Abstract
Objective(s):
In some previous studies, the extract of embryonic carcinoma cells (ECCs) and embryonic stem cells (ESCs) have been used to reprogram somatic cells to more dedifferentiated state. The aim of this study was to investigate the effect of mouse ESCs extract on the expression of some pluripotency markers in human adipose tissue-derived stem cells (ADSCs).
Materials and Methods:
Human ADSCs were isolated from subcutaneous abdominal adipose tissue and characterized by flow cytometric analysis for the expression of some mesenchymal stem cell markers and adipogenic and osteogenic differentiation. Frequent freeze-thaw technique was used to prepare cytoplasmic extract of ESCs. Plasma membranes of the ADSCs were reversibly permeabilized by streptolysin-O (SLO). Then the permeabilized ADSCs were incubated with the ESC extract and cultured in resealing medium. After reprogramming, the expression of some pluripotency genes was evaluated by RT-PCR and quantitative real-time PCR (qPCR) analyses.
Results:
Third-passaged ADSCs showed a fibroblast-like morphology and expressed mesenchymal stem cell markers. They also showed adipogenic and osteogenic differentiation potential. QPCR analysis revealed a significant upregulation in the expression of some pluripotency genes including OCT4, SOX2, NANOG, REX1 and ESG1 in the reprogrammed ADSCs compared to the control group.
Conclusion:
These findings showed that mouse ESC extract can be used to induce reprogramming of human ADSCs. In fact, this method is applicable for reprogramming of human adult stem cells to a more pluripotent sate and may have a potential in regenerative medicine.
Keywords: Adipose tissue-derived-stem cells, Dedifferentiation, Embryonic stem cells- extract, Reprogramming
Introduction
The fate of differentiated somatic cells may be altered by either nuclear or cytoplasmic extracts from other cell types. Through this process, the recipient cells acquire the phenotypic and functional characteristics of donor cells (1-3). For transport of substances from the extract into the cytoplasm and subsequent nuclear reprogramming, cells usually require to be reversibly permeabilized, exposed to reprogramming extract and resealed (3, 4). For some cell types, such as adipose tissue-derived stem cells (ADSCs) which can take the components of extract by endocytosis easily, permeabilization is not necessary but is preferred (5). Using this technique, cell-free extract of pluripotent cell, such as embryonic carcinoma cells (ECCs) and embryonic stem cells (ESCs), have been used to reprogram somatic cells to a more dedifferentiated state (6-8). In one of these studies, extract of xenopus eggs induced the expression of pluripotency genes in porcine fibroblasts (6). Moreover, extract of undifferentiated mouse ESCs resulted in partial reprogramming of NIH3T3 cells nuclei (7). Exposure to the extract of ESCs also reprogrammed primary rabbit corneal epithelial cells and induced the expression of ESC-specific genes, OCT4 and SSEA1. The reprogrammed cells showed ESC-like morphology and growth characteristics (8).
ADSCs have several important features that make them valuable for dedifferentiation and redifferentiation studies. ADSCs can be isolated easily from subcutaneous fat tissue. These cells can be expanded in vitro for several passages, with a stable doubling time and without significant levels of senescence (9). Multipotential differentiation capability of the ADSCs has been reported previously (6, 9-14). In addition, several studies have demonstrated a low-level expression of key pluripotency genes, including OCT4, SOX2, NANOG and REX1, in the freshly isolated and early cultured ADSCs (15-18), which may be in favor of dedifferentiation. In the current study, we reprogrammed human ADSCs by the extract of mouse ESCs and showed significant alterations in the expression of some pluripotency genes.
Materials and Methods
Culture of undifferentiated mouse ESCs
Mouse ESC line Royan B1 (Royan Stem Cell Bank, Royan Institute, Tehran, Iran) was cultured on top of mitomycin C-inactivated mouse embryonic fibroblast (MEF) feeder layer in a medium containing Dulbecco’s Modified Eagles Medium (DMEM, Gibco, Thermo Fisher Scientific, Waltham, USA), 15% Fetal bovine serum (FBS, Gibco), 2 mM L-glutamine (Gibco), 0.1 mM nonessential amino acids (Sigma-Aldrich Chemie GmbH, Germany), 0.1 mM 2-β-mercaptoethanol (2β-ME, Sigma) and 1000 IU/ml leukemia inhibitory factor (ESGRO® LIF, Merck Millipore). The culture medium was renewed every day.
