Abstract
Objective(s):
Increasing evidence suggests that regular physical exercise improves type 2 diabetes mellitus (T2DM). However, the potential beneficial effects of swimming on insulin resistance and lipid disorder in T2DM, and its underlying mechanisms remain unclear.
Materials and Methods:
Rats were fed with high fat diet and given a low dosage of Streptozotocin (STZ) to induce T2DM model, and subsequently treated with or without swimming exercise. An 8-week swimming program (30, 60 or 120 min per day, 5 days per week) decreased body weight, fasting blood glucose and fasting insulin.
Results:
Swimming ameliorated lipid disorder, improved muscular atrophy and revealed a reduced glycogen deposit in skeletal muscles of diabetic rats. Furthermore, swimming also inhibited the activation of Wnt3a/β-catenin signaling pathway, decreased Wnt3a mRNA and protein level, upregulated GSK3β phosphorylation activity and reduced the expression of β-catenin phosphorylation in diabetic rats.
Conclusion:
The trend of the result suggests that swimming exercise proved to be a potent ameliorator of insulin resistancein T2DM through the modulation of Wnt3a/β-catenin pathway and therefore, could present a promising therapeutic measure towards the treatment of diabetes and its relatives.
Keywords: GSK3β, Insulin resistance, Swimming training, Type 2 diabetes mellitus, Wnt3a/β-catenin – signaling
Introduction
Type 2 diabetes mellitus (T2DM) is a complex, chronic metabolic disease of multiple etiologies charac- terized by chronic hyperglycemia with disturbed carbo-hydrate, fat and protein metabolism. Insulin resistance (IR) is a hallmark in the majority of individuals with obesity or T2DM (1). Insulin primarily acts on liver and fatty tissues, as well as skeletal muscles. It is well established that skeletal muscle plays a central role in the whole body IR (2, 3). Several cytokines have been evidenced that can be synthesized by skeletal muscle cells in T2DM and are possibly involved in IR.
The Wnt signaling system, comprising extracellu-lar factor (Wnt), transmembrane receptors (frizzled), cytoplasmic protein (β-catenin), nuclear transcrip-tion factor (TCFS/LEF), and a series of proteins, plays a critical role during embryonic development and oncogenesis (4). Many recent studies have shown that the canonical Wnt/β-catenin signaling pathway plays a well-established role in the metabolic syndrome, especially T2DM, which regulating pancreas develop-ment as well as islet function, insulin production and secretion both in vitro and in vivo (5, 6). In Streptozotocin (STZ)-induced diabetic rat models, the expression of Wnt/β-catenin signaling pathway is activated, and β-catenin was upregulated in the islet regeneration process to promote the regeneration of damaged pancreatic islet cell (7). Rulifson et al. proved that there was an increase in β-catenin expression by Wnt3a treated islet β-cells, which provoked the proliferation of β-cells and ultimately signaled for increased insulin synthesis and secretion (8). The results showed that the activation of Wnt/β-catenin signaling pathway can promote the healing of diabetic wounds.
Exercise represents a physical stress that transient - ly disrupts homeostasis, and the working skeletal muscle is clearly the organ most directly affected during physical activity (9, 10). It is well established that continuous physical exercises could enhance the body’s propensity to use and absorb glucose by interfering with some molecular signaling pathways. One of the most credible mechanisms that explain the molecular antidote of diabetes type 2 is the improve-ment of metabolic system with exercise in order to reduce insulin resistance prevalence in skeletal muscle (11-13). However, to date, little is known about whether insulin resistance alleviated through physical exercises is associated with Wnt/β- catenin signaling in T2DM, and the detailed mechanisms involved remain to be verified.
In the present study, we investigated this hypothesis that Wnt/β-catenin signaling pathway may play an important role in skeletal muscle ameliorating insulin resistance in T2DM mice and aimed at providing a reliable experiment basis for clinical practice.
Materials and Methods
Animals
Fifty-six male Sprague Dawley rats, weighing 200-220 g were bred in the Center of Experimental Animal, Xuzhou Medical University, where an SPF level laboratory (24 ± 1 °C; humidity of 45% to 55%, 12:12 dark/light cycle) was founded, as authorized by the Jiangsu province government. Animals were allowed food and water ad libitum before and during the experiment. Experiments were performed on rats aged between 8-10 weeks.
One week after feeding adaptation, the rats were randomly divided into normal group (N) and diabetic model (DM), and were given standard diet and high fat diet for 8 weeks. The rats were fasted for 12 hr and subjected to a single intraperitoneal injection of 30 mg/kg STZ, which was freshly dissolved in 100 mmol/l sodium citrate buffer (pH 4.5). Normal rats received sodium citrate buffer only. On the third day of STZ administration, the fasting blood glucose (FBG) value was determined by a reagent kit. Rats with FBG levels higher than 13.9 mmol/l were considered to be diabetic. Fat diet was purchased from the Laboratory Animal Hayes Laikang Co. and the feed formulation include 74% basal mouse material, 10% lard, 7% sucrose, 5% casein, 2% fish meal, 2% maltodextrin and 0.1% methionine. All experiments were performed in accordance with Guide to Laboratory Animal Ethics Examination of Xuzhou Medical University, and relative animal experiments are permitted.
