Abstract
Objective(s):
We investigated the relationship between the expression of tumor necrosis factor-inducible gene 6 (TSG-6) with inflammation and integrity of the bladder epithelium in the bladder tissues of patients with bladder pain syndrome/interstitial cystitis (BPS/IC) and the mechanism of action using a rat model of BPS/IC.
Materials and Methods:
Expression of TSG-6 and uroplakin III was determined by immuno- histochemistry of bladder biopsy samples from control human subjects and patients with verified BPS/IC. Our rat model of BPS/IC was employed to measure the perfusion of bladders with hyaluronidase, and assessment of the effect of TSG-6 administration on disease progression. Treatment effects were assessed by measurement of metabolic characteristics, RT-PCR of TGR-6 and interleukin-6, bladder histomorphology, and immunohistochemistry of TGR-6 and uroplakin III.
Results:
The bladders of patients with BPS/IC had lower expression of uroplakin III and higher expression of TSG-6 than controls. Rats treated with hyaluronidase for 1 week developed the typical signs and symptoms of BPS/IC, and rats treated with hyaluronidase for 4 weeks had more serious disease. Administration of TSG-6 reversed the effects of hyaluronidase and protected against disease progression.
Conclusion:
Our results indicate that TSG-6 plays an important role in maintaining the integrity of the bladder epithelial barrier.
Keywords: Bladder pain syndrome/-interstitial cystitis, Immunofluorescence – staining, Interleukin-6, TSG-6, Uroplakin III
Introduction
Bladder pain syndrome/interstitial cystitis (BPS/IC) is a chronic inflammatory disease of the bladder that causes pain and discomfort, and is more common in females (prevalence: 2.7%) than males (prevalence: 1.3%) (1). The pathogenesis of BPS/IC is unclear.; however, potential causes of BPS/IC include infection, increased epithelial permeability, autoimmune disease, and neurogenic inflammation (2), and these can lead to chronic bladder inflammation, infiltration of mononuc-lear inflammatory cells and mast cells, release of inflammatory mediators, and ultimately bladder fibrosis (3). The main treatments include dietary control, oral medications, and intravesical surgery. Although intra-vesical heparin, hyaluronic acid (HA), and lidocaine are the main treatments, these methods generally have poor efficacy (4-9). Thus, it is important to establish new and effective treatments for BPS/IC (3, 10).
Uroplakin III and the glycosaminoglycan (GAG) layer of the bladder epithelium form a protective barrier that prevents entry of toxic substances in healthy subjects, but not in those with BPS/IC (1, 2, 11, 12). Thus, disruption of uroplakin III expression may indicate disrupted integrity of the bladder barrier (13, 14). Lee et al. (15) first identified tumor necrosis factor-inducible gene 6 protein (TSG-6) by screening a cDNA library of skin fibroblasts incubated with tumor necrosis factor-α (TNF-α). TSG-6 is a 35-kDa secreted glycoprotein, which has a LINK domain that binds to hyaluronan, and a complement C1r/C1s, Uegf, Bmp1 (CUB) domain that may mediate interactions with other proteins or carbohydrates (15). Numerous cell types (chondrocytes, synovial cells, and vascular smooth muscle cells) secrete TSG-6 upon exposure to pro-inflammatory cytokines, such as TNF-α and interleukin-1 (IL-1). TSG-6 is expressed in diverse inflammatory conditions, including osteoarthritis, inflammatory bowel disease, systemic lupus erythematosus, bacterial abscess, keratitis, myocardial infarction, and cerebral ischemia (16-19). Other research indicates that TSG-6 has significant protective and anti-inflammatory effects (20). In particular, TSG-6 contributes to remodeling of the extracellular matrix (ECM), synthesis of HA (21), and repair of defective bone and articular cartilage (22).
Recent research has highlighted the important role of TSG-6 as a protein with anti-inflammatory and tissue-protective effects (21, 23). In particular, activation of mesenchymal stem cells leads to production of TSG-6, and this reduces signaling through the Toll-like receptor 2/Nuclear factor κB (TLR2/NF-κB) pathway (21, 24). Other research indicated that injection of recombinant TSG-6 stabilized retinal lesions in a murine model of macular degeneration (25), and that TSG-6 counteracted the matrix metalloproteinase-mediated proteolytic degradation in conjunctivochalasis (26). Moreover, TSG-6 has potential therapeutic value in treatment of other inflammatory disorders (27-29).
The documented therapeutic and anti-inflamma-tory effects of TSG-6 led us to hypothesize that injection of TSG-6 may promote repair of the bladder epithelial barrier in BPS/IC. Thus, this study investigated the expression of TSG-6 and uroplakin III in the bladder tissues of control human subjects and those with BPS/IC, and examined the mechanism of TSG-6 action in an animal model of BPS/IC.
Materials and Methods
Clinical study
All clinical procedures were approved by the Institutional Review Board of the First Affiliated Hospital of Fujian Medical University, China. Thirteen patients with IC were recruited into the patient group, with diagnosis based on previously described criteria (30), and 8 patients without cystitis or lower urinary tract symptoms were recruited into the control group. Bladder biopsies were performed with a cystoscope, and the bladder tissues were fixed in 10% neutral formalin for 24 hr, embedded in paraffin, and then subjected to immunofluorescence staining.
