Abstract
Monocyte chemoattractant protein-1 (MCP-1) stimulates the migration of monocytes to inflammatory sites, leading to the progression of many diseases. Recently, we described a monocyte-targeting peptide amphiphile micelle (MCP-1 PAM) incorporated with the chemokine receptor CCR2 binding motif of MCP-1, which has a high affinity for monocytes in atherosclerotic plaques. We further report here the biomimetic components of MCP-1 PAMs and the influence of the nanoparticle upon binding to monocytes. We report that MCP-1 PAMs have enhanced secondary structure compared to the MCP-1 peptide. As a result, MCP-1 PAMs displayed improved binding and chemoattractant properties to monocytes, which upregulated the inflammatory signaling pathways responsible for monocyte migration. Interestingly, when MCP-1 PAMs were incubated in the presence of prostate cancer cells in vitro, the particle displayed anticancer efficacy by reducing CCR2 expression. Given that monocytes play an important role in tumor cell migration and invasion, our results demonstrate that PAMs can improve the native biofunctional properties of the peptide and may be used as an effective inhibitor to prevent chemokine–receptor interactions that promote disease progression.
Keywords: monocyte, peptide, peptide amphiphile micelle, nanoparticle, prostate cancer, atherosclerosis
Graphical Abstract
1. INTRODUCTION
Monocyte chemoattractant protein-1 (MCP-1) is part of the family of C–C chemokines that promote monocyte and macrophage migration to sites of inflammation.1,2 MCP-1 protein consists of 76 amino acids with three antiparallel β-sheets followed by an α-helix surrounding the sheets; however, only the first loop composed of residue 13–35 is involved in the MCP-1 chemotactic activity.3 MCP-1 contributes to arterio-genesis, as its upregulation promotes monocyte adhesion to the endothelium and infiltration into the subendothelial space within the vessel walls.4,5 In tumor cells, the binding of the MCP-1 protein to its receptor, C–C chemokine receptor type 2 (CCR2), promotes monocyte migration from the bone marrow to cancer metastatic sites.6–8 MCP-1-CCR2 signaling also contributes to the upregulation of tumor-promoting genes and the recruitment of tumor-associated monocytes and macrophages, which further enhances cancer metastasis.6,9 It was reported that elevated MCP-1 and CCR2 expressions in patients with prostate, breast, colorectal, pancreatic, and ovarian cancers correlate with disease progression.10–15 Therefore, there is a clear clinical relevance and need to develop an effective therapeutic that inhibits the interaction between MCP-1 and CCR2 in order to control cancer development and progression.
Over the past few decades, synthetic peptides have made significant clinical advancements in cancer therapy, particularly as angiogenesis inhibitors, tumor targeting moieties, and vaccines.16,17 Among their diverse functionalities (e.g., cell binding, enzymatic reactions, and immune responses), peptides can mimic aspects of the original protein and inhibit protein-receptor interactions for combating tumor growth and progression. Moreover, synthetic peptides offer selective binding to receptors and enhanced penetration to tumors as potential advantages over other therapeutics, and have been shown to promote monocyte chemotactic activity by attracting monocytes to tumor sites, activating increased cytostatic and cytotoxic activity, thereby inhibiting tumor growth.18–21 Additionally, peptide sequences that correlate to functional epitopes derived from the full protein can be easily synthesized and have the potential to maintain the mimetic properties and functions of the protein.22 Despite these advantages, efficient delivery of synthetic peptides faces many challenges, including poor bioavailability and instability in systemic circulation due to protease cleavage.23
To mitigate these disadvantages, peptides have been encapsulated within a nanocarrier.24–28 Therapeutic nanoparticles have several advantages over traditional small molecules, including higher payloads, sustained release of drugs, and improved pharmacokinetic profiles.29–33 To maximize drug delivery, we can tailor nanoparticles with specific physiochemical properties, such as their size and surface chemistry, while including multiple diagnostic or therapeutic agents.34–39 To stabilize particles against rapid degradation, we can encapsulate them within a lipid layer containing water-soluble polymers such as polyethylene glycol (PEG).40 This coating shields the nanoparticle from the immune system, prevents degradation, and increases circulation to improve drug accumulation and control drug release.41 Over recent years, nanoparticle-based drug delivery systems have proven to promote diagnostic and therapeutic efficacy and reduce side effects by improving tissue specificity and bioavailability.29,42–47 We and other researchers have developed nanoparticles with peptide moieties that successfully target monocytes for diagnostic and therapeutic treatments of inflammatory diseases like atherosclerosis.48–52 Specifically, peptide amphiphile micelles (PAMs), self-assembled from a biologically active peptide “headgroup” and a hydrophobic “tail,” were previously developed for monocyte targeting to monitor different stages of atherosclerosis in the aorta.48 PAMs are advantageous over other nanoparticle-based platforms because of their intrinsic biocompatibility and modularity, and have been tailored for a wide range of applications.53,54 Moreover, the ability of PAMs to include a multivalent display of peptides and disassemble under certain stimuli allows for avidity, specific binding to target sites, and sustained drug release.55,56
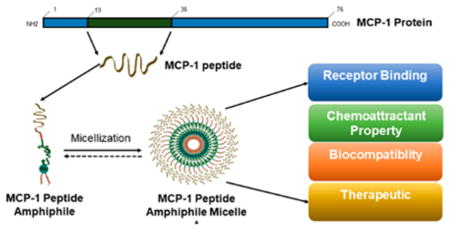
To date, while successful, these investigations have been largely focused on the feasibility of nanoparticles for targeting applications in disease states, and the protein mimetic and biofunctional properties of monocyte-targeting PAMs themselves remain unknown. To bridge this knowledge gap, our current work aims to evaluate monocyte-targeting PAMs (MCP-1 PAMs) and its protein mimetic components through binding activity, chemoattractant property, and biocompatibility with monocytes. MCP-1 PAM-treated monocytes were also analyzed for differential gene expression in cytokine interaction, chemotaxis, and immune response by microarray. Given the significant role of MCP-1 and CCR2 in promoting cancer progression, we also extended our investigation on the inhibitory and cytotoxic effects of monocyte-targeting PAMs using prostate cancer cell lines in vitro. Our results suggest that the delivery of synthetic MCP-1 peptides within a micelle enhances the biofunctional properties of the peptide and has the potential to act as an effective therapeutic to hinder the interactions between chemokines and receptors that promote disease progression.
2. MATERIALS AND METHODS
2.1. Cell Culture Materials and Methods
Cell culture supplies including fetal bovine serum (FBS, Gibco, USA), RPMI-1640 growth medium (Gibco, USA), penicillin-streptomycin (Gibco, USA), 2-mercaptoethanol (Gibco, USA), and phosphate buffered saline (Gibco, USA) were purchased from ThermoFisher Scientific. Dulbecco’s modified Eagle’s medium (DMEM) was purchased from Sigma-Aldrich. WEHI-247.1 (ATCC# CRL-1679) murine monocytes and 22Rv1 (ATCC# CRL-2505) and PC3 (ATCC# CRL-1435) prostate cancer cells were purchased through ATCC, used within six months, and subjected to mycoplasma testing.
WEHI-274.1 murine monocytes were cultured and expanded in DMEM supplemented with 0.05 × 10−3 M 2-mercaptoethanol, 1% penicillin-streptomycin, and 10% FBS. 22Rv1 and PC3 prostate cancer cells were maintained in RPMI-1640 growth medium supplemented with 10% FBS and 1% penicillin-streptomycin. All cell types were cultured in a humidified incubator at 37 °C under 5% CO2. Cells at passage five were used and media was changed every 2–3 days.
2.2. Synthesis of MCP-1 and Scrambled Peptides
MCP-1 peptides were synthesized according to previous methods.48 Residues 13–35 of the MCP-1 protein [YNFTNRKISVQRLASYRRITSSK] comprising the CCR2-binding motif of the protein or a scrambled peptide sequence [YNSLVFRIRNSTQRKYRASIST] was used. In order to achieve covalent conjugation to the micelle lipid tail, the motif was modified with a cysteine residue to the N-terminus. MCP-1 peptides (0.25 mmol) were synthesized with an automated peptide synthesizer (PS3, Protein Technologies, Tucson, AZ) using standard Fmoc-mediated solid phase peptide synthesis methods on a Wang resin. A cleavage cocktail of 94:2.5:2.5:1 vol % trifluoroacetic acid:1,2-ethanedithiol:water:triisopropylsilane was used for peptide cleavage from the resin. Deprotected peptides were precipitated and washed twice with cold diethyl ether, dissolved in water, lyophilized, and stored at −20 °C. Reverse-phase high-performance liquid chromatography (HPLC, Prominence, Shimadzu, Columbia, MD) was used to purify crude peptide mixtures. HPLC was performed on a Luna C4 column (Phenomenex, Torrance, CA) at 50 °C using 0.1% formic acid in acetonitrile/water mixtures. Purified peptides were characterized by matrix-assisted laser desorption/ionization (MALDI) mass spectral analysis (Bruker, Billerica, MA). The expected mass peak for both peptides is [M + H]+ = 2892 (Figures S1 and S2).