Isolation of human ADSCs
Adipose tissue was obtained from female patients who underwent elective abdominoplasty or lipoaspiration in Shariati or Erphan Hospitals, Tehran, Iran. The study was approved by the Local Institutional Ethics Committee (7-8-93/NIGEB), and all donors gave their informed consent. These women were all 40 to 50 years old without any type of dietary restriction or disease background before surgery.
The ADSCs were isolated and characterized as described previously (19). In brief, adipose tissue was washed by phosphate-buffered saline (PBS, Gibco) solution containing antibiotics. Then, the tissue was minced by a sterile surgical scalpel and digested by 2 mg/ml collagenase I (Thermo Fisher Scientific) in PBS containing 2% bovine serum albumin (BSA, Sigma). The achieved cell suspension was centrifuged and stromal vascular fraction (SVF) was resuspended and cultured in a growth medium containing DMEM, 20% FBS and 1% penicillin and streptomycin solution (all from Gibco). The cells were passaged after reaching 80-90% confluency. The culture medium was renewed every two days.
Characterization of human ADSCs
For flow cytometric analysis, third-passaged ADSCs were detached using trypsin-EDTA solution (Gibco) and were fixed in 70% ice-cold ethanol. After washing with cold PBS, the cells were incubated with primary antibodies against CD90, CD105, CD73 and CD45 proteins (all from Abcam, Cambridge, UK) for 30 min. Then, the cells were stained with fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse IgG (Sigma-Aldrich) or phycoerythrin (PE)-conjugated donkey anti-rabbit IgG (Abcam) for 30 min. The cells only stained with the secondary antibodies were considered as the negative control. Flow cytometry was performed by a BD FACSCalibur™ (BD Biosciences, San Jose, USA). Analysis of the data was performed by FlowJo vX.0.6 (Tree Star Inc., Ashland, USA).
For adipogenic differentiation, third-passaged ADSCs were cultured in a medium containing DMEM, 10% FBS, 1 μM dexamethasone, 100 μM indomethacin, 5 μg/ml insulin and 500 μM isobutylmethylxanthine (BIO-IDEA, BI1007). Three weeks after induction, lipid accumulation in the cytoplasm was detected by Oil Red O staining. For osteogenic differentiation, third-passaged ADSCs were cultured in a medium containing DMEM, 10% FBS, 10-8 M dexamethasone, 10-3 M glycerol phosphate, 3.7 g/l sodium bicarbonate and 0.05 g/l ascorbic acid (BIO-IDEA, BI1008). Three weeks after initiation of differentiation, calcium deposits were assessed by Alizarin Red S staining.
Cell extract preparation
To prepare ESC extracts, Royan B1 cells were isolated from the MEF feeder layer, washed with calcium and magnesium-free PBS and centrifuged at 100 g for 5 min. the cell pellet was resuspended in ice-cold PBS containing 1 mM dithiothreitol (DTT), 0.1 mM phenylmethylsulfonyl fluoride (PMSF, Sigma) and 0.1 mM protease inhibitor cocktail (Sigma) and incubated for 1 hr on ice. Cells were lysed with repeated cycles of freezing in liquid nitrogen and thawing in water bath. Then the lysate was centrifuged at 18,000 g for 15 min at 4 °C, and the supernatant was collected and frozen in liquid nitrogen. Before reprogramming of the ADSCs, the concentration of proteins in the ESC extract was adjusted to 2 mg/ml.
Permeabilization of the ADSCs
Third-passaged ADSCs were washed in cold PBS three times. 5×105 cells were suspended in 500 µl cold PBS and were placed at 37 °C for 2 min. Then, Streptolysin O (SLO, Sigma) was added with final concentrations of 500, 1000 and 2000 ng/ml, and the ADSCs were incubated in a water bath for 50 min at 37 °C with occasional agitation. Then, the cell suspension was placed on ice, diluted by adding 500 µl cold PBS and centrifuged at 100 g for 5 min at 4 °C.
To determine the appropriate concentration of SLO for permeabilization of mouse ADSCs, the permeabilized cells were resuspended in growth medium and cultured for 15 min. Then the cells were stained with trypan blue and visualized under the inverted microscope. For each concentration of SLO, the percentage of blue-stained cells was calculated by counting 100 cells from four independent replicates.
Viability of the permeabilized cells was also determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. For this purpose, the permeabilized ADSCs were seeded in a 96-well tissue culture plate with 5×10³ cells/well and cultured in resealing medium containing 20% FBS and 2 mM CaCl2. After 24 hr, medium of each well was replaced with 100 µl RPMI 1640 (Gibco), and 10 µl of 12 mM MTT stock solution was added. The cells were incubated for 3 hr at 37 °C. The MTT tetrazolium crystals were then solubilized in 100 µl DMSO, and the absorbance was read at 570 nm using a microplate reader (LabSystems Multiskan MS). All experiments were performed in quadruplicate. The percentage of viability (%) for every SLO treatment group was calculated by dividing the absorbance of that group to absorbance of the control group.