Exercise programs
Weight-unloaded swimming training was perform- ed in this study, with slight modifications. Swimming took place in a 200 cm container filled with water to a depth of 50 cm and maintained at 30 °C. Rats were acclimatized to the training pool for one-week prior to swimming training. Acclimation entailed swimming for gradually increasing times (10, 20, 30, 40, 50, 60, and 70 min/d). After adaptation training, 38 rats were randomly divided into three groups: less-intensive group (LM group, rats swimming for 30 min), moderate-intensive group (MM group, rats swimming for 60 min), high-intensive group (HM group, rats swimming for 120 min). These groups of rats were allowed to swim five times a week and training applied for 8 weeks.
Sample collection and preparation
After 8 weeks of swimming exercise, rats were sacrificed after fasting them for 12 hr. Samples of blood was collected from the abdominal aorta, and the serum samples were harvested and stored at -20 °C for detection of blood biochemical indicators. At the time of euthanasia, the gastrocnemius was isolated after which a fraction of the samples was fixed in 4% paraformaldehyde while the remaining tissues were stored at -80 °C for subsequent biochemical analysis.
Blood biochemical indicators detection
Rats fasted insulin levels (FINs) was detected by ELISA method and the level of fasting blood glucose (FBG), triglyceride (TG), total cholesterol (TC), low density lipoprotein (LDL), high density lipoprotein (HDL) were evaluated by semi-automatic biochemical analyzer.
Histology analysis
The gastrocnemius were fixed in 4% paraformal-dehyde and embedded in paraffin. Sections of 3 μM thickness were cut perpendicularly to the long axis of the gastrocnemius for HE and periodic acid-Sciff (PAS) staining. HE staining was used to observe the changes in morphological structure of the gastrocnemius. For the degree of glycogen deposited in skeletal muscle, the sections were stained with PAS. The sections were examined using Olympus BX43F microscope (Tokyo, Japan). The three most central sections of each defect were analyzed.
Western blotting analysis
Gastrocnemius were harvested in lysis buffer containing 50 mmol/l Tris (pH 7.6), 150 mmol/l NaCl, 1 mmol/l EDTA, 1% NP-40, 1 mmol/l PMSF, 1 mmol/l Na3VO4 and 20 mmol/l NaF. The supernatant was decanted after centrifugation at 12,000×g at 4 °C for 15 min. The protein concentration was determined using bicinchoninic acid protein assay (BCA Protein Assay Kit, Pierce Thermo-Scientific, Rockford, IL, USA) according to the manufacturer’s instructions. For immunoblotting, an equal amount of protein (60 μg) was loaded into 8% SDS-PAGE and transferred onto Immobilon-NC Transfer Membrane (Millipore, Bedford, MA, USA). The memb-ranes were blocked in PBS containing 2% BSA for 1 hr at room temperature, followed by incubation with primary antibodies overnight at 4 °C. The primary antibodies against β-catenin, phospho-β-catenin (Ser33/37/Thr41), GSK3β, phospho-GSK3β (Ser9), were obtained from Cell Signaling (Beverly, MA, USA), Wnt3a antibody was purchased from Abcam (Cambridge, UK) and β-actin antibody was obtained from Bioworld Technology (St. Louis, USA). The membranes were colorimetrically developed using fluorescent marked secondary antibody (Beyotime Institute of Biotechnology, Nantong, China). The quantification was performed through the measurement of the signal intensity using ImageJ software (National Institutes of Health, Bethesda, MD, USA).
RNA isolation and real-time quantitative RT-PCR
Total RNA was isolated from gastrocnemius using trizol reagent (Invitrogen, Carlsbad, CA, USA) accor-ding to the manufacturer’s instructions. The cDNA was synthesized using 1 μg of total RNA and reverse transcribed using the RT-ReverTra Ace qPCR RT Kit (Toyobo, Osaka, Japan). Gene expression was measure-ed through real-time PCR using 0.2 μmol/l gene-specific primers and 1×LightCycler 480 SYBR Green I Master Mix (Roche Applied Science, Mannheim, Germany) in a total volume of 10 μl. The PCR reactions were performed on a Light Cycle 480 System (Roche Applied Science, Mannheim, Germany) using a thermal profile of 10 min at 95 °C, followed by 40 cycles of 15 sec at 95 °C, 30 sec at 60 °C, a melting curve of 15 sec at 95 °C, 60 sec at 60 °C, heating to 95 °C and cooling for 30 sec at 4 °C. PCR primers sequences used were as follows: Wnt3a, forward TCC GAC TCT TGG CAG AAC TT and reverse AAT GGA ATA GGT CCC GAA CA; β-catenin, forward TGC TGA AGG TGC TGT CTG TC and reverse TCG GTA ATG TCC TCC CTG TC; GSK3β, forward TTT GCT CCC TTG TTG GTG TT and reverse AGG CTG TGT GTT GGC TGA AT; β-actin, forward CCC ATC TAT GAG GGT TAC GC and reverse TTT AAT GTC ACG CAC GAT TTC. The results were analyzed using the LightCycler 480 software (version 1.5, Roche Applied Science, Mannheim, Germany). The relative levels of mRNA were analyzed using the ΔΔCt method.
