Abstract
Objective(s):
Paclitaxel-induced peripheral neuropathy is a common adverse effect of cancer chemo -therapy. This neuropathy has a profound impact on quality of life and patient’s survival. Preventing and treating paclitaxel-induced peripheral neuropathy is a major concern. First- and second-generation antipsychotics have shown analgesic effects both in humans and animals. Quetiapine is a novel atypical antipsychotic with low propensity to induce extrapyramidal or hyperprolactinemia side effects. The present study was designed to investigate the effects of quetiapine on the development and expression of neuropathic pain induced by paclitaxel in mice and the role of α2-adrenoceptors on its antinociception.
Materials and Methods:
Paclitaxel (2 mg/kg IP) was injected for five consecutive days which resulted in thermal hyperalgesia and mechanical and cold allodynia.
Results:
Early administration of quetiapine from the 1st day until the 5th day (5, 10, and 15 mg/kg PO) did not affect thermal, mechanical, and cold stimuli and could not prevent the development of neuropathic pain. In contrast, when quetiapine (10 and 15 mg/kg PO) administration was started on the 6th day after the first paclitaxel injections, once the model had been established, and given daily until the 10th day, heat hyperalgesia and mechanical and cold allodynia were significantly attenuated. Also, the effect of quetiapine on heat hyperalgesia was reversed by pretreatment with yohimbine, as an alpha-2 adrenergic receptor antagonist.
Conclusion:
These results indicate that quetiapine, when administered after nerve injury can reverse the expression of neuropathic pain. Also, we conclude that α2-adrenoceptors participate in the antinociceptive effects of quetiapine.
Keywords: Hyperalgesia, Mice, Neuropathic pain, Paclitaxel, Quetiapine, Yohimbine
Introduction
Peripheral neuropathy is a common disorder of cancer chemotherapeutic agents, specifically paclitaxel (1). Peripheral neuropathy, which is predominantly sensory neuropathy presents as paresthesia, numbness and/or pain, which can severely affect the quality of life and results in chemotherapy dose reduction and/or premature treatment discontinuation (2).
The mechanisms responsible for the development of neurotoxicity are often linked to the cytotoxicity mechanisms, implying the obvious difficulty in reducing neurotoxicity without diminishing anticancer efficacy (3).
Preventing and treating paclitaxel-induced peri-pheral neuropathy are major concerns in clinical cancer therapy. A wide variety of neuroprotective agents have been examined in both animals and humans to prevent paclitaxel neurotoxicity (4).
The most widely used drugs include the tricyclic antidepressants (TCAs), serotonin/norepinephrine reuptake inhibitors (SNRIs) and antiepileptic agents such as pregabalin and gabapentin, (5-8). However, there is still a need to find newer additional thera-peutic agents for the management of this complicated and debilitating disorder.
Recently it has been demonstrated that first- and second-generation antipsychotics have analgesic effects, both in humans and animals (9). Some second-generation antipsychotics have antidepressant and anxiolytic effects. Several old and new antipsychotics improve sleep parameters in healthy subjects (10).
Quetiapine was approved for the treatment of refractory major depression and bipolar depression (11, 12). Quetiapine belongs to newer atypical antipsychotics with a dopamine, serotonin, adrenergic, and histamine antagonistic properties. Quetiapine occupies serotonin receptors and acts as an antagonist at 5-HT2A receptors (13). It has been shown that 5-HT2A receptors in the peripheral sensory terminals are responsible for serotonin-induced hyperalgesia and pretreatment with 5-HT2A receptor antagonist attenu- ates the pain (14).
Also, growing evidence suggests that specific cytokines and chemokines play an important role in pain signaling and behavioral changes (15). It has been demonstrated that atypical antipsychotics modulate cytokines production (16). Quetiapine sig- nificantly reduced TNF-α release from microglia and ’its anti-inflammatory effects are well established (17). Current evidence suggests that quetiapine is able to control fibromyalgia (18).
mong the antipsychotic drugs, quetiapine very rapidly dissociates from the D2 receptors. So physiological surges of dopamine in the nigrostriatal and tuberoinfundibular pathways will occur and risk of side-effects such as extrapyramidal and hyperprolac-tinemia will diminish (19).
Each of these properties indicates that quetiapine could be a novel alternative for the management of paclitaxel-induced neuropathic pain.
lso, quetiapine and N-Desalkylquetiapine are potent norepinephrine reuptake inhibitors and increase noradrenergic transmission. Norepinephrine reuptake inhibition caused the stimulation of the descending noradrenergic pain inhibitory pathway that produced antinociception through alpha-2 adrenergic receptors (20). So, we assumed that this drug produced anti -nociceptive effects through the stimulation of the descending noradrenergic pain inhibitory pathway and α2-adrenoceptor.
The aim of this study was to evaluate the anti-nociceptive effect of quetiapine in attenuating the behavioral scores of neuropathic pain and to determine the role of α2-adrenoceptors in the antinociceptive effect of quetiapine.
