AMD.73 Rare variants in the functional domains of CFH were enriched in the advanced AMD cohort and also reduced levels of the protein factor H in the blood.58,68 Rare variants found in CFH and CFI strongly implicate complement activation in AMD etiopathogenesis, because these genes interact to inhibit the alternative pathway.
Figure 4.
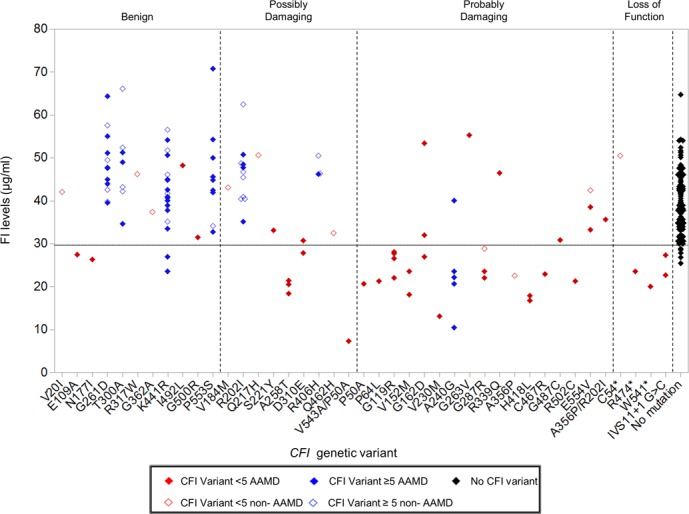
Serum FI levels of individuals stratified by CFI genetic variant. CFI variants with counts <5 in the sequencing panel are colored red. Variants with counts ≥5 are blue. Genetic variants in individuals with advanced age-related macular degeneration (AAMD) are represented by a filled diamond and comparison group without AAMD are shown with an unfilled diamond. Genetic variants are grouped into benign, possibly damaging, probably damaging, or loss of function as predicted by PolyPhen2. Two individuals were compound heterozygotes (p.V543A/p.P50A; p.A356P/p.R202I). p.R202I and p.V543Awere predicted to be possibly damaging, while A356P and P50A were predicted to be probably damaging. The black filled diamonds represent 95 individuals (47 non-AAMD, 48 AAMD) with no CFI variants. The lower limit of normal is demonstrated by a dotted line (29.3 μg/mL). Reprinted with permission from Kavanagh D, Yu Y, Schramm EC, et al. Rare genetic variants in the CFI gene are associated with advanced age-related macular degeneration and commonly result in reduced serum factor I levels. Hum Mol Genet. 2015;24:3861–3870. © The Author 2015. Published by Oxford University Press, under the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0/).
More investigation is warranted to dissect the functional impact of these variants. Gain-of-function mutations confer new or enhanced activity of the protein, whereas loss-of-function variants reduce or abolish the function of the protein. They collectively lead to ongoing activation of the alternative complement pathway in AMD, and loss of the regulatory activity tilts the balance toward amplification and excessive activation of this pathway. When working well, this system assists in favorable immune responses, and increased immune activity is advantageous in reducing the risk of infections. However, chronic dysregulation and overactivity can be damaging to cells rendered vulnerable in the retina, leading to AMD in susceptible individuals. The mechanism related to the amplification of the alternative complement pathway is schematically depicted in Figure 5.69 Potential new treatments for AMD that target various points along this pathway are now being tested in clinical trials.74
Figure 5.
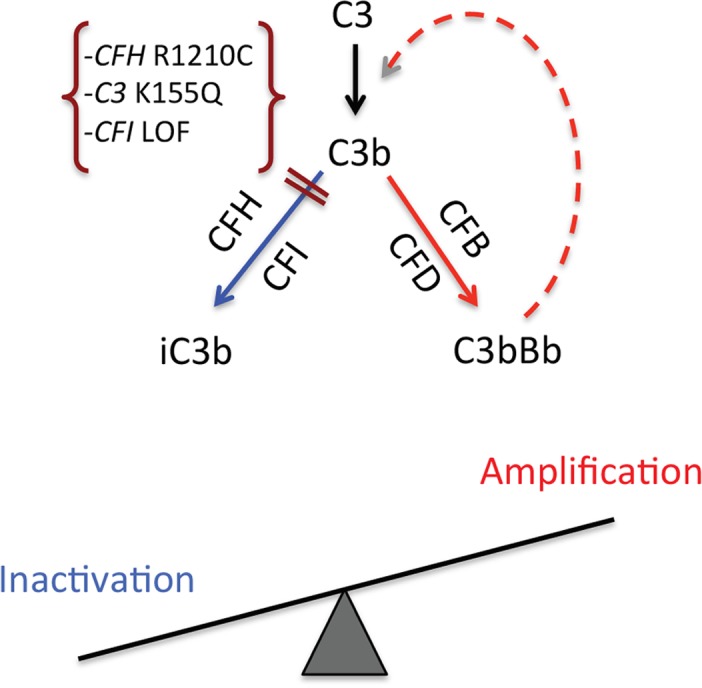
The two fates of C3b generated in the retina. On C3 activation, the resulting C3b may be either proteolytically cleaved (inactivated) or generate a C3 convertase (C3bBb) to produce more C3b (a feedback or amplification loop). On the left, the combined action of regulatory proteins factor H (CFH) and factor I (CFI) cleave and inactivate C3b, resulting in iC3b, which does not participate in the amplification process. On the right, C3b, together with factor B (FB) and factor D (FD), forms a C3 convertase, which, in turn, cleaves C3 and generates more active C3b. Thus, variants seen in AMD, such as a loss of function (LOF) in CFH (e.g., R1210C), LOF in CFI, or a gain of function in C3 (e.g., K155Q) (e.g., Refs. 65, 69, 73), would alter the balance toward excessive complement activation and thereby could enhance tissue damage. Not shown in this diagram is that properdin stabilizes (positive regulator) the alternative pathway C3 convertase, increasing its half-life up to 10-fold (20–30 seconds to 3–4 minutes). Reprinted with permission from Seddon JM, Yu Y, Miller EC, et al. Rare variants in CFI, C3 and C9 are associated with high risk of advanced age-related macular degeneration. Nat Genet. 2013;45:1366–1370. Copyright 2013 Nature America, Inc., part of Springer Nature.
