Abstract
Background
Limited efficacy of IV recombinant tissue plasminogen activator (rt-PA) for large vessel occlusions (LVO) raises doubts about its utility prior to endovascular therapy.
Purpose
To compare outcomes and hospital costs for anterior circulation LVOs (middle cerebral artery, internal carotid artery terminus (ICA-T)) treated with either primary endovascular therapy alone (EV-Only) or bridging therapy (IV+EV)).
Methods
A single-center retrospective analysis was performed. Clinical and demographic data were collected prospectively and relevant cost data were obtained for each patient in the study.
Results
90 consecutive patients were divided into EV-Only (n=52) and IV+EV (n=38) groups. There was no difference in demographics, stroke severity, or clot distribution. The mean (SD) time to presentation was 5:19 (4:30) hours in the EV-Only group and 1:46 (0:52) hours in the IV+EV group (p<0.0001). Recanalization: EV-Only 35 (67%) versus IV+EV 31 (81.6%) (p=0.12). Favorable outcome: EV-Only 26 (50%) versus IV+EV 22 (58%) (p=0.45). For patients presenting within 4.5 hours (n=64): Recanalization: EV-Only 21/26 (81%) versus IV+EV 31/38 (81.6%) (p=0.93). Favorable outcome: EV-Only 14/26 (54%) versus IV+EV 22/38 (58%) (p=0.75). There was no significant difference in rates of hemorrhage, mortality, home discharge, or length of stay. A stent retriever was used in 67 cases (74.4%), with similar recanalization, outcomes, and number of passes in the EV-Only and IV+EV groups. The mean (SD) total hospital cost was $33 810 (13 505) for the EV-Only group and $40 743 (17 177) for the IV+EV group (p=0.02). The direct cost was $23 034 (8786) for the EV-Only group and $28 711 (11 406) for the IV+EV group (p=0.007). These significantly higher costs persisted for the subgroup presenting in <4.5 hours and the stent retriever subgroup. IV rt-PA administration independently predicted higher hospital costs.
Conclusions
IV rt-PA did not improve recanalization, thrombectomy efficacy, functional outcomes, or length of stay. Combined therapy was associated with significantly higher total and direct hospital costs than endovascular therapy alone.
Keywords: Stroke, Economics, Thrombectomy, Intervention
Introduction
An estimated 24 patients per 100 000 people per year in the USA have an acute ischemic stroke (AIS) secondary to a large vessel occlusion (LVO).1 These strokes are more severe and are less likely to respond to IV administered therapies than other types of ischemic stroke.2–5 A number of trials have shown improved outcomes following endovascular therapy for LVO strokes compared with standard therapies alone.6–10 However, the number of patients treated without prior IV recombinant tissue plasminogen activator (rt-PA) was small. The question of whether IV rt-PA before rapid endovascular treatment helps or harms the patient with LVO stroke therefore remains unanswered. It is possible that IV rt-PA prior to mechanical therapies has no effect on the outcome of LVO strokes, as shown by two recent meta-analyses.11 12 If this is the case, then the additional cost of IV rt-PA may be burdensome to the patients and their insurers as a 100 mg vial of alteplase (Activase, Genentech, San Francisco, California, USA) used in the preparation of an IV dose for stroke thrombolysis costs our hospital $7800; 2 years ago the cost was $6000.
Patients with an LVO may do better with IV thrombolysis compared with no treatement7 8 13 so, in the absence of endovascular therapy, IV rt-PA for LVO strokes may be justified. However, when endovascular therapy is readily available, the rationale for administering this drug to all patients with LVO without a proven benefit can be questioned. Trials confirming the superior efficacy of mechanical thrombectomy did not show an increased risk of symptomatic intracranial hemorrhage with endovascular therapy following IV thrombolysis.6–8 This lack of harm may not be sufficient justification to maintain the current standard since a complete definition of harm includes the cost to society—a true measure of which also includes the opportunity cost of the therapeutic approach. In total these can be substantial, considering the multiple other steps in the stroke chain that can be improved with additional resources. In the evolving landscape for ischemic stroke treatment, a paradigm change might be required to reflect the current evidence—a paradigm that includes socioeconomic aspects of delivering that care. There may be an adjunctive role for pharmacological thrombolysis; however, it is possible that the thrombolytic agent, such as rt-PA, is more efficacious when administered IA in conjunction with mechanical thrombectomy than the IV administration of a larger dose.
