Abstract
Objective(s):
The aim of this study was to explore the effects of Squid ink polysaccharide (SIP) on prevention of autophagy and oxidative stress induced by cyclophosphamide (CP) in Leydig cells of mice.
Materials and Methods:
Examination of reproductive organ exponents, abnormal sperm rate, activities of superoxide dismutase (SOD), catalase (CAT), contents of malondialdehyde (MDA), and histological structure were performed to detect the optimal dose of SIP against oxidative stress damage in vivo, and autophagy-associated protein LC3 and Beclin-1 were examined by immunofluorescence, and their expression was detected by Western blot analysis. Leydig cells ultrastructural changes were observed by transmission fluorescent microscope.
Results:
SIP significantly inhibited sperm aberration, histological structure and injury of seminiferous tubules caused by CP, as well as the antioxidant activity of SOD and CAT were increased; contents of MDA were decreased. The optimal dose of SIP for prevention of oxidative stress injury by CP was 80 mg/kg. In addition, LC3 and Beclin-1 fluorescent granules were much less in the Leydig cell layer after treatment via SIP compared with the CP-treated group, and the expression levels of LC3 and Beclin-1 were also decreased. Furthermore, characteristics of cell autophagy such as mitochondrial swelling, autophagic vacuoles, and chromatin pyknosis were observed in CP-treated Leydig cells, but SIP could effectively weaken injury of Leydig cell ultrastructure by CP.
Conclusion:
SIP, as an antioxidant, prevents the cytoskeleton damage through up-regulation antioxidant capacity and inhibition autophagy caused by CP.
Keywords: Autophagy, Cyclophosphamide, Leydig cells, Oxidative stress, Squid ink polysaccharide
Introduction
Cyclophosphamide (CP) is one of the primary chemotherapy drugs for treatment of cancer. However, reports found that CP had toxic side effects with strong alkylating activity to induce DNA double-strand breaks and germ cell apoptosis (1-3). It was also found that the testicular toxicity of CP was primarily connected with its mechanism of oxidative stress injury (4, 5). Excessive accumulation of oxygen free radical would induce apoptosis and autophagy, and eventually programmed cell death (6, 7). In recent years, there were a lot of studies on cell death; autophagy and apoptosis were shown to be two important ways of programmed cell death. Autophagy became the most popular field in life sciences after apoptosis. Autophagy possessed protective effects in some pathophysiology situations; it helps to resist stress, maintain cellular energy supplement, and homeostasis(8-10). However, the concrete mechanism of autophagy remained unclear and still remains pending further investigation. It was generally accepted that autophagy was closely related to cellular damages induced by exogenous factors such as reactive oxygen species, radiation, and chemotherapy (11-15).
Squid ink polysaccharides (SIP), a natural marine product, possesses antioxidant ability and eliminating free radical activity in vivo and in vitro (16, 17). It is a type of glycosaminoglycan with a unique structure –[3GlcAβ1-4(GalNAc α1-3)-Fucα1]n– (18). In our previous research (4, 5, 19), SIP has been proven to have antioxidant capabilities and chemotherapy-protective activities on model animals. These studies showed CP-induced, serious negative changes on testicular antioxidant ability, sexual hormone contents, activities of marker enzymes, functions of mitochondria, apoptosis in spermatogonial cells, and Sertoli cells, which were all significantly impaired by SIP. Results also suggest that SIP reduces CP-induced testicular damage via Nrf2/ARE activation pathway and inhibition of p38 MAPK signal pathway. To learn more details about the protection of testicular germ cells and the regulatory mechanisms from SIP, in this paper, effects of SIP as a cytoprotective factor on CP-induced autophagy and possible mechanism were explored, which would help to protect and treat the male reproductive system during chemotherapy.
