Abstract
Objective(s):
Arachidonic Acid/5-lipoxygenase (AA/5-LOX) pathway connects lipid metabolism and proinflammatory cytokine, which are both related to the development and progression of nonalcoholic fatty liver disease (NAFLD). Therefore, the present study was designed to investigate the role of AA/5-LOX pathway in progression of NAFLD, and the effect of zileuton, an inhibitor of 5-LOX, in this model.
Materials and Methods:
Animal model for progression of NAFLD was established via feeding high saturated fat diet (HFD). Liver function, HE staining, NAFLD activity score (NAS) were used to evaluate NAFLD progression. We detected the lipid metabolism substrates: free fatty acids (FFA) and AA, products: cysteinyl-leukotrienes (CysLTs), and changes in gene and protein level of key enzyme in AA/5-LOX pathway including PLA2 and 5-LOX. Furthermore, we determined whether NAFLD progression pathway was delayed or reversed when zileuton (1-[1-(1-benzothiophen-2-yl)ethyl]-1-hydroxyurea) was administrated.
Results:
Rat model for progression of NAFLD was well established as analyzed by liver transaminase activities, hematoxylin-eosin (HE) staining and NAS. The concentrations of substrates and products in AA/5-LOX pathway were increased with the progression of NAFLD. mRNA and protein expression of PLA2 and 5-LOX were all enhanced. Moreover, administration of zileuton inhibited AA/5-LOX pathway and reversed the increased transamine activities and NAS.
Conclusion:
AA/5-LOX pathway promotes the progression of NAFLD, which can be reversed by zileuton.
Keywords: Arachidonic acid, Lipid metabolism, 5-lipoxygenase, Nonalcoholic fatty liver-disease, Proinflammatory- mediators, Zileuton
Introduction
Non-alcoholic fatty liver disease (NAFLD) represents a spectrum of liver disease encompassing steatosis (fatty change), non-alcoholic steatohepatitis (NASH) and cirrhosis in the absence of alcohol abuse (1, 2). NASH is the key stage in progression of NAFLD, which begins to require drug treatment in clinic. In Europe and North America, NAFLD has been increasingly recognized as the leading cause of liver dysfunction and cirrhosis in non-alcoholic and viral-negative hepatitis patients. In light of the increasing prevalence and health consequences of NAFLD, it is necessary to identify the mechanisms that mediate the development and progression of this disease.
Research over the last decade has greatly enhanced our understanding of NAFLD. The “Two-hit hypothesis” is the most widely accepted mechanism of this disease (3). The first hit accounts for its strong links with over-nutrition, insulin resistance and genetic factors. Lipotoxicity, oxidative stress, cytokines, and other proinflammatory mediators may each play a role in transition of steatosis to NASH, which is related to the second hit. Especially, oxidative stress correlated with the levels of NASH activity
markers, while the antioxidative function and liver function was preserved in younger individuals (4, 5). However, the pathogenic mechanism of NAFLD is complicated, and the exact molecular mediators and biochemical processes that mediate the transition from simple steatosis to NASH have not been completely characterized.
Arachidonic Acid (AA), one of the main free fatty acids (FFA) produced by metabolism of phospholipase A2 (PLA2), was metabolized by at least 3 types of enzymes including 5-lipoxygenase (5-LOX). 5-LOX converts AA to cysteinyl-leukotrienes (cysLTs). This course is called AA/5-LOX pathway, which connects lipid metabolism and proinflammatory cytokines (6). The disorder of lipid metabolism in patients is a common clinical feature of NAFLD (7). An increased ratio of saturated-to-unsaturated fatty acids delivered to or stored within the liver may mediate the progression from simple steatosis to NASH (8). It was reported that phospholipase A2 (PLA2) activity was increased during the progression of NASH (7), positively correlated with the severity of this disease, and 5-LOX contributes to the progression of hepatocellular carcinoma (9). Thus, it is of great importance to investigate the role of AA/5-LOX pathway in the progression of NAFLD.