Reprogramming of the ADSCs by ESC extract
After permeabilization, the ADSCs were resuspended in 500 µl of ESC extract containing an ATP-regenerating system (1 mM ATP, 10 mM creatine phosphate, 25 µg/ml creatine kinase, 100 µM GTP), and 1 mM of each nucleotide triphosphate (NTP, all from Sigma). The cell containing-tube was incubated for 1 hr at 37 °C with occasional agitation. Then, the cells were cultured in resealing medium containing DMEM, 20% FBS, 1% of antibiotics and 2 mM CaCl2. Viability of the reprogrammed ADSCs was determined by MTT assay as described in the previous section.
Reverse transcription-PCR and qPCR analyses
Total RNA was isolated by High Pure RNA Isolation Kit (Roche, Germany). Synthesis of cDNA was performed using RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher Scientific), according to the manufacturer’s instructions. Assessment of gene expression was performed using Taq DNA Polymerase 2x Master Mix Red (Ampliqon A/S, Denmark) and PCR products were size fractioned on a 2% agarose gel.
Quantification of gene expression was performed using specific primers for the target genes (Table 1) and RealQ PCR Master (Ampliqon A/S, Denmark) on a Rotor-Gene™ 6000 real time analyzer (Corbett Research, Australia). Assessment of gene expression was performed by relative comparative method using REST 2009 software (Qiagen, Germany) based on Pair Wise Reallocation Randomization Test (20). At least, three biologic replicates of each group were included in the qPCR experiments, and β2 microglobulin (B2M) and GAPDH (Table 1) were used for normalization of the quantitative data.
Table 1.
Primers used for RT-PCR and quantitative real-time PCR
| Target | Forward | Reverse | Size (bp) | Accession |
|---|---|---|---|---|
| ACTB | 5’-CCTGGGCATGGAGTCCTGT-3’ | 5’-ATCTCCTTCTGCATCCTGTCG-3’ | 153 | NM_001101 |
| GAPDH | 5’-CAAATGAGCCCCAGCCTTCT-3’ | 5’-TCACCATCTTCCAGGAGCGA-3’ | 116 | NM_002046 |
| Β2M | TCCAGCGTACTCCAAAGATTCA | GTCAACTTCAATGTCGGATGGAT | 113 | NM_004048 |
| OCT4A | 5’-GATGTGGTCCGAGTGTGGTT-3’ | 5’-AAGGGACCGAGGAGTACAGT-3’ | 202 | NM_002701 |
| NANOG | 5’-GAAGCATCCGACTGTAAAGAATC-3’ | 5’-TGGTGGAAGAATCAGGGCTGT-3’ | 169 | NM-024865 |
| SOX2 | 5’-AGAACCCCAAGATGCACAACT-3’ | 5’-TCCTTCTTCATGAGCGTCTTG-3’ | 184 | NM-003106 |
| KLF4 | 5’-ATTACCAAGAGCTCATGCCACC-3’ | 5’-GTGTGCCTTGAGATGGGAACT-3’ | 158 | NM-004235 |
| LIN28 | 5’-TTCCATGTGCAGCTTACTCT-3’ | 5’-CAGCAGTTTGCAGGTGGC-3’ | 228 | NM_024674 |
| REX1 | 5’-GAAACCCATTATCCCCAAAGAGT-3’ | 5’-CCTCGTTCAGTGCCTTCTCTA-3’ | 137 | NM_020695 |
| ESG1 | 5’-GCGAGGGATGCTCAAACTTG-3’ | 5’-TCTGGCCACAACCTAATCTCT-3’ | 120 | NM_001025290 |
| UTF1 | 5’-TCGACGAGCAGATCCGGAAG-3’ | 5’-AGCGGGGTGGCGTCTGGTT-3’ | 157 | NM_003577 |
Results
The ADSC culture and characterization
The ADSCs attached to the growth surfaces of tissue culture plates within 3-4 hr. After 2-3 passages, the ADSCs showed a fibroblast-like morphology (Figure 1A). As determined by flow cytometric analysis, 98.4%, 87.3% and 80.5% of the third-passaged ADSCs expressed CD105, CD73, and CD90 proteins, respectively, while CD45 was expressed in only 0.3% of the cells (Figure 1D-G).