Common and Rare Variants and AMD Progression
Genes conferring AMD risk are not only related to the occurrence of AMD as found in case-control studies, but we also found they are important in determining the rate of progression of disease over time, from early and intermediate stages to advanced clinical phenotypes. We reported in 2007 that CFH Y402H and ARMS2/HTRA1 were independently and significantly associated with progression, the first longitudinal report of AMD genes and progression.75 Subsequently, additional common variants, including CFH rs1410996, C2 E318D, CFB R32Q, C3 R102G, RAD51B, and COL8A1, as well as the rare variants CFH R1210C and C3 K155Q, were also evaluated, and these common and rare variants conferred a higher risk of transitioning from nonadvanced to advanced stages of disease.76–79
Gene-Environment-Biomarker Interactions
Discordant Twins
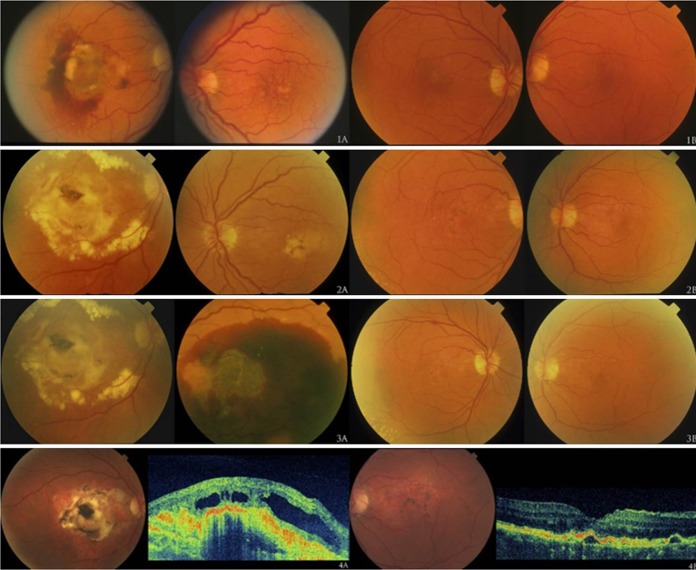
The combined impact of modifiable and genetic factors on AMD is particularly evident in our epigenetic studies of monozygotic (MZ) twins. We evaluated MZ twin pairs with discordant AMD phenotypes to assess differences in their modifiable risk factors, specifically behavioral and nutritional components.80 The impact of nature and nurture is clear in the representative twin pair shown in Figure 6.80 The images for twin A and his MZ co-twin twin B appear sequentially over the course of several years. At each time point, twin A (left) is more advanced than his co-twin (right). To explore the reasons for this difference, we evaluated nongenetic factors. We observed that pack-years of smoking were higher for twins with more advanced disease (P = 0.05), and their diets were different. Lower intake of the nutrients vitamin D (P = 0.01) and betaine and methionine (P = 0.009) were significantly associated with a more advanced AMD stage.80 Smoking and these dietary factors are associated with epigenetic mechanisms, and these mechanisms could also influence occurrence of the disease. Our twin studies of epigenetics suggested a role for gliosis, a process that is associated with neovascular disease.81 Observing a differential effect of diet and smoking on AMD outcomes in MZ twins sharing the same genetic susceptibility provided support for additional studies of gene-environment interactions.
Figure 6.
Discordant macular phenotypes within one monozygotic twin pair. The fundus appearance of twin A is represented in (1A) to (4A), and that of twin B is depicted in (1B) to (4B) on the right. Photos were taken at clinic visits when twins were age 64 (March 1997), 68 (April 2001), 68.5 (July 2001), and 74 (August 2007). (4A) and (4B) are representative ultra– high-resolution optical coherence tomography scans. Twin A had advanced neovascular disease and twin B had drusen and RPE irregularities. Reprinted with permission from Seddon JM, Reynolds R, Shah HR, Rosner B. Smoking, dietary betaine, methionine, and vitamin D in monozygotic twins with discordant macular degeneration: epigenetic implications. Ophthalmology. 2011;118:1386–1394. Copyright 2011 American Academy of Ophthalmology.
Diet-Gene Interactions
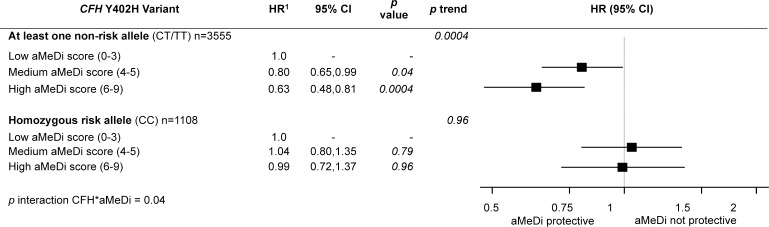
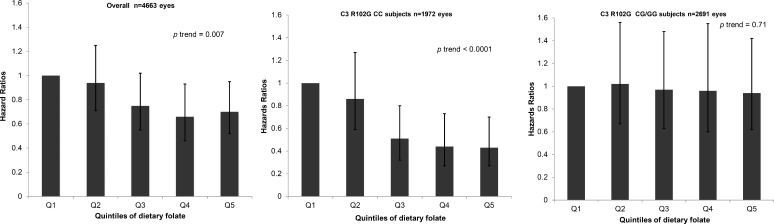
We found the highest quintile of omega-3 intake was associated with a lower risk of progression to geographic atrophy, when compared with the lowest intake, and this beneficial effect was noted particularly among individuals who carried the homozygous risk genotype for ARMS2 (hazard ratio [HR] 0.4; P = 0.002; P interaction = 0.05).82 No protective effect was observed for the ARMS2 homozygous nonrisk genotype. Additional gene-diet differences were observed with regard to high adherence to a Mediterranean diet (Fig. 7). High adherence reduced the risk of progression to advanced AMD, and specifically among those individuals carrying at least one nonrisk allele at CFH Y402H (HR 0.63; 95% CI 0.48–0.81; P trend = 0.0004; P interaction = 0.04).83 There was no effect of the Mediterranean diet on risk of progressing to advanced AMD among individuals carrying the CFH homozygous risk genotype (CC). In our diet-gene evaluation of dietary folate, high consumption of dietary folate was significantly associated with a lower risk of progression (HR 0.43; 95% CI 0.27–0.70; P = 0.0005).84 We found a protective effect of higher folate intake against progression to geographic atrophy, particularly among individuals carrying the C3 R102G homozygous nonrisk genotype (Fig. 8). The beneficial effect of folate was not observed for those carrying at least one risk allele (G) at this locus. We also recently reported that participants with the highest quintile of dietary vitamin D intake had a significantly lower risk of progression to advanced stages of AMD, and especially NV.85 This effect also may vary according to genotype.
Figure 7.
Effect of adherence to the alternate Mediterranean diet (aMeDi) on progression to advanced AMD according to CFH Y402H genotypes. HRs and 95% CIs were estimated by using Cox proportional hazards models with individual eye as the unit of analysis, adjusted for age, sex, AMD status at baseline for both eyes, AREDS treatment, total energy intake, educational level, smoking, BMI, supplement use, and the other nine genetic variants (CFH rs1410996, CFH rs121913059 [R1210C], ARMS2/HTRA1 rs10490924, C2 rs9332739 [E318D], CFB rs641153 [R32Q], C3 rs2230199 [R102G], C3 rs147859257 [K155Q], COL8A1 rs13095226, and RAD51B rs8017304). P trend was calculated by using median values within each category. Low aMeDi score (0–3) was the referent. Reprinted with permission from Merle BM, Silver RE, Rosner B, Seddon JM. Adherence to a Mediterranean diet, genetic susceptibility, and progression to advanced macular degeneration: a prospective cohort study. Am J Clin Nutr. 2015;102:1196–1206. Copyright 2015 American Society for Nutrition.
Figure 8.
Effect of dietary folate on progression to geographic atrophy according to C3 R102G genotype. HRs, 95% CIs, and P trend were calculated by using Cox proportional hazards models adjusted for age, sex, education, smoking, BMI, AREDS treatment, multivitamin supplement use, AMD status at baseline in both eyes, total energy intake, and nine genetic variants (CFH: rs1061170 [Y402H], CFH: rs1410996, CFH: rs121913059 [R1210C], ARMS2/HTRA1: rs10490924, C2: rs9332739 [E318D], CFB: rs641153 [R32Q], C3: rs147859257 [K155Q], COL8A1: rs13095226, RAD51B: rs8017304). Reprinted with permission from Merle BM, Silver RE, Rosner B, Seddon JM. Dietary folate, B vitamins, genetic susceptibility and progression to advanced nonexudative age-related macular degeneration with geographic atrophy: a prospective cohort study. Am J Clin Nutr. 2016;103:1135–1144. Copyright 2016 American Society for Nutrition.