CysLTs, the products of AA/5-LOX pathway, are a family of inflammatory mediators including LTC4, LTD4, and LTE4. CysLTs have important proinflammatory and profibrotic effects, which have been proven to contribute to the pathophysiologic features of many diseases (10, 11). For instance, competitive binding to CysLT1 receptor using antagonist drugs, blocks the effects of CysLTs, improving the symptoms of CysLTs-related chronic respiratory diseases (12). Another type of antileukotriene drugs is LT synthesis inhibitor such as zileuton, which has been approved for clinical use (13) and produces similar beneficial effects seen in 5-LOX knockout mice (14). However, whether inhibitor of cysLTs can block the progression of NAFLD to NASH is still unknown. Therefore in this study, we aimed to detect the alteration of 5-LOX pathway in the progression of NAFLD, and evaluate whether it could be inhibited by zileuton. The study will shed light on future research on AA/5-LOX pathway and its relation to the pathology of NAFLD.
Materials and Methods
Animals and treatment procedure
Sprague–Dawley (SD) rats (8 weeks old, half male and half female) were used for the experiments. All procedures on animals were carried out in accordance with the guidelines of China for animal care, which is approved by Ethics Committee of Zhejiang University (Approval number 2015-237) and conformed to the internationally accepted principles in the care and use of experimental animals. Briefly, SD rats were divided into two groups, and taking normal or high fat diet (HFD) freely (purchased from Beijing Botai Hondar Biotechnology Co Ltd.), at 20±2 °C and with a humidity of 60%. Food and water were supplied ad libitum. Six rats in each group were euthanized at the 4th, 8th, 12th and 16th week respectively, after overnight fasting. Serum, liver homogenate and liver tissue paraffin sections were prepared for the study.
Serum, liver homogenate and liver tissue paraffin sections were prepared in the following way:
Serum
Taking blood, coagulated for 1 to 2 hr (without anticoagulant) at 37 °C, stay overnight at 4 °C. When the serum is naturally separated, centrifuged it for 10 min at 4 °C, 3000 rev / min, discarded the insoluble substance, move serum to a clean test tube, and sub packed into small pieces, store at -80 °C.
Liver homogenate
Collect the liver tissue of mice in each group, then rinse with ice salt water and dry with absorbent paper. Take 0.2 g tissue, cut the crushed tissue as soon as possible and pour it into the homogenizer. Add 1.8 ml PBS to prepare the homogenate and then hold it to 10 ml. Bene tritum the tissue 10 times using stamp stem make it homogenized, followed by 3000-4000 r/min centrifugal 10-15 min, gently absorb the supernatant, keep in -20 degree or test for relevant index.
Liver tissue paraffin sections
The liver tissue is cut into small pieces after dissected, and fixed in 10% formalin solution, the fixed tissue was dehydrated with gradient ethanol of 60%, 70%, 80%, 90%, 95%, and 100%, each 1 hr, transparent 30 min by xylene, 2 times, put transparent tissue blocks at 55 °C soft wax 1, 55 °C soft wax 2 and 57 °C soft wax 3 respectively, each 20 min, ensure that the wax is fully penetrated into the tissue. After the paraffin wax completely enters the tissue block, embedded them in a metal embedding basket filled with melted paraffin. Remove the wax block when it is completely solidified, then prepare paraffin section by microtome.
In order to study whether AA/5-LOX pathway is involved in the progression of NAFLD, 3 mg/kg of zileuton, an inhibitor of 5-LOX, was intravenously injected everyday for one week by tail at the stage of possible NAFL (4 weeks) or NASH (12 weeks), which are marked as zileuton-early and zileuton-late group respectively in our following study. The rats were decapitated after being fed for 16 weeks, and samples of serum, liver homogenate and liver tissue were harvested as mentioned above.
Evaluation of NAFLD progression
HE staining was employed to observe the histological changes, which were observed by the liver pathology specialist in the light microscope. Alanine transaminase (ALT) and aspartate transaminase (AST) activities were assayed using automatic biochemical analyzer (AU2700, Olympus, Japan). NAFLD activity score (NAS) was evaluated by pathological professionals according to steatosis, lobular inflammation, hepatocellular ballooning and liver fibrosis (15), which was used to assess progression of NAFLD and classify the progression of NAFLD into different stages, including NAFL, “borderline,” and “NASH”.