Animal model of BPS/IC
All animal experiments were approved by the Institutional Animal Care and Use Committee of the First Affiliated Hospital of Fujian Medical University. Forty-two healthy adult female Sprague-Dawley rats (250-300 gram) were used. The rats’ bladders were perfused with hyaluronidase (HAase) to establish the BPS/IC model (12), and animals were then anesthetized with isoflurane/oxygen (5% isoflurane for induction, 2% isoflurane for maintenance). Rats were then divided into 6 groups, with 6 rats per group: (i) the Control 1W group received bladder perfusion with 0.5 ml of normal saline (NS) once every 2 days for 1 week; (ii) the Control 4W group received bladder perfusion with NS for 4 weeks; (iii) the HAase 1W group were catheterized with PE50 tubes and received bladder perfusion with 4 mg/ml HAase (0.5 ml) for 30 minutes once every 2 days for 1 week; (iv) the HAase 4W group received bladder perfusion with HAase for 4 weeks; (v) the HAase 1W/TSG-6 group received TSG-6 (100 μg in 0.1 ml, from R&D Systems, Minneapolis MS, USA) and the HAase treatment for 1 week (Lee at al. 2009); and (vi) the HAase 1W/PBS group received phosphate-buffered saline (PBS) (instead of TSG-6) with the HAase treatment for 1 week. TSG-6 and PBS were injected via the tail veins at 6 hr after bladder perfusion with HAase, and injections were performed once every two days for 1 week. At 48 hr after the last treatment, animals were sacrificed for further analysis.
Measurement of rat metabolism
Rat metabolism was measured as previously described (31). In brief, rats in each group were placed into metabolic cages that were connected to a sensor and a computer, and were given ad libitum access to water and food. The urination frequency and total urine volume were recorded every 24 hr.
Histological examination
After sacrifice, rat bladders were collected and divided into two parts along the longitudinal axis. One part was stored at -80 °C and processed for RT-PCR and the other part was fixed in 10% neutral formalin for 48 hr. Tissues were fixed in paraffin and cut into 5-mm transverse sections (3 sections per animal), followed by hematoxylin and eosin (H&E) staining. Five fields were randomly selected from each section for analysis.
The inflammation score and number of inflamma-tory cells per field were assessed as previously described (2). In brief, bladder inflammation was assessed using a 4-point scoring system (0, morpholo-gically unremarkable with no or minimal inflammation or epithelial changes; 1, mild inflammatory infiltrate within the lamina propria with scattered lymphocytes or monocytes, accompanied by mild chronic edema, hemorrhage or urothelial changes [altered epithelial thickness]; 2, moderate inflammatory infiltrate in the lamina propria and focal extension of the inflammation into the muscularis propria, accompanied by moderate chronic edema, hemorrhage, fibrin deposition or urothelial changes; 3, severe inflammation in the lamina propria and muscularis propria in association with other significant findings, such as urothelial ulceration, severe chronic edema, hemorrhage, and fibrin deposition.
RT-PCR
Total RNA was extracted from the bladder tissue with TRIzol® reagent (Takara, Otsu, Shiga, Japan) according to the manufacturer’s instructions, reverse transcribed into cDNA, and then synthesized by reverse transcription (Fermentas, Waltham, MA, USA). The primers used for RT-PCR were as follows: TSG-6, AAGCAGCCAGAAAGATTGGA (forward), TTCGGG-TTGTAGCAATAGGC (reverse); IL-6, TCTGTCTCGAGCCC-ACCAGGA (forward), GTCCCAAGAAGGCAACTGGCTGG (reverse); GAPDH, GCACCGTCAAGGCTGAGAAC (forward), TGGTGAAGACGCCAGTGGA (reverse). The expression of TSG-6 and IL-6 mRNAs is reported relative to that of GAPDH, as calculated by the 2-ΔΔCT method.
Enzyme-linked immunosorbent assay
The IL-6 level in the bladder tissue was measured by ELISA according to manufacturer’s instructions (R&D Systems, Minneapolis, MN, USA). The reported level of IL-6 was normalized to the fresh weight of bladder tissue.
Immunofluorescence staining of the bladder
Immunofluorescence staining was performed with a standard two-step method. An uroplakin III polyclonal antibody (1:50), a TSG-6 polyclonal antibody (1:50) (Santa Cruz Biotechnology, CA, USA), an Alexa Fluor® 594 conjugated rabbit secondary antibody (1:200), an FITC conjugated goat second antibody (1:100), and the ChemMate Envision™ + Detection Kit (DakoCytomation, Dako, Denmark) were used for these experiments. DAPI-positive cells (blue), uroplakin III-positive positive cells (red), and TSG-6 positive cells (green) were observed under a fluorescence microscope within 2 hr, and representa-tive photographs are shown. Image-Pro Plus 6.0 was used to determine the distribution and expression of these proteins, and expression was given as integrated optical density (IOD).
Statistical analysis
Continuous variables are presented as medians and inter-quartile ranges (IQRs). The Mann-Whitney U test was used to compare 2 groups, and the Kruskal- Wallis test was used to compare more than 2 groups. For post hoc analysis, the Mann-Whitney test with the Bonferroni correction was used for multiple comparisons. Statistical analyses were performed with IBM SPSS statistical software version 22 for Windows (IBM Corp., Amonk, NY, USA). A two-tailed P-value less than 0.05 indicated statistical significance.