This study asks two questions: (1) Is there a significant difference in hospital costs for patients receiving endovascular stroke therapy following IV thrombolysis compared with endovascular therapy alone? (2) Are the procedural and clinical outcomes better if a patient receives IV rt-PA prior to endovascular therapy?
Methods
The study was performed after institutional review board approval as a single-center retrospective analysis. This is primarily a pilot study conducted in order to evaluate feasibility, potentially determine effect size, and guide larger scale studies.
Patient selection
A prospectively maintained database was searched for endovascular therapy (with or without prior IV rt-PA) for anterior circulation LVO over a 3-year period. We restricted the study to the recent 3-year period because of improved and more consistent thrombectomy techniques and the use of next generation devices. Another reason to limit the study to the recent 3-year period was implementation of a new, robust and accurate financial analytics system that was introduced at the beginning of this study period. All patients with suspected AIS at our institution undergo an immediate non-contrast CT (NCCT), a CT angiogram (CTA), and a CT perfusion (CTP) study regardless of their eligibility for IV rt-PA. Patients presenting with an occlusion involving the internal carotid artery terminus (ICA-T) or the main stem of the middle cerebral artery (MCA) with or without bifurcation branch involvement prospectively identified on the CTA were included. Patients with posterior circulation occlusions were excluded because the efficacy of endovascular therapy has not been shown to be superior to IV thrombolysis in randomized trials. Patients in both groups, regardless of time from symptom onset, were selected for endovascular therapy based on stroke severity, baseline functional status, comorbid conditions, and imaging findings of salvageable brain based on NCCT Alberta Stroke Program Early CT Score (ASPECTS)14 as well as CTP.15 16 Patients with an LVO presenting within 4.5 hours of symptom onset received IV rt-PA (unless contraindicated) followed by endovascular treatment. If there were contraindications to IV rt-PA (as listed in the Results section), the patients were treated with endovascular therapy directly. The patients were divided into two groups: endovascular therapy following IV rt-PA administration (IV+EV) and endovascular therapy alone (EV-Only).
Cost analysis
Our hospital assigns costs on an observed cost methodology using Vizient's Clinical Data Base (https://vizientinc.com). Every item on a master price list of devices, medications, supplies, procedures, and other services has a corresponding revenue code. These revenue codes are periodically updated by the Centers for Medicare and Medicaid and the National Uniform Billing Committee. The total charges for each discharge are grouped by the revenue code and multiplied by the cost–charge ratio, giving an unadjusted observed direct cost. The cost–charge ratios are calculated from an individual facility's cost report data reflected in the Medicare Cost Reports (MCR) (https://www.cms.gov/Research-Statistics-Data-and-Systems/Downloadable-Public-Use-Files/Cost-Reports/), with separate cost–charge ratios for direct and total costs. The unadjusted observed direct cost is multiplied by a wage index, relevant to the geographic location of the hospital, applied to the labor portion of the unadjusted cost to obtain the adjusted observed cost.
Hospital costs for all patients in the study were obtained using the above described methodology. The total hospital costs are made up of direct and indirect costs. Direct costs specific to each case profile include the costs that are directly related to patient care such as medications (eg, alteplase), devices (eg, stent retrievers), procedures, supplies, and technologists' and nurses' compensation. Indirect costs are not directly related to patient care and generally include capital and building costs, utilities, support services, and overheads. The following parameters constituted endpoints for cost analysis and were compared between the IV+EV and the EV-Only groups: total hospital costs; direct hospital costs; and indirect hospital costs. Since the length of stay is a significant predictor of hospital costs,17 we also included length of hospital stay and length of intensive care unit (ICU) stay.
Outcome analyses
Functional outcome
The primary endpoint for treatment efficacy was the 90-day modified Rankin score dichotomized into a favorable outcome (0–2) and poor outcome (3–6).
Procedural outcomes
The primary procedural endpoint was vascular recanalization, defined as Thrombolysis In Cerebral Ischemia (TICI), with a grade of 2B or higher considered as successful recanalization.
The number of thrombectomy ‘passes’ was evaluated for patients in whom a stent retriever was used. We did this for the stent retriever group as it was the overwhelmingly dominant device used in our practice.
Safety outcomes
The safety parameters assessed were intracranial hemorrhage based on the ECASS-II criteria18 19 (parenchymal hematoma PH-1 or PH-2 defined as significant hemorrhage) and 90-day mortality.