2.3. Synthesis of Peptide Amphiphiles
Peptides modified with a cysteine residue (10 mg, 3.5 × 10−3 mmol) were conjugated to 1,2-distearoyl-sn-glycero-3phosphoethanolamine-N-[maleimide-(polyethylene glycol)-2000] (DSPE-PEG(2000)-maleimide, 11.2 mg, 3.8 × 10−3 mmol) though a thioether linkage in 3 mL of water. The reaction was allowed to proceed at room temperature for 24 h, after which the resulting product was purified and characterized as described above. The expected mass peak for both MCP-1 and scrambled peptide amphiphiles (PAs) are [M + H]+ = 5830 (Figures S3 and S4). Five mg of Cy5 was conjugated to 1,2-distearoyl-sn-glycero-3phosphoethanol-amine-N-[amino(polyethylene glycol)-2000] (DSPE-PEG(2000)-amine) via an amide bond by adding an equal molar equivalent of Cy5 mono-N-hydroxysuccinimide ester to the lipid dissolved in 10 mM aqueous sodium carbonate buffer (pH 8.5, 2 mL). After 24 h, the product was purified and characterized as described above.
2.4. Self-Assembly of Micelles
MCP-1 and scrambled micelles were constructed by dissolving DSPE-PEG(2000)-MCP-1, scrambled, and/or Cy5 in methanol, mixing, and evaporating the mixture under nitrogen. The resulting lipid film was dried overnight under vacuum, hydrated for 30 min at 80 °C in water or PBS, and allowed to cool to room temperature.
2.5. Transmission Electron Microscopy
To prepare negative staining samples for transmission electron microscopy (TEM), 50 μM PAM solutions were placed on carbon grids for 2 min. Filter paper was used to wick away excess liquid, after which the grids were washed with Milli-Q water. One weight percent phosphotungstic acid solution was placed on the grids for 2 min. The grids were then washed with Milli-Q water once again. Dried samples were immediately imaged on a JEM 2100-F TEM (JEOL Ltd., Tokyo Japan).
2.6. Particle Size and Zeta Potential Analysis
To confirm the presence of spheroidal micelles, stock solutions of 100 μM PAMs in PBS were analyzed using dynamic light scattering (DLS). Measurements were determined at 90° and 637 nm using a Dynapro Nanostar system (Wyatt, Santa Barbara, CA). 100 μM PAMs dissolved in water were used to measure zeta potential (Zetasizer Nano ZS, Malvern, Worcestershire, United Kingdom, n = 3).
2.7. Circular Dichroism
A Jasco J-815 spectropolarimeter (Easton, MD) was used to perform circular dichroism (CD) spectroscopy. Peptide and PA solutions (100 μM) were analyzed at room temperature using 0.2 mm path-length cuvettes. Measurements from 190 to 265 nm with a 1 s averaging time and 1 nm bandwidth were recorded at 0.5 nm intervals. Data from five scans were obtained and averaged. CD spectra were fit using the Provencher & Glockner Method in order to quantify secondary structure.
2.8. Critical Micelle Concentration Assay
1,6-Diphenylhexa-1,3,5-triene (DPH) fluorescence was used to determine the critical micelle concentration (CMC). Concentrations of MCP-1 and scrambled PA varied from 316 μM to 0.01 μM. A small amount of DPH dissolved in tetrahydrofuran (THF) was added to individual PA solutions to generate 1 μM DPH with a residual THF volume percentage of approximately 0.1. Solutions containing PA and DPH were allowed to equilibrate for 1 h and then measured using a Tecan Infinite 200 plate reader (Männedorf, Switzerland). DPH was excited at 350 nm and the fluorescence emission was measured at 428 nm.
2.9. In Vitro Micelle Binding
Cy5-labeled amphiphiles were incorporated into MCP-1 PAMs and scrambled PAMs during synthesis at a 90:10 mol ratio of PAs:Cy5 amphiphiles. WEHI 274.1 murine monocytes (nonadherent cell type) were seeded at a cell density of 5 × 105 cells/well in 6-well plates. Fluorescently labeled micelles at 100 μM were incubated with monocytes for 1 h at 37 °C. The cell suspensions were centrifuged, washed with PBS and fixed with 2.5% glutaraldehyde/1% paraformaldehyde (Electron Microscopy Sciences) onto glass coverslips. The cells were observed by a Confocal Laser Scanning Microscope 780 (Zeiss) at an excitation wavelength of 650 nm to visualize the micelle internalization from Cy5 (red fluorescence). The Cy5 fluorescence signal was quantified by ImageJ by measuring the average pixel intensity of each cell and subtracting from the background intensity.
2.10. Cytoskeleton Staining
Actin microfilament staining was performed to assess the ability of MCP-1 PAMs to promote migration through cytoskeletal reorganization.57 WEHI 274.1 were seeded on a 12-well plate for 18 h in growth media with 10 μM of MCP-1 or scrambled PAMs. After nonadherent cells were aspirated, the adherent fraction was washed and imaged using light microscopy. Murine macrophages were derived by culturing WEHI-274.1 in the presence of macrophage colony-stimulating factor and by collecting the adherent cells. These macrophages were plated on glass coverslips overnight and treated with 100 μM MCP-1 or scrambled PAMs for 4 h. Cells were washed three times with PBS, fixed (with 4% paraformaldehyde, 5 mM PIPES pH 6.8, 129 mM KCl, 20% sucrose), and stained for nucleus with DAPI and for actin with Alexa-568 phalloidin. The phallodin fluorescence signal was quantified by ImageJ by measuring the average pixel intensity of each cell and subtracting the background intensity. The units are arbitrary, pixel intensity, using the same staining conditions and image acquisition conditions (exposure, illumination intensity) in the same session. The averages for each condition were then normalized against the PBS control.
2.11. Chemotaxis Assay
A chemotaxis assay was performed in order to measure chemoattractant properties of MCP-1 PAMs. 50 000 monocytes suspended in 15 μL were briefly placed on the transwells of 96-transwell plates with 8 μm pores. The number of cells that migrated to the bottom of the plate containing PBS or 1, 10, 100, or 1000 μM MCP-1 PAMs, scrambled PAMs, MCP-1 peptides, scrambled peptides, or DSPE-PEG(2000)-methoxy suspended in PBS after 4 h was measured by lysing the cells via sonication and quantifying DNA via Quant-it Pico Green (Invitrogen, Carlsbad, CA).
2.12. In Vitro Cell Viability
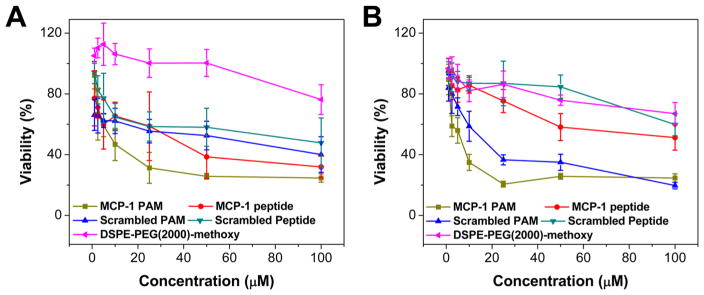
In a 96-well plate, WEHI 274.1 murine monocytes were seeded at a density of 4000 cells/well in supplemented DMEM and incubated for 24 h. Different concentrations of MCP-1 PAMs, scrambled PAMs, MCP-1 peptides, scrambled peptides, and DSPE-PEG(2000)-methoxy in 10 μL of PBS were added to the wells. After 24 or 72 h, cell viability was determined by a (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium) MTS assay (BioVision, Milpitas, CA), according to the manufacturer’s instructions. IC50 values were determined from the best fit of the cell viability (%) versus concentration (μM) plot. In the analysis, 100% viability using nontreated, healthy cells was used as a positive control and to normalize the data. For each assay, 6 different wells per concentration per treatment group were assessed (n = 6).
In vitro cell viability of 22Rv1 and PC3 prostate cancer cells were performed as described above, except they were seeded at 2000 cells/well in supplemented RPMI 1640 media.