Results
Comparison of human subjects with and without BPS/IC
Figure 1 shows representative immunohisto-chemical staining images for uroplakin III and TSG-6 in bladder biopsies of control subjects (top) and patients with BPS/IC (bottom). Uroplakin III is expressed on the bladder epithelial cells of control subjects, where it contributes to the protective layer. However, uroplakin III expression is discontinuous in the bladder epithelial cells of patients with IC, suggesting disruption of this protective layer. Similarly, TSG-6 is only expressed on the bladder epithelial cells of control subjects, suggesting that it contributes to the protective layer. However, TSG-6 is expressed in all layers of the bladder in IC subjects, indicative of severe bladder inflammation. These results imply that TSG-6 and uroplakin III are important components of normal bladder epithelial cells, but patients with BPS/IC exhibit disrupted uroplakin III and TSG-6 expression in all layers of the bladder. Table 1 shows a quantitative analysis of these results, based on 8 control patients and 13 BPS/IC patients.
Figure 1.
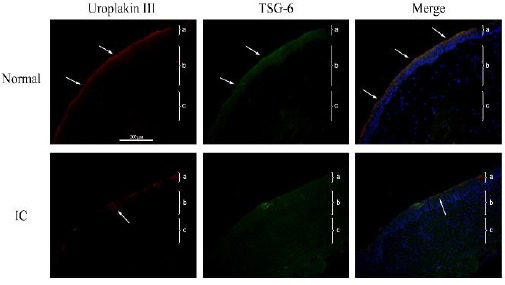
Representative immunofluorescence results (red, uroplakin III; green: tumor necrosis factor-inducible gene 6 (TSG-6); blue: DAPI) of the bladders of control human subjects (top row) or patients with bladder pain syndrome/interstitial cystitis (BPS/IC) (bottom row) (200×). Each tissue layer is labeled with a bracket “}” to indicate its range. a: transitional cell epithelium, b: lamina propria mucosae, c: muscle layer
Top row: note continuous expression of uroplakin III on the surface of the bladder epithelium (arrow), TSG-6 expression in and on the surface of the bladder epithelium (arrow), and significant overlapping expression of these 2 proteins in the merged image. Bottom row: note discontinuous expression of uroplakin III on the surface of the bladder epithelium with a focal deficit (arrow), and higher TSG-6 expression in the bladder epithelium, lamina propria mucosa, and tunica muscularis layers
Table 1.
Expression of uroplakin III and tumor necrosis factor-inducible gene 6 (TSG-6) based on immunohistochemical analysis and integrated optical density (IOD) of bladder biopsies from control human subjects and those with bladder pain syndrome/ interstitial cystitis (BPS/IC). Values show means and inter-quartile ranges (IQRs)
| Control (N=8) | BPS/IC (N=13) | P-value | |
|---|---|---|---|
| Uroplakin III (IOD) | 1579.5(1505-1645) | 573(509-605.5) | <0.001 |
| TSG-6 (IOD) | 1117.5(1016.3-1244.8) | 5466(5337-5580) | <0.001 |
Establishment of a rat model of BPS/IC
We initially validated the rat model of BPS/IC by random allocation of rats into 4 groups (6 rats per group): Control 1W, Control 4W, HAase 1W, and HAase 4W (Table 2). The median urination frequency, inflammation score, number of inflammatory cells, TSG-6 mRNA, IL-6 mRNA, IL-6 protein, and TSG-6 IOD were significantly higher in HAase 1W group than the control 1W group (P=0.002 for all comparisons). In contrast, the median daily urine volume and uroplakin III levels were significantly lower in the HAase 1W group than the control 1W group (P=0.002 for both comparisons). Rats in the control 1W and HAase 1W groups had similar body weight (Table 2).
Table 2.
Comparisons of control rats and rats given hyaluronidase (HAase) at 1 week and 4 weeks. Values show means and inter-quartile ranges (IQRs)
| 1W | 4W | |||||
|---|---|---|---|---|---|---|
| Control (N=6) | HAase (N=6) | P-value | Control (N=6) | HAase (N=6) | P-value | |
| Weight(g) | 271.5 (257-279.3) | 268.5 (258.8-286) | 0.818 | 268 (254.5-275.5) | 268 (260.8-274.5) | 0.937 |
| Urination frequency (per day) | 11.5 (10.5-13) | 21.5 (19.8-23.5) | 0.002 | 12.5 (11.8-13.3) | 38.5 (34.5-42)* | 0.002 |
| Daily urine volume (ml) | 1.6 (1.5-1.9) | 0.8 (0.7-0.9) | 0.002 | 1.6 (1.5-1.8) | 0.3 (0.2-0.4)* | 0.002 |
| Inflammation score | 0 (0-0.3) | 2(1.8-2) | 0.002 | 0 (0-1) | 3(2.8-3)* | 0.002 |
| Number of inflammatory cells | 3 (2-5) | 181.5 (171-217.8) | 0.002 | 4 (2-7) | - | - |
| TSG-6 mRNA | 0.2 (0.2-0.3) | 0.6 (0.6-0.7) | 0.002 | 0.2 (0.2-0.3) | 0.8 (0.7-0.8)* | 0.002 |
| IL-6 mRNA | 0.4 (0.4-0.5) | 0.7 (0.6-0.7) | 0.002 | 0.5 (0.4-0.5) | 0.9 (0.8-0.9)* | 0.002 |
| IL-6 protein | 0.5 (0.5-0.6) | 1.3 (1.2-1.5) | 0.002 | 0.5 (0.5-0.6) | 2.1 (1.9-2.2)* | 0.002 |
| Uroplakin III(IOD) | 690 (659.8-720.8) | 83.5 (75-89.3) | 0.002 | 688.5 (672.3-722.3) | 22.5 (20.3-28)* | 0.002 |
| TSG-6 (IOD) | 1094.5 (998.8-1157.3) | 12932.5 (12141-13291.8) | 0.002 | 1148 (998.3-1208.5) | 21443 (19973.3-22330.8)* | 0.002 |
P<0.05, significantly different from the HAase 1W group. TSG-6: tumor necrosis factor-inducible gene 6
The median urination frequency, inflammation score, TSG-6 mRNA, IL-6 mRNA, IL-6 protein, and TSG-6 IOD were significantly higher in the HAase 4W group than in the control 4W group (P= 0.002 for all comparisons). The median daily urine volume and uroplakin III IOD were significantly lower in the HAase 4W group than in the control 4W group (P= 0.002 for both comparisons). Rats in the control 4W and HAase 4W groups had similar body weight (Table 2).