Determination of substrate and product levels of AA/5-LOX pathway
Liver homogenate in different groups were harvested to detect substrates and products of AA/5-LOX pathway. Liver homogenate is pre-treated by free fatty acid quantitation kit (MAK044, Sigma-Aldrich Co.Ltd.) and analyzed by automatic biochemical analyzer (AU2700, Olympus, Japan). AA, a substrate of AA/5-LOX pathway, was detected by ELISA (CSB-E13008r, cusabio biotech CO., Ltd.) according to the manufacturer’s instructions. CysLTs, representative products of AA/5-LOX pathway, were also determined by ELISA kit (FM010230, Shanghai Hufeng Chemical Co. Ltd.).
mRNA expression detected by real time-PCR
PCR primers for tested genes were designed by primer 5. Primers (Nanjing Sirui Technology Co. Ltd.) and application parameters for PCR analysis of 5-LOX, iPLA2 and GAPDH are listed in Table 1. Real-time PCR reactions were carried out using MultiGene Gradient System (Labnet, America) in a 96-well, clear optical reaction plate with optical adhesive covers.
Table 1.
Primers and application parameters for PCR analysis
| Gene | Primer sequence (5’-3’) | Designed size (bp) |
|---|---|---|
| GAPDH | F: TGTTGCCATCAACGACCCCTT R: CTCCACGACATACTCAGCA |
202 |
| 5-LOX | F: TCTGGTGTCTGAGGTGTTCG R: AACCTCACATGGGCTACCAG |
90 |
| iPLA2 | F: CTCCCGACTGACAGAGAGCTA R: TCACACTTCCCAGGTGTTCTC |
109 |
Reactions were run in duplicated, and were carried out in a volume of 5 μl containing 2 μl of cDNA solution and 3 μl of a homemade target-specific mix composed of 5/6 2× Power SYBR Green Master Mix (TOYOBO, Japan) and 1/6 of 100 mM primers solution. The PCR program was: 95 °C for 10 min, followed by 40 cycles of (15 sec at 95 °C; 1 min at 60 °C). Fast real-time PCR system was used for real-time PCR and data were analyzed with software v2.0.1 (Applied Biosystems). Results were normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression.
Protein expression detected by Western blot
Liver was treated with specific lysis buffers (10 mM Tris buffer, pH 7.4, 150 mM NaCl, 24 mM sodium deoxycholate, 1.0 mM EDTA, 1% NP40, 1mMPMSF, 2 mg/ml leupeptin, 10μg/ml aprotinin) at 4 °C. Protein analyses by Western blot were performed routinely. Briefly, total protein was prepared and analyzed for protein content. SDS containing sample buffer (5×) was added to an aliquot of proteins (25 mg), subsequently heated for 5 min at 95 °C, and electrophoresed through 15% SDS-polyacrylamide gels. After separation, proteins were transferred onto a nitrocellulose membrane (NC membrane). Membranes were incubated with a polyclonal primary antibody (5-LOX(1:200), iPLA2(1:200), overnight at 4 °C). Blots were then exposed to the secondary antibody (peroxidase-conjugated rabbit anti-goat IgG, Beijing Zhongshan Bio; final dilution 1:5000, 1 hr at room temperature), followed by detection with an enhanced chemiluminescent substrate (ECL, Pierce, USA). β-actin was used as internal control.
Statistical analysis
Statistical Product and Service Solutions (SPSS) 19 was used for statistical analyses. Data are expressed as means±SD. Student’s t test was applied for comparing paired data, and one-way analysis of variance (ANOVA) was used for multiple group comparisons. A probability of P<0.05 was considered statistically significant.