Statistical analysis
The significance of simple bivariate associations was assessed using Fisher's exact test for categorical variables, Student's t-test for continuous variables, or logistic regression, as appropriate. The normality of the cost distribution data assessed with the Shapiro-Wilk W test showed a non-normal distribution of cost (p<0.0001). The differences in cost between the two groups were thus assessed using the non-parametric Wilcoxon rank sum test. All data analysis was performed using JMP statistical software V.11 (SAS Institute, Cary, North Carolina, USA).
Analyses comparing costs and outcomes were performed for the following groups: (1) the entire cohort; (2) patients presenting within 4.5 hours of symptom onset (ie, those eligible for IV thrombolysis); and (3) patients in whom a stent retriever was used.
Results
Outcome analyses for the entire cohort and the ≤4.5 hours subgroup
A total of 90 consecutive patients satisfied the above described inclusion criteria. Of these, 52 patients (58%) were in the EV-Only group and 38 (42%) were in the IV+EV group. The baseline statistics presented in table 1 show that the two groups were similar in demographic make-up, stroke severity, and comorbidities. The only significant difference was a longer time to presentation from symptom onset for the EV-Only group. The CT-Interventional Neuroradiology (INR) Lab time trended to be lower in the EV-Only group, but the procedure duration was similar for both groups. For the entire cohort, successful recanalization was achieved in 66 patients (73.3%, 95% CI 63% to 81%) and a favorable outcome was seen in 48 (53.3%, 95% CI 43% to 63%). Recanalization was a significant predictor of outcome, with a favorable outcome seen in 42/66 patients (64%) with successful recanalization compared with 6/24 patients (25%) with failed recanalization (OR 0.19, 95 CI 0.07 to 0.54, p=0.001). There was no difference in outcomes between the IV+EV and EV-Only groups either in patients with successful recanalization or those with failed recanalization: among patients with successful recanalization (n=66), a favorable outcome was seen in 22/35 patients (63%) in the EV-Only group versus 20/31 patients (64.5%) in the IV+EV group (p=0.88). In patients with failed recanalization (n=24), a favorable outcome was seen in 4/17 patients (23.5%) in the EV-Only group versus 2/7 patients (28.6%) in the IV+EV group (p=0.8).
Table 1.
Baseline demographics, comorbidities and treatment times
| EV-Only (n=52) | IV+EV (n=38) | ||
|---|---|---|---|
| Age, mean (SD) | 69 (18) | 63 (19) | 0.15 |
| Female patients, n (%) | 32 (61) | 18 (47) | 0.18 |
| ICA-T, n (%)/MCA, n (%) | 8 (15)/44 (85) | 4 (10.5)/34 (89.5) | 0.5 |
| Diabetes, n (%) | 15 (29) | 7 (18) | 0.25 |
| Hypertension, n (%) | 38 (73) | 21 (55) | 0.08 |
| Hyperlipidemia, n (%) | 27 (52) | 17 (45) | 0.5 |
| Atrial fibrillation, n (%) | 24 (46) | 10 (26) | 0.052 |
| Smoking, n (%) | 12 (23) | 5 (13.) | 0.23 |
| NIHSS, median (IQR) | 16 (10–22) | 18 (13–23) | 0.08 |
| CT ASPECTS | 7.5 (6–9) | 8 (7–9) | 0.09 |
| Mean (SD) time from symptom onset, hour:min | 5:19 (4:30) | 1:46 (0:52) | <0.0001* |
| Mean (SD) CT–INR, hour:min | 0:47 (0:32) | 1:00 (0:33) | 0.054 |
| Mean (SD) procedure duration, hour:min | 1:11 (0:33) | 1:16 (0:37) | 0.47 |
*Significance level is set at 0.05.
EV-Only, endovascular therapy alone group; ICA-T, internal carotid artery terminus; IV+EV, endovascular therapy following IV rt-PA administration group; MCA, middle cerebral artery; NIHSS, NIH Stroke Scale.
A comparison of the clinical, procedural, and safety outcomes did not show any difference between the two groups for recanalization, favorable outcome, significant hemorrhage, mortality, or home discharge—either for the entire cohort or for the subgroup presenting within 4.5 hours of symptom onset (ie, those within the therapeutic time window for IV rt-PA) (table 2). The reasons for patients who presented within 4.5 hours undergoing endovascular therapy without IV rt-PA (n=26) included recent stroke or hemorrhage (n=6), anticoagulation therapy (n=5), recent surgical procedure (n=3), abnormal platelets or coagulation (n=2), metastatic cancer (n=1), a nonagenarian presenting at 4 hours (n=1), and patients who were barely within the time window on arrival but would have been outside before the bolus could be administered (n=8).