These data validated our rat model of BPS/IC, and indicated that signs and symptoms of BPS/IC are evident after 1 week, and that rats in the HAase 4W group had more severe disease than those in the HAase 1W group.
TSG-6 ameliorates HAase-induced BPS/IC symptoms
We also examined the effect of TSG-6 administration in our rat model of BPS/IC by comparison of the control 1W, HAase 1W/TSG-6, and HAase 1W/PBS groups (Table 3). The median inflammation score, TSG-6 mRNA, and TSG-6 IOD were significantly greater in the HAase 1W/TSG-6 than in the control 1W group.
Table 3.
Comparisons of rats in the control 1W, HAase 1W/TSG-6, and HAase 1W/PBS groups
| Control 1W (N=6) | HAase 1W/TSG-6 (N=6) | HAase 1W/PBS (N=6) | P-value | |
|---|---|---|---|---|
| Weight(g) | 271.5 (257-279.3) | 265.5 (254.8-277) | 267 (258.8-276.8) | 0.884 |
| Urination frequency (per day) | 11.5 (10.5-13) | 14.5 (13.8-15.3) | 22 (20.3-26.5)* | 0.001 |
| Daily urine volume (ml) | 1.6 (1.5-1.9) | 1.6 (1.4-1.7) | 0.9 (0.7-0.9)*† | 0.003 |
| Inflammation score | 0 (0-0.3) | 1 (1-1.3)* | 1.5 (1-2)* | 0.004 |
| Number of inflammatory cells | 3 (2-5) | 77.5 (64.3-112.5) | 184 (175.3-205.3)* | <0.001 |
| TSG-6 mRNA | 0.2 (0.2-0.3) | 0.6 (0.6-0.7)* | 0.5 (0.5-0.6) | 0.001 |
| IL-6 mRNA | 0.4 (0.4-0.5) | 0.6 (0.5-0.6) | 0.6 (0.5-0.8)* | 0.005 |
| IL-6 protein | 0.5 (0.5-0.6) | 0.9 (0.8-1.1) | 1.3 (1.2-1.4)* | 0.001 |
| Uroplakin III (IOD) | 690 (659.8-720.8) | 676.5 (651.8-703.8) | 80.5 (74.5-87.3)*† | 0.003 |
| TSG-6 (IOD) | 1094.5 (998.8-1157.3) | 14110.5 (13748.3-14362.3)* | 12781 (12040-13239.8) | 0.001 |
P<0.05, significantly different with control 1W
P<0.05, significantly different with HAase 1W/TSG-6. TSG-6: tumor necrosis factor-inducible gene 6, HAase: hyaluronidase, PBS: phosphate-buffered saline
The median urination frequency, inflammation score, number of inflammatory cells, IL-6 mRNA, and IL-6 protein were significantly greater in the HAase 1W/PBS group than in the control 1W group. On the other hand, the median daily urine volume and uroplakin III level were significantly lower in the HAase 1W/PBS group than in the control 1W group.
Effect of HAase and TSG-6 on bladder histology
Figure 2 shows representative H&E staining images of the bladders of rats in the control 1W, HAase 1W, HAase 1W/TSG-6, and HAase 1W/PBS groups. In agreement with the results above, the bladders of the HAase 1W and HAase 1W/PBS groups had definite signs of pathology, but the bladders of the control 1W and HAase 1W/TSG-6 groups had more normal histology. However, there is no focal deficit in the epithelium of the control 1W and HAase 1W/PBS groups, and there is only moderate cell infiltration in the submucosa in the HAase 1/PBS group, reflecting an early stage of disease in these rats.
Figure 2.