Statistical analysis
All data were presented as means±SEM. Groups comparisons were achieved using one-way ANOVA, followed by the Newman-Keuls test. Statistical significance was considered at P<0.05. The statistical analysis was performed using SPSS statistical software (Version 16.0).
Results
Effects of swimming on body weights, FBG, FINs levels and HOMA-IR of diabetic rats
The body weights, FBG and FINs of experimental rats were measured after swim-training for 8 weeks. Compared with the normal rats, the body weights of the diabetic rats increased significantly (P<0.05). However, low, intermediate and high intensive swimming markedly reduced the body weights (P<0.01). FBG, FINs levels and HOMA-IR of the diabetic rats were significantly higher than normal rats (P<0.05 or P<0.01). Swim-training had reduced levels of FINs and HOMA-IR. Furthermore, intermediate and high intensive swimming decreased obviously the levels of FBG in diabetic rats (P<0.05) (Table 1).
Table 1.
Effects of swim-training on body weights, FBG, FINs and HOMA-IR levels of diabetic rats
| Groups | Body weight (g) | FBG (mmol/l) | FINs (mmol/l) | HOMA-IR |
|---|---|---|---|---|
| N | 430.0±9.6 | 7.80±0.36 | 0.11±0.01 | 1.02±0.12 |
| DM | 456.3±11.6* | 25.50±0.68** | 0.36±0.05* | 10.39±1.16** |
| LM | 414.7±3.8## | 22.79±0.65 | 0.13±0.01# | 3.45±1.09## |
| MM | 377.0±21.0## | 21.66±1.06# | 0.14±0.02# | 3.53±0.43## |
| HM | 384.5±6.1## | 20.91±1.88# | 0.13±0.02# | 3.53±0.63## |
Notes:
P <0.05,
P <0.01, compared with N;
P<0.05,
P<0.01, compared with DM. N: normal group; DM: diabetic group; LM: diabetic rats with low-intensive swimming, 30 min/d, five times a week; MM: diabetic rats with moderate-intensive swimming, 90 min/d, five times a week; HM: diabetic rats with high-intensive swimming, 120 min/d, five times a week. Data are presented as the means±SEM, n= 6
Effects of swim-training on lipid profile of diabetic rats
TC, TG, LDL, HDL are important blood lipid parameter of obesity T2DM. We examined the blood lipid parameter after swim-training for 8 weeks. The results showed that the levels of TC, TG and LDL in diabetic rats were obviously elevated compared with normal rats. Low and high intensive swimming ameliorated the high levels of TC resulting from diabetes (P<0.05) (Figure 1A). In addition, the levels of TG and LDL in rats showed a remarkable decrease in the groups treated with low, intermediate and highintensive swimming (P<0.05 or P<0.01) (Figure 1B, D). Moreover, although the HDL of diabetic rats declined after STZ administration while swimming training had increased levels of HDL in diabetic rats (P<0.05 or P<0.01) (Figure 1C).
Figure 1.
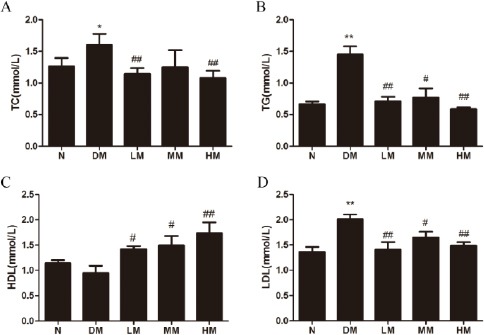
Effects of swimming training on triglyceride (TG), total cholesterol (TC), low density lipoprotein (LDL), high density lipoprotein (HDL) of diabetic rats.