Biomarker-Gene Associations
Interactions between genes and other biomarkers provide additional insights. We found that CRP and genetic variants in the genes CFH and ARMS2 were independently associated with risk of AMD, and that higher CRP levels tended to confer higher risk of AMD in most genotype groups.86 We also explored the effect of HDL levels and the HDL pathway gene LIPC on risk of AMD. We found that the HDL-raising allele of the LIPC gene (T) was associated with a reduced risk of AMD, higher total cholesterol and low-density lipoprotein levels were associated with increased risk, and higher HDL levels tended to reduce risk.87 We also evaluated LIPC in relation to other known modifiable factors for AMD. Smoking and higher BMI increased risk of AMD and higher intake of lutein reduced risk, adjusting for genetic variants. LIPC was associated with reduced risk of advanced AMD independent of the demographic and environmental variables.88 The associations between LIPC and other HDL pathway genes related to AMD and the underlying mechanisms are under investigation.
Treatment-Gene Interactions
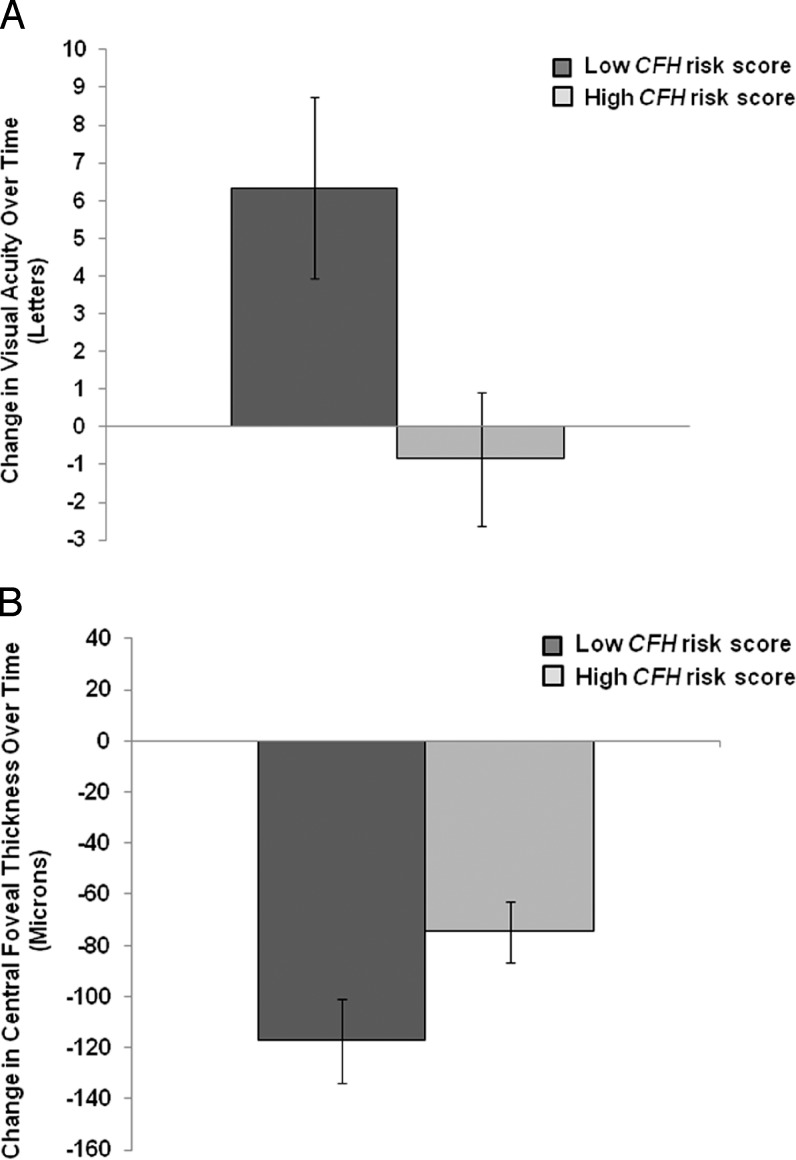
Pharmaco-genetics has been explored by many groups. We evaluated the effect of genes on outcomes following anti-VEGF treatment, including visual acuity (Fig. 9A) and central foveal thickness (Fig. 9B).89 Significant improvement in visual acuity was observed for the nonrisk CFH Y402H genotype (P < 0.001) and for a low CFH risk score (P = 0.019). Improvement in central foveal thickness was observed after treatment among individuals with a low CFH risk score (P = 0.033).
Figure 9.
(A) Change in visual acuity (VA) over time for low versus high CFH risk scores after intravitreal anti-VEGF treatment for AMD. Among subjects with a low CFH risk score, there was a notable improvement in VA over time (6.33 ± 2.40 letters) compared with no demonstrable improvement for subjects in the high-risk group (Pheterogeneity = 0.019). VA is measured by Early Treatment Diabetic Retinopathy Study acuity in letters. Standard error (SE) is illustrated by vertical error bars. (B) Change in central foveal thickness as measured by optical coherence tomography for low versus high CFH risk scores after intravitreal anti-VEGF treatment for AMD. Among subjects with a low CFH risk score, there was greater reduction in central foveal thickness over time compared with the high-risk group (Pheterogeneity = 0.033). SE is illustrated by vertical error bars. Reprinted with permission from Shah AR, Williams S, Baumal CR, Rosner B, Duker JS, Seddon JM. Predictors of response to intravitreal anti-vascular endothelial growth factor treatment of age-related macular degeneration. Am J Ophthalmol. 2016;163:154–166.e8. Copyright 2016 Elsevier, Inc.
To determine if genotype could modify the risk of developing advanced AMD after supplementation with antioxidants and zinc, we evaluated the genotypes for CFH Y402H and ARMS2.90 Among antioxidant and zinc supplement users, patients with the nonrisk genotype for CFH (TT) had a lower risk of progression to advanced AMD (HR 0.55; 95% CI 0.32–0.95; P = 0.033). However, there was no significant treatment effect among patients who were homozygous for the CFH risk allele (CC). A protective effect was observed among patients with the high-risk ARMS2 (TT) genotype (HR 0.52; 95% CI 0.33–0.82; P = 0.005).
Prediction Models for Progression to Advanced AMD
Given the emergence of personalized medicine and targeted therapies, it is important to consider the utility of evaluating individual genotypes to inform the selection of patient-specific strategies. As in many chronic diseases, the potential for personalized medicine is evolving in ophthalmology. AMD stands alone in having numerous lifestyle and genetic risk factors with confirmed, high impact on disease development and progression.79 Over the past 11 years, we have developed a series of algorithms that predict risk for progression to advanced stages of AMD over time, combining known demographic, behavioral, ocular, and genetic factors.44,75–79,91–93 These models have achieved high predictive values and can discriminate between progressors and nonprogressors to advanced stages of disease.
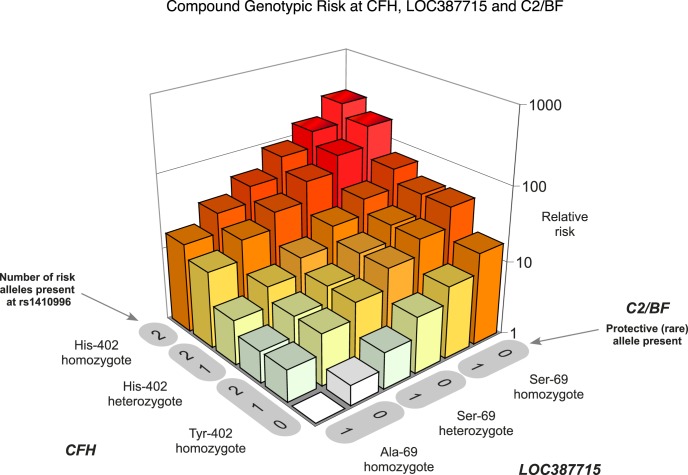
We developed the initial polygenic risk model in 2006,44 and showed that individuals carrying homozygous risk variants at each of three loci (CFH Y402H, CFH rs1410996, and ARMS2 A69S) had the highest level of AMD risk. These high-risk individuals had more than a 250-fold greater risk compared with those individuals who carried the homozygous nonrisk genotype at all three loci. The relative risk of AMD as a result of genetic burden is depicted in Figure 10.44
Figure 10.