Materials and Methods
Preparation of SIP
Fresh squid (Sepia esculenta) were caught from the east coast of Beibu Gulf and the ink sacs were removed by the fishermen, the ink was then stored at –70°C in an ultra-low temperature freezer until use. SIP was prepared with a slightly modified method as described by Chen et al (18). Briefly, the frozen squid ink thawed at 4 °C was diluted with an equal volume of PBS (0.01 mol/l, pH 7.4) and then treated by sonication in an ice bath. After storage at 4 °C for more than 8 hr, the mixture was centrifuged (8000 rpm) at 4 °C for 50 min, and the supernatant was collected and hydrolyzed with papain (1.5 ‰) at 50 °C for 90 min, and it was then heated in boiling water to denature the protease. The proteins in the treated supernatant were removed by the Sevag method (20). The aqueous phase was mixed with four volumes of ethanol to precipitate the polysaccharides. Crude polysaccharides were obtained from the precipitate and then separated into three fractions by DEAE-52 cellulose column chromatography. The first fraction (the peak area was far larger than the others) was collected, dialyzed, concentrated, and further purified in a Sephacryl S-300HR column. One elution peak was obtained from S-300HR column and the fraction from that peak was named SIP. Then, the collected SIP was dialyzed, concentrated, freeze-dried, and stored at -20 °C.
Experimental animals
Sexually mature male Kunming mice aged 6 weeks were purchased from the Experimental Animal Centre of Guangxi Medical University (certificate of conformity: SCXK (Gui) 2009-002). They were adaptively domesticated for one week under the following constant experimental conditions: a relative humidity of 55 ± 5%, a temperature of 22±2 °C, a quasi-diurnal cycle of 12 hr light/12 hr darkness, under a free-feeding and drinking regime.
Determination of optimal dose of SIP
Animals in the experiment and grouping
Fifty mice were randomly divided into five groups: control group (CON, normal saline), CP-treated group (CP, 120 mg/kg CP), low dose SIP-treated group (L-SIP, 40 mg/kg SIP+120 mg/kg CP), medium dose SIP-treated group (M-SIP, 60 mg/kg SIP+120 mg/kg CP), and high-dose SIP-treated group (H-SIP, 80 mg/kg SIP+120 mg/kg CP). The control group was administered orally and injected abdominally with normal saline, The CP-treated group was administered orally with normal saline and injected abdominally with CP, all SIP-treated groups were administered orally with SIP and injected abdominally with CP. The SIP was administered once a day for two weeks with 40 mg/kg (low dose), 60 mg/kg (medium dose) and 80 mg/kg (high dose) body weight. The CP was injected intraperitoneally only once at the 7th day with 120 mg/kg body weight in accordance with previous research (19). After treatment, all animals were weighed and sacrificed at 24 hr after the last administration, and then bilateral testes and epididymis were collected and stored.
Reproductive organ exponent
The bilateral testes and epididymides were rapidly removed, the surrounding adipose tissue, was weighed and stored for later use. To calculate the testis and epididymis indices by weighting the immune organs and body of mice in the same day, the formula for calculation was as follows: the testis or epididymis indices=the weight of testis or epididymis/the weight of body ×100%.
Sperm analysis
The epididymis was washed twice with PBS, immediately incubated in preheated normal saline, cut it in thirds lengthwise, and the epididymis gently shaken to fully free sperm at 37 °C for 5 min. 10 μl sperm suspension was smeared on a slide and colored with 2% eosin. Abnormal sperm were distinguished in a 200 sperm sample and were used to calculate their abnormality ratio.
Histopathological observation
The testis tissues were fixed with 10% formaldehyde solution, then dehydrated with ethanol of gradient concentration, and cleared in xylene and finally embedded in paraffin. The tissue blocks were sectioned at 5 um thickness, and after HE staining testicular structure was observed under a light microscope.
Detection of testicular oxidative stress level
The samples were homogenized quickly in ice-cold PBS at low temperature. The homogenate was centrifuged at 2000 g for 10 min. The supernatant was then determined by measuring the activity of superoxide dismutase (SOD) and catalase (CAT), and contents of malondialdehyde (MDA) were determined with detection kits according to manufacturer’s protocols. SOD, CAT, and MDA Kit were purchased from Nanjing Jiancheng Bioengineering Institute (Assay Kit, China).