2.14. Microarray
Murine monocytes (1 × 106) were cultured within 24-well plates and 200 μL of PBS or 1 mM MCP-1 PAMs were added to 1800 μL of media (100 μM final micelle concentration) for 1 h at 37 °C in a humidified incubator under 5% CO2 (n = 4). Cells were washed with PBS and total RNA was extracted using the combination of TRIzol (Life Technologies, Carlsbad, CA) and the RNeasy Mini Kit (QIAGEN, Venlo, The Netherlands). The quality of the RNA was assessed via a 2100 Bioanalyzer (Agilent, Santa Clara, CA). High-quality RNA (RNA integrity number >8.0) was used for the array experiment and 250 ng of total RNA was used to synthesize cRNA. cRNA was prepared using the Illumina TotalPrep-96 RNA Amplification Kit (Life Technologies, Carlsbad, CA) and was profiled on an Illumina BeadChip MouseWG-6 V2.0 expression array (Illumina, San Diego, CA). After scanning by an Illumina HiScan, the raw probe intensities were background subtracted, log2 transformed and quantile normalized using the Illumina GenomeStudio (v2011.1) expression module. Probe level data were further filtered by detection p-value of ≤0.01 on at least 80% of the samples and batch effects among slides were removed by ComBat R program.58 Processed data were analyzed using Partek Genomics Suite (v6.6, St. Louis, MO). ANOVA and multiple testing corrections were used to detect differentially expressed probes/genes. Gene expression was considered significantly altered when the false discovery rate (FDR corrected p-value) was q ≤0.05 and the fold change was ≥1.5.59 Top GO terms and pathways enriched with the differentially expressed genes were identified using the Database for Annotation, Visualization and Integrated Discovery (DAVID) v6.7 tools (http://david.abcc.ncifcrf.gov/).60,61
2.15. Quantitative Real-Time Polymerase Chain Reaction
cDNA was generated by using a High Capacity cDNA Reverse Transcription Kit (Life Technologies, Carlsbad, CA) for the microarray confirmation analysis and RT2 First Strand Kit (Qiagen, Germany) for the CCR2 analysis based on manufacturer’s instructions. cDNA was quantified using a LightCycler 480 SYBR Green I Master (Roche, Indianapolis, IN) for the microarray confirmation analysis and RT2 SYBR Green qPCR Mastermix (Qiagen, Germany) for the CCR2 analysis. Beta actin (Actb) or Glyceraldehyde 3-phospahte dehydrogenase (GAPDH) served as the house keeping gene to determine relative abundances (fold change) of gene expression. The 2ΔΔCq method was used to quantify mRNA expression level. DNA primer sequences of amplified PCR products are listed in Table S1.
2.16. Statistical Analysis
Data are expressed as mean ± SEM. A Student’s t test was used to compare means of pairs and analysis of variance (ANOVA) using Tukey multiple comparison test posthoc analysis to determine significant differences among three or more means. A p-value of 0.05 or less was considered to be statistical significant.
3. RESULTS
3.1. Preparation, Self-Assembly, and Characterization of MCP-1 PAMs
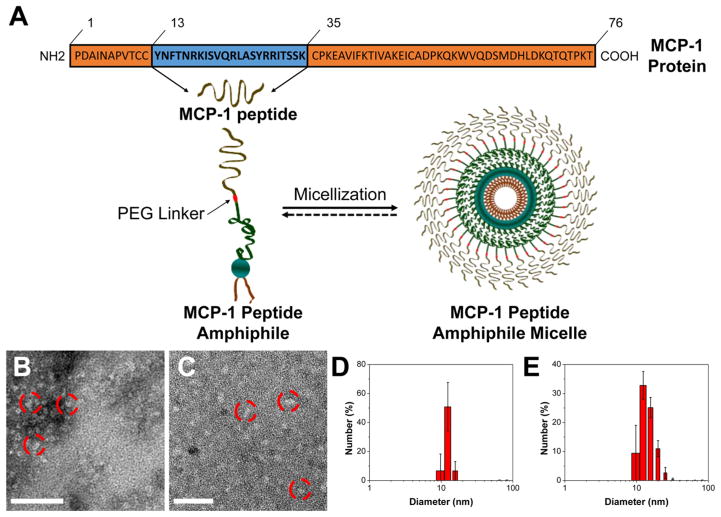
The MCP-1 peptide sequence containing the CCR2-binding motif (residues 13–35) and an additional N-terminal cysteine, was synthesized and conjugated to the DSPE-PEG(2000) lipid tail via a thioether bond to yield MCP-1 PAs as previously described.48 We designed and synthesized a scrambled peptide, lacking monocyte-targeting specificity but consisting of a similar secondary structure to the MCP-1 peptide (Table 1, Figure S5). Monocyte-targeting PAMs were self-assembled through thin-film hydration (Figure 1A). On the basis of the DPH assay, MCP-1 PAMs displayed a CMC of 2.7 μM (Table 1). TEM confirmed spherical micelles and DLS confirmed a hydrodynamic diameter of 13.6 ± 2.4 nm (Figure 1B, D, Table 1). MCP-1 PAMs displayed a slightly positive zeta potential of 11.0 ± 0.1 mV. The self-assembly of MCP-1 PAs within the micelle decreased the random coil content from 55.3 to 32.9%, whereas increasing the β-sheet composition from 36.2 to 60.4% (Figure S6 and Table 1). Scrambled PAMs exhibited similar CMC, particle size, zeta potential, and enhanced secondary structure composition as MCP-1 PAMs (Figure 11C, E, Figure S5, and Table 1).
Table 1.
Materials Characterization of PAMs and Peptides
| MCP-1 PAM | scrambled PAM | MCP-1 Peptide | scrambled Peptide | |
|---|---|---|---|---|
| MW (g/mol) | 5830 | 5830 | 2894 | 2894 |
| CMC (μM) | 2.7 | 2.2 | ||
| size (nm) | 13.6 ± 2.4 | 13.7 ± 2.2 | ||
| PDI | 0.11 ± 0.02 | 0.23 ± 0.01 | ||
| zeta potential (mV) | 11.0 ± 0.1 | 5.3 ± 2.6 | 8.7 ± 1.9 | 7.2 ± 3.4 |
| secondary structure | ||||
| β sheet (%) | 60.4 | 60.2 | 36.2 | 33.7 |
| random coil (%) | 32.9 | 30.7 | 55.3 | 48.3 |
| α helix (%) | 6.6 | 8.9 | 8.5 | 8.0 |
Figure 1.
Preparation and characterization of MCP-1 PAMs. (A) Schematic representation of MCP-1 PAMs. Synthetic MCP-1 peptides corresponding to the CCR2-binding motif (residues 13–35) of the MCP-1 protein were conjugated with DSPE-PEG(2000) to form MCP-1 PAs. (B) Representative TEM images of MCP-1 PAMs and (C) scrambled PAMs. Scale bar = 100 nm. (D) Particle size distribution of MCP-1 PAMs and (E) scrambled PAMs measured by DLS.
3.2. MCP-1 PAM Binding, Chemoattractant Property, and Biocompatibility on Monocytes
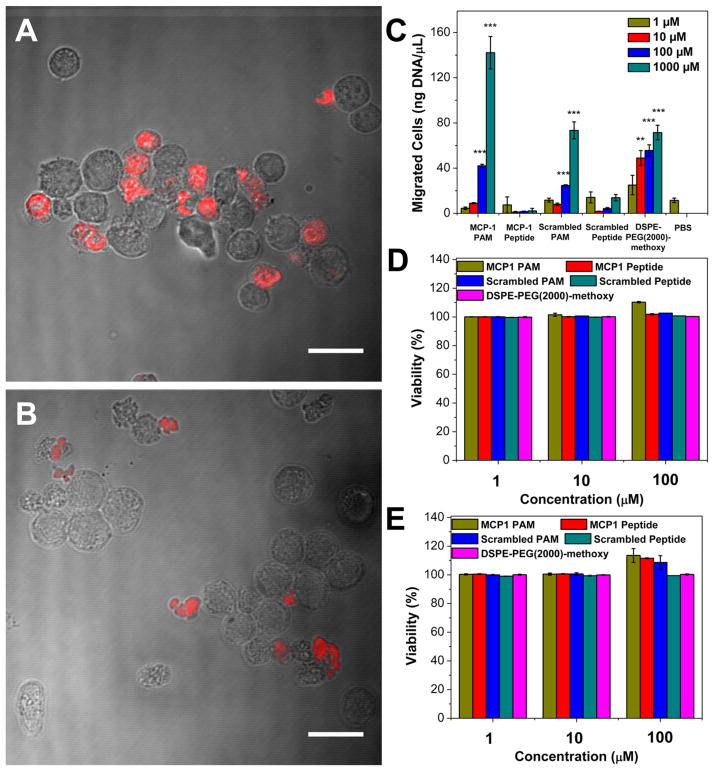
Micelle binding of MCP-1 PAMs and scrambled PAMs in the murine monocyte cell line WEHI 274.1 was visualized by CLSM imaging. To construct fluorescently labeled PAMs, we conjugated Cy5 onto DSPE-PEG(2000)-amine and mixed the DSPE-PEG(2000)-Cy5 with MCP-1 PAs or scrambled PAs at a 10:90 molar ratio, respectively. Previously, we determined that 10 mol % fluorophore-amphiphile provides the maximal fluorescence signal without quenching effects.48 The Cy5-containing PAMs showed similar particle size and zeta potential to nonlabeled PAMs (Figure S7 and Table S2). WEHI 274.1 cells were incubated with Cy5 fluorescently labeled PAMs for 1 h, and MCP-1 PAMs showed a 1.9-fold increase in binding of monocytes vs scrambled PAMs, which is in agreement with our previous reports (Figure 2A and B).48 We further examined the ability of MCP-1 PAMs to induce monocyte migration, similar to the native MCP-1 protein, by assessing actin-containing microfilaments (Figure S8). We found a 20% increase in fluorescence Alexa-568 phalloidin staining indicating enhanced cellular actin polymerization in monocytes incubated with MCP-1 PAMs. In contrast, no difference in phallodin fluorescence intensity was observed in monocytes incubated with scrambled PAMs and the PBS control.