Table 2.
Functional and safety outcomes for the entire cohort and for the subgroup presenting within 4.5 hours of symptom onset
| EV-Only vs IV+EV Entire cohort (n=90) |
EV-Only vs IV+EV Onset to presentation ≤4.5 hours (n=64) |
|||||
|---|---|---|---|---|---|---|
| EV-Only (n=52) | IV+EV (n=38) | p Value | EV-Only (n=26) | IV+EV (n=38) | p Value | |
| Recanalization | 35 (67) | 31 (81.6) | 0.12 | 21 (81) | 31 (81.6) | 0.93 |
| Favorable outcome | 26 (50) | 22 (58) | 0.45 | 14 (54) | 22 (58) | 0.75 |
| Hemorrhage (PH1/PH2) | 3 (5.8) | 1 (2.6) | 0.46 | 1 (3.8) | 1 (2.6) | 0.78 |
| Mortality | 13 (25) | 4 (10.5) | 0.07 | 5 (19.2) | 4 (10.5) | 0.33 |
| Home discharge | 19 (36.5) | 11 (29) | 0.45 | 11 (42) | 11 (29) | 0.27 |
All values shown are n (%).
EV-Only, endovascular therapy alone group; IV+EV, endovascular therapy following IV rt-PA administration group.
Cost comparison
A comparison of the total, direct, and indirect hospital costs for the entire cohort as well as the ≤4.5 hours subgroup is presented in table 3. The table shows that the direct and total hospital costs were significantly higher for the IV+EV group. There was no significant difference in the indirect costs, indicating that the driver for increased hospitals costs is the direct cost component. The length of hospital stay was significantly correlated with total hospital cost (R2 0.59, p<0.0001). The hospital costs were higher in the IV+EV group than in the EV-Only group despite a similar length of total hospital stay and stay in the ICU. Among the outcomes, recanalization, length of stay and treatment type, IV+EV treatment (p=0.002 for total cost, p=0.0007 for direct cost), and longer length of hospital stay (p<0.0001 for both total and direct costs) remained independent predictors of higher total and direct costs on logistic regression.
Table 3.
Comparison of length of stay and hospital costs between the two treatment groups
| EV-Only vs IV+EV Entire cohort (n=90) |
EV-Only vs IV+EV Onset to presentation ≤4.5 hours (n=64) |
|||||
|---|---|---|---|---|---|---|
| EV-Only (n=52) | IV+EV (n=38) | p Value | EV-Only (n=26) | IV+EV (n=38) | p Value | |
| Total cost, $ | 33 810 (13 505) | 40 743 (17 177) | 0.024* | 31 621 (12 874) | 40 743 (17 177) | 0.027* |
| Direct cost, $ | 23 034 (8786) | 28 711 (11 406) | 0.007* | 22 087 (9228) | 28 711 (11 406) | 0.017* |
| Indirect cost, $ | 10 777 (5104) | 12 032 (6311) | 0.39 | 9534 (3928) | 12 032 (6311) | 0.09 |
| Length of stay, days | 8 (6) | 8 (6) | 0.86 | 6 (4) | 8 (6) | 0.34 |
| Length of ICU stay, days† | 2.1 (2.1) | 2.2 (1.5) | 0.48 | 2 (2.2) | 2.2 (1.5) | 0.23 |
All values shown are mean (SD).
*Significance level is set at 0.05.
†ICU stay comparison is for 39 patients in the EV-Only group and 30 patients in the IV+EV group for the entire cohort and 20 patients in the EV-Only group and 30 patients in the IV+EV group for the ≤4.5 hours cohort.
EV-Only, endovascular therapy alone group; ICU, intensive care unit; IV+EV, endovascular therapy following IV rt-PA administration group.