Notes: N: normal group; DM: diabetic group; LM: diabetic rats with low-intensive swimming, 30 min/d, five times a week; MM: diabetic rats with moderate intensive swimming, 90 min/d, five times a week; HM: diabetic rats with high-intensive swimming, 120 min/d, five times and a week. Data are presented as the means±SEM, n= 6. *P < 0.05, **P < 0.01, compared with N; #P<0.05, ##P< 0.01, compared with DM
Effects of swimming on skeletal muscle in diabetic rats
To further determine the effects of swimming on skeletal muscle in diabetic rats, we examined the morphology of muscle bundle by HE staining in Figure 2A. Diabetic rats showed a marked decrease in skeletal muscle fiber cross-sectional area and muscular atrophy compared to normal rats, while low, intermediate and high intensive swimming obviously increased muscle fiber cross-sectional area and improved the muscular atrophy condition in diabetic rats. Moreover, PAS-stained positive areas in muscle of diabetic rats were significantly increased compared with normal controls. Different intensities of swimming effectively reduced glycogen deposited in skeletal muscle (Figure 2B).
Figure 2.
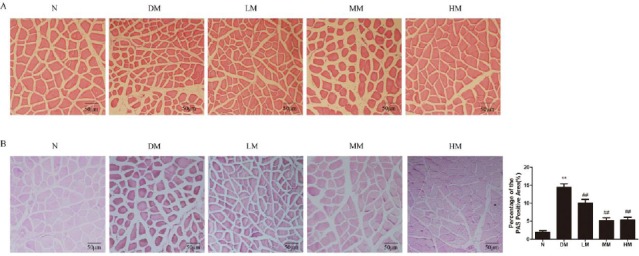
Effects of swim-training on the accumulation of area (A) and glycogen (B) in gastrocnemius of diabetic rats
Notes: N: normal group; DM: diabetic group; LM: diabetic rats with low-intensive swimming, 30 min/d, five times a week; MM: diabetic rats with moderate intensive swimming, 90 min/d, five times a week; HM: diabetic rats with high-intensive swimming, 120 min/d, five times in a week. Data are presented as means±SEM, n= 6. **P <0.01, compared with N; ##P <0.01, compared with DM
Effects of swimming on Wnt/β-catenin/GSK3β signaling in skeletal muscle of diabetic rats
To examine possible signaling mechanism involveed in the skeletal muscle of diabetic rats, the protein and mRNA expressions of Wnt/β-catenin signaling, including Wnt3a, the downstream signaling molecules β-catenin and GSK3β, were evaluated. Diabetic rats revealed elevated mRNA levels of Wnt3a in the skeletal muscle, while the mRNA level of β-catenin and GSK3β did not significantly change. Low, intermediate and high-intensive training of swimming blocked the high levels of Wnt3a (Figure 3A). However, intensive swimming does not obviously alter the mRNA levels of β-catenin and GSK3β induced by diabetic state (Figure 3B, C).
Figure 3.
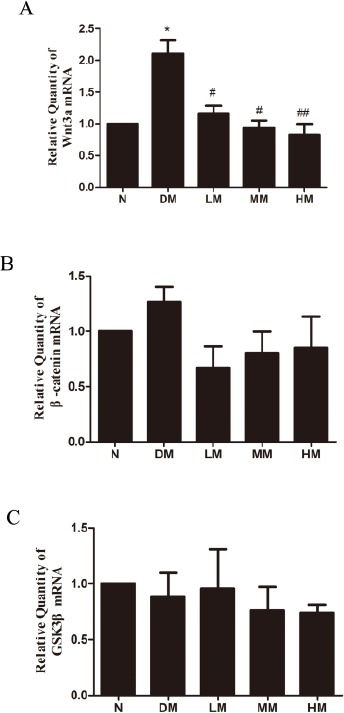
Effects of swimming on mRNA levels of Wnt3a (A), β-catenin (B) and GSK3β (C) in gastrocnemius of diabetic rats through RT-PCR
Notes: N: normal group; DM: diabetic group; LM: diabetic rats with low-intensive swimming, 30 min/d, five times a week; MM: diabetic rats with moderate intensive swimming, 90 min/d, five times a week; HM: diabetic rats with high-intensive swimming, 120 min/d, five times in a week. Data are presented as the means±SEM, n= 6 *P<0.05, compared with N; #P<0.05, ##P<0.01, compared with DM
The levels of Wnt3a and phosphorylation levels of β-catenin (Ser33/37/Thr41) and GSK3β (Ser9) were also examined in the skeletal muscle of diabetic rats. Compared with the normal group, the total Wnt3a and the phosphorylation of β-catenin rose notably in diabetic rats (P<0.01), whereas low, intermediate or high intensive swimming downregulated Wnt3a and its downstream proteins phosphorylation of β-catenin (P<0.05 or P<0.01) (Figure 4A-D). Moreover, the phosphorylation of GSK3β (Ser9) was also decreased in diabetic rats while elevated after swimming training (Figure 4E, F).
Figure 4.