Figure 2.
MCP-1 PAM binding, chemoattractant property, and biocompatibility with WEHI 274.1 murine monocytes. (A) CLSM images of monocytes incubated with MCP-1 PAMs and (B) scrambled PAMs with 10 mol % Cy5 PA (red fluorescence) after 1 h. Scale bar = 20 μm. (C) Monocyte migration after 4 h incubation (n = 5; **p < 0.01, ***p < 0.001 compared to the PBS-treated group). In vitro viability of monocytes after (D) 24 h and (E) 72 h exposure to MCP-1 PAMs, MCP-1 peptides, scrambled PAMs, scrambled peptides, and DSPE-PEG(2000)-methoxy assessed through an MTS assay (n = 6).
Next, we quantified the chemoattractant properties of MCP-1 PAMs. After 4 h incubation with 1–1000 μM of MCP-1 or scrambled peptides and PAMs, the amount of migrated monocytes through a transwell was measured via DNA quantification. As shown in Figure 2C, scrambled PAMs had 24.7 ± 0.7 and 73.4 ± 7.5 ng DNA/mL at 100 μM and 1000 μM, respectively. In contrast, MCP-1 PAMs had 42.1 ± 1.4 and 142.1 ± 14.3 ng DNA/mL at 100 μM and 1000 μM. In addition, the amount of migrated cells incubated with MCP-1 and scrambled peptides were only 2.2 ± 2.2 and 13.9 ± 2.8 ng DNA/mL at 1000 μM. At lower concentrations, no statistical difference was observed between PAMs and peptides, possibly due to the particle at near or below the CMC. DSPE-PEG(2000)-methoxy controls were similar to scrambled PAMs. Taken altogether, these data showed that when the MCP-1 peptide is within the micelle, chemoattractant properties are enhanced at higher concentrations compared to the free peptide. In vitro biocompatibility of the peptides and micelles with WEHI 274.1 using MTS assay (Figure 2D and E) showed no decrease in cell viability when incubated with varying concentrations of MCP-1 or scrambled peptides and PAMs at 24 and 72 h, indicating the biocompatible nature of the peptide and micelles with monocytes.
3.3. DNA Microarray Analysis of Monocyte Treated with MCP-1 PAMs
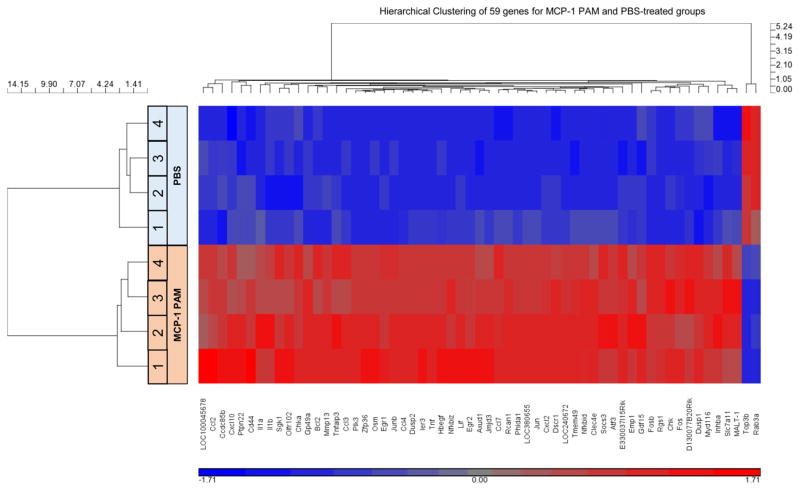
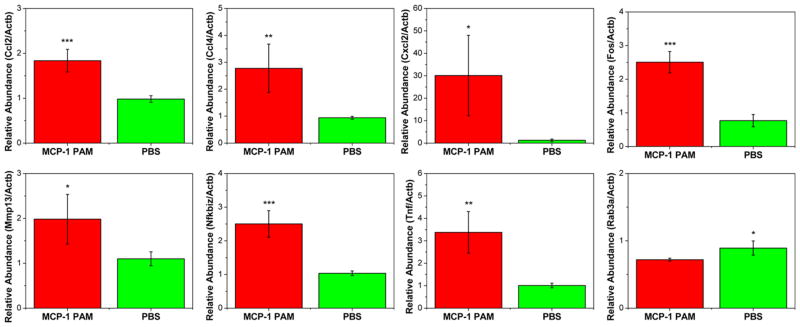
To gain molecular insight into the global biofunctional properties of MCP-1 PAMs, gene expression profiles of monocytes treated with MCP-1 PAMs or PBS were assessed using DNA microarrays. The mRNAs were isolated independently and were subjected to an analysis with the Illumina BeadChip MouseWG-6 v2.0 expression array. Of the 45 281 transcripts analyzed, 59 genes were differentially expressed between MCP-1 PAM vs PBS-treated groups. Hierarchical clustering of the 59 genes was visualized to demonstrate the coregulated genes (Figure 3 and Table S3). Of the 59 genes visualized, 57 genes have shown to have upregulation levels (≥1.5, red shade) when monocytes were induced with MCP-1 PAMs compared to monocytes treated from PBS, while only 2 genes (Topoisomerase III β, Top3b and member RAS oncogene family, Rab3a) were shown to have decreased in expression (blue shade). The highest genes upregulated after MCP-1 PAM treatment were chemokine (C-X-C motif) ligand 2 (Cxcl2) and tumor necrosis factor-α (Tnf-α), which exhibited 7.3- and 4.1-fold increases compared to the PBS-treated group (Table S3). In addition, chemokine (C–C motif) ligands 2 and 4 (Ccl2, Ccl4), v-Fos FBJ murine osteosarcoma viral oncogene homologue (Fos), matrix metallopeptidase 13 (Mmp13), nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, zeta (Nfkbiz) also showed high upregulation for monocytes treated with MCP-1 PAMs (Table S4). Quantitative real-time PCR (qRT-PCR) was performed on 8 genes responsible for cytokine receptor interaction, chemotaxis, or immune response to validate the microarray data (Figure 4). In agreement with the DNA microarray analysis, MCP-1 PAMs effciently upregulated Ccl2, Ccl4, Cxcl2, Fos, Mmp13, Nfkbiz, and Tnf-α gene expression in monocytes, whereas Rab3a was downregulated. Regulators and pro-inflammatory mediators such as chemokines and interleukins were upregulated, while pathways involving monocyte effector functions were augmented with the treatment with MCP-1 PAMs. These data are consistent with previously published work that show relevant genes including Ccl2, Tnf-α, and interleukin-1β (Ilβ), were also upregulated by the native MCP-1 protein.1,62,63 Taken together, this confirmed the ability of MCP-1 PAMs to mimic elements of the native MCP-1 protein.
Figure 3.
Evaluation of gene expression changes induced by MCP-1 PAMs. Whole genome microarray analysis was performed on murine monocytes (WEHI.274.1) cultured with MCP-1 PAMs for 1 h and compared to PBS-treated samples on an Illumina BeadChip MouseWG-6 v2.0 expression array (n = 4). Out of 45 281 transcripts analyzed, 59 genes were differentially expressed between MCP-1 PAM vs PBS-treated groups and only Top3b and Rab3a showed decreased expression with FDR corrected p-value, q ≤0.05 and the fold change was ≥1.5.
Figure 4.