Materials and Methods
Chemicals
Quetiapine (Seroquel®) was purchased from AstraZeneca Pharmaceuticals and dissolved in saline (0.9%) solution. Paclitaxel was purchased from Stragen Pharma SA (Chemin du Pré-Fleuri 3, 1228 Plan-les-Ouates, Switzerland).
Animals
NMRI mice weighing 20–30 g were used. They were housed under a 12 hr light/dark cycle and had free access to food and water. Animals were randomly assigned to different treatment groups (n=8 in each group).
General procedures for drug administration
To induce the neuropathic pain, paclitaxel (2 mg/kg) was injected IP on the 1st day and given daily for 4 additional days with a cumulative dose of 10 mg/kg (paclitaxel group) (21, 22). Vehicle group received only the saline solution.
To investigate the effects of quetiapine on the development and expression of neuropathic pain we designed two different treatment protocols; early treatment which started in the development phase of neuropathic pain and late treatment which initiated after the nerve injury and when the pain had established.
Quetiapine was dissolved in saline 0.9% solution. Quetiapine’s solution was prepared with different concentrations (0.5, 1, and 1.5 mg/ml). Quetiapine was administered orally (PO), in a volume of 10 ml/kg.
Quetiapine was administered directly into the stomach of mice. In this method, a bulb-tipped gastric gavage needle was attached to a syringe and used to administer the quetiapine into the stomach. The animal was gently restrained but not such that the animal vocalized or showed other signs of distress. The animals were maintained in an upright (vertical) position and the gavage needle passed along the side of the mouth.
To assess the effects of quetiapine on the deve -lopment of neuropathic pain, this drug (5, 10, and 15 mg/kg P.O) was administered daily from the 1st day until the 5th day in the different mouse groups.
These doses were selected based on the Kim and his colleagues study. Considering their quetiapine (10 mg/kg) shows anti-inflammatory effects in an animal model of collagen-induced arthritis (23).
To determine the effects of quetiapine on the expression of neuropathic pain, quetiapine was administered (5, 10, and 15 mg/kg PO) once daily from the 6th day until the 10th day.
Considering our previous study, neuropathic pain following paclitaxel injections starts on the 6th day after first paclitaxel injections and lasts at least for two weeks. But the highest intensity of pain can be observed on the 11th day. So we choose this day to evaluate the pain scores (21).
Heat hyperalgesia, mechanical and cold allodynia were assessed on the 11th day after the first paclitaxel injections.
Evaluation of the role of α2-adrenergic receptor
To identify the role of the α2-adrenergic receptor in the anti-nociceptive effect of quetiapine, animals were treated with yohimbine (5 mg/kg IP) on the 10th day, 15 min before last administration of quetiapine (10 mg/kg PO).
Behavioral tests of neuropathic pain
Cold allodynia (acetone test)
Animals were placed on top of an aluminum mesh table. They were allowed to adapt for 15 min. Cold allodynia was assessed by applying an acetone drop via a needle to the plantar surface of the hind paw five times with a 60 sec interval. Paw withdrawal frequency was calculated and stated as a percentage with the following formula (number paw withdrawal /total number of trial) × 100 (24).
Mechanical allodynia (von Frey filament stimulation)
For mechanical allodynia assessment, von Frey filaments (Steeling, Wood Dale, IL, USA) ranging from 0.16 to 10 g were used as previously described. The animals were placed on a wire mesh in a plexiglass box (18×18×25 cm). After the animals were adapted to the new environment, von Frey filaments, in an ascending order of forces, were used to measure mechanical allodynia. Each filament was applied to the plantar surface of the hind paw three consecutive times for 1 sec with 5 sec intervals. The smallest filament size which evoked 3 withdrawal responses was considered as withdrawal threshold (25, 26).
Thermal hyperalgesia (plantar test)
Using the plantar test and infrared radiation from the plexiglass surface to the animal’s hind paw, heat hyperalgesia to the radiant heat stimulus was measured.
Paw withdrawal latency was expressed as the latency (sec) between the radiant stimulus start and paw withdrawal. Thermal stimulation was repeated three times with 5 to 10 min intervals. A cut-off time of 22 sec was used to avoided skin surface damage (27).
Data analysis
Statistical analysis was done by one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparisons test. Results are expressed as Mean±SEM.
Results
Effect of quetiapine on the paw withdrawal frequency in vehicle-treated mice
Quetiapine (15 mg/kg PO) administration did not alter paw withdrawal frequency compared to the vehicle-treated group (Figure 1).
Figure 1.