Results
Establishment and evaluation of NAFLD progression model by feeding a HFD
Animal model for progression of NAFLD was established by feeding a HFD. HE staining showed no signs of lipid accumulation in normal structure of hepatic lobules in the control group, and there were also no signs of inflammatory cell infiltration in portal area and hyperplastic fibrous tissue. In contrast, in the group fed with HFD, steatohepatitis, ballooning degeneration, significant macrosteatosis extending beyond the periportal area to the lobule and the central zone, and foci of lobular inflammation were observed. Histological changes were aggravated along with HFD feeding time (Figure 1A). NAS was 0, 2, 2.75, 3.25 and 4.25 respectively, which indicated “Possible NAFL” at week 4, “NAFL” at week 8, “Possible NASH” at week 12 and “NASH” at week 16 (Figure 1B). Liver transaminase activities were increased along with the feeding time of HFD, exhibiting a significant difference compared with control group since week 8 (P<0.05) (Figure 1C).
Figure 1.
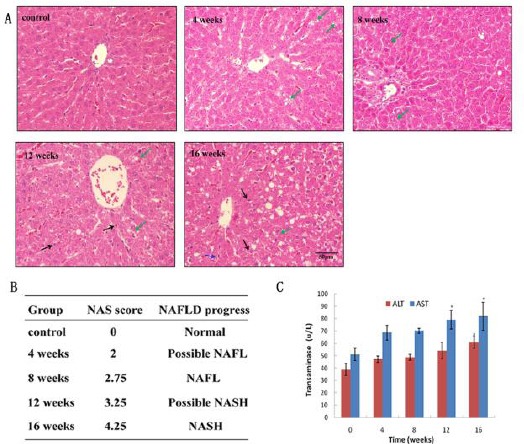
Progression of NAFLD model as evaluated by HE, NAFLD activity score and transamines activities. A: HE staining of liver tissue paraffin sections prepared from rats administrated with high fat diet for 0, 4, 8, 12, 16 weeks. Green arrow: hepatocellular ballooning; Black arrow: lobular inflammation. B: NAS was evaluated by pathological professionals according to steatosis, lobular inflammation, hepatocellular ballooning and liver fibrosis. C: Determination of alanine aminotranferease (ALT) and aspartate aminotransferase (AST) activities in different groups
Changes of substrates and products of AA/5-LOX metabolism pathway in progression of NAFLD model
Liver FFA was increased along with the feeding time of HFD. AA was increased most dramatically at week 8, and cysLTs were elevated with the feeding time (2.68 times higher than that of control group at week 16) (Figure 2). The results suggested that both of the substrates and products of AA/5-LOX metabolism pathway are changed in the progression of NAFLD model.
Figure 2.
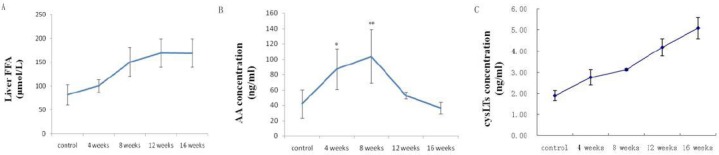
Changes of substrates and products of AA/5-LOX metabolism pathway in progression of NAFLD model. A: liver FFA; B: liver AA; C: liver cysLTs. Data were represented as mean±SD (4 rats per group, 3 replicates for each rat). ** P<0.01, ***P<0.001 vs. control
Expression of iPLA2 and 5-LOX in progression of NAFLD model
Real time PCR was used to detect the mRNA level of 5-LOX and iPLA2. The result showed that mRNA expression of 5-LOX and iPLA2 in the liver were increased since week 4. At 12 weeks, they were increased by 2.88 and 3.37 times compared to those in the control group, respectively (Figure 3A). Western blot was used to determine the protein level changes of iPLA2 and 5-LOX in progression of NAFLD. iPLA2 protein level was found to be gradually increased with the progression of NAFLD, and reached the maximum value of 2.55 times higher than that of control at week 16. Similarly, the protein level of 5-LOX was also found to be gradually increased, and reached the maximum value of 2.4 times higher than that of control at week 16 (Figure 3 B, C).