Microarray data was validated by performing qRT-PCR on mRNA levels of chemokine (C–C) ligand 2 (Ccl2), chemokine (C–C motif) ligand 4 (Ccl4), chemokine (C-X-C motif) ligand 2 (Cxcl2), v-Fos FBJ murine osteosarcoma viral oncogene homologue (Fos), matrix metallopeptidase 13 (Mmp13), nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, zeta (Nfkbiz), tumor necrosis factor (Tnf-α), and RAB3A, member RAS oncogene family (Rab3a) (n = 3; *p < 0.05, **p < 0.01, ***p < 0.001 compared to the PBS-treated group).
3.4. In Vitro Cytotoxicity on Prostate Cancer Cells
Targeting of the MCP-1-CCR2 axis has been proposed as a therapy for metastatic cancer.6 Given that MCP-1 PAMs have protein-mimetic chemoattractant properties with monocytes, we investigated the use of MCP-1 PAMs on neoplastic cells, which could open a wide range of possibilities for cancer treatment. First, in vitro cytotoxicity assays of peptides and PAMs were carried out against 22Rv1 prostate cancer cells (Figure 5A, Table 2). After 72 h of incubation with free MCP-1 and scrambled peptides, the IC50 value of MCP-1 peptide was 23.8 μM, which was 3.2-fold lower than that of the scrambled peptide, confirming anticancer potential. Although the MCP-1 and scrambled peptides contained the same amino acids but in a different order, the cytotoxic effect in prostate cancer cells was found to be specific to the MCP-1 sequence (residue 13–35). Notably, this cytotoxic effect was not observed in monocytes (Figure 2D, E). Cytotoxicity was further enhanced when the MCP-1 peptide was incorporated into the micelle. MCP-1 PAMs exhibited significantly increased anticancer efficacy, with IC50 value of 10.3 μM, which was 2.3-times lower than that of free MCP-1 peptide (p = 0.0017). On the other hand, DSPE-PEG(2000)-methoxy controls showed no toxicity (IC50 > 100 μM), confirming that the PAM toxicity was caused by the MCP-1 peptide. The enhanced cytotoxicity of MCP-1 PAM could be attributed to increased cellular uptake of the nanoparticle compared to free peptide as reported in other systems.64
Figure 5.
In vitro viability of prostate cancer cells. (A) In vitro cytotoxicity profiles of 22Rv1 and (B) PC3 after 72 h exposure to MCP-1 peptides, MCP-1 PAMs, scrambled peptides, and scrambled PAMs (n = 6).
Table 2.
IC50 Values of MCP-1 PAMs, MCP-1 Peptides, Scrambled PAMs, Scrambled Peptides, and DSPE-PEG(2000)-Methoxy against 22Rv1 and PC3 Cellsa
| IC50 (μM)
|
||
|---|---|---|
| 22Rv1 | PC3 | |
| MCP-1 PAM | 10.3 ± 3.1 | 1.7 ± 0.7 |
| MCP-1 peptide | 23.8 ± 6.5 | >100 |
| scrambled PAM | 48.4 ± 10.8 | 14.1 ± 3.9 |
| scrambled Peptide | 76.1 ± 15.8 | >100 |
| DSPE-PEG(2000)-methoxy | >100 | >100 |
22Rv1: MCP-1 PAM v. MCP-1 Peptide p < 0.01; MCP-1 PAM v. scrambled PAM p < 0.001; MCP-1 peptide v. scrambled peptide p < 0.001; scrambled PAM v. scrambled peptide p < 0.05; PC3: MCP-1 PAM v. scrambled PAM p < 0.001.
Next, we also studied the in vitro anticancer efficacy on another prostate cancer cell line, PC3, which is used as a model for late-stage prostate cancer (Figure 5B, Table 2), to further examine the therapeutic potential of MCP-1 PAMs for cancer applications. Both free MCP-1 and scrambled peptides showed little toxicity, with IC50 > 100 μM. However, when the peptides were loaded within the micelles, the IC50 value of MCP-1 PAMs decreased considerably to 1.7 μM, even more potent than in 22Rv1. Like 22Rv1, DSPE-PEG(2000)-methoxy micelles showed minimal cytotoxicity. Given that cancer cells display great morphological and phenotypical heterogeneity with respect to the cellular uptake of nanoparticles, MCP-1 PAMs may offer the ability to globally target a wide range of tumors and bypass these differences.65
3.5. CCR2 Expression in Prostate Cancer Cells
Because the binding of MCP-1 is mediated by CCR2, and CCR2 expression has been shown to correlate with prostate cancer progression, CCR2 expression was evaluated on 22Rv1 prostate cancer cells when treated with peptides and PAMs via qRT-PCR.11 22Rv1 prostate cancer cells were incubated with 5 μM of peptides and PAMs, a concentration that does not exhibit cytotoxicity, in order to assess for their effects on CCR2. As shown in Figure 6, MCP-1 peptides downregulated CCR2 gene expression in 22Rv1 cells by 41%. MCP-1 PAMs further enhanced the downregulation of CCR2 by 65%, while unappreciable CCR2 changes were observed between PBS and scrambled peptide-treated groups. A slight decrease in CCR2 expression levels (30%) for scrambled PAMs was found. As CCR2 expression is lower in 22Rv1 than in PC3 prostate cancer cells, this could explain why MCP-1 PAMs were more effective in PC3, which is supported by the lower IC50 value in PC3 cells (Table 2).66 Together these results indicated that MCP-1 PAMs could limit the progression of prostate cancers by suppressing CCR2 expression.
Figure 6.
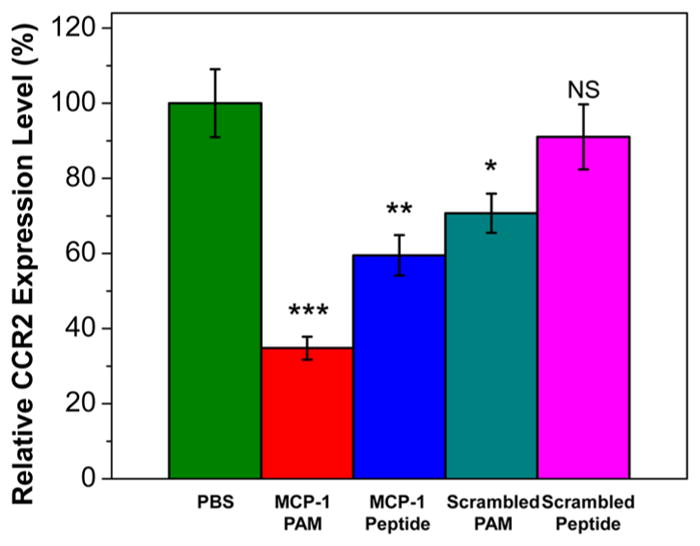
Relative mRNA expression of CCR2 in 22Rv1 prostate cancer cells upon treatment with PBS, MCP-1 PAMs, MCP-1 peptides, scrambled PAMs, or scrambled peptides at a noncytotoxic concentration of 5 μM. Data are expressed as mean ± SEM (n = 3; *p < 0.05, ** p < 0.01, ***p < 0.001 compared to PBS-treated group).
4. DISCUSSION
MCP-1 is the first tumor-derived factor that promotes monocyte migration in response to CCR2 signals.67 Monocytes are recruited from the bone marrow and spleen to pro-inflammatory sites in tumors because of the upregulation of MCP-1-CCR2 interaction.7 Tumor cells also produce MCP-1, which further stimulates monocyte proliferation, infiltration, and adhesion to cancer cells, thus leading to cancer metastasis. Considering the involvement of chemokines and their receptors in inflammation correlates with cancer progression, effective inhibitors that can block these interactions could provide a means to control the disease.
We have previously reported that MCP-1 micelles have preferential binding to monocytes and can detect varying levels monocytes that correspond to different stages of atherosclerotic plaques.48 To expand this work and assess their utility in other diseases affected by monocytes, herein, we studied the biological properties of MCP-1 and its potential in cancer therapeutics. To that end, we incorporated the CCR2-binding motif (residue 13–35) of MCP-1 within the construct of a micelle. Our results demonstrate that the secondary structure of the peptide within the micelle was enhanced to reflect the elevated β-sheet composition of the native protein’s secondary structure.68 Enhanced in vitro micelle binding and chemoattractant properties were observed for MCP-1 PAMs (Figure 2). In contrast, we found that free MCP-1 peptides did not possess any chemoattractant capability. This can be explained by the enhanced secondary structure of MCP-1 within micelles, as well as the presence of PEG, which has been shown to enhance receptor binding and the chemotactic activity of peptides.69 In addition, the increase in monocyte binding and chemotactic activity upregulated the expression of different chemokine and receptors like CCR2 and promoted inflammatory responses, which is consistent with the role of the endogenous MCP-1 protein (Figure 3 and 4).3,20,70,71 Therefore, the biomimetic properties of MCP-1 was maintained within a micelle.