Relative risk plotted as a function of the genetic load of the five variants that influence risk of AMD. Two variants are in the CFH gene on chromosome 1: Y402H and rs1410996. Another common variant (A69S) is in the gene on chromosome 10. Two less common variants are observed in the C2 and CFB genes on chromosome 6. There was no evidence for interaction between any of these variants, suggesting an independent mode of action. Reprinted with permission from Maller J, George S, Purcell S, et al. Common variation in three genes, including a noncoding variant in CFH, strongly influences risk of age-related macular degeneration. Nat Genet. 2006;38:1055–1059. Copyright 2006 Nature America, Inc., part of Springer Nature.
As these loci were associated with AMD onset, we hypothesized that they also might be associated with disease progression. We subsequently evaluated the impact of genetic variants in CFH and ARMS2 on rates of progression from early or intermediate disease to advanced stages of AMD.75 Our initial prospective model determined that polymorphisms in these genes were significantly and independently associated with progression to advanced disease. Carriers of the homozygous risk genotypes for CFH Y402H had more than a 2-fold higher risk of progression (OR 2.6; 95% CI 1.7–3.9; P trend < 0.001) and ARMS2 had a 4-fold higher risk of progression to advanced AMD (OR 4.1; 95% CI 2.7–6.3; P trend < 0.001).
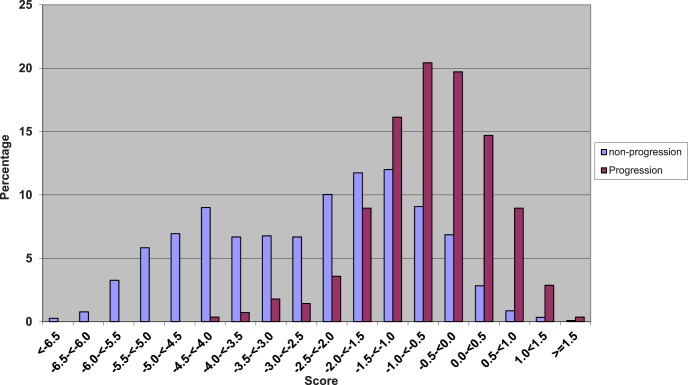
Over the years, we created several risk models to predict progression to advanced stages of disease.76–79 The initial composite risk score to predict advanced AMD endpoints was based on demographic, environmental, ocular, genetic, and treatment variables. We observed that the risk scores for progressors were higher compared with those for nonprogressors (Fig. 11).76 With additions of newly discovered common and rare genetic variants to our models, we assessed how well these models performed with regard to predicting progression. We also assessed which genes might affect specific transitions between the early, intermediate and advanced AMD stages using a multistate Markov model (Fig. 12).92
Figure 11.
Distribution of composite risk scores among progressors and nonprogressors to incident advanced AMD. Risk scores for progressors and nonprogressors are based on six genetic variants, as well as demographic, ocular, environmental, and treatment variables. Reprinted with permission from Seddon JM, Reynolds R, Maller J, Fagerness JA, Daly MJ, Rosner B. Prediction model for prevalence and incidence of advanced age-related macular degeneration based on genetic, demographic, and environmental variables. Invest Ophthalmol Vis Sci. 2009;50:2044–2053. Copyright 2009 Association for Research in Vision and Ophthalmology.
Figure 12.
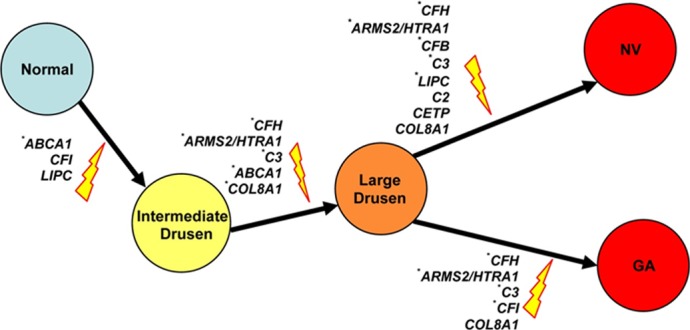
The genetic effects on different stages of AMD progression. Genes that are significantly associated with the risk of a specific transition in the univariate or the multivariate Markov model are shown. SNPs that are significantly associated with risk of AMD progression in the multivariate Markov model are indicated by an asterisk. Reprinted with permission from Yu Y, Reynolds R, Rosner B, Daly MJ, Seddon JM. Prospective assessment of genetic effects on progression to different stages of age-related macular degeneration using multistate Markov models. Invest Ophthalmol Vis Sci. 2012;53:1548–1556. Copyright 2012 Association for Research in Vision and Ophthalmology.
Age-adjusted area under the curve (AUC) statistics were calculated using methods described previously.94,95 These AUCs estimated how well the risk scores could separate progressors from nonprogressors. The AUCs for our prediction models began at 0.70,91 and have since achieved excellent separation of groups, indicated by an AUC over 0.90, on incorporating additional common and rare variants.77–79 The highest AUC observed was 0.94 in the model that incorporated plasma complement biomarkers as a predictive measure.72 In contrast, AUCs for the Framingham Heart Study, a large longitudinal cohort evaluating risk factors for cardiovascular disease, reach 0.65.96
Our latest prediction model included 10 common and rare variants in seven genes, as well as demographic, environmental, and ocular factors, and evaluates the likelihood of patient-specific progression to advanced AMD over time.79 Patients who have the same baseline AMD stage may have variable risk based on their genetic burden. This differential risk is apparent in Figure 13, where two patients have similar ages and intermediate disease at baseline. One patient has a more than 80% chance of progressing, whereas the other has an approximately 14% risk of progression. The distribution of risk scores according to quintile of the score is shown in Figure 14. The likelihood of progressing over 5 years was quite variable: 0.34% to 55%, depending on the composite risk score. Information about our risk calculator can be found online at www.seddonamdriskscore.org (available in the public domain). New and emerging diagnostic and ocular imaging technologies, such as optical coherence tomography (OCT) and OCT angiography, are providing new markers that precede the onset of advanced disease,97 and these parameters may become useful for inclusion in predictive modeling.
Figure 13.
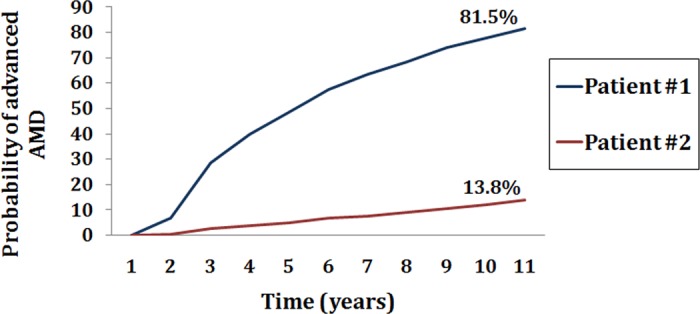
The probability of progression to advanced AMD in two patients classified as intermediate-stage AMD and with similar ages at baseline, according to their composite risk score (based on demographic, environmental, ocular, and 10 genetic factors). Risk score category was very high for patient 1 with about an 81% risk, and very low for patient 2 with approximately 14% risk of progression over 11 years.