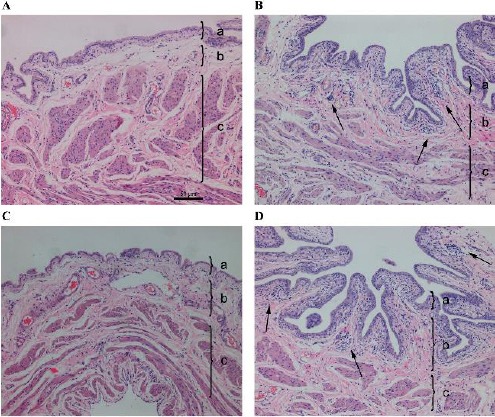
Representative hematoxylin & eosin staining results of the bladders of rats in the control 1W group (A), hyaluronidase (HAase) 1W group (B), HAase 1W/ tumor necrosis factor-inducible gene 6 (TSG-6) group (C), and HAase 1W/PBS group (D) (100×). Each tissue layer is labeled with a bracket “}” to indicate its range. a: transitional cell epithelium, b: lamina propria mucosae, c: muscle layer. Note the focal deficit of bladder epithelium and infiltration of inflammatory cells in the submucosa and muscularis (arrow in B) and infiltration of inflammatory cells (arrow in D)
Figure 3 shows representative immunohistochemical images of uroplakin III (red) and TSG-6 (green) in the bladders of rats in the same 4 groups. In the control 1W rats (top row), TSG-6 and uroplakin III were mainly expressed in the GAG layer of the bladder epithelium, and these proteins form a barrier that protects the bladder epithelium; however, we observed no TSG-6 expression in the lamina propria or tunica muscularis. In the HAase 1W rats (second row), uroplakin III expression was much weaker and
Figure 3.
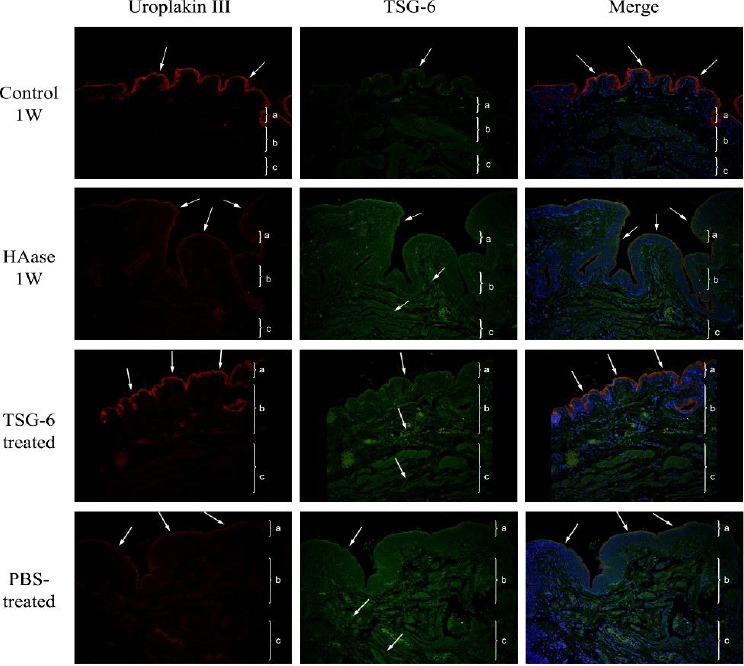
Representative immunofluorescence results (red, uroplakin III; green: tumor necrosis factor-inducible gene 6 (TSG-6); blue: DAPI) of the bladders of rats in the untreated control group (top row), control 1W group (second row), hyaluronidase (HAase) 1W group (third row), HAase 1W/TSG-6 group (fourth row), and HAase 1W/PBS group (bottom row) (200×). Each tissue layer is labeled with a bracket “}” to indicate its range. a: transitional cell epithelium, b: lamina propria mucosae, c: muscle layer. Top row: Note uroplakin III expression on the surface of the bladder epithelium and weak TSG-6 expression. In the merged image, there is co-expression of uroplakin III and TSG-6 on the surface of the bladder epithelium (arrows). Second row: note reduced uroplakin III expression on the surface of the bladder epithelium, and increased expression of TSG-6 in the bladder epithelium, lamina propria mucosa, and tunica muscularis (arrows). Third row: note increased and more continuous expression of uroplakin III (arrow), increased TSG-6 expression in the full-thickness bladder wall (arrow), and co-expression of uroplakin III and TSG-6 on the surface of the bladder epithelium with a repaired bladder barrier in the merged image (arrow). Bottom row: note a focal deficit of uroplakin III expression (arrow) and increased TSG-6 expression in the full-thickness of the bladder wall (arrow)
TSG-6 was strongly expressed in the bladder epithelium, submucosal layer, and muscularis layer, but not on the surface of the bladder epithelium. In the HAase 1W/TSG-6 rats (third row), there was high expression of uroplakin III on the surface of the bladder epithelium and high expression of TSG-6 in the full-thickness of the bladder wall. In the HAase 1W/PBS rats (bottom row), expression of uroplakin III and TSG-6 were similar to that observed in the HAase 1W rats.
Discussion
Our results showed expression of TSG-6 on the GAG layer of the bladder epithelium of healthy rats and control human subjects, consistent with the view that this protein has a role in maintaining the integrity of the bladder epithelium. In patients and rats with BPS/IC, TSG-6 expression was elevated and was also present in the bladder epithelium, submucosa, and tunica muscularis. However, TSG-6 expression was reduced on the surface of the bladder epithelium. This overall increase of TSG-6 expression may be a protective response to BPS/IC. In agreement with this interpretation, we also found that TSG-6 administra-tion had an anti-inflammatory effect in the bladders of rats with BPS/IC; in addition, prevented damage of the bladder epithelium barrier, and normalized urinary metabolism.