In contrast, MCP-1 PAMs exhibited significantly enhanced cytotoxicity in prostate cancer cells. Previously, Zachariae et al. demonstrated MCP-1 has anticancer activity in vitro by upregulation of FAS ligand protein expression, leading cancer cells into apoptosis.19 Furthermore, Rafei et al. found that the binding of MCP-1 (residue 6–76) with CCR2 initiated pro-apoptotic responses through calcium flux, dephosphorylation of STAT3, and decreased pAKT pathways.21 Koga et al. showed the MCP-1 mutant lacking amino acids 2–8 was able to prevent tumor angiogenesis and tumor growth in vivo by inhibiting the expressions of native MCP-1 and CCR2 in melanoma cells.72 We further support these findings by showing that specifically, residue 13–35 of MCP-1 had antitumor efficacy in prostate cancer cells in vitro because of the downregulation of CCR2 (Figure 6). When MCP-1 was incorporated within a nanocarrier, the IC50 values were decreased by more than 4.5-fold and the CCR2 silencing effect was enhanced by 1.5-fold, likely due to its improved cellular uptake.73
The MCP-1-CCR2 axis has been reported to trigger a series of signaling cascade that promotes chemotactic migration of monocytes.74 The binding of MCP-1 to CCR2 induces MCP-induced protein MCPIP that results in cell death by inducing apoptotic genes, including subsets of the Bcl2 and Tnf family members.62 Similarly, MCP-1 PAMs may provide a similar outcome, where the binding of MCP-1 PAMs to CCR2 initiates an inflammatory response and upregulates other transcripts involved in cell death. Future studies will assess whether MCP-1 PAMs can competitively inhibit the interaction of native MCP-1 protein with CCR2, which is known to elicit cancer progression and metastasis.
5. CONCLUSION
We investigated the protein mimetic properties of MCP-1 PAMs. The binding affinity and chemoattractant properties of MCP-1 PAMs were enhanced compared to the free peptide, and MCP-1 PAM upregulated inflammatory pathways upon microarray analysis in monocytes. For cancer cells, MCP-1 PAMs reduced the proliferation of prostate cancer cells by decreased CCR2 expression. Unlike conventional small molecule therapy, which lacks specificity and bioavailability, synthetic peptides, which is based on naturally occurring proteins, can be used to control biological functions and offer enhanced chemical and structural flexibility. Our findings show therapeutic potential of combining synthetic peptides into micelles to mimic the ability of the protein in the treatment of various diseases.
Supplementary Material
Acknowledgments
We thank Mr. Johan Joo and Miss Shivani Gupta for experimental assistance. We also thank Dr. Matthew Tirrell for his helpful discussion. This work was supported by the University of Southern California, the National Heart, Lung, and Blood Institute (NHLBI), R00HL124279, Eli and Edythe Broad Innovation Award, and the L.K. Whittier Foundation Non-Cancer Translational Research Award granted to E.J.C. We thank the Center for Research Informatics at the Univeristy of Chicago for experimental assistance and the Center for Electron Microscopy and Microanalysis, Center of Excellence in Nano-Biophysics, Center of Excellence for Molecular Characterization, and Translational Imaging Center at the University of Southern California for assistance in instrument setups.
Footnotes
Author Contributions
C.P. and E.J.C. conceived the project and designed the experiments; C.P., S.C., C.-H.K., Y.F., F.J.A., D.H., and E.J.C. performed the experiments and analyzed the data; C.P., K.K, M.E.G., and E.J.C. interpreted data; C.P. and E.J.C wrote the manuscript.
Notes
The authors declare no competing financial interest.
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsbiomaterials.7b00600.
Characterization (MALDI-TOF spectra, hydropathy plot, CD analysis, TEM images, and confocal microscopy) of PAMs and its precursors; primers and microarray/genomics and GO analysis table (PDF)
References
- 1.Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte Chemoattractant Protein-1 (MCP-1): An Overview. J Interferon Cytokine Res. 2009;29(6):313–326. doi: 10.1089/jir.2008.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goser S, Ottl R, Brodner A, Dengler TJ, Torzewski J, Egashira K, Rose NR, Katus HA, Kaya Z. Critical role for monocyte chemoattractant protein-1 and macrophage inflammatory protein-1alpha in induction of experimental autoimmune myocarditis and effective anti-monocyte chemoattractant protein-1 gene therapy. Circulation. 2005;112(22):3400–7. doi: 10.1161/CIRCULATIONAHA.105.572396. [DOI] [PubMed] [Google Scholar]
- 3.Steitz SA, Hasegawa K, Chiang S-L, Cobb RR, Castro MA, Lobl TJ, Yamada M, Lazarides E, Cardarelli PM. Mapping of MCP-1 functional domains by peptide analysis and site-directed mutagenesis. FEBS Lett. 1998;430(3):158–164. doi: 10.1016/s0014-5793(98)00637-1. [DOI] [PubMed] [Google Scholar]
- 4.Jaipersad AS, Lip GYH, Silverman S, Shantsila E. The Role of Monocytes in Angiogenesis and Atherosclerosis. J Am Coll Cardiol. 2014;63(1):1–11. doi: 10.1016/j.jacc.2013.09.019. [DOI] [PubMed] [Google Scholar]
- 5.Scholz D, Ito W, Fleming I, Deindl E, Sauer A, Wiesnet M, Busse R, Schaper J, Schaper W. Ultrastructure and molecular histology of rabbit hind-limb collateral artery growth (arteriogenesis) Virchows Arch. 2000;436(3):257–70. doi: 10.1007/s004280050039. [DOI] [PubMed] [Google Scholar]
- 6.Lim SY, Yuzhalin AE, Gordon-Weeks AN, Muschel RJ. Targeting the CCL2-CCR2 signaling axis in cancer metastasis. Oncotarget. 2016;7(19):28697–710. doi: 10.18632/oncotarget.7376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nat Rev Immunol. 2011;11(11):762–774. doi: 10.1038/nri3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu Y, Xiao G, Galson DL, Nishio Y, Mizokami A, Keller ET, Yao Z, Zhang J. PTHrP-induced MCP-1 production by human bone marrow endothelial cells and osteoblasts promotes osteoclast differentiation and prostate cancer cell proliferation and invasion in vitro. Int J Cancer. 2007;121(4):724–733. doi: 10.1002/ijc.22704. [DOI] [PubMed] [Google Scholar]
- 9.Smith HA, Kang Y. The metastasis-promoting roles of tumor-associated immune cells. J Mol Med (Heidelberg, Ger) 2013;91(4):411–429. doi: 10.1007/s00109-013-1021-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Craig MJ, Loberg RD. CCL2 (Monocyte Chemoattractant Protein-1) in cancer bone metastases. Cancer Metastasis Rev. 2007;25(4):611–619. doi: 10.1007/s10555-006-9027-x. [DOI] [PubMed] [Google Scholar]
- 11.Lu Y, Cai Z, Xiao G, Liu Y, Keller ET, Yao Z, Zhang J. CCR2 expression correlates with prostate cancer progression. J Cell Biochem. 2007;101(3):676–685. doi: 10.1002/jcb.21220. [DOI] [PubMed] [Google Scholar]
- 12.Neumark E, Sagi-Assif O, Shalmon B, Ben-Baruch A, Witz IP. Progression of mouse mammary tumors: MCP-1-TNFα cross-regulatory pathway and clonal expression of promalignancy and antimalignancy factors. Int J Cancer. 2003;106(6):879–886. doi: 10.1002/ijc.11337. [DOI] [PubMed] [Google Scholar]
- 13.Saji H, Koike M, Yamori T, Saji S, Seiki M, Matsushima K, Toi M. Significant correlation of monocyte chemoattractant protein-1 expression with neovascularization and progression of breast carcinoma. Cancer. 2001;92(5):1085–91. doi: 10.1002/1097-0142(20010901)92:5<1085::aid-cncr1424>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 14.Hefler L, Tempfer C, Heinze G, Mayerhofer K, Breitenecker G, Leodolter S, Reinthaller A, Kainz C. Monocyte chemoattractant protein-1 serum levels in ovarian cancer patients. Br J Cancer. 1999;81(5):855–859. doi: 10.1038/sj.bjc.6690776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu H, Sun L, Guo C, Liu Q, Zhou Z, Peng L, Pan J, Yu L, Lou J, Yang Z, Zhao P, Ran Y. Tumor Cell-Microenvironment Interaction Models Coupled with Clinical Validation Reveal CCL2 and SNCG as Two Predictors of Colorectal Cancer Hepatic Metastasis. Clin Cancer Res. 2009;15(17):5485–5493. doi: 10.1158/1078-0432.CCR-08-2491. [DOI] [PubMed] [Google Scholar]