Figure 14.
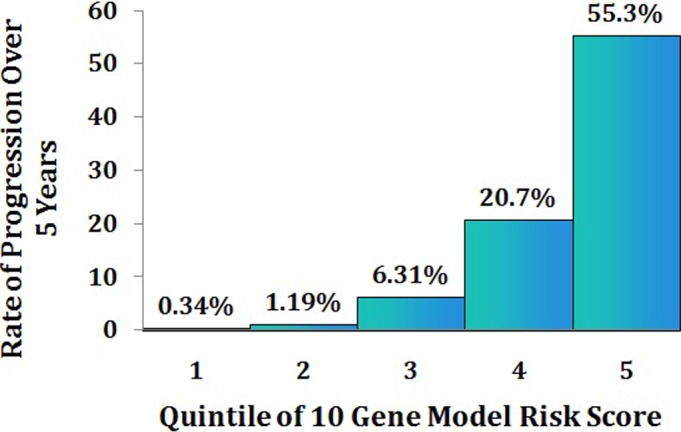
Rate of progression to advanced stages of AMD (GA and NV) over 5 years among participants without advanced disease at baseline, according to quintile of the polygenic, composite model risk score.
Looking to the Future of AMD Research
We are entering the realm of precision medicine: stratifying patients with this heterogeneous disease into different behavioral, clinical, and genetic risk groups to guide individual clinical management decisions and delivering treatments based on genetic susceptibilities. We will move from genetic research to genomic medicine and new therapies based on this new understanding of the genetic and biologic pathways. Behaviors and lifestyles and specifically gene-environment interactions will play a role in response to therapies and will guide precision medicine approaches. Prevention will be started earlier for those predicted to be at greater risk. Associations between AMD and other diseases,26,98 as well as between AMD genes and other systemic pathways will emerge.
As a young ophthalmologist and epidemiologist, I was inspired by those who went before me, looking for the reasons behind this blinding disease. Back then, we knew only that the disease was related to aging. Years of searching, using different roadmaps, led us to the complicated nature and nurture roots of macular degeneration.
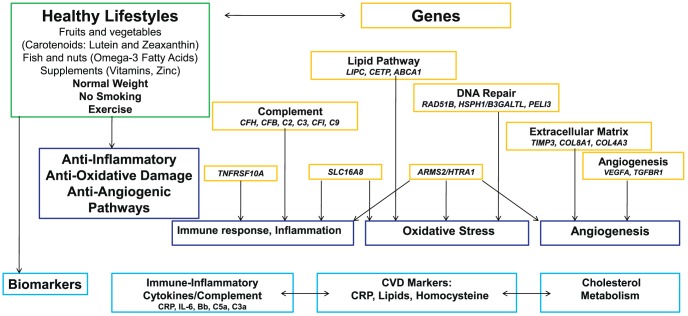
Together with many of my colleagues in other laboratories, and with the work of other scientists around the world, we put many of these pieces of the AMD puzzle together. We now know that AMD is complex, and there are common genetic variants with low to moderate effect and rare or low-frequency genetic variants with high impact. Biomarkers in the blood that reflect inflammation and cardiovascular disease are related to these genetic pathways. Behaviors like smoking and diet can increase or decrease your risk and modify your genetic susceptibility. Genes may alter your response to treatment. The specific clinical appearance of the disease in your eyes can reflect your genetic makeup. The interplay between and among all of these markers and pathways that mediate AMD risk is depicted in Figure 15.
Figure 15.
Diagram of interplay between environmental and genetic risk factors and biomarkers that mediate AMD risk. Adapted and reprinted with permission from Sobrin L, Seddon JM. Nature and nurture-genes and environment-predict onset and progression of macular degeneration. Prog Retin Eye Res. 2014;40:1–15. Copyright 2014 Published by Elsevier Ltd.
Collectively, we have had key moments of discovery about diet and lifestyles that helped change the way we manage the disease around the world. Gene discoveries have provided insights into new mechanisms, new treatments are emerging as a result, and knowledge of behavioral risks and how they interact with genes will tailor the application and indications for these treatments. Therapies developed by other groups, like VEGF inhibition, have made a big difference, and new treatments, like regenerating cells in the retina, offer promise.
Now, thanks to the efforts of many, we understand AMD better, thousands keep their sight, and the work continues in many promising ways. We are all working together so that patients with this disease can continue to see the faces of the people they love.
Acknowledgments
I thank the following individuals and groups for their support and contributions to the research reported in this article: Mark Daly (Massachusetts General Hospital and Broad Institute, Boston, MA, USA), Bernard Rosner (Channing Laboratory, Harvard Medical School, Boston, MA, USA), Yi Yu (Tufts Medical Center and Amgen, Boston, MA, USA), Soumya Raychaudhuri (Brigham and Women's Hospital, Boston, MA, USA), Lucia Sobrin (Massachusetts Eye and Ear Infirmary, Boston, MA, USA), John Atkinson (Washington University, St. Louis, MO, USA), David Kavanagh (Newcastle University, Newcastle upon Tyne, England, UK), Eric Souied (University of Cretiel, Cretiel, France), Ilkka Immonen (Helsinki, Finland), Mary Elizabeth Hartnett (Moran Eye Institute, Salt Lake City, UT, USA), Paul Bernstein (Moran Eye Institute, Salt Lake City, UT, USA), Benedicte Merle (University of Bordeaux, Bordeaux, France), Daniela Ferrara (Tufts Medical Center and Genentech, Boston, MA, USA), Ingrid Kreissig (Tuebingen, Germany), Jules Baum (New York, NY, USA), Daniel Albert (Portland, OR, USA), Martine Jager (Leiden, The Netherlands), Alan Laties (University of Pennsylvania, Philadelphia, PA, USA), Hugh Taylor (Melbourne, Australia); my husband, Ralph Hingson (Boston, MA, USA); numerous collaborators for the genetic studies in the United States, United Kingdom, Europe, Asia, and Australia; and thousands of families, twins, and individual participants who provided information and samples that made these studies possible.
Dedicated to my parents and brothers who encouraged me to pursue a career in medicine.
The Weisenfeld Award lecture was presented at the annual meeting of the Association for Research in Vision and Ophthalmology, Baltimore, Maryland, United States, May 8, 2017.
Supported by NEI/NIH Grant R01 EY011309 (JMS), Massachusetts Lions Research Fund, American Macular Degeneration Foundation, and the Macular Degeneration Fund, Tufts Medical Center, and Tufts University School of Medicine.
Disclosure: J.M. Seddon, Novartis (F), Théa (F), Apellis (C), Gemini (S)
References
- 1. Ross RD. Pioneering women in opthalmology. In: DM, Albert, Edwards DD, eds The History of Ophthalmology. Cambridge, MA: Blackwell Science; 1996: 275–278, 282–273.