Figure 1.
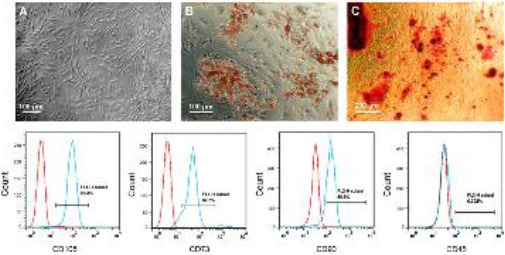
Third-passaged ADSCs showed a fibroblast-like morphology (A). After three-week differentiation of the ADSCs in adipogenic and osteogenic media, lipid accumulation was confirmed using Oil Red O staining (B) and calcium deposits were stained by Alizarin Red (C). The third-passaged ADSCs were characterized by flow cytometry analysis for the expression of mesenchymal markers, CD105 (D), CD73 (E) and CD90 (F), and hematopoietic marker, CD45 (G)
To show multipotential differentiation capability of the ADSCs, the third-passaged cells were induced to differentiate into adipogenic and osteogenic lineages. Within the first week of adipogenic differentiation, some small fat droplets appeared in cytoplasm of the ADSCs. Lipid accumulation in three-week differentiated cells was confirmed by Oil Red O staining (Figure 1B). Also, osteogenic differentiation of three-week differentiated ADSCs was confirmed by Alizarin Red S (Figure 1C).
Reprograming of the ADSCs by ESC extract
After trypan blue staining, SLO at concentrations of 500, 1000 and 2000 ng/ml resulted in about 38%, 76% and 93% blue-stained cells, respectively. Nearly 5% of the control ADSCs were also stained blue (Figure 2A-E). To determine viability of the permeabilized cells, SLO-treated ADSCs were cultured in resealing medium for 24 hr (Figure 2F-I). As determined by MTT assay, viability of the ADSCs after permeabilization with 500, 1000 and 2000 ng/ml SLO was about 86.3%, 72.3% and 38.9%, respectively (Figure 2J). Altogether, SLO at concentration of 2000 ng/ml resulted in the best permeabilization but a decreased attachment and viability of the ADSCs. Therefore, 1000 ng/ml SLO was selected for other experiments.
Figure 2.
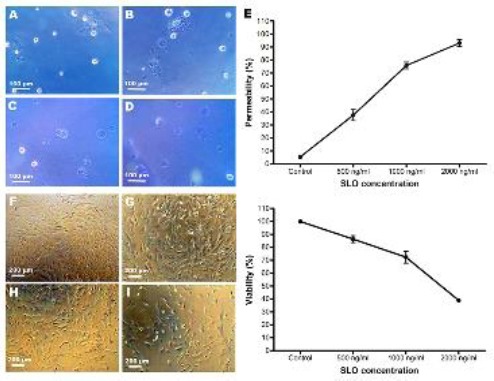
Permeability and viability of the control and permeabilized ADSCs. A to D show trypan blue staining of the control and 500, 1000 and 2000 ng/ml SLO-treated ADSCs, respectively. (E) Permeability of the cells was assessed by trypan blue staining. F to I show phase contrast images of the control and 500, 1000 and 2000 ng/ml SLO-treated ADSCs after 24 hr culture in resealing medium, respectively. (J) Assessment of the cell viability after 24 hr culture in resealing medium by MTT assay
After permeabilization, the ADSCs were reprogrammed by incubation with ESC extract and cultured in resealing medium containing 20% FBS and 2 mM CaCl2. The reprogrammed ADSCs showed a fibroblast-like morphology (Figure 3A), and viability of the reprogrammed ADSCs after 24 hr culture in resealing medium was about 68.08% (Figure 3B).
Figure 3.