Figure 3.
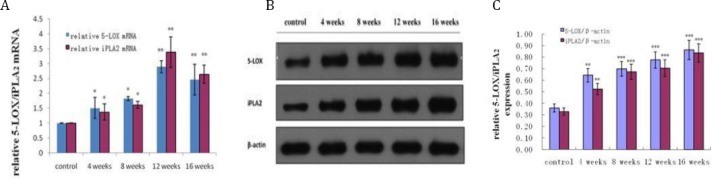
Time course of 5-LOX and iPLA2 mRNA and protein level changes in progression of NAFLD. A: mRNA expression of 5-LOX and iPLA2 detected by real time-PCR; B: Protein expression of 5-LOX and iPLA2 detected by Western blot. C: Quantitative analysis of the band was performed by densitometric analysis of immunoblots. Data were represented as means±SD (6 rats per group, 3 replicates for each rat). *P<0.05,** P< 0.01, ***P< 0.001 vs. control group
The results further support the notion that AA/5-LOX pathway was altered in progression of NAFLD, considering that 5-LOX and iPLA2 were key enzymes in AA/5-LOX metabolic pathway.
Inhibition of AA/5-LOX pathway by zileuton
AA/5-LOX pathway was inhibited by intravenous injection of zileuton. Liver cysLTs in rats fed with HFD for 16 weeks were significantly reduced when zileuton was injected at week 4 (zileuton-early) or 12 (zileuton-late) (Figure 4A). Meanwhile, mRNA and protein expressions of 5-LOX were also declined by zileuton administration both at week 4, or 12. (Figure 4 B-D)
Figure 4.
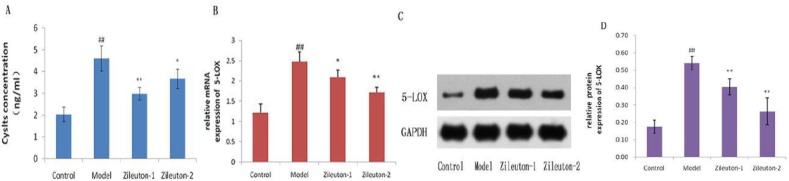
Inhibition of AA/5-LOX pathway by zileuton. A: Change of cysLTs concentration in rat after intravenously injected with zileuton. B: mRNA expression of 5-LOX detected by real time-PCR. C: Protein expression of 5-LOX detected by Western blot. D: Quantitative analysis of the band was performed by densitometric analysis of immunoblots. n=6, ##P<0.01 vs. control group; *P<0.05, **P<0.01, vs. Model
Inhibition of NAFLD progression by zileuton
As zileuton could potently inhibit AA/5-LOX pathway, we studied whether it could also reverse or delay the progression to NASH in a rat model. The result showed that transamines were significantly reduced. NAS was restricted to 2.83 and 3.33 when rats were injected with zileuton for a week at week 4 and 12, respectively, indicating that zileuton delayed the progression to NASH in a rat model. It also indicated that the effect would be better if zileuton was injected earlier when NAFLD was at a slight stage. (Figure 5)
Figure 5.
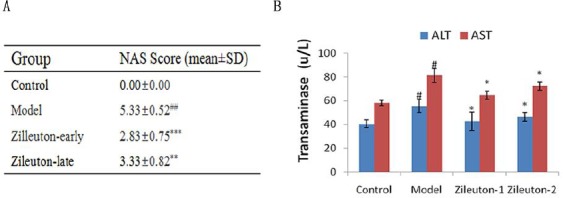
Inhibition of progression to NAFLD by zileuton. A: NAS was evaluated by pathological professionals. B: Determination of alanine aminotranferease (ALT) and aspartate aminotransferase (AST) in rat intravenous injected with zileuton. n=6, ##P<0.01 vs control group; **P<0.01,***P<0.001, vs Model
Discussion
Animal models of NAFLD may be divided into two broad categories: those caused by genetic mutation and those with an acquired phenotype produced by dietary or pharmacological manipulation (16). In this study, a rat model for development and progression of NAFLD was established via feeding a HFD. Increased dietary supply of fat to the liver could promote steatosis by increasing hepatic lipid uptake (17). Feeding with HFD gradually increased lipid accumulation and inflammatory foci deposition in the rat liver. Liver FFA showed an increasing trend accompanied by the development of disease and grows at a relatively rapid rate before 8 weeks. Meanwhile, the express of cysLTs synthesis related to protein 5-LOX and PLA2 are both increased with the progression of the disease. Correspondingly, NAS showed a time-related increase, indicating “Possible NAFL” at week 4, “NAFL” at week 8, “Possible NASH” at week 12 and “NASH” at week 16. The results showed that steatosis was mainly observed at an earlier stage, whereas, inflammation occurred gradually after 8 weeks, which subsequently progressed into NASH.