- 2. Seddon JM,, Schwartz B,, Flowerdew G. Case-control study of ocular hypertension. Arch Ophthalmol. 1983; 101: 891–894. [DOI] [PubMed] [Google Scholar]
- 3. Seddon JM,, Albert DM,, Lavin PT,, Robinson N. A prognostic factor study of disease-free interval and survival following enucleation for uveal melanoma. Arch Ophthalmol. 1983; 101: 1894–1899. [DOI] [PubMed] [Google Scholar]
- 4. Egan KM,, Seddon JM,, Glynn RJ,, Gragoudas ES,, Albert DM. Epidemiologic aspects of uveal melanoma. Surv Ophthalmol. 1988; 32: 239–251. [DOI] [PubMed] [Google Scholar]
- 5. Seddon J,, Gragoudas E,, Egan K,, Polivogianis L,, Finn S,, Albert D. Standardized data collection and coding in eye disease epidemiology: the Uveal Melanoma Data System. Ophthalmic Surg. 1991; 22: 127–136. [PubMed] [Google Scholar]
- 6. Seddon JM,, Gragoudas ES,, Glynn RJ,, Egan KM,, Albert DM,, Blitzer PH. Host factors, UV radiation, and risk of uveal melanoma. A case-control study. Arch Ophthalmol. 1990; 108: 1274–1280. [DOI] [PubMed] [Google Scholar]
- 7. Seddon JM,, Polivogianis L,, Hsieh CC,, Albert DM,, Gamel JW,, Gragoudas ES. Death from uveal melanoma. Number of epithelioid cells and inverse SD of nucleolar area as prognostic factors. Arch Ophthalmol. 1987; 105: 801–806. [DOI] [PubMed] [Google Scholar]
- 8. Friedman DS,, O'Colmain BJ,, Munoz B,, et al. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004; 122: 564–572. [DOI] [PubMed] [Google Scholar]
- 9. Lim LS,, Mitchell P,, Seddon JM,, Holz FG,, Wong TY. Age-related macular degeneration. Lancet. 2012; 379: 1728–1738. [DOI] [PubMed] [Google Scholar]
- 10. Sobrin L,, Seddon JM. Nature and nurture-genes and environment-predict onset and progression of macular degeneration. Prog Retin Eye Res. 2014; 40: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wong WL,, Su X,, Li X,, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014; 2: e106–e116. [DOI] [PubMed] [Google Scholar]
- 12. Seddon JM,, Sharma S,, Adelman RA. Evaluation of the clinical age-related maculopathy staging system. Ophthalmology. 2006; 113: 260–266. [DOI] [PubMed] [Google Scholar]
- 13. Bessho H,, Kondo N,, Honda S,, Kuno S,, Negi A. Coding variant Met72Thr in the PEDF gene and risk of neovascular age-related macular degeneration and polypoidal choroidal vasculopathy. Mol Vis. 2009; 15: 1107–1114. [PMC free article] [PubMed] [Google Scholar]
- 14. Sun E,, Lim A,, Liu X,, Snellingen T,, Wang N,, Liu N. Apolipoprotein E gene and age-related macular degeneration in a Chinese population. Mol Vis. 2011; 17: 997–1002. [PMC free article] [PubMed] [Google Scholar]
- 15. Seddon JM,, Cote J,, Page WF,, Aggen SH,, Neale MC. The US twin study of age-related macular degeneration: relative roles of genetic and environmental influences. Arch Ophthalmol. 2005; 123: 321–327. [DOI] [PubMed] [Google Scholar]
- 16. Seddon JM,, Willett WC,, Speizer FE,, Hankinson SE. A prospective study of cigarette smoking and age-related macular degeneration in women. JAMA. 1996; 276: 1141–1146. [PubMed] [Google Scholar]
- 17. Ajani UA,, Willett WC,, Seddon JM. Reproducibility of a food frequency questionnaire for use in ocular research. Eye Disease Case-Control Study Group. Invest Ophthalmol Vis Sci. 1994; 35: 2725–2733. [PubMed] [Google Scholar]
- 18. Seddon JM, Ajani UA, Sperduto RD, et al. for the Eye Disease Case-Control Study Group. . Dietary carotenoids, vitamins A, C, and E, and advanced age-related macular degeneration. JAMA. 1994; 272: 1413–1420. [PubMed] [Google Scholar]
- 19. Seddon JM,, Rosner B,, Sperduto RD,, et al. Dietary fat and risk for advanced age-related macular degeneration. Arch Ophthalmol. 2001; 119: 1191–1199. [DOI] [PubMed] [Google Scholar]
- 20. Seddon JM,, Cote J,, Rosner B. Progression of age-related macular degeneration: association with dietary fat, transunsaturated fat, nuts, and fish intake. Arch Ophthalmol. 2003; 121: 1728–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Seddon JM,, George S,, Rosner B. Cigarette smoking, fish consumption, omega-3 fatty acid intake, and associations with age-related macular degeneration: the US Twin Study of Age-Related Macular Degeneration. Arch Ophthalmol. 2006; 124: 995–1001. [DOI] [PubMed] [Google Scholar]
- 22. Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2001; 119: 1417–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. SanGiovanni JP, Chew EY, Clemons TE, et al. for the Age-Related Eye Disease Study Research Group. . The relationship of dietary carotenoid and vitamin A, E, and C intake with age-related macular degeneration in a case-control study: AREDS Report No. 22. Arch Ophthalmol. 2007; 125: 1225–1232. [DOI] [PubMed] [Google Scholar]
- 24. Chew EY, Clemons TE, Danis RP, et al. for the Age-Related Eye Disease Study 2 Research Group. . Secondary analyses of the effects of lutein/zeaxanthin on age-related macular degeneration progression: AREDS2 report No. 3. JAMA Ophthalmol. 2014; 132: 142–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Seddon JM,, Cote J,, Davis N,, Rosner B. Progression of age-related macular degeneration: association with body mass index, waist circumference, and waist-hip ratio. Arch Ophthalmol. 2003; 121: 785–792. [DOI] [PubMed] [Google Scholar]
- 26. Snow KK,, Seddon JM. Do age-related macular degeneration and cardiovascular disease share common antecedents? Ophthalmic Epidemiol. 1999; 6: 125–143. [DOI] [PubMed] [Google Scholar]
- 27. Seddon JM,, Gensler G,, Milton RC,, Klein ML,, Rifai N. Association between C-reactive protein and age-related macular degeneration. JAMA. 2004; 291: 704–710. [DOI] [PubMed] [Google Scholar]
- 28. Seddon JM,, Gensler G,, Klein ML,, Milton RC. C-reactive protein and homocysteine are associated with dietary and behavioral risk factors for age-related macular degeneration. Nutrition. 2006; 22: 441–443. [DOI] [PubMed] [Google Scholar]
- 29. Seddon JM,, George S,, Rosner B,, Rifai N. Progression of age-related macular degeneration: prospective assessment of C-reactive protein, interleukin 6, and other cardiovascular biomarkers. Arch Ophthalmol. 2005; 123: 774–782. [DOI] [PubMed] [Google Scholar]
- 30. Thompson JT,, Seddon JM. Eat Right for Your Sight: Simple, Tasty Recipes that Help Reduce the Risk of Vision Loss from Macular Degeneration. New York, NY: The Experiment, LLC; 2015. [Google Scholar]
- 31. Seddon JM,, Ajani UA,, Mitchell BD. Familial aggregation of age-related maculopathy. Am J Ophthalmol. 1997; 123: 199–206. [DOI] [PubMed] [Google Scholar]
- 32. Haddad S,, Chen CA,, Santangelo SL,, Seddon JM. The genetics of age-related macular degeneration: a review of progress to date. Surv Ophthalmol. 2006; 51: 316–363. [DOI] [PubMed] [Google Scholar]
- 33. Weeks DE,, Conley YP,, Tsai HJ,, et al. Age-related maculopathy: an expanded genome-wide scan with evidence of susceptibility loci within the 1q31 and 17q25 regions. Am J Ophthalmol. 2001; 132: 682–692. [DOI] [PubMed] [Google Scholar]
- 34. Seddon JM,, Santangelo SL,, Book K,, Chong S,, Cote J. A genomewide scan for age-related macular degeneration provides evidence for linkage to several chromosomal regions. Am J Hum Genet. 2003; 73: 780–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fisher SA,, Abecasis GR,, Yashar BM,, et al. Meta-analysis of genome scans of age-related macular degeneration. Hum Mol Genet. 2005; 14: 2257–2264. [DOI] [PubMed] [Google Scholar]
- 36. Santangelo SL,, Yen CH,, Haddad S,, Fagerness J,, Huang C,, Seddon JM. A discordant sib-pair linkage analysis of age-related macular degeneration. Ophthalmic Genet. 2005; 26: 61–67. [DOI] [PubMed] [Google Scholar]