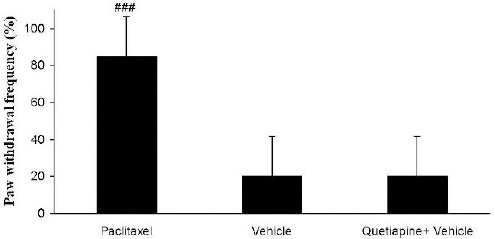
Effects of quetiapine (15 mg/kg PO) administration on paw withdrawal frequency compared to the vehicle-treated group. The paclitaxel group only received paclitaxel (2 mg/kg IP) for five consecutive days, which resulted in neuropathic pain. The vehicle group was only treated with saline 0.9% solution and ’did not receive paclitaxel. The quetiapine+vehicle group received quetiapine (15 mg/kg PO) dissolved in a saline solution. The results are expressed as Mean±SEM, ###P<0.001 versus the vehicle group, n=8 in all groups
Effect of quetiapine on the development of cold allodynia
As shown in Figure 2 paclitaxel injections significantly induced cold allodynia in comparison with the vehicle group (P<0.001). Quetiapine (5, 10, and 15 mg/kg PO) administration during the development phase did not alter cold allodynia.
Figure 2.
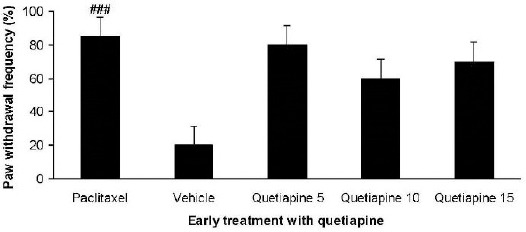
Effect of quetiapine (5, 10 and 15 mg/kg PO) administration from 1st day to the 5th day on the development of cold allodynia. The paclitaxel group only received paclitaxel (2 mg/kg IP) for five consecutive days, which resulted in neuropathic pain. The vehicle group was only treated with a saline 0.9% solution and ’did not receive paclitaxel. The results are expressed as Mean±SEM, ###P<0.001 versus the vehicle group, n=8 in all groups
Effect of quetiapine on the mechanical allodynia in vehicle-treated mice
Quetiapine (15 mg/kg PO) administration did not alter paw withdrawal threshold compared to the vehicle-treated group (Figure 3).
Figure 3.
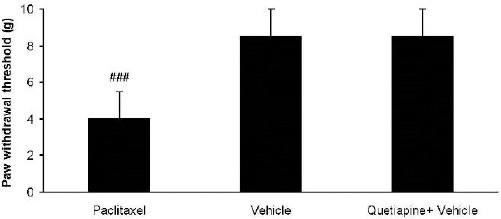
Effects of quetiapine (15 mg/kg PO) administration on paw withdrawal threshold compared to vehicle-treated group. The paclitaxel group only received paclitaxel (2 mg/kg IP) for five consecutive days, which resulted in neuropathic pain. The vehicle group was only treated with a saline 0.9% solution and ’did not receive paclitaxel. The quetiapine+vehicle group received quetiapine (15 mg/kg PO), which was dissolved in a saline solution. The results are expressed as Mean ± SEM, ###P<0.001 versus the vehicle group, n=8 in all groups
Effect of quetiapine on the development of mechanical allodynia
Paclitaxel injection significantly reduced paw withdrawal threshold compared to the vehicle group (P<0.01). However early treatment with quetiapine (5, 10, and 15 mg/kg PO) did not reduce the mechanical allodynia (Figure 4).
Figure 4.
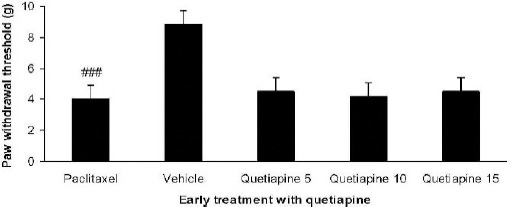
Effect of quetiapine (5, 10 and 15 mg/kg PO) administration from 1st day to the 5th day on the development of mechanical allodynia. The paclitaxel group only received paclitaxel (2 mg/kg IP) for five consecutive days, which resulted in neuropathic pain. The vehicle group was only treated with a saline 0.9% solution and ’did not receive paclitaxel. The results are expressed as Mean ± SEM, ###P<0.001 versus the vehicle group, n=8 in all groups
Effect of quetiapine on the paw withdrawal latency in vehicle-treated mice
Quetiapine (15 mg/kg PO) administration did not alter paw withdrawal latency compared to the vehicle-treated group (Figure 5).
Figure 5.
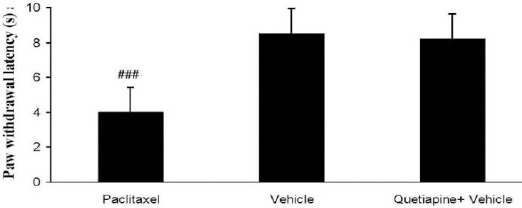
Effects of quetiapine (15 mg/kg PO) administration on paw withdrawal latency compared to the vehicle-treated group. The paclitaxel group only received paclitaxel (2 mg/kg IP) for five consecutive days, which resulted in neuropathic pain. The vehicle group was only treated with a saline 0.9% solution and ’did not receive paclitaxel. The quetiapine+vehicle group received quetiapine (15 mg/kg PO,) which was dissolved in a saline solution. The results are expressed as Mean±SEM, ###P<0.001 versus vehicle group, n=8 in all groups
Effect of quetiapine on the development of heat hyperalgesia
Paclitaxel injection decreased paw withdrawal latency (Figure 6). Early treatment with quetiapine did not significantly affect paw withdrawal latency.