SIP prevents autophagy affected by CP in Leydig cells
Animals in the experiment and grouping
Forty mice were randomly divided into five groups: control group (CON, normal saline), CP-treated group (CP), SIP-treated group (SIP), and co-treated group (SIP+CP). The control group was administered orally and injected abdominally with normal saline, the CP-treated group was administered orally with normal saline and injected abdominally with CP, the SIP-treated group was administered orally with SIP, and the co-treated group was administered orally with SIP and injected abdominally with CP. The SIP was administered once a day for two weeks with the optimal dose based on the frontal research. The CP was injected intraperitoneally only once on the 7th day with 120 mg/kg body weight. After treatment, all animals were sacrificed at 24 hr after the last administration, and then bilateral testes were collected; the surrounding adipose tissue rapidly removed and stored.
Immunofluorescence
Left side testes were immediately fixed in 10% paraformaldehyde and dehydrated with ethanol of gradient concentration, then cleared in xylene, finally embedded in paraffin. The tissue blocks were sectioned at 5 um thickness. De-waxed and hydrated paraffin section testis were immersed in methanol solution at room temperature for 10 min after washing twice with PBS: they were blocked with 1% BSA at room temperature for 10 min. The target protein in each section was probed with a monoclonal antibody that would be captured by the fluorescence-labeled secondary antibody at 37 °C for 30 min. To each section, a drop of buffered glycerol was added, before they were covered with a cover-slip and observed under a laser scanning confocal microscope.
Transmission electron microscopy detection
The testis was fixed with 5% glutaraldehyde in PBS followed by post-fixation with 1% osmic acid formulated with PBS. After washing three times with PBS, the tissue was dehydrated with ethanol and acetone, embedded in Epon 812 and sectioned with an ultramicrotome. These sections were stained with 2% uranyl acetate and lead citrate, and they were then examined under JEM-1400 transmission electron microscope (JEOL LTD, Tokyo, Japan) at 80 kV.
Western blotting analysis
The supernatant of testis homogenate added to the protein sample buffer was denatured in boiling water for 5 min. After SDS-PAGE, the protein was electrotransferred to a nitrocellulose membrane and then probed with a monoclonal antibody that would be captured by the secondary antibody conjugated with horseradish peroxidase. The membrane was visualized with SuperSignal West Pico chemiluminescence detection system: β-actin was used as the internal reference.
Statistical analysis
All experimental data were presented as the mean±standard error of the mean: data were analyzed using the JMP 7.0.2 program (SAS Institute Inc., SAS Campus Drive, Cary, NC, USA 27513). One-way analysis of variance (ANOVA) and the post hoc Tukey HSD test were used to evaluate differences between groups: *P<0.05, #P<0.05 were considered significant.
Results
Optimal dose of SIP for preventing oxidative stress on injection CP
In Figure 1-A, The rate of sperm deformity in the CP group was remarkably higher than those of all the other groups (P<0.05). But in Figure 1-B and Figure 1-C, the indices of testis and epididymis in each group were not different (P>0.05). The activity of SOD, CAT, and content of MDA in testis were shown in Figure 1-D, E, F. There were significant increases of MDA content, but reduction of SOD and CAT activity in the CP group compared with other groups (P<0.05). After SIP treatment, SOD activity of all SIP-treated mice was obviously higher than that of the CP group (P<0.05); CAT activity and MDA content were also greatly improved at the SIP dose of more than 60 mg/kg and 80 mg/kg (P<0.05). In Figure 2, most of the spermatogenic cells were necrotic and seminiferous tubules had a hollow cross in the CP group compared with the CON group. However, the testicular histological structure in M-SIP and H-SIP groups was visibly better than that in the CP group. To sum up, the optimal dose of SIP to prevent, abnormal sperm rate, oxidative stress, and histological structure affected by CP was 80 mg/kg.
Figure 1.