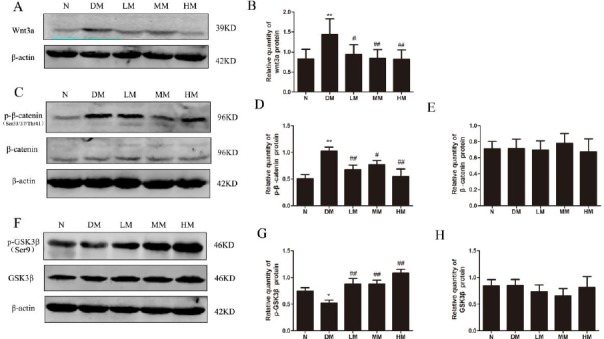
Activation of Wnt/β-catenin signaling in gastrocnemius of dibetic rats.
Notes: (A) Expression of wnt3a in dibetic rats through western blotting. (B) Statistical analysis of Wnt3a in dibetic rats. (C and E) Expression of p-β-catenin and p-GSK3β in dibetic rats through western blotting. (D and F) Relative phosphorylation levels of β-catenin and GSK3β. N: normal group; DM: diabetic group; LM: diabetic rats with low-intensive swimming, 30 min/d, five times a week; MM: diabetic rats with moderate intensive swimming, 90 min/d, five times a week; HM: diabetic rats with high-intensive swimming, 120 min/d, five times a week. Data are presented as the means±SEM, n = 6. *P< 0.05, **P<0.01, compared with N; #P<0.05, ##P <0.01, compared with DM
Discussion
In this study, we demonstrate that swimming training alleviates insulin resistance in type 2 diabetic rats. The beneficial effects are as follows: 1. Attenuation of high fat diet-induced BW gain, reduced levels of FBG, serum FINs release and HOMA-IR, and reversed IR; 2. Decreased blood lipid parameter, including TC, TG, LDL levels, and increased levels of HDL; 3. Reduced glycogen deposited in skeletal muscle and alleviated muscular atrophy in diabetes condition; 4. Inhibition the protein and mRNA expressions of Wnt3a, reduction its downstream proteins phosphorylation of β-catenin, and upregulation of GSK3β phosphorylation.
Insulin resistance is the important pathophysio-logical basis of diseases such as T2DM, hypertension, hyperlipidemia, and obesity (14-16). It has been widely accepted that plasma lipoprotein abnormalities are strongly correlated with the IR in muscle and liver tissues, which include reduced HDL cholesterol, increased TC, LDL particles and TG (17-19). Long-time excessive intake of high fat could lead to massive fat accumulation in fat cells, consequently resulting in fats flowing to other tissues (20). Excessive TG and TC accumulations have been known to cause cells damage and to reduce sensitivity to insulin, eventually leading to diabetes and metabolic syndrome (21). In this study, we fed the rats with high fat and high glucose diet for 8 weeks, after which a low dosage of STZ was administered to induce T2DM. We noticed that high-fat diet induced the increase body weight, lead to massive TC and TG in the blood, serum LDL-C significantly increased simultaneously. We also investigated the effect of exercise on blood lipids and lipoproteins. Comparing with the diabetic ones, the different intensities of swimming significantly reduced body weight, LDL-C, TG and TC and elevated serum HDL-C, which showed that physical training could improve lipid disorder in type 2 diabetic rats. Furthermore, swimming also obviously reduced the levels of FBG, FINs and HOMA-IR in diabetic rats, implying that IR genesis and progress in T2DM rats can be effectively relieved by swimming exercise. This might be due to the fact that swimming could inhibit some reactions that induce the secretion of insulin, or the swimming exercise could improve insulin sensitivity and also lend a hand to glucose homeostasis, therefore ameliorating the symptoms related to T2DM (22-24).
Skeletal muscle serves as the largest reservoir of glycogen and it is responsible for more than 30% of energy consumption (19, 25). So it is the major peripheral tissue of glucolipid metabolism under insulin stimulation. During exercise, glycogen meta-bolism is been regulated to ensure the immediate availability of glycogen from skeletal muscle store (26). In this study, we observed the morphological changes of skeletal muscle by HE staining, and the PAS staining was used to evaluate glycogen deposition in the tissues. In the diabetic model, skeletal muscle fiber cross-sectional area were obviously decreased and showed a marked muscular atrophy. Swimming could increase muscle fiber cross-sectional area and improve the muscular atrophy in diabetic rats. Glycogen is the main components of extracellular matrix (ECM) in skeletal muscle tissue. The accumulation of ECM is linked to overall metabolic dysfunction in humans (27). Further, mechanistic studies in rodent models demonstrate that skeletal muscle ECM expansion is a contributing factor to the development of insulin resistance (28, 29). Animals in the diabetic group showed more glycogen accumulation, while different intensities of swimming effectively reduced glycogen deposited in skeletal muscle. These results suggest that endurance training increased glucose uptake and utilization, and further ameliorate ECM-mediated insulin resistance.