Two recent review articles of TSG-6 (19, 20) reported abundant evidence for upregulation of this protein following inflammation, but no previous studies reported expression of TSG-6 in healthy bladder tissues. Thus, a novel finding of our study is that TSG-6 expression occurred on the surface of the bladder epithelium of healthy rats and of control human subjects. Based on our experience, improper preparation of tissue samples may damage the GAG layer of the bladder epithelium, and this could result in non-detection of TSG-6. This may explain, in part, the reasons for the variable results of previous studies (16, 27, 31). In the healthy rat bladder, TSG-6 and uroplakin III had similar cellular distributions on the surface of bladder epithelium, although we did not assess their precise subcellular distributions. The GAG layer is an important physical barrier (32, 33). Thus, we speculate that toxic substances in the urine stimulate the production of TSG-6, which interacts with components of the GAG (HA, heparin, and chondroitin sulfate), and this helps to restore the bladder function. In control human subjects, TSG-6 is also found in the GAG layer of the bladder epithelium, in contrast to the expression pattern in rats. Nonetheless, TSG-6 also has a role in maintenance of the bladder epithelial barrier in humans.
The important role of TSG-6 in maintaining the bladder epithelium led to our hypothesis that administration of TSG-6 might reverse the damage from BPS/IC. Previous studies indicated that TSG-6 can cross-link with the components of the ECM (including HA, heparin, and chondroitin sulfate) to stabilize the GAG layer, and is a potential regulator of HA synthesis (21). Recent clinical studies have used bladder perfusion with exogenous HA to repair the GAG layers of patients with BPS/IC (3, 10). The present study showed that the bladders of rats with BPS/IC had disrupted uroplakin III expression, and that TSG-6 therapy normalized expression of this protein, repaired the structure of bladder epithelium, and restored bladder function.
TSG-6 expression is increased in diverse acute and chronic inflammatory conditions, and this appears to be a protective response (19, 20). Our clinical results indicated that controls had TSG-6 expression on the bladder epithelia, but not on the submucosa; however, in the presence of BPS/IC, TSG-6 expression was significantly increased in the bladder epithelia, submucosa, and tunica muscularis. TSG-6 expression was also elevated in rats with BPS/IC. These observations are consistent with the observation that TSG-6 expression is elevated in osteoarthritis (22), and suggest that TSG-6 may be useful as a marker of inflammation in BPS/IC. Although TSG-6 is expressed in the bladder epithelia, submucosa, and tunica muscularis of rats with BPS/IC, expression was low on the surface of the bladder epithelium. These results are consistent with our clinical findings. The low expression of TSG-6 in the bladder epithelia of BPS/IC rats may be due to HAase-induced degradation of the GAG layer, and the subsequent disruption of the bladder barrier. This also supports the view that TSG-6 is important in maintenance of the GAG layer.
Previous studies have confirmed the therapeutic efficacy of TSG-6 in several models of inflammatory diseases. For example, in rats with corneal injury, injection of the aqueous chamber with TSG-6 improved corneal transparency and reduced angiogenesis (27). In mice with lipopolysaccharide-induced lung injury, administration of TSG-6 attenuated lung injury (34). In mice with myocardial infarction, intravenous injection of TSG-6 reduced the infarct area and improved cardiac function (16). Previous studies of BPS/IC indicated the presence of hyperplastic mast cells (31), and that TSG-6 may exert anti-inflammatory effects by inhibiting the tryptase activity of these cells (35). In the present study, rats with BPS/IC had bladder inflammation, altered urination patterns, and related symptoms, and TSG-6 therapy led to significant resolution of these histological and metabolic changes. Our previous study (2) showed that IL-6 expression in the bladder of rats with HAase-induced cystitis correlated with disease severity, suggesting that IL-6 may be a useful marker for chronic cystitis (2). In the present study, similar to that reported in other inflammatory disease models (27), bladder expression of IL-6 declined significantly after TSG-6 therapy, confirming the anti-inflammatory effects of this protein.
A limitation of this study is that we do not have data for mice given 4 weeks of HAase + TSG, so cannot assess the effect of TSG-6 in mice with more advanced disease. Another limitation is that the control group of human subjects consisted of only 8 patients in whom there was no diagnosis of BPS/IC.
Conclusion
Our results indicate that TSG-6 plays an important role in the integrity of the bladder epithelial barrier. BPS/IC leads to disruption of this barrier, and increased TSG-6 expression throughout the bladder is a protective response. This increased TSG-6 expression appears to have anti-inflammatory effects, so this protein may be a useful marker of inflammation in BPS/IC. TSG-6 administration ameliorated the HAase-induced cystitis and urination behavior in rats, and repaired the bladder barrier. These results suggest the potential of using TSG-6 for therapy of BPS/IC.
Acknowledgment
This study was supported by the Foundation for Young Scientists of the Education Bureau of Fujian Province, China (Grant No.:JA14148).
Conflict of interest
The authors declare that there are no conflicts of interest.