Figure 6.
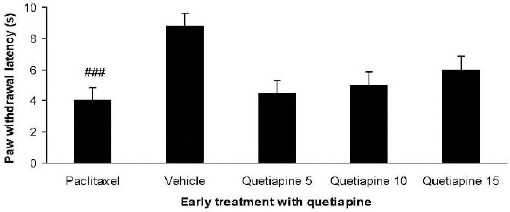
Effect of quetiapine (5, 10, and 15 mg/kg PO) administration from 1st day to the 5th day on the development of heat hyperalgesia. The paclitaxel group only received paclitaxel (2 mg/kg IP) for five consecutive days, which resulted in neuropathic pain. The vehicle group was only treated with a saline 0.9% solution and ’did not receive paclitaxel. The results are expressed as Mean±SEM, ###P<0.001 versus the vehicle group, n=8 in all groups
Effect of quetiapine on the expression of cold allodynia
Quetiapine (10 and 15 mg/kg PO) administration from the 6th day until the 10th day, i.e. during the expression phase significantly reduced cold allodynia compared to the paclitaxel-treated group (P<0.001) (Figure 7).
Figure 7.
Effect of quetiapine (5, 10 and 15 mg/kg PO) administration from 6th day to the 10th day on the expression of cold allodynia. The paclitaxel group only received paclitaxel (2 mg/kg IP) for five consecutive days, which resulted in neuropathic pain. The vehicle group was only treated with a saline 0.9% solution and ’did not receive paclitaxel. The results are expressed as Mean±SEM, ###P<0.001 versus the vehicle group,***P<0.001 versus the paclitaxel group, n=8 in all groups
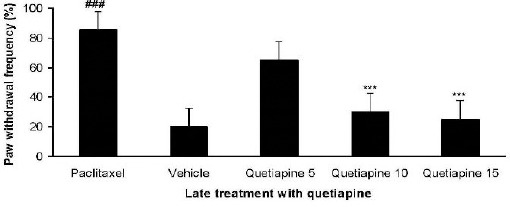
Effect of quetiapine on the expression of mechanical allodynia
Administration of quetiapine (10 and 15 mg/kg PO) during the expression phase augmented paw withdrawal threshold and attenuated mechanical allodynia in von Frey monofilament test (P<0.001) (Figure 8).
Figure 8.
Effect of quetiapine (5, 10, and 15 mg/kg PO) administration from 6th day to the 10th day on the expression of mechanical allodynia. The paclitaxel group only received paclitaxel (2 mg/kg IP) for five consecutive days, which resulted in neuropathic pain. The vehicle group was only treated with a saline 0.9% solution and ’did not receive paclitaxel. The results are expressed as Mean ± SEM, ###P<0.001 versus the vehicle group,***P<0.001 versus the paclitaxel group, n=8 in all groups
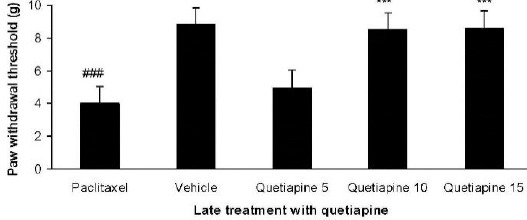
Effect of quetiapine on the expression of heat hyperalgesia
Quetiapine (10 and 15 mg/kg PO) administration from day 6 until the 10th day after paclitaxel injection produced significant anti-hyperalgesic effects com -pared to the paclitaxel group (Figure 9).
Figure 9.
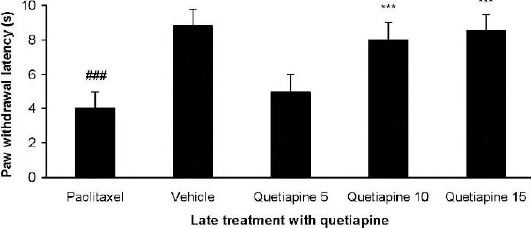
Effect of quetiapine (5, 10, and 15 mg/kg PO) administration from 6th day to the 10th day on the expression of heat hyperalgesia. The paclitaxel group only received paclitaxel (2 mg/kg IP) for five consecutive days, which resulted in neuropathic pain. The vehicle group was only treated with a saline 0.9% solution and ’did not receive paclitaxel. The results are expressed as Mean±SEM, ###P<0.001 versus the vehicle group,***P<0.001 versus the paclitaxel group, n=8 in all groups
The effects of yohimbine on quetiapine-induced effects
Yohimbine reduced the antihyperalgesic effect of quetiapine (10 mg/kg daily from day six until the 10th day) (Figure 10). Nevertheless it could not signi- ficantly change the effect of quetiapine in the mechanical and cold allodynia. Moreover, yohimbine (5 mg/kg IP) solely did not alter the nociceptive thresholds.