AA is one of the FFA produced by metabolism of PLA2. It has been known that iPLA2 is activated in progression of NASH (18). However, how AA is metabolized in NAFLD remains unknown. CysLTs, as the main products of AA/5-LOX metabolism pathway, are potent pro-inflammatory mediators. We found that cysLTs were increased with the progression of NAFLD, and mRNA and protein level of iPLA2 and 5-LOX were also elevated, which indicated AA/5-LOX pathway was activated and may play a role in progression of NAFLD. In order to further identify the importance of AA/5-LOX pathway, zileuton was injected at stage of possible NAFL and NASH. Zileuton was reported to inhibit the production of cysLTs and 5-LOX pathway similar to these observed in 5-LOX knockout mice (14). Our result showed that zileuton injected at week 4 inhibited AA/5-LOX pathway and delayed the progression to NASH in a rat model, which indicated that inhibition of 5-LOX may be effective in the therapy for NAFLD. However, administration of zileuton did not completely stop NASH progression, which suggests that other pathways may also play a role in the progression. Further studies are needed to elucidate detailed mechanisms how AA/5-LOX pathway mediates liver injury, and how zileuton and other intervening drugs can be used most effectively in therapies.
Conclusion
In this study, we provide an evidence in animal studies that an involvement of AA/5-LOX pathway in the progression of NAFLD and 5-LOX inhibitor zileuton may be effective in the treatment of NAFLD. But the detailed mechanisms of AA/5-LOX pathway mediates liver injury and zileuton decrease disease process need further studies.
Acknowledgment
The project was supported by National Natural Science Foundation of China (No 81100277, 81402862, 81503048) and Natural Science Foundation of Zhejiang Province (LY15H030007).
References
- 1.Koo SH. Nonalcoholic fatty liver disease:molecular mechanisms for the hepatic steatosis. Clin Mol Hepatol. 2013;19:210–215. doi: 10.3350/cmh.2013.19.3.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Verbeek J, Cassiman D, Lannoo M, Laleman W, van der Merwe S, Verslype C, et al. Treatment of non-alcoholic fatty liver disease:can we already face the epidemic? Acta Gastroenterol Belg. 2013;76:200–209. [PubMed] [Google Scholar]
- 3.Gentile CL, Pagliassotti MJ. The role of fatty acids in the development and progression of nonalcoholic fatty liver disease. J nutr biochem. 2008;19:567–576. doi: 10.1016/j.jnutbio.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shimomura Y, Takaki A, Wada N, Yasunaka T, Ikeda F, Maruyama T, et al. The serum oxidative/anti-oxidative stress balance becomes dysregulated in patients with non-alcoholic steatohepatitis associated with hepatocellular carcinoma. Intern Med. 2017;56:243–251. doi: 10.2169/internalmedicine.56.7002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ayd N S, Atukeren PN, Akatay U, Uzun H, Altu T. Gender-dependent oxidative variations in liver of aged rats. BIOGERONTOLOGY. 2010;11:335–346. doi: 10.1007/s10522-009-9257-8. [DOI] [PubMed] [Google Scholar]