Stent retriever subgroup
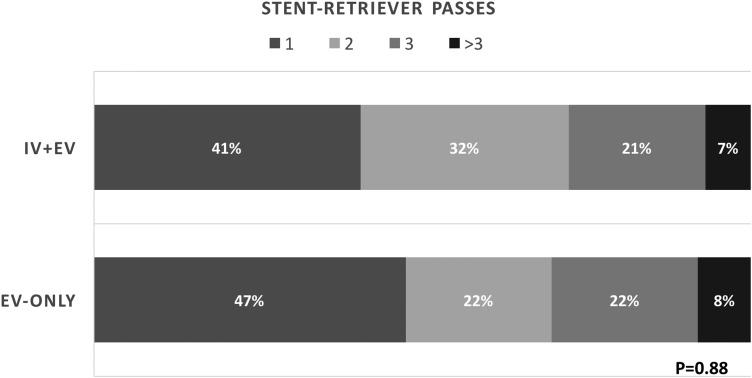
Sixty-seven patients (74.4%) were treated using a stent retriever. An aspiration catheter was used as the primary thrombectomy device in seven patients (7.8%). In 16 patients (17.8%) no thrombectomy device was used either due to inaccessible anatomy (n=11) or clot lysis/migration (n=5). Clot lysis prior to intervention was observed in three patients (5.7%) in the EV-Only group and two (5.3%) in the IV+EV group. The distribution of the devices was stent retriever in 36 patients (69.2%), aspiration in 4 (7.7%), and no device in 12 patients (23.1%) in the EV-Only group versus stent retriever in 31 patients (81.6%), aspiration in 3 (7.9%), and no device in 4 patients (10.5%) in the IV+EV group (p=0.28). In order to remove the effects of device choice on outcomes and cost, we conducted a separate analysis for only those patients in whom a stent retriever was used (n=67, table 4). This analysis showed that, for similar stroke severity and clot distribution, there was no significant difference between the rate of recanalization and favorable outcomes between the two groups; however, the total and direct costs remained significantly higher for the IV+EV group. There was no difference in the number of stent retriever thrombectomy passes made between the two groups (figure 1).
Table 4.
Comparison of outcomes and costs for patients in whom a stent retriever was the primary thrombectomy device (n=67)
| EV-Only (n=36) | IV+EV (n=31) | ||
|---|---|---|---|
| NIHSS, median (IQR) | 17 (10–24) | 18 (11–24) | 0.38 |
| CT ASPECTS, median (IQR) | 7 (6–8) | 8 (7–9) | 0.02* |
| ICA-T/MCA, n (%) | 5 (14)/31 (86) | 3 (9.7)/28 (90.3) | 0.59 |
| Recanalization, n (%) | 29 (80.6) | 27 (87.1) | 0.46 |
| Favorable outcome, n (%) | 19 (53) | 18 (58) | 0.66 |
| Mean (SD) total cost, $ | 33 999 (11 394) | 41 420 (17 155) | 0.027* |
| Mean (SD) direct cost, $ | 23 429 (7534) | 29 269 (11 287) | 0.007* |
| Mean (SD) indirect cost, $ | 10 570 (4355) | 12 150 (6443) | 0.38 |
*Significance level is set at 0.05.
ASPECTS, Alberta Stroke Program Early CT Score; EV-Only, endovascular therapy alone group; ICA-T, internal carotid artery terminus; IV+EV, endovascular therapy following IV rt-PA administration group; MCA, middle cerebral artery; NIHSS, NIH Stroke Scale.
Figure 1.
Comparison of the number of passes made using a stent retriever in the endovascular therapy following IV rt-PA administration group (IV+EV) and endovascular therapy alone group (EV-Only).
Discussion
The systems of care for endovascular stroke therapy are evolving following positive trials.6–10 Successful outcomes after mechanical thrombectomy depend to a large degree on the speed and efficiency with which recanalization is achieved. The key determinant in triaging a patient with AIS is rapid identification of an LVO, which is now possible with modern multidetector CT scanners. Once an LVO is identified in a patient with favorable clinical and imaging parameters, all resources should be directed at achieving revascularization. In this setting, diverting attention and resources to administering IV rt-PA, which may or may not have an effect on the outcome but does add to the costs, needs to be re-examined. IV rt-PA did not improve recanalization or favorable outcomes in our patients, despite the significantly longer time to presentation for the EV-Only group. This is because we rely heavily on tissue-based patient selection using NCCT and CTP as opposed to a time clock. To remove the effect of time, we conducted a separate analysis of patients presenting within 4.5 hours and found nearly identical rates of recanalization and favorable outcomes.
Frequent arguments in favor of administering IV rt-PA before endovascular therapy include possible enhancement of the interventional procedure and at least some possibility of a good outcome in case of unsuccessful recanalization. In our study, administration of IV rt-PA did not improve revascularization or functional outcomes. There was higher mortality in the EV-Only group, although this was not statistically significant. This difference could be partly due to the complexity of the case mixture between the groups or procedural variables that we did not measure. It is possible that the difference in mortality would become significant in a larger sample or that the difference would be diminished in a larger, better matched sample. There was no difference in the number of thrombectomy passes or procedure duration. The failure to improve outcomes was not dependent on whether or not recanalization was achieved. Thus, the notion that IV rt-PA could provide some benefit in cases where there is failure of recanalization was not borne out by our data. These results did not change for patients presenting within 4.5 hours or those treated with a stent retriever. Our findings are in keeping with a recent study20 and meta-analyses11 12 showing no benefit of IV thrombolysis prior to endovascular therapy.