Figure 10.
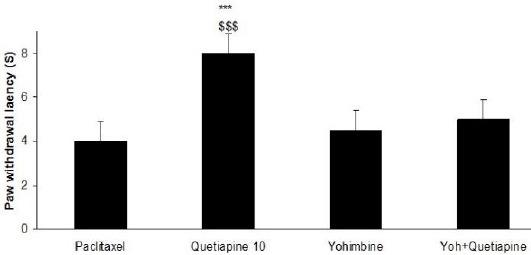
The effects of yohimbine on the anti-hyperalgesic effect of quetiapine administration (10 mg/kg daily from day six until the 10th day). The paclitaxel group only received paclitaxel (2 mg/kg IP) for five consecutive days, which resulted in neuropathic pain. the quetiapine 10 group received paclitaxel (2 mg/kg IP) for five consecutive days and quetiapine (10 mg/kg PO daily from day six until the 10th day). The yoh+quetiapine group received paclitaxel (2 mg/kg IP) for five consecutive days, quetiapine (10 mg/kg PO daily from day six until the 10th day) and yohimbine (5 mg/kg IP) on the 10th day, 15 min before last administration of quetiapine (10 mg/kg PO). The results are expressed as Mean±SEM,***P<0.001 versus the paclitaxel group, $$$P<0.001 versus the yoh+quetiapine group, n=8 in all groups
Discussion
In this experimental research, 5 day paclitaxel injection induced neuropathic pain and mice demonstrated a severe sensitivity to heat, cold, and mechanical stimulus. This is in accordance with results obtained by Nieto et al who have reported that paclitaxel (2 mg/kg) injections (IP), in a volume of 10 ml/kg, once per day for 5 consecutive days produces a painful neuropathy in rodents (22).
Considering our results, early treatment with quetiapine did not affect heat hyperalgesia and mechanical and cold allodynia and quetiapine could not inhibit the development of neuropathic pain.
Neuropathy is one of the most common and serious disorders of paclitaxel treatment and usually is the main reason for cancer chemotherapy discontinuation (28).
Axonal damage induced by paclitaxel is the suppos- ed pathogenesis of this neuropathy (29).
However, the exact pathogenesis of this disorder is not clear and various mechanisms are invsolved in this complication. The cytotoxicity of these drugs is often responsible for the development of neurotoxicity (30). Due to the Lack of an effective protective layer, Dorsal Root Ganglions (DRG) are more sensitive to this neurotoxic effects (31). It has been suggested that disrupting energy mechanisms in the axon through damage of mitochondria are involved in the nerve degeneration induced by paclitaxel (32, 2). Considering our results early treatment with quetiapine did not affect the development of this neuropathy. Therefore, it is proposed that quetiapine did not interfere with cytotoxic effects of paclitaxel or did not show any neuroprotective effects.
However when quetiapine (10 and 15 mg/kg PO) administration was started on the 6th day after first paclitaxel injections, once the neuropathy had been induced and administered daily until the 10th day, thermal hyperalgesia and mechanical and cold allodynia were significantly attenuated and quetiapine relieved the expression of neuropathic pain.
It has been shown that atypical anti-psychotics inhibit TNF-α and IL-6 production (33). Quetiapine considerably reduces NO generation and inhibits TNF-α release from activated microglia (34). In the central nervous system (CNS) these cytokines mediate the inflammatory process and contribute to the progress of the neuropathic pain (35, 36). Releasing of these pro-inflammatory cytokines by sensitization of nociceptors caused mechanical hypersensitivity (37).
Moreover, quetiapine can block 5-HT2A receptors with great affinity (13, 14). Following nerve damage, serotonin release occurs from mast cells which stimulate 5-HT2A receptors in the DRG (38, 39). It has been shown that 5-HT2A receptors are involved in the development of hyperalgesia (40). Additionally, quetiapine modulates glutamate receptor activity. This would decrease the neurotoxicity following cancer chemotherapy (41)
N-desalkylquetiapine is a 5-HT1A receptor agonist with high affinity for this receptor (42, 43).
At the spinal level, presynaptical activation of this receptor modulates pain sensation (44).
According to our results and mechanisms involved in pain modulation, it could be suggested that quetiapine is able to control the pain expression.
Also, norquetiapine acts as a norepinephrine reuptake inhibitor and stimulates the descending noradrenergic pain inhibitory pathway (45, 46). Stimulation of ɑ2-adrenoceptors in the dorsal root ganglia (DRG) modulates pain sensitivity (47, 48). Role of α2-adrenoceptor in the anti-nociceptive effect of quetiapine especially in neuropathic pain remains elusive.
Considering our results yohimbine (5 mg/kg IP), an alpha 2- adrenoceptor antagonist, reversed the antihyperalgesic effect of quetiapine in the plantar test but couldn’t influence the effect of quetiapine on the cold and mechanical allodynia. These data suggest that alpha 2- adrenoceptor participates in the antinociceptive effect of quetiapine.