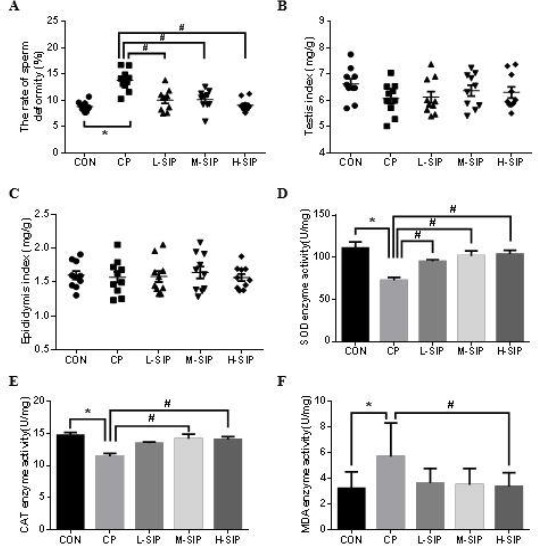
Effects of SIP on CP-mediated rate of sperm deformity (A), testis index (B), epididymis index (C), SOD enzyme activity(D), CAT enzyme activity(E), and MDA content(F) of mice. CON: control mice, CP: CP-treated mice, L-SIP, M-SIP, and H-SIP: mice treated with CP, low dose SIP, medium dose SIP, and high dose SIP. SIP was administered once a day for two weeks 40 mg/kg (low dose), 60 mg/kg (medium dose), and 80 mg/kg (high dose) body weight. The CP was injected intraperitoneally only once on the 7th day with 120 mg/kg body weight, correspondingly, control mice were orally/injected with saline. Data are presented as the mean± standard error of the mean. *P<0.05, vs. the control group; #P<0.05, vs. the CP-treated group
Figure 2.
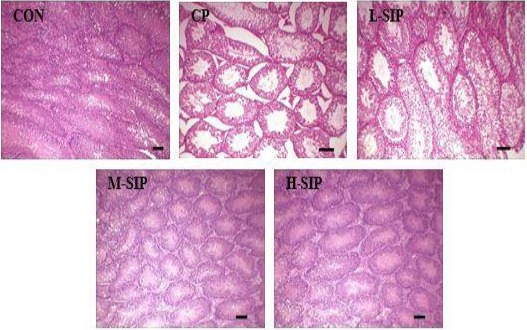
Histopathological observation of testicular seminiferous tubules. Photographs were taken at 200× magnification. CON: control mice, CP: CP-treated mice, L-SIP, M-SIP, and H-SIP: mice treated with CP, low dose SIP, medium dose SIP, and the high dose SIP. SIP was administered once a day for two weeks 40 mg/kg (low dose), 60 mg/kg (medium dose), and 80 mg/kg (high dose) body weight. CP was injected intraperitoneally only once on the 7th day with 120 mg/kg body weight, correspondingly, control mice were given orally/injected with saline. Scale bar 100 µm
Effect of SIP on the expression of LC3 and Beclin-1 against CP
As shown in Figures 3-A and 4-A, there were a few strong fluorescent particles present in the cell layers of all groups except the CP-treated group. In the testes of CP-treated mice, LC3-positive cells and Beclin-1-positive cells were observed in greater number than in the control group, and they were mainly distributed in the Leydig cell layer. However, LC3 and Beclin-1 fluorescent granules were much less in spermatogenic cells and the Leydig cell layer after treatment via SIP in the co-treated group. Moreover, no such fluorescence was found in the SIP group, which had a similar result as the control group.
Figure 3.
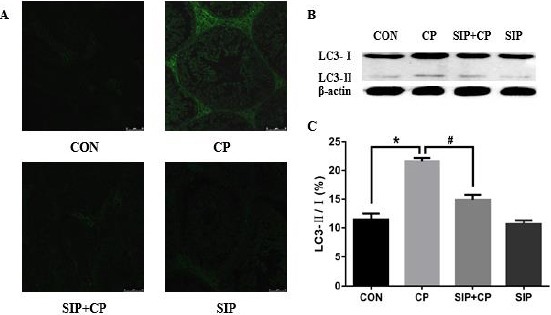
Effects of SIP on CP-mediated expression and activation of the LC3 gene. CON: control mice, CP: CP-treated mice, SIP+CP: mice treated with SIP and CP, and SIP: SIP-treated mice. Mice were administered orally 80 mg/kg SIP once a day for two weeks (SIP+CP and SIP); After 2 hr on the 7th day of SIP-treatment, mice were injected with 120 mg/kg cyclophosphamide (CP and SIP+CP); correspondingly, control mice were given orally/injected with saline. Data are presented as the mean±standard error of the mean. *P<0.05, vs. the control group; #P<0.05, vs. the CP-treated group. Scale bar 50 µm
Figure 4.