This study shows significantly higher total and direct hospital costs for patients who received IV rt-PA before endovascular therapy. These costs remained significantly higher for patients presenting within 4.5 hours and for those treated with a stent retriever. There was no difference in indirect costs between these groups, indicating that the reason for higher total costs is the direct rather than the indirect cost. As mentioned in the Methods section, direct costs represent the costs of services and supplies directly involved in patient care. This is important because the cost of medications such as alteplase and devices such as stent retrievers is reflected in the direct costs. The fact that direct costs remained significantly higher for the IV+EV group despite similar demographics, similar length of total and ICU stay, similar device use, and similar outcomes indicates that the cost of IV alteplase ($7800 for our hospital) was the main driver of higher direct costs in the group receiving bridging therapy. Along with a longer length of stay, the use of IV rt-PA independently predicted higher hospital costs. In fact, the difference in average total and direct costs was roughly equal to the cost of alteplase. The price of stent retrievers is similar to that of alteplase and it is possible that, as the stent retriever market saturates, a product may differentiate itself with a lower price. Additionally, aspiration-only techniques may incur lower direct costs than stent retrievers,21 thus both a lower-priced thrombectomy device and increased use of aspiration techniques can further increase the cost gap with bridging therapy. Of course, the use of multiple devices or a decrease in the price of alteplase can offset these dynamics.
The majority of payer mix in our catchment population is Medicare, so the cost of care is an important consideration when expanding services or expediting delivery of stroke interventions.22 Endovascular therapy for LVO strokes has been associated with a financial benefit to the hospital over IV thrombolysis.17 A detailed analysis from the UK showed mechanical thrombectomy to be cost effective based on the quality adjusted gain in life years,23 and a similar cost analysis from the USA showed a significant reduction in the financial burden of stroke with endovascular therapy compared with IV rt-PA for large vessel strokes.24 A randomized clinical trial evaluating the efficacy of IV rt-PA for large vessel strokes versus medical management has never been done and is probably not possible anymore. It may not be prudent to completely eliminate IV rt-PA since there is indirect evidence that, in the absence of anything else, IV rt-PA could be beneficial for LVO strokes. Endovascular interventions are resource-intensive around the clock therapies that are ideally suited for large comprehensive centers, not just because of the cost of care involved but also because of the critical supportive services required for optimal postoperative management and improved functional outcomes. Operator experience in higher volume comprehensive centers also correlates with better outcomes after stroke interventions.25 The neurointerventional workforce is estimated to be more than sufficient to meet the current and expected demand for endovascular stroke coverage.26 27 It may therefore be practical to organize stroke care along the ST-elevation myocardial infarction guidelines28 29—that is, develop systems for efficient transfer of patients to endovascular-capable hospitals where primary mechanical thrombectomy is the preferred treatment modality and reserve IV thrombolysis if access to a comprehensive center is not readily available. A randomized clinical trial showing clear benefit of thrombectomy alone over thrombectomy following IV thrombolysis may be required to take this next step in large vessel stroke care.
Study limitations
This is not a cost–utility analysis and therefore we cannot comment on the long-term cost effectiveness of one thrombectomy approach against another. The sample size was small, especially in the <4.5 hours subgroup, so it is possible that a larger sample may yield a statistically significant treatment or safety effect not discernible in our study. There may be selection biases in our data that we have not accounted for, and this is a single-center experience with a large rural catchment population. Hospital costs vary based on geography, size, and level of care, so our costs are therefore not generalizable across the board.
Conclusion
IV thrombolysis prior to endovascular therapy did not improve recanalization rates, procedure duration, number of thrombectomy passes, length of admission, or functional outcomes over endovascular therapy alone in this pilot study. These observations held true for patients presenting within 4.5 hours of symptom onset. IV rt-PA use was associated with significantly higher total and direct hospital costs, with direct costs being the driver for this difference. As systems evolve, a tiered treatment paradigm similar to acute coronary care may serve as a useful model for endovascular stroke therapy.