- 37. Klein RJ,, Zeiss C,, Chew EY,, et al. Complement factor H polymorphism in age-related macular degeneration. Science. 2005; 308: 385–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Edwards AO,, Ritter R, III,, Abel KJ,, Manning A,, Panhuysen C,, Farrer LA. Complement factor H polymorphism and age-related macular degeneration. Science. 2005; 308: 421–424. [DOI] [PubMed] [Google Scholar]
- 39. Hageman GS,, Anderson DH,, Johnson LV,, et al. A common haplotype in the complement regulatory gene factor H (HF1/CFH) predisposes individuals to age-related macular degeneration. Proc Natl Acad Sci U S A. 2005; 102: 7227–7232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Haines JL,, Hauser MA,, Schmidt S,, et al. Complement factor H variant increases the risk of age-related macular degeneration. Science. 2005; 308: 419–421. [DOI] [PubMed] [Google Scholar]
- 41. Jakobsdottir J,, Conley YP,, Weeks DE,, Mah TS,, Ferrell RE,, Gorin MB. Susceptibility genes for age-related maculopathy on chromosome 10q26. Am J Hum Genet. 2005; 77: 389–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Dewan A,, Liu M,, Hartman S,, et al. HTRA1 promoter polymorphism in wet age-related macular degeneration. Science. 2006; 314: 989–992. [DOI] [PubMed] [Google Scholar]
- 43. Rivera A,, Fisher SA,, Fritsche LG,, et al. Hypothetical LOC387715 is a second major susceptibility gene for age-related macular degeneration, contributing independently of complement factor H to disease risk. Hum Mol Genet. 2005; 14: 3227–3236. [DOI] [PubMed] [Google Scholar]
- 44. Maller J,, George S,, Purcell S,, et al. Common variation in three genes, including a noncoding variant in CFH, strongly influences risk of age-related macular degeneration. Nat Genet. 2006; 38: 1055–1059. [DOI] [PubMed] [Google Scholar]
- 45. Gold B,, Merriam JE,, Zernant J,, et al. Variation in factor B (BF) and complement component 2 (C2) genes is associated with age-related macular degeneration. Nat Genet. 2006; 38: 458–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Maller JB,, Fagerness JA,, Reynolds RC,, Neale BM,, Daly MJ,, Seddon JM. Variation in complement factor 3 is associated with risk of age-related macular degeneration. Nat Genet. 2007; 39: 1200–1201. [DOI] [PubMed] [Google Scholar]
- 47. Yates JR,, Sepp T,, Matharu BK,, et al. Complement C3 variant and the risk of age-related macular degeneration. N Eng J Med. 2007; 357: 553–561. [DOI] [PubMed] [Google Scholar]
- 48. Fagerness JA,, Maller JB,, Neale BM,, Reynolds RC,, Daly MJ,, Seddon JM. Variation near complement factor I is associated with risk of advanced AMD. Eur J Hum Genet. 2009; 17: 100–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Peter I,, Huggins GS,, Ordovas JM,, Haan M,, Seddon JM. Evaluation of new and established age-related macular degeneration susceptibility genes in the Women's Health Initiative Sight Exam (WHI-SE) Study. Am J Ophthalmol. 2011; 152: 1005–1013.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Neale BM,, Fagerness J,, Reynolds R,, et al. Genome-wide association study of advanced age-related macular degeneration identifies a role of the hepatic lipase gene (LIPC). Proc Natl Acad Sci U S A. 2010; 107: 7395–7400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chen W,, Stambolian D,, Edwards AO,, et al. Genetic variants near TIMP3 and high-density lipoprotein-associated loci influence susceptibility to age-related macular degeneration. Proc Natl Acad Sci U S A. 2010; 107: 7401–7406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yu Y,, Bhangale TR,, Fagerness J,, et al. Common variants near FRK/COL10A1 and VEGFA are associated with advanced age-related macular degeneration. Hum Mol Genet. 2011; 20: 3699–3709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Arakawa S,, Takahashi A,, Ashikawa K,, et al. Genome-wide association study identifies two susceptibility loci for exudative age-related macular degeneration in the Japanese population. Nat Genet. 2011; 43: 1001–1004. [DOI] [PubMed] [Google Scholar]
- 54. Sobrin L,, Ripke S,, Yu Y,, et al. Heritability and genome-wide association study to assess genetic differences between advanced age-related macular degeneration subtypes. Ophthalmology. 2012; 119: 1874–1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Fritsche LG,, Chen W,, Schu M,, et al. Seven new loci associated with age-related macular degeneration. Nat Genet. 2013; 45: 433–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Peter I,, Seddon JM. Genetic epidemiology: successes and challenges of genome-wide association studies using the example of age-related macular degeneration. Am J Ophthalmol. 2010; 150: 450–452.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Yu Y,, Triebwasser MP,, Wong EK,, et al. Whole-exome sequencing identifies rare, functional CFH variants in families with macular degeneration. Hum Mol Genet. 2014; 23: 5283–5293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wagner EK,, Raychaudhuri S,, Villalonga MB,, et al. Mapping rare, deleterious mutations in Factor H: Association with early onset, drusen burden, and lower antigenic levels in familial AMD. Sci Rep. 2016; 6: 31531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Yu Y,, Wagner EK,, Souied EH,, et al. Protective coding variants in CFH and PELI3 and a variant near CTRB1 are associated with age-related macular degeneration. Hum Mol Genet. 2016; 25: 5276–5285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Fritsche LG,, Igl W,, Bailey JN,, et al. A large genome-wide association study of age-related macular degeneration highlights contributions of rare and common variants. Nat Genet. 2016; 48: 134–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Yu Y,, Reynolds R,, Fagerness J,, Rosner B,, Daly MJ,, Seddon JM. Association of variants in the LIPC and ABCA1 genes with intermediate and large drusen and advanced age-related macular degeneration. Invest Ophthalmol Vis Sci. 2011; 52: 4663–4670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. De La Paz MA,, Pericak-Vance MA,, Lennon F,, Haines JL,, Seddon JM. Exclusion of TIMP3 as a candidate locus in age-related macular degeneration. Invest Ophthalmol Vis Sci. 1997; 38: 1060–1065. [PubMed] [Google Scholar]
- 63. Seddon JM,, Reynolds R,, Rosner B. Peripheral retinal drusen and reticular pigment: association with CFHY402H and CFHrs1410996 genotypes in family and twin studies. Invest Ophthalmol Vis Sci. 2009; 50: 586–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Sobrin L,, Reynolds R,, Yu Y,, et al. ARMS2/HTRA1 locus can confer differential susceptibility to the advanced subtypes of age-related macular degeneration. Am J Ophthalmol. 2011; 151: 345–352.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Raychaudhuri S,, Iartchouk O,, Chin K,, et al. A rare penetrant mutation in CFH confers high risk of age-related macular degeneration. Nat Genet. 2011; 43: 1232–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ferrara D,, Seddon JM. Phenotypic characterization of complement factor H R1210C rare genetic variant in age-related macular degeneration. JAMA Ophthalmol. 2015; 133: 785–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Sobrin L,, Maller JB,, Neale BM,, et al. Genetic profile for five common variants associated with age-related macular degeneration in densely affected families: a novel analytic approach. Eur J Hum Genet. 2010; 18: 496–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Triebwasser MP,, Roberson ED,, Yu Y,, et al. Rare variants in the functional domains of complement factor H are associated with age-related macular degeneration. Invest Ophthalmol Vis Sci. 2015; 56: 6873–6878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Seddon JM,, Yu Y,, Miller EC,, et al. Rare variants in CFI, C3 and C9 are associated with high risk of advanced age-related macular degeneration. Nat Genet. 2013; 45: 1366–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Helgason H,, Sulem P,, Duvvari MR,, et al. A rare nonsynonymous sequence variant in C3 is associated with high risk of age-related macular degeneration. Nat Genet. 2013; 45: 1371–1374. [DOI] [PubMed] [Google Scholar]