- 16.Thundimadathil J. Cancer treatment using peptides: current therapies and future prospects. J Amino Acids. 2012;2012:967347. doi: 10.1155/2012/967347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo C, Manjili MH, Subjeck JR, Sarkar D, Fisher PB, Wang XY. Therapeutic cancer vaccines: past, present, and future. Adv Cancer Res. 2013;119:421–475. doi: 10.1016/B978-0-12-407190-2.00007-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xiao YF, Jie MM, Li BS, Hu CJ, Xie R, Tang B, Yang SM. Peptide-Based Treatment: A Promising Cancer Therapy. J Immunol Res. 2015;2015:761820. doi: 10.1155/2015/761820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zachariae COC, Anderson AO, Thompson HL, Appella E, Mantovani A, Oppenheim JJ, Matsushima K. Properties of monocyte chemotactic and activating factor (MCAF) purified from a human fibrosarcoma cell line. J Exp Med. 1990;171(6):2177–82. doi: 10.1084/jem.171.6.2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Valente AJ, Rozek MM, Schwartz CJ, Graves DT. Characterization of monocyte chemotactic protein-1 binding to human monocytes. Biochem Biophys Res Commun. 1991;176(1):309–314. doi: 10.1016/0006-291x(91)90925-w. [DOI] [PubMed] [Google Scholar]
- 21.Rafei M, Deng J, Boivin MN, Williams P, Matulis SM, Yuan S, Birman E, Forner K, Yuan L, Castellino C, Boise LH, MacDonald TJ, Galipeau J. A MCP1 fusokine with CCR2-specific tumoricidal activity. Mol Cancer. 2011;10:121. doi: 10.1186/1476-4598-10-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gross A, Hashimoto C, Eichler J, Sticht H. Synthetic Peptides as Protein Mimics. Front Bioeng Biotechnol. 2016;3:211. doi: 10.3389/fbioe.2015.00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bruno BJ, Miller GD, Lim CS. Basics and recent advances in peptide and protein drug delivery. Ther Delivery. 2013;4(11):1443–1467. doi: 10.4155/tde.13.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chung EJ. Targeting and therapeutic peptides in nanomedicine for atherosclerosis. Exp Biol Med (London, U K) 2016;241(9):891–8. doi: 10.1177/1535370216640940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Acar H, Srivastava S, Chung EJ, Schnorenberg MR, Barrett JC, LaBelle JL, Tirrell M. Self-assembling peptide-based building blocks in medical applications. Adv Drug Delivery Rev. 2017;110–111:65–79. doi: 10.1016/j.addr.2016.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Black KA, Lin BF, Wonder EA, Desai SS, Chung EJ, Ulery BD, Katari RS, Tirrell MV. Biocompatibility and Characterization of a Peptide Amphiphile Hydrogel for Applications in Peripheral Nerve Regeneration. Tissue Eng, Part A. 2015;21(7–8):1333–1342. doi: 10.1089/ten.tea.2014.0297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoo SP, Barrett JC, Tirrell M, Chung EJ, Pineda F, Poon C. Gadolinium-Functionalized Peptide Amphiphile Micelles for Multimodal Imaging of Atherosclerotic Lesions. ACS Omega. 2016;1(5):996–1003. doi: 10.1021/acsomega.6b00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang J, Masehi-Lano JJ, Chung EJ. Peptide and antibody ligands for renal targeting: nanomedicine strategies for kidney disease. Biomater Sci. 2017;5(8):1450–1459. doi: 10.1039/c7bm00271h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol. 2007;2(12):751–760. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- 30.Li S-D, Huang L. Pharmacokinetics and Biodistribution of Nanoparticles. Mol Pharmaceutics. 2008;5(4):496–504. doi: 10.1021/mp800049w. [DOI] [PubMed] [Google Scholar]
- 31.Ma X, Zhao Y, Liang X-J. Theranostic Nanoparticles Engineered for Clinic and Pharmaceutics. Acc Chem Res. 2011;44(10):1114–1122. doi: 10.1021/ar2000056. [DOI] [PubMed] [Google Scholar]
- 32.Banerjee A, Qi J, Gogoi R, Wong J, Mitragotri S. Role of nanoparticle size, shape and surface chemistry in oral drug delivery. J Controlled Release. 2016;238:176–185. doi: 10.1016/j.jconrel.2016.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mitragotri S, Anderson DG, Chen X, Chow EK, Ho D, Kabanov AV, Karp JM, Kataoka K, Mirkin CA, Petrosko SH, Shi J, Stevens MM, Sun S, Teoh S, Venkatraman SS, Xia Y, Wang S, Gu Z, Xu C. Accelerating the Translation of Nanomaterials in Biomedicine. ACS Nano. 2015;9(7):6644–6654. doi: 10.1021/acsnano.5b03569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nel AE, Madler L, Velegol D, Xia T, Hoek EMV, Somasundaran P, Klaessig F, Castranova V, Thompson M. Understanding biophysicochemical interactions at the nano-bio interface. Nat Mater. 2009;8(7):543–557. doi: 10.1038/nmat2442. [DOI] [PubMed] [Google Scholar]
- 35.Albanese A, Tang PS, Chan WCW. The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annu Rev Biomed Eng. 2012;14:1–16. doi: 10.1146/annurev-bioeng-071811-150124. [DOI] [PubMed] [Google Scholar]
- 36.Chen F, Ehlerding EB, Cai W. Theranostic nanoparticles. J Nucl Med. 2014;55(12):1919–1922. doi: 10.2967/jnumed.114.146019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen Q, Wang X, Wang C, Feng L, Li Y, Liu Z. Drug-Induced Self-Assembly of Modified Albumins as Nano-theranostics for Tumor-Targeted Combination Therapy. ACS Nano. 2015;9(5):5223–5233. doi: 10.1021/acsnano.5b00640. [DOI] [PubMed] [Google Scholar]
- 38.Chou LYT, Zagorovsky K, Chan WCW. DNA assembly of nanoparticle superstructures for controlled biological delivery and elimination. Nat Nanotechnol. 2014;9(2):148–155. doi: 10.1038/nnano.2013.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hall SS, Mitragotri S, Daugherty PS. Identification of Peptide Ligands Facilitating Nanoparticle Attachment to Erythrocytes. Biotechnol Prog. 2007;23(3):749–754. doi: 10.1021/bp060333l. [DOI] [PubMed] [Google Scholar]
- 40.Immordino ML, Dosio F, Cattel L. Stealth liposomes: review of the basic science, rationale, and clinical applications, existing and potential. Int J Nanomed. 2006;1(3):297–315. [PMC free article] [PubMed] [Google Scholar]
- 41.Knop K, Hoogenboom R, Fischer D, Schubert U. Poly(ethylene glycol) in Drug Delivery: Pros and Cons as Well as Potential Alternatives. Angew Chem, Int Ed. 2010;49(36):6288. doi: 10.1002/anie.200902672. [DOI] [PubMed] [Google Scholar]
- 42.Danhier F, Feron O, Preat V. To exploit the tumor microenvironment: Passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J Controlled Release. 2010;148(2):135–146. doi: 10.1016/j.jconrel.2010.08.027. [DOI] [PubMed] [Google Scholar]
- 43.Devalapally H, Chakilam A, Amiji MM. Role of nanotechnology in pharmaceutical product development. J Pharm Sci. 2007;96(10):2547–2565. doi: 10.1002/jps.20875. [DOI] [PubMed] [Google Scholar]