References
- 1.Nordling J, Fall M, Hanno P. Global concepts of bladder pain syndrome (interstitial cystitis) World J Urol. 2012;30:457–464. doi: 10.1007/s00345-011-0785-x. [DOI] [PubMed] [Google Scholar]
- 2.Lv YS, Yao YS, Lin ME, Rong L, Deng BH, Huang J, et al. Interleukin-6 levels in female rats with protamine sulfate-induced chronic cystitis treated with hyaluronic acid. Int J Urol. 2013;20:1017–1022. doi: 10.1111/iju.12090. [DOI] [PubMed] [Google Scholar]
- 3.Bassi PF, Costantini E, Foley S, Palea S. Glycosaminoglycan therapy for bladder diseases:Emerging new treatments. Eur Urol Suppl. 2011;10:451–459. [Google Scholar]
- 4.Anger JT, Zabihi N, Clemens JQ, Payne CK, Saigal CS, Rodriguez LV. Treatment choice, duration, and cost in patients with interstitial cystitis and painful bladder syndrome. Int Urogynecol J. 2011;22:395–400. doi: 10.1007/s00192-010-1252-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gülpınar O, Kayis A, Suer E, Gokce MI, Guclu AG, Arikan N. Clinical comparision of intravesical hyaluronic acid and hyaluronic acid-chondroitin sulphate therapy for patients with bladder pain syndrome/interstitital cystitis. Can Urol Assoc J. 2014;8:E610–E614. doi: 10.5489/cuaj.2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lv YS, Zhou HL, Mao HP, Gao R, Wang YD, Xue XY. Intravesical hyaluronic acid and alkalinized lidocaine for the treatment of severe painful bladder syndrome/interstitial cystitis. Int Urogynecol J. 2012;23:1715–1720. doi: 10.1007/s00192-012-1802-3. [DOI] [PubMed] [Google Scholar]
- 7.Nomiya A, Naruse T, Niimi A, Nishimatsu H, Kume H, Igawa Y, et al. On- and post-treatment symptom relief by repeated instillations of heparin and alkalized lidocaine in interstitial cystitis. Int J Urol. 2013;20:1118–1122. doi: 10.1111/iju.12120. [DOI] [PubMed] [Google Scholar]
- 8.Riedl C, Engelhardt P, Schwarz B. Treatment costs of bladder pain syndrome/interstitial cystitis in Austria:a pharmacoeconomic approach following current guidelines. Clin Drug Investig. 2013;33:737–742. doi: 10.1007/s40261-013-0119-4. [DOI] [PubMed] [Google Scholar]
- 9.Wu EQ, Birnbaum H, Mareva M, Parece A, Huang Z, Mallett D, et al. Interstitial Cystitis:Cost, treatment and co-morbidities in an employed population. Pharmacoeconomics. 2006;24:55–65. doi: 10.2165/00019053-200624010-00005. [DOI] [PubMed] [Google Scholar]
- 10.Madersbacher H, van Ophoven A, van Kerrebroeck PE. GAG layer replenishment therapy for chronic forms of cystitis with intravesical glycosaminoglycans--a review. Neurourol Urodyn. 2013;32:9–18. doi: 10.1002/nau.22256. [DOI] [PubMed] [Google Scholar]
- 11.GuhaSarkar S, Banerjee R. Intravesical drug delivery:Challenges, current status, opportunities and novel strategies. J Control Release. 2010;148:147–159. doi: 10.1016/j.jconrel.2010.08.031. [DOI] [PubMed] [Google Scholar]
- 12.Lv YS, Yao YS, Rong L, Lin ME, Deng BH, Xie Y, et al. Intravesical hyaluronidase causes chronic cystitis in a rat model:a potential model of bladder pain syndrome/interstitial cystitis. Int J Urol. 2014;21:601–607. doi: 10.1111/iju.12358. [DOI] [PubMed] [Google Scholar]
- 13.Slobodov G, Feloney M, Gran C, Kyker KD, Hurst RE, Culkin DJ. Abnormal expression of molecular markers for bladder impermeability and differentiation in the urothelium of patients with interstitial cystitis. J Urol. 2004;171:1554–1558. doi: 10.1097/01.ju.0000118938.09119.a5. [DOI] [PubMed] [Google Scholar]
- 14.Keay S, Leitzell S, Ochrzcin A, Clements G, Zhan M, Johnson D. A mouse model for interstitial cystitis/painful bladder syndrome based on APF inhibition of bladder epithelial repair:a pilot study. BMC Urol. 2012;12:17. doi: 10.1186/1471-2490-12-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee TH, Wisniewski HG, Vilcek J. A novel secretory tumor necrosis factor-inducible protein (TSG-6) is a member of the family of hyaluronate binding proteins, closely related to the adhesion receptor CD44. J Cell Biol. 1992;116:545–557. doi: 10.1083/jcb.116.2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee RH, Pulin AA, Seo MJ, Kota DJ, Ylostalo J, Larson BL, et al. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009;5:54–63. doi: 10.1016/j.stem.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roddy GW, Oh JY, Lee RH, Bartosh TJ, Ylostalo J, Coble K, et al. Action at a distance:systemically administered adult stem/progenitor cells (MSCs) reduce inflammatory damage to the cornea without engraftment and primarily by secretion of TNF-alpha stimulated gene/protein 6. Stem Cells. 2011;29:1572–1579. doi: 10.1002/stem.708. [DOI] [PubMed] [Google Scholar]