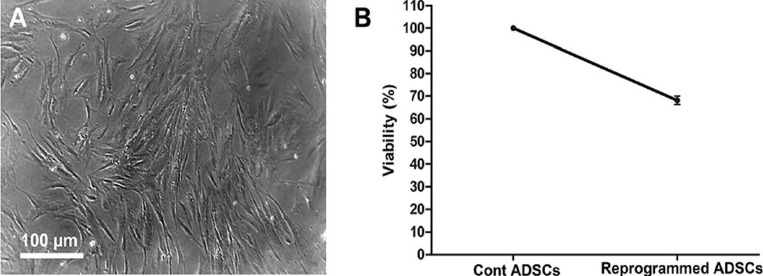
Viability of the reprogrammed ADSCs. (A) Reprogrammed ADSCs showed a fibroblast-like morphology. (B) Assessment of the cell viability after 24 hr culture in resealing medium by MTT assay
Gene expression analysis
RT-PCR analysis showed the expression of OCT4A, SOX2 and NANOG mRNAs in the control, SLO-treated and reprogrammed ADSCs (Figure 4). Based on qPCR analysis, three weeks after reprogramming, the expression levels of OCT4A, SOX2 and NANOG mRNAs were upregulated by 5.65, 2.302, 4.72 folds in the reprogrammed ADSCs compared to the control ADSCs (P<0.05). Also, the expression of REX1 and ESG1 mRNAs in the reprogrammed ADSCs was 1.36 and 1.93 folds higher than the control group (P<0.05). The expression of UTF1 and LIN28 was not significantly different between the reprogrammed and control ADSCs (Figure 5).
Figure 4.
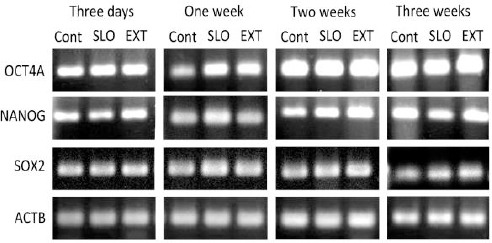
RT-PCR analysis for the expression of OCT4A, SOX2 and NANOG mRNAs. The gene expression was studied in the control, SLO-treated and reprogrammed ADSCs, 3 days and one, two and three weeks after reprogramming
Figure 5.
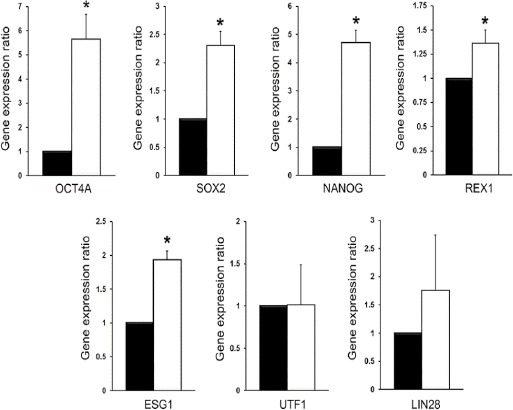
Quantitative real-time PCR analysis of the expression of some pluripotency genes in the control (black bars) and reprogrammed ADSCs (white bars). * P<0.05 (Pair Wise Fixed Reallocation Randomization Test® performed by REST 2009 software)
Discussion
Reprogramming of somatic cells has been induced by several methods including somatic cell nuclear transfer (SCNT) (21, 22), cell fusion (23, 24) and delivery of ESC-specific transcription factors (25, 26). As an alternative to these methods, somatic cells can be reprogrammed using cytoplasmic and nuclear extracts of the ESCs. Previously, several studies have demonstrated the usefulness of ESC-derived cell-free extracts for reprogramming of somatic cells toward a more ESC-like state (7, 8, 27-29). Some researchers believe that the ESC extract contains regulatory components such as ESC-specific transcription factors, chromatin modifying proteins and small RNAs which are necessary for dedifferentiation toward pluripotency (30).
In the current study, we tested the hypothesis that the mouse ESC extract can induce reprogramming of human ADSCs to a more dedifferentiated state. Repeated freezing and thawing technique was used to prepare cytoplasmic extract of mouse ESCs. This technique is easier than other extract preparation methods like sonication and high-speed centrifugation, and requires less instruments (4).
Most cell types need to be reversibly permeabilized to take up components of extract and start nuclear reprogramming (3, 4). SLO is a cholesterol-binding cytolysin frequently used for cell permeabilization which forms 30 nm pores in the plasma membrane (31). Different cell types show varying degrees of sensitivity to SLO and examining a concentration range of 100-1000 ng/ml has been recommended for permeabilization of any given cell type. In this study, SLO at the concentrations of 500, 1000 and 2000 ng/ml was assessed for permeabilization of the ADSCs. After determining the permeability and viability of the cells by trypan blue staining and MTT assay, respectively, 1000 ng/ml SLO was selected as the optimum concentration.
After permeabilization, the ADSCs were incubated with ESC extract containing an ATP-regenerating system which is necessary for remodelling of mammalian chromatin (32). Then, the permeabilization of plasma membrane was reversed by culturing the ADSCs in resealing medium containing 20% FBS and 2 mM CaCl2.