- 44.Haley B, Frenkel E. Nanoparticles for drug delivery in cancer treatment. Urol Oncol: Semin Orig Invest. 2008;26(1):57–64. doi: 10.1016/j.urolonc.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 45.Chung EJ, Cheng Y, Morshed R, Nord K, Han Y, Wegscheid ML, Auffinger B, Wainwright DA, Lesniak MS, Tirrell MV. Fibrin-binding, peptide amphiphile micelles for targeting glioblastoma. Biomaterials. 2014;35(4):1249–1256. doi: 10.1016/j.biomaterials.2013.10.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Poon C, He C, Liu D, Lu K, Lin W. Self-assembled nanoscale coordination polymers carrying oxaliplatin and gemcitabine for synergistic combination therapy of pancreatic cancer. J Controlled Release. 2015;201:90–99. doi: 10.1016/j.jconrel.2015.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Poon C, Duan X, Chan C, Han W, Lin W. Nanoscale Coordination Polymers Codeliver Carboplatin and Gemcitabine for Highly Effective Treatment of Platinum-Resistant Ovarian Cancer. Mol Pharmaceutics. 2016;13(11):3665–3675. doi: 10.1021/acs.molpharmaceut.6b00466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chung EJ, Mlinar LB, Nord K, Sugimoto MJ, Wonder E, Alenghat FJ, Fang Y, Tirrell M. Monocyte-Targeting Supra-molecular Micellar Assemblies: A Molecular Diagnostic Tool for Atherosclerosis. Adv Healthcare Mater. 2015;4(3):367–376. doi: 10.1002/adhm.201400336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Leuschner F, Dutta P, Gorbatov R, Novobrantseva TI, Donahoe JS, Courties G, Lee KM, Kim JI, Markmann JF, Marinelli B, Panizzi P, Lee WW, Iwamoto Y, Milstein S, Epstein-Barash H, Cantley W, Wong J, Cortez-Retamozo V, Newton A, Love K, Libby P, Pittet MJ, Swirski FK, Koteliansky V, Langer R, Weissleder R, Anderson DG, Nahrendorf M. Therapeutic siRNA silencing in inflammatory monocytes in mice. Nat Biotechnol. 2011;29(11):1005–1010. doi: 10.1038/nbt.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lobatto ME, Fayad ZA, Silvera S, Vucic E, Calcagno C, Mani V, Dickson SD, Nicolay K, Banciu M, Schiffelers RM, Metselaar JM, van Bloois L, Wu H-S, Fallon JT, Rudd JH, Fuster V, Fisher EA, Storm G, Mulder WJM. Multimodal Clinical Imaging To Longitudinally Assess a Nanomedical Anti-Inflammatory Treatment in Experimental Atherosclerosis. Mol Pharmaceutics. 2010;7(6):2020–2029. doi: 10.1021/mp100309y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Khodabandehlou K, Masehi-Lano JJ, Poon C, Wang J, Chung EJ. Targeting cell adhesion molecules with nanoparticles using in vivo and flow-based in vitro models of atherosclerosis. Exp Biol Med (London, U K) 2017;242(8):799–812. doi: 10.1177/1535370217693116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mlinar LB, Chung EJ, Wonder EA, Tirrell M. Active targeting of early and mid-stage atherosclerotic plaques using self-assembled peptide amphiphile micelles. Biomaterials. 2014;35(30):8678–8686. doi: 10.1016/j.biomaterials.2014.06.054. [DOI] [PubMed] [Google Scholar]
- 53.Acar H, Srivastava S, Chung EJ, Schnorenberg MR, Barrett JC, LaBelle JL, Tirrell M. Self-assembling peptide-based building blocks in medical applications. Adv Drug Delivery Rev. 2017;110–111:65. doi: 10.1016/j.addr.2016.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Busseron E, Ruff Y, Moulin E, Giuseppone N. Supra-molecular self-assemblies as functional nanomaterials. Nanoscale. 2013;5(16):7098–7140. doi: 10.1039/c3nr02176a. [DOI] [PubMed] [Google Scholar]
- 55.Trent A, Marullo R, Lin B, Black M, Tirrell M. Structural properties of soluble peptide amphiphile micelles. Soft Matter. 2011;7(20):9572–9582. [Google Scholar]
- 56.Toughrai S, Malinova V, Masciadri R, Menon S, Tanner P, Palivan C, Bruns N, Meier W. Reduction-Sensitive Amphiphilic Triblock Copolymers Self-Assemble Into Stimuli-Responsive Micelles for Drug Delivery. Macromol Biosci. 2015;15(4):481–489. doi: 10.1002/mabi.201400400. [DOI] [PubMed] [Google Scholar]
- 57.Alenghat FJ, Baca QJ, Rubin NT, Pao LI, Matozaki T, Lowell CA, Golan DE, Neel BG, Swanson KD. Macrophages require Skap2 and Sirpα for integrin-stimulated cytoskeletal rearrangement. J Cell Sci. 2012;125(22):5535–5545. doi: 10.1242/jcs.111260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Johnson WE, Li C, Rabinovic A. Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics. 2007;8(1):118–127. doi: 10.1093/biostatistics/kxj037. [DOI] [PubMed] [Google Scholar]
- 59.Storey JD. A direct approach to false discovery rates. Journal of the Royal Statistical Society: Series B (Statistical Methodology) 2002;64(3):479–498. [Google Scholar]
- 60.Huang DW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2008;4(1):44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 61.Huang DW, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009;37(1):1–13. doi: 10.1093/nar/gkn923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhou L, Azfer A, Niu J, Graham S, Choudhury M, Adamski FM, Younce C, Binkley PF, Kolattukudy PE. Monocyte Chemoattractant Protein-1 Induces a Novel Transcription Factor That Causes Cardiac Myocyte Apoptosis and Ventricular Dysfunction. Circ Res. 2006;98(9):1177–1185. doi: 10.1161/01.RES.0000220106.64661.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gavrilin MA, Deucher MF, Boeckman F, Kolattukudy PE. Monocyte Chemotactic Protein 1 Upregulates IL-1β Expression in Human Monocytes. Biochem Biophys Res Commun. 2000;277(1):37–42. doi: 10.1006/bbrc.2000.3619. [DOI] [PubMed] [Google Scholar]
- 64.Stefanick JF, Ashley JD, Bilgicer B. Enhanced Cellular Uptake of Peptide-Targeted Nanoparticles through Increased Peptide Hydrophilicity and Optimized Ethylene Glycol Peptide-Linker Length. ACS Nano. 2013;7(9):8115–8127. doi: 10.1021/nn4033954. [DOI] [PubMed] [Google Scholar]
- 65.Sykes EA, Dai Q, Sarsons CD, Chen J, Rocheleau JV, Hwang DM, Zheng G, Cramb DT, Rinker KD, Chan WCW. Tailoring nanoparticle designs to target cancer based on tumor pathophysiology. Proc Natl Acad Sci U S A. 2016;113(9):E1142–E1151. doi: 10.1073/pnas.1521265113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Maxwell PJ, Neisen J, Messenger J, Waugh DJJ. Tumor-derived CXCL8 signaling augments stroma-derived CCL2-promoted proliferation and CXCL12-mediated invasion of PTEN-deficient prostate cancer cells. Oncotarget. 2014;5(13):4895–908. doi: 10.18632/oncotarget.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Richards DM, Hettinger J, Feuerer M. Monocytes and Macrophages in Cancer: Development and Functions. Cancer Microenviron. 2013;6(2):179–191. doi: 10.1007/s12307-012-0123-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang YJ, Rutledge BJ, Rollins BJ. Structure/activity analysis of human monocyte chemoattractant protein-1 (MCP-1) by mutagenesis. Identification of a mutated protein that inhibits MCP-1-mediated monocyte chemotaxis. J Biol Chem. 1994;269(22):15918–15924. [PubMed] [Google Scholar]
- 69.Jiarpinitnun C, Kiessling LL. Unexpected Enhancement in Biological Activity of a GPCR Ligand Induced by an Oligoethylene Glycol Substituent. J Am Chem Soc. 2010;132(26):8844–8845. doi: 10.1021/ja102640c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mack M, Cihak J, Simonis C, Luckow B, Proudfoot AEI, Plachy J, Bruhl H, Frink M, Anders H-J, Vielhauer V, Pfirstinger J, Stangassinger M, Schlondorff D. Expression and characterization of the chemokine receptors CCR2 and CCR5 in mice. J Immunol. 2001;166(7):4697–4704. doi: 10.4049/jimmunol.166.7.4697. [DOI] [PubMed] [Google Scholar]
- 71.Han KH, Han KO, Green SR, Quehenberger O. Expression of the monocyte chemoattractant protein-1 receptor CCR2 is increased in hypercholesterolemia: differential effects of plasma lipoproteins on monocyte function. J Lipid Res. 1999;40(6):1053–1063. [PubMed] [Google Scholar]
- 72.Koga M, Kai H, Egami K, Murohara T, Ikeda A, Yasuoka S, Egashira K, Matsuishi T, Kai M, Kataoka Y, Kuwano M, Imaizumi T. Mutant MCP-1 therapy inhibits tumor angiogenesis and growth of malignant melanoma in mice. Biochem Biophys Res Commun. 2008;365(2):279–284. doi: 10.1016/j.bbrc.2007.10.182. [DOI] [PubMed] [Google Scholar]
- 73.Fleischer CC, Payne CK. Secondary Structure of Corona Proteins Determines the Cell Surface Receptors Used by Nanoparticles. J Phys Chem B. 2014;118(49):14017–14026. doi: 10.1021/jp502624n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Aragay AM, Mellado M, Frade JMR, Martin AM, Jimenez-Sainz MC, Martinez-A C, Mayor F. Monocyte chemoattractant protein-1-induced CCR2B receptor desensitization mediated by the G protein-coupled receptor kinase 2. Proc Natl Acad Sci U S A. 1998;95(6):2985–2990. doi: 10.1073/pnas.95.6.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.