Conclusion
Our results have demonstrated that quetiapine prevents the expression of paclitaxel-induced neuropathic pain. In contrast considering our results, quetiapine cannot prevent the development of this neuropathy. Quetiapine is a novel antipsychotic agent with low propensity to induce extrapyramidal or hyperprolactinemia side effects and this study identifies quetiapine as a new potential treatment for paclitaxel-induced neuropathic pain.
Acknowledgment
This paper has been supported by Vice Chancellor of Research, Shiraz University of Medical Sciences, Shiraz, Iran.
Conflict of interest
The authors declare no conflicts of interest.
References
- 1.Landowski LM, Dyck PJ, Engelstad J, Taylor BV. Axonopathy in peripheral neuropathies:Mechanisms and therapeutic approaches for regeneration. J Chem Neuroanat. 2016;76(Pt A):19–27. doi: 10.1016/j.jchemneu.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 2.Scripture CD, Figureg WD, Sparreboom A. Peripheral neuropathy induced by paclitaxel:recent insights and future perspectives. Curr Neuropharmacol. 2006;4:165–172. doi: 10.2174/157015906776359568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hershman DL, Lacchetti C, Dworkin RH, Lavoie Smith EM, Bleeker J, Cavaletti G, Chauhan C, et al. Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers:American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2014;32:1941–1967. doi: 10.1200/JCO.2013.54.0914. [DOI] [PubMed] [Google Scholar]
- 4.Albers JW, Chaudhry V, Cavaletti G, Donehower RC. Interventions for preventing neuropathy caused by cisplatin and related compounds. Cochrane Database Syst Rev. 2014;31:CD005228. doi: 10.1002/14651858.CD005228.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xiao W, Boroujerdi A, Bennett GJ, Luo ZD. Chemotherapy-evoked painful peripheral neuropathy:analgesic effects of gabapentin and effects on expression of the alpha-2- delta type-1 calcium channel subunit. Neuroscience. 2007;144:714–720. doi: 10.1016/j.neuroscience.2006.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nihei S, Sato J, Kashiwaba M, Itabashi T, Kudo K, Takahashi K. Efficacy and safety of pregabalin for oxaliplatin- and paclitaxel-induced peripheral neuropathy. Gan To Kagaku Ryoho. 2013;40:1189–93. [PubMed] [Google Scholar]
- 7.Kaley TJ, Deangelis LM. Therapy of chemotherapy-induced peripheral neuropathy. Br J Haematol. 2009;145:3–14. doi: 10.1111/j.1365-2141.2008.07558.x. [DOI] [PubMed] [Google Scholar]
- 8.Aziz MT, Good BL, Lowe DK. Serotonin-norepinephrine reuptake inhibitors for the management of chemotherapy-induced peripheral neuropathy. Ann Pharmacother. 2014;48:626–632. doi: 10.1177/1060028014525033. [DOI] [PubMed] [Google Scholar]
- 9.Seidel S, Aigner M, Ossege M, Pernicka E, Wildner B, Sycha T. Antipsychotics for acute and chronic pain in adults. Cochrane Database Syst Rev. 2013;29:CD004844. doi: 10.1002/14651858.CD004844.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohrs S. Sleep disturbances in patients with schizophrenia :impact and effect of antipsychotics. CNS Drugs. 2008;22:939–962. doi: 10.2165/00023210-200822110-00004. [DOI] [PubMed] [Google Scholar]
- 11.Roberts RJ, Lohano KK, El-Mallakh RS. Antipsychotics as antidepressants. Asia Pac Psychiatry. 2016;8:179–188. doi: 10.1111/appy.12186. [DOI] [PubMed] [Google Scholar]
- 12.Nishiyama A, Matsumoto H. Quetiapine reduces irritability and risk of suicide in patients with agitated depression. Tokai J Exp Clin Med. 2013;38:93–96. [PubMed] [Google Scholar]
- 13.Kondo MA, Tajinda K, Colantuoni C, Hiyama H, Seshadri S, Huang B, et al. Unique pharmacological actions of atypical neuroleptic quetiapine:possible role in cell cycle/fate control. Transl Psychiatry. 2013;3:e243. doi: 10.1038/tp.2013.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tokunaga A, Saika M, Senba E. 5-HT2Areceptor subtype is involved in the thermal hyperalgesic mechanism of serotonin in the periphery. Pain. 1998;76:349–355. doi: 10.1016/S0304-3959(98)00066-9. [DOI] [PubMed] [Google Scholar]