- 6.Nakamura Y, Kozuka M, Naniwa K, Takabayashi S, Torikai K, Hayashi R, et al. Arachidonic acid cascade inhibitors modulate phorbol ester-induced oxidative stress in female ICR mouse skin:differential roles of 5-lipoxygenase and cyclooxygenase-2 in leukocyte infiltration and activation. Free Radic Biol Med. 2003;35:997–1007. doi: 10.1016/s0891-5849(03)00440-4. [DOI] [PubMed] [Google Scholar]
- 7.Colak Y, Senates E, Ozturk O, Doganay HL, Coskunpinar E, Oltulu YM, et al. Association of serum lipoprotein-associated phospholipase A2 level with nonalcoholic fatty liver disease. Metab Syndr Relat Disord. 2012;10:103–109. doi: 10.1089/met.2011.0111. [DOI] [PubMed] [Google Scholar]
- 8.Carmiel-Haggai M, Cederbaum AI, Nieto N. A high-fat diet leads to the progression of non-alcoholic fatty liver disease in obese rats. faseb J. 2005;19:136–138. doi: 10.1096/fj.04-2291fje. [DOI] [PubMed] [Google Scholar]
- 9.Xu XM, Deng JJ, Yuan GJ, Yang F, Guo HT, Xiang M, et al. 5-Lipoxygenase contributes to the progression of hepatocellular carcinoma. mol med rep. 2011;4:1195–200. doi: 10.3892/mmr.2011.547. [DOI] [PubMed] [Google Scholar]
- 10.Martinez-Clemente M, Claria J, Titos E. The 5-lipoxygenase/leukotriene pathway in obesity, insulin resistance, and fatty liver disease. Curr Opin Clin Nutr Metab Care. 2011;14:347–353. doi: 10.1097/MCO.0b013e32834777fa. [DOI] [PubMed] [Google Scholar]
- 11.Back M. Inhibitors of the 5-lipoxygenase pathway in atherosclerosis. Curr Pharm Des. 2009;15:3116–3132. doi: 10.2174/138161209789058020. [DOI] [PubMed] [Google Scholar]
- 12.Cingi C, Muluk NB, Ipci K, Sahin E. Antileukotrienes in upper airway inflammatory diseases. Curr Allergy Asthma Rep. 2015;15:64. doi: 10.1007/s11882-015-0564-7. [DOI] [PubMed] [Google Scholar]
- 13.Zhou GX, Ding XL, Wu SB, Zhang HF, Cao W, Qu LS, et al. Inhibition of 5-lipoxygenase triggers apoptosis in pancreatic cancer cells. oncol rep. 2015;33:661–668. doi: 10.3892/or.2014.3650. [DOI] [PubMed] [Google Scholar]
- 14.Collin M, Rossi A, Cuzzocrea S, Patel NS, Di Paola R, Hadley J, et al. Reduction of the multiple organ injury and dysfunction caused by endotoxemia in 5-lipoxygenase knockout mice and by the 5-lipoxygenase inhibitor zileuton. J Leukoc Biol. 2004;76:961–970. doi: 10.1189/jlb.0604338. [DOI] [PubMed] [Google Scholar]
- 15.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 16.Anstee QM, Goldin RD. Mouse models in non-alcoholic fatty liver disease and steatohepatitis research. int J exp pathol. 2006;87:1–16. doi: 10.1111/j.0959-9673.2006.00465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferraioli G, Tinelli C, Lissandrin R, Zicchetti M, Faliva M, Perna S, et al. Correlation of the controlled attenuation parameter with indices of liver steatosis in overweight or obese individuals:a pilot study. Eur J Gastroenterol Hepatol. 2015;27:305–312. doi: 10.1097/MEG.0000000000000287. [DOI] [PubMed] [Google Scholar]
- 18.Ferraioli G, Tinelli C, Lissandrin R, Zicchetti M, Faliva M, Perna S, et al. Correlation of the controlled attenuation parameter with indices of liver steatosis in overweight or obese individuals. eur J gastroen hepat. 2015;27:305–312. doi: 10.1097/MEG.0000000000000287. [DOI] [PubMed] [Google Scholar]