Acknowledgments
The authors acknowledge and appreciate the contribution of Aaron Seldon for helping with the financial data for the study.
Footnotes
Contributors: ATR: Study design, data analysis, manuscript preparation. AKA: Manuscript preparation. SHB, CB, ART, MMM, TDR, JRD: Data collection. JSC: Data collection, manuscript preparation.
Competing interests: ATR has a consulting agreement with Stryker Neurovascular who make the Trevo ProVue device which is used for mechanical thrombectomy in acute ischemic stroke.
Ethics approval: Ethics approval was obtained from the IRB.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Rai AT, Seldon AE, Boo S, et al. A population-based incidence of acute large vessel occlusions and thrombectomy eligible patients indicates significant potential for growth of endovascular stroke therapy in the USA. J Neurointerv Surg. Published Online First: 15 July 2016 10.1136/neurintsurg-2016-012515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rai A, Cline B, Williams E, et al. Intravenous thrombolysis outcomes in patients presenting with large vessel acute ischemic strokes—CT angiography-based prognosis. J Neuroimaging 2015;25:238–42. 10.1111/jon.12126 [DOI] [PubMed] [Google Scholar]
- 3.Saqqur M, Molina CA, Salam A, et al. Clinical deterioration after intravenous recombinant tissue plasminogen activator treatment: a multicenter transcranial Doppler study. Stroke 2007;38:69–74. 10.1161/01.STR.0000251800.01964.f6 [DOI] [PubMed] [Google Scholar]
- 4.Saqqur M, Uchino K, Demchuk AM, et al. Site of arterial occlusion identified by transcranial Doppler predicts the response to intravenous thrombolysis for stroke. Stroke 2007;38:948–54. 10.1161/01.STR.0000257304.21967.ba [DOI] [PubMed] [Google Scholar]
- 5.Murphy A, Symons SP, Hopyan J, et al. Factors influencing clinically meaningful recanalization after IV-rtPA in acute ischemic stroke. AJNR Am J Neuroradiol 2013;34:146–52. 10.3174/ajnr.A3169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berkhemer OA, Fransen PS, Beumer D, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med 2015;372:11–20. 10.1056/NEJMoa1411587 [DOI] [PubMed] [Google Scholar]
- 7.Campbell BC, Mitchell PJ, Kleinig TJ, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med 2015;372:1009–18. 10.1056/NEJMoa1414792 [DOI] [PubMed] [Google Scholar]
- 8.Goyal M, Demchuk AM, Menon BK, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med 2015;372:1019–30. 10.1056/NEJMoa1414905 [DOI] [PubMed] [Google Scholar]
- 9.Jovin TG, Chamorro A, Cobo E, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med 2015;372:2296–306. 10.1056/NEJMoa1503780 [DOI] [PubMed] [Google Scholar]
- 10.Saver JL, Goyal M, Bonafe A, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med 2015;372:2285–95. 10.1056/NEJMoa1415061 [DOI] [PubMed] [Google Scholar]
- 11.Broeg-Morvay A, Mordasini P, Bernasconi C, et al. Direct mechanical intervention versus combined intravenous and mechanical intervention in large artery anterior circulation stroke: a matched-pairs analysis. Stroke 2016;47:1037–44. 10.1161/STROKEAHA.115.011134 [DOI] [PubMed] [Google Scholar]
- 12.Tsivgoulis G, Katsanos AH, Mavridis D, et al. Mechanical thrombectomy improves functional outcomes independent of pretreatment with intravenous thrombolysis. Stroke 2016;47:1661–4. 10.1161/STROKEAHA.116.013097 [DOI] [PubMed] [Google Scholar]
- 13.González RG, Furie KL, Goldmacher GV, et al. Good outcome rate of 35% in IV-tPA-treated patients with computed tomography angiography confirmed severe anterior circulation occlusive stroke. Stroke 2013;44:3109–13. 10.1161/STROKEAHA.113.001938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pexman JH, Barber PA, Hill MD, et al. Use of the Alberta Stroke Program Early CT Score (ASPECTS) for assessing CT scans in patients with acute stroke. AJNR Am J Neuroradiol 2001;22:1534–42. [PMC free article] [PubMed] [Google Scholar]
- 15.Rai AT, Raghuram K, Carpenter JS, et al. Pre-intervention cerebral blood volume predicts outcomes in patients undergoing endovascular therapy for acute ischemic stroke. J Neurointerv Surg 2013;5(Suppl 1):i25–32. 10.1136/neurintsurg-2012-010293 [DOI] [PubMed] [Google Scholar]