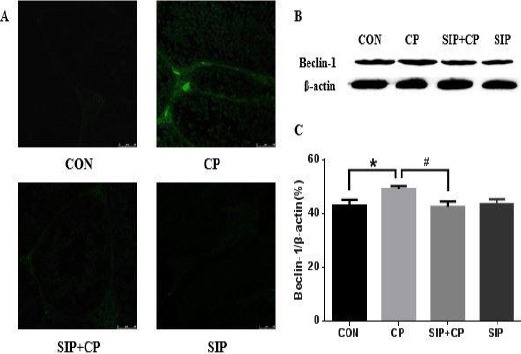
Effects of SIP on CP-mediated expression of Beclin-1 gene. CON: control mice, CP: CP-treated mice, SIP+CP: mice treated with SIP and CP, and SIP: SIP-treated mice. Mice were administered orally 80 mg/kg SIP once a day for two weeks (SIP+CP and SIP); After 2 hr on the 7th day of SIP-treatment, mice were injected 120 mg/kg cyclophosphamide (CP and SIP+CP); correspondingly, control mice were given orally/injected with Saline. Data are presented as mean ± standard error of the mean. *P<0.05, vs. the control group; #P<0.05, vs. the CP-treated group. Scale bar 50 µm
SIP prevents the expression of LC3 and Beclin-1 induced by CP
In Figures 3-B and 3-C and Figures 4-B and 4-C, CP up-regulated the expression of Beclin-1 and improved LC3-I into LC3-II, and there were obvious differences compared with the other groups (P<0.05). After administration of SIP, testes of CP-exposed mice showed a significant reduction of the Beclin-1 protein and decreased conversion rate of LC3-II/I; the contents of Beclin-1 protein and the LC3-II/I ratio in the co-treated group were much less than in the CP group (P<0.05).
SIP weakened the destruction of ultra-structure of testicular Leydig cells caused by CP
As shown in Figure 5, in control mice, Leydig cells were healthy, there were large nuclei, homogeneous electron density in the nucleoplasm, prominent nucleoli, and spherical mitochondria with dense cristae in the cytoplasm. However, after treatment with CP alone, Leydig cells appeared to have many autophagic vacuoles and chromatin pyknosis, and mitochondria were swollen with lost cristae. Under administration of SIP and CP, cells returned to normality, nucleoli were clear and evident, and clear cristae were observed in many mitochondria. In Leydig cells of SIP-treated mice, nucleoli were clear and obvious, the karyoplasm was light in color and of uniform density, and the cytoplasm had many mitochondria with rich cristae.
Figure 5.
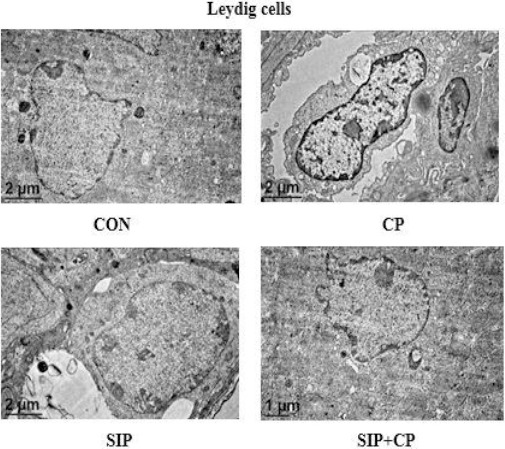
Ultrastructure of testicular spermatogonia, Sertoli cells, and Leydig cells in the 4 mouse groups by TEM. CON: control mice, CP: CP-treated mice, SIP+CP: mice treated with SIP and CP, and SIP: SIP-treated mice. Mice were administered orally 80 mg/kg SIP once a day for two weeks (SIP+CP and SIP); after 2 hr on the 7th day of SIP-treatment, mice were injected with 120 mg/kg cyclophosphamide (CP and SIP+CP); correspondingly, control mice were given orally/injected with saline. Photographs were taken at 15000× magnification
Discussion
The main physiological functions of testes are spermatogenesis and endocrine regulation, they are both interlinked, but generally the former is limited by the latter. Physiologically, Leydig cells are the main secretor of testosterone, which is an indispensable steroid hormone for both spermatogenesis and sex characteristics in males (21, 22). Low-level autophagy is crucial for maintaining sperm production and intracellular homeostasis of testis under normal conditions, but such dynamic balance is often broken during the course of chemotherapy. Testicular cells will produce excessive free radicals and oxidation products with a chemical therapeutic drug like cyclophosphamide, then cause testicular oxidative stress (23). This leads to inhibition of synthesis of the protective enzymes, active oxygen accumulates and damages proteins, membrane lipids, DNA, and other cellular components, causes DNA mutation, lipid peroxidation, and mitochondrial membrane channel opening, resulting in injury of the male reproductive system (24, 25).