- 18.Lin QM, Zhao S, Zhou LL, Fang XS, Fu Y, Huang ZT. Mesenchymal stem cells transplantation suppresses inflammatory responses in global cerebral ischemia:contribution of TNF-alpha-induced protein 6. Acta Pharmacol Sin. 2013;34:784–792. doi: 10.1038/aps.2012.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Milner CM, Day AJ. TSG-6:a multifunctional protein associated with inflammation. J Cell Sci. 2003;116:1863–1873. doi: 10.1242/jcs.00407. [DOI] [PubMed] [Google Scholar]
- 20.Prockop DJ. Concise review:two negative feedback loops place mesenchymal stem/stromal cells at the center of early regulators of inflammation. Stem Cells. 2013;31:2042–2046. doi: 10.1002/stem.1400. [DOI] [PubMed] [Google Scholar]
- 21.Lauer ME, Cheng G, Swaidani S, Aronica MA, Weigel PH, Hascall VC. Tumor necrosis factor-stimulated gene-6 (TSG-6) amplifies hyaluronan synthesis by airway smooth muscle cells. J Biol Chem. 2013;288:423–431. doi: 10.1074/jbc.M112.389882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bárdos T, Kamath RV, Mikecz K, Glant TT. Anti-inflammatory and chondroprotective effect of TSG-6 (tumor necrosis factor-alpha-stimulated gene-6) in murine models of experimental arthritis. Am J Pathol. 2001;159:1711–1721. doi: 10.1016/s0002-9440(10)63018-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Milner CM, Higman VA, Day AJ. TSG-6:a pluripotent inflammatory mediator? Biochem Soc Trans. 2006;34:446–450. doi: 10.1042/BST0340446. [DOI] [PubMed] [Google Scholar]
- 24.Liu Y, Zhang R, Yan K, Chen F, Huang W, Lv B, et al. Mesenchymal stem cells inhibit lipopolysaccharide-induced inflammatory responses of BV2 microglial cells through TSG-6. J Neuroinflammation. 2014;11:135. doi: 10.1186/1742-2094-11-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tuo J, Cao X, Shen D, Wang Y, Zhang J, Oh JY, et al. Anti-inflammatory recombinant TSG-6 stabilizes the progression of focal retinal degeneration in a murine model. J Neuroinflammation. 2012;9:59. doi: 10.1186/1742-2094-9-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guo P, Zhang SZ, He H, Zhu YT, Tseng SC. TSG-6 controls transcription and activation of matrix metalloproteinase 1 in conjunctivochalasis. Invest Ophthalmol Vis Sci. 2012;53:1372–1380. doi: 10.1167/iovs.11-8738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oh JY, Roddy GW, Choi H, Lee RH, Ylöstalo JH, Rosa RH Jr, et al. Anti-inflammatory protein TSG-6 reduces inflammatory damage to the cornea following chemical and mechanical injury. Proc Natl Acad Sci USA. 2010;107:16875–16880. doi: 10.1073/pnas.1012451107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Watanabe J, Shetty AK, Hattiangady B, Kim DK, Foraker JE, Nishida H, et al. Administration of TSG-6 improves memory after traumatic brain injury in mice. Neurobiol Dis. 2013;59:86–99. doi: 10.1016/j.nbd.2013.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Foskett AM, Bazhanov N, Ti X, Tiblow A, Bartosh TJ, Prockop DJ. Phase-directed therapy:TSG-6 targeted to early inflammation improves bleomycin-injured lungs. Am J Physiol Lung Cell Mol Physiol. 2014;306:L120–L131. doi: 10.1152/ajplung.00240.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Homma Y, Ueda T, Tomoe H, Lin AT, Kuo HC, Lee MH, et al. Clinical guidelines for interstitial cystitis and hypersensitive bladder syndrome. Int J Urol. 2009;16:597–615. doi: 10.1111/j.1442-2042.2009.02326.x. [DOI] [PubMed] [Google Scholar]
- 31.Kitta T, Tanaka H, Mitsui T, Moriya K, Nonomura K. Type 4 phosphodiesterase inhibitor suppresses experimental bladder inflammation. BJU Int. 2008;102:1472–1476. doi: 10.1111/j.1464-410X.2008.07662.x. [DOI] [PubMed] [Google Scholar]
- 32.Rajasekaran M, Stein P, Parsons CL. Toxic factors in human urine that injure urothelium. Int J Urol. 2006;13:409–414. doi: 10.1111/j.1442-2042.2006.01301.x. [DOI] [PubMed] [Google Scholar]
- 33.Soler R, Bruschini H, Freire MP, Alves MT, Srougi M, Ortiz V. Urine is necessary to provoke bladder inflammation in protamine sulfate induced urothelial injury. J Urol. 2008;180:1527–1531. doi: 10.1016/j.juro.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 34.Danchuk S, Ylostalo JH, Hossain F, Sorge R, Ramsey A, Bonvillain RW, et al. Human multipotent stromal cells attenuate lipopolysaccharide-induced acute lung injury in mice via secretion of tumor necrosis factor-alpha-induced protein 6. Stem Cell Res Ther. 2011;2:27. doi: 10.1186/scrt68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nagyeri G, Radacs M, Ghassemi-Nejad S, Tryniszewska B, Olasz K, Hutas G, et al. TSG-6 protein, a negative regulator of inflammatory arthritis, forms a ternary complex with murine mast cell tryptases and heparin. J Biol Chem. 2011;286:23559–23569. doi: 10.1074/jbc.M111.222026. [DOI] [PMC free article] [PubMed] [Google Scholar]