- 71. Zhan X,, Larson DE,, Wang C,, et al. Identification of a rare coding variant in complement 3 associated with age-related macular degeneration. Nat Genet. 2013; 45: 1375–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Reynolds R,, Hartnett ME,, Atkinson JP,, Giclas PC,, Rosner B,, Seddon JM. Plasma complement components and activation fragments: associations with age-related macular degeneration genotypes and phenotypes. Invest Ophthalmol Vis Sci. 2009; 50: 5818–5827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Kavanagh D,, Yu Y,, Schramm EC,, et al. Rare genetic variants in the CFI gene are associated with advanced age-related macular degeneration and commonly result in reduced serum factor I levels. Hum Mol Genet. 2015; 24: 3861–3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Grassmann F,, Fauser S,, Weber BH. The genetics of age-related macular degeneration (AMD)-Novel targets for designing treatment options? Eur J Pharm Biopharm. 2015; 95: 194–202. [DOI] [PubMed] [Google Scholar]
- 75. Seddon JM,, Francis PJ,, George S,, Schultz DW,, Rosner B,, Klein ML. Association of CFH Y402H and LOC387715 A69S with progression of age-related macular degeneration. JAMA. 2007; 297: 1793–1800. [DOI] [PubMed] [Google Scholar]
- 76. Seddon JM,, Reynolds R,, Maller J,, Fagerness JA,, Daly MJ,, Rosner B. Prediction model for prevalence and incidence of advanced age-related macular degeneration based on genetic, demographic, and environmental variables. Invest Ophthalmol Vis Sci. 2009; 50: 2044–2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Seddon JM,, Reynolds R,, Yu Y,, Daly MJ,, Rosner B. Risk models for progression to advanced age-related macular degeneration using demographic, environmental, genetic, and ocular factors. Ophthalmology. 2011; 118: 2203–2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Seddon JM,, Reynolds R,, Yu Y,, Rosner B. Three new genetic loci (R1210C in CFH, variants in COL8A1 and RAD51B) are independently related to progression to advanced macular degeneration. PLoS One. 2014; 9: e87047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Seddon JM,, Silver RE,, Kwong M,, Rosner B. Risk prediction for progression of macular degeneration: 10 common and rare genetic variants, demographic, environmental, and macular covariates. Invest Ophthalmol Vis Sci. 2015; 56: 2192–2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Seddon JM,, Reynolds R,, Shah HR,, Rosner B. Smoking, dietary betaine, methionine, and vitamin D in monozygotic twins with discordant macular degeneration: epigenetic implications. Ophthalmology. 2011; 118: 1386–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Hutchinson JN,, Fagerness J,, Kirby A,, et al. (Epi)Genetic analyses of age-related macular degeneration: case-control and discordant twin studies. Hum Hered. 2014; 78: 59–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Reynolds R,, Rosner B,, Seddon JM. Dietary omega-3 fatty acids, other fat intake, genetic susceptibility, and progression to incident geographic atrophy. Ophthalmology. 2013; 120: 1020–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Merle BM,, Silver RE,, Rosner B,, Seddon JM. Adherence to a Mediterranean diet, genetic susceptibility, and progression to advanced macular degeneration: a prospective cohort study. Am J Clin Nutr. 2015; 102: 1196–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Merle BM,, Silver RE,, Rosner B,, Seddon JM. Dietary folate, B vitamins, genetic susceptibility and progression to advanced nonexudative age-related macular degeneration with geographic atrophy: a prospective cohort study. Am J Clin Nutr. 2016; 103: 1135–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Merle BM,, Silver RE,, Rosner B,, Seddon JM. Associations between vitamin D intake and progression to incident advanced age-related macular degeneration. Invest Ophthalmol Vis Sci. 2017; 58: 4569–4578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Seddon JM,, Gensler G,, Rosner B. C-reactive protein and CFH, ARMS2/HTRA1 gene variants are independently associated with risk of macular degeneration. Ophthalmology. 2010; 117: 1560–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Reynolds R,, Rosner B,, Seddon JM. Serum lipid biomarkers and hepatic lipase gene associations with age-related macular degeneration. Ophthalmology. 2010; 117: 1989–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Seddon JM,, Reynolds R,, Rosner B. Associations of smoking, body mass index, dietary lutein, and the LIPC gene variant rs10468017 with advanced age-related macular degeneration. Mol Vis. 2010; 16: 2412–2424. [PMC free article] [PubMed] [Google Scholar]
- 89. Shah AR,, Williams S,, Baumal CR,, Rosner B,, Duker JS,, Seddon JM. Predictors of response to intravitreal anti-vascular endothelial growth factor treatment of age-related macular degeneration. Am J Ophthalmol. 2016; 163: 154–166.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Seddon JM,, Silver RE,, Rosner B. Response to AREDS supplements according to genetic factors: survival analysis approach using the eye as the unit of analysis. Br J Ophthalmol. 2016; 100: 1731–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Seddon JM,, George S,, Rosner B,, Klein ML. CFH gene variant, Y402H, and smoking, body mass index, environmental associations with advanced age-related macular degeneration. Human Hered. 2006; 61: 157–165. [DOI] [PubMed] [Google Scholar]
- 92. Yu Y,, Reynolds R,, Rosner B,, Daly MJ,, Seddon JM. Prospective assessment of genetic effects on progression to different stages of age-related macular degeneration using multistate Markov models. Invest Ophthalmol Vis Sci. 2012; 53: 1548–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Seddon JM,, Reynolds R,, Yu Y,, Rosner B. Validation of a prediction algorithm for progression to advanced macular degeneration subtypes. JAMA Ophthalmol. 2013; 131: 448–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Hanley JA,, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982; 143: 29–36. [DOI] [PubMed] [Google Scholar]
- 95. Rosner B,, Glynn RJ. Power and sample size estimation for the Wilcoxon rank sum test with application to comparisons of C statistics from alternative prediction models. Biometrics. 2009; 65: 188–197. [DOI] [PubMed] [Google Scholar]
- 96. D'Agostino RB Sr, Grundy S, Sullivan LM, Wilson P; for the CHD Risk Prediction Group. . Validation of the Framingham coronary heart disease prediction scores: results of a multiple ethnic groups investigation. JAMA. 2001; 286: 180–187. [DOI] [PubMed] [Google Scholar]
- 97. Ferrara D,, Silver RE,, Louzada RN,, Novais EA,, Collins GK,, Seddon JM. Optical coherence tomography features preceding the onset of advanced age-related macular degeneration. Invest Ophthalmol Vis Sci. 2017; 58: 3519–3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Weiner DE,, Tighiouart H,, Reynolds R,, Seddon JM. Kidney function, albuminuria and age-related macular degeneration in NHANES III. Nephrol Dial Transplant. 2011; 26: 3159–3165. [DOI] [PMC free article] [PubMed] [Google Scholar]