- 15.Zhang JM, An J. Cytokines, inflammation, and pain. Int Anesthesiol Clin. 2007;45:27–37. doi: 10.1097/AIA.0b013e318034194e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sugino H, Futamura T, Mitsumoto Y, Maeda K, Marunaka Y. Atypical antipsychotics suppress production of proinflammatory cytokines and up-regulate interleukin-10 in lipopolysaccharide-treated mice. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:303–307. doi: 10.1016/j.pnpbp.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 17.Bian Q, Kato T, Monji A, Hashioka S, Mizoguchi Y, Horikawa H, Kanba S. The effect of atypical antipsychotics, perospirone, ziprasidone and quetiapine on microglial activation induced by interferon-gamma. Prog Neuro -psychopharmacol Biol Psychiatry. 2008;32:42–48. doi: 10.1016/j.pnpbp.2007.06.031. [DOI] [PubMed] [Google Scholar]
- 18.Calandre EP, Rico-Villademoros F. The role of anti - psychotics in the management of fibromyalgia. CNS Drugs. 2012;26:135–53. doi: 10.2165/11597130-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 19.Seeman P. Atypical antipsychotics:mechanism of action. Can J Psychiatry. 2002;47:27–38. [PubMed] [Google Scholar]
- 20.Hajhashemi V, Banafshe HR, Minaiyan M, Mesdaghinia A, Abed A. Antinociceptive effects of venlafaxine in a rat model of peripheral neuropathy:role of alpha2- adrenergic receptors. Eur J Pharmacol. 2014;738:230–236. doi: 10.1016/j.ejphar.2014.04.046. [DOI] [PubMed] [Google Scholar]
- 21.Naji-Esfahani H, Vaseghi G, Safaeian L, Pilehvarian AA, Abed A, Rafieian-Kopaei M. Gender differences in a mouse model of chemotherapy-induced neuropathic pain. Lab Anim. 2016;50:15–20. doi: 10.1177/0023677215575863. [DOI] [PubMed] [Google Scholar]
- 22.Nieto FR, Entrena JM, Cendán CM, Pozo ED, Vela JM, Baeyens JM. Tetrodotoxin inhibits the development and expression of neuropathic pain induced by paclitaxel in mice. Pain. 2008;137:520–531. doi: 10.1016/j.pain.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 23.Kim H, Bang J, Chang HW, Kim JY, Park KU, Kim SH, Lee KJ, Cho CH, Hwang I, Park SD, Ha E, Jung SW. Anti-inflammatory effect of quetiapine on collagen-induced arthritis of mouse. Eur J Pharmacol. 2012;678:55–60. doi: 10.1016/j.ejphar.2011.12.017. [DOI] [PubMed] [Google Scholar]
- 24.Banafshe HR, Hajhashemi V, Minaiyan M, Mesdaghinia A, Abed A. Antinociceptive effects of maprotiline in a rat model of peripheral neuropathic pain:possible involvement of opioid system. Iran J Basic Med Sci. 2015;18:752–7. [PMC free article] [PubMed] [Google Scholar]
- 25.Abed A, Hajhashemi V, Banafshe HR, Minaiyan M, Mesdaghinia A. Venlafaxine attenuates heat hyperalgesia independent of adenosine or opioid system in a rat model of peripheral neuropathy. Iran J Pharm Res. 2015;14:843–50. [PMC free article] [PubMed] [Google Scholar]
- 26.Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- 27.Hamidi GA, Jafari-Sabet M, Abed A, Mesdaghinia A, Mahlooji M, Banafshe HR. Gabapentin enhances anti-nociceptive effects of morphine on heat, cold, and mechanical hyperalgesia in a rat model of neuropathic pain. Iran J Basic Med Sci. 2014;17:753–9. [PMC free article] [PubMed] [Google Scholar]
- 28.Brzeziński K. Chemotherapy-induced polyneuropathy. Part I. Pathophysiology. Contemp Oncol (Pozn) 2012;16:72–78. doi: 10.5114/wo.2012.27341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carozzi VA, Canta A, Chiorazzi A. Chemotherapy-induced peripheral neuropathy:What do we know about mechanisms? Neurosci Lett. 2015;596:90–107. doi: 10.1016/j.neulet.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 30.Sisignano M, Baron R, Scholich K, Geisslinger G. Mechanism-based treatment for chemotherapy-induced peripheral neuropathic pain. Nat Rev Neurol. 2014;10:694–707. doi: 10.1038/nrneurol.2014.211. [DOI] [PubMed] [Google Scholar]
- 31.Dumontet C, Jordan MA. Microtubule-binding agents:a dynamic field of cancer therapeutics. Nat Rev Drug Discov. 2010;9:790–803. doi: 10.1038/nrd3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boyette-Davis JA, Walters ET, Dougherty PM. Mechanisms involved in the development of chemotherapy-induced neuropathy. Pain Manag. 2015;5:285–296. doi: 10.2217/pmt.15.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pollmächer T, Haack M, Schuld A, Kraus T, Hinze-Selch D. Effects of antipsychotic drugs on cytokine networks. J Psychiatr Res. 2000;34:369–382. doi: 10.1016/s0022-3956(00)00032-7. [DOI] [PubMed] [Google Scholar]