Based on qPCR analysis, three weeks after reprogramming, the expression levels of OCT4A, SOX2, NANOG, REX1 and ESG1 were significantly upregulated while UTF1 and LIN28 expression were not affected. OCT4, SOX2 and NANOG are three important transcription factors essential for self-renewal of ESCs (33, 34). These factors together with KLF4, C-MYC and LIN28 are required for maintenance of pluripotency state (35). ESG1 is another important pluripotency-regulating gene with a similar expression profile to OCT4 and SOX2 (36). REX1 and UTF1 are two well-known target genes of OCT4A; transcriptional activity of these genes is mediated by an OCT4-binding octamer element in their regulatory regions (37). Taken together, all the genes assessed in this study including OCT4A, SOX2, NANOG, REX1, ESG1, UTF1 and LIN28 have critical roles in regulation of pluripotency and their upregulation indicates the capability of ESC extract for nuclear reprogramming of adult stem cells and generation of cells with a more pluripotency state. Although the molecular mechanisms of somatic nuclear reprogramming by ESC extract is not precisely clear, previous studies show changes in DNA methylation and histone modification status of OCT4 and NANOG promoters following treatment with ESC extracts (38). These epigenetic modifications could induce the expression of pluripotency genes and are critical for dedifferentiation of somatic cells to a pluripotency state (39).
In our study, upregulation of some but not all of the pluripotency genes by mouse ESC extract indicates dedifferentiation of human ADSCs toward partial pluripotency. Moreover, since we used a mouse ESC extract to reprogram human cells, our findings shows that the reprogramming effect of ESC extract is not species-specific, as previously suggested by Miyamoto et al (6).
Conclusion
The findings of this study suggest that the nuclei of human ADSCs can be reprogrammed by mouse ESC extract to produce more dedifferentiated cells with partial pluripotency. This strategy may be useful in generating cells with improved plasticity for cell replacement therapy purposes. However further investigation will be required in order to assess the differentiation potential of the reprogrammed ADSCs.
Acknowledgment
This study was financially supported by Iran National Science Foundation (INSF) (Grant No. 78040521).
Conflict of interests
The authors declare that there is no conflict of interests.
References
- 1.Hakelien AM, Gaustad KG, Collas P. Transient alteration of cell fate using a nuclear and cytoplasmic extract of an insulinoma cell line. Biochem Biophys Res Commun. 2004;316:834–841. doi: 10.1016/j.bbrc.2004.02.127. [DOI] [PubMed] [Google Scholar]
- 2.Hakelien AM, Gaustad KG, Collas P. Modulation of cell fate using nuclear and cytoplasmic extracts. Methods Mol Biol. 2006;325:99–114. doi: 10.1385/1-59745-005-7:99. [DOI] [PubMed] [Google Scholar]
- 3.Hakelien AM, Landsverk HB, Robl JM, Skalhegg BS, Collas P. Reprogramming fibroblasts to express T-cell functions using cell extracts. Nat Biotechnol. 2002;20:460–466. doi: 10.1038/nbt0502-460. [DOI] [PubMed] [Google Scholar]
- 4.Xu YN, Guan N, Wang ZD, Shan ZY, Shen JL, Zhang QH, et al. ES cell extract-induced expression of pluripotent factors in somatic cells. Anat Rec (Hoboken) 2009;292:1229–1234. doi: 10.1002/ar.20919. [DOI] [PubMed] [Google Scholar]
- 5.Gaustad KG, Boquest AC, Anderson BE, Gerdes AM, Collas P. Differentiation of human adipose tissue stem cells using extracts of rat cardiomyocytes. Biochem Biophys Res Commun. 2004;314:420–427. doi: 10.1016/j.bbrc.2003.12.109. [DOI] [PubMed] [Google Scholar]
- 6.Miyamoto K, Furusawa T, Ohnuki M, Goel S, Tokunaga T, Minami N, et al. Reprogramming events of mammalian somatic cells induced by Xenopus laevis egg extracts. Mol Reprod Dev. 2007;74:1268–1277. doi: 10.1002/mrd.20691. [DOI] [PubMed] [Google Scholar]