- 16.Rai AT, Raghuram K, Domico J, et al. Pre-intervention triage incorporating perfusion imaging improves outcomes in patients undergoing endovascular stroke therapy: a comparison with the device trials. J Neurointerv Surg 2013;5:121–7. 10.1136/neurintsurg-2011-010189 [DOI] [PubMed] [Google Scholar]
- 17.Rai AT, Evans K. Hospital-based financial analysis of endovascular therapy and intravenous thrombolysis for large vessel acute ischemic strokes: the ‘bottom line’. J Neurointerv Surg 2015;7:150–6. 10.1136/neurintsurg-2013-011085 [DOI] [PubMed] [Google Scholar]
- 18.Larrue V, von Kummer RR, Müller A, et al. Risk factors for severe hemorrhagic transformation in ischemic stroke patients treated with recombinant tissue plasminogen activator: a secondary analysis of the European-Australasian Acute Stroke Study (ECASS II). Stroke 2001;32:438–41. 10.1161/01.STR.32.2.438 [DOI] [PubMed] [Google Scholar]
- 19.Hacke W, Kaste M, Fieschi C, et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Second European-Australasian Acute Stroke Study Investigators. Lancet 1998;352:1245–51. [DOI] [PubMed] [Google Scholar]
- 20.Weber R, Nordmeyer H, Hadisurya J, et al. Comparison of outcome and interventional complication rate in patients with acute stroke treated with mechanical thrombectomy with and without bridging thrombolysis. J Neurointerv Surg. Published Online First: 22 Feb 2016 10.1136/neurintsurg-2015-012236 [DOI] [PubMed] [Google Scholar]
- 21.Turk AS, Turner R, Spiotta A, et al. Comparison of endovascular treatment approaches for acute ischemic stroke: cost effectiveness, technical success, and clinical outcomes. J Neurointerv Surg 2015;7:666–70. 10.1136/neurintsurg-2014-011282 [DOI] [PubMed] [Google Scholar]
- 22.Rai AT, Smith MS, Boo S, et al. The ‘pit-crew’ model for improving door-to-needle times in endovascular stroke therapy: a Six-Sigma project. J Neurointerv Surg 2016;8:447–52. 10.1136/neurintsurg-2015-012219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ganesalingam J, Pizzo E, Morris S, et al. Cost-utility analysis of mechanical thrombectomy using stent retrievers in acute ischemic stroke. Stroke 2015;46:2591–8. 10.1161/STROKEAHA.115.009396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mangla S, O'Connell K, Kumari D, et al. Novel model of direct and indirect cost-benefit analysis of mechanical embolectomy over IV tPA for large vessel occlusions: a real-world dollar analysis based on improvements in mRS. J Neurointerv Surg. Published Online First: 20 Jan 2016 10.1136/neurintsurg-2015-012152 [DOI] [PubMed] [Google Scholar]
- 25.Gupta R, Horev A, Nguyen T, et al. Higher volume endovascular stroke centers have faster times to treatment, higher reperfusion rates and higher rates of good clinical outcomes. J Neurointerv Surg 2013;5:294–7. 10.1136/neurintsurg-2011-010245 [DOI] [PubMed] [Google Scholar]
- 26.Rai AT. The stroke interventionalist. J Neurointerv Surg 2016;8:333–4. 10.1136/neurintsurg-2016-012341 [DOI] [PubMed] [Google Scholar]
- 27.Zaidat OO, Lazzaro M, McGinley E, et al. Demand-supply of neurointerventionalists for endovascular ischemic stroke therapy. Neurology 2012;79(Suppl 1):S35–41. 10.1212/WNL.0b013e31826957ef [DOI] [PubMed] [Google Scholar]
- 28.Levine GN, Bates ER, Blankenship JC, et al. 2015 ACC/AHA/SCAI focused update on primary percutaneous coronary intervention for patients with ST-elevation myocardial infarction: an update of the 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention and the 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction. J Am Coll Cardiol 2016;67:1235–50. http://dx.doi.org/10.1016/j.jacc.2015.10.005 [DOI] [PubMed] [Google Scholar]
- 29.Joy ER, Kurian J, Gale CP. Comparative effectiveness of primary PCI versus fibrinolytic therapy for ST elevation myocardial infarction: a review of the literature. J Comp Eff Res 2016;5:217–26. 10.2217/cer-2015-0011 [DOI] [PubMed] [Google Scholar]