The Canonical Wnt/β-catenin pathway signaling plays a well-established role in the metabolic syndrome, especially T2DM (30). Recent data puts Wnt/β-catenin signaling pathway in a pivotal role in regulating pancreas development as well as islet function, insulin produc-tion and secretion (7, 30). Wnt3a has been demons-trated to mediate increases in growth and glucose stimulated insulin secretion in the pancreatic β-cell (31). β-catenin, as the important downstream target of Wnt signaling pathway, controls the transcription of TCF/LEF and other Wnt target genes. Glycogen synthase kinase 3 (GSK3), a key component of the β-catenin destruction complex, phosphorylates β-catenin, leading to its degradation by the proteasome (32). Our results showed that, in the normal ratskeletal muscle, there was silenced canonical Wnt signaling. The mRNA and protein level of Wnt3a was significantly increased in diabetic rats, however, the expression of GSK3β and β-catenin did not change significantly. Interestingly, the upregulation of Wnt3a led to the decrease of GSK3β phosphorylation activity and inhibition of β-catenin phosphorylation, in which it regulates the expression of insulin in pancreatic β-cells. Our result presented data that justifies the inability of the Wnt3a signaling activation to involve the downstream GSK3β and β-catenin genes, but the magnitude of their phosphorylation could be strong enough to regulate glucose and lipid metabolisms.
It is well known that exercise could regulate the metabolism and transcription of the body’s skeletal muscle tissue. It was also established that GSK3β and β-catenin signaling are regulated by exercise in human skeletal muscle which therefore underscores their significance as mediators of metabolic and transcriptional processes in this tissue (33). Hence, Wnt/β-catenin signaling pathway was selected as the target pathway in this study and we questioned whether exercise could regulate insulin resistance via the Wnt/β-catenin pathway in T2DM. In present study, low, medium and high intensity swimming decreased Wnt3a mRNA levels and protein expression, which subsequently elevated GSK3β phosphorylation and reduced β-catenin phosphorylation levels in skeletal muscle of diabetic rats. Our results indicated that swimming could regulate the activation of Wnt/β-catenin pathway in diabetes condition.
Conclusion
In summary, we showed that swimming may be an adjunct or alternative therapy for insulin resistance and metabolic syndrome in T2DM. Insulin resistance and lipid disorder induced by high-fat diet can be effectively improved during an 8-week swimming exercise. The possible mechanism implied that swimming exercise could reduce Wnt3a mRNA and protein expression in type 2 diabetic rat skeletal muscle, and subsequently upregulate GSK3β phosphorylation activity and inhibit the expression of β-catenin phosphorylation. As a result, the inactivation of Wnt3a/ β-catenin signaling reduces the synthesis of fat, improves lipid metabolism, increases glucose uptake and utilization, and relieves muscular atrophy, then finally improving insulin resistance.
Acknowledgment
This work was supported by Jiangsu Key Laboratory of New Drug Research and Clinical Pharmacy Research Foundation at Xuzhou Medical University [No. KF-XY201409], the Qian Lan project, and was a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Conflict of interest
No potential conflict of interest was reported by the authors. Compliance with ethical standards.
References
- 1.Tangvarasittichai S. Oxidative stress, insulin resistance, dyslipidemia and type 2 diabetes mellitus. World J Diabetes. 2015;6:456–480. doi: 10.4239/wjd.v6.i3.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brøns C, Grunnet LG. Mechanisms in endocrinology:Skeletal muscle lipotoxicity in insulin resistance and type 2 diabetes:a causal mechanism or an innocent bystander? Eur J Endocrinol. 2017;176:R67–R78. doi: 10.1530/EJE-16-0488. [DOI] [PubMed] [Google Scholar]
- 3.Pachori AS, Madan M, Nunez Lopez YO, Yi F, Meyer C, Seyhan AA. Reduced skeletal muscle secreted frizzled-related protein 3 is associated with inflammation and insulin resistance. Obesity. 2017;25:697–703. doi: 10.1002/oby.21787. [DOI] [PubMed] [Google Scholar]