- 34.Kato TA, Monji A, Mizoguchi Y, Hashioka S, Horikawa H, Seki Y, Kasai M, Utsumi H, Kanba S. Anti-Inflammatory properties of antipsychotics via microglia modulations:are antipsychotics a ‘fire extinguisher’ in the brain of schizophrenia? Mini Rev Med Chem. 2011;11:565–574. doi: 10.2174/138955711795906941. [DOI] [PubMed] [Google Scholar]
- 35.Luo XG, Chen SD. The changing phenotype of microglia from homeostasis to disease. Transl Neurodegener. 2012;1:9–14. doi: 10.1186/2047-9158-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Janes K, Little JW, Li C, Bryant L, Chen C, Chen Z, Kamocki K, Doyle T, Snider A, Esposito E, Cuzzocrea S, Bieberich E, Obeid L, Petrache I, Nicol G, Neumann WL, Salvemini D. The development and maintenance of paclitaxel-induced neuropathic pain require activation of the sphingosine 1-phosphate receptor subtype 1. J Biol Chem. 2014;289:21082–21097. doi: 10.1074/jbc.M114.569574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fregnan F, Muratori L, Simões AR, Giacobini-Robecchi MG, Raimondo S. Role of inflammatory cytokines in peripheral nerve injury. Neural Regen Res. 2012;7:2259–2266. doi: 10.3969/j.issn.1673-5374.2012.29.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang F, Stefano GB, Kream RM. Epigenetic modification of DRG neuronal gene expression subsequent to nerve injury:etiological contribution to complex regional pain syndromes (Part I) Med Sci Monit. 2014;20:1067–1077. doi: 10.12659/MSM.890702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Okamoto K, Imbe H, Morikawa Y, Itoh M, Sekimoto M, Nemoto K, Senba E. 5-HT2A receptor subtype in the peripheral branch of sensory fibers is involved in the potentiation of inflammatory pain in rats. Pain. 2002;99:133–143. doi: 10.1016/s0304-3959(02)00070-2. [DOI] [PubMed] [Google Scholar]
- 40.Rahman W, Bannister K, Bee LA, Dickenson AH. A pronociceptive role for the 5- HT2 receptor on spinal nociceptive transmission:an in vivo electrophysiological study in the rat. Brain Res. 2011;1382:29–36. doi: 10.1016/j.brainres.2011.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tascedda F, Lovati E, Blom JM, Muzzioli P, Brunello N, Racagni G, Riva MA. Regulation of ionotropic glutamate receptors in the rat brain in response to the atypical antipsychotic seroquel (quetiapine fumarate) Neuropsycho-pharmacology. 1999;21:211–217. doi: 10.1016/S0893-133X(99)00034-2. [DOI] [PubMed] [Google Scholar]
- 42.Jensen NH, Rodriguiz RM, Caron MG, Wetsel WC, Rothman RB, Roth BL. Ndesalkylquetiapine, a potent norepinephrine reuptake inhibitor and partial 5-HT1A agonist, as a putative mediator of quetiapine's antidepressant activity. Neuropsycho- pharmacology. 2008;33:2303–2312. doi: 10.1038/sj.npp.1301646. [DOI] [PubMed] [Google Scholar]
- 43.López-Muñoz F, Alamo C. Active metabolites as antidepressant drugs:the role of norquetiapine in the mechanism of action of quetiapine in the treatment of mood disorders. Front Psychiatry. 2013;4:102–107. doi: 10.3389/fpsyt.2013.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jeong HJ, Mitchell VA, Vaughan CW. Role of 5-HT(1) receptor subtypes in the modulation of pain and synaptic transmission in rat spinal superficial dorsal horn. Br J Pharmacol. 2012;165:1956–1965. doi: 10.1111/j.1476-5381.2011.01685.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ossipov MH, Morimura K, Porreca F. Descending pain modulation and chronification of pain. Curr Opin Support Palliat Care. 2014;8:143–151. doi: 10.1097/SPC.0000000000000055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smith H, Elliott J. Alpha(2) receptors and agonists in pain management. Curr Opin Anaesthesiol. 2001;14:513–518. doi: 10.1097/00001503-200110000-00009. [DOI] [PubMed] [Google Scholar]
- 47.Kawasaki Y, Kumamoto E, Furue H, Yoshimura M. Alpha 2 adrenoceptor-mediated presynaptic inhibition of primary afferent glutamatergic transmission in rat substantiagelatinosa neurons. Anesthesiology. 2003;98:682–689. doi: 10.1097/00000542-200303000-00016. [DOI] [PubMed] [Google Scholar]
- 48.Kingery WS, Agashe GS, Guo TZ, Sawamura S, Davies MF, Clark JD, et al. Isoflurane and nociception:spinal alpha2A adrenoceptors mediate antinociception while supraspinal alpha1 adrenoceptors mediate pronociception. Anesthesiology. 2002;96:367–374. doi: 10.1097/00000542-200202000-00023. [DOI] [PubMed] [Google Scholar]