This research demonstrated that CP-induced excessive MDA, decreased SOD and CAT activity in testes of mice, all-round declined sperm quality and number, and severely damaged Leydig cells; plentiful phagocytic vacuoles and swelling mitochondria were all observed in their cytoplasm. Mitochondria are highly sensitive to stressful stimulation, damaged mitochondrial inner membrane by the collapse of mitochondrial △Ψ m and the generation of ROS oxidative stress could induce autophagy by the mitochondrial pathway (26, 27). Meanwhile, LC-3 and Beclin-1, which are expressional levels of two key autophagy-related proteins in mammalian cells, involved in the formation of autophagosomes, and reflect the degree of autophagy (28, 29), were up-regulated and were mainly found. Therefore, CP can result in autophagy of Leydig cells and oxidative stress may be the mechanism.
SIP can effectively weaken the testicular toxicity induced by CP in vivo and protect the testicular reproductive function of the male mice. Improvement of cellular antioxidant ability, prevents abnormality of sperm, reduces expressional levels of autophagy-related LC-3 and Beclin-1 genes, inhibits autophagy of Leydig cells, and protects the cellular structure against oxidative damage. So, this paper attempts to analyze the following three aspects of testicular protection of SIP against toxic resistance of CP: Firstly, the most direct performance was weakening the histopathological damage in testis, then testicular atrophy was prevented, the number of spermatogenic cells was increased, and abnormal sperm rate was reduced. Secondly, it enhanced the antioxidant ability of the testis tissue. SOD and CAT are important antioxidant enzymes; the contents of MDA in the body directly reflect the degree of membrane lipid peroxidation. SIP could inhibit the oxidative damage of testes and effectively eliminate excessive free radicals by improving SOD and CAT activity and reduce the MDA contents in the testes. Finally, it inhibits autophagy of Leydig cells, which are the most important cells that synthesize and secrete male hormones such as testosterone. The protective effect of SIP on Leydig cells could ensure that the body holds on to its normal levels of male hormones, so as to keep the normal regulatory function of hormones on gonads and help the stability of the male reproductive system indirectly. Results suggest that antioxidation effect of SIP may underlie these protective benefits in mice. However, the mechanism of autophagy in the Leydig cells is not yet known well; its idiographic mechanism of action and signal conduction pathway is to be addressed.
Conclusion
This paper studies the preventive effects of SIP on oxidative lesion and autophagy induced by CP in mice testes. We have demonstrated that Leydig cells present the phenomenon of autophagy in CP-mediated testicular damage and SIP; as an antioxidant, they can inhibit testis injury and autophagy of Leydig cells caused by CP. Consequently, SIP protects testicular cells from oxidative damage and prevents the cytoskeleton impairment through up-regulation of antioxidant capacity and inhibition autophagy.
Acknowledgment
This work was supported by the National Natural Science Foundation of China (31171667), Special Fund for Distinguished Experts in Guangxi and Guangxi talent highland of preservation and deep processing research in fruit and vegetables. The results presented in this paper were part of a student thesis.