- 7.Taranger CK, Noer A, Sorensen AL, Hakelien AM, Boquest AC, Collas P. Induction of dedifferentiation, genomewide transcriptional programming, and epigenetic reprogramming by extracts of carcinoma and embryonic stem cells. Mol Biol Cell. 2005;16:5719–5735. doi: 10.1091/mbc.E05-06-0572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhan W, Liu Z, Liu Y, Ke Q, Ding Y, Lu X, et al. Modulation of rabbit corneal epithelial cells fate using embryonic stem cell extract. Mol Vis. 2010;16:1154–1161. [PMC free article] [PubMed] [Google Scholar]
- 9.Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, et al. Multilineage cells from human adipose tissue:implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 10.Khaleghi M, Taha MF, Jafarzadeh N, Javeri A. Atrial and ventricular specification of ADSCs is stimulated by different doses of BMP4. Biotechnol Lett. 2014;36:2581–2589. doi: 10.1007/s10529-014-1637-8. [DOI] [PubMed] [Google Scholar]
- 11.Taha MF, Javeri A. The expression of NPPA splice variants during mouse cardiac development. DNA Cell Biol. 2015;34:19–28. doi: 10.1089/dna.2014.2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taha MF, Javeri A, Kheirkhah O, Majidizadeh T, Khalatbary AR. Neural differentiation of mouse embryonic and mesenchymal stem cells in a simple medium containing synthetic serum replacement. J Biotechnol. 2014;172:1–10. doi: 10.1016/j.jbiotec.2013.11.028. [DOI] [PubMed] [Google Scholar]
- 13.Halvorsen YD, Franklin D, Bond AL, Hitt DC, Auchter C, Boskey AL, et al. Extracellular matrix mineralization and osteoblast gene expression by human adipose tissue-derived stromal cells. Tissue Eng. 2001;7:729–741. doi: 10.1089/107632701753337681. [DOI] [PubMed] [Google Scholar]
- 14.Mizuno H, Zuk PA, Zhu M, Lorenz HP, Benhaim P, Hedrick MH. Myogenic differentiation by human processed lipoaspirate cells. Plast Reconstr Surg. 2002;109:199–209. doi: 10.1097/00006534-200201000-00030. discussion 210-191. [DOI] [PubMed] [Google Scholar]
- 15.Greco SJ, Liu K, Rameshwar P. Functional similarities among genes regulated by OCT4 in human mesenchymal and embryonic stem cells. Stem Cells. 2007;25:3143–3154. doi: 10.1634/stemcells.2007-0351. [DOI] [PubMed] [Google Scholar]
- 16.Peroni D, Scambi I, Pasini A, Lisi V, Bifari F, Krampera M, et al. Stem molecular signature of adipose-derived stromal cells. Exp Cell Res. 2008;314:603–615. doi: 10.1016/j.yexcr.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 17.Taha MF, Javeri A, Rohban S, Mowla SJ. Upregulation of pluripotency markers in adipose tissue-derived stem cells by miR-302 and leukemia inhibitory factor. Biomed Res Int. 2014;2014:941486. doi: 10.1155/2014/941486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tat PA, Sumer H, Jones KL, Upton K, Verma PJ. The efficient generation of induced pluripotent stem (iPS) cells from adult mouse adipose tissue-derived and neural stem cells. Cell Transplant. 2010;19:525–536. doi: 10.3727/096368910X491374. [DOI] [PubMed] [Google Scholar]
- 19.Faghih H, Javeri A, Taha MF. Impact of early subcultures on stemness, migration and angiogenic potential of adipose tissue-derived stem cells and their resistance to in vitro ischemic condition. Cytotechnology. 2017 doi: 10.1007/s10616-017-0104-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pfaffl MW, Horgan GW, Dempfle L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002;30:e36. doi: 10.1093/nar/30.9.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wakayama T, Perry AC, Zuccotti M, Johnson KR, Yanagimachi R. Full-term development of mice from enucleated oocytes injected with cumulus cell nuclei. Nature. 1998;394:369–374. doi: 10.1038/28615. [DOI] [PubMed] [Google Scholar]
- 22.Wilmut I, Schnieke AE, McWhir J, Kind AJ, Campbell KH. Viable offspring derived from fetal and adult mammalian cells. Nature. 1997;385:810–813. doi: 10.1038/385810a0. [DOI] [PubMed] [Google Scholar]
- 23.Cowan CA, Atienza J, Melton DA, Eggan K. Nuclear reprogramming of somatic cells after fusion with human embryonic stem cells. Science. 2005;309:1369–1373. doi: 10.1126/science.1116447. [DOI] [PubMed] [Google Scholar]