- 4.Xi XH, Wang Y, Li J, Wang FW, Tian GH, Yin MS, Mu YL, Chong ZZ. Activation of Wnt/β-catenin/GSK3βsignaling during the development of diabetic cardiomyopathy. Cardiovasc Pathol. 2015;24:179–186. doi: 10.1016/j.carpath.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 5.Wang J, Zhao J, Zhang J, Luo X, Gao K, Zhang M, Li L, Wang C, Hu D. Association of canonical Wnt/β-Catenin pathway and type 2 diabetes:genetic epidemiological study in Han Chinese. Nutrients. 2015;7:4763–4777. doi: 10.3390/nu7064763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu X, Chen D, Wu Z, Li J, Li J, Zhao H, Liu T. Ghrelin inhibits high glucose-induced 16HBE cells apoptosis by regulating Wnt/β-catenin pathway. Biochem Biophys Res Commun. 2016;477:902–907. doi: 10.1016/j.bbrc.2016.06.156. [DOI] [PubMed] [Google Scholar]
- 7.Figeac F, Uzan B, Faro M, Chelali N, Portha B, Movassat J. Neonatal growth and regeneration of beta-cells are regulated by the Wnt/beta-catenin signaling in normal and diabetic rats. Am J Physiol Endocrinol Metab. 2010;298:E245–256. doi: 10.1152/ajpendo.00538.2009. [DOI] [PubMed] [Google Scholar]
- 8.Rulifson IC, Karnik SK, Heiser PW, ten Berge D, Chen H, Gu X, Taketo MM, Nusse R, Hebrok M, Kim SK. Wnt signaling regulates pancreatic beta cell proliferation. Proc Natl Acad Sci USA. 2007;104:6247–6252. doi: 10.1073/pnas.0701509104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Astrom MB, Feigh M, Pedersen BK. Persistent lowgrade inflammation and regular exercise. Front Biosci (Schol Ed) 2010;2:96–105. doi: 10.2741/s48. [DOI] [PubMed] [Google Scholar]
- 10.Qin L, Yao ZQ, Chang Q, Zhao YL, Liu NN, Zhu XS, Liu QQ, Wang LF, Yang AG, Gao CF, Li JT. Swimming attenuates inflammation, oxidative stress, and apoptosis in a rat model of dextran sulfate sodium-induced chronic colitis. Oncotarget. 2017;8:7391–7404. doi: 10.18632/oncotarget.14080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sigal RJ, Kenny GP, Wasserman DH, Castaneda-Sceppa C. Physical activity/ exercise and type 2 diabetes. Diabetes Care. 2004;27:2518–2539. doi: 10.2337/diacare.27.10.2518. [DOI] [PubMed] [Google Scholar]
- 12.Capaldo B, Gastaldelli A, Antoniello S, Auletta M, Pardo F, Ciociaro D, Guida R, Ferrannini E, Saccà L. Splanchnic and leg substrate exchange after ingestion of a natural mixed meal in humans. Diabetes. 1999;48:958–966. doi: 10.2337/diabetes.48.5.958. [DOI] [PubMed] [Google Scholar]
- 13.DeFronzo RA, Tripathy D. Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care. 2009;32:S157–163. doi: 10.2337/dc09-S302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patel TP, Rawal K, Bagchi AK, Akolkar G, Bernardes N, Dias Dda S, Gupta S, Singal PK. Insulin resistance:an additional risk factor in the pathogenesis of cardiovascular disease in type 2 diabetes. Heart Fail Rev. 2016;21:11–23. doi: 10.1007/s10741-015-9515-6. [DOI] [PubMed] [Google Scholar]
- 15.Hashemipour S, Esmailzadehha N, Hamid H, Oveisi S, Yakhchaliha P, Ziaee A. Association of metabolic syndrome components with insulin resistance in normal weight population:the Qazvin Metabolic Diseases study. J Endocrinol Invest. 2015;38:1111–1115. doi: 10.1007/s40618-015-0302-y. [DOI] [PubMed] [Google Scholar]
- 16.Hayakawa T, Takamura T, Abe T, Kaneko S. Association of the C825T polymorphism of the G-protein beta3 subunit gene with hypertension, obesity, hyperlipidemia, insulin resistance, diabetes, diabetic complications, and diabetic therapies among Japanese. Metabolism. 2007;56:44–48. doi: 10.1016/j.metabol.2006.08.020. [DOI] [PubMed] [Google Scholar]
- 17.Popovic DS, Del Prato S. 1h post-load blood glucose for detection of individuals at increased risk of diabetes and cardiovascular disease. Diabetes Res ClinPract. 2016;120:184–185. doi: 10.1016/j.diabres.2016.07.031. [DOI] [PubMed] [Google Scholar]
- 18.Wang J, Stančáková A, Soininen P, Kangas AJ, Paananen J, Kuusisto J, Ala-Korpela M, Laakso M. Lipoprotein subclass profiles in individuals with varying degrees of glucose tolerance:a population-based study of 9399 Finnish men. J Intern Med. 2012;272:562–572. doi: 10.1111/j.1365-2796.2012.02562.x. [DOI] [PubMed] [Google Scholar]