References
- 1.Mythili Y, Sudharsan PT, Selvakumar E, Varalakshmi P. Protective effect of DL-α-lipoic acid on cyclophosphamide induced oxidative cardiac injury. Chem-Biol Interact. 2004;151:13–19. doi: 10.1016/j.cbi.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 2.Zhang J, Ma K, Wang H. Cyclophosphamide suppresses thioredoxin reductase in bladder tissue and its adaptive response via inductions of thioredoxin reductase and gluththione peroxidase. Chem-Biol Interact. 2006;162:24–30. doi: 10.1016/j.cbi.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 3.Aguilar-Mahecha A, Hales BF, Robaire B. Effects of acute and chronic cyclophosphamide treatment on meiotic progression and the induction of DNA double-strand breaks in rat spermatocytes. Biol Reprod. 2005;72:1297–1304. doi: 10.1095/biolreprod.104.038620. [DOI] [PubMed] [Google Scholar]
- 4.Le XY, Luo P, Gu YP, Tao YX, Liu HZ. Interventional effects of squid ink polysaccharides on cyclophosphamide-associated testicular damage in mice. Bratisl Med J. 2015;116:334–339. doi: 10.4149/bll_2015_063. [DOI] [PubMed] [Google Scholar]
- 5.Le XY, Luo P, Gu Y P, Tao YX, Liu HZ. Squid ink polysaccha-rides reduces cyclophosphamide-induced testicular damage via Nrf2/ARE activation pathway in mice. Iran J Basic Med Sci. 2015;18:827–831. [PMC free article] [PubMed] [Google Scholar]
- 6.Tan C, Lai S, Wu S, Hu S, Zhou L, Chen Y, et al. Nuclear permeable ruthenium (II) β-carboline complexes induce autophagy to antagonize mitochondrial-mediated apoptosis. J Med Chem. 2010;53:7613–7624. doi: 10.1021/jm1009296. [DOI] [PubMed] [Google Scholar]
- 7.Faleiro L, Lazebnik Y. Caspases disrupt the nuclear-cytoplasmic barrier. J Cell Biol. 2000;151:951–960. doi: 10.1083/jcb.151.5.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klionsky DJ, Abdalla FC, Abeliovich H, Abraham RT, Acevedo-Arozena A, Adeli K, et al. Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy. 2012;8:445–544. doi: 10.4161/auto.19496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451:1069–1075. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carchman EH, Rao J, Loughran PA, Rosengart MR, Zuckerbraun BS. Heme oxygenase-1-mediated autophagy protects against hepatocyte cell death and hepatic injury from infection/sepsis in mice. Hepatology. 2011;53:2053–2062. doi: 10.1002/hep.24324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Serrone L, Hersey P. The chemoresistance of human malignant melanoma:an update. Melanoma Res. 1999;9:51–58. doi: 10.1097/00008390-199902000-00007. [DOI] [PubMed] [Google Scholar]
- 12.Kourtis N, Tavernarakis N. Autophagy and cell death in model organisms. Cell Death Differ. 2009;16:21–30. doi: 10.1038/cdd.2008.120. [DOI] [PubMed] [Google Scholar]
- 13.Ito H, Daido S, Kanzawa T, Kondo S, Kondo Y. Radiation-induced autophagy is associated with LC3 and its inhibition sensitizes malignant glioma cells. Int J Oncol. 2005;26:1401–1410. [PubMed] [Google Scholar]
- 14.Tang D, Kang R, Cheh CW, Livesey KM, Liang X, Schapiro N E, et al. HMGB1 release and redox regulates autophagy and apoptosis in cancer cells. Oncogene. 2010;29:5299–5310. doi: 10.1038/onc.2010.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dalby K, Tekedereli I, Lopez-Berestein G, Ozpolat B. Targeting the pro-death and pro-survival functions of autophagy as novel therapeutic strategies in cancer. Autophagy. 2010;6:322–329. doi: 10.4161/auto.6.3.11625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luo P, Liu H. Antioxidant ability of squid ink polysaccharides as well as their protective effects on deoxyribonucleic acid DNA damage in vitro . Afr J Pharm Pharmacol. 2013;7:1382–1388. [Google Scholar]