- 24.Tada M, Takahama Y, Abe K, Nakatsuji N, Tada T. Nuclear reprogramming of somatic cells by in vitro hybridization with ES cells. Curr Biol. 2001;11:1553–1558. doi: 10.1016/s0960-9822(01)00459-6. [DOI] [PubMed] [Google Scholar]
- 25.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 26.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 27.Rajasingh J, Lambers E, Hamada H, Bord E, Thorne T, Goukassian I, et al. Cell-free embryonic stem cell extract-mediated derivation of multipotent stem cells from NIH3T3 fibroblasts for functional and anatomical ischemic tissue repair. Circ Res. 2008;102:e107–117. doi: 10.1161/CIRCRESAHA.108.176115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bru T, Clarke C, McGrew MJ, Sang HM, Wilmut I, Blow JJ. Rapid induction of pluripotency genes after exposure of human somatic cells to mouse ES cell extracts. Exp Cell Res. 2008;314:2634–2642. doi: 10.1016/j.yexcr.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neri T, Monti M, Rebuzzini P, Merico V, Garagna S, Redi CA, et al. Mouse fibroblasts are reprogrammed to Oct-4 and Rex-1 gene expression and alkaline phosphatase activity by embryonic stem cell extracts. Cloning Stem Cells. 2007;9:394–406. doi: 10.1089/clo.2006.0011. [DOI] [PubMed] [Google Scholar]
- 30.Mostafavi-Pour Z, Keihani S, Talaei-Khozani T, Mokaram P, Fardaei M, Rohani L, et al. Expression of a2, a5 and a6 subunits of integrin in de-differentiated NIH3T3 cells by cell-free extract of embryonic stem cells. Mol Biol Rep. 2012;39:7339–7346. doi: 10.1007/s11033-012-1565-4. [DOI] [PubMed] [Google Scholar]
- 31.Walev I, Hombach M, Bobkiewicz W, Fenske D, Bhakdi S, Husmann M. Resealing of large transmembrane pores produced by streptolysin O in nucleated cells is accompanied by NF-kappaB activation and downstream events. FASEB J. 2002;16:237–239. doi: 10.1096/fj.01-0572fje. [DOI] [PubMed] [Google Scholar]
- 32.Kikyo N, Wade PA, Guschin D, Ge H, Wolffe AP. Active remodeling of somatic nuclei in egg cytoplasm by the nucleosomal ATPase ISWI. Science. 2000;289:2360–2362. doi: 10.1126/science.289.5488.2360. [DOI] [PubMed] [Google Scholar]
- 33.Mitsui K, Tokuzawa Y, Itoh H, Segawa K, Murakami M, Takahashi K, et al. The homeoprotein Nanog is required for maintenance of pluripotency in mouse epiblast and ES cells. Cell. 2003;113:631–642. doi: 10.1016/s0092-8674(03)00393-3. [DOI] [PubMed] [Google Scholar]
- 34.Nichols J, Zevnik B, Anastassiadis K, Niwa H, Klewe-Nebenius D, Chambers I, et al. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell. 1998;95:379–391. doi: 10.1016/s0092-8674(00)81769-9. [DOI] [PubMed] [Google Scholar]
- 35.Voutsadakis IA. The network of pluripotency, epithelial-mesenchymal transition, and prognosis of breast cancer. Breast Cancer (Dove Med Press) 2015;7:303–319. doi: 10.2147/BCTT.S71163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Western P, Maldonado-Saldivia J, van den Bergen J, Hajkova P, Saitou M, Barton S, et al. Analysis of Esg1 expression in pluripotent cells and the germline reveals similarities with Oct4 and Sox2 and differences between human pluripotent cell lines. Stem Cells. 2005;23:1436–1442. doi: 10.1634/stemcells.2005-0146. [DOI] [PubMed] [Google Scholar]
- 37.Chew JL, Loh YH, Zhang W, Chen X, Tam WL, Yeap LS, et al. Reciprocal transcriptional regulation of Pou5f1 and Sox2 via the Oct4/Sox2 complex in embryonic stem cells. Mol Cell Biol. 2005;25:6031–6046. doi: 10.1128/MCB.25.14.6031-6046.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cho HJ, Lee CS, Kwon YW, Paek JS, Lee SH, Hur J, et al. Induction of pluripotent stem cells from adult somatic cells by protein-based reprogramming without genetic manipulation. Blood. 2010;116:386–395. doi: 10.1182/blood-2010-02-269589. [DOI] [PubMed] [Google Scholar]
- 39.Freberg CT, Dahl JA, Timoskainen S, Collas P. Epigenetic reprogramming of OCT4 and NANOG regulatory regions by embryonal carcinoma cell extract. Mol Biol Cell. 2007;18:1543–1553. doi: 10.1091/mbc.E07-01-0029. [DOI] [PMC free article] [PubMed] [Google Scholar]