- 19.Qi J, Yang B, Ren C, Fu J, Zhang J. Swimming exercise alleviated insulin resistance by regulating tripartite motif family protein 72 expression and AKT signal pathway in Sprague-Dawley rats fed with high-fat diet. J Diabetes Res. 2016;2016:1564386. doi: 10.1155/2016/1564386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.da Rocha GL, Crisp AH, de Oliveira MR, da Silva CA, Silva JO, Duarte AC, Sene-Fiorese M, Verlengia R. Effect of high intensity interval and continuous swimming training on body mass adiposity level and serum parameters in high-fat diet fed rats. ScientificWorldJournal. 2016;2016:2194120. doi: 10.1155/2016/2194120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barel M, Perez OA, Giozzet VA, Rafacho A, Bosqueiro JR, do Amaral SL. Exercise training prevents hyperinsulinemia, muscular glycogen loss and muscle atrophy induced by dexamethasone treatment. Eur J ApplPhysiol. 2010;108:999–1007. doi: 10.1007/s00421-009-1272-6. [DOI] [PubMed] [Google Scholar]
- 22.vanDijk JW, Tummers K, Stehouwer CD, Hartgens F, van Loon LJ. Exercise therapy in type 2 diabetes:is daily exercise required to optimize glycemic control? Diabetes Care. 2012;35:948–954. doi: 10.2337/dc11-2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yfanti C, Nielsen AR, Akerström T, Nielsen S, Rose AJ, Richter EA, Lykkesfeldt J, Fischer CP, Pedersen BK. Effect of antioxidant supplementation on insulin sensitivity in response to endurance exercise training. Am J Physiol Endocrinol Metab. 2011;300:E761–770. doi: 10.1152/ajpendo.00207.2010. [DOI] [PubMed] [Google Scholar]
- 24.Palm DC, Rohwer JM, Hofmeyr JH. Regulation of glycogen synthase from mammalian skeletal muscle--a unifying view of allosteric and covalent regulation. FEBS J. 2013;280:2–27. doi: 10.1111/febs.12059. [DOI] [PubMed] [Google Scholar]
- 25.Rudrappa SS, Wilkinson DJ, Greenhaff PL, Smith K, Idris I, Atherton PJ. Human skeletal muscle disuse atrophy:effects on muscle protein synthesis, breakdown, and insulin resistance-aqualitative review. Front Physiol. 2016;7:361. doi: 10.3389/fphys.2016.00361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Onat. A Metabolic syndrome:nature, therapeutic solutions and options. Expert Opin Phar-macother. 2011;12:1887–1900. doi: 10.1517/14656566.2011.585462. [DOI] [PubMed] [Google Scholar]
- 27.Richardson DK, Kashyap S, Bajaj M, Cusi K, Mandarino SJ, Finlayson J, DeFronzo RA, Jenkinson CP, Mandarino LJ. Lipid infusion decreases the expression of nuclear encoded mitochondrial genes and increases the expression of extracellular matrix genes in human skeletal muscle. J Biol Chem. 2005;280:10290–10297. doi: 10.1074/jbc.M408985200. [DOI] [PubMed] [Google Scholar]
- 28.Kang L, Ayala JE, Lee-Young RS, Zhang Z, James FD, Neufer PD, Pozzi A, Zutter MM, Wasserman DH. Diet-induced muscle insulin resistance is associated with extracellular matrix remodeling and interaction with integrin alpha2beta1 in mice. Diabetes. 2011;60:416–426. doi: 10.2337/db10-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kang L, Mokshagundam S, Reuter B, Lark DS, Sneddon CC, Hennayake C, Williams AS, Bracy DP, James FD, Pozzi A, Zent R, Wasserman DH. Integrin-linked kinase in muscle is necessary for the development of insulin resistance in diet-induced obese mice. Diabetes. 2016;65:1590–1600. doi: 10.2337/db15-1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schinner S. Wnt-signalling and the metabolic syndrome. Horm Metab Res. 2009;41:159–163. [Google Scholar]
- 31.Rulifson IC, Karnik SK, Heiser PW, ten Berge D, Chen H, Gu X, Taketo MM, Nusse R, Hebrok M, Kim SK. Wnt signaling regulates pancreatic beta cell proliferation. Proc Natl AcadSci USA. 2007;104:6247–6252. doi: 10.1073/pnas.0701509104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gaudio A, Privitera F, Battaglia K, Torrisi V, Sidoti MH, Pulvirenti I, Canzonieri E, Tringali G, Fiore CE. Sclerostin levels associated with inhibition of the Wnt/β-catenin signaling and reduced bone turnover in type 2 diabetes mellitus. J Clin Endocrinol Metab. 2012;97:3744–3750. doi: 10.1210/jc.2012-1901. [DOI] [PubMed] [Google Scholar]
- 33.Sakamoto K, Arnolds DE, Ekberg I, Thorell A, Goodyear LJ. Exercise regulates Akt and glycogen synthase kinase-3 activities in human skeletal muscle. Biochem Biophys Res Commun. 2004;319:419–425. doi: 10.1016/j.bbrc.2004.05.020. [DOI] [PubMed] [Google Scholar]