- 17.Zhong JP, Guang W, Shang JH, Pan JQ, Li K, Huang Y, et al. Protective effects of squid ink extract towards hemopoietic injuries induced by cyclophosphamide. Mar Drugs. 2009;7:9–18. doi: 10.3390/md7010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen S, Xu J, Xue C, Dong P, Sheng W, Yu G, et al. Sequence determination of a non-sulfated glycos- aminoglycan-like polysaccharide from melanin-free ink of the squid Ommastrephes bartrami by negative-ion electrospray tandemmass spectrometry and NMR spectroscopy. Glycoconjugate J. 2008;25:481–492. doi: 10.1007/s10719-007-9096-2. [DOI] [PubMed] [Google Scholar]
- 19.Liu HZ, Tao YX, Luo P, Deng CM, Gu YP, Yang L, et al. Preventive effects of a novel polysaccharide from Sepia esculenta ink on ovarian failure and its action mechanisms in cyclophosphamide-treated mice. J Agr Food Chem. 2016;64:5759–5766. doi: 10.1021/acs.jafc.6b01854. [DOI] [PubMed] [Google Scholar]
- 20.Staub AM. Removal of protein-Sevag method. Methods Carbohyd Chem. 1965;5:5–6. [Google Scholar]
- 21.Holdcraft RW, Braun RE. Hormonal regulation of spermatogenesis. Int J Androl. 2004;6:335–342. doi: 10.1111/j.1365-2605.2004.00502.x. [DOI] [PubMed] [Google Scholar]
- 22.Handelsman DJ. Hormonal regulation of spermato- genesis:insights from constructing genetic models. Reprod Fert Develop. 2011;4:507–19. doi: 10.1071/RD10308. [DOI] [PubMed] [Google Scholar]
- 23.Iuchi Y, Kaneko T, Matsuki S, Ishii T, Ikeda Y, Uchida K, et al. Carbonyl stress and detoxification ability in the male genital tract and testis of rats. Histochem Cell Biol. 2004;2:123–130. doi: 10.1007/s00418-003-0607-3. [DOI] [PubMed] [Google Scholar]
- 24.Selvakumar E, Prahalathan C, Sudharsan PT, Varalakshmi P. Protective effect of lipoic acid on cyclophosphamide-induced testicular toxicity. Clin Chim Acta. 2006;1(2):114–119. doi: 10.1016/j.cca.2005.11.034. [DOI] [PubMed] [Google Scholar]
- 25.Motawi TMK, Sadik NAH, Refaat A. Cytoprotective effects of DL-alpha-lipoic acid or squalene on cyclophos- phamide-induced oxidative injury:An experimental study on rat myocardium, testicles and urinary bladder. Food Chem Toxicol. 2010;48:2326–2336. doi: 10.1016/j.fct.2010.05.067. [DOI] [PubMed] [Google Scholar]
- 26.Tinari A, Garofalo T, Sorice M, Esposti M D, Malorni W. Mitoptosis:Different Pathways for Mitochondrial Execution. Autophagy. 2007;3:282–284. doi: 10.4161/auto.3924. [DOI] [PubMed] [Google Scholar]
- 27.Lemasters JJ, Nieminen AL, Qian T, Trost LC, Elmore SP, Nishimura Y, et al. The mitochondrial permeability transition in cell death:A common mechanism in necrosis, apoptosis and autophagy. BBA-Bioenergetics. 1998;1366:177–196. doi: 10.1016/s0005-2728(98)00112-1. [DOI] [PubMed] [Google Scholar]
- 28.Kihara A, Kabeya Y, Ohsumi Y, Yoshimori T. Beclin-phosphatidylinositol 3-kinase complex functions at the trans -Golgi network. EMBO Rep. 2001;2:330–335. doi: 10.1093/embo-reports/kve061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karim MR, Kanazawa T, Daigaku Y, Fujimura S, Miotto G, Kadowaki M. Cytosolic LC3 ratio as a sensitive index of macroautophagy in isolated rat hepatocytes and H4-II-E cells. Autophagy. 2007;3:553–560. doi: 10.4161/auto.4615. [DOI] [PubMed] [Google Scholar]


